Introduction
Esophageal cancer occurs in humans worldwide with a
variable geographic distribution, and it ranks eighth among cancers
in the order of occurrence (1).
Esophageal squamous cell carcinoma (ESCC) is the most common type
of esophageal cancer in Japan. Despite recent advances in cancer
therapy, esophageal cancer remains one of the least responsive
malignancies (2). The overall
5-year survival rate for esophageal cancer is approximately 20–25%
for all stages (3), therefore, the
development of a molecular oncogenic therapy that can provide a
higher response rate than the current combinations of chemotherapy
and radiotherapy is urgently required.
Epigenetics is a rapidly expanding field that
focuses on stable changes in gene expression that are not
accompanied by any changes in the DNA sequence, and that are
mediated primarily by DNA methylation, histone modifications and
small non-coding RNA molecules (4). Histone deacetylation is known to
correlate with transcriptional silencing and with the
downregulation of the expression of proapoptotic genes, especially
in cancer cells. The histone deacetylase inhibitors (HDACIs) were
mainly thought to act by modulating the gene expression patterns,
including those of genes associated with cell cycle arrest and
apoptosis, by inhibiting the activity of histone deacetylases
(HDACs)(5). Previous we reported
that depsipeptide (FK228) and cyclic hydroxamic acid-containing
peptide 31 (CHAP31) have potent antitumor effects against ESCC
in vitro and in vivo (6–8).
Numerous studies have demonstrated that microRNAs
(miRNAs), non-coding RNAs 21–25 nucleotides in length, control gene
expression by targeting mRNAs for cleavage or translational
repression (9). These miRNAs are
associated with important biological processes, including
development, differentiation, apoptosis, and proliferation
(9,10). A growing body of evidence indicates
that the miRNA expression profiles associated with particular types
of cancer could serve as useful biomarkers for both disease
prognosis and diagnosis (11,12).
The purpose of this study was to determine whether
histone acetylation is associated with the regulation of the
expression of tumor-suppressive miRNAs in ESCC, and to identify the
target genes that are regulated by these miRNAs.
Materials and methods
Clinical ESCC specimens
RNA extraction was performed for 19 pairs of primary
ESCC and corresponding normal esophageal epithelium. All specimens
were obtained from patients who underwent surgical treatment at the
Department of Frontier Surgery, Graduate School of Medicine, Chiba
University, Japan from 2004 to 2005. The clinicopathological
characteristics of the patients and samples are listed in (Table I). The staging of the tumors was
carried out according to the TNM classification. The tissues were
frozen in liquid nitrogen immediately, and stored at −80°C.
 | Table IThe clinicopathological features of
patients with ESCC. |
Table I
The clinicopathological features of
patients with ESCC.
| No. | Gender | Age | Locationa | UICCb T | UICCb N | UICCb Stage |
|---|
| 1 | F | 48 | Mt | 4 | 4 | 4a |
| 2 | M | 69 | Mt | 1b | 0 | 1 |
| 3 | M | 75 | Mt | 3 | 3 | 3 |
| 4 | M | 73 | Mt | 3 | 2 | 3 |
| 5 | M | 67 | Lt | 4 | 1 | 4a |
| 6 | F | 70 | Mt | 1b | 0 | 1 |
| 7 | M | 65 | Mt | 1b | 0 | 1 |
| 8 | M | 53 | Ae | 1b | 3 | 3 |
| 9 | M | 65 | Mt | 3 | 1 | 3 |
| 10 | M | 67 | Lt | 2 | 2 | 3 |
| 11 | M | 71 | Mt | 3 | 2 | 3 |
| 12 | M | 77 | Mt | 3 | 0 | 2 |
| 13 | M | 70 | Lt | 3 | 1 | 3 |
| 14 | M | 66 | Lt | 1b | 0 | 1 |
| 15 | M | 47 | Mt | 3 | 0 | 2 |
| 16 | M | 68 | Lt | 3 | 3 | 3 |
| 17 | M | 53 | Ae | 4 | 1 | 4a |
| 18 | M | 60 | Lt | 4 | 0 | 3 |
| 19 | M | 71 | Lt | 3 | 1 | 3 |
Immunohistochemical staining was performed for 94
patients wh underwent surgical resection from 1997 to 2005. Normal
esophageal epithelial tissue specimens were obtained far from the
cancer in the specimens. All patients gave their informed consent
for tissue donation. Surgical treatments were performed without any
preoperative radiotherapy or chemotherapy.
ESCC cell culture and reagents
The human ESCC cell lines were cultured in DMEM
(Sigma-Aldrich, St. Louis, MO, USA) supplemented with 10% FCS in a
humidified incubator containing 5% CO2 at 37°C. The T.Tn
cells were provided by the Japanese Cancer Research Resources Bank.
TE2 cells were provided by Tohoku University. CHAP31 was provided
by Dr M. Yoshida (RIKEN Advanced Science Institute, Wako, Saitama,
Japan), and was dissolved in dimethyl sulfoxide.
Total RNA preparation and miRNA
analysis
The cells were seeded into 225 cm2 flasks
and incubated for 48 h, then treated with or without an
IC50 concentration of CHAP31 and harvested after 12 h of
treatment. The cells were washed with PBS and processed for RNA
extraction with TRIzol. The miRNA expression patterns were
evaluated using the TaqMan Low Density Array Human MicroRNA Panel
v1.0 (Applied Biosystems, Foster City, CA). The assay was conducted
in 2 steps: generation of cDNA by reverse transcription, and a
TaqMan real-time PCR assay. Briefly, the miRNAs in the samples were
converted into cDNA using 365 specific stem-loop reverse
transcription primers. The quantity of mature miRNAs was evaluated
using specific TaqMan real-time PCR primers and probes. Real-time
PCR was performed in duplicate using the GeneAmp Fast PCR Master
Mix (Applied Biosystems) and the ABI 7900HT Real-Time PCR System.
The comparative CT method was used to determine the expression
levels. The relative miRNA expression data were analyzed using the
GeneSpring GX version 7.3.1 software package (Agilent
Technologies), as previously described (13). Normalization to an endogenous gene
(RNA48) was used to normalize the expression data.
RNA isolation
The tissue specimens and cells were treated with the
TRIzol reagent (Invitrogen, Carlsbad, CA), according to the
manufacturer’s protocol, for total RNA extraction. The RNA
concentrations were determined spectrophotometrically, and the
molecular integrity was checked by gel electrophoresis. The RNA
quality was confirmed using an Agilent 2100 Bioanalyzer (Agilent
Technologies, Santa Clara, CA).
Mature miRNA transfection and small
interfering RNA treatment
The RNA sequences used in this study included mature
miR-375, Pre-miR™ miRNA precursors (hsa-miR-375; Pre-miR ID:
PM10327), miRNA-control (P/N: AM17111; Applied Biosystems), small
interfering RNA [Stealth Select RNAi™ siRNA; si-LDHB Cat#;
HSS106003 and HSS106005 (Invitrogen)], and siRNA-control (Stealth™
RNAi negative control medium GC Duplex; 12935-300). The RNA
sequences were incubated with Opti-MEM (Invitrogen) and
Lipofectamine™ RNAiMax reagent (Invitrogen) as previously described
(14).
Cell proliferation assay
The cells were transfected with 10 nM miRNA or siRNA
by reverse transfection and plated in 96-well plates at
3×103 cells per well. Cell proliferation was evaluated
by the XTT assay after 72 h, using the Cell proliferation kit II
(Roche Molecular Biochemicals, Mannheim, Germany). Triplicate wells
were measured for cell viability in each treatment group.
