Introduction
Tumor-induced lymphangiogenesis has primarily been
investigated concentrating on peritumoral and intratumoral
lymphatic vessels at primary sites. Studies in animal tumor models
have shown that lymphatic vessels promote the metastatic spread of
tumors (1–3), and that the induction of
lymphangiogenesis could even be used as a prognostic indicator for
metastatic risk of human malignant melanoma of the skin (4). The vascular endothelial growth
factors (VEGF)-C and -D have been identified as factors
predominantly lymphangiogenic via the VEGF receptor-3 (VEGFR-3)
(5). Studies have shown that this
receptor is expressed on lymphatic endothelial cells of lymphatic
vessels (5) and on lymphatic
sinuses within lymph nodes (6).
Moreover, it has been shown that blocking VEGFR-3 signaling can
decrease tumor lymphangiogenesis and cancer spread (7–9).
Previously, were able to show that lymphangiogenesis
of sentinel lymph nodes might also play a role in cancer
progression. VEGF-A and VEGF-C expressing skin tumors maintained
their lymphangiogenic activity after metastasizing to the sentinel
lymph node and even induced sentinel lymph node (LN)
lymphangiogenesis before the tumor has metastasized (10,11).
LN-lymphangiogenesis was also identified in melanoma models after
subcutaneous implantation prior to metastasis (12).
In clinical oncology, the sentinel lymph nodes play
an important role in diagnosis, staging and management of disease.
Especially in breast cancer and melanoma the involvement of
regional lymph nodes is an excellent prognostic indicator. But
about two thirds of the invasive cancers have no regional lymph
node involvement and of those another third will recur (13,14).
Studies evaluating the prognostic value of tumor-free sentinel
lymph nodes are contradictory (15–17).
Lymph nodes constitute a critical crossroad between
drained proteins, antigen-presenting cells, lymphocytes and even
tumor cells. Also in the absence of tumor metastasis draining lymph
nodes can undergo hyperplasia in number and size (18), because an immune response is also
associated with changes of various parameters, such as fluid
accumulation, migration and proliferation (19,20).
To investigate the earliest changes of the sentinel
lymph nodes we injected genetically modified fluorescent
MDA-MB-435/green fluorescent protein human melanoma cancer cells
transfected to overexpress VEGF-C or control vector in nude mice.
The MDA-MB-435 cell line, which has been reclassified from breast
to melanoma, has been derived from the M14 melanoma cell line
(21,22). In tumor-free sentinel lymph nodes
we determined the lymphangiogenesis, angiogenesis and lymph node
size of sentinel lymph nodes. VEGF-C overexpression significantly
induced lymphatic sinus hyperplasia in sentinel lymph nodes even
before the tumor metastasis. At early time-points, the expansion of
the lymphatic network was observed even though no difference of
blood vessel area and lymph node size was detected. These results
suggest that primary tumors, via secretion of lymphangiogenic
factors, can induce hyperplasia of the sentinel lymph node
lymphatic network and in case of tumor free non-enlarged lymph
nodes might provide a new prognostic indicator.
Materials and methods
Cell lines
As tumor cells we used a previously published cell
line (1). The MDA-MB-435 cell
line, which has been reclassified from breast to melanoma, was
derived from the M14 melanoma cell line (21, 22). The two MDA-MB-435 cell lines
(American Type Culture Collection, Rockville, MD, USA) were grown
in DMEM with 10% fetal bovine serum (FBS) and transfected with the
expression construct (pcDNA3.1/EGFP) using the Superfect reagent
(Qiagen, Chatsworth, CA, USA). Clone 6 had the highest tumor take
and produced lymph node and lung metastasis. Clone 6 was
transfected with the human VEGF-C cDNA (23) into a pcDNA3.1/ZEO vector. The
transfected cell lines were maintained in media containing 600
μg/ml zeocin and 400 μg/ml geneticin. All animal
studies were approved by the Massachusetts General Hospital
Subcommittee on Research Animal Care.
