Introduction
Oral squamous cell carcinoma (OSCC) is the sixth
most common type of cancer worldwide: a total of 36,540 people in
the United States are affected annually (1). The most common sites for early OSCC
lesions are the ventral aspect of the tongue and the floor of the
mouth in the oral cavity (1,2).
Although the diagnosis and surgical treatment of OSCC have
advanced, survival rates of OSCC patients have not improved over
the last 30 years, and only 40–50% of patients will survive for 5
years (1,3). Moreover, since OSCC arises in the
oral cavity, pharynx or larynx, it can cause severe dysfunction in
swallowing, speech, and physical appearance, markedly decreasing
the quality of life (QOL).
It has been well recognized that lymph node
metastasis strongly correlates with the risk to survival in
patients with OSCC. The incidence of neck lymph node metastasis in
oral carcinoma varies from 25–65% (1,3).
Although patients without lymph node metastases have a cumulative
survival rate of approximately 50–70%, this rate drops drastically
to 30–50% in patients with documented nodal metastases (4,5).
Thus, understanding the pathophysiology of lymph node metastasis of
OSCC is important for early diagnosis and treatment. However,
precise molecular mechanisms of lymph node metastasis have not been
elucidated, partly due to the lack of consistent and reproducible
animal models.
The formation of new lymphatic vessels,
lymphangiogenesis, is a critical step during the development of
lymph node metastasis. Two major lymphangiogenic factors, namely
vascular endothelial growth factor-C (VEGF-C) and -D, have been
identified, and are linked to the promotion of lymphangiogenesis in
animal models (6–8). In addition, the increase in lymphatic
vessel density by VEGF-C greatly facilitates the spreading of tumor
cells to lymph nodes (9). The
occurrence of lymphangiogenesis can be detected using several
lymphatic vessel-specific markers, such as VEGF receptor (VEGFR)-3,
LYVE-1, Prox-1, podoplanin, and desmoplakin (10). VEGF-C and -D bind to the receptor
VEGFR-3, and induce proliferation of lymphatic endothelial cells
in vitro and lymphangiogenesis in vivo through the
mitogen-activated protein kinase and phosphatidylinositol 3-kinase
signaling pathways (11–14). Importantly, ablation of the VEGF-R3
mediated pathway by neutralizing antibodies against VEGF-R3 has
been shown to inhibit lymph node metastasis (12,13,15).
Although the elevation of VEGF-C expression strongly correlates
with the formation of metastases in regional lymph nodes in human
thyroid, prostate, gastric, colorectal, breast, melanoma, and lung
carcinoma (16–18), the pathological role of VEGF-C and
VEGF-D in lymph node metastasis of OSCC is still unclear.
Cyclooxygenase-2 (COX-2), the inducible form of the
COX enzymes (19), catalyzes the
synthesis of prostaglandins with diverse biological activities, and
its dysregulation plays a pivotal role in inflammation, tissue
damage, and tumorigenesis (20,21).
The involvement of COX-2 in tumor activity is well documented: it
is significantly increased in a range of human malignancies
(22). Studies from transgenic
animals have shown that the COX-2 gene is involved in the
early stages of the oncogenic process of colorectal tumors
(23). Moreover, a large amount of
evidence points to a close association of COX-2 upregulation
with tumor invasion and metastasis in human colorectal, breast, and
lung tumors (24,25).
COX-2 has been implicated in several processes of
cancer metastasis, especially angiogenesis, in which its major role
is thought to be induction of the synthesis of prostanoids, which
then stimulate the secretion of pro-angiogenic factors, including
VEGF-A and fibroblast growth factor-2, from cancer cells and/or
stromal fibroblasts (26,27). In addition, COX-2 stimulates the
proliferation, migration, and tube formation of vascular
endothelial cells (26,28). Several clinical studies have shown
a correlation between the level of COX-2 expression and the extent
of angiogenesis in cancer (29).
Despite its evident importance in angiogenesis, the precise role of
COX-2 in tumor lymphangiogenesis of OSCC remains poorly
understood.
Here, we have successfully developed a model of OSCC
that spontaneously metastases to the lymph nodes, enabling us to
monitor the process using fluorescent-labeled human OSCC cells in a
non-invasive manner. We found that elevated expression of VEGF-C
under the control of COX-2 is critical for the development of
lymphangiogenesis and lymph node metastasis and that inactivation
of COX-2 clearly inhibited these metastatic properties in OSCC.
Materials and methods
Cell culture
Human OSCC SAS cells, which were originally isolated
from the surgical specimens of a Japanese woman with a tongue
primary lesion, were used in this study (30). The cells were cultured at 37°C
under a 5% CO2 atmosphere in Dulbecco’s modified Eagle’s
medium (DMEM, Sigma, St. Louis, MO, USA) supplemented with 10%
fetal bovine serum (FBS, Equitech-Bio Inc., Kerrville, TX, USA) and
100 μg/ml kanamycin (Meiji-Seika, Tokyo). Cells were
regularly certified free of mycoplasma contamination.
Animal model of lymph node
metastasis
All experiments were conducted according to the
ethical guidelines of the Institutional Review Boards, and approved
by the Institutional Animal Use Committee of the Osaka University
Graduate School of Dentistry.
Five-week-old, male, immunodeficient BALB/c nu/nu
mice were anesthetized with pentobarbital (0.05 mg/g body weight;
Dainippon Pharmaceutical Co., Ltd.), and Venus-labeled SAS cells
(1×106 in 0.1 ml phosphate-buffered saline) were
injected into the tongue (31).
Metastatic lymph nodes could then be visualized by fluorescence
stereoscopic microscopy (Leica Microsytems, Wetzlar, Germany).
To establish highly metastatic OSCC, tumor cells
were isolated from metastatic lymph node lesions and re-inoculated
after expansion in culture. Venus-positive SAS cells were purified
by FACS Aria.
