Introduction
Worldwide, breast cancer is one of the most common
forms of malignancy in females. Most breast cancer deaths are
caused by distant metastasis from the primary tumor site and breast
cancer is a leading cause of mortality from such metastases
(1). Despite recent advances in
breast cancer treatment, subsequent metastasis can still occur
through blood vessels or lymphatic channels. In recent years,
attention has been focused on the anticancer properties of natural
products, which play an important role in the prevention of
disease. Thus, effective chemo-preventive treatments for breast
cancer metastasis would have an important impact on breast cancer
morbidity and mortality (2).
In metastasis, malignant tumor cells move from the
primary tumor to a secondary distant organ through a complex
multistage process involving the coordination of several signaling
pathways that allow changes in cell migration and invasion
(3). ECM interactions,
disconnection of intercellular adhesion, degradation of the ECM,
and the invasion of lymph and blood vessels are important steps in
cancer invasion and metastasis. The degradation of ECM, mediated by
the concerted action of matrix metalloproteinases (MMPs), plays an
important role in tumor invasion and metastasis (4,5). The
MMPs play a substantial role in several physiologic processes,
including tissue development, remodeling, and wound healing.
However, they are also involved in some tissue-destruction
diseases, such as arthritis, inflammation, cardiovascular diseases,
invasion, and metastasis. The MMP family of zinc- and
calcium-dependent endoproteinases is divided into four subclasses
based on their substrates. These include collagenase, gelatinase,
stromelysin, and membrane-associated MMPs (6). MMPs process a broad spectrum of cell
surface molecules and function in several important biological
processes. Activating MMPs enables the degradation of ECM by cancer
cells, allowing vasculature access, invasion, and migration into
organs. MMP-2 (also known as gelatinase A and 72-kDa type IV
collagenase) and MMP-9 (also known as gelatinase B and 92-kDa type
IV collagenase) are the most important enzymes in the degradation
of the basement membrane due to their ability to degrade type IV
collagen. Therefore, they are of pivotal importance for cancer
invasion and metastasis (7).
Increased expression of MMP-9 is associated with the progression
and invasion of tumors and MMP-2 is constitutively overexpressed in
highly metastatic tumors. MMP-9 is strongly expressed in invasive
breast cancer and may be used as a marker for the metastatic
potential of breast cancer (8).
Interestingly, the activity of MMPs tends to inhibit the endogenous
tissue inhibitors of metalloproteinases (TIMPs), which are specific
physiological inhibitors of MMPs. In particular, TIMP-1 binds to
the hemopexin domain of MMP-9. Disturbance in the balance between
MMPs and TIMPs may contribute to degradation or deposition of ECM.
Therefore, TIMP activators may be useful chemo-preventive
treatments for malignant cancer (7,9,10).
The expression of MMP-9 can be stimulated by various
agents, such as 12-O-tetradecanoylphorbol-13-acetate (TPA),
inflammatory cytokines, or growth factors, through the activation
of various intracellular signaling pathways. TPA is one of the most
commonly utilized agents for studying the mechanisms of
carcinogenesis (11,12). In addition to carcinogenesis, TPA
induces MMP-9 expression via the PI3K/Akt and MAPK signaling
pathways, increasing the invasiveness of cell lines. PI3K
activation leads to phosphorylation of phosphatidylinositides,
which then activates Akt. Akt appears to play various important
roles in regulating cellular growth, inflammatory reactions, and
metastasis. Mitogen-activated protein kinases (MAPKs) are members
of a highly conserved family of protein serine/threonine kinases
that includes extracellular signal-regulated kinases (ERK1/2),
c-jun N-terminal or stress-regulated protein kinases (JNK/SAPK),
and p38 MAPKs. These kinases are involved in numerous cellular
activities and are important regulators of inflammatory reactions
and metastasis (13–15).
The promoter region of MMP-9 is highly conserved and
contains AP-1 and NF-κB binding sites. TPA regulates the expression
of MMP-9 by modulating the activation of AP-1 and NF-κB through
PI3K/Akt and MAPK signaling pathways. AP-1 and NF-κB are inducible
transcription factors that play a central role in regulating the
expression of a wide variety of genes associated with cell
proliferation, immune response, inflammation and malignant
transformation (14,15). Several authors have reported that
the human MMP-9 promoter contains a cis-acting regulatory
element and NF-κB and AP-1 binding sites. These sites regulate
transcription of the MMP-9 gene. NF-κB is predominantly a
heterodimeric complex of p65/Rel A and p50. The NF-κB transcription
factor is found in the cytoplasm as an inactive homodimer or
heterodimer. The dimer associates through the Rel homology domain
with an inhibitory molecule, IκBα, a member of the IκB protein
family. Stimulation by TPA causes IκBα to dissociate from NF-κB and
phosphorylation and degradation via the
ubiquitin/proteaseome-dependent pathway. AP-1 consists of
homodimers and heterodimers of members from Fos and Jun families.
After activation, AP-1 and NF-κB move into the nucleus and promote
metastasis (11,16).
Recent studies of new anti-metastatic agents have
demonstrated that some natural compounds with chemopreventive
capability attenuate the metastasis of several types of cancer.
Frondoside A is a triterpenoid saponin, the major component of sea
cucumbers, which is known to exhibit a variety of biological
activities. Sea cucumbers have been traditionally used for food
delicacy in East Asia, as well as a dietary health supplement in
United States and Canada. Recently the anti-tumor and
anti-angiogenic activities of sea cucumbers have attracted
considerable attention (17–19).
In addition, some authors have reported that frondoside A exerts
anti-proliferative and apoptotic effects on the growth of cancer
cells (20–22). Although various bioactivity studies
of frondoside A have been performed, the molecular mechanisms by
which frondoside A affects the expression of MMP-9 and the invasion
of breast cancer cells remain unclear. In this study, human breast
cancer MDA-MB-231 cell line was used to investigate both the effect
of frondoside A on TPA-induced MMP-9 expression and the underlying
molecular mechanism of that effect. We show that frondoside A
inhibits the colony formation, migration, and invasion of breast
cancer cells by suppressing MMP-9 expression and blocking the
activation of AP-1 and NF-κB transcription factors and PI3K/Akt,
ERK1/2 and p38 MAPK signaling.
Materials and methods
Materials
BD BioCoat™ Matrigel™ Invasion Chambers were
obtained from BD Biosciences (San Jose, CA, USA). Cell culture
medium, RPMI-1640, and fetal bovine serum (FBS) were purchased from
Gibco-BRL (now part of Invitrogen Corporation, Carlsbad, CA, USA).