Metastasis assay
Cells were injected bilaterally into the second
mammary fat pads of athymic, female, eight-week-old NCR nu/nu mice
(2×106 cells/100 μl serum-free culture medium).
Two mice of each group (VEGF-C transfected MDA-MB-435 and control
vector transfected MDA-MB-435) were sacrificed every two weeks
until week ten. The two sentinel lymph nodes and the superficial
inguinal lymph nodes were removed from each mouse and paraffin
embedded (24). Tumor volume was
determined as previously published (25). The smallest and largest tumor
diameter were measured every other week, using a digital caliper,
and tumor volumes were calculated using the following formula:
volume = 4/3 × (1/2 × smaller diameter)2 × 1/2 × larger diameter.
Tumor data were statistically analyzed by the two-sided unpaired
t-test.
Immunostainings and immunofluorescence
analysis
Sections were stained using antibodies to mouse
LYVE-1 (kindly provided by Dr D. Jackson, Oxford, UK; Upstate
Biotechnology, Lake Placid, NY, USA), CD31 (BD Biosciences
Pharmingen, San Diego, CA, USA), Prox-1 (Covance, Berkeley, CA,
USA), F4/80 (Serotech, Raleigh, NC, USA) and corresponding
secondary antibodies labeled with Alexa Fluor 488 or 594 (Molecular
Probes, Eugene, OR, USA). Cell nuclei were counterstained with
Hoechst-bisbenzimide (Sigma-Aldrich). Specimens were examined by
using a Nikon E-600 microscope (Nikon, Melville, NY, USA) and
images captured with a SPOT digital camera (Diagnostic Instruments,
Sterling Heights, MI, USA). At autopsy, all axillary and inguinal
lymph nodes were examined for the presence of metastases. In
addition, we determined the presence of metastases by fluorescence
microscopic analysis and H&E staining for each lymph node.
Computer-assisted lymph node and
lymphatic/medullary sinuses analysis
Representative H&E stained slides of the
inguinal and axillary lymph nodes, obtained from the two groups
(n=10 for each group) and 4 controls, were analyzed for the highest
