Introduction
Although the incidence and mortality rates
attributable to gastric cancer have recently been declining, it
remains the fourth most common cancer and the second leading cause
of cancer-related mortality worldwide (1). Data from the National Cancer Registry
at Tawam Hospital and the Center of Arab Genomic Studies in the
United Arab Emirates have shown that gastric cancer is more common
than previously believed and that there is an increasing trend in
its incidence locally. A greater understanding of gastric
carcinogenesis may lead to the design of new modalities for its
early detection and prevention (2).
The majority of gastric neoplasms (95%) are
adenocarcinomas which develop more commonly in the pyloric antrum
and are usually of the differentiated intestinal type (3). The pathogenesis of intestinal
adenocarcinoma has not yet been fully elucidated. It is usually
associated with Helicobacter pylori (H. pylori)
infection and is preceded by prolonged pre-cancerous changes which
progress through a number of sequential steps: superficial mild
gastritis, atrophic gastritis, intestinal metaplasia, dysplasia and
finally, invasive carcinoma (4).
In addition, previous studies using animal models demonstrating the
early stages of gastric cancer development have suggested a role
for local (gastric) or distant (bone marrow) stem/progenitor cells
in carcinogenesis (5,6).
Limited data are available concerning pyloric antral
stem/progenitor cells in humans and rodents. Previous studies aimed
at identifying these cells, analyzing their dynamics and following
their fate in mice, have demonstrated their location at the
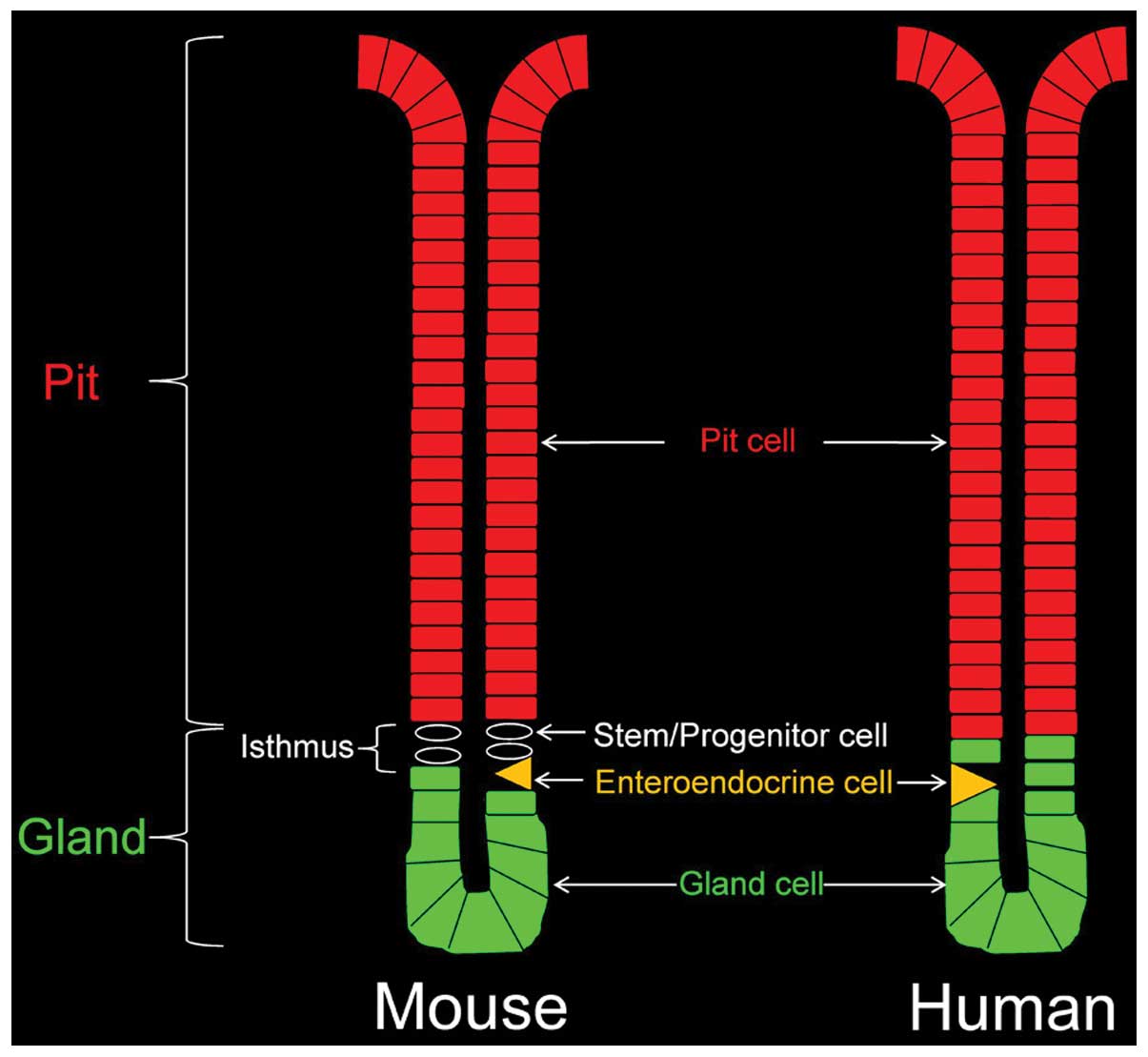
pit-gland junction (isthmus region; Fig. 1) of the epithelial units and their
differentiation pathways with a bidirectional migration mode
(7,8). However, a recent functional genetic
analysis demonstrated the presence of a pluripotent stem cell, not
in the isthmus, but at the bottom of the antral glands, which is
capable of populating the whole epithelium of the pit-gland unit,
implying a unidirectional mode of migration (9).
Regardless of their location and direction of
migration of their progeny, it is not yet known whether these
stem/progenitor cells play a role in the development of gastric
cancer in humans. Experimental studies in mice have recently
provided growing evidence suggesting that gastric epithelial
stem/progenitor cells are involved in carcinogenesis (6). For example, a deficiency in trefoil
factor 1, known to control the commitment program of gastric
progenitors (10), has been
associated with the gradual amplification of these progenitors
leading to the development of gastric adenoma and carcinoma
(11). Recently, in a previous
study, we examined gastric biopsies showing mild-to-severe chronic
gastritis, as well as gastric cancer tissues obtained from the safe
resection margin and tumor edge, and we observed an amplification
of gastric epithelial progenitors in pre-cancerous tissues
(12). These data support the
long-standing postulate that cancer arises from mutated adult stem
cells (13,14).
A number of molecules are involved in the signaling
pathways for maintaining the proliferation and differentiation of
normal gastrointestinal stem cells (15). The octamer-binding transcription
factor 4 (Oct4) is a transcription factor which belongs to the POU
family of proteins and binds octamer DNA motifs in the promoters of
several genes to regulate the pluripotency of embryonic stem cells
(16). Further studies have shown
that Oct4 expression is not restricted to the embryonic stem cells
(17–20).
The ectopic expression of Oct4 in mice inhibits the
differentiation of progenitor cells and promotes epithelial
dysplasia, suggesting a role for Oct4 in tumor induction (21). Indeed, the expression of Oct4 has
been demonstrated in tumors of various origin (22–25).
Oct4 is also expressed in a number of cell lines established from
tumors of the cervix, colon, liver, mammary gland and pancreas
(20). The expression of the Oct4
gene in embryonic stem cells, tissue stem cells, and a tumor
arising in these tissues, suggests a critical role for Oct4 in
maintaining not only the pluripotency of embryonic stem cells, but
also the homeostasis of somatic stem cells, as well as the possible
role of these cells in the tumorigenesis and tumorigenenicity of
cancer.
This study was conducted to determine whether Oct4
is expressed in the microscopically normal antrum of the human
stomach and, consequently, to examine whether there is an
alteration in the expression pattern of Oct4 during the multi-step
process of gastric carcinogenesis.
Materials and methods
The design and protocols of this study were approved
by the Ethics Committees for Research on Human Subjects in the
Faculty of Medicine and Health Sciences, UAE University, Al-Ain,
United Arab Emirates.
Human tissues
In the present study, gastric mucosal tissues were
obtained from the pyloric antrum of patients at Tawam Hospital
undergoing endoscopy (n=89) for the investigation of recurrent
upper gastrointestinal symptoms, or gastrectomy (n=3) for
adenocarcinoma. All patients gave written informed consent prior to
the study. The patients were of both genders and aged 20–90 years.
Following the endoscopic or surgical procedures, biopsies or cancer
tissues (taken from 3 regions: tumor center, tumor edge and from
the safe resection margin) were immediately processed for
immunohistochemistry and protein analyses.