FuGENE 6 transfection reagent and X-treme GENE siRNA Transfection
Reagent were purchased from Roche (Indianapolis, IN, USA).
Antibodies against for phosphorylated p38 (p-p38), p-JNK, p-ERK,
p-IκBα MMP-2, and MMP-9 were purchased from Cell Signaling
Technology (Beverly, MA, USA). MMP-9 small interfering RNA (siRNA),
and antibodies for ERK, JNK, p38, c-Jun, c-Fos, NF-κB, IκBα, and
Histone H1 were purchased from Santa Cruz Biotechnology (Santa
Cruz, CA, USA). Frondoside A and other chemicals were purchased
from Sigma-Aldrich (St. Louis, MO, USA).
Cell culture
Human breast cell lines MDA-MB-231 were obtained
from the American Type Culture Collection (Manassas, VA, USA).
These cells were grown in RPMI-1640 supplemented with 10%
heat-inactivated FBS and 1% penicillin-streptomycin at 37°C in a
humidified incubator with 5% CO2.
Clonogenic assay using soft agar colony
formation
Colony formation ability was examined by an
anchorage-independent soft agar assay. Briefly, 0.5% agar gel with
10% FBS and 1% penicillin-streptomycin in RPMI-1640 was prepared
and added as a base agar to each well in a six-well culture dish.
MDA-MB-231 cells were plated for anchorage-independent growth
analysis in 0.4% agar gel with 10% FBS and 1%
penicillin-streptomycin in RPMI-1640 that was supplemented with the
target treatment. Plated cells were placed on top of the base agar.
The medium was replaced once every day. Cell colonies were counted
at weeks two and three using a microscope and digital camera.
Cell invasion assay
The cell invasion assay was conducted using BioCoat™
Matrigel™ Invasion Chambers according to the manufacturer’s
instructions. Briefly, the Matrigel coating was re-hydrated in 0.5
ml RPMI-1640 for 30 min immediately before the experiments. Cells
(5×104) suspended in 0.5 ml of serum-free medium were
added to the upper chamber of Matrigel-coated filter inserts. After
treatment with frondoside A for 1 h, 0.5 ml of serum-free medium
containing 50 nM of PMA was added to the bottom well as
chemoattractant. The chambers were incubated for 24 h. After
incubation, cells on the upper side of the chamber were removed
using cotton swabs, and cells that had migrated were fixed and
stained with 2% ethanol containing 0.2% crystal violet powder.
Invading cells were enumerated under a light microscope using a ×10
objective.
In vitro wound-healing repair assay
For the cell migration assay, the cells were seeded
into a 24-well culture dish until 90% confluent. The cells were
then maintained in serum-free medium for 12 h. The monolayers were
carefully scratched using a 200-μl pipette tip. Cellular
debris was removed by washing with PBS, and then the cells were
incubated in medium without serum. The migrated cells were fixed in
cold 75% methanol for 30 min and washed three times with PBS. The
cultures were photographed at 0 and 24 h to monitor the migration
of cells into the wounded area, and the closure of wounded area was
calculated.
Gelatin zymography assay
The activity of MMP-2 and MMP-9 in the conditioned
medium was determined by gelatin zymography protease assay.
Briefly, cells (2×105) were seeded in 6-well plates and
allowed to grow to 80% confluence. The cells were then maintained
in serum-free medium for 12 h prior to designated treatments with
frondoside A and TPA for 24 h. Conditioned media were collected,
cleared by centrifugation and mixed with 2X sodium dodecyl sulfate
(SDS) sample buffer (Invitrogen Corporation), and electrophoresed
in a polyacrylamide gel containing 0.1% (w/v) gelatin. Following
electrophoresis, the gels were incubated in a renaturing buffer
(2.5% Triton X-100) with gentle agitation to remove SDS and then
incubated in a developing buffer (50 mM Tris-HCl buffer, pH 7.4,
and 10 mM CaCl2) overnight at 37°C to allow digestion of
the gelatin. Gels were then stained with SimplyBlue SafeStain
(Invitrogen Corporation) until clear bands suggestive of gelatin
digestion appeared.
Western blot analysis
Cells were harvested in ice-cold lysis buffer
consisting of 1% Triton X-100, 1% deoxycholate, and 0.1% SDS. The
protein content of the cell lysates was determined using Bradford
reagent (Bio-Rad, Hercules, CA, USA). Proteins in each sample (50
μg total proteins) were resolved by 12% SDS-polyacrylamide
gel electrophoresis, transferred to a polyvinylidene difluoride
membrane, and exposed to the appropriate antibodies. The proteins
were visualized by the enhanced chemiluminescence detection system
(Amersham Biosciences, Piscataway, NJ, USA) using horseradish
peroxidase-conjugated anti-rabbit or anti-mouse secondary
antibodies. Images were acquired using an ImageQuant 350 analyzer
(Amersham Biosciences).
Immunofluorescence confocal
microscopy
MDA-MB-231 cells were cultured directly on glass
cover-slips in 35 mm-diameter dishes. Cells were fixed with 3.5%
paraformaldehyde in PBS for 10 min at room temperature and
permeabilized with 100% methanol for 10 min. To investigate the
cellular localization of NF-κB, we treated cell with a 1:100
dilution of polyclonal antibody against NF-κB for 24 h. After
extensive washing with PBS, cells were further incubated for 4 h at
room temperature with a 1:1000 dilution of secondary fluorescein
isothiocyanate (FITC)-conjugated donkey anti-rabbit IgG antibody.
Cell nuclei were stained with 1 μg/ml of
4′,6-diamidino-2-phenylindole (DAPI), and then analyzed by confocal
microscopy using an LSM 510 Meta microscope (Zeiss, Jena,
Germany).
Chromatin immunoprecipitation assay
To detect the in vitro association of nuclear
proteins with human MMP-9 promoter, chromatin immunoprecipitation
(ChIP) analysis was conducted as described previously (23) with some modifications. Briefly,
2×107 MBA-MB-231 cells were incubated in a culture
medium containing 1% formaldehyde for 10 min at room temperature,
and the cross-linking reaction was quenched by adding glycine to
0.125 M. Isolated nuclei were digested with 200 U of MNase at 37°C
for 15 min followed by sonication to produce chromatin of primarily
mononucleosome size. Fragmented chromatin was reacted with
antibodies for 3 h at 4°C. Protein-DNA complexes were recovered
with protein A agarose beads, washed, and then eluted with elution
buffer. Crosslinks were reversed at 65°C in 0.25 M NaCl overnight
and DNA was digested with proteinase K for 2 h at 50°C. The DNA was
isolated using a DNA purification kit (Qiagen). Immunoprecipitated
DNA was used for each PCR. PCR primers for the MMP-9 promoter (373
bp, including the NF-κB/AP-1 cluster, Gene Bank accession no.