diameter, sections were examined by a Nikon E-600 microscope
(Nikon) and images captured with a SPOT digital camera (Diagnostic
Instruments). Further sections were stained with lymphatic and
blood vessel markers (LYVE-1/CD31) to examine the lymphatic and
blood vessel network. Images were captured with a Spot digital
camera (Diagnostic Instruments). Computer-assisted morphometric
analyses of the lymph node size, lymphatic network (lymphatic plus
medullary sinuses) and blood vessel area were performed using the
IP-LAB software (Scanalytics, Billerica, MA, USA). Statistical
analyses were performed using the two-sided, unpaired Student’s
t-test.
Results
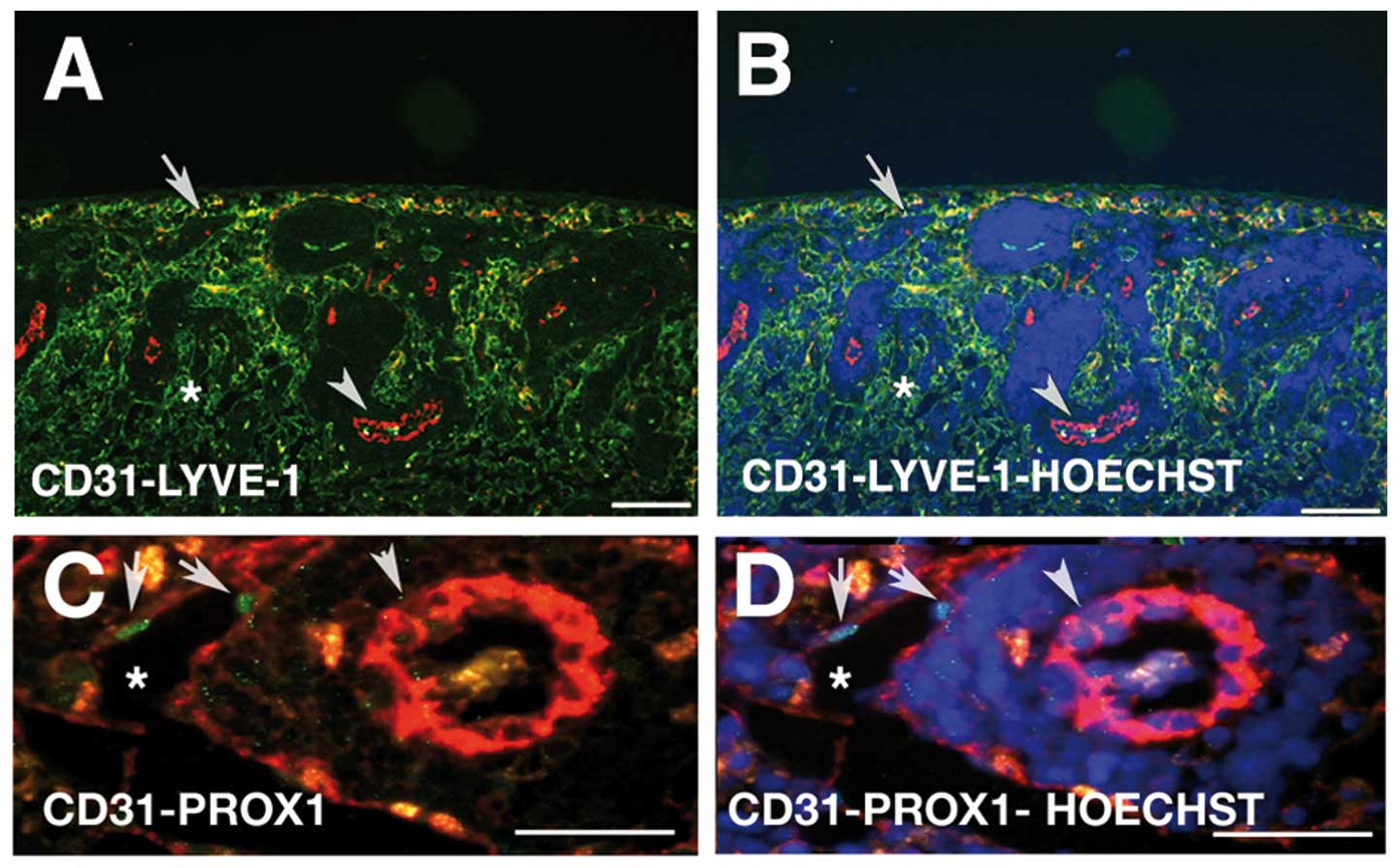
Lymphatic endothelial markers of the
lymph node sinus endothelium
For distinguishing lymphatic endothelium from blood
vessel endothelium in lymph nodes for computer-assisted evaluation
we identified the lymphatic endothelial cell expression profile. We
investigated the expression of known lymphatic markers, using
antibodies against Lyve-1, Prox-1 and PECAM-1 (Fig. 1) (reviewed in ref. 26). The lymph node consists of a
lymphatic labyrinth filled with lymphocytes (27). Within the lymph node the afferent
lymphatic vessel enters the capsule and empties into the
subcapsular sinus, which is connected to the trabecular and
medullary sinuses (Fig. 1)
(28,29). It could be shown that those sinuses
are lined with a continued endothelium with long and elaborate
intercellular junctions supported by reticular cells (30). Immunofluorescence staining revealed
that the lining endothelium of the subcaspsular, lymphatic and
medullary sinuses express a lymphatic endothelial profile. Lining
endothelium is positive for Lyve-1 (Fig. 1A and B) and Prox-1 (Fig. 1C and D, arrow). Especially, the
expression of the most reliable marker for lymphatic endothelial
cells, Prox-1, revealed the lymphatic phenotype of the lining
endothelium.