Cell migration assay
Cell migration was evaluated using modified Boyden
Chambers (Transwells; Corning/Costar #3422, NY, USA) containing
uncoated transwell-polycarbonate membrane filters with 8 μm pores
in 24-well tissue culture plates. Cells were transfected with 10 nM
miRNA or siRNA by reverse transfection and plated in 10 cm dishes
at 8×105 cells. The cells were cultured for 48 h, and
then 5×104 cells were added to the upper chamber of each
well and allowed to migrate for 48 h. The non-migratory cells were
gently removed from the filter surface of the upper chamber, and
the cells that migrated to the lower side were fixed and stained
with Diff-Quick (Sysmex Corporation, Tokyo, Japan). The number of
cells migrating to the lower surface was determined microscopically
by counting 4 constant areas per well. Triplicate wells were
measured for cell migration in each treatment group.
Cell invasion assays
The cell invasion assays were carried out using
modified Boyden Chambers containing transwell-precoated Matrigel
membrane filter inserts with 8 μm pores in 24-well tissue culture
plates (BD Biosciences, Bedford, MA) as previously described
(13). All experiments were
performed in triplicate.
Screening for miRNA-375 target genes by a
microarray analysis
The expression profiles of TE2 and T.Tn cells
transfected with miRNA-375 were displayed and compared against
miRNA-negative control transfectants using the Oligo-microarray
human 44K platform (Agilent Technologies) as previously described
(15). The hybridization and
washing steps were performed as previously described (16). The arrays were scanned using a
Packard GSI Lumonics ScanArray 4000 (Perkin Elmer, Boston, MA, USA)
and the data were analyzed. Data from each microarray study were
subjected to global normalization (16).
The predicted target genes and their target miRNA
site seed regions were explored using the TargetScan software
program (release 5.1, http://www.targetscan.org/). The sequences of
predicted mature miRNAs were confirmed using the miRBase software
program, release 13.0 (http://microrna.sanger.ac.uk/).
Real-time quantitative RT-PCR
First-strand cDNA was synthesized from 1 μg of total
RNA using a High Capacity cDNA reverse transcription kit (Applied
Biosystems). The gene-specific PCR products were assayed
continuously using a 7900-HT Real-Time PCR system according to the
manufacturer’s protocol. The first PCR step was a 10 min
denaturation at 95°C, followed by 40 cycles of a 15 sec
denaturation at 95°C and a 1 min annealing/extension at 63°C. The
TaqMan probes and primers used to amplify LDHB (Hs00929956_m1),
MTDH (Hs00757841_m1), PRDX1 (Hs03044567_g1), CXCL1 (Hs00236937_m1),
MAL2 (Hs00294541_m1), CHSY1 (Hs00208704_m1) and GAPDH
(Hs02758991_g1) were Assay-On-Demand Gene Expression Products
(Applied Biosystems). All reactions were performed in triplicate
and included a negative control lacking cDNA. The expression levels
of miRNA-375 (Assay ID: 000564) were analyzed by TaqMan
quantitative real-time PCR (TaqMan MicroRNA Assay; Applied
Biosystems) and normalized to RNU48 (Assay ID, 001006).
Western blot analysis
The cells were harvested and lysed 72 h after
transfection. Each cell lysate (50 μg of protein) was separated by
electrophoresis using Mini-PROTEAN TGX gels (Bio-Rad, Hercules, CA,
USA) and transferred to PVDF membranes. Immunoblotting was
performed with a monoclonal antibody against LDHB (1:10000; 2090-1;
Epitomics, Burlingame, CA, USA) and a polyclonal antibody against
MTDH (1:200; HPA015104; Sigma-Aldrich). A GAPDH antibody (1:1000;
ab8245; AbCam, Cambridge, UK) was used as an internal loading
control. The membranes were washed and incubated with a goat
anti-rabbit IgG (H+L)-HRP conjugate (Bio-Rad). The complexes were
visualized with the Immun-Star WesternC chemiluminescence kit
(Bio-Rad), and the expression levels of these proteins were
evaluated using the ImageJ software package (version 1.44;
http://rsbweb.nih.gov/ij/index.html).
Expression of LDHB and MTDH determined by
immunohistochemistry in clinical esophageal squamous cell carcinoma
specimens
Immunohistochemical staining was performed to detect
the expression of LDHB and MTDH in the cancerous and normal
epithelial regions in 19 ESCC clinical specimens. The LDHB
expression was also evaluated for 94 cases to assess the
immunohistochemical features of LDHB during the progression of
ESCC. Paraffin blocks were cut into 3 μm-thick sections, and
mounted after staining with hematoxylin and eosin.
Immunohistochemical staining was performed with a
monoclonal LDHB antibody (1:250; 2090-1; Epitomics, Burlingame, CA,
USA) and a polyclonal AEG-1/MTDH antibody (1:350; HPA015104;
Sigma-Aldrich). Secondary antibodies (biotinylated rabbit
anti-rabbit immunoglobulins, Dako K4003) were applied to all slides
for 60 min at 37°. The color was developed by 2 min of incubation
with DAB chromogen on the slides. The slides were all
counterstained with hematoxylin.
The proportion of the specimen showing positive
staining for LDHB in five representative fields at magnification
×100 was evaluated independently by two observers who were blinded
to the clinicopathological characteristics and prognosis of the
patients. The LDHB expression was graded according to the
percentage of LDHB positive cells using the scale: 0–10% (−),
10–50% (1+), 50–100% (2+).
Comparisons between the groups were performed using
the chi-square test. The overall survival was calculated from the
time of the surgical treatment until mortality or the last
follow-up date. The correlation between the overall survival and
LDHB expression was computed by the log-rank test and presented as
curves determined using the Kaplan-Meier method.
Results
Identification of upregulated miRNAs in
ESCC cell lines treated with CHAP31
The raw data were normalized using an internal
reference, RNU44, and 11 upregulated and 9 downregulated miRNAs we
identified using a cutoff p-value of <0.05 and a fold-change of
the with/without CHAP31 treatment value <0.5 (Log2 ratio;
Table II). The expression of
miR-375 in CHAP31-treated cells was upregulated more than 400-fold
in both cell lines, and was then identified to be a potential tumor
suppressor.
 | Table IIUpregulated and downregulated miRNAs
in ESCC cell lines treated with an IC50 concentration of
CHAP31. |
Table II
Upregulated and downregulated miRNAs
in ESCC cell lines treated with an IC50 concentration of
CHAP31.