Lentiviral vector preparation and
transductions
The lentiviral vector pLenti6/V5-Venus was
constructed by sub-cloning PCR products of a Venus fragment into
pLenti6/V5 TOPO vector. Venus cDNA was kindly provided by Professor
Atsushi Miyawaki (Riken, Japan). The vectors were packaged in 293FT
cells using FuGENE 6 with ViraPower packaging mix (Invitrogen), and
vector particles were harvested from the medium 49 h after
transfection. SAS cells and vector particles were incubated with 6
μg/ml polybrene (Sigma) and Venus-labeled SAS cells were
isolated by FACS Aria and cloned.
Immunohistochemistry
Mice were anesthetized with pentobarbital (0.05 mg/g
body weight) and fixed by perfusion with 4% paraformaldehyde in 0.1
M phosphate buffer through the left cardiac ventricle. The tongues
were removed and post-fixed for 24 h, and then 7-μm frozen
sections were cut following a conventional method.
Immunohistochemical staining of LYVE-1 (1:500 dilution; Abcam) and
COX-2 (1:200 dilution; Cayman Chemical) were performed at 4°C
overnight. As secondary antibodies, Alexa Fluor 555-conjugated
anti-rabbit IgG (1:500; Invitrogen) were incubated for 1 h at room
temperature. The sections were coverslipped with Vectashield Hard
Set mounting medium with DAPI (Vector Laboratories, Burlingame,
CA).
In vitro wound healing assay
For the wound-healing assay, 1×105 SAS
cells per well were plated in DMEM containing 10% FBS in 10 cm
plates and incubated for 24 h. After confirming that a complete
monolayer had formed, the monolayers were wounded by scratching
lines in them with a standard 200-μl plastic tip. Migration
and cell movement throughout the wound area was observed with a
phase-contrast microscope after 24 h. The distance that the cells
had migrated was measured on the photograph.
Histomorphometric analysis of
lymphangiogenesis
In the model of lymph node metastasis,
lymphangiogenesis was evaluated by lymphatic vessel density using
LYVE-1 antibodies according to previous reports (32). Briefly, five hotspots (fields with
the highest vascular density) in tumor areas were photographed at
magnification of 200×, and digital images of LYVE-1-positive
lymphatic vessels were captured. Area densities (percentage of
total tumor area) of lymphatic vessels were then calculated using
ImageJ software (NIH, Bethesda, MD, USA).
RNA preparation and quantitative
real-time polymerase chain reaction
Total RNA from SAS cells was extracted using the
Total RNA isolation system (NucleoSpin RNAII; Macherey-Nagel GmbH
& Co., Germany). First-strand cDNAs were synthesized using the
Prime Script 1st strand cDNA synthesis kit (Takara) with Oligo-dT
primers. Quantitative real-time reverse transcription-PCR (qRT-PCR)
analysis was performed using the SYBR Green PCR protocol and a 7300
Real-Time PCR system (Applied Biosystems, Branchburg, New Jersey,
USA). SYBR Green primers used for the amplification were as
follows: human VEGF-C, sense 5′-GGAGGCTGGCAACATAACAG-3′ and
antisense 5′-ACGTCTTGCTGAGGTAGCTC-3′; human VEGF-D, sense
5′-AGCGATCATCTCAGTCCACA-3′ and antisense
5′-AGGTGCTGGTGTTCATACAG-3′; human COX-2, sense
5′-TGCATTCTTTGCCCAGCACT-3′ and antisense
5′-AAAGGCGCAGTTTACGCTGT-3′; human β-actin, sense
5′-AGCGGGAAATCGTGCGTG-3′ and antisense 5′-CAGGGT
ACATGGTGGTGGTGCC-3′. mRNA expression levels were normalized to that
of β-actin.
Enzyme-linked immunosorbent assay
(ELISA)
SAS cells were seeded in 48-well plates and
incubated in serum-free DMEM for 48 h. The conditioned medium was
collected, and human VEGF-C protein levels quantified using the
Quantikine VEGF-C ELISA kit (R&D Systems, Minneapolis, MN, USA)
according to the manufacturer’s instructions.
COX-2 inhibitor
NS-398
(N-[2-(cyclohexyloxy)-4-nitrophenyl]-methanesulfonamide), a COX-2
inhibitor, was purchased from Cayman Chemical (Ann Arbor, MI,
USA).
Statistical analysis
The data are presented as the mean ± SD, except
morphometric analysis data, which are expressed as mean ± SE.
Student’s t-test was used to compare data between two groups.
P-values of <0.05 were considered to have statistical
significance.