Immunohistochemical studies
Tissues with the dimension of approximately 3x3 mm
(biopsy) or 10x5 mm (cancer) were immediately immersed overnight in
Bouin’s solution and then processed for paraffin embedding. Tissue
sections were stained with hematoxylin and periodic acid Schiff
(PAS) and examined with an Olympus microscope. To categorize
gastric mucosal biopsies, the updated Sydney system was utilized
(26). Some tissue sections of
biopsies representing normal mucosa, as well as mild and severe
gastritis were mounted on the same slide along with the cancerous
tissues from the safe margin, tumor edge and tumor center.
The tissue sections were deparaffinized, rehydrated
and washed in phosphate-buffered saline (PBS). To inhibit
endogenous peroxidase activity, the sections were incubated in 3%
hydrogen peroxide in methanol for 1 h. To ensure equal conditions
on all biopsy and cancer tissue sections mounted on the same slide,
they were circled with a hydrophobic film using a PAP pen (Dako,
Glostrup, Denmark). To block non-specific binding, the sections
were incubated in 1% bovine serum albumin (BSA) containing 0.5%
Tween-20 in PBS for 45 min. The sections were then incubated
overnight at 4°C with well-characterized goat polyclonal or mouse
monoclonal anti-Oct4 antibodies (Santa Cruz Biotechnology, Inc.;
final dilution, 1:50). Following the PBS wash, the tissue sections
were incubated with biotinylated donkey anti-goat or anti-mouse
immunoglobulin G (1:100; Jackson ImmunoResearch) for 1 h. Tissue
sections were washed in PBS and then incubated in
extravidin/peroxidase conjugate (1:500; Sigma, St. Louis, MO, USA)
for 1 h. The antigen-antibody binding sites were revealed by using
3,3′-diaminobenzidine tetrahydrochloride (DAB; Sigma).
Some slides with biopsy and cancer tissue sections
were processed for double fluorescent labeling using anti-Oct4
antibodies and lectins. In these cases, the secondary antibody was
biotinylated donkey anti-goat or anti-mouse immunoglobulin G.
Antigen-antibody binding sites were visualized using avidin
conjugated to fluorescein isothiocyanate (FITC) or rhodamine. After
washing with PBS, the tissue sections were incubated with
fucose-specific Ulex europaeus agglutinin I (UEA) or
N-acetyl-D-glucosamine-specific Griffonia
simplicifolia II (GSII) lectins conjugated to FITC or
rhodamine. The tissue sections were finally washed with PBS and
mounted. Negative control slides were prepared in parallel by
either omitting the primary antibody, or by replacing it with
normal serum or by pre-incubating the primary antibody with the
blocking peptide (Oct4).
Quantification of Oct4 expression
Two methods were used to provide a numerical
assessment of the Oct4 expression in immunoperoxidase-labeled
tissue sections. These were as follows:
Manually using the x40 magnification of the light
microscope, cells were categorized according to the intensity of
immunostaining. At least 3 glands with the best longitudinal
orientation were examined in each tissue section of biopsies and
cancerous tissues. A score of 3 was given for the most intensely
brown-stained cells, intermediate brown staining was given a score
of 2 and a score of 1 was given for light brown staining. Unstained
cells were given a 0 score. The number of labeled cells was
multiplied by the corresponding score. To estimate the average
level of Oct4 expression, the total score of the 3 glands was
divided by the total number of cells examined. This was termed the
‘expression score’ and expressed as the mean ± SEM value. The means
of Oct4 expression scores were compared by using the ANOVA test and
Tukey’s post hoc test (PASW statistics 18). P<0.05 was
considered to indicate statistically significant differences.
To automate the quantification, a computerized
method was used to estimate the area of immunolabeled cells per
image. This digitalized approach also provides another parameter
for quantification of Oct4 expression and speeds up the task of
sample examination by several orders of magnitude. The Matlab
(Matlab; http://www.mathworks.com/products/matlab/) image
processing library was used for manipulating micrographs of
different stomach tissue samples. The following steps for
identification and measurement of the labeled areas were applied:
a) Image resizing: pixel sizes for the images were reduced and
unified. b) Artifact removal: some images included small but
varying-size dark spots. The pixels below an (empirically
determined) intensity level were replaced by pixels representing
the average intensity of the image. c) Color intensity enhancement
by using the Matlab imadjust function (MathWorks: Documentation,
Image Processing Toolbox imresize; http://www.mathworks.com/access/helpdesk/help/toolbox/images/imresize.html).
Application of this function made the images sharper and removed
background colors. d) Identification of the Oct4-labeled area by
performing red-green-blue color separation and then using different
‘threshold’ values to identify the ‘brown’ areas of the images. The
percentage of the area labeled by Oct4 was finally determined and
expressed as a mean ± SEM value. The means of Oct4 labeled areas
were compared using the ANOVA test and Tukey’s post hoc test.
P<0.05 was considered to indicate statistically significant
differences.
Protein extraction and western blot
analysis
Frozen tissue samples from biopsies and the 3
regions of gastric cancer were homogenized and lysed using the
CelLytic™ NuCLEAR™ extraction kit (Sigma). Briefly, sample tissues
were rinsed in cold PBS and then homogenized in ice-cold hypotonic
lysis buffer (10 mM HEPES, pH 7.9, with 1.5 mM MgCl2 and
10 mM KCl) containing 0.1 M DTT and protease inhibitor cocktail
made of 4-(2-aminoethyl) benzenesulfonyl fluride (AEBSF), pepstatin
A, bestatin, leupeptin, aprotinin, and
transepoxysuccinyl-L-leucylamido (4-guanidino)-butane. The crude
homogenates were centrifuged for 20 min at 11,000 × g at 4°C. The
supernatant represented the cytoplasmic fraction. The remaining
pellet was re-suspended in an extraction buffer (20 mM HEPES, pH
7.9, with 1.5 mM MgCl2, 0.42 M NaCl, 0.2 mM EDTA, 0.1 M
DTT and 25% glycerol) containing protease inhibitor cocktail for 30
min at 4°C. To collect the nuclear fractions, the homogenates were
centrifuged for 5 min at 21,000 × g at 4°C. Cytoplasmic and nuclear
fractions of each of the tissue samples were processed for protein
estimation using the BioRad assay kit (BioRad Laboratories,
Hercules, CA, USA) and the DU-700 spectrophotometer (Beckman).
Equal amounts of protein (5 or 20 µg) of extracted
tissue samples were mixed with 5X Laemmli buffer (60 mM Tris-HCl,
pH 6.8, 2% SDS, 10% glycerol, 5% β-mercaptoethanol, and 0.01%
bromophenol blue) and separated by sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) using 10%
acrylamide at 150 V for 90 min. Protein gels were subsequently
transblotted on to nitrocellulose membranes (Schleicher &
Schuell BioScience, Dassel, Germany) at 90 V for 120 min.
Non-specific binding was blocked by 5% non-fat dry milk in PBS for
1 h. The membranes were then washed with PBS containing 0.1%
Tween-20 (PBST). Subsequently, the membranes were probed overnight
at 4°C with mouse monoclonal Oct4 (Santa Cruz Biotechnology, Santa
Cruz, CA, USA) diluted to 1:500 in PBST and 5% milk. Following the
PBST wash, the blots were incubated with horseradish
peroxidase-conjugated goat anti-mouse immunoglobulin G (Jackson
ImmunoResearch) diluted 1:10,000 in PBST for 2 h. Immunoreactive
proteins in the blots were detected using SuperSignal West Pico
chemiluminescent substrate on CL-Xposure Film (Thermo Scientific,
Barrington, IL, USA.). To control equal loading of proteins in the
different lanes, the blots were probed with mouse monoclonal
anti-β-actin antibody (Santa Cruz).