| No. | microRNA | Accession no. | Fold change
(CHAP31/control)
| Average |
|---|
| | | T.Tn | TE2 | |
|---|
| 1 | miR-375 | MIMAT0000728 | 1724.872 | 473.217 | 1099.045 |
| 2 | miR-449a | MIMAT0001541 | 184.607 | 9.238 | 96.922 |
| 3 | miR-449b | MIMAT0003327 | 27.045 | 19.514 | 23.280 |
| 4 | miR-192 | MIMAT0000222 | 17.483 | 15.143 | 16.313 |
| 5 | miR-497 | MIMAT0002820 | 28.623 | 2.569 | 15.596 |
| 6 | miR-132 | MIMAT0000426 | 13.077 | 10.885 | 11.981 |
| 7 | miR-194 | MIMAT0000460 | 12.253 | 8.502 | 10.378 |
| 8 | miR-146b-5p | MIMAT0002809 | 2.813 | 3.394 | 3.103 |
| 9 | miR-183 | MIMAT0000261 | 3.222 | 2.697 | 2.959 |
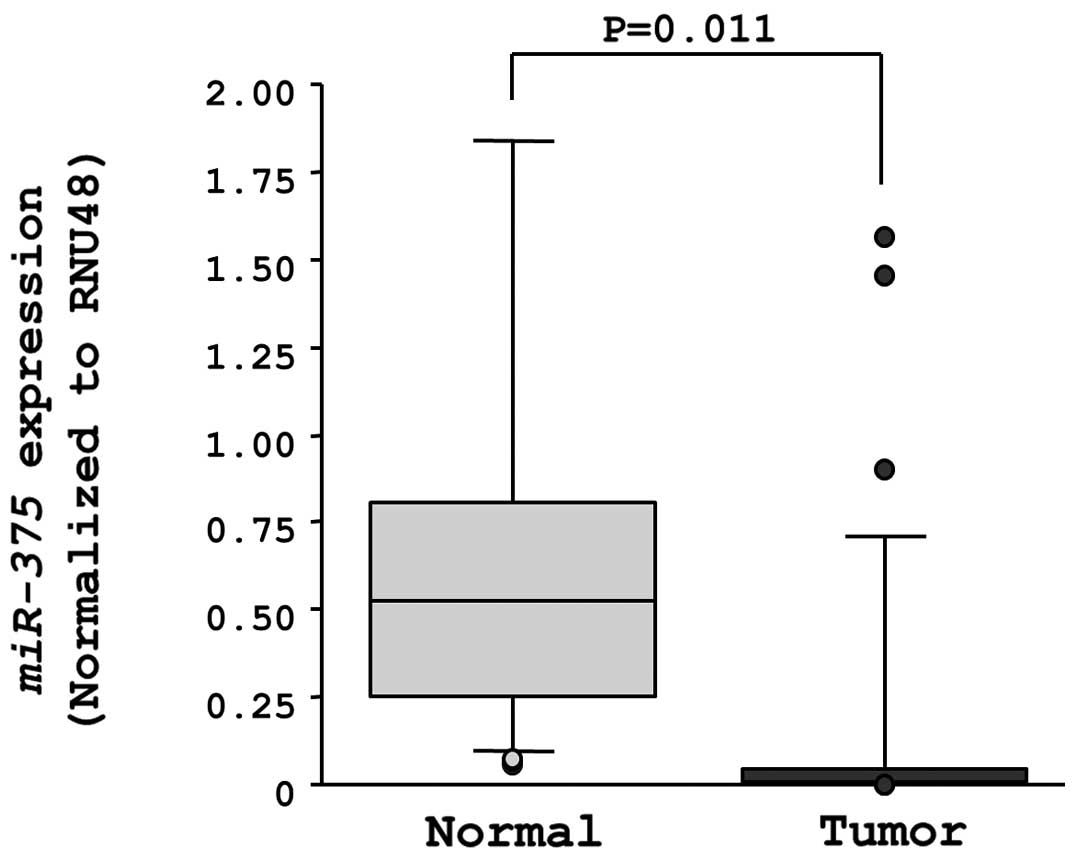
Expression of miR-375 in ESCC clinical
specimens
The expression levels of miR-375 were significantly
downregulated in clinical ESCC specimens in comparison to
neighboring normal tissue sections (Fig. 1).
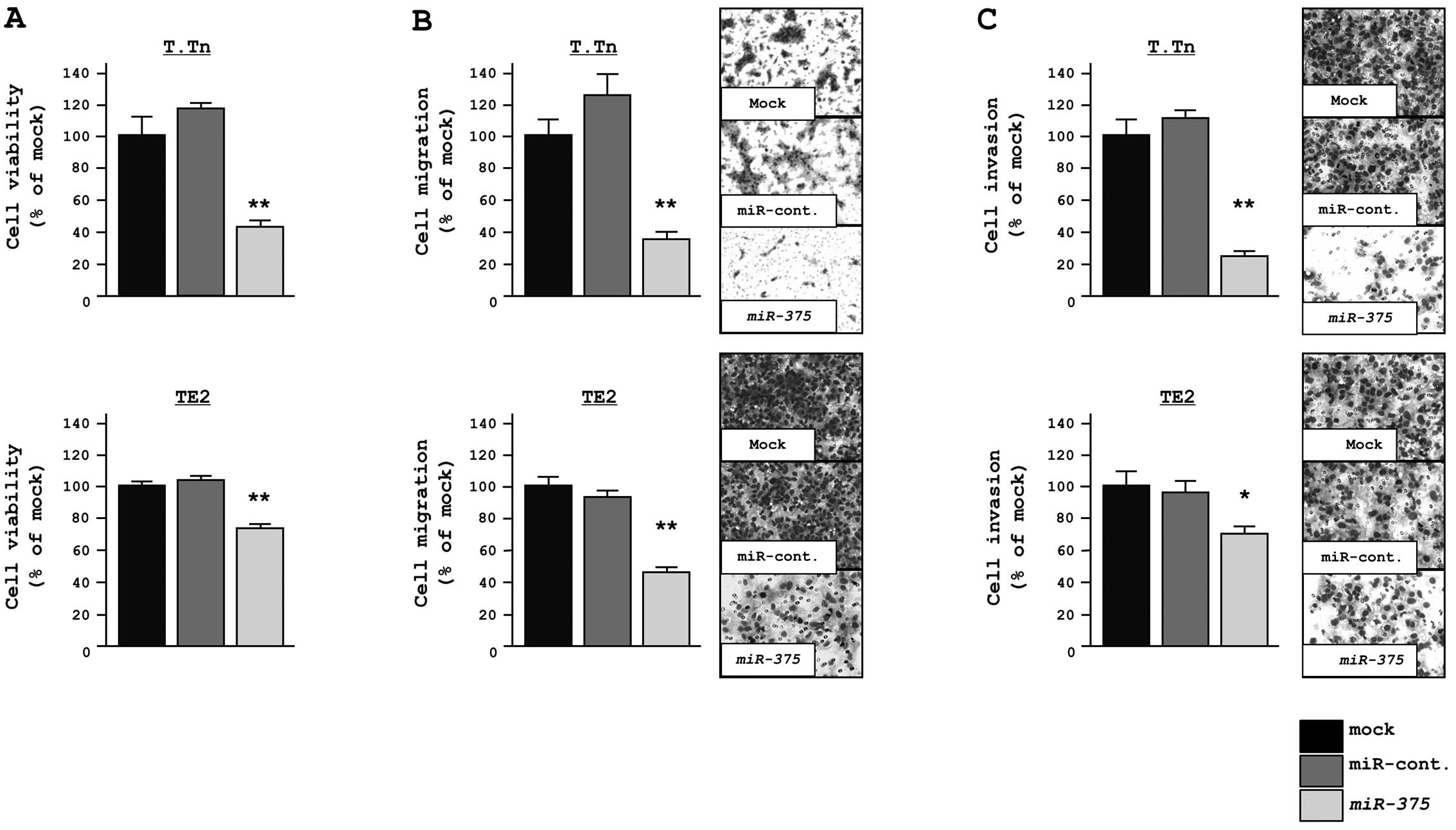
Effect of miR-375 transfection on the
proliferation, migration and invasion in ESCC cells
The functional significance of miR-375 was evaluated
with a gain-of-function assay using miR-375 transfectants. The XTT
assay showed significant inhibition of cell proliferation in
miR-375 transfectants in comparison with mock and miRNA-control
transfectants after a 72-h treatment (% cell proliferation, T.Tn;
43.7±1.4, 100.0±1.0 and 120.0±3.2, respectively, P<0.0001, TE2;
75.5±2.3, 100.0±1.5 and 101.5±1.7, respectively, P<0.0001;
Fig. 2A).