Results
Establishment of highly metastatic oral
squamous cell carcinoma
To understand the underlying mechanism of lymph node
metastasis of OSCC, we first aimed to establish a highly metastatic
model of OSCC using fluorescent-labeled OSCC cell lines. We
inoculated several OSCC cell types into mouse tongue, and found
that SAS cells that had originally been isolated from oral cancer
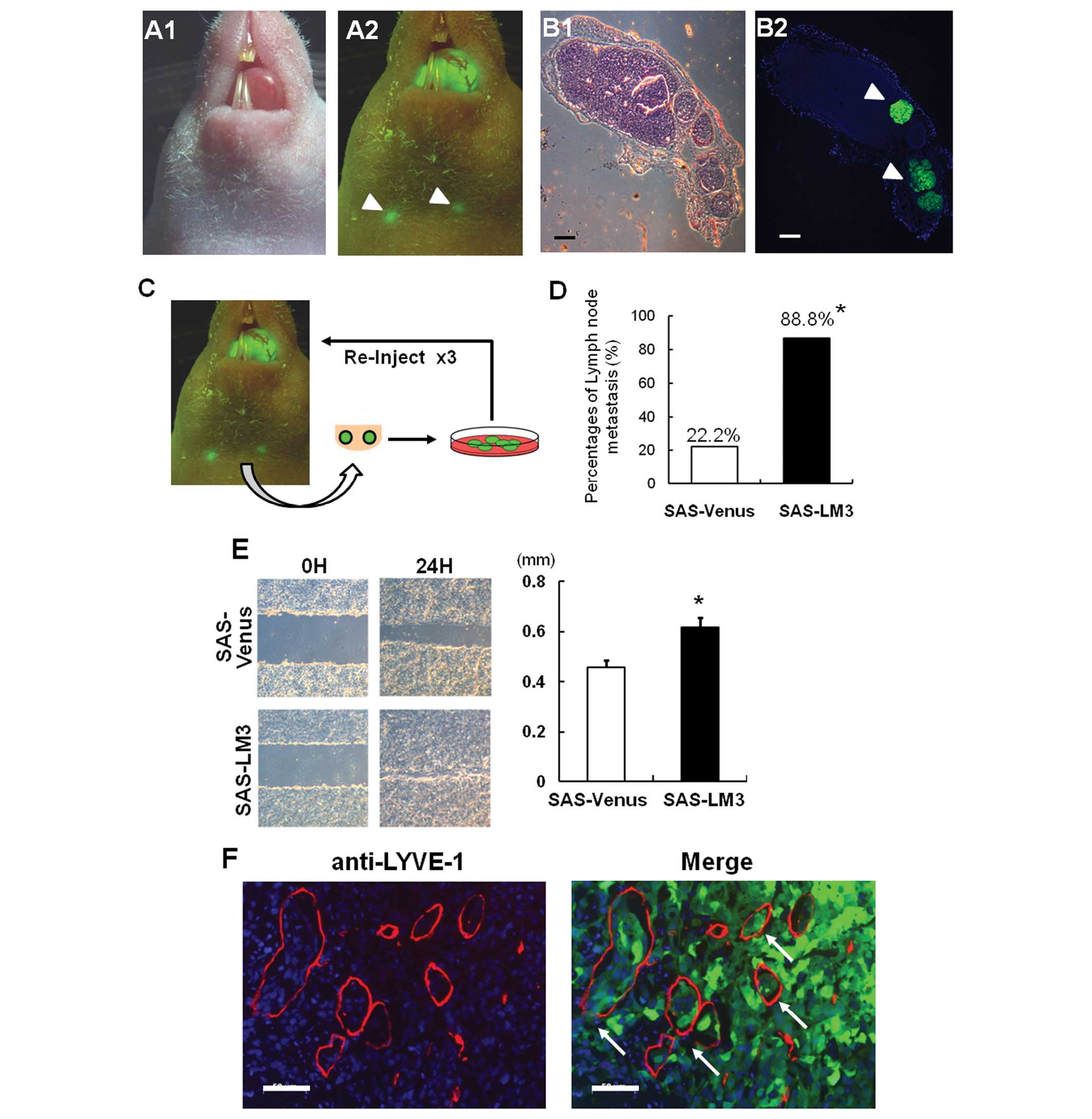
showed discernible tumorigenesis in vivo. We stably
overexpressed Venus protein, an improved version of GFP, into SAS
cells (SAS-Venus). Venus-labeled SAS cells spontaneously
metastasized to the cervical lymph nodes 3 weeks after tongue
inoculation, and fluorescent labeling enabled us to perform in
vivo monitoring of lymph node metastasis under a fluorescence
stereomicroscope (Fig. 1A).
Histological examination also showed the metastasis of SAS-Venus
cells to the lymph nodes (Fig.
1B). To obtain highly metastatic cells by in vivo
selection, tumors were recovered from metastatic lesions, expanded
in culture, and re-inoculated into mice (Fig. 1C). Finally, we established highly
metastatic SAS cells (named SAS-LM3) after three rounds of in
vivo selection using the poorly metastatic human cell line
SAS-Venus as a starting point. The incidence of lymph node
metastasis reached 88.8% in the fourth generation of the mouse
model (Fig. 1D). In vitro
wound healing assay revealed that migration activity was also
increased in SAS-LM3 cells compared to SAS-Venus cells (Fig. 1E). Interestingly,
immunohistochemical analysis using LYVE-1 antibody, a specific
lymphatic vessel marker, demonstrated abundant lymph node vessels
in SAS-LM3 tumor tissues (Fig.
1F). Moreover, we found that SAS-LM3 cancer cells in this model
invaded into peri-tumoral lymphatic vessels and spread along
lymphatic vessels toward the regional lymph nodes (Fig. 1F). These data suggest that SAS-LM3
cancer cells are a relevant preclinical animal cancer model for
non-invasive imaging of lymph node metastases and the determination
of underlying molecular mechanism.
