Introduction
In a recent study, the leucine-rich repeat G
protein-coupled receptor 5 (LGR5, also known as GPR49) was
identified as a potential marker of intestinal stem cells in human
(1–3). LGR5 is expressed mainly in crypt base
columnar (CBC) cells at the bottom of intestinal crypts and it is a
markers for both cancer and normal intestinal stem cells (1). Cancer stem cells (CSCs) are a small
population of cancer cells that have the ability to affect tumor
initiation, differentiation and metastasis. The self-renewal and
differentiation capabilities of CSCs are similar to those of normal
embryonic or adult stem cells (4,5). For
isolation of CSCs, some cell surface markers, so-called cancer stem
cell markers, can be used. In the intestine, functional stem cells
are located at the bottom of the crypts, especially at the +4
position (1–3), and they can be isolated and studied
via molecular markers such as CD34, CD44, CD133, and LGR5 (6–8).
CSCs play an important role in cancer recurrence after radio- or
chemotherapy (9–11), and CSC-mediated resistance to
radiation and chemotherapeutic agents is influenced by the cellular
level of reactive oxygen species (ROS) (11,12).
Many ROS, including O2−,
OH−, and H2O2, are produced by
physiological intracellular reactions and function not only as a
byproduct of cellular metabolisms but also as a mediator of cell
signaling pathways (13).
Diehn et al demonstrated that CSCs in breast
and head/neck tumors have lower ROS levels and higher expression
levels of antioxidant genes or proteins, compared to levels in
nontumorigenic progeny, which were similar to levels in normal stem
cells (12). However, the effects
of ROS on CSCs have not been fully described.
The above-mentioned LGR5 is a Wnt target gene and
its expression pattern has been related to β-catenin mutation
(2,14,15).
It has been shown that H2O2 can inhibit the
Wnt/β-catenin signaling pathway through decreases in the amount of
nuclear β-catenin and Tcf/Lef dependent transcription (16). In contrast, Funato et al
showed that H2O2 induces β-catenin
stabilization and increases the expression of endo genous Wnt
target genes (17). Despite these
studies, little is known about the effect of
H2O2 on the Wnt/β-catenin signaling
pathway.
The purpose of this study is to reveal the effects
of ROS on various aspects of CSCs, in colorectal cancer cells. To
study this, colorectal cancer cells were treated with exogenous
H2O2, and then examined for expression of the
CSC marker, LGR5, and for cellular responses.
Materials and methods
Cell culture
Thirty of the 32 human colorectal cancer cell lines
were maintained in RPMI-1640 media. The CACO-2 cell line was
maintained in minimum essential medium and the WiDr cells were
maintained in Dulbecco’s modified Eagle’s medium (cells obtained
from Korean Cell line Bank, Seoul, Korea). All media were
supplemented with 10% fetal bovine serum (FBS), 100 U/ml of
penicillin, and 0.1 mg/ml of streptomycin. Cells were maintained in
humidified incubators at 37°C, with an atmosphere of 5%
CO2 and 95% air.
Cell proliferation assay by MTT
Cell proliferation was analyzed by using MTT
[3-(4,5-dimethylthiazol-2-yl)-2,5 diphenyltetrazolium bromide]
(Sigma Chemical Co.) assay.
Initially, 3×106 cells were seeded on
96-well plates and cultured for 1 day. Subsequently, media
containing 50–900 μM of H2O2 were added to
the plates and the cells incubated for 24 h before being treated
with 50 μl of MTT solution for 4 h at 37°C. Absorbance at 540 nm
was measured by an ELISA reader (Molecular Devices Co., CA,
USA).
Total RNA extraction, cDNA synthesis and
reverse transcriptase-PCR (RT-PCR)
Total RNA was isolated by using easy-BLUE™ kits
(Intron Biotechnology, Gyeonggi, Korea). For cDNA synthesis, 2 μg
of total RNA were combined with a random primer, dNTP, and 200 U of
Superscript II™ reverse transcriptase (Invitrogen, Carlsbad, CA,
USA) in a final volume of 20 μl. The mixture incubated for 60 min
at 42°C, after that incubated for 15 min at 70°C for denaturation.
Next, 80 μl of distilled water was added to the
reverse-transcription reaction mixture. PCR was performed to obtain
mRNA expression data. The prepared 15 μl PCR mixtures included 1 μl
of cDNA, 10X buffer, 2.5 mM of dNTP, 0.1 pM of primers and 1 U of
Taq DNA polymerase (Intron Biotechnology). PCR amplification was
conducted using the described primer sets (Table I). The β-actin was used as an
endogenous reference for the normalization of expression levels.
The following PCR conditions were used: initial denaturation for 5
min at 94°C, cycling at 94°C (30 sec), 55°C (30 sec), and 72°C (30
sec) for 35 cycles, with final elongation for 7 min at 72°C. PCR
was performed in a thermal cycler (PCR System 9700, Applied
Biosystems, Foster City, CA, USA). The PCR products were divided in
2% agarose gel stained with ethidium bromide.
 | Table I.Sequences of specific primers used in
the study. |
Table I.
Sequences of specific primers used in
the study.
| Gene | | Primer
sequence | Size (bp) | Refs. |
|---|
| h LGR5 | Forward |
5′-AGGATCTGGTGAGCCTGAGAA-3′ | 151 | (25) |
| Reverse |
5′-CATAAGTGATGCTGGAGCTGGTAA-3′ | | |
| β-catenin | Forward |
5′-TCTTGGCTATTACGACAG-3′ | 459 | (16) |
| Reverse |
5′-CCTCTATACCACCCACTT-3′ | | |
| HO-1 | Forward |
5′-GCTCAACATCCAGCTCTTTGAGG-3′ | 282 | - |
| Reverse |
5′-GACAAAGTTCATGGCCCTGGGA-3′ | | |
| β-actin | Forward |
5′-GACCACACCTTCTACAATGAG-3′ | 301 | (17) |
| Reverse |
5′-GCATACCCCTCGTAGATGGG-3′ | | |
Treatment of hydrogen peroxide
For exogenous H2O2 treatment,
cells were seeded in 75 cm2 culture flasks at a rate of
0.5×106 cells per flask. After 1 day, the cells treated
with 0, 50, 100, 150, 300, 600, or 900 μM of
H2O2 (Sigma Chemical Co.) for 24 h.
Subsequently, the cells were harvested for protein, RNA isolation,
and cell cycle analysis.