Results
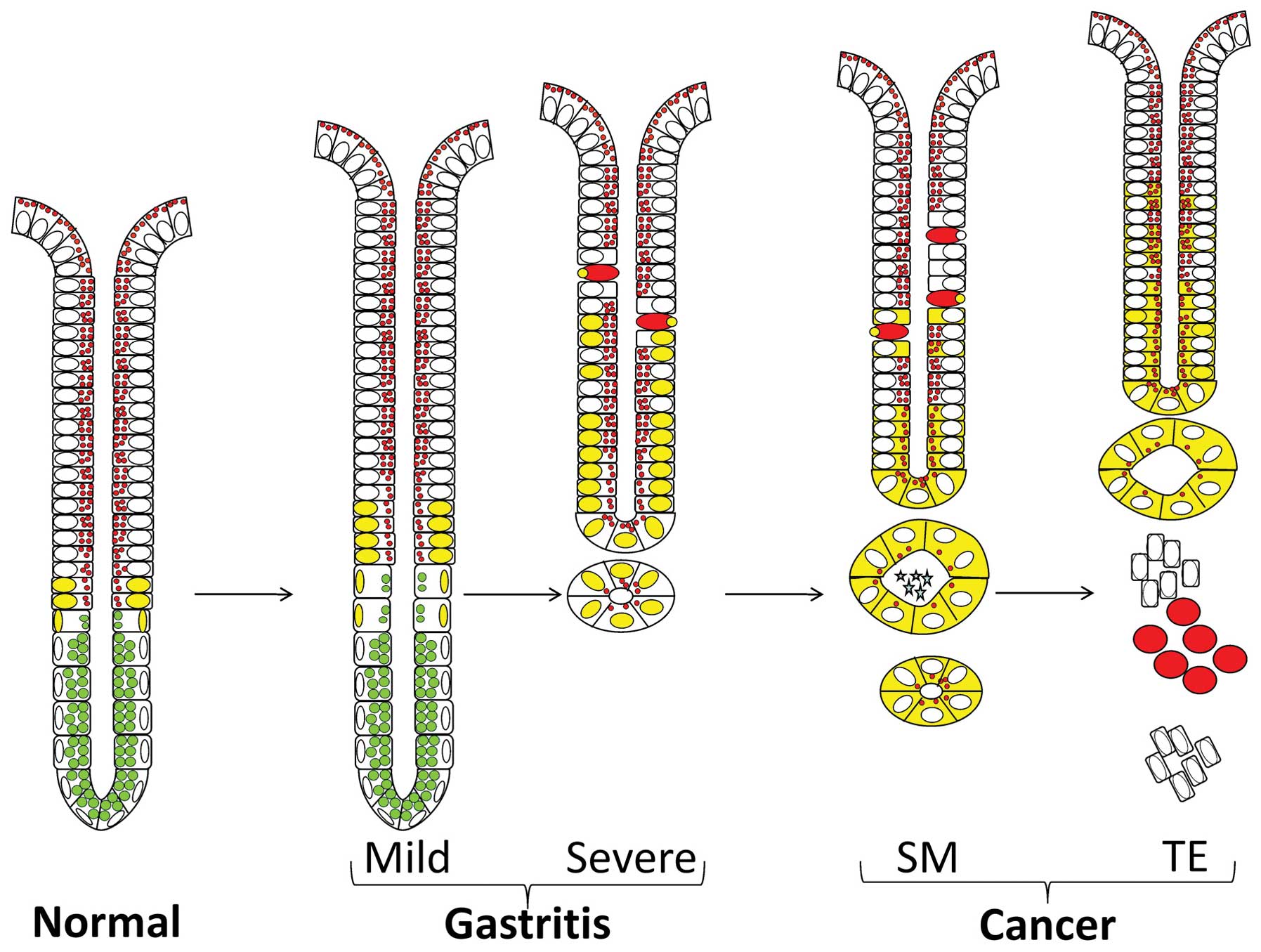
Morphological analysis of the pyloric antral tissues
obtained from the mucosal biopsies and from the 3 regions of the
resected cancerous stomachs revealed progressive epithelial
changes, as previously described (12). While some antral mucosal biopsies
appeared normal, others were infiltrated with variable numbers of
lymphocytes and plasma cells with evidence of mild or severe
gastritis. The latter was commonly associated with atrophic changes
in the gastric glands and occasionally showed evidence of
metaplasia or even dysplasia. The safe resection margin was
determined by cancerous tissues that were hyper-plastic and were
also associated with metaplastic and dysplastic changes. Tissues
from the both tumor edge and center were characterized by a massive
increase in mucosal thickness and invasive cancer cells. These
series of antral mucosal tissues with progressive alterations
provided material to investigate the expression pattern of Oct4 in
the normal pyloric antrum and during multistep carcinogenesis.
Immunohistochemical localization of Oct4
in normal gastric mucosa
In the tissue sections of normal antral biopsies
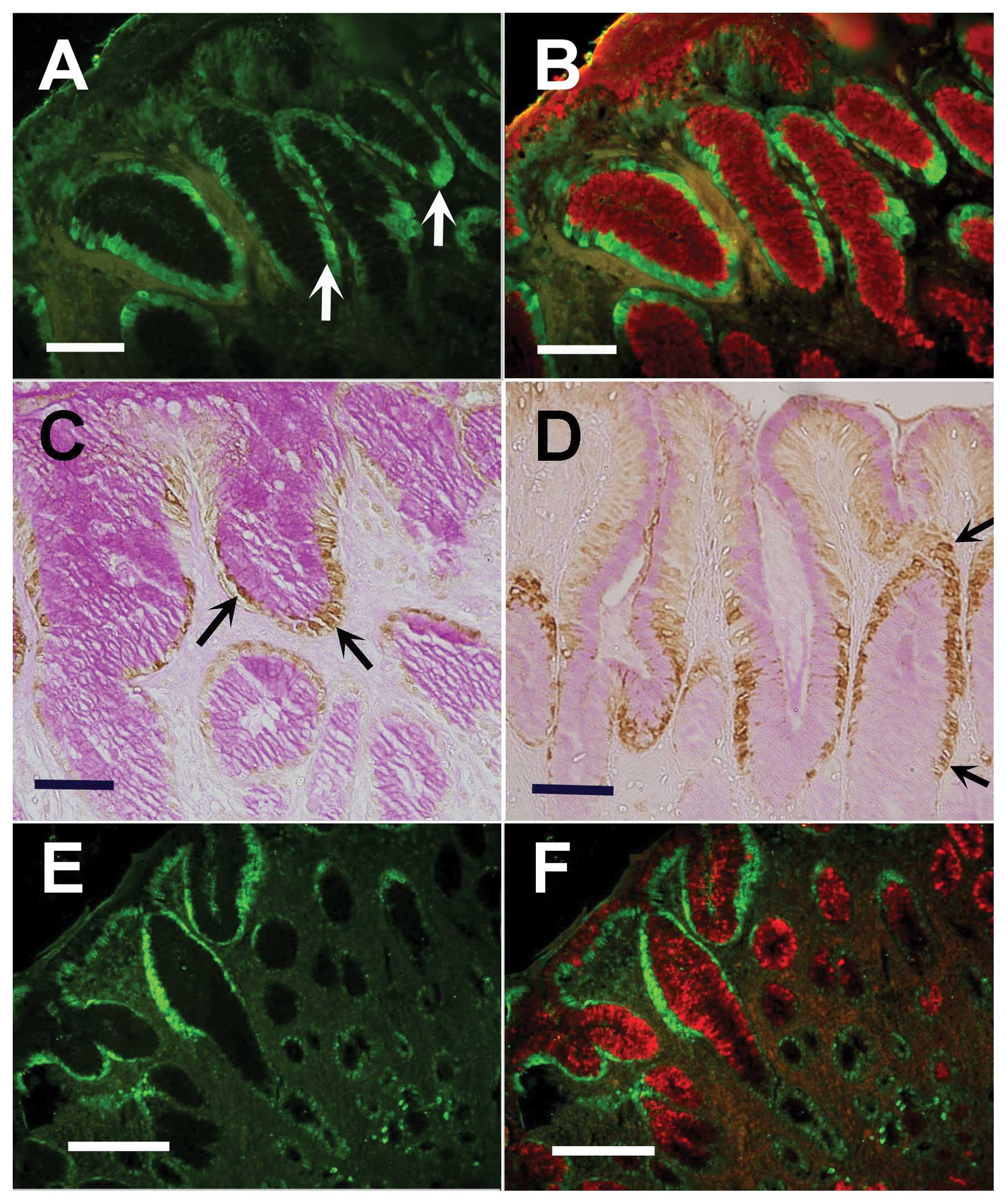
processed for immunofluorescence histochemistry, Oct4 expression
was detected in the basal (nuclear) portion of epithelial cells
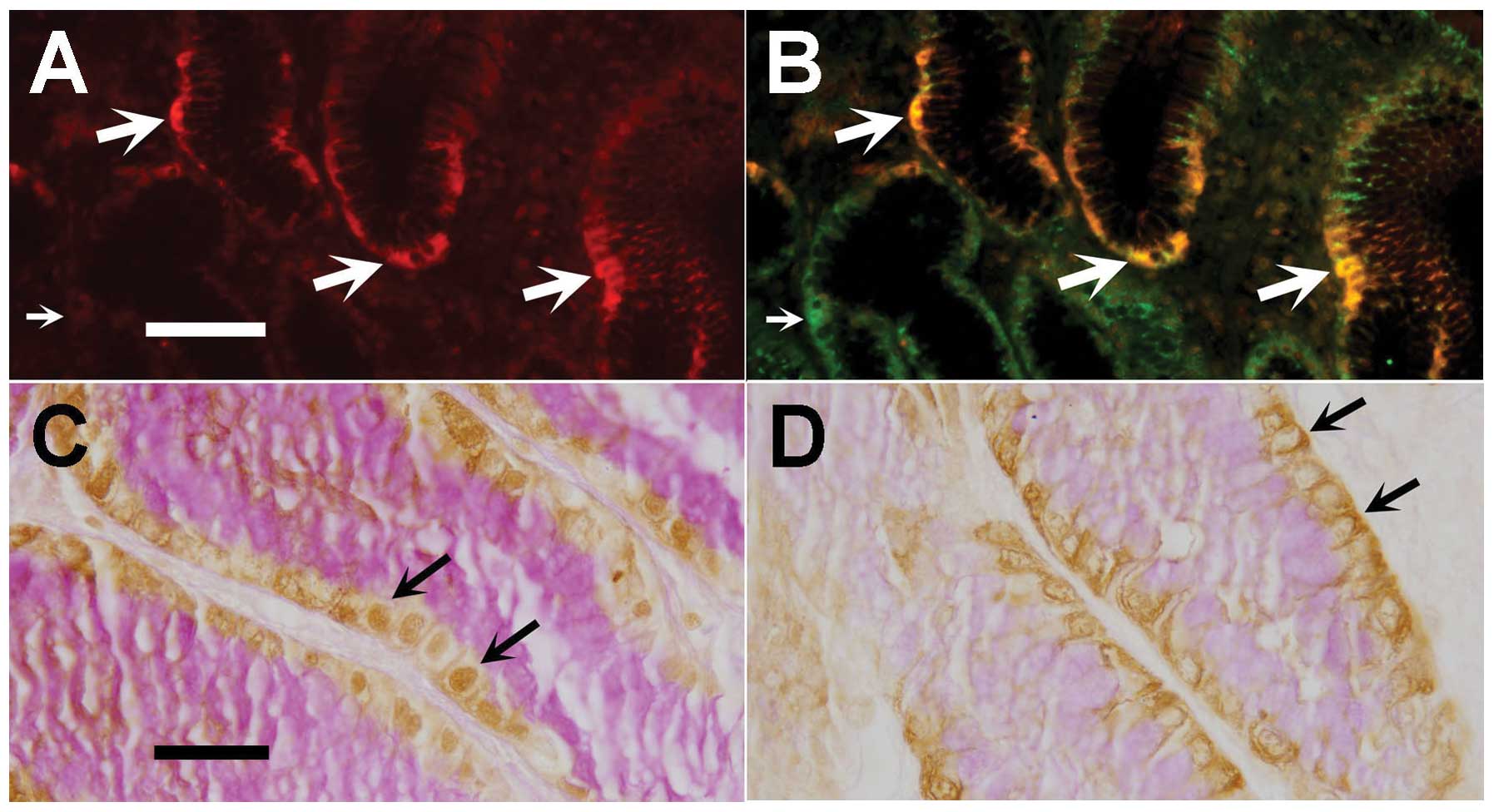
lining the lower gastric pits next to the gland junction (Fig. 2A). A decreasing gradient of Oct4
labeling toward the luminal surface and glandular bottom was noted.
When the same section was also probed with UEA lectin which binds
to fucose of pit mucous cell lineage, Oct4-labeled cells also
became labeled with UEA. Image overlay showed the co-localization
of Oct4 and UEA in the same cells, but in different portions; Oct4
was basal and UEA, apical (Fig.
2B).
The localization of Oct4 in normal gastric mucosa
was also demonstrated by using the immunoperoxidase technique. A
similar pattern of labeling at the bottom of the gastric pits next
to the gland junction was revealed (Fig. 2C). Tissue sections were
counterstained with PAS which stained mucus in the apical cytoplasm
of Oct4-labeled cells.
Microscopic scoring and quantification of the mean
Oct4 expression score in all the control biopsies examined (n=6)
was 85±6.8 (Table I). The
percentages of Oct4 immunolabeled areas were calculated in binary
images and found to have a mean value of 2.19±0.73 (Table II).
 | Table I.Manual quantification of the
expression scores of Oct4 in control, gastritis and cancer
tissues. |
Table I.
Manual quantification of the