Lymphatic sinuses of the cortical and paracortical
zone are indicated by the asterisk (Fig. 1A and B). Interestingly, the
lymphatic sinuses and medullary sinuses were in close proximity to
the high endothelial venules (Fig. 1A
and B, arrowhead), but we detected no direct communication.
VEGF-C does not significantly increase
the size of the sentinel lymph nodes
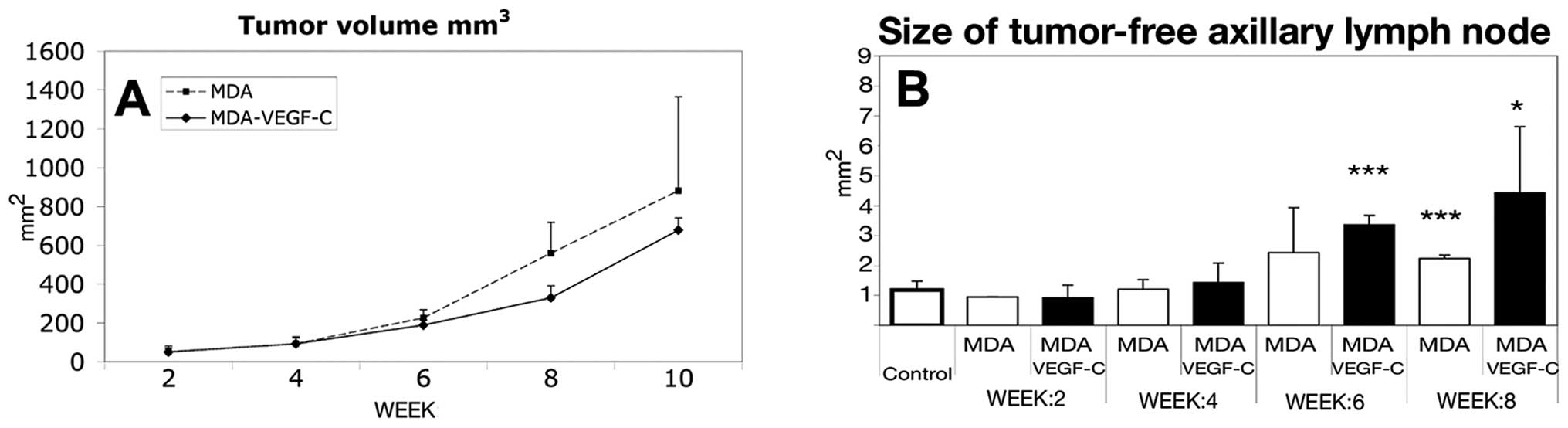
To investigate the effect of VEGF-C on tumor growth
and sentinel lymph node size we used the previously published human
cell line MDA-MB-435 transfected with human VEGF-C (1). We examined the growth of the tumor
volume (Fig. 2A) and found that
the average tumor growth rate revealed no significant difference
between the VEGF-C transfected and the control cell line (Fig. 2A). To determine if overexpression
of VEGF-C leads to differences in sentinel lymph node size, we
evaluated the tumor-free sentinel axillary lymph nodes every two
weeks (Fig. 2B). The axillary
lymph nodes were enlarged at the beginning of week 6, but until