Western blotting
For protein analysis, harvested cells were washed in
PBS, suspended in Pro-Prep™ kits (Intron Biotechnology) and placed
on ice for 30 min. After centrifuging at 13,000 x g for 30 min at
4°C, the supernatant was collected. Protein quantitative analysis
was accomplished by using Bradford’s method using BSA (Sigma
Chemical Co.). Protein (20 μg) and 4X SDS sample buffer
(Invitrogen) mixtures were boiled at 95°C for 5 min and loaded on
4–12% Bis-Tris gel (Invitrogen) at 100 V for 3 h. Proteins were
transferred to a PVDF membrane (Invitrogen) by electroblotting at a
constant 270 mA current for 2 h. For membrane blocking, the
membrane was incubated for 1 h at room temperature in 1.5% non-fat
dry milk and 0.5% Tween-20 TBS buffer containing 1 mM of
MgCl2. Primary antibodies against hLGR5 (GPR49) (Abcam,
Cambridge, UK) (1:500), heme oxygenase-1 (Abcam) (1:1,000), jun
(BD, New Jersey, USA) (1:1,000), PARP (BD) (1:1,000), β-catenin
(Abcam) (1:2,000), HSP60 (Abcam) (1:1,000), and Lamin-B (Abcam)
(1:1,000) were incubated overnight at 4°C. For secondary antibody
incubation, peroxidase conjugated mouse or rabbit IgG antibody
(Jackson Immunoresearch, Baltimore, MD, USA) was diluted 1:5,000.
After incubation at room temperature for 1 h, a chemiluminescent
working solution, WESTZOL™ (Intron Biotechnology) was decanted to
the membrane. The membrane was then covered with a thin plastic
wrap and exposed to Fuji RX film for 1–60 min.
Cell cycle analysis
For cell cycle distribution analysis, cells treated
with H2O2 were fixed overnight in 70% ethanol
at −20°C. Subsequently, cellular DNA was stained with 100 μg/ml of
propidium iodide (PI) (Sigma Chemical Co.) for 30 min on ice. After
staining, cells were subjected to fluorescence-activated cell
sorter (FACs CantoII™, BD, NJ, USA) analysis of DNA content to
determine the percentage of the cells in different cell phases and
in apoptosis.
Immunocytochemical staining
For visual cell examination, 0.5×105
cells were grown on cover slips in 12-well culture plates. After 1
day, the cell-covered slips were treated with
H2O2 for 24 h, then rinsed briefly in PBS and
fixed with 3.7% formaldehyde (Sigma Chemical Co.) in PBS for 15 min
at room temperature. For permeabilization, samples were incubated
for 10 min with 0.25% Triton X-100 (Merck, Darmstadt, Germany) in
PBS at room temperature. Samples were then incubated with 1% BSA
(Invitrogen) in PBS-T for 30 min at room temperature for blocking,
and then treated with 1:1,000 diluted LGR5 and β-catenin primary
antibody in PBS-T overnight at 4°C. Subsequently, the samples were
rinsed and incubated with 1:500 diluted goat anti-rabbit secondary
antibody for 2 h at room temperature in the dark.
4′,6-diamidino-3-phenylindole dihydrochloride hydrate (DAPI, 100
μg/ml) (Sigma Chemical Co.) was used for counter staining. Cells
were visualized using a Pascal confocal microscope (Carl Zeiss,
Oberkochen, Germany).
Preparation of nuclear extraction
The cells were removed from the culture flask by
scraping and washed in PBS. For cytoplasmic fraction collection,
cells were resuspended in 500 μl of 1X hypotonic buffer and 25 μl
of detergent (Active Motif, Shinjuku, Japan). Following
centrifuging at 14,000 × g for 30 sec at 4°C, the supernatant (the
cytoplasmic fraction), to a new tube. Remaining nuclear pellet was
resuspended in 50 μl of complete lysis buffer (Active Motif) and
incubated for 30 min on ice. Following which it was centrifuged for
10 min at 14,000 × g at 4°C. The supernatant (the nuclear
fraction), was then transferred to a new tube.
Co-immunoprecipitation of LGR5
LGR5 primary antibody (5 μg) was added to the
microcentrifuge tube that contained the SNU-C2A lysate (200 μg);
the mixture was then incubated overnight at 4°C. Subsequently 20 μl
of anti-rabbit IgG beads (eBioscience, Iceland, UK) were added and
the mixture incubated for 1 h at 4°C. Next, the tube was
centrifuged for 1 min at 10,000 × g at 4°C. The supernatant was
then removed and the bead pellet resuspended using Pro-Prep™
(Intron Biotechnology). After washing, add 20 μl of 2X SDS sample
buffer (Invitrogen) were added and the mixture boiled at 95°C for
10 min. While avoiding loading of anti-rabbit IgG beads, the
supernatant was loaded on to 4–12% Bis-Tris gel.
Results
Increased apoptotic cell death at high
dose of H2O2
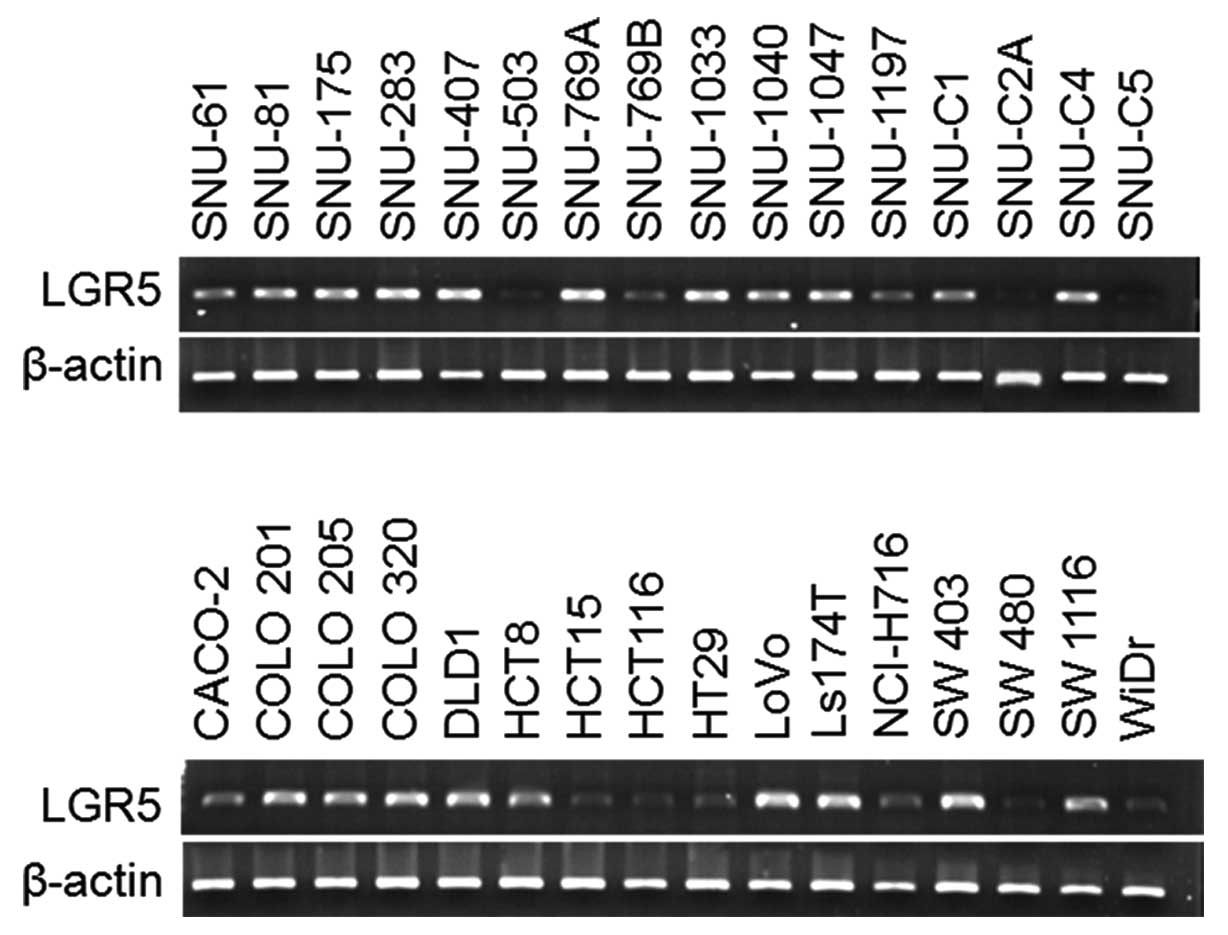
For the analysis of expression level of LGR5, we
investigated expression in 32 human colorectal cancer cell lines by
RT-PCR. LGR5 was highly expressed in most of cell lines (29/32 cell
lines; 90.63%) and expressed at a low level in 3 cell lines
(SNU-503, SNU-C2A, and SNU-C5) (Fig.