AF538844) were: sense (5′-CACTTCAAAGTGGTAAGA-3′), antisense
(5′-GAAAGTGATGGAAGACTCC-3′).
Transient transfection and dual
luciferase assay
To determine promoter activity, we used a
dual-luciferase reporter assay system (Promega, Madison, WI).
MBA-MB-231 cells were transfected with NF-κB luciferase reporter
plasmid and AP-1 luciferase reporter plasmid (Stratagene, Grand
Island, NY) using FuGENE 6 reagent (Roche Applied Science) by
according to the manufacturer’s instructions. A Renilla
luciferase control plasmid pRL-CMV (Promega) was cotransfected as
an internal control for transfection efficiency. Twenty-four hours
after transfection, the cells were incubated with indicated
reagents for 1 h and then treated with TPA for 24 h. Luciferase
activity was assayed using a dual-luciferase assay kit (Promega)
according to the manufacturer’s instructions. Luminescence was
measured using a GloMaxTM 96-microplate luminometer
(Promega).
Transient transfection of siRNA
Transfection of MDA-MB-231 cells with siRNA was
performed using X-treme GENE siRNA Transfection Reagent (Roche
Applied Science, Basel, Switzerland), according to the
manufacturer’s instructions. Commercially available human
MMP-9-specific siRNAs, and negative control siRNAs were used for
transfection. In brief, X-treme GENE siRNA transfection reagent (10
μl) was added to 100 μl serum-free medium containing
2 μg of each siRNA oligo, and was incubated for 20 min at
room temperature. Gene silencing was measured after 72 h by western
blotting.
Statistical analysis
The results are expressed as the means ± SE. Each
experiment was repeated at least three times. Differences between
treatment groups were compared using paired Student’s t-tests.
Comparisons with p<0.05 were considered statistically
significant.
Results
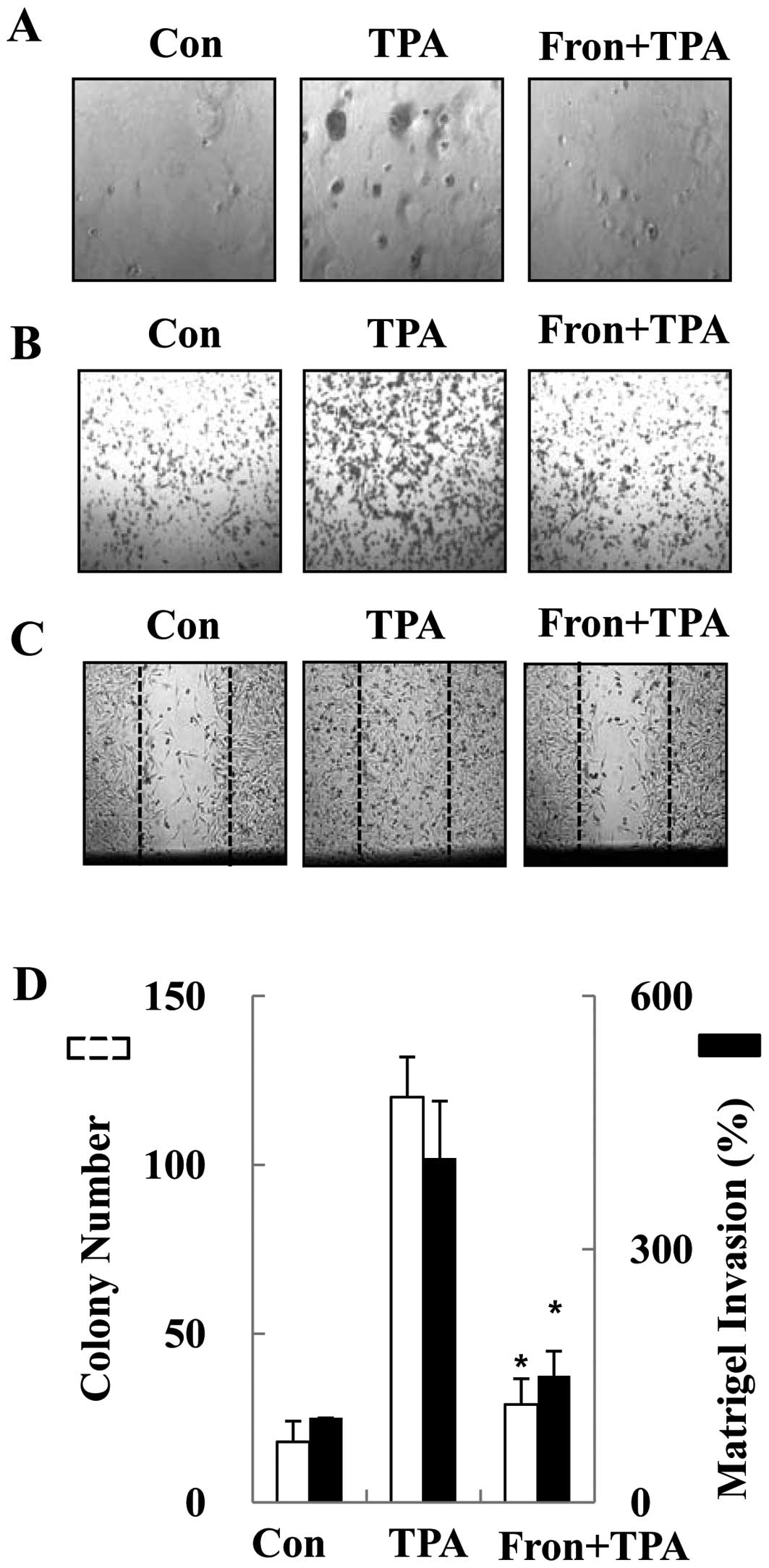
Frondoside A inhibited TPA-induced colony
formation, migration and invasion of human breast cancer cells
In vitro invasion and migration assays
including trans-well, soft agar, and wound-healing assays were used
to investigate the differential inhibitory effects of frondoside A
on the human breast cancer MDA-MB-231 cells. The anti-invasive
potential of frondoside A was first investigated by studying its
effects on the TPA-induced clonogenicity of breast cancer cells
using a soft agar clonogenic assay. Frondoside A exhibited an
inhibitory effect on the TPA-induced clonogenic ability of breast
cancer cells (Fig. 1A and D). We
next assessed the effect of frondoside A on invasion using a
trans-well migration assay. Treatment with 1 μM of
frondoside A inhibited 62% of cell invasion (Fig. 1B and D). The wound-healing assay
also indicated that TPA-induced migration of breast cancer cells
was inhibited by frondoside A (Fig.