expression scores of Oct4 in control, gastritis and cancer
tissues.
| A, Expression
scores of Oct4 in control, gastritis and cancer tissues |
|---|
|
|---|
| Tissues | No. of cells
examined | Total score | Mean expression
score ± SEM |
|---|
| Control | 963 | 812 | 85.4±6.8 |
| Gastritis | | | |
| Mild | 1,219 | 1,291 | 110.3±15.8 |
| Severe | 1,094 | 1,589 | 130.4±9.7 |
| All | 2,313 | 2,880 | 122.1±8.7 |
| Cancer | | | |
| Safe margin | 854 | 1,110 | 129.1±16.6 |
| Tumor edge | 823 | 1,218 | 148.9±18.3 |
| Tumor center | 814 | 1,248 | 163.5±23.4 |
| All | 2,491 | 3,576 | 147.1±11.0 |
| B, Significance
(P-value) of the differences in Oct4 expression scores between the
controls and gastritis tissues and the 3 regions of gastric
cancer |
|---|
|
|---|
| Gastritis
| Cancer
|
|---|
| Tissues | All | Mild | Severe | All | Safe margin | Tumor edge | Tumor center |
|---|
| Control | 0.027 | 0.32 | 0.02 | 0.002 | 0.096 | 0.049 | 0.015 |
| Gastritis | | | | | | | |
| Mild | | | 0.648 | 0.129 | 0.861 | 0.647 | 0.271 |
| Severe | | | | 0.611 | 0.999 | 0.974 | 0.698 |
| All | | | | 0.146 | 0.861 | 0.647 | 0.271 |
| Cancer | | | | | | | |
| Safe margin | | | | | | 0.984 | 0.776 |
| Tumor edge | | | | | | | 0.93 |
 | Table II.Computerized estimation of the areas
of Oct4 labeling in control, gastritis and cancer tissues. |
Table II.
Computerized estimation of the areas
of Oct4 labeling in control, gastritis and cancer tissues.
| A, Areas of Oct4
labeling in control, gastritis and cancer tissues |
|---|
|
|---|
| Tissues | No. of images
examined | % Labeled area | SEM |
|---|
| Control | 6 | 2.19 | 0.73 |
| All gastritis | 12 | 6.46 | 0.650 |
| All cancer | 5 | 16.13 | 1.22 |
| B, Significance
(P-value) of the differences between the areas of Oct4 labeling in
control, gastritis and cancer tissues |
|---|
|
|---|
| Tissues | All gastritis | All cancer |
|---|
| Control | 0.009 | <0.001 |
| All gastritis | | 0.013 |
Immunohistochemical localization of Oct4
in biopsies with gastritis
The probing of gastric tissues obtained from
patients with mild gastritis revealed a tendency of pronounced Oct4
immunolabeling in the mucus-producing cells in some biopsies. The
area labeled with Oct4 was slightly wider than in the controls
(Fig. 2D). Counts revealed that in
mild gastritis more cells expressed Oct4 compared to the control
tissues. The estimated mean score of Oct4 expression in the
biopsies examined with mild gastritis was 110±15.8 (Table I). However, a comparison of the
Oct4 expression scores in the control subjects with those seen in
patients with mild gastritis showed no significant difference
(P=0.32).