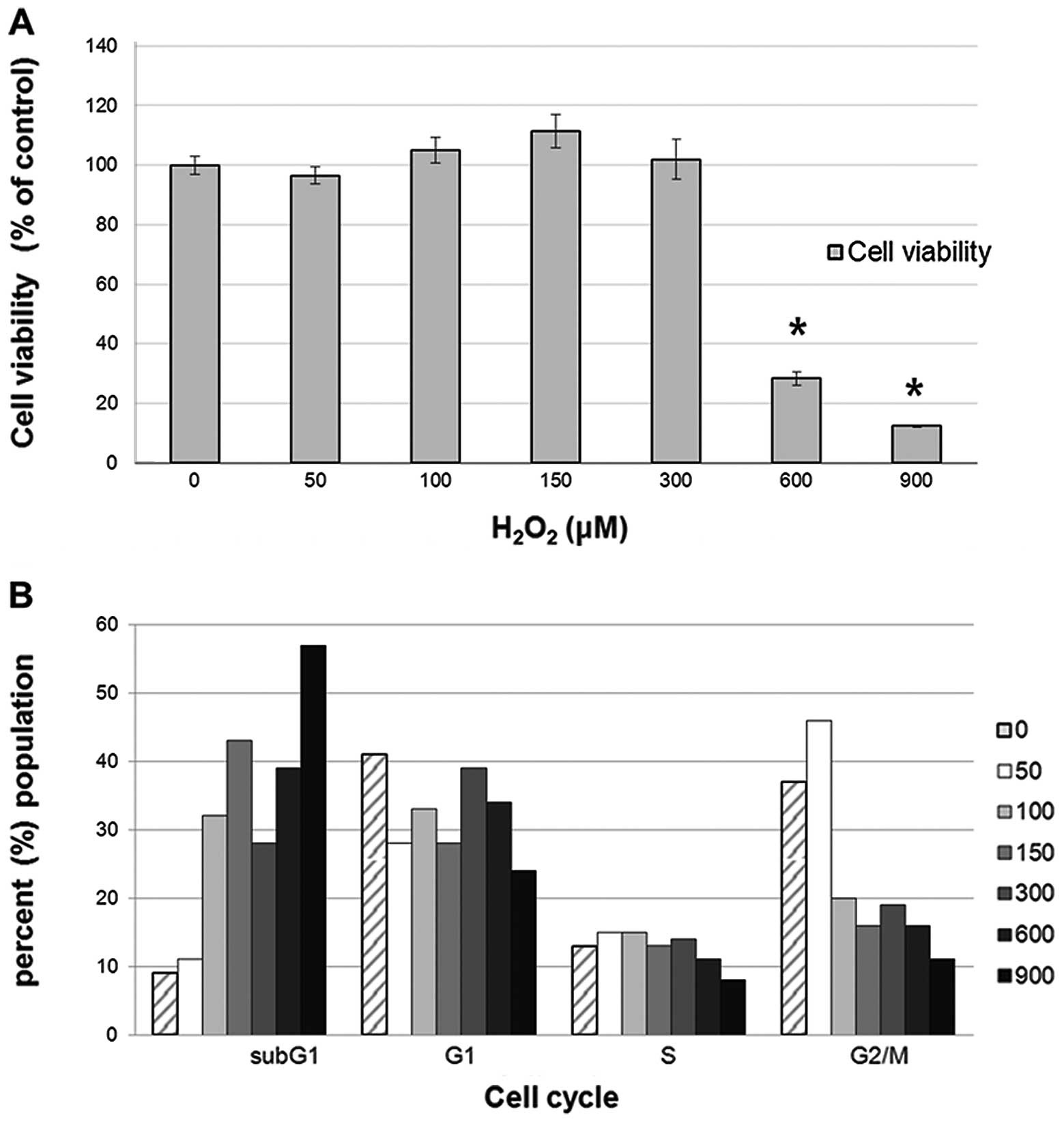
1). To determine whether ROS treatment would affects the cell
proliferation in SNU-C2A, which has wild-type β-catenin (18), SNU-C2A cells were treated with 50,
100, 150, 300, 600, or 900 µM of H2O2 and
incubated for 24 h. As shown in Fig.
2A, the number of SNU-C2A cells increased when treated with a
low dose of H2O2, but the relationship dosage
and cell increase had no significant correlation. However, SNU-C2A
cell viability was significantly decreased when treated with 600 or
900 μM of H2O2 (p<0.005). Park et
al suggested that a low level of H2O2
promotes cancer cell proliferation, compared to that in the control
cells, while at a higher H2O2 concentration
the cancer cells exhibited an 80% growth inhibition with apoptosis
triggered via activation of the AMP-activated protein kinase (AMPK)
pathway (19). Here, since SNU-C2A
cell viability increased at the low concentration of
H2O2, the percentage of subG1, G1, S, and
G2/M cell cycle phases were determined by FACs in order to
investigate the effect of H2O2 on the cell
cycle. As shown in Fig. 2B, the
percentage of cells in the G1 stage increased under treatment with
50–300 μM of H2O2, which indicates that the
cell cycle was arrested to evade cell death by the ROS. However, at
higher H2O2 concentration the percentage of
cells in the G2/M stage decreased, and that in the subG1 stage
increased. The increase in the subG1 stage is mediated through cell
death at the higher concentration of
H2O2.