1C). To confirm that the inhibitory activity of frondoside A
was not due to direct cytotoxicity of frondoside A, we examined the
toxicity of frondoside A in breast cancer cells. The breast cancer
cells were treated with various concentrations (0.1, 0.5, 1 and 2
μM) of frondoside A for 24 h. Frondoside A had no cytotoxic
effect on breast cancer cells at concentrations of 0.1–1 μM
(data not shown), indicating that frondoside A was not toxic to
breast cancer cells at these dosages. These results suggest cell
clonogenicity, invasion and migration of human breast cancer cells
are inhibited by frondoside A at non-cytotoxic concentrations.
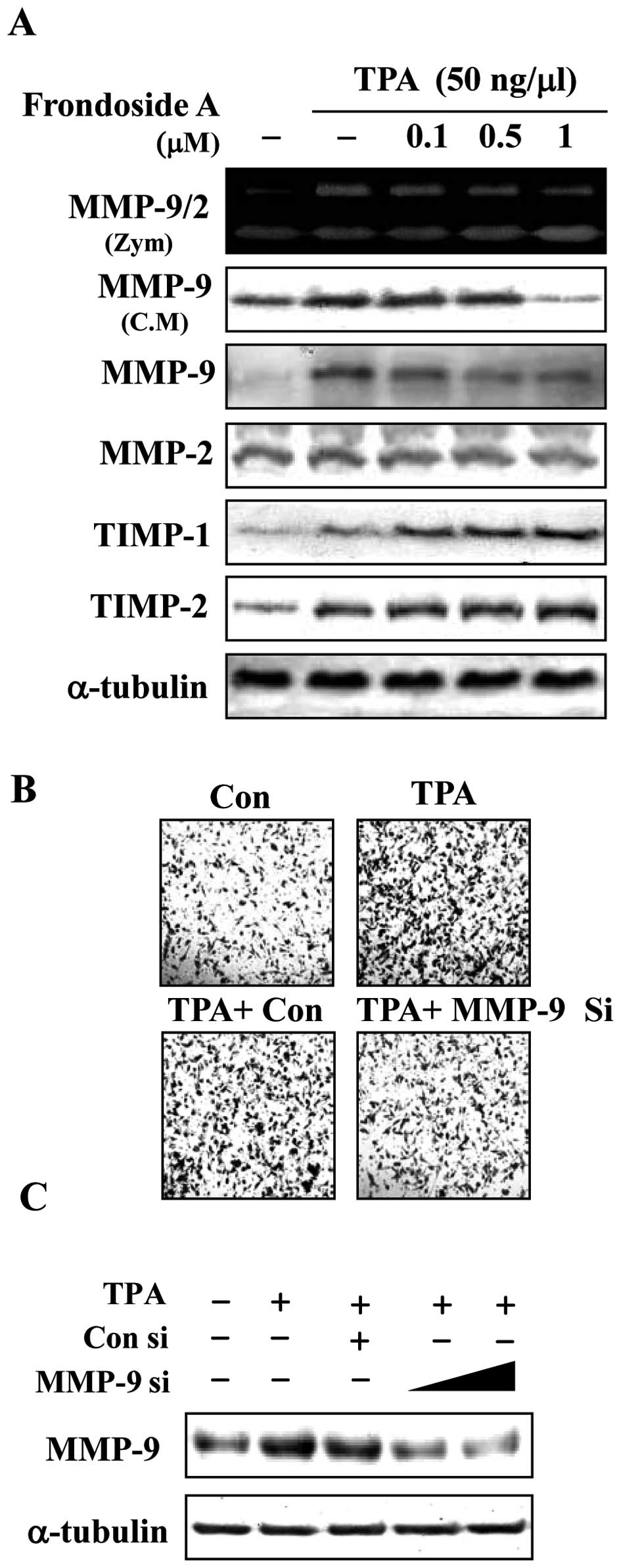
The effect of frondoside A on MMP-9,
TIMP-1 and TIMP-2 in human breast cancer cells
The upregulation of MMP-9 has been reported to play
an essential role in invasion and metastasis in breast cancer cells
(8). We therefore examined whether
the inhibitory effect of frondoside A against breast cancer cell
invasion was associated with regulation in MMP-9 enzyme activity
and secretion. Cells were treated with frondoside A 1 h prior to
the addition of TPA, and incubation for an additional 24 h.
Conditioned medium was collected and enzyme activity of MMP-9 was
analyzed using gelatin zymography. Treatment with TPA for 24 h
dramatically increased MMP-9 enzyme activity, whereas the MMP-2
enzyme activity was not affected by TPA. As shown in Fig. 2A, treatment of breast cancer cells
with frondoside A at doses above 0.1 μM suppressed
TPA-induced MMP-9 activity. Furthermore, treatment of breast cancer
cells with frondoside A decreased TPA-stimulated secretion and
intracellular expression of MMP-9 in a dose-dependent manner.
Because physiological activity of MMP-9 is closely related to that
of its specific endogenous inhibitors, TIMP-1 and TIMP-2 (7), we explored the potential effects of
frondoside A on TIMP-1 and TIMP-2 expression. As shown in Fig. 2A, frondoside A increased the levels
of TIMP-1 and TIMP-2 expression. TIMP-1 and TIMP-2 levels probably
increase as a result of the inhibition of MMP-9 activation by
frondoside A. These data indicate that TIMP-1 and TIMP-2 are
involved in the downregulation of MMP in TPA-stimulated human
breast cancer cells that have been treated with frondoside A. To
examine whether TPA induced invasion by regulating the MMP-9, a
small interference RNA (siRNA) approach was employed. Knockdown of
endogenous MMP-9 in MDA-MB-231 cells suppressed TPA-induced MMP
expression and invasion when compared to control siRNA (Fig. 2B and C). These results suggest
frondoside A inhibits MMP-9 activation and are consistent with
frondoside A inhibition of cell invasion. Further, the
anti-metastatic effect of frondoside A may be related to the
inhibition of the enzymatic ECM degradation processes in breast
cancer cells.
