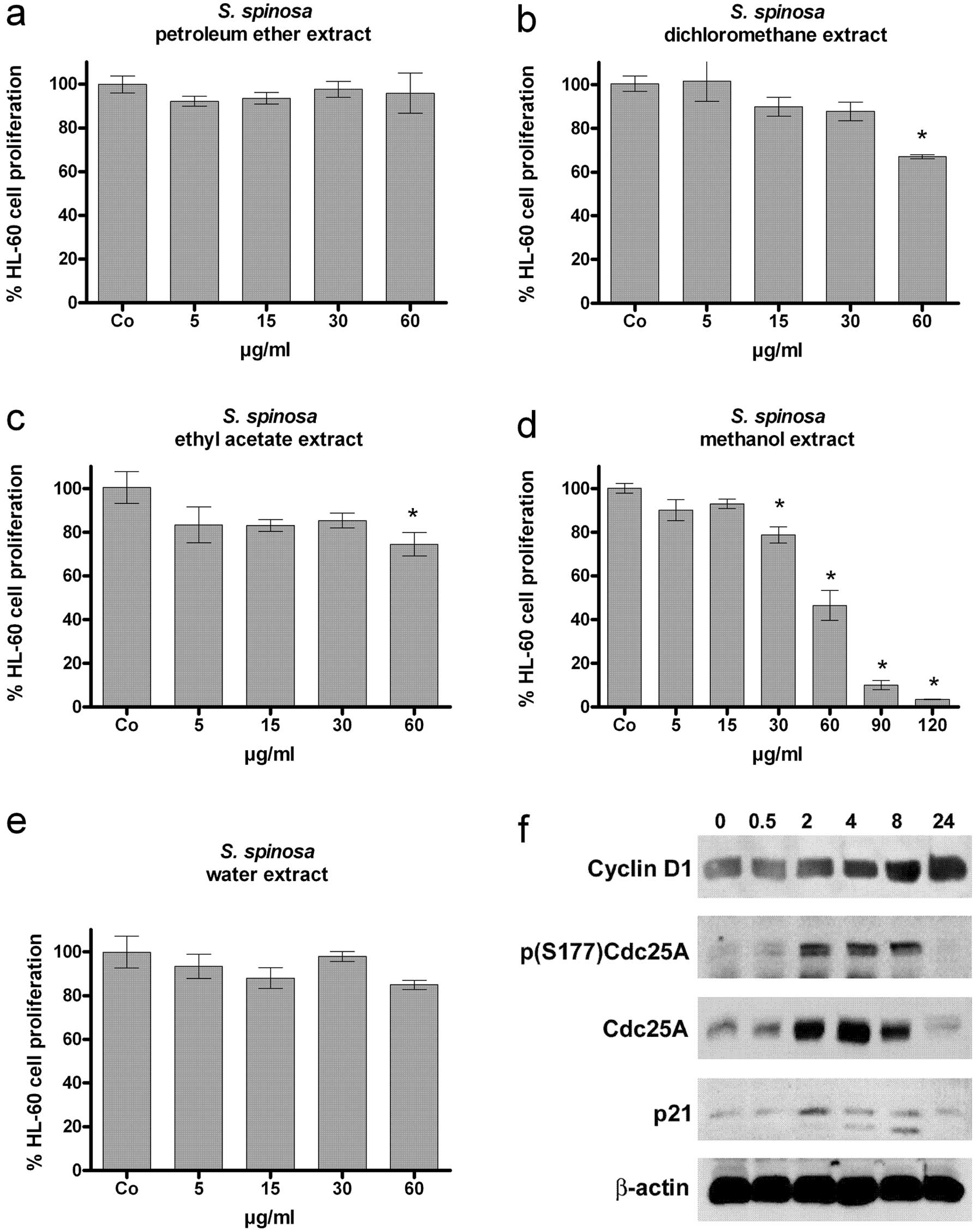
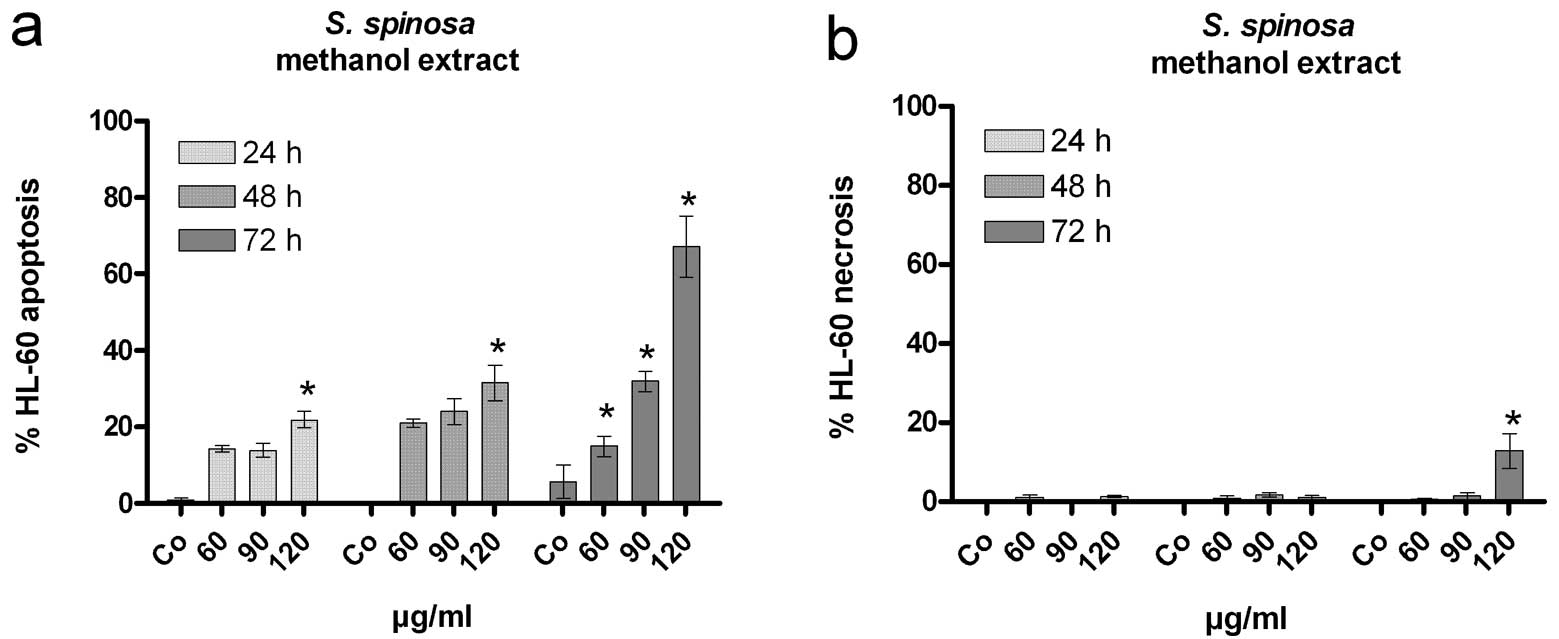
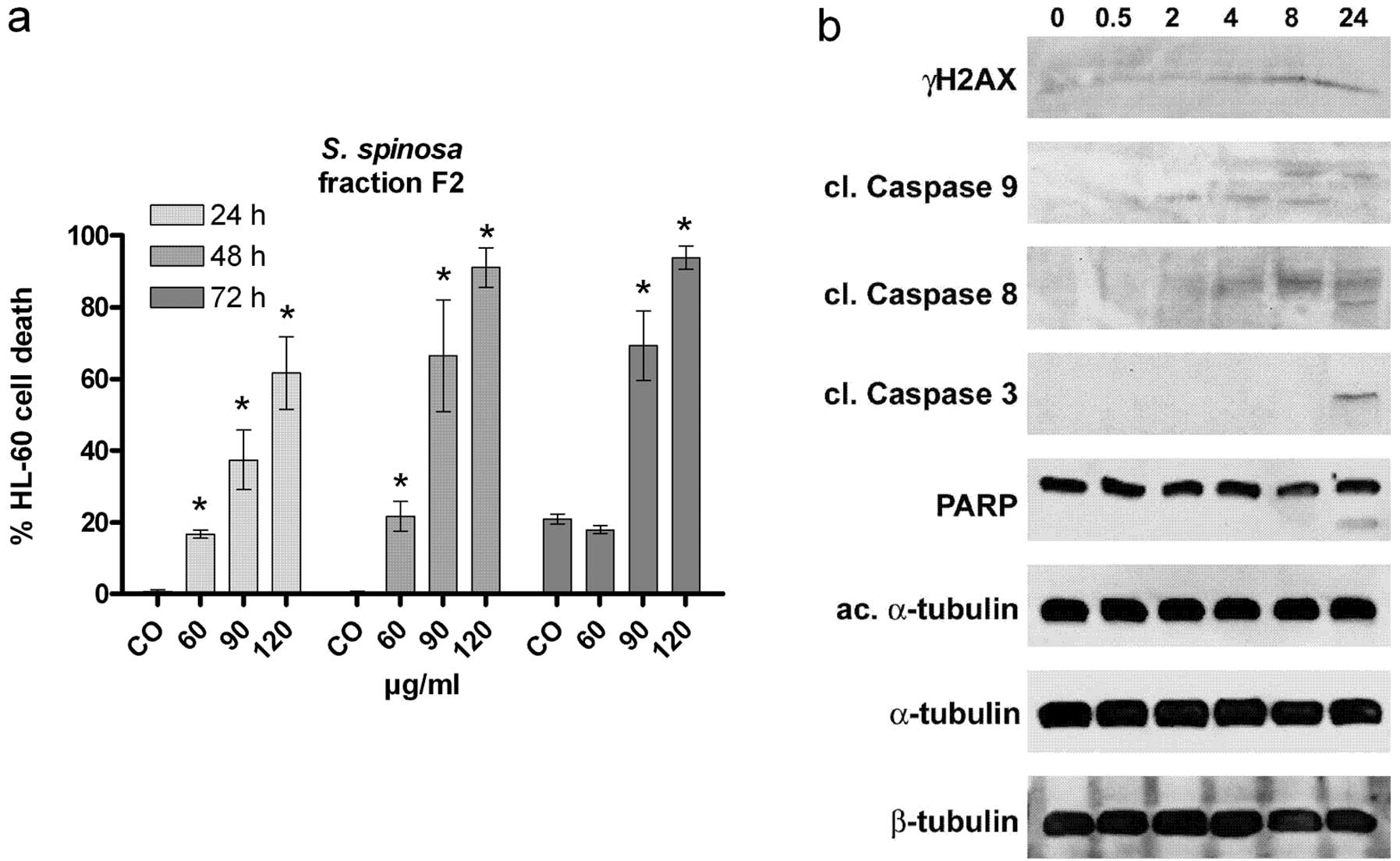
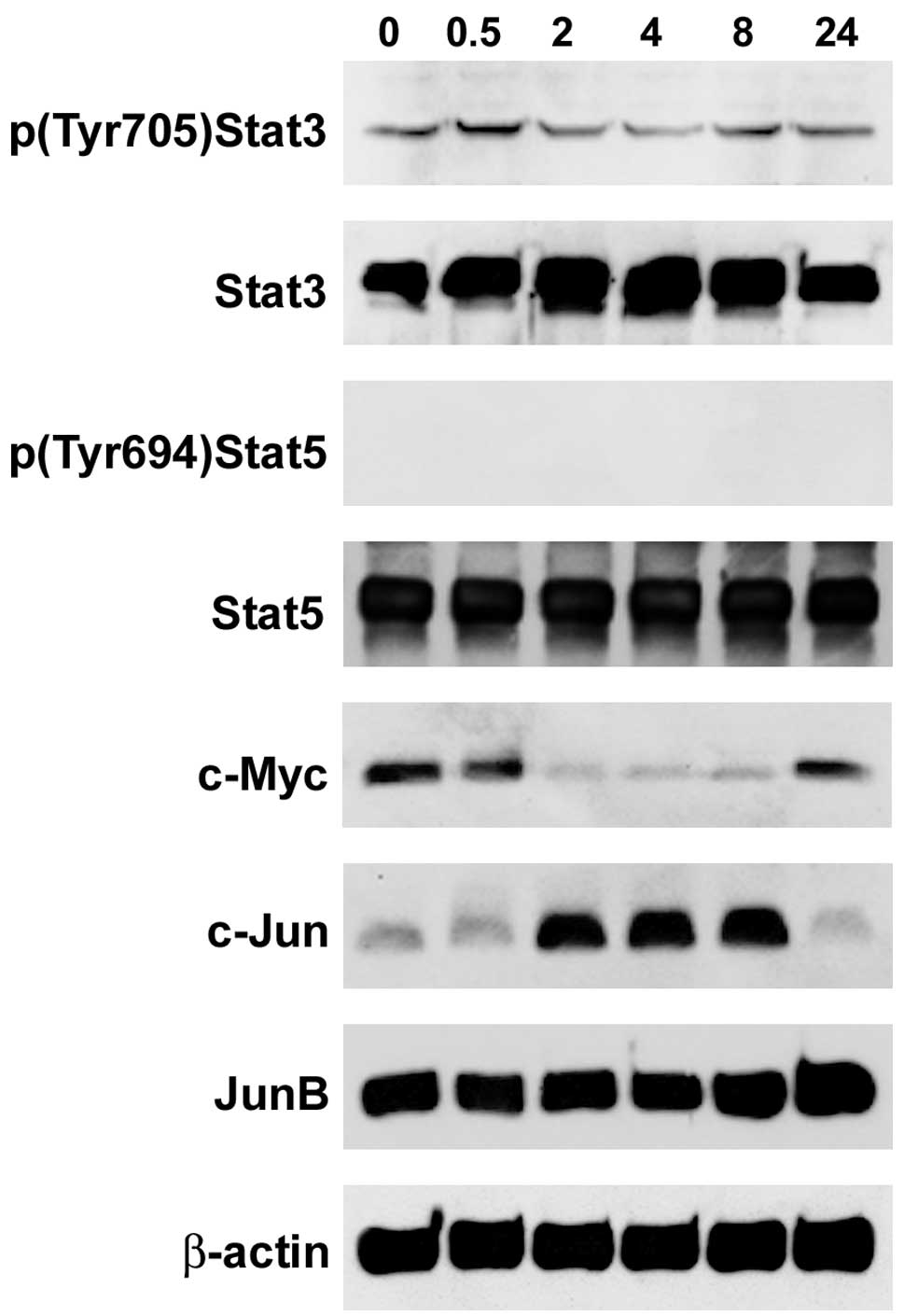
|
1.
|
Pecorino L: Molecular Biology of Cancer.
2nd edition. Oxford University Press; New York, NY: 2008
|
|
2.
|
Stewart BW and Kleihues P: World Cancer
Report. IARC Press; Lyon: 2003
|
|
3.
|
Cragg GM and Newman DJ: Plants as a source
of anti-cancer agents. J Ethnopharmacol. 100:72–79. 2005.PubMed/NCBI
|
|
4.
|
Fabricant DS and Farnsworth NR: The value
of plants used in traditional medicine for drug discovery. Environ
Health Perspect. 109:69–75. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Rastogi RP and Dhawan BN: Research on