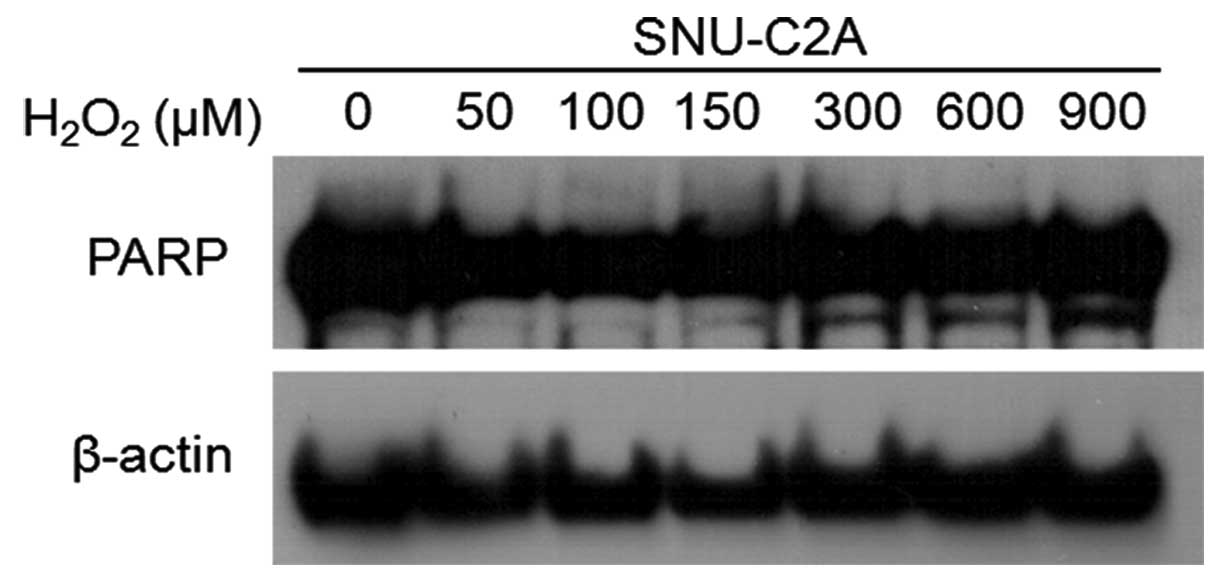
As PARP [poly(ADP-ribose) polymerase] cleavage is
considered a marker of apoptosis (20), we performed western blotting to
detect the presence of PARP cleavage. The level of the 85 kDa
cleavage form of PARP, was increased at the higher concentrations
of H2O2 (Fig.
3, lower bands).
These results suggest that ROS treatment of over 300
μM of H2O2, will arrest the cell cycle, with
the cells then falling into apoptosis in the SNU-C2A cell line. As
a result, the SNU-C2A proliferation rate significantly decreased
when treated with 600 or 900 μM of H2O2.
JNK signaling pathway regulates the
expression level of LGR5 by ROS
To investigate whether ROS affected expression of a
cancer stem cell marker, we examined LGR5 expression level after
H2O2 treatment of SNU-C2A cells for 24 h, by
using RT-PCR, western blotting, and immunocytochemical staining
(Figs. 4 and 5A).
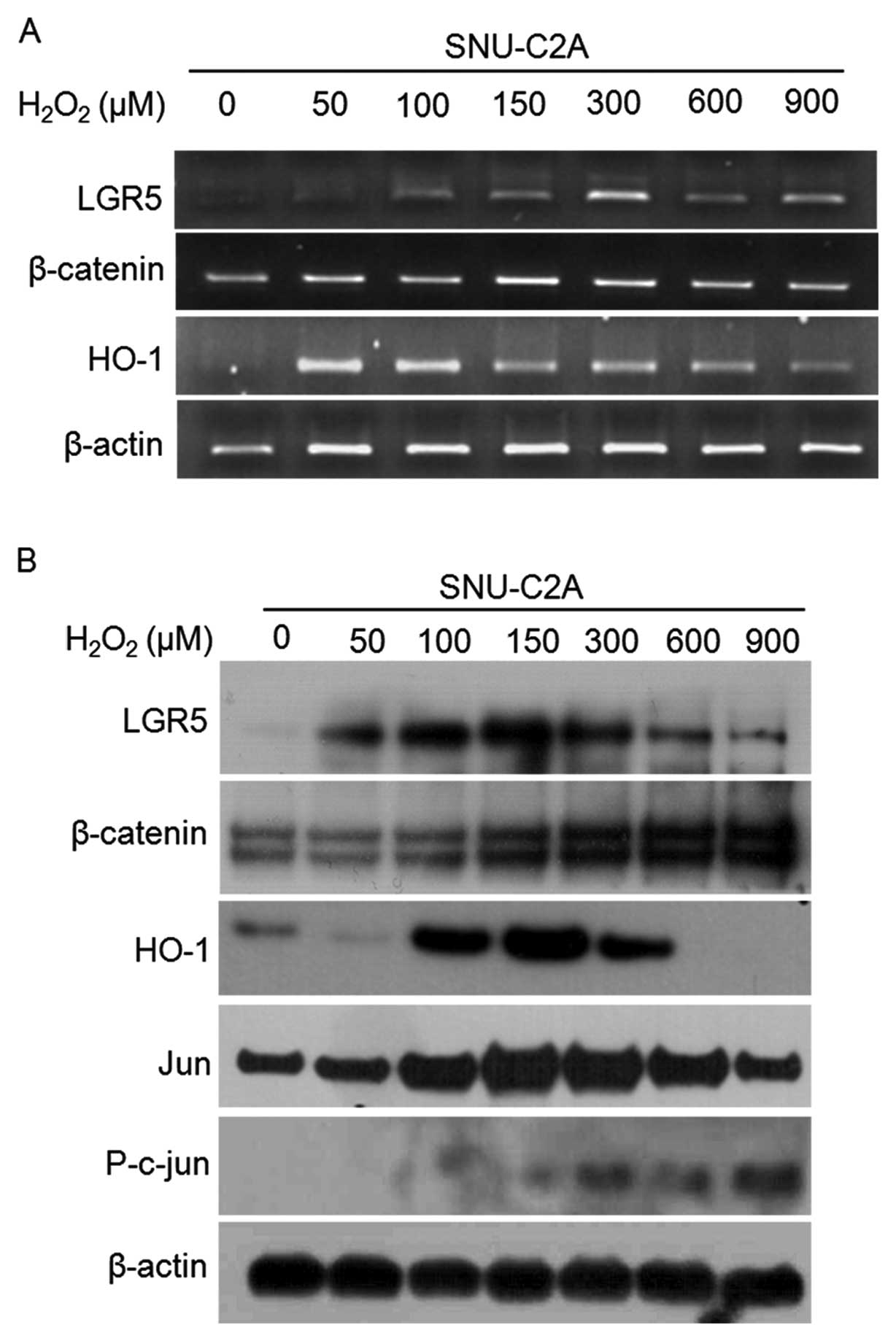 | Figure 4.Induction of β-catenin and LGR5
expression following treatment with H2O2.