week 10 the size of the lymph nodes was not indicative of the
presence of metastases. To obtain accurate quantitative analysis of
metastases and lymph node involvement, we used cancer cells
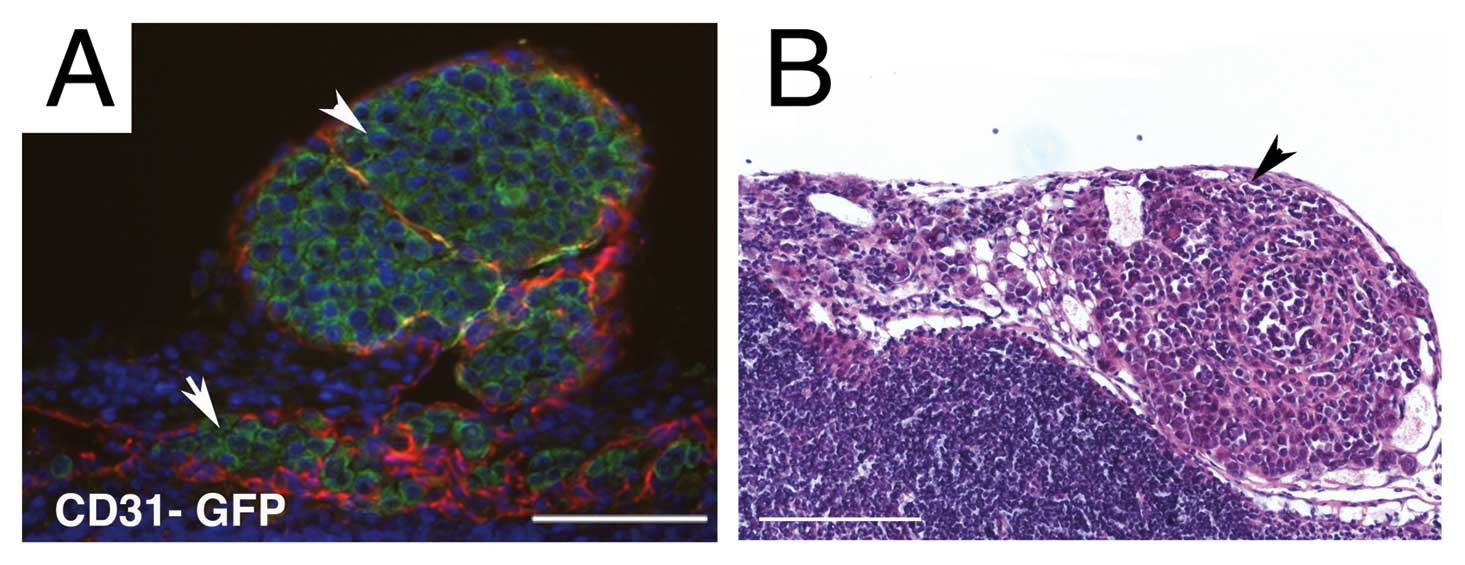
genetically labeled with GFP, a sensitive method for the direct
visualization of micro-metastases. Immunofluorescence for example
revealed GFP expressing tumor cells entering the subcapsular sinus
(Fig. 3A, arrow) from the tumor
containing afferent lymphatic (Fig.
3A, arrowhead). To verify that tumor cells while in vivo
did not lose their GFP vector every section was in addition
evaluated by an H&E stain (Fig.
3B). Metastasis analysis, in agreement with our previously
published data, revealed that the incidence of metastases was
increased in VEGF-C- overexpressing tumors, as compared with the
control tumors. The earliest metastasis in the VEGF-C
overexpressing MDA cell line occurred at week 4, while in the
control MDA cell line the first lymph node involvement was observed
at week 8 (data not shown).