The migration assay demonstrated that the number of
cells that migrated was significantly decreased in miR-375
transfectants in comparison to mock and miRNA-control transfectants
(% cell migration, T.Tn; 36.9±4.3, 100.0±11.1 and 125.2±14.8,
respectively, P<0.0001, TE2; 44.1±2.4, 100.0±2.7 and 93.7±5.3,
P<0.0001; Fig. 2B).
Similarly, the Matrigel invasion assay demonstrated
that the number of invading cells was significantly decreased in
miR-375 transfectants in comparison to mock and miRNA-control
transfectants (% cell invasion, T.Tn; 24.8±3.4, 100.0±13.8 and
112.2±5.9, respectively, P<0.0001, TE2; 70.4±6.1, 100.0±10.3 and
96.1±6.0, respectively, P=0.0132; Fig.
2C).
Screening of miR-375 target genes by a
genome-wide gene expression analysis
The effect of miR-375 on protein-coding genes was
examined to identify candidate molecular targets of miR-375 in ESCC
cells. A comprehensive gene expression analysis was performed with
miR-375 transfectants in both the T.Tn and TE2 cell lines.
MiR-control transfectants that produced raw signal values of
<3,000 were excluded before comparisons were made. Sixteen genes
were downregulated by <−1.0 (Log2 ratio) in the miR-375
transfectants in both the T.Tn and TE2 cell lines (Table III). The 3′UTR of these genes were
screened for miR-375 target sites using the TargetScan database.
Six of these 16 genes had miR-375 target sites in their 3′UTR, and
were identified as putative target genes of miR-375.
 | Table IIIGenes downregulated by miR-375
treatment in ESCC cell lines. |
Table III
Genes downregulated by miR-375
treatment in ESCC cell lines.
| No. | Entrez gene ID | Gene name | Gene symbol | Log2 ratio
| miR-375 |
|---|
| | | | T.Tn | TE2 | Average | Target |
|---|
| 1 | 8000 | Prostate stem cell
antigen | PSCA | −1.50 | −1.95 | −1.72 | - |
| 2 | 642587 | NPC-A-5 | LOC642587 | −1.58 | −1.60 | −1.59 | - |
| 3 | 3945 | Lactate
dehydrogenase B | LDHB | −1.48 | −1.60 | −1.54 | 1 |
| 4 | 8581 | Lymphocyte antigen
6 complex, locus D | LY6D | −1.59 | −1.33 | −1.46 | - |
| 5 | 92140 | Metadherin | MTDH | −1.24 | −1.63 | −1.43 | 1 |
| 6 | 5052 | Peroxiredoxin
1 | PRDX1 | −1.43 | −1.35 | −1.39 | 1 |
| 7 | 218 | Aldehyde
dehydrogenase 3 family, memberA1 | ALDH3A1 | −1.36 | −1.32 | −1.34 | - |
| 8 | 2919 | Chemokine (C-X-C
motif) ligand 1 (melanoma Growth stimulating activity, α) | CXCL1 | −1.24 | −1.37 | −1.30 | 1 |
| 9 | 1789 | DNA
(cytosine-5-)-methyltransferase 3 β | DNMT3B | −1.28 | −1.31 | −1.29 | - |
| 10 | 1475 | Cystatin A (stefin
A) | CSTA | −1.02 | −1.57 | −1.29 | - |
| 11 | 114569 | Mal, T-cell
differentiation protein 2 | MAL2 | −1.23 | −1.34 | −1.29 | 1 |
| 12 | 445 | Argininosuccinate
synthetase 1 | ASS1 | −1.26 | −1.18 | −1.22 | - |
| 13 | 216 | Aldehyde
dehydrogenase 1 family, member A1 | ALDH1A1 | −1.11 | −1.31 | −1.21 | - |
| 14 | 84958 | Synaptotagmin-like
1 | SYTL1 | −1.20 | −1.21 | −1.20 | - |
| 15 | 10857 | Progesterone
receptor membrane component 1 | PGRMC1 | −1.32 | −1.02 | −1.17 | - |
| 16 | 22856 | Chondroitin sulfate
synthase 1 | CHSY1 | −1.09 | −1.01 | −1.05 | 1 |
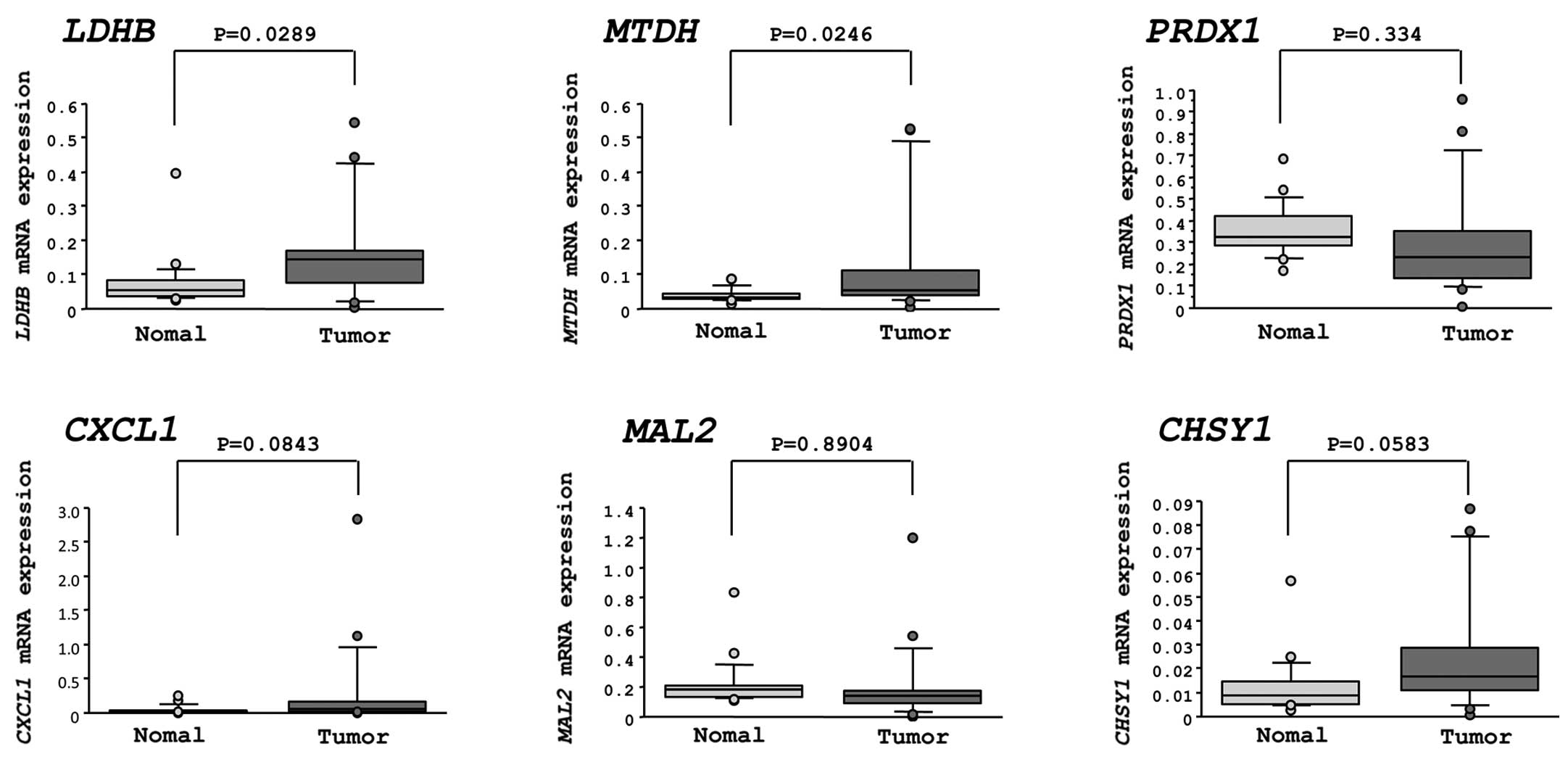
Expression levels of candidate miR-375
target genes in ESCC clinical specimens
The mRNA expression levels of the six candidate
genes were measured in clinical specimens of ESCC by quantitative
real-time reverse-transcription-PCR. Two genes, lactate
dehydrogenase B (LDHB) and astrocyte elevated gene-1/metadherin
(AEG-1/MTDH), were significantly upregulated in cancer tissues
(P=0.0289 and P=0.0246, respectively). The other four genes (PRDX1,
CXCL1, MAL2 and CHSY1) were not significantly upregulated in the
specimens of ESCC (Fig. 3).