(A), RT-PCR was performed after treatment with
H2O2. SNU-C2A cancer cells were treated with
50, 100, 150, 300, 600, or 900 μM of H2O2 and
mRNA expressions of LGR5, β-catenin, and HO-1 were assessed. Each
sample was normalized by β-actin level. (B), Protein levels
determined by western blotting after treatment with
H2O2. In each lane, 20 μg of protein was
loaded. |
To clarify the effect of ROS in these cells, we
assessed the expression level of HO-1, which is induced by various
stimuli and is known to play a significant role in protection of
cells against oxidative injury, heat shock, and ROS. In addition,
HO-1 may be involved in anti-apoptotic activity through activation
of the Akt pathway (21). We
detected overexpression of HO-1 at treatment of 100-300 μM
H2O2 in SNU-C2A cells (Fig. 4). We also investigated jun
expression, considered to be another indicator that affected by
ROS. The expression pattern of jun, a JNK signaling pathway
component, was similar to HO-1. Jun expression resulted from
activation of the JNK signaling pathway, which is also involved in
H2O2 induced upregulation of HO-1 (22). When jun is activated by
phosphorylation, the JNK signaling pathway is stimulated (23). Here, phospho-jun increased in a
dose-dependent manner with H2O2 treatment
(Fig. 4B), indicating that JNK
signaling is activated by H2O2. Our results
support previous MTT results (Fig.
2) and suggest that JNK signaling has a protective effect
against ROS (22). In addition,
the result indicate that ROS induce cell proliferation through
upregulation of jun and via cross-talk with the PI3-K/PKB pathway
(24). Furthermore, a recent study
has shown that LGR5 is a novel target of c-jun/Mbd3 (25). Based on our results, we can confirm
a similar expression pattern in LGR5 and jun, leading to the
suggestion that activation of JNK signaling is associated with
alteration of LGR5 expression by ROS.
Induction of β-catenin and LGR5 as
treated with H2O2
LGR5 expression increased following cell treatment
with 50–300 μM of H2O2; a finding that is
similar to the proliferation increase noted in Fig. 2. In contrast, LGR5 expression
decreased at doses higher than 600 μM of
H2O2, which were the same level at which
apoptosis was induced (Figs. 4 and
5A).
Since LGR5 is a Wnt target gene (2,14,15),
we analyzed the correlation between LGR5 and β-catenin, a Wnt
signaling component. As shown in Fig.
4, the expression level of β-catenin increased with
H2O2 treatment in a dose-dependent manner.
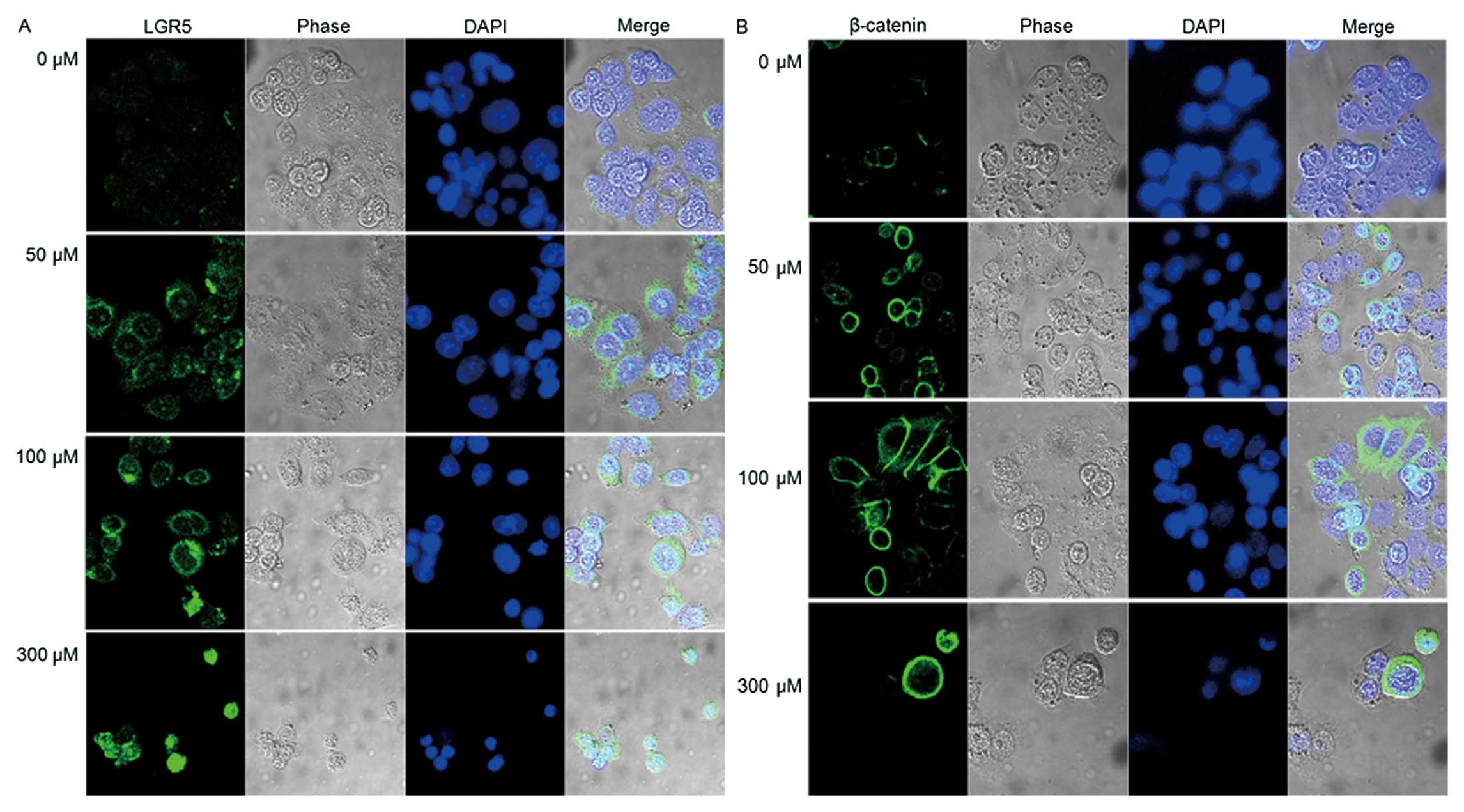
Immunocytochemistry was performed in SNU-C2A cells that were
treated with the same dosage levels of H2O2
as shown in Fig. 4 (Fig. 5). The expression patterns of LGR5
and β-catenin were found to be similar (Fig. 4).