medicinal plants at the Central Drug Research Institute, Lucknow
(India). Indian J Med Res. 76:27–45. 1982.PubMed/NCBI
|
|
6.
|
Cseke LJ: Natural products from plants.
2nd edition. CRC Press Taylor and Francis; Boca Raton, FL: 2006
|
|
7.
|
Shoeb M: Anticancer agents from medicinal
plants. Bangladesh J Pharmacol. 1:35–41. 2006.
|
|
8.
|
Kundu JK and Surh YJ: Inflammation:
gearing the journey to cancer. Mutat Res. 659:15–30. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Taylor L: The Healing Power of Rainforest
Herbs: A Guide to Understanding and Using Herbal Medicinals. Square
One Publishers; New York, NY: 2005
|
|
10.
|
Caceres A, Cano O, Samayoa B and Aguilar
L: Plants used in Guatemala for the treatment of gastrointestinal
disorders. 1 Screening of 84 plants against enterobacteria. J
Ethnopharmacol. 30:55–73. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Jiang J and Xu Q: Immunomodulatory
activity of the aqueous extract from rhizome of Smilax glabra in
the later phase of adjuvant-induced arthritis in rats. J
Ethnopharmacol. 85:53–59. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Alam MI and Gomes A: Adjuvant effects and
antiserum action potentiation by a (herbal) compound
2-hydroxy-4-methoxy benzoic acid isolated from the root extract of
the Indian medicinal plant ‘sarsaparilla’ (Hemidesmus indicus R.
Br.). Toxicon. 36:1423–1431. 1998.PubMed/NCBI
|
|
13.
|
Navarro MC, Montilla MP, Cabo MM, Galisteo
M, Cáceres A, Morales C and Berger I: Antibacterial, antiprotozoal
and antioxidant activity of five plants used in Ibazal for
infectious. Phytother Res. 17:325–329. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Arvigo R and Balick M: Rainforest
Remedies. One Hundred Healing Herbs of Belize. 2nd edition. Lotus
Press; Twin Lakes, WI: pp. 72–73. 1998
|
|
15.
|
Gridling M, Stark N, Madlener S, Lackner
A, Popescu R, Benedek B, Diaz R, Tut FM, Nha Vo TP, Huber D, et al:
In vitro anti-cancer activity of two ethno-pharmacological
healing plants from Guatemala Pluchea odorata and Phlebodium
decumanum. Int J Oncol. 34:1117–1128. 2009.