Increased lymphangiogenesis and VEGF-C
expression in SAS-LM3 tumors
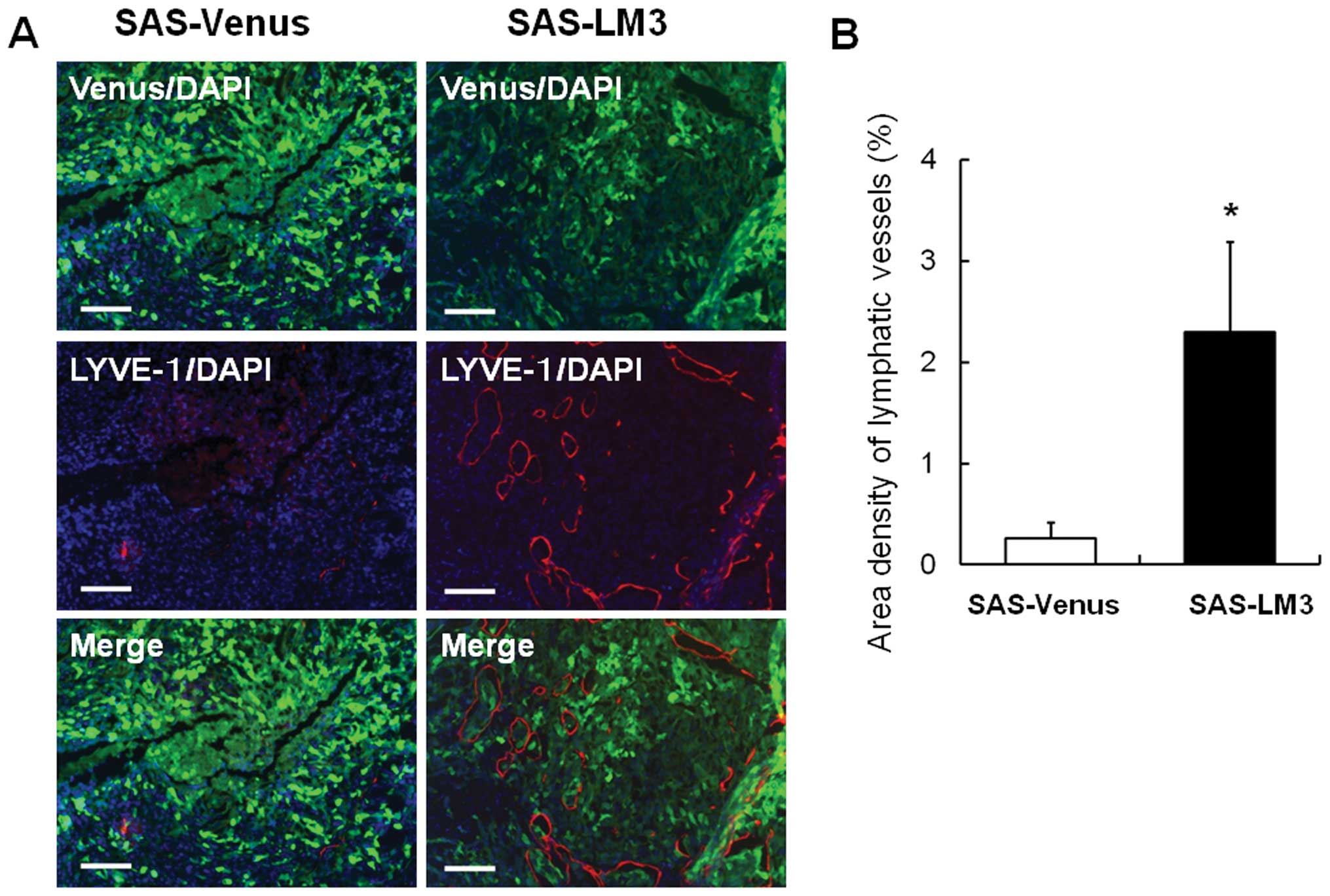
Since abundant lymphangiogenesis was observed in
SAS-LM3 tumors (Fig. 1G), we next
quantified tumor lymphangiogenesis in SAS-Venus and SAS-LM3 tumors.
Immunohistochemical staining of LYVE-1 revealed that the size and
number of lymphatic vessels were dramatically increased in mice
inoculated with SAS-LM3 cells compared with those inoculated with
SAS-Venus cells (Fig. 2A).
Increased lymphangiogenesis in SAS-LM3 tumors was validated by the
quantification of LYVE-1-positive areas (Fig. 2B). These data raised the
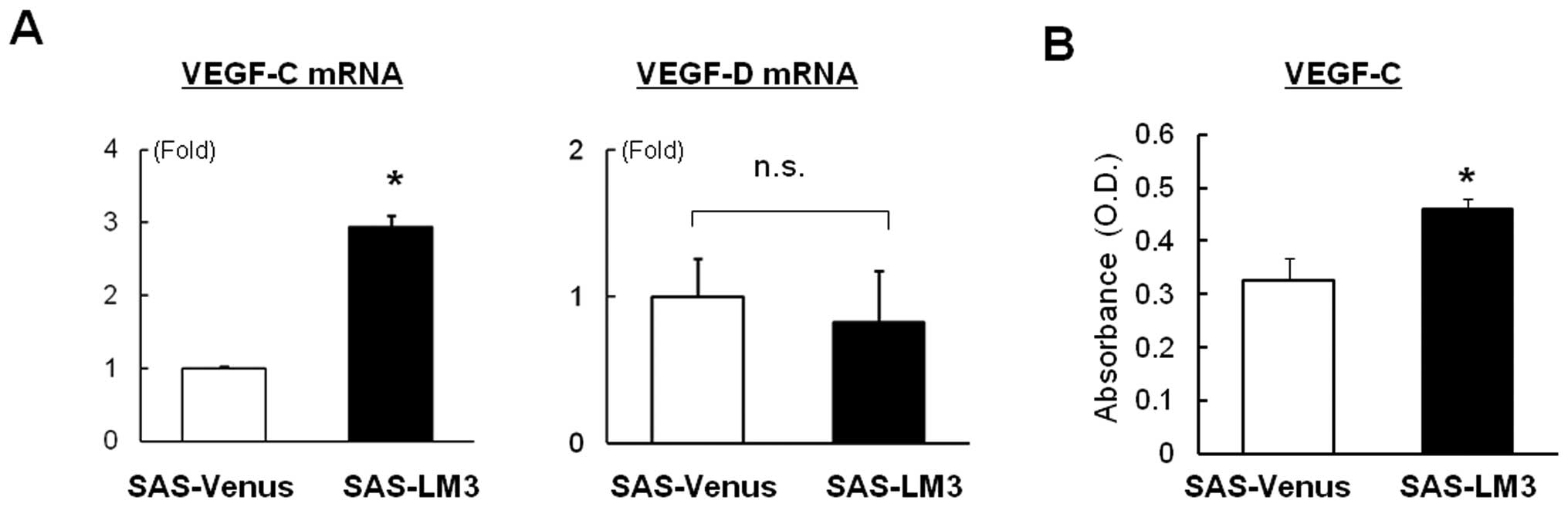
possibility that SAS-LM3 cells produce the lymphangiogenic growth
factors VEGF-C and -D. We found that mRNA expression of VEGF-C, but
not VEGF-D, was elevated in SAS-LM3 cells compared with SAS-Venus
cells (Fig. 3A). VEGF-C production
in SAS-LM3 cells was significantly higher than in SAS-Venus cells
(Fig. 3B). These data suggest that
elevated expression of VEGF-C seems to be an important feature of
highly metastatic OSCC.
Involvement of COX-2 in tumor
lymphangiogenesis and lymph node metastasis of OSCC
Recent studies have suggested that COX-2 is involved
in lymphangiogenesis (32).