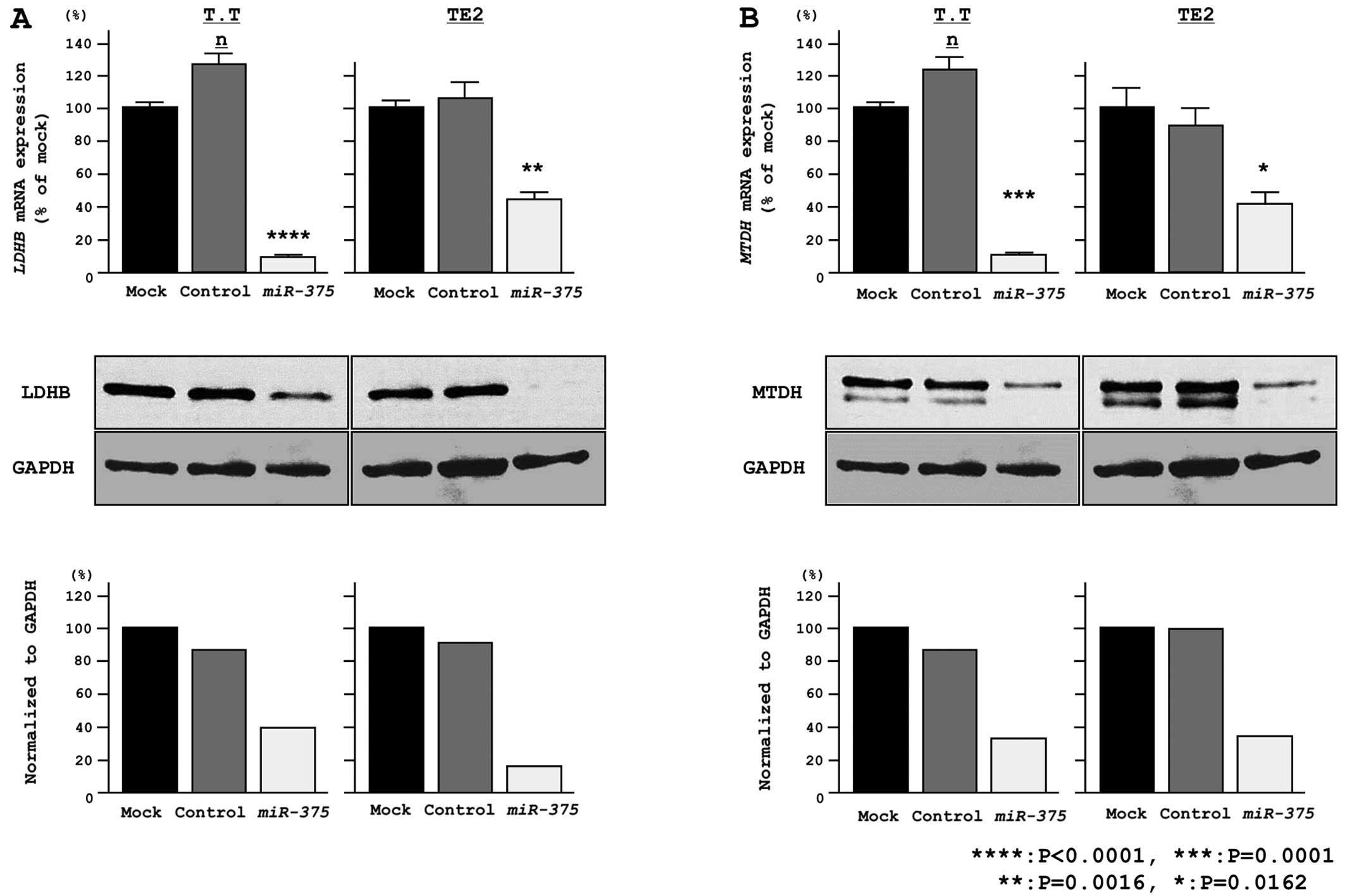
LDHB and MTDH mRNA and protein levels are
repressed by miR-375
Gain-of-function studies were conducted using
miR-375-transfected T.Tn and TE2 cells, and the mRNA and protein
expression levels of LDHB (Fig.
4A) and MTDH (Fig. 4B) were
found to be markedly downregulated in the transfectants in
comparison to the mock controls.