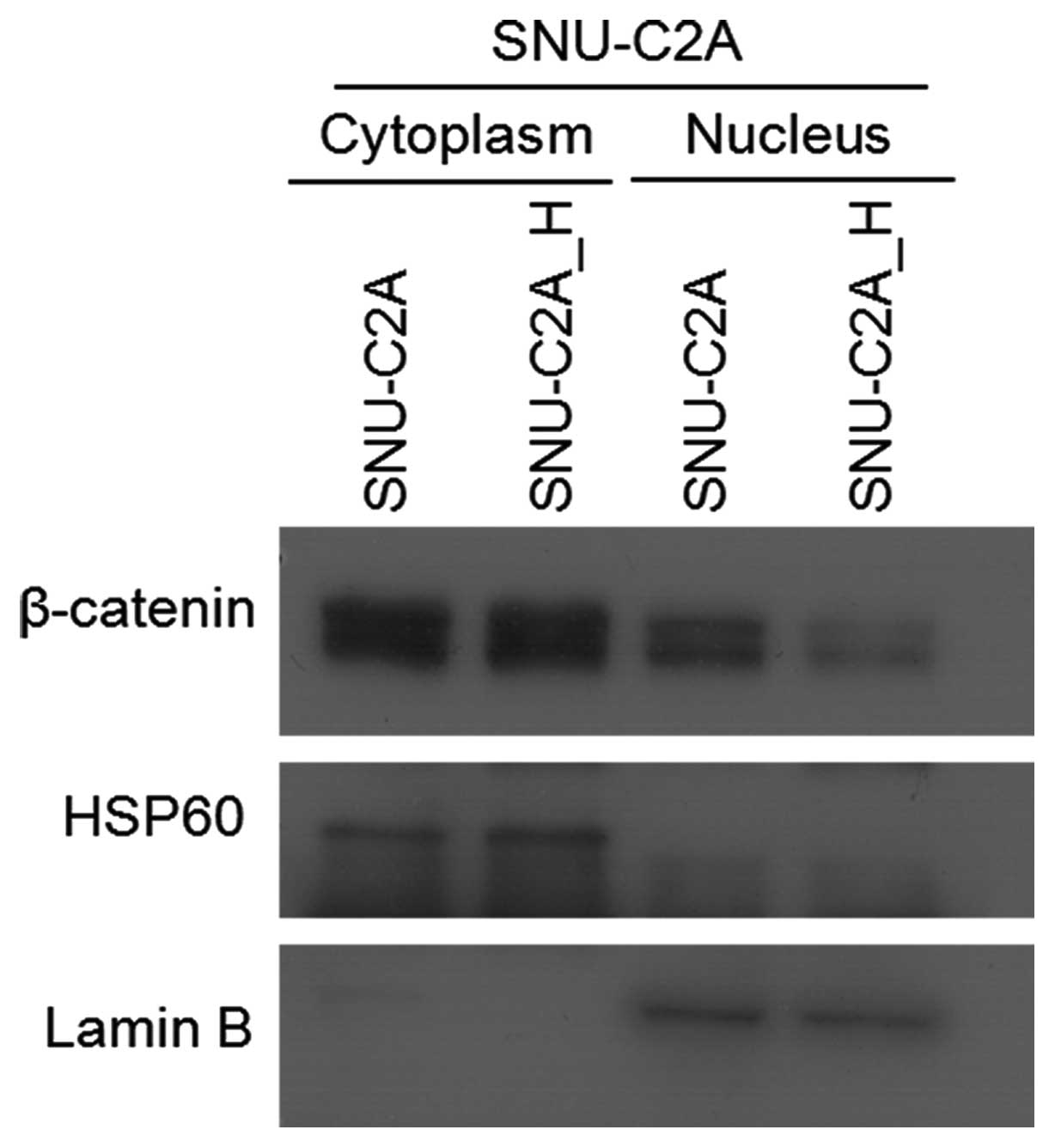
To determine whether induction of LGR5 is the result
of β-catenin-mediated Wnt signaling, the location of β-catenin
within the cells was determined. As shown in Fig. 5, β-catenin was mainly present in
the cytoplasm. To confirm the immunocytochemistry results, we
separated and isolated cytoplasmic and nuclear proteins, and
performed western blotting. The β-catenin levels were lower in the
nucleus of H2O2-treated cells than in the
cytoplasm (Fig. 6). For activation
of β-catenin-mediated Wnt signaling, stabilized β-catenin needs to
be translocated to the nucleus and not degraded in the cytoplasm.
Our results show that nuclear β-catenin levels were low; therefore,
the expression of LGR5 could be regulated by factors other than
those in Wnt signaling.
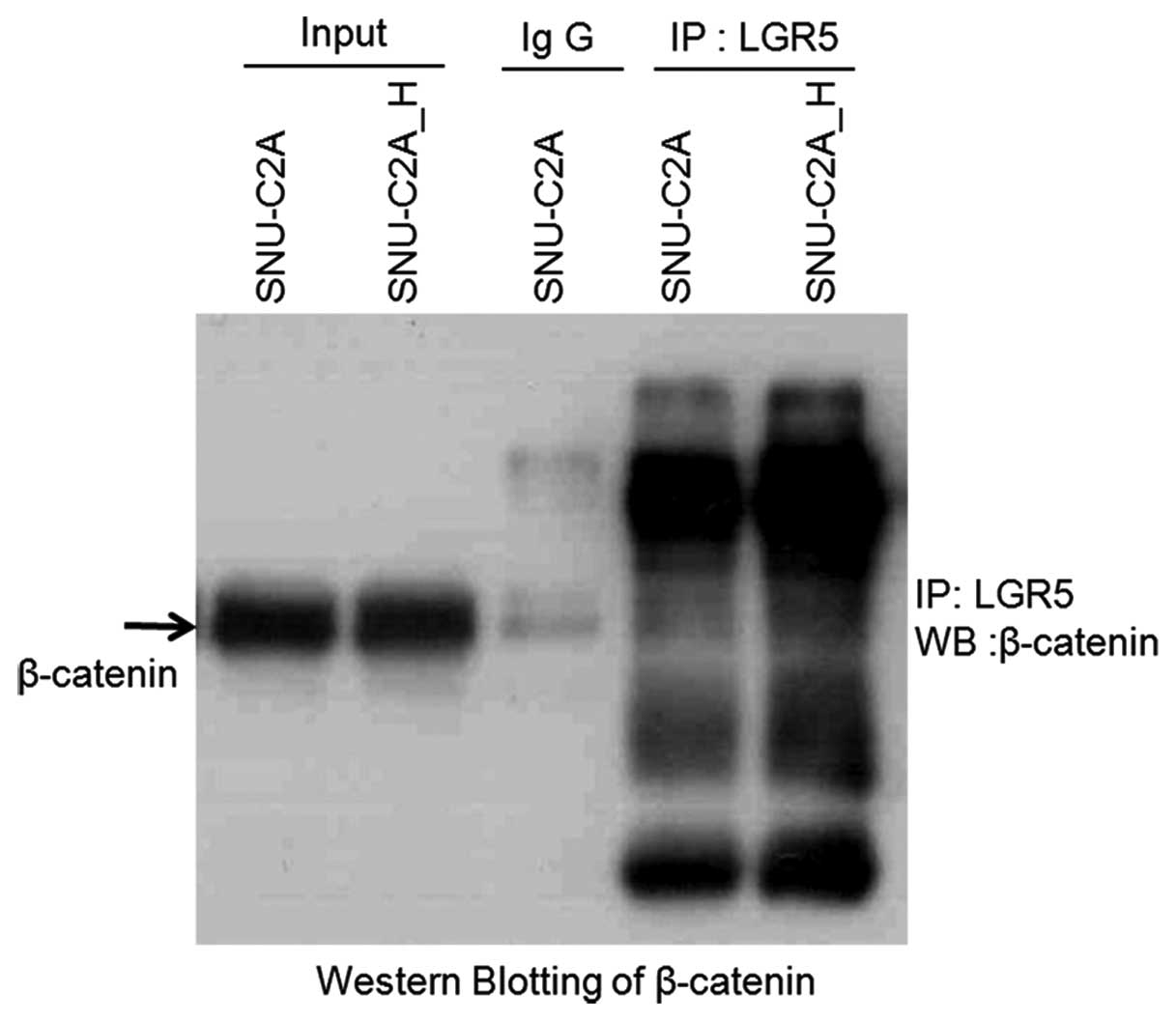
To investigate this, we performed
co-immunoprecipitation to determine if LGR5 directly interacts with
β-catenin. Western blotting with anti-β-catenin was used to analyze
the co-immuno-precipitation obtained by using the LGR5 antibody. As
shown in Fig. 7, β-catenin was not
detected. Therefore, we concluded that LGR5 did not interact with
β-catenin.