Accordingly, we examined whether expression of COX-2 was associated
with the increase in VEGF-C expression and lymphangiogenesis in
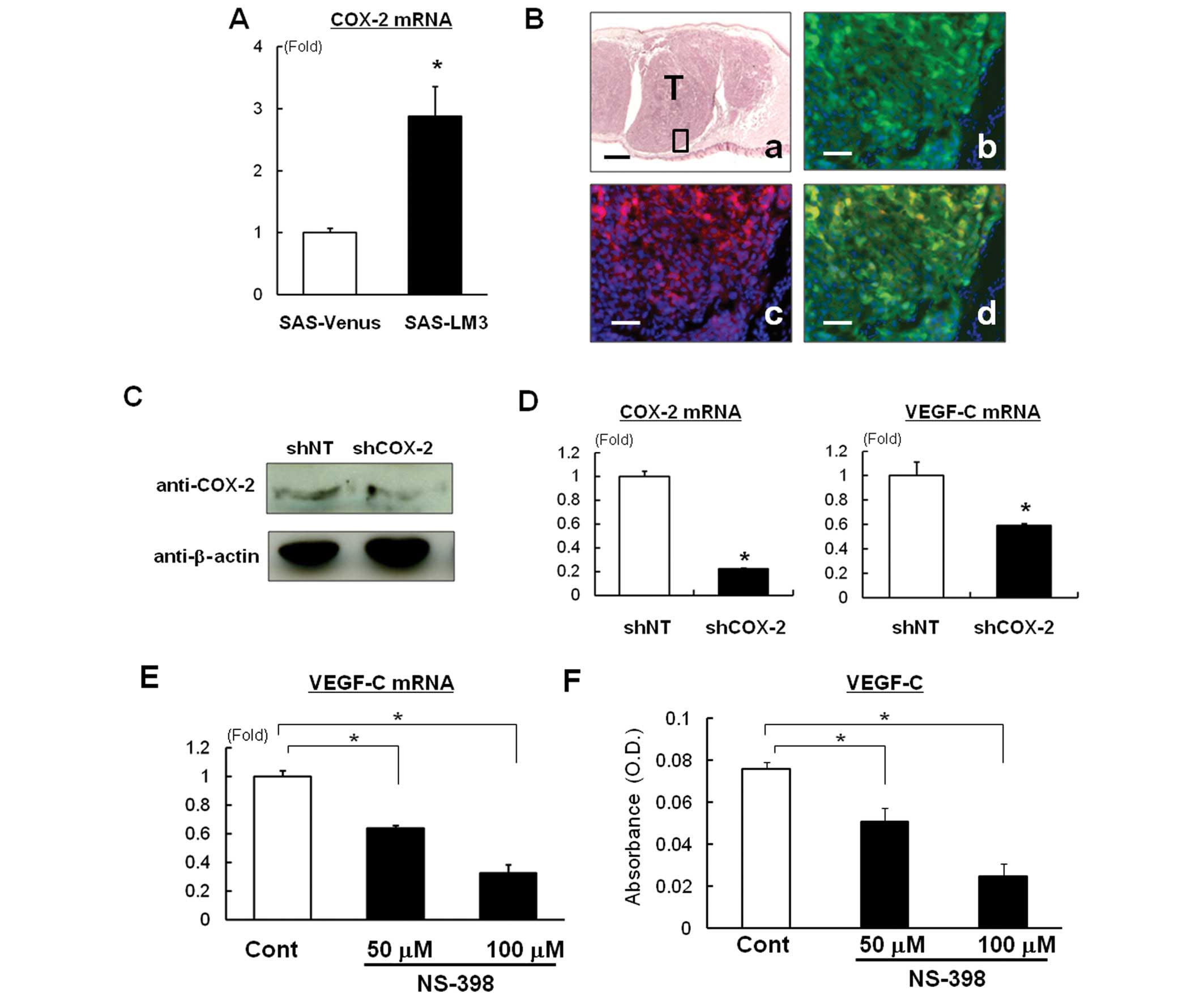
SAS-LM3 cells. To approach this, we first compared COX-2 expression
in SAS-LM3 and SAS-Venus cells, and found that COX-2 mRNA
expression was elevated in SAS-LM3 compared with SAS-Venus cells
(Fig. 4A). Immunohistochemical
staining detected COX-2 protein in Venus-positive OSCC inoculated
in mice (Fig. 4B). To confirm the
direct effects of COX-2 on VEGF-C expression in SAS-LM3 cells, we
investigated the effect of COX-2 knockdown on VEGF-C
expression. We confirmed that COX-2 shRNA decreased COX-2
expression at the protein level (Fig.
4C). Importantly, knockdown of COX-2 significantly
decreased the expression of VEGF-C mRNA in SAS-LM3 cells (Fig. 4D). Moreover, the COX-2-selective
inhibitor NS-398 also decreased VEGF-C mRNA expression (Fig. 4E) and production (Fig. 4F).