The expression levels of LDHB and MTDH by
IHC in ESCC clinical specimens
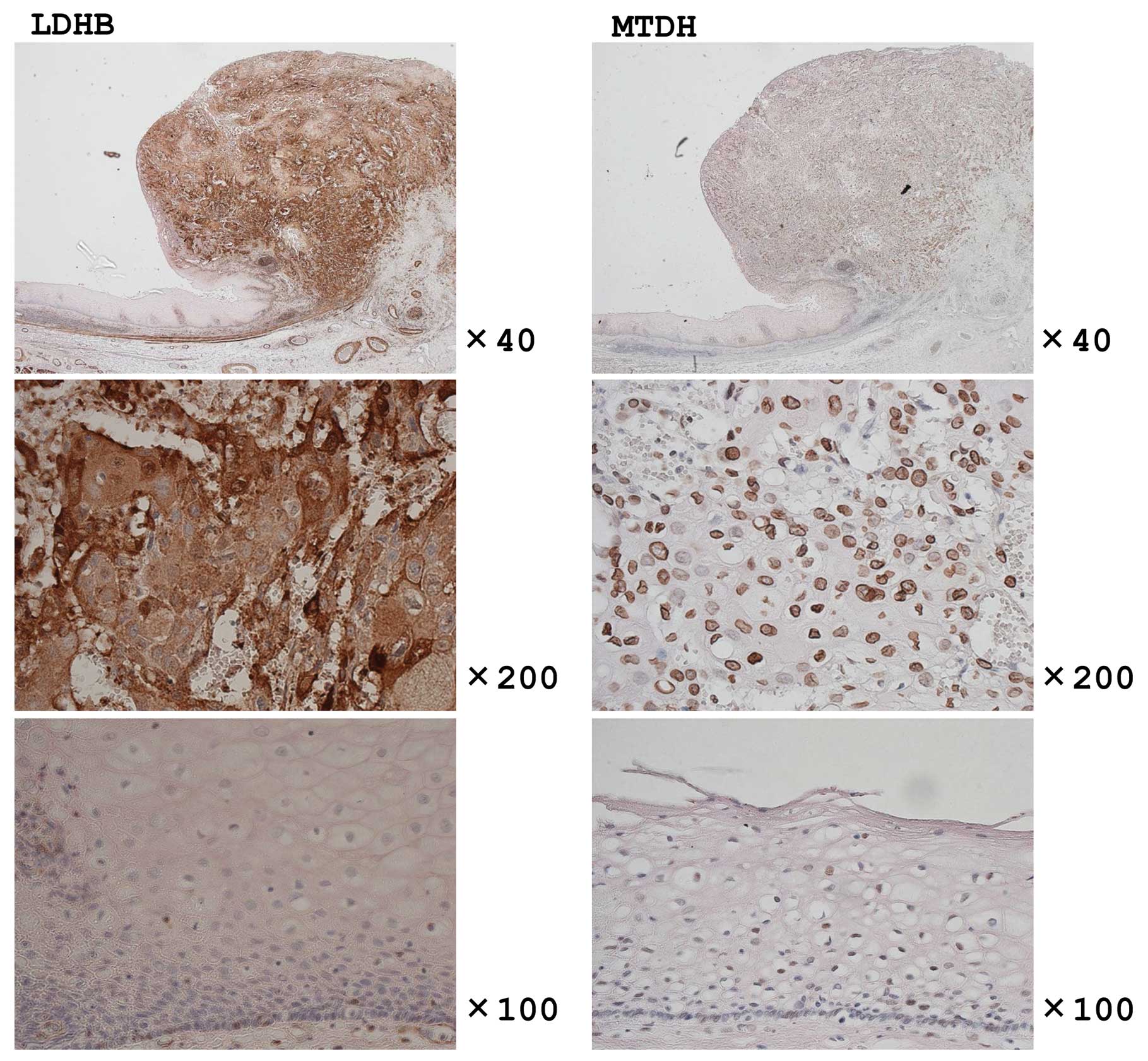
The expression of LDHB and MTDH was observed in all
the specimens examined, but the expression in tumors was much
higher in comparison to that in the corresponding normal epithelium
(Fig. 5).
The correlation between LDHB expression
and the clinicopathological characteristics
Positive staining for LDHB was found in 68% of the
cases. The correlation between LDHB expression and the
clinicopathological features, including patient age, gender, tumor
depth, lymph node metastasis, distant metastasis, tumor stage and
tumor differentiation was investigated (Table IV).
 | Table IVCorrelation between LDHB expression
and clinicopathological characteristics. |
Table IV
Correlation between LDHB expression
and clinicopathological characteristics.
| Clinicopathological
features |
LDHB− |
LDHB+ | P-value |
|---|
| Age | | | |
| ≤65 years | 18 | 39 | 0.9309 |
| >65 years | 12 | 25 | |
| Gender | | | |
| Male | 27 | 54 | 0.6775 |
| Female | 3 | 10 | |
| Tumor depth | | | |
| Tis/T1 | 15 | 20 | 0.0796 |
| T2/T3/T4 | 15 | 44 | |
| Lymph node
metastasis | | | |
| N0 | 19 | 23 | <0.05 |
| N1 | 11 | 41 | |
| Distant
metastasis | | | |
| M0 | 25 | 56 | 0.8219 |
| M1 | 5 | 8 | |
| Stage | | | |
| 0/I | 13 | 10 | <0.005 |
| II/III/IV | 16 | 55 | |
| Tumor
differentation | | | |
| Well | 10 | 16 | 0.3558 |
| Moderate | 11 | 37 | |
| Poor | 9 | 10 | |
| Other | 0 | 1 | |
The level of LDHB staining correlated significantly
with lymph node metastasis (P<0.05) and the tumor stage
(P<0.005). There was no significant correlation between LDHB
staining and other factors.
Relationship between LDHB expression and
patient prognosis
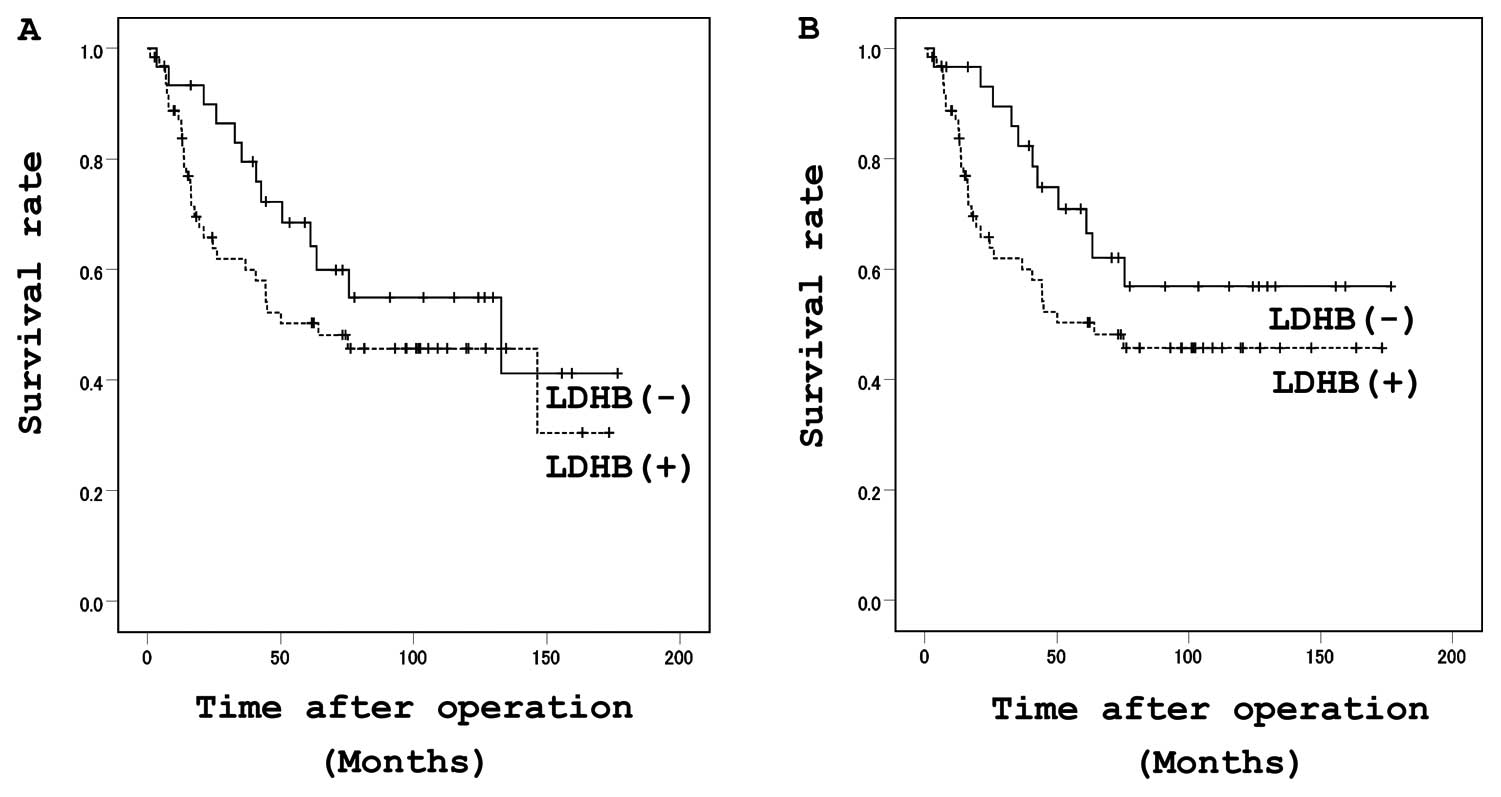
No significant differences in survival were observed
according to the LDHB expression levels, although there was a
tendency for the patients with high immunoreactivity for LDHB to
have a poorer prognosis (Fig. 6A and
B).