In the cases of severe gastritis, Oct4-immunolabeled
cells were found along the gastric pits and even at the luminal
surface. Moreover, a few Oct4-labeled cells were seen scattered
deep in the gastric glands (Fig. 2E
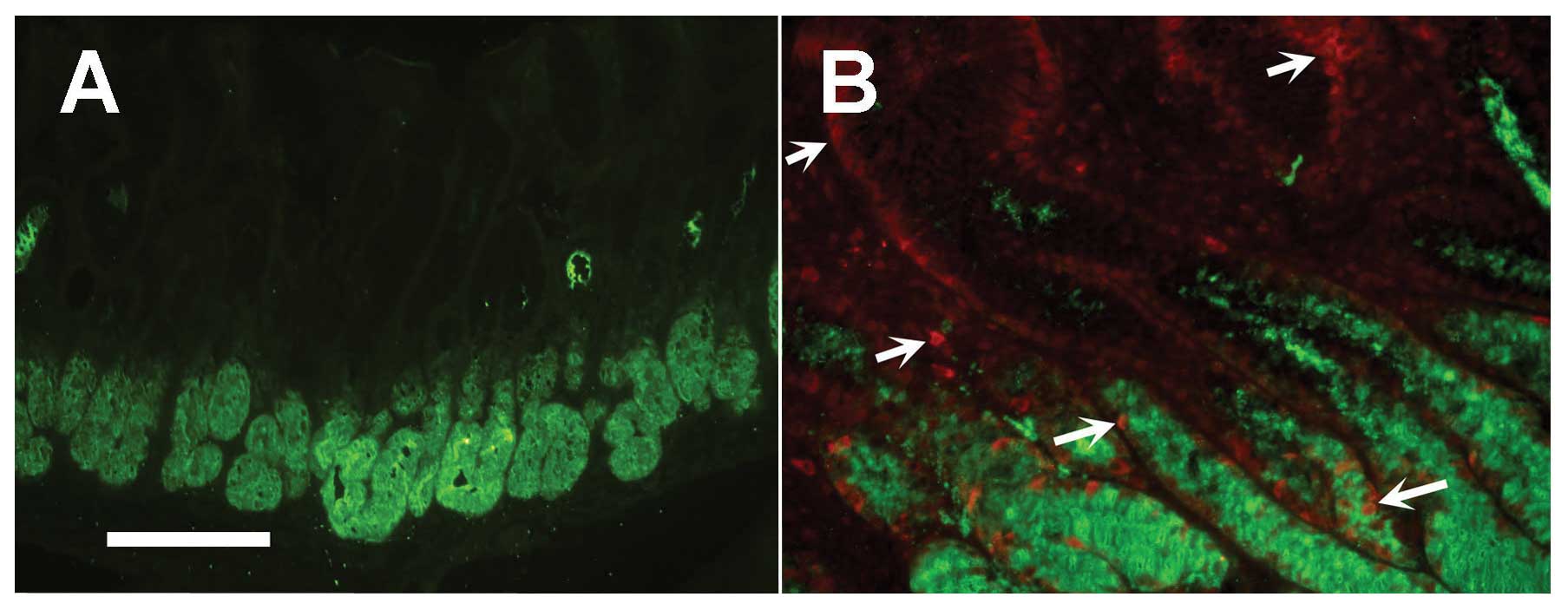
and F). Since GSII lectin bound to the gland mucous cells deep
in the gastric mucosa (Fig. 3A),
the question arose as to whether the Oct4-labeled cells deep in the
gland were indeed some of those that also bound to GSII. Double
labeling of tissue sections with the antibody and lectin revealed
the co-localization of Oct4 in some GSII-labeled gland mucous cells
(Fig. 3B).
Measurements showed that the expression score of
Oct4 in tissues with severe gastritis (130±9.7) was significantly
increased when compared to the control patients (P=0.02). There
was, however, no significant difference between the Oct4 expression
scores comparing mild with severe gastritis (Table I). When the mean Oct4-labeled
percentage areas were estimated for all gastritis specimens, they
were found to be significantly different from those of the control
patients (P=0.009; Table II).
Immunohistochemical localization of Oct4
in gastric cancer tissues
Since the morphological features of gastric cancer
tissues obtained from the 3 sites (safe margin, tumor edge and
tumor center) were different, the immunolocalization of Oct4 was
examined in each of these sites separately. At the safe (resection)
margin of cancerous tissues (an area of the gastric mucosa which is
several centimeters away from the tumor), areas of intestinal
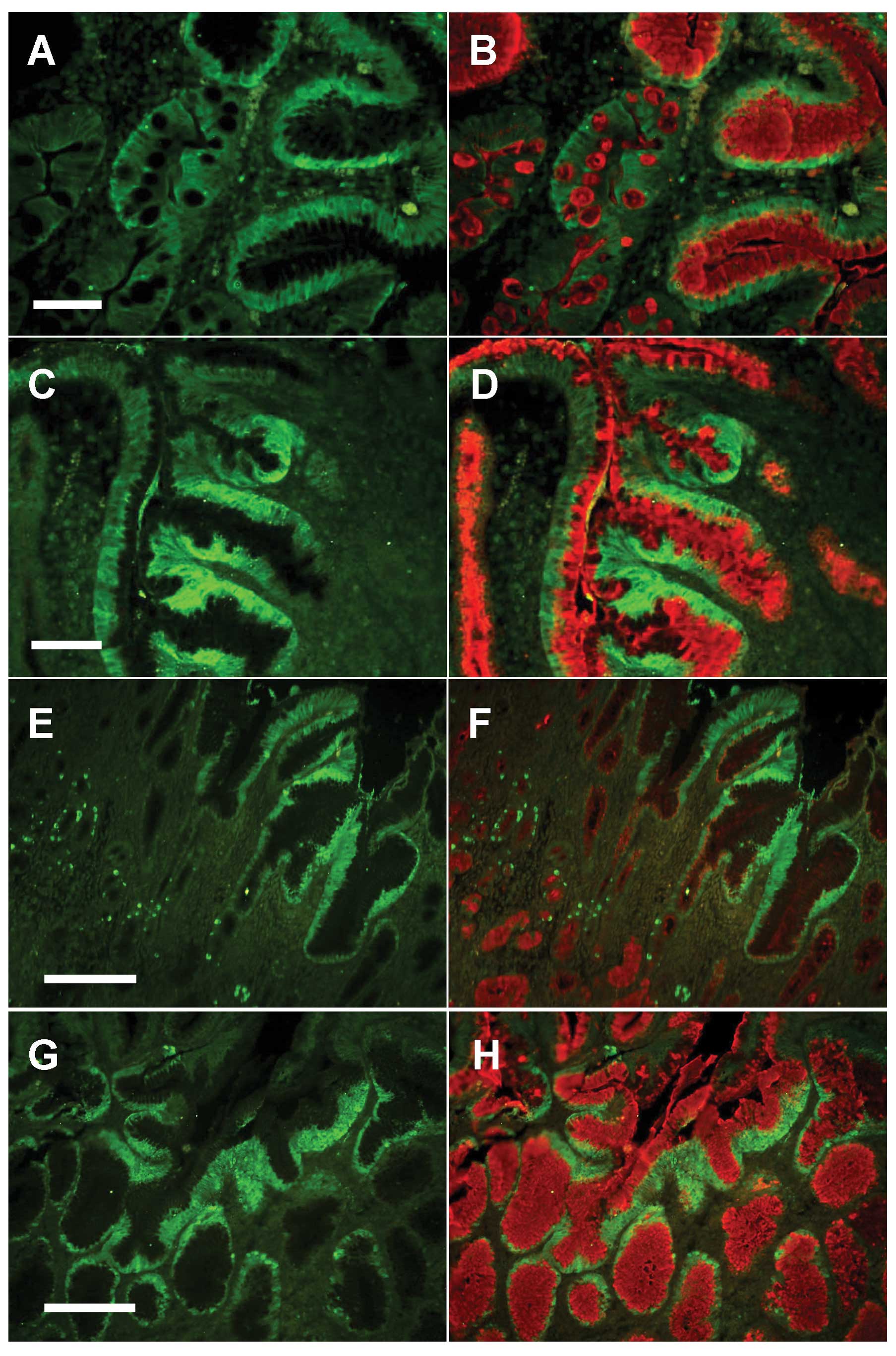
metaplasia were common. In a tissue section of the safe margin,
Oct4 expression was seen along the walls of the pits and also in
the scattered metaplastic goblet cells and their associated
absorptive cells (Fig. 4A).
Mucus-secreting cells at the luminal surface were also
immunolabeled with anti-Oct4 antibody. In this tissue, mucus not
only in pit cells, but also in goblet cells was labeled with UEA
lectin (Fig. 4B).
In the dysplastic areas of the safe margin tissues,
there was intensified Oct4 expression (Fig. 4C). Double immunofluorescence
labeling using anti-Oct4 and UEA lectin confirmed the presence of
Oct4 and fucose in the dysplastic mucus-secreting cells (Fig. 4D).
In the safe margin, measurements revealed that the
Oct4 expression score was 129±16.6 (Table I). This value was not significantly
different from that obtained from the tissues of the control
biopsies and from mild or severe gastritis tissues (P=0.096, 0.861
and 0.999, respectively).
At the tumor edge, Oct4 expression was associated
with the mucus-secreting PAS- and UEA-labeled cells located along
the hyperplastic pits and the luminal surface (Fig. 4E). In addition, a large number of
labeled cells was found deep in the gastric glands. UEA binding was
less extensive than in the control or gastritis tissues. The lectin
labeling of pit cells tended to be mainly at the supranuclear Golgi
area (Fig. 4F). Measurements of
Oct4 labeling in the tumor edge revealed that the mean expression
score was 149±18.3. This value was significantly different from the
score of the control tissues, P=0.049 (Table I).
Since the morphological features of the tumor center
were variable, this also showed an inconsistent Oct4 expression
profile. Tissue sections with evidence of necrosis and loss of
surface epithelium did not reveal Oct4-labeled cells. Tissue
sections with intact surface epithelium and many groups of invasive
cancer cells showed Oct4 expression on the surface cells which were
also positive for PAS or UEA staining. Tissue sections with an
intact luminal surface and many glandular profiles showed massive
labeling with PAS or UEA in the cells (Fig. 4G and H).