To further clarify the role of COX-2 in lymph node
metastasis of OSCC, we subsequently investigated the effect of
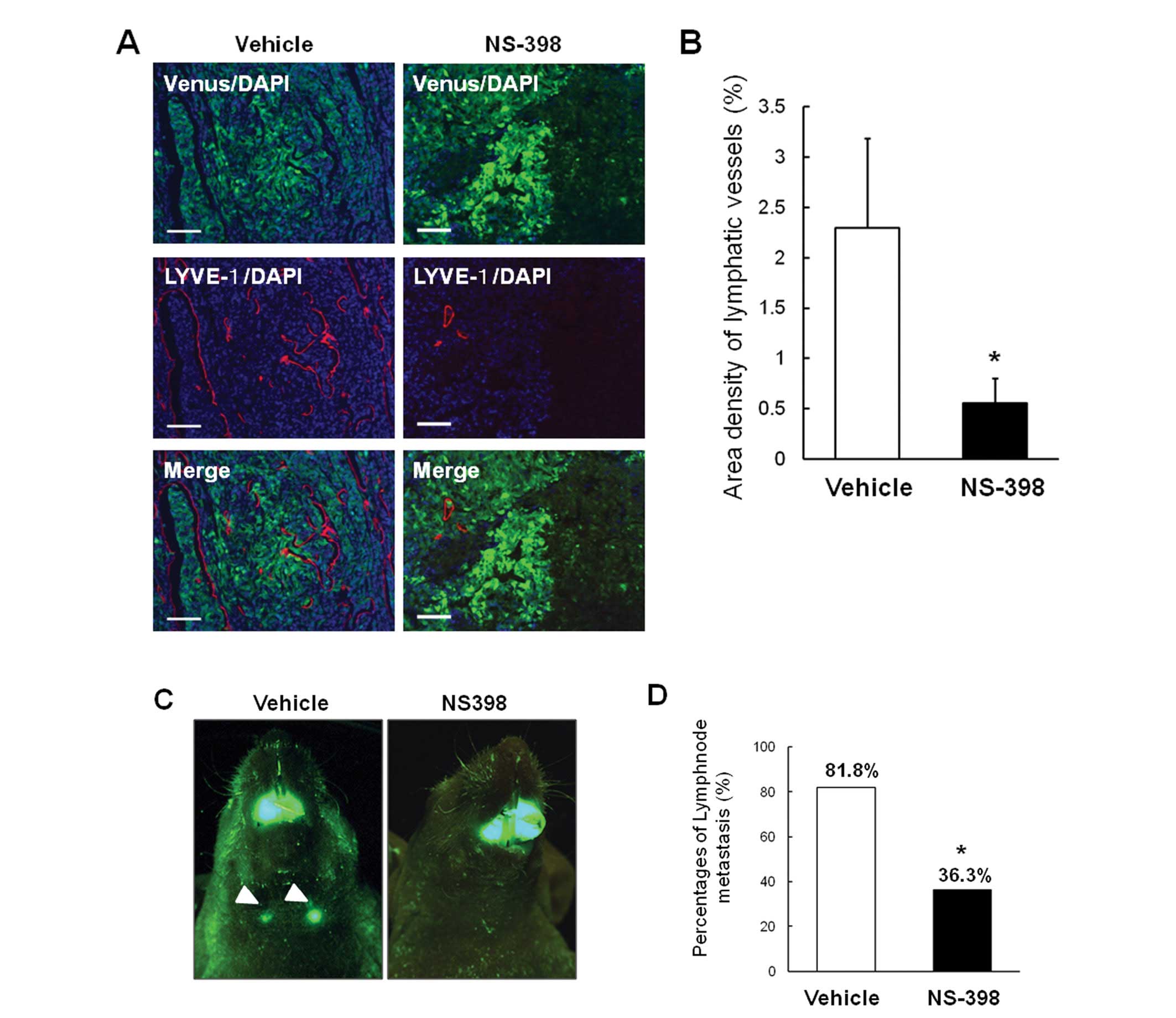
NS-398 on tumor lymphangiogenesis in our animal model. As shown in
Fig. 5A, tumor lymphangiogenesis
was significantly decreased in NS-398-treated mice compared with
control mice. Quantification of LYVE-1-positive areas in hotspots
confirmed these findings (Fig.
5B). Importantly, NS-398 significantly suppressed the rate of
lymph node metastasis (Fig. 5C and
D). These data suggest that COX-2 contributes to the
development of lymphangiogenesis and lymph node metastasis of
OSCC.
Discussion
Cervical lymph node metastasis significantly
correlates with poor survival in patients with OSCC. A better
understanding of the molecular mechanisms underlying lymph node
metastasis is important for the early diagnosis and development of
effective treatments of OSCC, which in turn leads to improved QOL
and survival. However, little attention has been paid to the
understanding of the pathophysiology of OSCC lymph node metastasis.
Here, we have established an animal model of OSCC lymph node
metastasis in an attempt to uncover its mechanism. We generated a
highly metastatic OSCC cell line, SAS-LM3, which exhibited enhanced
lymphangiogenesis. We demonstrated that increased VEGF-C under the
control of COX-2 is critical for lymph node metastasis, and that
inhibition of COX-2 clearly decreased lymphangiogenesis and lymph
node metastases. Our results suggest that COX-2 is involved in
lymphangiogenesis and lymph node metastases, and thus is a
potential therapeutic target in the treatment of OSCC.
COX-2 expression has been reported to be
significantly increased in a variety of human cancer cells. High
expression of COX-2 is associated with tumor growth, apoptosis,
angiogenesis and metastasis (33–36).
Previous studies have also shown that blockage of the COX-2 pathway
is a promising antitumor strategy, and COX-2 inhibitors have
potential as chemopreventive agents in OSCC (37,38).
Our preclinical studies clearly showed COX-2 to be critical for
tumor lymphangiogenesis and lymph node metastasis of OSCC. Although
previous clinical studies described a correlation between COX-2
expression and lymph node metastasis in tumors including gastric
(39), lung (40), breast (41) and prostate (42), the role of COX-2 in tumor
lymphangiogenesis and lymphatic metastasis in OSCC has remained
poor. Here, we showed that highly metastatic OSCC exhibited
augmented expression of COX-2, which significantly correlated with
increased lymph node metastasis. Lymphangiogenesis is regulated by
various growth factors including VEGF-C/D, transforming growth
factor β (TGF-β), platelet-derived growth factor and fibroblast
growth factor 2 (FGF2) (43,44),
which can all be potential therapeutic targets. Recent studies
suggest that inhibition of VEGF-C/D and their cognitive receptor
VEGFR-3 is an alternative therapeutic approach for lymph node
metastasis, and that neutralizing antibodies against VEGFR-3
inhibited lymph node metastasis (12,13,45).
Our results showing that VEGF-C expression was highly correlated
with lymphatic metastasis of OSCC suggest that therapeutic
approaches targeting VEGFR-3 using neutralizing antibody could also
be beneficial for patients with OSCC.
