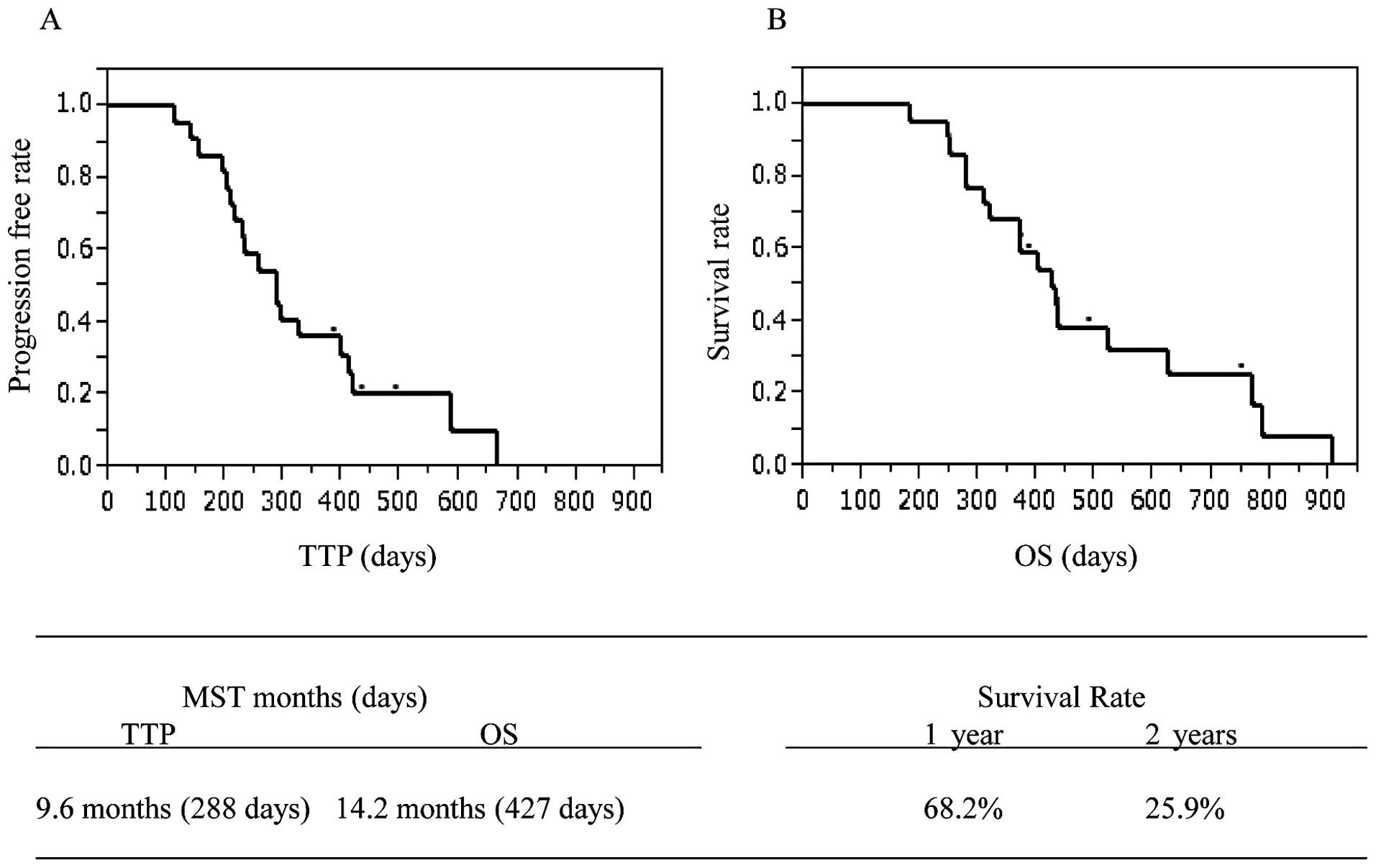
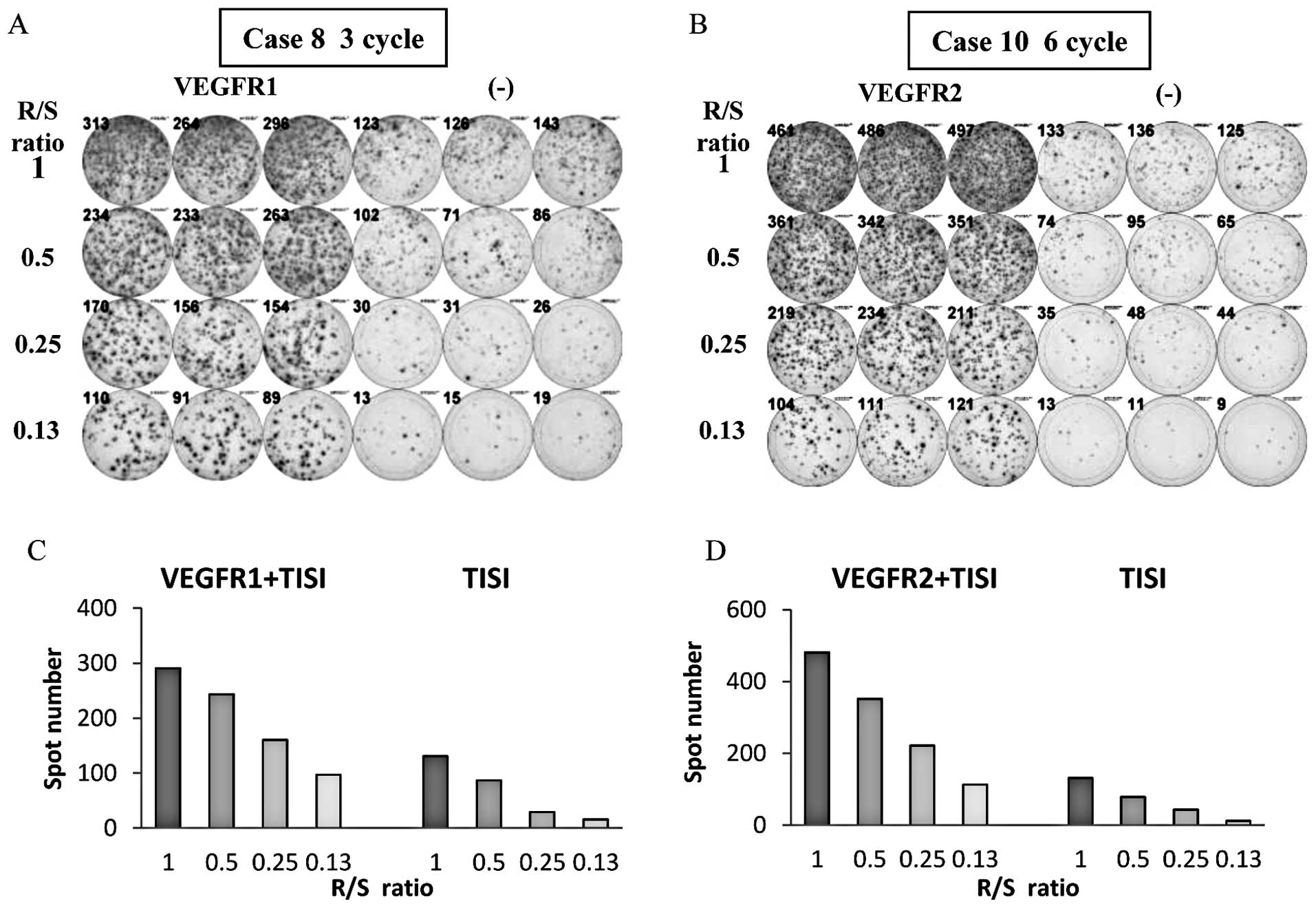
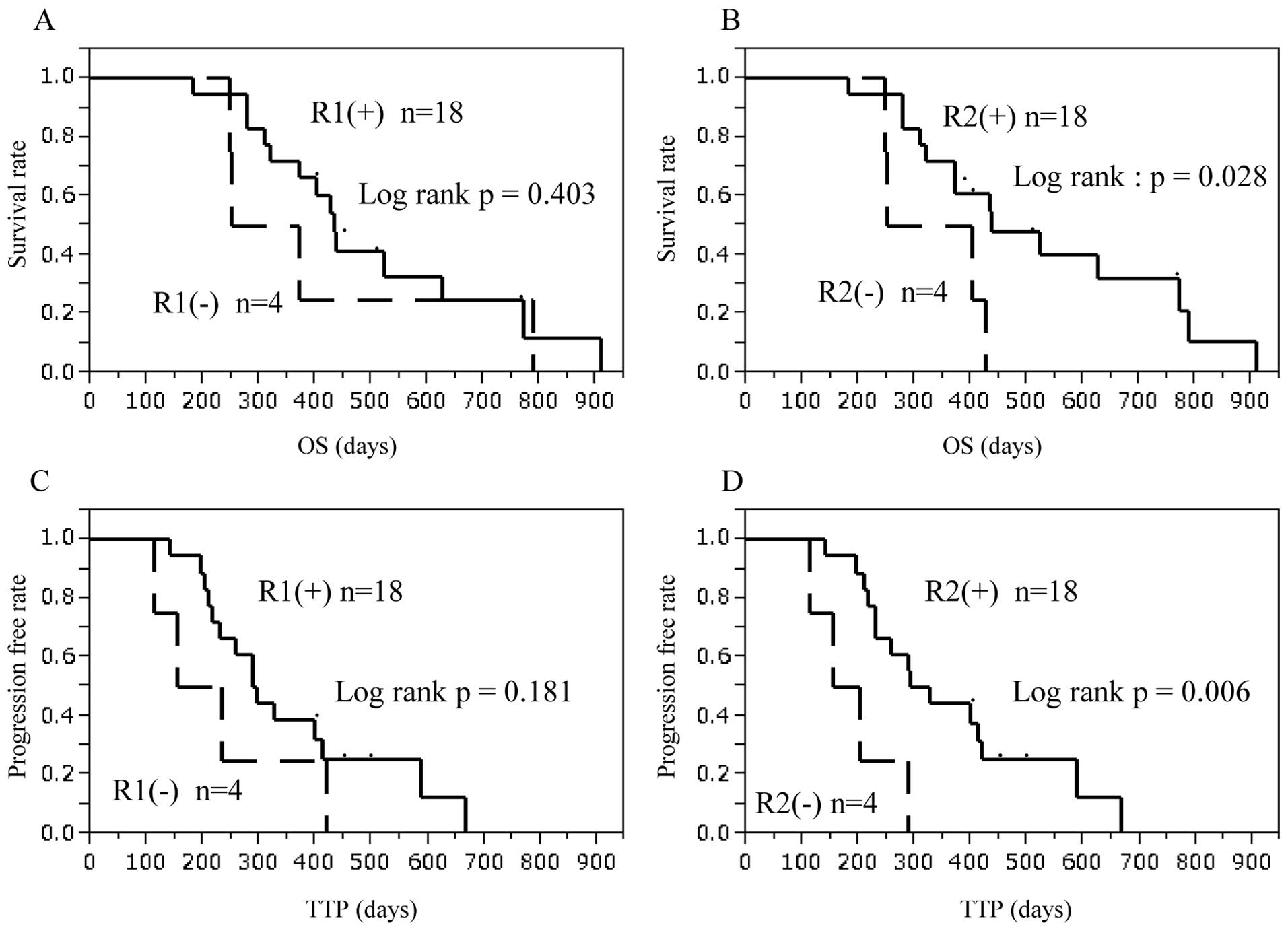
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
2
|
Narahara H, Fujitani K, Takiuchi H, et al:
Phase II study of a combination of S-1 and paclitaxel in patients
with unresectable or metastatic gastric cancer. Oncology. 74:37–41.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Iwase H, Shimada M, Tsuzuki T, et al: A
phase II multi-center study of triple therapy with paclitaxel, S-1
and cisplatin in patients with advanced gastric cancer. Oncology.
80:76–83. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sulkes A, Smyth J, Sessa C, et al:
Docetaxel (Taxotere) in advanced gastric cancer: results of a phase
II clinical trial. EORTC Early Clinical Trials Group. Br J Cancer.