Measurements of Oct4 expression in the tumor center
areas without necrotic tissues revealed the highest score
(163.5±23.4) which was significantly different from that of the
control tissues (P=0.015; Table
I). When the percentage of Oct4-labeled areas was estimated in
the tissue sections obtained from all 3 tumor areas, these revealed
a mean value (16.13), which was significantly different when
compared with the control (P≤0.001) and all gastritis tissues
(P=0.013), as shown in Table
II.
Western blot analysis of Oct4 expression
in gastric mucosal biopsies and cancerous tissues
To confirm the immunohistochemical localization of
Oct4 in control gastric mucosal biopsies and the changes observed
during the development of gastritis and gastric cancer, some
biopsies (n=6) and cancerous tissues (n=3) were frozen and then
processed for western blot analysis.
The specificity of the anti-Oct4 antibody was first
examined by using 2 different control tissue extracts believed to
be adequate sources of gastric stem/progenitor cells and, hence,
rich in Oct4 protein. The first positive control was the gastric
mucosal tissue of a newborn mouse which was already shown to have
plenty of stem/progenitor cells (27). For comparison, an extract of adult
mouse gastric mucosa was also used. When equal amounts of proteins
of the newborn and adult gastric mucosal homogenates were loaded
for SDS-PAGE, transferred onto a nitrocellulose membrane and
subsequently incubated with anti-Oct4 antibody, a protein band was
detected for each of the adult and neonatal extracts at
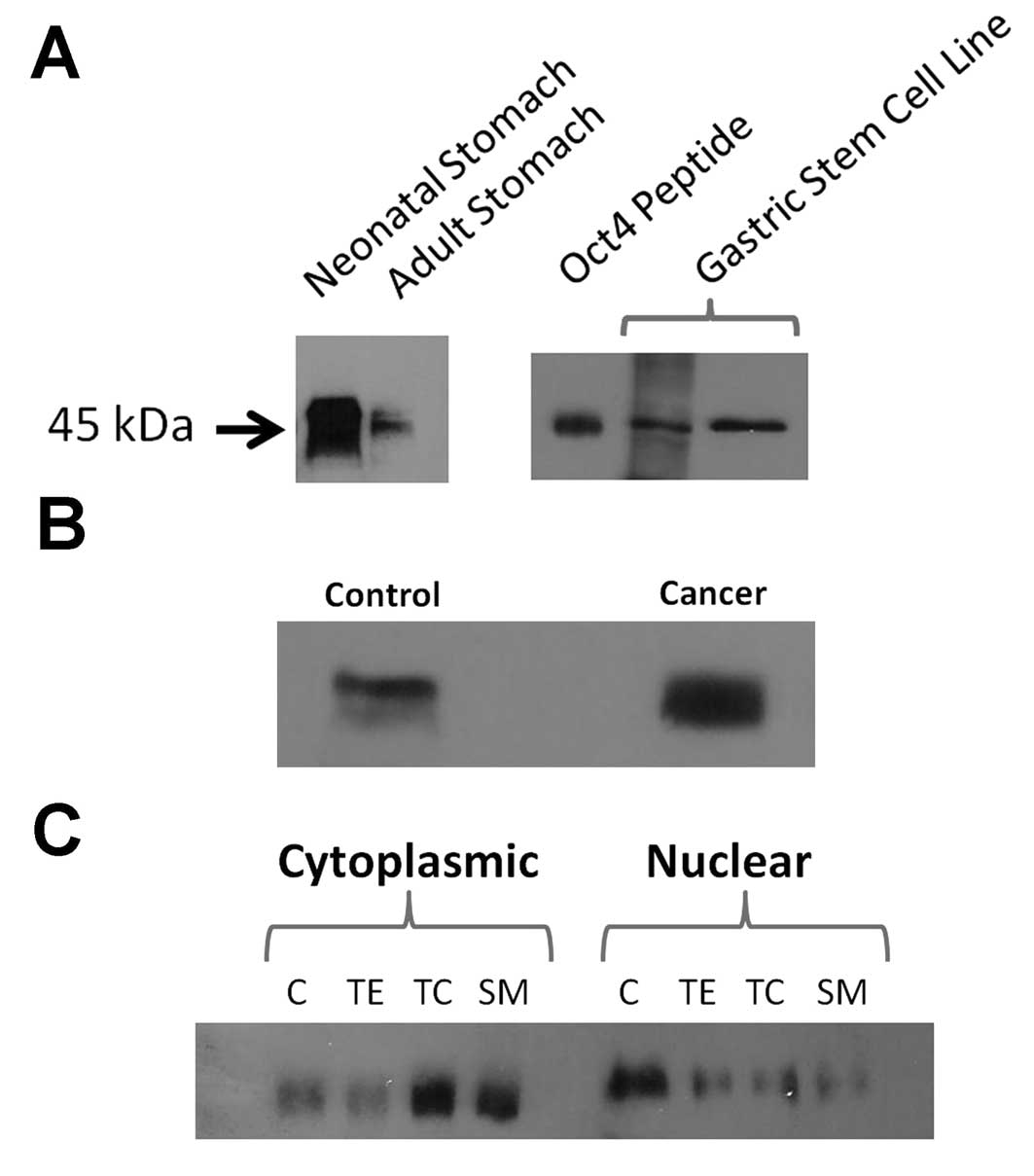
approximately 45 kDa (Fig. 5A). As
expected, the band intensity (reflecting the amount of protein
present) was much higher in the neonatal than the adult protein
extracts.
The second positive control was an epithelial cell
line which was previously shown to be representative of gastric
stem/progenitor cells (28). This
cell line was found to express stem cell-specific genes (e.g.,
Notch3 and DcamKl1). A frozen aliquot of this cell line was allowed
to thaw and then plated in a culture flask. When 2 different
passages of these cells became semi-confluent, they were processed
for protein extraction and then separation on a SDS-polyacrylamide
gel. Western blot analysis using anti-Oct4 antibodies revealed a
single protein band separated at 45 kDa, the expected molecular
weight of Oct4 (Fig. 5A). The
identity of this band was confirmed by pre-incubating the primary
antibody with the Oct4 peptide; the band did not develop.
For the human antral tissue samples, to determine
whether Oct4 was upregulated in cancerous tissue, the crude
homogenates of a control biopsy and tumor tissue (containing equal
amounts of proteins) were initially probed for Oct4. The results
demonstrated the upregulation of Oct4 expression in cancerous
tissue (Fig. 5B).
Since Oct4 has more than one isoform with debatable
subcellular distribution (29–31),
it was also essential to clarify its localization using different
subcellular fractions. The crude homogenates of human gastric
mucosal tissues were processed using the Thermo Scientific
Subcellular Protein Fractionation kit. The following subcellular
protein fractions were obtained: cytoplasmic, membrane, soluble
nuclear, chromatin-bound and cytoskeletal. Equal quantities of
proteins from the fractions were loaded on a SDS gel for separation
and subsequent transfer to nitrocellulose membranes. Probing with
anti-Oct4 antibody revealed that both the cytoplasmic and nuclear
fractions were rich in Oct4.
To confirm these findings, another subcellular
fractionation protocol was applied using the Sigma CelLytic NuCLEAR
extraction kit which has another advantage over the Thermo
Scientific protocol. The Sigma procedure is suitable for analysis
of small biopsy samples. The homogenized tumor tissues and control
biopsies were processed to obtain 2 protein fractions, nuclear and
cytoplasmic.
Fractions of the control biopsies were first
processed in parallel with those of the cancerous tissues. The
results showed an increase in the amount of Oct4 protein in the
cancerous tissues as demonstrated in Fig. 5B. The nuclear and cytoplasmic
fractions of the control and the cancerous tissues (safe margin,
tumor edge and tumor center) were processed separately for western
blot analysis using monoclonal anti-Oct4 antibody. The results
revealed a surprising distribution and alteration in Oct4
expression in the various fractions of cancerous tissues. While in
the control tissue, the majority of Oct4 protein was localized in
the nuclear fraction, the situation was reversed in the tumor
tissues and the majority of Oct4 protein was identified in the
cytoplasmic fractions (Fig. 5C).
These results suggest that cancer development is associated not
only with the upregulation of Oct4 expression but also with its
accumulation in the cytoplasm which could possibly be due to a
block in its translocation to the nucleus.
Discussion
In the present study, the expression profiles of
Oct4 were investigated in an array of human tissues composed of
normal, pre-cancerous and cancerous antral mucosae by using both
immunohistochemistry and western blot analysis. In addition, the
subcellular localization of Oct4 was examined in different tissues
using 2 fractionation protocols from Sigma and Thermo
Scientific.