Effect of LDHB loss-of-function in ESCC
cell lines
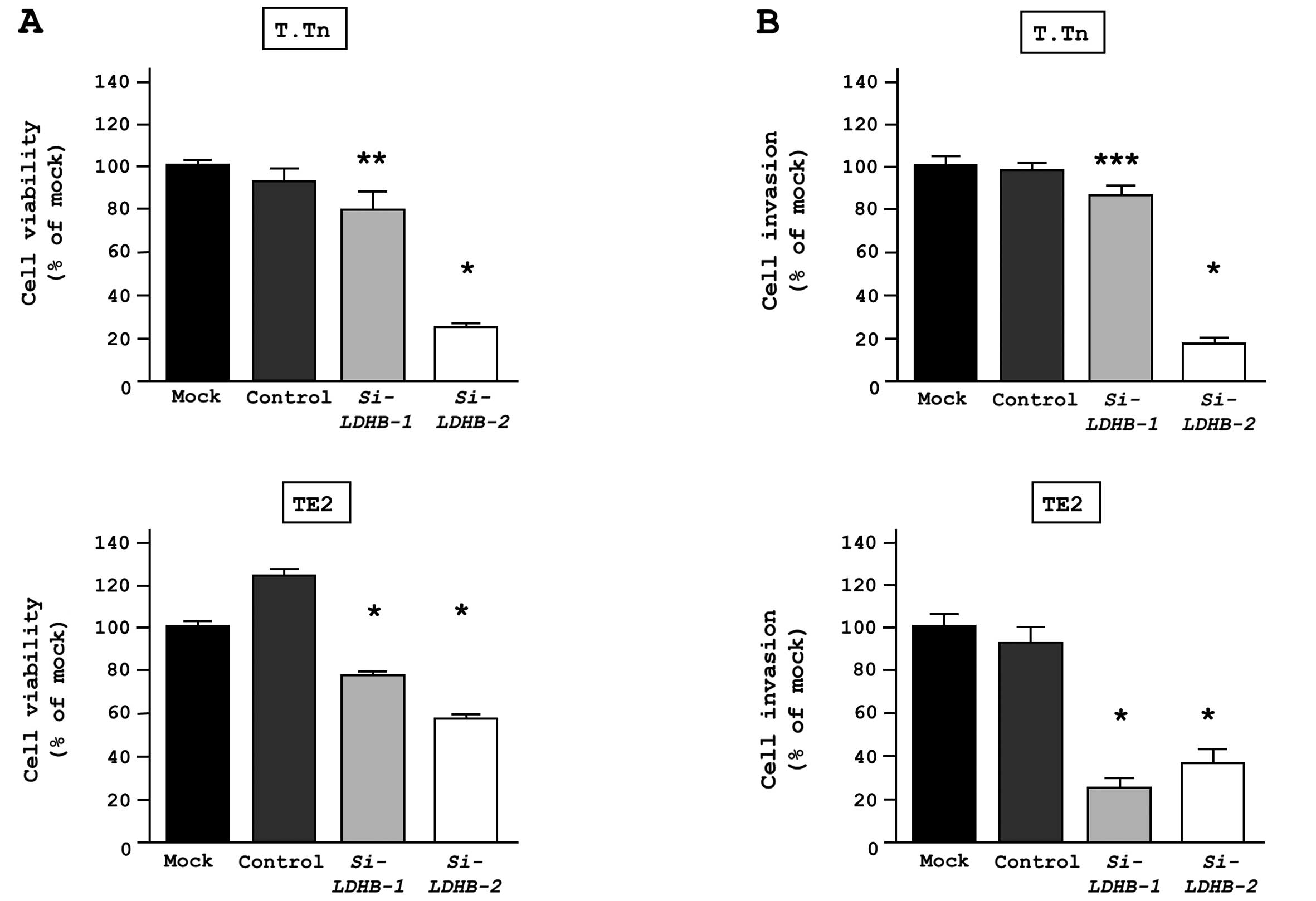
A loss-of function assay using a siRNA analysis was
performed to examine the oncogenic function of LDHB. The effects of
si-LDHB on the mRNA and protein expression levels were evaluated 72
h after transfection into both T.Tn and TE2 cells. The LDHB mRNA
and protein levels were both reduced after transfection. The XTT
assay revealed significant inhibition of cell proliferation in
si-LDHB transfectants in comparison to mock and si-control
transfectants after 72 h. The Matrigel invasion assay demonstrated
that the number of invading cells was significantly lower in the
si-LDHB transfectants compared to mock and si-control
transfectants (Fig. 7).
Discussion
This study showed that HDACIs induced miR-375
overexpression in ESCC cell lines, and that miR-375 downregulated
LDHB and AEG-1/MTDH in ESCC cell lines. HDACs are
associated with numerous types of cancer and regulate cancer
development (5). Histone
deacetylation correlates with transcriptional silencing and with
the downregulation of the expression of proapoptotic genes,
especially in cancer cells (5–8).
HDACIs cause changes in the acetylation status of chromatin,
resulting in changes in gene expression, induction of apoptosis,
cell cycle arrest, and inhibition of angiogenesis and metastasis
(17,18).
Dysregulation of miRNAs is associated with
dysregulated gene expression of tumor suppressors and oncogenes in
several types of cancer (9).
miRNAs are differentially expressed in several cancers, including
ESCC, as indicated by their expression signatures (13–15,19–21).
A previous study analyzed the function of miR-375 as a tumor
suppressor in head and neck squamous cell carcinoma and maxillary
sinus squamous cell carcinoma, and investigated the target genes
and their function (15,22). Another study showed significantly
lower expression of miR-375 in ESCC (19).
The correlation(s) between DNA demethylation or
histone acetylation and miRNAs has not been fully elucidated, and
few reports exist on this correlation regarding miR-375 (23–25).
The downregulation of miR-375 is caused by promoter
hypermethylation.
A computational analysis revealed that miR-375 is
located in a CpG island on chromosome 2q35 (National Center for
Biotechnology Information) (24).
The acetylation of lysine residues in the N-terminal histone tail
of the unmethylated CpG island induces an open structure of the
chromatin and increased the transcription of that region (5,26).
Although histone acetylation might directly upregulate miR-375,
further experiments are required to confirm this.
We showed that the reinstatement of miR-375 could
inhibit cancer cell proliferation and invasion in ESCC cell lines.
In a recent study, it was shown that miR-375 inhibits tumor growth
and metastasis in ESCC in vivo and in vitro. That
study also revealed that the downregulation of miR-375
significantly correlated with a poor prognosis in ESCC (25).
In the present study, the genome-wide gene
expression analysis revealed six candidate genes that were
regulated by miR-375(LDHB, MTDH, PRDX1,
CXCL1, MAL2 and CHSY1). In this analysis, the
criterion used for selection was upregulation in cancer tissues.
Two genes, LDHB and MTDH, were of particular interest
as they had also been identified in a search for miR-375 targets in
HNSCC, an indication that these genes may have a role in the
oncogenesis of human squamous cell carcinoma (15,22).