70:380–383. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Van Cutsem E, Moiseyenko VM, Tjulandin S,
et al: Phase III study of docetaxel and cisplatin plus fluorouracil
compared with cisplatin and fluorouracil as first-line therapy for
advanced gastric cancer: a report of the V325 Study Group. J Clin
Oncol. 24:4991–4997. 2006.PubMed/NCBI
|
|
6
|
Boku N, Ohtsu A, Shimada Y, et al: Phase
II study of a combination of irinotecan and cisplatin against
metastatic gastric cancer. J Clin Oncol. 17:319–323.
1999.PubMed/NCBI
|
|
7
|
Bang YJ, Van Cutsem E, Feyereislova A, et
al: Trastuzumab in combination with chemotherapy versus
chemotherapy alone for treatment of HER2-positive advanced gastric
or gastro-oesophageal junction cancer (ToGA): a phase 3,
open-label, randomised controlled trial. Lancet. 376:687–697. 2010.
View Article : Google Scholar
|
|
8
|
Cunningham D, Starling N, Rao S, et al:
Capecitabine and oxaliplatin for advanced esophagogastric cancer. N
Engl J Med. 358:36–46. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Amarantidis K, Xenidis N, Chelis L, et al:
Docetaxel plus oxaliplatin in combination with capecitabine as
first-line treatment for advanced gastric cancer. Oncology.
80:359–365. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Koizumi W, Narahara H, Hara T, et al: S-1
plus cisplatin versus S-1 alone for first-line treatment of
advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet
Oncol. 9:215–221. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hong YS, Song SY, Lee SI, et al: A phase
II trial of capecitabine in previously untreated patients with
advanced and/or metastatic gastric cancer. Ann Oncol. 15:1344–1347.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Japanese Gastric Cancer Association:
Japanese classification of gastric carcinoma: 3rd English edition.
Gastric Cancer. 14:101–112. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Boon T, De Plaen E, Lurquin C, et al:
Identification of tumour rejection antigens recognized by T
lymphocytes. Cancer Surv. 13:23–37. 1992.PubMed/NCBI
|
|
14
|
Rosenberg SA, Yang JC and Restifo NP:
Cancer immunotherapy: moving beyond current vaccines. Nat Med.
10:909–915. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mackensen A, Herbst B, Chen JL, et al:
Phase I study in melanoma patients of a vaccine with peptide-pulsed
dendritic cells generated in vitro from CD34(+) hematopoietic
progenitor cells. Int J Cancer. 86:385–392. 2000.PubMed/NCBI
|
|
16
|
Bender A, Karbach J, Neumann A, et al: LUD
00-009: phase 1 study of intensive course immunization with
NY-ESO-1 peptides in HLA-A2 positive patients with
NY-ESO-1-expressing cancer. Cancer Immun. 7:16–23. 2007.PubMed/NCBI
|
|
17
|
Nestle FO, Alijagic S, Gilliet M, et al:
Vaccination of melanoma patients with peptide- or tumor
lysate-pulsed dendritic cells. Nat Med. 4:328–332. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dunn GP, Bruce AT, Ikeda H, Old LJ and
Schreiber RD: Cancer immunoediting: from immunosurveillance to
tumor escape. Nat Immunol. 3:991–998. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Khong HT and Restifo NP: Natural selection
of tumor variants in the generation of ‘tumor escape’ phenotypes.
Nat Immunol. 3:999–1005. 2002.
|
|
20
|
Fong TA, Shawver LK, Sun L, et al: SU5416
is a potent and selective inhibitor of the vascular endothelial
growth factor receptor (Flk-1/KDR) that inhibits tyrosine kinase
catalysis, tumor vascularization, and growth of multiple tumor
types. Cancer Res. 59:99–106. 1999.
|
|
21
|
Gerber HP, Kowalski J, Sherman D, Eberhard
DA and Ferrara N: Complete inhibition of rhabdomyosarcoma xenograft
growth and neovascularization requires blockade of both tumor and
host vascular endothelial growth factor. Cancer Res. 60:6253–6258.
2000.
|
|
22
|
Hurwitz H, Fehrenbacher L, Novotny W, et
al: Bevacizumab plus irinotecan, fluorouracil, and leucovorin for
metastatic colorectal cancer. N Engl J Med. 350:2335–2342. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kang Y, Ohtsu A, Van Cutsem E, et al:
AVAGAST: A randomized, double-blind, placebo-controlled, phase III
study of first-line capecitabine and cisplatin plus bevacizumab or
placebo in patients with advanced gastric cancer (AGC). ASCO Annual
Meeting. 2010
|
|
24
|
Shibuya M, Yamaguchi S, Yamane A, et al:
Nucleotide sequence and expression of a novel human receptor-type
tyrosine kinase gene (flt) closely related to the fms family.