Expression of Oct4 protein
Oct4 is a transcription factor which is prominently
expressed in embryonic stem cells and adult stem/progenitor cells
anchored in a number of tissues (16–20).
The expression of Oct4 is not only detected in embryonic/adult stem
cells, but also in various cancerous tissues and cell lines
(20,22–25).
During stem cell differentiation, the expression of
Oct4 is downregulated. The ectopic expression of Oct4 has been
shown to inhibit the differentiation of progenitor cells and
promote the dysplastic growth of the gastrointestinal tract and the
skin of adult mice (21).
Knowledge in the field of stem cell biology
especially of transcription factors responsible for pluripotency,
such as Oct4 is rapidly advancing. During the conduct of this
study, a brief report was published by Chen et al on the
expression of Oct4 in gastric cancer tissues and in biopsies with
atrophic gastritis (32). The
authors found that Oct4 is upregulated in gastric cancer and not
expressed in adjacent non-cancerous tissues. They further
recommended the use of Oct4 as a biomarker for the diagnosis of
gastric cancer. However, the authors demonstrated a single image of
Oct4 immunohistochemical localization in non-cancerous gastric
tissue which showed only a small part of the basal region of the
gastric mucosa. The authors may have missed the luminal region of
the mucosa which we found to be immunolabeled with anti-Oct4
antibody.
While Chen et al proposed the use of Oct4 as
a possible biomarker for gastric cancer, their study did not
provide an answer to a number of questions: i) Whether Oct4 is
expressed in the normal human antrum, and if so, which cells along
the pit-gland unit are involved in the production of Oct4. ii)
Whether Oct4 expression is affected during the development of mild
gastritis. iii) Clarification as to how this is related to severe
gastritis and to H. pylori infection. iv) If Oct4 is
upregulated in gastric cancer, it should be clarified whether there
are any differences in its expression pattern between the safe
(resection) margin, tumor edge and tumor center. v) In which part
of the cell Oct4 is localized in normal tissues and during cancer
development.
Oct4 is expressed in the proliferative
zone of the antrum in normal human stomach
In order to answer the question as to whether or not
Oct4 is expressed in the normal antrum of the human stomach, and if
so, to identify the location of the cells responsible for its
expression (isthmus or the bottom of the gland), tissue sections of
normal ‘control’ biopsies were first examined. As it is not usually
possible to find normal (healthy) volunteers for endoscopy, we
examined biopsies from individuals with upper gastrointestinal
symptoms, but with macroscopically normal gastric mucosa. When some
of these biopsies were examined microscopically, they had features
similar to those of normal control individuals. As regards H.
pylori, many (n=15) of these control individuals tested
negative, very few (n=2) tested positive and the remainder (n=8)
were not tested.
In normal control antral mucosal biopsies, the
expression of Oct4 was demonstrated by both immunoperoxidase and
immunofluorescence techniques. Oct4 expression was found in the
basal portion of PAS-positive cells lining the lower part of the
gastric pits at the pit-gland junction (isthmus region). These
double PAS-Oct4-positive cells also bind UEA lectin, and therefore,
are fucose-rich. Furthermore, some of these Oct4 positive cells
also express the proliferating cell nuclear antigen (PCNA) and are
hence, proliferative (Fig. 6A and
B). Thus, based on their location and proliferative potential,
in the normal (control) human stomach, Oct4 is expressed in
dividing mucus-producing pre-pit and differentiating pit cells
located in the isthmus region of the pit-gland units.
It has been previously shown that in the mouse
stomach, pre-pit cells and even differentiating pit cells maintain
some capacity of self renewal which gradually decreases as the
cells migrate up toward the luminal surface (7,8). In
the pyloric antrum of the human stomach, the epithelial lining
includes progenitor cells which contain some mucous granules and
are capable of self-renewal. These progenitors act as committed
stem cells and give rise to the mucus-secreting pit and gland cells
(12). It appears that Oct4 is
required for the self-renewal of pre-pit cells and also to maintain
some capacity of division in differentiating pit cells which
gradually decreases with migration, as indicated by the decreasing
gradient of Oct4 expression towards the luminal surface.
To quantify the expression of Oct4 in the tissue
sections of normal human stomach, 2 methods were used: manual and
computer-based. The former method provided an estimation of the
expression score and the computerized images provided a rapid
measurement of the percentage of labeled areas. These 2 parameters
may be useful in providing complementary ways to investigate the
expression of Oct4 in tissue sections.
In the present study, 4 of the control patients with
microscopically normal gastric mucosa showed slightly different
patterns of Oct4 expression. Oct4 was localized in both the lower
and upper parts of the gastric pits. One possible explanation may
be linked to the ingestion by these patients of iron or omeprazole
which were found to induce histopathological changes in the gastric
mucosa (33,34). Another less likely explanation is
that Oct4 may be normally expressed in certain mature
differentiated mucous cells in some individuals. These results are
in agreement with those from previous studies which demonstrated
Oct4 expression in fully differentiated peripheral blood
mononuclear cells. This finding provides a new perspective
challenging the use of Oct4 as a marker specific for stem cells
(35). Finally, it should be
stated that in these microscopically normal individuals (n=17), we
found only 2 H. pylori-positive cases and they did not
reveal any alteration in the pattern of Oct4 expression when
compared with the H. pylori-negative cases.
Oct4 expression is altered during the
multistep process of gastric carcinogenesis
The development of gastritis was associated with the
accumulation of lymphoid cells in the connective tissue between the
pit-gland units. These cells were substantially increased in number
with the development of severe gastritis and were associated with
atrophy of some glands. Adjacent to these atrophied glands, the
glandular profiles were lined with poorly differentiated mucous
cells. It was therefore not surprising to find a significant
increase in Oct4 immunolabeling in the biopsies with evidence of
severe gastritis. Some scattered Oct4-labeled cells (possibly the
poorly differentiated cells) were observed deep in the mucosa
(Figs. 2F and 3B). Thus, during chronic (severe)
gastritis there is an enhancement of cell proliferation and
pluripotency as evidenced by the upregulation of Oct4
immunolabeling. Quantification by the two different methods
confirmed this observation (Tables
I and II).
An immunohistochemical analysis of the tissues
obtained from the 3 locations of the resected gastric cancer
tissues showed an upregulation of Oct4 expression. These findings
confirm recently published data (32). Quantifications of immunoprobed
tissue sections confirmed these findings particularly when all the
cancerous tissues were considered as one group and compared with
the controls and the group with gastritis (Tables I and II).
Subcellular localization of Oct4 in
normal and cancerous tissues
In the present study, we also attempted to provide
an answer to the question regarding Oct4 intracellular
localization; we aimed to identify whether Oct4 expression is
nuclear or cytoplasmic. There has been some debate regarding this
issue (29,30,36).
An attempt to examine the detailed immunolabeling of Oct4 in normal
control mucosal biopsies showed its localization in the nuclei with
little cytoplasmic immunostaining of UEA-labeled cells lining the
lower part of the gastric pits (Figs.
2A and 6C). The UAE- and
PAS-positive labeling of the apical cytoplasm of these cells
indicated that they belong to the mucus-secreting pit cell lineage.
Therefore, Oct4 is mostly localized in the nuclei of the
mucus-secreting pre-pit cells and their immediate descendents, the
differentiating pit cells.
Of note, the cancerous tissues did not only show
overexpression and intensification of Oct4 immunostaining, but also
showed an alteration in the subcellular localization of Oct4.
Microscopic examination revealed that the Oct4 immunolabeling was
mostly cytoplasmic (Figs. 6D and
7). This observation was supported
by subcellular fractionation and western blot analysis, wherein the
majority of Oct4 was localized in the nuclear fraction of normal
biopsies, but was mostly localized in the cytoplasmic fraction of
cancerous tissues (Fig. 5C).
Although the mechanisms that regulate the expression of Oct4 are
not yet well understood (37,38),
mutation could possibly explain its accumulation in the cytoplasm
during carcinogenesis with the inhibition of its translocation into
the nucleus.
The data from the present study support the concept
of ‘cancer stem cells’ and the theory of the stem cell origin of
cancer (13,14,38,39).
Self-renewing progenitor and differentiating cells expressing Oct4
may be the target for cancer initiation and progression, through
the induction of symmetrical cell division. The data from the
present study also support the view that Oct4 may represent a
diagnostic biomarker for gastric cancer. However, further human
oncology and mechanistic studies are recommended. Understanding the
mechanism by which adult stem or progenitor cells initiate gastric
carcinogenesis may well provide greater opportunities for cancer
prevention and lead to more effective cancer treatment
strategies.
Acknowledgements
This study was supported by a research
grant from the Terry Fox Fund for Cancer Research.