Discussion
LGR5 (GPR49) has been identified as a marker of
tumor initiation (2). The LGR5
gene encodes a G protein coupled receptor that is associated with a
glycoprotein ligand. LGR5 gene marks small cells, especially
cycling crypt-base columnar (CBC) cells that are interspersed
between Paneth cells (1).
Moreover, physiological expression of LGR5 is restricted to cells
at the base of crypts, a location known as a stem cell niche. LGR5
is one of the Wnt signaling target genes in colorectal cancer and
is critically involved in the development of carcinoma associated
with β-catenin mutation (14,15).
Wnt signaling regulates intestinal epithelium cell lineage
proliferation, differentiation, and maintenance (26). When mutated, such as APC mutation,
the Wnt signaling pathway is activated, and it plays a role in
tumorigenesis in humans (26).
Therefore, it has been suggested that activation of Wnt signaling
and expression of Wnt target genes are involved in tumor initiation
(27).
In our study, high expression of LGR5 was observed
in 90.6% the human colon cancer cell lines tested (Fig. 1). However, in a few cell lines,
including SNU-C2A, the LGR5 expression level was low. In order to
examine the effect of ROS on colorectal cancer cells and on the
cancer stem cell marker LGR5, experiments with the treatment of the
H2O2 were perform. H2O2
is reported to regulate the intensity of growth factor signaling,
metabolism, aging and apoptosis (28). In addition, it acts as either a
negative or positive regulator in the Wnt signaling pathway. Shin
et al have shown that H2O2 can inhibit
Wnt signaling through the down-regulation of β-catenin (16). In contrast, Funato et al
showed that treatment of cells with a low dose of
H2O2 induces stabilization of β-catenin and
increased expression of endogenous Wnt target genes (17). Even in our results, the effects of
H2O2 on Wnt signaling and a target gene were
not clear. However, based on such findings, LGR5 expression is
expected to be affected by H2O2.
In our study, SNU-C2A colon cancer cells, which have
wild-type β-catenin (17) and a
low expression of LGR5, were used to determine the correlation
between ROS and LGR5.
To that end, SNU-C2A cells were treated with 50,
100, 150, 300, 600, or 900 μM of H2O2 for 24
h to examine the ROS effects. According to Park et al, low
doses of H2O2 resulted in enhanced cell
proliferation accompanied by an increase in COX-2 expression,
whereas apoptosis was induced by high doses of
H2O2 and was correlated with AMPK activation
(19,29). There were similarities in our
results. As shown in Fig. 2A, the
number of SNU-C2A cells increased to a greater extent when treated
with low doses of H2O2, indicating that cell
proliferation increased with low dose treatment of
H2O2; however, there was no statistical
significance to the difference. With high doses of
H2O2, however, cell viability significantly
decreased with increased cell death with treatment of 600 and 900
μM of H2O2. As cell proliferation changed
with different H2O2 dosage, we performed cell
cycle analysis using the same dosages of ROS. As shown in Fig. 2B, the percentage of cells in the G1
stage increased following treatment with 50–300 μM
H2O2. At higher H2O2
concentrations percentage of the cells in the G2/M phase decreased
and those in the subG1 phase increased. The results indicate that,
at low levels of H2O2 treatment, the cell
cycle is arrested at the G1 phase to evade cell death. However, at
the higher H2O2 dosage the increase in the
subG1 stage is mediated through apoptotic cell death, which we
confirmed through assessment of the amount of PARP cleavage
(Fig. 3, lower bands).
We also investigated whether
H2O2 affects expression of LGR5. Expression
of HO-1 was induced in H2O2-treated SNU-C2A
in a dose-dependent manner (Fig.
4). The HO-1 is involved in heme catabolism and plays an
anti-apoptotic role against various stimuli, including those
related to ROS and heat shock (21). Therefore, our result suggest that
an increase in HO-1 would indicate that H2O2
is a potent inducer of cellular responses to ROS and that HO-1 was
expressed in order to reduce oxidative stress in the treated
SNU-C2A cells. JNK signaling, related to the promotion of cell
survival, was also induced by H2O2 treatment
(22). Following
H2O2 treatment, the expression of jun, a JNK
signaling component, increased in a pattern similar to that of HO-1
(Fig. 4). This result suggests
that an increase in jun expression can be regulated by the cellular
ROS level; thus, aiding in the prevention of cell death, as was
mentioned for HO-1. The LGR5 expression level significantly
increased following low dose treatment of
H2O2 in colon cancer cells. A recent study
showed that LGR5 is a novel target of c-jun/Mbd3 (25), and our results confirm a
correlative pattern between LGR5 and jun expression, leading to the
suggestion that activation of JNK signaling by ROS is associated
with LGR5 expression.
In conclusion, induced HO-1 and jun expression by
H2O2 treatment results in a reduction of
cellular ROS; thereby, LGR5 expression is increased. According to
these results, LGR5 is one of the stress induced genes. Since LGR5
is a Wnt target gene (2,14,15),
we supposed that LGR5 expression increases through
H2O2 activation of β-catenin-mediated Wnt
signaling. Fig. 4 shows that
β-catenin expression levels, associated with the Wnt signaling
pathway, also increased in H2O2-treated colon
cancer cells in a dose-dependent manner. However, an increased
level of β-catenin was detected only in cytoplasm, not in the
nucleus (Figs. 5 and 6). As a result, Wnt signaling would not
be involved in H2O2-mediated expression of
LGR5; thus, further investigation is needed. Cross-talk between JNK
and Wnt signaling has been reported (30) and we speculated that there could be
a correlation between β-catenin and jun. However, nuclear
β-catenin, which acts as a Wnt signaling transcriptional factor,
was not detected. Therefore, there was no relevance to the
association between JNK and Wnt signaling. Since induction of LGR5
seemed not to be regulated by Wnt signaling, we performed
co-immunoprecipitation in order to determine if LGR5 directly
interacts with β-catenin (Fig. 7).