|
|
16.
|
Stark N, Gridling M, Madlener S, Bauer S,
Lackner A, Popescu R, Diaz R, Tut FM, Vo TP, Vonach C, et al: A
polar extract of the Maya healing plant Anthurium schlechtendalii
(Aracea) exhibits strong in vitro anticancer activity. Int J
Mol Med. 24:513–521. 2009.PubMed/NCBI
|
|
17.
|
Strasser S, Maier S, Leisser C, Saiko P,
Madlener S, Bader Y, Bernhaus A, Gueorguieva M, Richter S, R. Mader
RM, et al: 5-FdUrd-araC heterodinucleoside re-establishes
sensitivity in 5-FdUrd- and AraC- resistant MCF-7 breast cancer
cells overexpressing ErbB2. Differentiation. 74:488–498. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Maier S, Strasser S, Saiko P, Leisser C,
Sasgary S, Grusch M, Madlener S, Bader Y, Hartmann J, Schott H, et
al: Analysis of mechanisms contributing to AraC-mediated
chemoresistance and re-establishment of drug sensitivity by the
novel heterodinucleoside phosphate 5-FdUrd-araC. Apoptosis.
11:427–440. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Hüttenbrenner S, Maier S, Leisser C,
Polgar D, Strasser S, Grusch M and Krupitza G: The evolution of
cell death programs as prerequisites of multicellularity. Rev Mutat
Res. 543:235–249. 2003.PubMed/NCBI
|
|
20.
|
Grusch M, Fritzer-Szekeres M, Fuhrmann G,
Rosenberger G, Luxbacher C, Elford HL, Smid K, Peters GJ, Szekeres
T and Krupitza G: Activation of caspases and induction of apoptosis
by amidox and didox. Exp Haematol. 29:623–632. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Madlener S, Rosner M, Krieger S, Giessrigl
B, Gridling M, Vo TP, Leisser C, Lackner A, Raab I, Grusch M, et
al: Short 42 degrees C heat shock induces phosphorylation and
degradation of Cdc25A which depends on p38MAPK, Chk2 and 14.3.3.
Hum Mol Genet. 18:1990–2000. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Kastan MB and Bartek J: Cell-cycle
checkpoints and cancer. Nature. 432:316–323. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Blomberg I and Hoffmann I: Ectopic
expression of Cdc25A accelerates the G(1)/S transition and leads to
premature activation of cyclin E- and cyclin A-dependent kinases.
Mol Cell Biol. 19:6183–6194. 1999.PubMed/NCBI
|
|
24.
|
Kiyokawa H and Ray D: In vivo roles of
CDC25 phosphatases: biological insight into the anti-cancer
therapeutic targets. Anticancer Agents Med Chem. 8:832–836. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Meeran SM and Katiyar SK: Cell cycle
control as a basis for cancer chemoprevention through dietary
agents. Front Biosci. 13:2191–2202. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Wolf D and Rotter V: Major deletions in
the gene encoding the p53 tumor antigen cause lack of p53
expression in HL-60 cells. Proc Natl Acad Sci USA. 82:790–794.
1985. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Abukhdeir AM and Park BH: P21 and p27:
roles in carcinogenesis and drug resistanc. Expert Rev Mol Med.
10:e192008. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Coller HA, Grandori C, Tamayo P, Colbert
T, Lander ES, Eisenman RN and Golub TR: Expression analysis with
oligonucleotide microarrays reveals that MYC regulates genes
involved in growth, cell cycle, signaling, and adhesion. Proc Natl
Sci USA. 97:3260–3265. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Chang C, Zhu YQ, Mei JJ, Liu SQ and Luo J:
Involvement of mitochondrial pathway in NCTD-induced cytotoxicity
in human hepG2 cells. J Exp Clin Cancer Res. 29:145–154. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Paull TT, Rogakou EP, Yamazaki V,
Kirchgessner CU, Gellert M and Bonner WM: A critical role for
histone H2AX in recruitment of repair factors to nuclear foci after
DNA damage. Curr Biol. 10:886–895. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Piperno G, LeDizet M and Chang J:
Microtubules containing acetylated alpha-tubulin in mammalian cells
in culture. J Cell Biol. 104:289–302. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Jackson CB and Giraud AS: Stat3 as a
prognostic marker in human gastric cancer. J Gastroenterol Hepatol.