References
|
1.
|
Brenner H, Rothenbacher D and Arndt V:
Epidemiology of stomach cancer. Methods Mol Biol. 472:467–477.
2009. View Article : Google Scholar
|
|
2.
|
Correa P: Is gastric cancer preventable?
Gut. 53:1217–1219. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Kushima R, Vieth M, Borchard F, Stolte M,
Mukaisho K and Hattori T: Gastric-type well-differentiated
adenocarcinoma and pyloric gland adenoma of the stomach. Gastric
Cancer. 9:177–184. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Correa P, Piazuelo MB and Camargo MC:
Etiopathogenesis of gastric cancer. Scand J Surg. 95:218–24.
2006.
|
|
5.
|
Fox JG and Wang TC: Inflammation, atrophy,
and gastric cancer. J Clin Invest. 117:60–69. 2007. View Article : Google Scholar
|
|
6.
|
Karam SM: Mouse models demonstrating the
role of stem/progenitor cells in gastric carcinogenesis. Front
Biosci. 15:595–603. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Lee ER and Leblond CP: Dynamic histology
of the antral epithelium in the mouse stomach: II. Ultrastructure
and renewal of isthmal cells. Am J Anat. 172:205–224. 1985.
View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Karam SM: Lineage commitment and
maturation of epithelial cells in the gut. Front Biosci.
4:D286–D298. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Barker N, Huch M, Kujala P, van de
Wetering M, Snippert HJ, van Es JH, Sato T, Stange DE, Begthel H,
van den Born M, Danenberg E, van den Brink S, Korving J, Abo A,
Peters PJ, Wright N, Poulsom R and Clevers H: Lgr5(+ve) stem cells
drive self-renewal in the stomach and build long-lived gastric
units in vitro. Cell Stem Cell. 6:25–36. 2010.
|
|
10.
|
Karam SM, Tomasetto C and Rio MC: Trefoil
factor 1 is required for the commitment programme of mouse oxyntic
epithelial progenitors. Gut. 53:1408–1415. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Karam SM, Tomasetto C and Rio MC:
Amplification and invasiveness of gastric epithelial progenitors
during carcinogenesis in TFF1-knockout mice. Cell Prolif.
41:923–935. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Al-Awadhi H, John R, Al-Marzooqi F, Vincze
A, Branicki F and Karam SM: Sequential alterations in gastric
biopsies and tumor tissues support the multistep process of
carcinogenesis. Histol Histopath. 26:1153–1164. 2011.PubMed/NCBI
|
|
13.
|
Trosko JE and Chang CC: Stem cell theory
of carcinogenesis. Toxicol Lett. 49:283–295. 1989. View Article : Google Scholar
|
|
14.
|
Sell S: On the stem cell origin of cancer.
Am J Pathol. 176:2584–2594. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Katoh M and Katoh M: WNT signaling pathway
and stem cell signaling network. Clin Cancer Res. 13:4042–4045.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Okamoto K, Okazawa H, Okuda A, Sakai M,
Muramatsu M and Hamada H: A novel octamer binding transcription
factor is differentially expressed in mouse embryonic cells. Cell.
60:461–472. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Thomas T, Nowka K, Lan L and Derwahl M:
Expression of endoderm stem cell markers: evidence for the presence
of adult stem cells in human thyroid glands. Thyroid. 16:537–544.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Yu H, Fang D, Kumar SM, Li L, Nguyen TK,
Acs G, Herlyn M and Xu X: Isolation of a novel population of
multipotent adult stem cells from human hair follicles. Am J
Pathol. 168:1879–1888. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Sotomayor P, Godoy A, Smith GJ and Huss
WJ: Oct4A is expressed by a subpopulation of prostate
neuroendocrine cells. Prostate. 69:401–410. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Tai MH, Chang CC, Kiupel M, Webster JD,
Olson LK and Trosko JE: Oct4 expression in adult human stem cells:
evidence in support of the stem cell theory of carcinogenesis.
Carcinogenesis. 26:495–502. 2005.PubMed/NCBI
|
|
21.
|
Hochedlinger K, Yamada Y, Beard C and
Jaenisch R: Ectopic expression of Oct-4 blocks progenitor-cell
differentiation and causes dysplasia in epithelial tissues. Cell.
121:465–477. 2005. View Article : Google Scholar
|
|
22.
|
Looijenga LH, Stoop H, de Leeuw HP, de
Gouveia Brazao CA, Gillis AJ, van Roozendaal KE, van Zoelen EJ,
Weber RF, Wolffenbuttel KP, van Dekken H, Honecker F, Bokemeyer C,
Perlman EJ, Schneider DT, Kononen J, Sauter G and Oosterhuis JW:
POU5F1 (OCT3/4) identifies cells with pluripotent potential in
human germ cell tumors. Cancer Res. 63:2244–2250. 2003.PubMed/NCBI
|
|
23.
|
Ezeh UI, Turek PJ, Reijo RA and Clark AT:
Human embryonic stem cell genes OCT4, NANOG, STELLAR, and GDF3 are
expressed in both seminoma and breast carcinoma. Cancer.
104:2255–2265. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Iki K and Pour PM: Expression of Oct4, a
stem cell marker, in the hamster pancreatic cancer model.
Pancreatology. 6:406–413. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Gibbs CP, Kukekov VG, Reith JD,
Tchigrinova O, Suslov ON, Scott EW, Ghivizzani SC, Ignatova TN and
Steindler DA: Stem-like cells in bone sarcomas: implications for
tumorigenesis. Neoplasia. 7:967–976. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Stolte M and Meining A: The updated Sydney
system: classification and grading of gastritis as the basis of
diagnosis and treatment. Can J Gastroenterol. 15:591–598.
2001.PubMed/NCBI
|
|
27.
|
Karam SM, Li Q and Gordon JI: Gastric
epithelial morphogenesis in normal and transgenic mice. Am J
Physiol. 272:G1209–G1220. 1997.PubMed/NCBI
|
|
28.
|
Farook VS, Alkhalaf M and Karam SM:
Establishment of a gastric epithelial progenitor cell line from a
transgenic mouse expressing the simian virus 40 large T antigen
gene in the parietal cell lineage. Cell Prolif. 41:310–320. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Liedtke S, Stephan M and Kogler G: Oct4
expression revisited: potential pitfalls for data misinterpretation
in stem cell research. Biol Chem. 389:845–850. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Zuk PA: The intracellular distribution of
the ES cell totipotent markers OCT4 and Sox2 in adult stem cells
differs dramatically according to commercial antibody used. J Cell
Biochem. 106:867–877. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Wang X and Dai J: Concise review: isoforms
of OCT4 contribute to the confusing diversity in stem cell biology.
Stem Cells. 28:885–893. 2010.PubMed/NCBI
|
|
32.
|
Chen Z, Xu WR, Qian H, Zhu W, Bu XF, Wang
S, Yan YM, Mao F, Gu HB, Cao HL and Xu XJ: Oct4, a novel marker for
human gastric cancer. J Surg Oncol. 99:414–419. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Graham DY, Opekun AR, Yamaoka Y, Osato MS
and el-Zimaity HM: Early events in proton pump inhibitor-associated
exacerbation of corpus gastritis. Aliment Pharmacol Ther.
17:193–200. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Ji H and Yardley JH: Iron
medication-associated gastric mucosal injury. Arch Pathol Lab Med.
128:821–822. 2004.PubMed/NCBI
|
|
35.
|
Zangrossi S, Marabese M, Broggini M,
Giordano R, D’Erasmo M, Montelatici E, Intini D, Neri A, Pesce M,
Rebulla P and Lazzari L: Oct-4 expression in adult human
differentiated cells challenges its role as a pure stem cell
marker. Stem Cells. 25:1675–1680. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Asadi MH, Mowla SJ, Fathi F, Aleyasin A,
Asadzadeh J and Atlasi Y: OCT4B1, a novel spliced variant of OCT4,
is highly expressed in gastric cancer and acts as an antiapoptotic
factor. Int J Cancer. 128:2645–2652. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Niwa H, Miyazaki J and Smith AG:
Quantitative expression of Oct-3/4 defines differentiation,
dedifferentiation or self-renewal of ES cells. Nat Genet.
24:372–376. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Ratajczak MZ, Shin DM, Liu R, Marlicz W,
Tarnowski M, Ratajczak J and Kucia M: Epiblast/germ line hypothesis
of cancer development revisited: lesson from the presence of
Oct-4+ cells in adult tissues. Stem Cell Rev. 6:307–316.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
39.
|
Sell S: Cancer and stem cell signaling: a
guide to preventive and therapeutic strategies for cancer stem
cells. Stem Cell Rev. 3:1–6. 2007. View Article : Google Scholar : PubMed/NCBI
|