Lymph node lymphangiogenesis in sentinel
lymph nodes
To investigate the effect of VEGF-C on the draining
sentinel lymph node we determined differences of the lymphatic
vessel area between the VEGF-C transfected and the control cell
line. We evaluated the effect only on lymph nodes without presence
of metastatic cells until week 4, an observation point with no
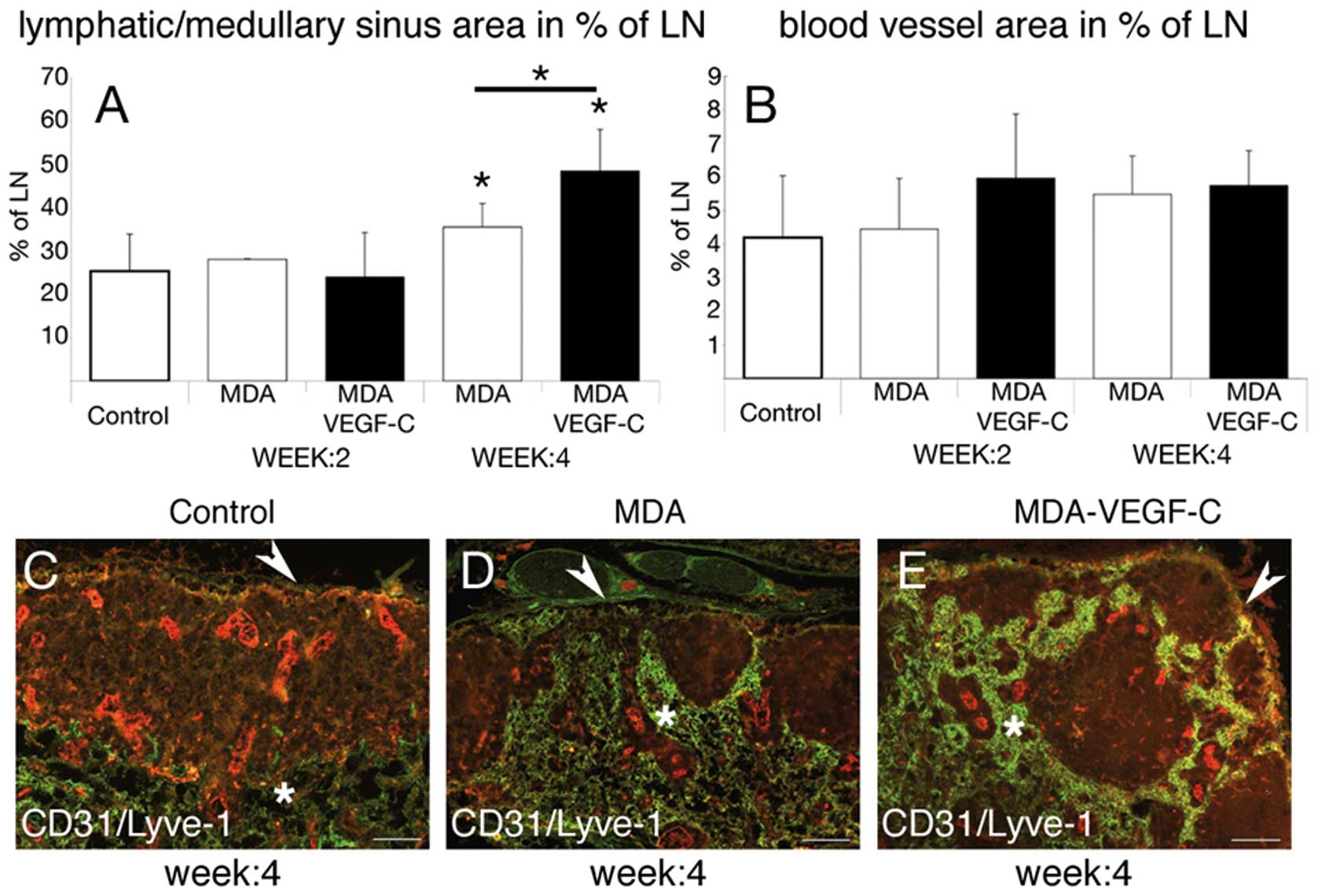
visible and statistical difference in general tumor (Fig. 2A) and lymph node size (Fig. 2B). We found that the lymphatic
vessel area in percent of the tumor-free lymph node was
significantly increased in mice carrying VEGF-C-overexpressing
tumors versus control tumors (Fig.
4A, *p<0.05). We further observed no evidence of
an increased blood vessel area in percent of the lymph node in mice
carrying VEGF-C overexpressing tumors versus control tumors
(Fig. 4B).
Double-immunofluorescence staining revealed histological changes of
the sentinel lymph nodes in mice carrying VEGF-C overexpressing
tumors versus control tumors (Fig.
4C–E). In lymph nodes draining VEGF-C transfected tumors the
profile of the lymphatic network had a globular appearance with an
empty germinal center (Fig. 4C–E).