However, we detected no direct interaction between LGR5 and
β-catenin.
In this study, we demonstrated that LGR5 and
β-catenin expression in colorectal cancer cells is increased by
H2O2 treatment. However, there remain
questions as to why the expression levels of LGR5 and β-catenin
increased at the same time and what their relative functions may
be. In order to answer these questions, further study is necessary;
we expect that our data will be fundamental to such a study.
Acknowledgements
This work was supported by Mid-career
Researcher Program (MEST R01-2008-000-20108-0) and Priority
Research Centers Program (2009-0093820) through National Research
Foundation of Korea grant funded by the MEST Ministry of
Educational Science and Technology.
References
|
1.
|
Barker N and Clevers H: Tracking down the
stem cells of the intestine: strategies to identify adult stem
cells. Gastroenterology. 133:1755–1760. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Barker N, van Es JH, Kuipers J, et al:
Identification of stem cells in small intestine and colon by marker
gene Lgr5. Nature. 449:1003–1007. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Becker L, Huang Q and Mashimo H:
Immunostaining of Lgr5, an intestinal stem cell marker, in normal
and premalignant human gastrointestinal tissue. Sci World J.
8:1168–1176. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Barker N and Clevers H: Leucine-rich
repeat-containing G-protein-coupled receptors as markers of adult
stem cells. Gastroenterology. 138:1681–1696. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Dalerba P, Cho RW and Clarke MF: Cancer
stem cells: models and concepts. Annu Rev Med. 58:267–284. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Ricci-Vitiani L, Lombardi DG, Pilozzi E,
et al: Identification and expansion of human
colon-cancer-initiating cells. Nature. 445:111–115. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Corbeil D, Röper K, Hellwig A, et al: The
human AC133 hematopoietic stem cell antigen is also expressed in
epithelial cells and targeted to plasma membrane protrusions. J
Biol Chem. 275:5512–5520. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Burkert J, Wright NA and Alison MR: Stem
cells and cancer: an intimate relationship. J Pathol. 209:287–297.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Bao S, Wu Q, McLendon RE, et al: Glioma
stem cells promote radioresistance by preferential activation of
the DNA damage response. Nature. 444:756–760. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Li X, Lewis MT, Huang J, et al: Intrinsic
resistance of tumorigenic breast cancer cells to chemotherapy. J
Natl Cancer Inst. 100:672–679. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Phillips TM, McBride WH and Pajonk F: The
response of CD24(-/low)/CD44+ breast cancer-initiating cells to
radiation. J Natl Cancer Inst. 98:1777–1785. 2006.
|
|
12.
|
Diehn M, Cho RW, Lobo NA, et al:
Association of reactive oxygen species levels and radioresistance
in cancer stem cells. Nature. 458:780–783. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Fridovich I: The biology of oxygen
radicals. Science. 201:875–880. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Yamamoto Y, Sakamoto M, Fujii G, et al:
Overexpression of orphan G-protein-coupled receptor, Gpr49, in
human hepato-cellular carcinomas with beta-catenin mutations.
Hepatology. 37:528–533. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Fan XS, Wu HY, Yu HP, Zhou Q, Zhang YF and
Huang Q: Expression of Lgr5 in human colorectal carcinogenesis and
its potential correlation with beta-catenin. Int J Colorectal Dis.
25:583–590. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Shin SY, Kim CG, Jho EH, et al: Hydrogen
peroxide negatively modulates Wnt signaling through downregulation
of beta-catenin. Cancer Lett. 212:225–231. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Funato Y, Michiue T, Asashima M and Miki
H: The thioredoxin-related redox-regulating protein nucleoredoxin
inhibits Wnt-beta-catenin signalling through dishevelled. Nat Cell
Biol. 8:501–508. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Oh JH, Ku JL, Yoon KA, et al:
Establishment and characterization of 12 human colorectal-carcinoma
cell lines. Int J Cancer. 81:902–910. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Park IJ, Hwang JT, Kim YM, Ha J and Park
OJ: Differential modulation of AMPK signaling pathways by low or
high levels of exogenous reactive oxygen species in colon cancer
cells. Ann NY Acad Sci. 1091:102–109. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Boulares AH, Yakovlev AG, Ivanova V,
Stoica BA, Wang G, Iyer S and Smulson M: Role of poly(ADP-ribose)
polymerase (PARP) cleavage in apoptosis. J Biol Chem.
274:22932–22940. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Busserolles J, Megias J, Terencio MC and
Alcaraz MJ: Heme oxygenase-1 inhibits apoptosis in Caco-2 cells via
activation of Akt pathway. Int J Biochem Cell Biol. 38:1510–1517.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Aggeli IK, Gaitanaki C and Beis I:
Involvement of JNKs and p38-MAPK/MSK1 pathways in
H2O2-induced upregulation of heme oxygenase-1
mRNA in H9c2 cells. Cell Signal. 18:1801–1812. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Myatt SS, Brosens JJ and Lam EW: Sense and
sensitivity: FOXO and ROS in cancer development and treatment.
Antioxid Redox Signal. 14:675–687. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Liu SL, Lin X, Shi DY, Cheng J, Wu CQ and
Zhang YD: Reactive oxygen species stimulated human hepatoma cell
proliferation via cross-talk between PI3-K/PKB and JNK signaling
pathways. Arch Biochem Biophys. 406:173–182. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Aguilera C, Nakagawa K, Sancho R,
Chakraborty A, Hendrich B and Behrens A: c-Jun N-terminal
phosphorylation antagonises recruitment of the Mbd3/NuRD repressor
complex. Nature. 469:231–235. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Reya T and Clevers H: Wnt signalling in
stem cells and cancer. Nature. 434:843–850. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Zeilstra J, Joosten SP, Dokter M, Verwiel
E, Spaargaren M and Pals ST: Deletion of the WNT target and cancer
stem cell marker CD44 in Apc(Min/+) mice attenuates intestinal
tumorigenesis. Cancer Res. 68:3655–3661. 2008.PubMed/NCBI
|
|
28.
|
Giorgio M, Trinei M, Migliaccio E and
Pelicci PG: Hydrogen peroxide: a metabolic by-product or a common
mediator of ageing signals? Nat Rev Mol Cell Biol. 8:722–728. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Lee YK, Lee MS, Kim YM and Park OJ:
Effects of cotreatment of 12-O-tetradecanoylphorbol-13-acetate and
H2O2 on apoptotic regulation via
AMP-activated protein kinase-cyclooxygenase-2 signals. Ann NY Acad
Sci. 1171:564–569. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Sancho R, Nateri AS, De Vinuesa AG, et al:
JNK signalling modulates intestinal homeostasis and tumourigenesis
in mice. EMBO J. 28:1843–1854. 2009. View Article : Google Scholar : PubMed/NCBI
|