Recent studies have shown that the
epithelial-mesenchymal transition (EMT) play important roles in the
process of cancer metastasis (46). EMT is associated with the increased
cellular motility which enables cancer cells to migrate into
distant organs. Although various cytokines are reported to induce
EMT in cancer cells, it is well established that TGF-β is the major
and potent inducer of EMT (47).
TGF-β activates Smad proteins and activated Smads regulate several
genes including Snail and Twist which causes EMT (47). It is likely that EMT was
upregulated in SAS-LM3 cells compared to SAS-Venus cells and
therefore showed high metastatic activity. To support this notion,
cellular motility of SAS-LM3 cells were increased (Fig. 1E) and SAS-LM3 cells showed high
expression of fibronectin which was described as an acquired
mesenchymal cell marker in EMT (data not shown). Further studies
are needed to clarify the role of EMT in lymph node metastasis of
OSCC.
In the present study, we focused on COX-2 as the
regulator of lymphangiogenesis and lymph node metastasis of OSCC.
However, various factors including signaling molecules, cytokines
and enzymes should be involved in metastatic events. Additional
studies using our cells established in this study would be useful
to determine the precise molecular mechanisms responsible for the
lymph node metastasis. For example, microarray analysis between
SAS-Venus and SAS-LM3 may lead to the identification of novel
regulator of lymphangiogenesis and non-invasive animal model of
lymph node metastasis enable us to examine the effect of new
therapeutic agents.
In conclusion, our results suggest that increased
expression of COX-2 is critical for the development of lymphatic
metastasis in OSCC. The results also suggest that COX-2 is a
potential therapeutic target in designing pharmacologic
interventions for the treatment of oral cancer.
Abbreviations:
|
COX-2
|
cyclooxygenase-2
|
|
OSCC
|
oral squamous cell carcinoma
|
|
VEGF-C
|
vascular endothelial growth
factor-C
|
|
LYVE-1
|
lymphatic vessel endothelial
hyaluronan receptor-1
|
Acknowledgements
We are grateful to Dr Riko Nishimura
(Osaka University Graduate School of Dentistry) for helpful
discussions. This study was supported by a Grant-in-Aid for
Scientific Research on Priority Areas (TY) from the Ministry of
Education, Culture, Sports, Science and Technology of Japan, and
the 21st Century COE Program (TY).
References
|
1.
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar
|
|
2.
|
Hunter KD, Parkinson EK and Harrison PR:
Profiling early head and neck cancer. Nat Rev Cancer. 5:127–135.
2005. View
Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Forastiere A, Koch W, Trotti A and
Sidransky D: Head and neck cancer. N Engl J Med. 345:1890–1900.
2001. View Article : Google Scholar
|
|
4.
|
Gil Z, Carlson DL, Boyle JO, et al: Lymph
node density is a significant predictor of outcome in patients with
oral cancer. Cancer. 115:5700–5710. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Mamelle G, Pampurik J, Luboinski B, Lancar
R, Lusinchi A and Bosq J: Lymph node prognostic factors in head and
neck squamous cell carcinomas. Am J Surg. 168:494–498. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Joukov V, Pajusola K, Kaipainen A, et al:
A novel vascular endothelial growth factor, VEGF-C, is a ligand for
the Flt4 (VEGFR-3) and KDR (VEGFR-2) receptor tyrosine kinases.
EMBO J. 15:17511996.
|
|
7.
|
Mandriota SJ, Jussila L, Jeltsch M, et al:
Vascular endothelial growth factor-C-mediated lymphangiogenesis
promotes tumour metastasis. EMBO J. 20:672–682. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Stacker SA, Caesar C, Baldwin ME, et al:
VEGF-D promotes the metastatic spread of tumor cells via the
lymphatics. Nat Med. 7:186–191. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Skobe M, Hawighorst T, Jackson DG, et al:
Induction of tumor lymphangiogenesis by VEGF-C promotes breast
cancer metastasis. Nat Med. 7:192–198. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Sleeman JP and Thiele W: Tumor metastasis
and the lymphatic vasculature. Int J Cancer. 125:2747–2756. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Makinen T, Veikkola T, Mustjoki S, et al:
Isolated lymphatic endothelial cells transduce growth, survival and
migratory signals via the VEGF-C/D receptor VEGFR-3. EMBO J.
20:4762–4773. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Roberts N, Kloos B, Cassella M, et al:
Inhibition of VEGFR-3 activation with the antagonistic antibody
more potently suppresses lymph node and distant metastases than
inactivation of VEGFR-2. Cancer Res. 66:2650–2657. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Shimizu K, Kubo H, Yamaguchi K, et al:
Suppression of VEGFR-3 signaling inhibits lymph node metastasis in
gastric cancer. Cancer Sci. 95:328–333. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Veikkola T, Jussila L, Makinen T, et al:
Signalling via vascular endothelial growth factor receptor-3 is
sufficient for lymphangiogenesis in transgenic mice. EMBO J.
20:1223–1231. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Burton JB, Priceman SJ, Sung JL, et al:
Suppression of prostate cancer nodal and systemic metastasis by
blockade of the lymphangiogenic axis. Cancer Res. 68:7828–7837.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Alitalo K and Carmeliet P: Molecular
mechanisms of lymphangiogenesis in health and disease. Cancer Cell.
1:219–227. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Dadras SS, Paul T, Bertoncini J, et al:
Tumor lymphangiogenesis: a novel prognostic indicator for cutaneous
melanoma metastasis and survival. Am J Pathol. 162:1951–1960. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Karpanen T and Alitalo K: Lymphatic
vessels as targets of tumor therapy? J Exp Med. 194:F37–F42. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Wu KK: Inducible cyclooxygenase and nitric
oxide synthase. Adv Pharmacol. 33:179–207. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Tsujii M, Kawano S and DuBois RN:
Cyclooxygenase-2 expression in human colon cancer cells increases
metastatic potential. Proc Natl Acad Sci USA. 94:3336–3340. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Vane JR, Mitchell JA, Appleton I, et al:
Inducible isoforms of cyclooxygenase and nitric-oxide synthase in
inflammation. Proc Natl Acad Sci USA. 91:2046–2050. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Prescott SM and Fitzpatrick FA:
Cyclooxygenase-2 and carcinogenesis. Biochim Biophys Acta.