Frondoside A inhibits MMP-9 activity
through suppression of TPA-stimulated NF-κB and AP-1 activity
Expression of MMP-9 is regulated by the interaction
of transcription factors, such as AP-1 and NF-κB, with binding
elements in the MMP-9 gene promoter. To further determine whether
frondoside A inhibition of MMP-9 activity is mainly the result of
AP-1 and NF-κB signaling pathway inhibition, we investigated the
effects of specific inhibitors of AP-1 (curcumin) and of NF-κB
(Bay-11-7802) (24,25). Breast cancer cells were pretreated
with curcumin or Bay-11-7802 for 1 h and then stimulated with TPA
in the presence or absence of frondoside A for 24 h. The culture
media were subjected to gelatin zymography and western blotting.
Treatment with either curcumin or Bay 11-7802 inhibited TPA-induced
MMP-9 enzyme activity, secretion, and expression (Fig. 3A). Furthermore, the inhibitory
effects of curcumin and Bay-11-7082 were confirmed by the
prevention of TPA-stimulated AP-1 and NF-κB promoter activity and
nuclear translocation. This, in turn, resulted in the reversal of
TPA-stimulated AP-1 and NF-κB promoter activity and nuclear
translocation (data not shown). Because frondoside A decreased the
expression of MMP-9, we examined whether these transcription
factors are regulated by frondoside A in TPA-stimulated breast
cancer cells. The cells were treated with different concentrations
of frondoside A in the presence of TPA for 1 h, and nuclear
extracts were prepared for and analyzed by western blotting. TPA
induced the nuclear translocation of AP-1 and NF-κB, and frondoside
A inhibited the nuclear translocation of Ap-1 and NF-κB (Fig. 3B and C). Cells transiently
transfected with AP-1-Luc reporter or κB-Luc reporter plasmids were
treated with frondoside A in the presence of TPA. As shown in
Fig. 3D, TPA treatment increased
AP-1 and NF-κB promoter activity. AP-1 and NF-κB promoter activity
was suppressed by frondoside A in a dose-dependent manner. We found
two AP-1 binding sites (−79 and −533) and one NF-κB binding site
(−600) in the MMP-9 promoter. We used a CHIP-PCR assay to determine
whether NF-κB was involved in TPA-induced MMP-9 gene expression.
Chromatin was extracted and immunoprecipitated using anti-NF-κB
antibody, and the MMP-9 promoter regions (NF-κB/AP-1 cluster
−739/−358) were amplified by PCR and real-time PCR. As shown in
Fig. 3E, in vitro binding
of NF-κB to the MMP-9 promoter was increased by TPA, but the
increase was significantly inhibited by frondoside A. These results
indicate that the inhibition of AP-l and NF-κB signals mediates the
inhibitory effect of frondoside A on MMP-9 expression.
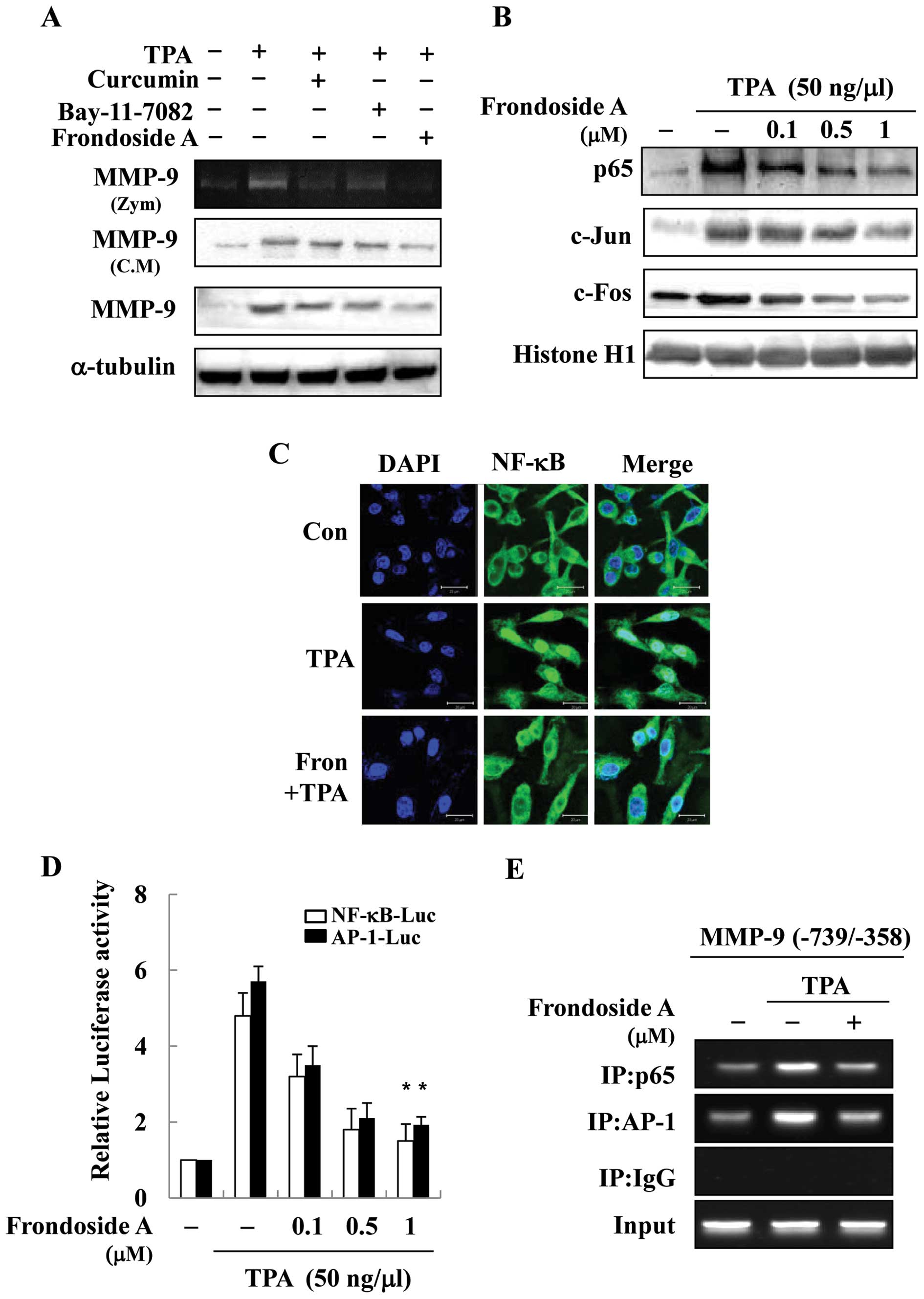 | Figure 3NF-κB and AP-1 are involved in
frondoside A-mediated downregulation of MMP-9. (A) MDA-MB-231 cells
were treated with TPA for 24 h in the presence of curcumin (AP-1
inhibitor, 10 μM), Bay-11-7802 (NF-κB inhibitor, 10
μM) and frondoside A (1 μM). Subsequently, MMP-9
enzymatic activity was analyzed by gelatin zymography (Zym).