LDHB is known to convert lactase to pyruvate, which
is then further oxidized (27). A
correlation between LDHB expression and cancer has been reported
(27). It was also revealed that
the serum levels of LDHB are specifically elevated in non-small
cell lung carcinoma patients, and are progressively increased with
clinical stage (28). Kinoshita
et al reported that the mRNA expression of LDHB might serve
as a predictor of a poor prognosis in maxillary sinus squamous cell
carcinoma (22). In our study, the
knockdown of LDHB by RNAi showed a tumor suppressive effect
in ESCC cells. In addition, ESCC clinical specimens exhibited a
high level of LDHB expression at both the mRNA and protein levels
compared with the normal esophageal epithelium. Kaplan-Meier curves
and log-rank tests revealed that positive immunoreactivity for the
LDHB protein had a tendency to indicate a poor prognosis. The
current results indicate that LDHB plays an important role in
cancer signaling pathways in ESCC.
Recent studies have shown that Metadherin
(MTDH)/Astrocyte Elevated Gene 1 (AEG-1) plays a key
role in tumor progression, invasion, metastasis, and resistance to
chemotherapies (29). There is
overexpression of AEG-1/MTDH in ESCC, and a multivariate analysis
indicated that AEG-1/MTDH expression is a valuable marker of ESCC
progression (30).
The current study suggested the possibility that
histone deacetylase inhibition, the downregulation of miR-375, and
the upregulation of LDHB and AEG-1/LDHB are involved in the
initiation and development of ESCC. Further studies are required to
elucidate the additional roles of miR-375-regulated molecular
networks and to characterize the epigenetic crosstalk between
histone acetylation and miRNAs, and also to determine the mechanism
underlying the involvement of LDHB and MTDH in human
oncogenesis.
Acknowledgements
This work was supported by
Grant-in-Aid for Scientific Research (KAKENHI) no. 23890031.
References
|
1.
|
Enzinger PC and Mayer RJ: Esophageal
cancer. N Engl J Med. 349:2241–2252. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Akutsu Y and Matsubara H: The significance
of lymph node status as a prognostic factor for esophageal cancer.
Surg Today. 41:1190–1195. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
National Cancer Institute (Bethesda, MD,
USA). The Surveillance, Epidemiology and End Results (SEER)
Program. Cancer Statistics Review. 2007.
|
|
4.
|
Boumber Y and Issa JP: Epigenetics in
cancer: what’s the future? Oncology (Williston Park). 25:220–226.
2282011.
|
|
5.
|
Hoshino I and Matsubara H: Recent advances
in histone deacetylase targeted cancer therapy. Surg Today.
40:809–815. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Hoshino I, Matsubara H, Ochiai T, et al:
Histone deacetylase inhibitor FK228 activates tumor suppressor
Prdx1 with apoptosis induction in esophageal cancer cells. Clin
Cancer Res. 11:7945–7952. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Murakami K, Matsubara H, Hoshino I, et al:
CHAP31 induces apoptosis only via the intrinsic pathway in human
esophageal cancer cells. Oncology. 78:62–74. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Hoshino I, Matsubara H, Ochiai T, et al:
Gene expression profiling induced by histone deacetylase inhibitor,
FK228, in human esophageal squamous cancer cells. Oncol Rep.
18:85–92. 2007.PubMed/NCBI
|
|
9.
|
Bartel DP: MicroRNAs: genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Kloosterman WP and Plasterk RH: The
diverse functions of microRNAs in animal development and disease.
Dev Cell. 11:441–450. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Lu J, Getz G, Miska EA, et al: MicroRNA
expression profiles classify human cancers. Nature. 435:834–838.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Calin GA and Croce CM: MicroRNA signatures
in human cancers. Nat Rev Cancer. 6:857–866. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Chiyomaru T, Enokida H, Nakagawa M, et al:
miR-145 and miR-133a function as tumour suppressors and directly
regulate FSCN1 expression in bladder cancer. Br J Cancer.
102:883–891. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Ichimi T, Enokida H, Seki N, et al:
Identification of novel microRNA targets based on microRNA
signatures in bladder cancer. Int J Cancer. 125:345–352. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Nohata N, Hanazawa T, Seki N, et al: Tumor
suppressive microRNA-375 regulates oncogene AEG-1/MTDH in head and
neck squamous cell carcinoma (HNSCC). J Hum Genet. 56:595–601.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Sugimoto T, Seki N, Hata A, et al: The
galanin signaling cascade is a candidate pathway regulating
oncogenesis in human squamous cell carcinoma. Genes Chromosomes
Cancer. 48:132–142. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Wagner JM, Hackanson B, Lübbert M, et al:
Histone deacetylase (HDAC) inhibitors in recent clinical trials for
cancer therapy. Clin Epigenetics. 1:117–136. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Ma X, Ezzeldin HH and Diasio RB: Histone
deacetylase inhibitors: current status and overview of recent
clinical trials. Drugs. 69:1911–1934. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Kano M, Seki N, Matsubara H, et al:
miR-145, miR-133a and miR-133b: Tumor-suppressive miRNAs target
FSCN1 in esophageal squamous cell carcinoma. Int J Cancer.
127:2804–2814. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Kikkawa N, Hanazawa T, Seki N, et al:
miR-489 is a tumour-suppressive miRNA target PTPN11 in
hypopharyngeal squamous cell carcinoma (HSCC). Br J Cancer.
103:877–884. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Yoshino H, Chiyomaru T, Nakagawa M, et al:
The tumour-suppressive function of miR-1 and miR-133a targeting
TAGLN2 in bladder cancer. Br J Cancer. 104:808–818. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Kinoshita T, Nohata N, Yoshino H, et al:
Tumor suppressive microRNA-375 regulates lactate dehydrogenase B in
maxillary sinus squamous cell carcinoma. Int J Oncol. 40:185–193.
2012.PubMed/NCBI
|
|
23.
|
Li X, Lin R and Li J: Epigenetic silencing
of microRNA-375 regulates PDK1 expression in esophageal cancer. Dig
Dis Sci. 56:2849–2856. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Tsukamoto Y, Nakada C, Moriyama M, et al:
MicroRNA-375 is downregulated in gastric carcinomas and regulates
cell survival by targeting PDK1 and 14-3-3zeta. Cancer Res.
70:2339–2349. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Kong KL, Kwong DL, Guan XY, et al:
MicroRNA-375 inhibits tumour growth and metastasis in oesophageal
squamous cell carcinoma through repressing insulin-like growth
factor 1 receptor. Gut. 61:33–42. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Rountree MR, Bachman KE, Baylin SB, et al:
DNA methylation, chromatin inheritance, and cancer. Oncogene.
20:3156–3165. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Zha X, Wang F, Zhang H, et al: Lactate
dehydrogenase B is critical for hyperactive mTOR-mediated
tumorigenesis. Cancer Res. 71:13–18. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Chen Y, Zhang H, Xiao X, et al: Elevation
of serum l-lactate dehydrogenase B correlated with the clinical
stage of lung cancer. Lung Cancer. 54:95–102. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Hu G, Wei Y and Kang Y: The multifaceted
role of MTDH/AEG-1 in cancer progression. Clin Cancer Res.
15:5615–5620. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Yu C, Chen K, Song L, et al:
Overexpression of astrocyte elevated gene-1 (AEG-1) is associated
with esophageal squamous cell carcinoma (ESCC) progression and
pathogenesis. Carcinogenesis. 30:894–901. 2009. View Article : Google Scholar : PubMed/NCBI
|