These cup-shaped structures of the lymphatic sinuses (Fig. 4D and E) drained to the medullary
sinuses just beneath the deep cortex (Fig. 4C–E). The number of follicles were
not statistical different between the two groups (data not shown).
In addition, we investigated if tumor associated macrophages
revealed any difference in their recruitment, because VEGF-C might
also have a direct impact on immune functions (8). VEGFR-3 was detected on macrophages
in vitro and in vivo, and VEGF-C might induce an
increased macrophage chemotaxis. To study this we stained the
tumors for F4/80, an antigen that is expressed by a majority of
mature macrophages. Immunofluorescence analyses revealed no
difference in the infiltration of tumor-associated macrophages
between the two groups (data not shown).
Discussion
Lymph nodes are the primary site of immune response
and are a critical crossroad, since tumor cells, inflammatory and
stroma cells could migrate towards and into them. Although
morphological changes of lymph nodes involved and uninvolved in
metastasis have been studied in various types of cancers, the
prognostic significance of immune response and lymph node size of
tumor-free sentinel lymph nodes of breast carcinoma patients is
unclear (15–17,31–37).
This prompted us to investigate the early morphological changes of
lymph vessels in sentinel lymph nodes in the premetastatic
situation. In our model using VEGF-C transfected tumor cells versus
control cells of the same cell line we observed that the lymph node
size and tumor-associated immune response is not predictive for an
subsequent metastasis. Both tumors, high- and low metastatic,
induced equal lymph node size enlargement. Interestingly, our
results suggest that LN-lymphangiogenesis in sentinel lymph nodes
still uninvolved in metastasis could be a new early predictor of
malignancy. LN-lymphangiogenesis induced by VEGF-C was revealed to
be a very early morphological change in sentinel lymph nodes before
overt metastasis. Previously, the function and role of VEGF-C was
primarily investigated with regard to peritumoral and intratumoral
tumor-lymphangiogenesis controlled by VEGFR-3 (5). VEGF-C, as the first lymphangiogenic
factor, is proven to be expressed in various cancer cell types
(reviewed in ref. 38) and it has
been proven to play an active role in the interaction between
tumors and lymphatics (1,3). It has been shown that the induction
of lymphangiogenesis is a prognostic indicator of the metastatic
risk of malignant melanoma of the skin (4), but only a few studies have
investigated the effect of tumor derived lymphangiogenic factors on
sentinel lymph nodes (6,10–12).
We found in a carcinogenesis model that transgenic overexpression
of VEGF-A and VEGF-C in the skin induced sentinel lymph node
lymphangiogenesis (10,11), also when released by chronically
inflamed tissue (39). Our study
reported here demonstrates that cancer derived VEGF-C induces
sentinel lymph node lymphatic hyperplasia without altering the
blood vessel area and lymph node size. Transgenic overexpression of
VEGF-C at early time points resulted in an increased lymphatic
hyperplasia in VEGF-C draining sentinel lymph nodes in comparison
to the control tumors not overexpressing VEGF-C even before the
tumor had metastasized.