Secretion and intracellular protein expression were analyzed by
western blotting (conditioned medium, C.M). (B) MDAMB-231 cells
were treated with frondoside A followed by TPA (50 ng/ml) treatment
for 1 h. Nuclear translocation of NF-κB and AP-1 complex (c-jun and
c-fos) was confirmed by western blotting. The nuclear extracts were
prepared and analyzed by western blotting. (C) Nuclear
translocation of NF-κB was assessed by confocal microscopy. The
cells were pre-treated with frondoside A (1 μM) for 1 h and
stimulated with TPA (50 ng/ml) for 1 h. Fixed cells were stained
with DAPI or anti-NF-κB p65 antibody, followed by incubation with
FITC-conjugated anti-rabbit IgG antibody. Images were obtained
using a confocal microscope. (D) Cells were co-transfected with the
AP-1 reporter, the κB-luc reporter, and the control Renilla
luciferase plasmid, pRL-CMV. After 24 h, cells were incubated with
the indicated concentrations of frondoside A for 1 h, and then
stimulated with TPA (50 ng/ml) for 24 h. Equal amounts of cell
extract were assayed for dual-luciferase activity. Expression of
the Renilla luciferase control was used to normalize
AP-1-luciferase activity and κB-luciferase activity. Each bar
represents the mean ± SE from 3 independent experiments.
*P<0.05 vs. the TPA-treated group. (E) Cells were
incubated with frondoside A for 1 h and the incubated with TPA for
4 h. DNA immunoprecipitated by an anti-NF-κB p65 antibody was
purified as described in Materials and methods. The precipitated
MMP-9 promoter region (−739 to −358 bp) was amplified by PCR and
real-time PCR. The input represents PCR products from chromatic
pellets prior to immunoprecipitation. |
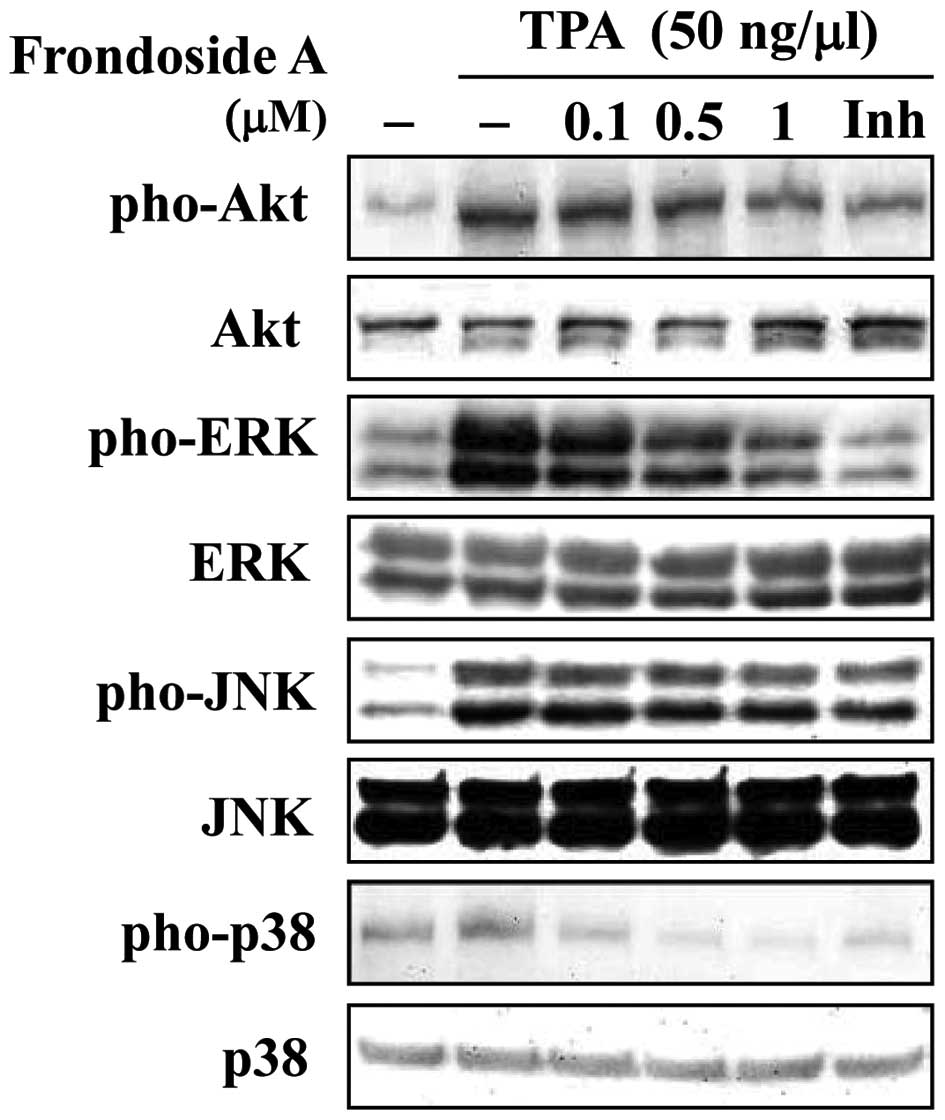
Frondoside A TPA-mediated invasion and
migration activation is through PI3K/Akt and ERK signaling
pathways
MMP-9 gene expression can be activated by a number
of signal transduction pathways, including those involving PI3K/Akt
and MAPKs, which are upstream modulators of AP-1 or NF-κB (12). We investigated whether frondoside A
inhibited the activation of these signaling pathways. As shown in
Fig. 4, phosphorylation of
PI3k/Akt, ERK1/2, JNK and P38 was significantly increased by
stimulation with TPA. Addition of frondoside A 1 h after TPA
treatment inhibited the phosphorylation of PI3K/Akt, ERK1/2 and p38
MAPK, but not JNK, in a dose-dependent manner. These results
suggest that frondoside A may be used to suppress MMP-9 activity
via modulation of PI3K/Akt, ERK1/2 and p38 MAPK signals that have
been stimulated by TPA.