Oncogene. 5:519–524. 1990.PubMed/NCBI
|
|
25
|
Matthews W, Jordan CT, Gavin M, Jenkins
NA, Copeland NG and Lemischka IR: A receptor tyrosine kinase cDNA
isolated from a population of enriched primitive hematopoietic
cells and exhibiting close genetic linkage to c-kit. Proc Natl Acad
Sci USA. 88:9026–9030. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ferrara N, Gerber HP and LeCouter J: The
biology of VEGF and its receptors. Nat Med. 9:669–676. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Plate KH, Breier G, Weich HA and Risau W:
Vascular endothelial growth factor is a potential tumour
angiogenesis factor in human gliomas in vivo. Nature. 359:845–848.
1992. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wada S, Tsunoda T, Baba T, et al:
Rationale for antiangiogenic cancer therapy with vaccination using
epitope peptides derived from human vascular endothelial growth
factor receptor 2. Cancer Res. 65:4939–4946. 2005. View Article : Google Scholar
|
|
29
|
Ishizaki H, Tsunoda T, Wada S, Yamauchi M,
Shibuya M and Tahara H: Inhibition of tumor growth with
antiangiogenic cancer vaccine using epitope peptides derived from
human vascular endothelial growth factor receptor 1. Clin Cancer
Res. 12:5841–5849. 2006. View Article : Google Scholar
|
|
30
|
Therasse P, Arbuck SG, Eisenhauer EA, et
al: New guidelines to evaluate the response to treatment in solid
tumors. European Organization for Research and Treatment of Cancer,
National Cancer Institute of the United States, National Cancer
Institute of Canada. J Natl Cancer Inst. 92:205–216. 2000.
View Article : Google Scholar
|
|
31
|
Sato Y, Fujiwara T, Mine T, et al:
Immunological evaluation of personalized peptide vaccination in
combination with a 5-fluorouracil derivative (TS-1) for advanced
gastric or colorectal carcinoma patients. Cancer Sci. 98:1113–1119.
2007. View Article : Google Scholar
|
|
32
|
Miyazawa M, Ohsawa R, Tsunoda T, et al:
Phase I clinical trial using peptide vaccine for human vascular
endothelial growth factor receptor 2 in combination with
gemcitabine for patients with advanced pancreatic cancer. Cancer
Sci. 101:433–439. 2010. View Article : Google Scholar
|
|
33
|
Suzuki E, Kapoor V, Jassar AS, Kaiser LR
and Albelda SM: Gemcitabine selectively eliminates splenic
Gr-1+/CD11b+ myeloid suppressor cells in tumor-bearing animals and
enhances antitumor immune activity. Clin Cancer Res. 11:6713–6721.
2005.PubMed/NCBI
|
|
34
|
Dauer M, Herten J, Bauer C, et al:
Chemosensitization of pancreatic carcinoma cells to enhance T
cell-mediated cytotoxicity induced by tumor lysate-pulsed dendritic
cells. J Immunother. 28:332–342. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Correale P, Cusi MG, Del Vecchio MT, et
al: Dendritic cell-mediated cross-presentation of antigens derived
from colon carcinoma cells exposed to a highly cytotoxic multidrug
regimen with gemcitabine, oxaliplatin, 5-fluorouracil, and
leucovorin, elicits a powerful human antigen-specific CTL response
with antitumor activity in vitro. J Immunol. 175:820–828. 2005.
|
|
36
|
Kaufman HL, Lenz HJ, Marshall J, et al:
Combination chemotherapy and ALVAC-CEA/B7.1 vaccine in patients
with metastatic colorectal cancer. Clin Cancer Res. 14:4843–4849.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kantoff PW, Higano CS, Shore ND, et al:
Sipuleucel-T immunotherapy for castration-resistant prostate
cancer. N Engl J Med. 363:411–422. 2010. View Article : Google Scholar : PubMed/NCBI
|