24:505–507. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Kanda N, Seno H, Konda Y, Marusawa H,
Kanai M, Nakajima T, Kawashima T, Nanakin A, Sawabu T, Uenoyama Y,
et al: Stat3 is constitutively activated and supports cell survival
in association with survivin expression in gastric cancer cells.
Oncogene. 23:4921–4929. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Gritsko T, Williams A, Turkson J, Kaneko
S, Bowman T, Huang M, Nam S, Eweis I, Diaz N, Sullivan D, et al:
Persistent activation of stat3 signaling induces survivin gene
expression and confers resistance to apoptosis in human breast
cancer cells. Clin Cancer Res. 12:11–19. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Deng JY, Sun D, Liu XY, Pan Y and Liang H:
Stat-3 correlates with lymph node metastasis and cell survival in
gastric cancer. World J Gastroenterol. 16:5380–5387. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Zhao M, Jiang B and Gao FH: Small molecule
inhibitors of STAT3 for cancer therapy. Curr Med Chem.
18:4012–4018. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Gündogdu MS, Liu H, Metzdorf D, Hildebrand
D, Aigner M, Aktories K, Heeg K and Kubatzky KF: The haematopoietic
GTPase RhoH modulates IL3 signalling through regulation of Stat
activity and IL3 receptor expression. Mol Cancer. 9:225–238.
2010.PubMed/NCBI
|
|
38.
|
Jinawath N, Vasoontara C, Jinawath A, Fang
X, Zhao K, Yap KL, Guo T, Lee CS, Wang W, Balgley BM, et al:
Oncoproteomic analysis reveals co-upregulation of RELA and Stat5 in
carboplatin resistant ovarian carcinoma. PLoS One. 5:e111982010.
View Article : Google Scholar : PubMed/NCBI
|
|
39.
|
Dominguez-Sola D, Ying CY, Grandori C,
Ruggiero L, Chen B, Li M, Galloway DA, Gu W, Gautier J and
Dalla-Favera R: Non-transcriptional control of DNA replication by
c-Myc. Nature. 448:445–451. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40.
|
Wang H, Birkenbach M and Hart J:
Expression of Jun family members in human colorectal
adenocarcinoma. Carcinogenesis. 21:1313–1317. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
41.
|
Mathiasen DP, Egebjerg C, Andersen SH,
Rafn B, Puustinen P, Khanna A, Daugaard M, Valo E, Tuomela S,
Bøttzauw T, et al: Identification of a c-Jun N-terminal
kinase-2-dependent signal amplification cascade that regulates
c-Myc levels in ras transformation. Oncogene. 31:390–401. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
42.
|
Ivanova A, Mikhova B, Klaiber I, Dinchev D
and Kostova I: Steroidal saponins from Smilax excelsa rhizomes. Nat
Prod Res. 23:916–924. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43.
|
Doyle BJ, Frasor J, Bellows LE, Locklear
TD, Perez A, Gomez-Laurito J and Mahady GB: Estrogenic effects of
herbal medicines from Costa Rica used for the management of
menopausal symptoms. Menopause. 16:748–755. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44.
|
Yu Z, Hu D and Li Y: Effects of
zearalenone on mRNA expression and activity of cytochrome P450 1A1
and 1B1 in MCF-7 cells. Ecotoxicol Environ Saf. 58:187–193. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
45.
|
Sergentanis TN and Economopoulos KP: Four
polymorphisms in cytochrome P450 1A1 (CYP1A1) gene and breast
cancer risk: a meta-analysis. Breast Cancer Res Treat. 122:459–469.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
46.
|
Sergentanis TN and Economopoulos KP:
Erratum to: Four polymorphisms in cytochrome P450 1A1 (CYP1A1) gene
and breast cancer risk: a meta-analysis. Breast Cancer Res Treat.
131:10832012. View Article : Google Scholar : PubMed/NCBI
|
|
47.
|
Kozak W, Mayfield KP, Kozak A and Kluger
MJ: Proadifen (SKF-525A), an inhibitor of cytochrome P-450,
augments LPS-induced fever and exacerbates prostaglandin-E2 levels
in the rat. J Therm Biol. 25:45–50. 2000. View Article : Google Scholar
|
|
48.
|
Sun J, Sui X, Bradbury JA, Zeldin DC,
Conte MS and Liao JK: Inhibition of vascular smooth muscle cell
migration by cytochrome p450 epoxygenase-derived eicosanoids. Circ
Res. 90:1020–1027. 2002. View Article : Google Scholar : PubMed/NCBI
|