Discussion
MMP-9 is an important regulatory molecule in a range
of physiological processes. Recent studies show that MMP-9 is
expressed differently in well-differentiated and poorly
differentiated tissue samples and may play a key role during the
development of breast cancer cells (26). Stimulation of breast cancer cells
by the TPA-induced release of MMP-9 is an important intermediary of
tumor metastasis. Therefore, the inhibition of MMP-9 would be an
effective therapeutic approach for breast carcinomas. Many recent
papers have reported the anti-metastatic effects of natural
products that are useful therapeutic agents which disrupt the MMP-9
activation associated with metastatic cancer cells (27). Frondoside A is purified from sea
cucumbers. Several studies have demonstrated the anti-cancer
effects of frondoside A. We investigated frondoside A suppression
of cell invasion via its inhibitory effect on MMP-9 expression. We
also provided detailed molecular mechanisms for the first time,
using TPA-treated breast cancer cells.
The activity of MMP-9 is regulated at three stages:
gene transcription, post-transcriptional activation of zymogens,
and endogenous expression of TIMPs (7). In our study, we showed that
frondoside A inhibits the activity, secretion, and expression of
MMP-9 in TPA-stimulated breast cancer cells at the transcriptional
and translational levels. TIMP-1 and -2 inhibit the catalytic
activity of MMP-9 by binding to activated MMP-9, controlling the
degradation of ECM. We investigated the effect of frondoside A on
the expression of TIMP-1 and TIMP-2 mRNA. TIMP-1 and TIMP-2 mRNA
levels gradually increased with frondoside A concentration (data
not shown). Additionally, the pharmacological actions of frondoside
A are associated with prevention of AP-1 and NF-κB activation. The
MMP-9 promoter region contains multiple DNA binding sites for
transcription factors, such as AP-1 (−533 bp and −79 bp) and NF-κB
(−600 bp). Upon stimulation by TPA, they are activated and bind to
the promoter region to regulate MMP-9 expression (28,29).
Activation of AP-1 and NF-κB plays a pivotal role in metastasis
because they induce transcription of metastatic-related genes.
NF-κB is then free to translocate to the nucleus and activate
target genes, including MMP-9. c-Jun and c-Fos are members of the
AP-1 family and translocate to the nucleus in TPA-stimulated breast
cancer cells. AP-1 is an important transcription factor for MMP-9
expression. Therefore, many current anti-metastatic therapies seek
to block AP-1 and NF-κB activity. The present study demonstrated
that frondoside A inhibited nuclear translocation and transactivity
of AP-1 and NF-κB, as assessed by western blot analyses and
promoter assays. We investigated the functional significance of
AP-1 and NF-κB transactivation in breast cancer cell MMP-9
activation. Treatment with curcumin and Bay-11-7802, potent
inhibitors of AP-1 and NF-κB transcriptional activation,
respectively, reduced the inductive effect of TPA on the activity,
secretion, and protein expression of MMP-9. Bay-1101 and curcumin
also reduced the TPA-induced transcriptional activity of AP-1 and
NF-κB. These findings collectively suggest that frondoside A
inhibits TPA-induced activation of MMP-9 by suppressing both AP-1
and NF-κB activation in breast cancer cells.
Frondoside A also significantly inhibited PI3K/Akt,
ERK1/2 and p38 MAPK activation in TPA-stimulated breast cancer
cells, indicating that frondoside A inhibits TPA-induced NF-κB and
AP-1 activation via inactivation of the PI3K/Akt, ERK1/2 and p38
MAPK signaling pathways. Researchers have recently demonstrated
that the PI3K/Akt and MAPK pathways are involved in the expression
of MMP-9 in breast cancer cells by way of their role in the
activation of AP-1 and NF-κB. Therefore, future experiments should
be performed to determine whether frondoside A induces
anti-metastatic effects in TPA-stimulated breast cancer cells
through tight regulation of PI3K/Akt and MAPK expression. In
response to TPA stimulation, PI3K/Akt and MAPKs are activated via
phosphorylation of both tyrosine and threonine residues, and this
phosphorylation leads to the activation of AP-1 and NF-κB. In this
study, frondoside A inhibited the phosphorylation of PI3K/Akt,
ERK1/2 and p38 MAPK, but had no effect on JNK phosphorylation. Our
results indicate that in breast cancer cells, frondoside A is a
potent inhibitor of the PI3K/Akt, ERK1/2 and p38 MAPK activation
that results from TPA stimulation. This suggests that the
anti-metastatic effects of frondoside A are due to inhibition of
the PI3K/Akt, ERK1/2 and p38 MAPK signaling pathways.
In conclusion, our study focused on the frondoside A
found in sea cucumbers. We evaluated the inhibitory effect of
frondoside A on TPA-induced clonogenicity, invasion, and migration
in breast cancer cells. Our data show that frondoside A inhibited
TPA-induced cell clonogenicity, invasion, and migration. We
demonstrated that frondoside A has an inhibitory effect on
TPA-induced breast cancer cells through a reduction of MMP-9
enzymatic activity, secretion, and expression. Furthermore,
frondoside A is a potent inhibitor of TPA-induced MMP-9 expression
and blocks the NF-κB, AP-1, PI3K/Akt, ERK1/2 and p38 MAPK signaling
pathways in breast cancer cells. Therefore, frondoside A is a
potential agent for prevention of the metastasis of breast
carcinoma in vivo.
References
|
1.
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar
|
|
2.
|
Weigelt B, Peterse JL and van ‘t Veer LJ:
Breast cancer metastasis: markers and models. Nat Rev Cancer.
5:591–602. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Liotta LA, Steeg PS and Stetler-Stevenson
WG: Cancer metastasis and angiogenesis: an imbalance of positive
and negative regulation. Cell. 64:327–336. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Johnson LL, Dyer R and Hupe DJ: Matrix
metalloproteinases. Curr Opin Chem Biol. 2:466–471. 1998.