1470:M69–M78. 2000.PubMed/NCBI
|
|
23.
|
Oshima M, Dinchuk JE, Kargman SL, et al:
Suppression of intestinal polyposis in Apc delta716 knockout mice
by inhibition of cyclooxygenase 2 (COX-2). Cell. 87:803–809. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Fujita T, Matsui M, Takaku K, et al: Size-
and invasion-dependent increase in cyclooxygenase 2 levels in human
colorectal carcinomas. Cancer Res. 58:4823–4826. 1998.PubMed/NCBI
|
|
25.
|
Rozic JG, Chakraborty C and Lala PK:
Cyclooxygenase inhibitors retard murine mammary tumor progression
by reducing tumor cell migration, invasiveness and angiogenesis.
Int J Cancer. 93:497–506. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Tsujii M, Kawano S, Tsuji S, Sawaoka H,
Hori M and DuBois RN: Cyclooxygenase regulates angiogenesis induced
by colon cancer cells. Cell. 93:705–716. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Williams CS, Tsujii M, Reese J, Dey SK and
DuBois RN: Host cyclooxygenase-2 modulates carcinoma growth. J Clin
Invest. 105:1589–1594. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Leahy KM, Ornberg RL, Wang Y, Zweifel BS,
Koki AT and Masferrer JL: Cyclooxygenase-2 inhibition by celecoxib
reduces proliferation and induces apoptosis in angiogenic
endothelial cells in vivo. Cancer Res. 62:625–631. 2002.PubMed/NCBI
|
|
29.
|
Masferrer JL, Leahy KM, Koki AT, et al:
Antiangiogenic and antitumor activities of cyclooxygenase-2
inhibitors. Cancer Res. 60:1306–1311. 2000.PubMed/NCBI
|
|
30.
|
Takahashi K, Kanazawa, Akiyama, et al:
Establishment and characterization of a cell line (SAS) from poorly
differentiated human squamous cell carcinoma of the tongue. J Jpn
Stomatol Soc. 38:20–28. 1989.
|
|
31.
|
Shintani S, Mihara M, Nakahara Y, et al:
Lymph node metastasis of oral cancer visualized in live tissue by
green fluorescent protein expression. Oral Oncol. 38:664–669. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Iwata C, Kano MR, Komuro A, et al:
Inhibition of cyclooxygenase-2 suppresses lymph node metastasis via
reduction of lymphangiogenesis. Cancer Res. 67:10181–10189. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Hida T, Yatabe Y, Achiwa H, et al:
Increased expression of cyclooxygenase 2 occurs frequently in human
lung cancers, specifically in adenocarcinomas. Cancer Res.
58:3761–3764. 1998.PubMed/NCBI
|
|
34.
|
Ristimaki A, Honkanen N, Jankala H,
Sipponen P and Harkonen M: Expression of cyclooxygenase-2 in human
gastric carcinoma. Cancer Res. 57:1276–1280. 1997.PubMed/NCBI
|
|
35.
|
Sano H, Kawahito Y, Wilder RL, et al:
Expression of cyclooxygenase-1 and -2 in human colorectal cancer.
Cancer Res. 55:3785–3789. 1995.PubMed/NCBI
|
|
36.
|
Zimmermann KC, Sarbia M, Weber AA,
Borchard F, Gabbert HE and Schror K: Cyclooxygenase-2 expression in
human esophageal carcinoma. Cancer Res. 59:198–204. 1999.PubMed/NCBI
|
|
37.
|
Choe MS, Zhang X, Shin HJ, Shin DM and
Chen ZG: Interaction between epidermal growth factor receptor- and
cyclooxygenase 2-mediated pathways and its implications for the
chemoprevention of head and neck cancer. Mol Cancer Ther.
4:1448–1455. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Mendes RA, Carvalho JF and Waal I: An
overview on the expression of cyclooxygenase-2 in tumors of the
head and neck. Oral Oncol. 45:e124–e128. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39.
|
Da MX, Wu XT, Wang J, et al: Expression of
cyclooxygenase-2 and vascular endothelial growth factor-C
correlates with lymphangiogenesis and lymphatic invasion in human
gastric cancer. Arch Med Res. 39:92–99. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40.
|
Su JL, Shih JY, Yen ML, et al:
Cyclooxygenase-2 induces EP1-and HER-2/Neu-dependent vascular
endothelial growth factor-C up-regulation: a novel mechanism of
lymphangiogenesis in lung adenocarcinoma. Cancer Res. 64:554–564.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
41.
|
Zhang XH, Huang DP, Guo GL, et al:
Coexpression of VEGF-C and COX-2 and its association with
lymphangiogenesis in human breast cancer. BMC Cancer. 8:42008.
View Article : Google Scholar : PubMed/NCBI
|
|
42.
|
Di JM, Zhou J, Zhou XL, et al:
Cyclooxygenase-2 expression is associated with vascular endothelial
growth factor-C and lymph node metastases in human prostate cancer.
Arch Med Res. 40:268–275. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43.
|
Oka M, Iwata C, Suzuki HI, et al:
Inhibition of endogenous TGF-beta signaling enhances
lymphangiogenesis. Blood. 111:4571–4579. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44.
|
Zwaans BM and Bielenberg DR: Potential
therapeutic strategies for lymphatic metastasis. Microvasc Res.
74:145–158. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
45.
|
Burton JB, Johnson M, Sato M, et al:
Adenovirus-mediated gene expression imaging to directly detect
sentinel lymph node metastasis of prostate cancer. Nat Med.
14:882–888. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46.
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
47.
|
Wendt MK, Allington TM and Schiemann WP:
Mechanisms of the epithelial-mesenchymal transition by TGF-beta.
Future Oncol. 5:1145–1168. 2009. View Article : Google Scholar : PubMed/NCBI
|