View Article : Google Scholar
|
|
5.
|
Stamenkovic I: Matrix metalloproteinases
in tumor invasion and metastasis. Semin Cancer Biol. 10:415–433.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Brinckerhoff CE and Matrisian LM: Matrix
metalloproteinases: a tail of a frog that became a prince. Nat Rev
Mol Cell Biol. 3:207–214. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Clark IM, Swingler TE, Sampieri CL and
Edwards DR: The regulation of matrix metalloproteinases and their
inhibitors. Int J Biochem Cell Biol. 40:1362–1378. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Sato H and Seiki M: Regulatory mechanism
of 92 kDa type IV collagenase gene expression which is associated
with invasiveness of tumor cells. Oncogene. 8:395–405.
1993.PubMed/NCBI
|
|
9.
|
Hall MC, Young DA, Waters JG, Rowan AD,
Chantry A, Edwards DR and Clark IM: The comparative role of
activator protein 1 and smad factors in the regulation of timp-1
and MMP-1 gene expression by transforming growth factor-beta 1. J
Biol Chem. 278:10304–10313. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Sato T, Koike L, Miyata Y, et al:
Inhibition of activator protein-1 binding activity and
phosphatidylinositol 3-kinase pathway by nobiletin, a polymethoxy
flavonoid, results in augmentation of tissue inhibitor of
metalloproteinases-1 production and suppression of production of
matrix metalloproteinases-1 and -9 in human fibrosarcoma HT-1080
cells. Cancer Res. 62:1025–1029. 2002.
|
|
11.
|
Cho HJ, Kang JH, Kwak JY, et al:
Ascofuranone suppresses PMA-mediated matrix metalloproteinase-9
gene activation through the Ras/Raf/MEK/ERK- and Ap1-dependent
mechanisms. Carcinogenesis. 28:1104–1110. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Weng CJ, Chau CF, Hsieh YS, Yang SF and
Yen GC: Lucidenic acid inhibits PMA-induced invasion of human
hepatoma cells through inactivating MAPK/ERK signal transduction
pathway and reducing binding activities of NF-kappaB and AP-1.
Carcinogenesis. 29:147–156. 2008. View Article : Google Scholar
|
|
13.
|
Huang X, Chen S, Xu L, Liu Y, Deb DK,
Platanias LC and Bergan RC: Genistein inhibits p38 map kinase
activation, matrix metalloproteinase type 2, and cell invasion in
human prostate epithelial cells. Cancer Res. 65:3470–3478.
2005.PubMed/NCBI
|
|
14.
|
Eberhardt W, Huwiler A, Beck KF, Walpen S
and Pfeilschifter J: Amplification of IL-1 beta-induced matrix
metalloproteinase-9 expression by superoxide in rat glomerular
mesangial cells is mediated by increased activities of NF-kappa B
and activating protein-1 and involves activation of the
mitogen-activated protein kinase pathways. J Immunol.
165:5788–5797. 2000.
|
|
15.
|
Kajanne R, Miettinen P, Mehlem A, et al:
EGF-R regulates MMP function in fibroblasts through MAPK and AP-1
pathways. J Cell Physiol. 212:489–497. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Garg A and Aggarwal BB: Nuclear
transcription factor-kappaB as a target for cancer drug
development. Leukemia. 16:1053–1068. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Zhang W, Lu Y, Xu B, et al: Acidic
mucopolysaccharide from holothuria leucospilota has
antitumor effect by inhibiting angiogenesis and tumor cell invasion
in vivo and in vitro. Cancer Biol Ther. 8:1489–1499. 2009.
|
|
18.
|
Roginsky AB, Ding XZ, Woodward C, et al:
Anti-pancreatic cancer effects of a polar extract from the edible
sea cucumber cucumaria frondosa. Pancreas. 39:646–652. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Han H, Xu QZ, Yi YH, Gong W and Jiao BH:
Two new cytotoxic disulfated holostane glycosides from the sea
cucumber pentacta quadrangularis. Chem Biodivers. 7:158–167.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Li X, Roginsky AB, Ding XZ, et al: Review
of the apoptosis pathways in pancreatic cancer and the
anti-apoptotic effects of the novel sea cucumber compound,
frondoside A. Ann N Y Acad Sci. 1138:181–198. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Janakiram NB, Mohammed A, Zhang Y, et al:
Chemopreventive effects of frondanol A5, a cucumaria
frondosa extract, against rat colon carcinogenesis and
inhibition of human colon cancer cell growth. Cancer Prev Res
(Phila). 3:82–91. 2010.PubMed/NCBI
|
|
22.
|
Jin JO, Shastina VV, Shin SW, et al:
Differential effects of triterpene glycosides, frondoside A and
cucumarioside A2-2 isolated from sea cucumbers on caspase
activation and apoptosis of human leukemia cells. FEBS Lett.
583:697–702. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Johnson KD and Bresnick EH: Dissecting
long-range transcriptional mechanisms by chromatin
immunoprecipitation. Methods. 26:27–36. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
O-charoenrat P, Wongkajornsilp A,
Rhys-Evans PH and Eccles SA: Signaling pathways required for matrix
metalloproteinase-9 induction by betacellulin in head-and-neck
squamous carcinoma cells. Int J Cancer. 111:174–183. 2004.
View Article : Google Scholar
|
|
25.
|
Wang HH, Hsieh HL, Wu CY, Sun CC and Yang
CM: Oxidized low-density lipoprotein induces matrix
metalloproteinase-9 expression via a p42/p44 and JNK-dependent AP-1
pathway in brain astrocytes. Glia. 57:24–38. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Hoover KB, Liao SY and Bryant PJ: Loss of
the tight junction MAGUK ZO-1 in breast cancer: relationship to
glandular differentiation and loss of heterozygosity. Am J Pathol.
153:1767–1773. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Chakraborti S, Mandal M, Das S, Mandal A
and Chakraborti T: Regulation of matrix metalloproteinases: an
overview. Mol Cell Biochem. 253:269–285. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Ratovitski EA: LKB1/PEA3/DeltaNp63 pathway
regulates PTGS-2 (COX-2) transcription in lung cancer cells upon
cigarette smoke exposure. Oxid Med Cell Longev. 3:317–324. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Chou YC, Sheu JR, Chung CL, et al:
Nuclear-targeted inhibition of NF-kappaB on MMP-9 production by
N-2-(4-bromophenyl) ethyl caffeamide in human monocytic cells. Chem
Biol Interact. 184:403–412. 2010. View Article : Google Scholar : PubMed/NCBI
|