Based on this observation it could be hypothesized
that LN-lymphangiogenesis facilitates tumor cell metastasis, an
early event of distant organ metastasis. Hirakawa et al
recently observed this in VEGF-C overexpressing skin tumors
(10), but the role of
LN-lymphangiogenesis and its inhibition in the further
dissemination of cancer remains largely unexplored. In clinical
oncology the size of sentinel lymph nodes has emerged as a
predictor next to the extracapsular growth, size of the primary
tumor and prescence of lymphovascular invasion, as reported by Van
Zee et al(40). It has been
observed that LN-lymphangiogenesis was associated with an increased
frequency of involved non-sentinel lymph nodes in humans (41). The findings suggest that primary
tumors, via secretion of lymphangiogenic factors such as VEGF-C
induce lymphatic sinus hyperplasia of the sentinel lymph node and
thereby might promote their further spread. Recent evidence even
indicated that LN-lymphangiogenesis increased lymph flow actively
about 20- to 30-fold (6). Although
overexpression of VEGF-C induced a more pronounced lymphatic
network of sentinel lymph nodes, also the control MDA cell line
induced LN-lymphangiogenesis, which indicates that there must be
different mechanisms in addition to VEGF-C release. Inflammatory
reactions due to tumor necrosis have already been postulated to
have an effect on lymph node hyperplasia (42). Especially for inflammatory breast
cancers it has been described that they have an angiogenic
phenotype and that factors such VEGF-C, VEGF-D and FGF-2 are
increased in comparison to non-inflammatory breast carcinomas
(43–45). In our xenograft model
lymphangiogenic factors produced by macrophages could be a possible
explanation, due to the fact that tumor associated macrophages have
been identified to produce a broad variety of lymphangiogenic and
angiogenic factors (46). We
determined the difference of tumor-associated macrophages to
exclude that VEGF-C induced a more pronounced secondary response by
inducing an increased macrophage chemotaxis, acting via the
VEGF-receptor 3 expressed by macrophages (8,46).
We found no difference in macrophage infiltration pattern. Studies
in which VEGF-A, VEGF-C, FGF-2 or other growth factors in this
setting are blocked might answer the question of relative
importance of one or more of these factors alone or in concert and
could be important for further cancer treatment approaches.
Importantly, our study reveals that the lymphatic
network of sentinel lymph nodes should be specifically evaluated by
using specific lymphatic endothelial markers. In agreement with the
previously published finding by Hattori (47), we recommend that the evaluation of
lymph node vessels should not be done by a PECAM-1 (CD31) staining.
CD31 is also expressed on the lymphatic sinus network endothelium,
but cannot distinguish between the lymphatic and blood vascular
system.
Our findings provide additional data to the
previously proposed ‘seed and soil hypothesis’ (11), inasmuch as primary tumors might
prepare their future metastatic site by producing lymphangiogenic
factors that support efficient transport to sentinel lymph nodes,
distant lymph nodes and organ sites.
Early changes of the lymphatic sinuses and
lymphangiogenesis might predict an unfavorable outcome of an
individual carcinoma patient. The lymphatic sinus network of
sentinel lymph nodes could even be an important target for future
therapies.
Acknowledgements
We thank M. Constant, L. Janes and L.
Nguyen for expert technical assistance. This work was supported by
NIH grants CA69184, CA86410, CA92644 (MD), American Cancer Society
Research Project Grant 99-23901 (MD), Swiss National Fund grant
3100A0-108207 (MD), Fonds für wissenschaftliche Förderung grant
S9408-B11 (MD), and by the Deutsche Krebshilfe (RL).
References
|
1.
|
M SkobeT HawighorstDG JacksonInduction of
tumor lymphangiogenesis by VEGF-C promotes breast cancer
metastasisNat Med7192198200110.1038/8464311175850
|
|
2.
|
SA StackerC CaesarME BaldwinVEGF-D
promotes the metastatic spread of tumor cells via the lymphaticsNat
Med7186191200110.1038/8463511175849
|
|
3.
|
SJ MandriotaL JussilaM JeltschVascular
endothelial growth factor-C-mediated lymphangiogenesis promotes
tumour metastasisEMBO
J20672682200110.1093/emboj/20.4.67211179212
|
|
4.
|
SS DadrasT PaulJ BertonciniTumor
lymphangiogenesis: a novel prognostic indicator for cutaneous
melanoma metastasis and survivalAm J
Pathol16219511960200310.1016/S0002-9440(10)64328-312759251
|
|
5.
|
L JussilaK AlitaloVascular growth factors
and lymphangiogenesisPhysiol Rev82673700200212087132
|
|
6.
|
A RuddellP MezquitaKA BrandvoldA FarrBM
IritaniB lymphocyte-specific c-Myc expression stimulates early and
functional expansion of the vasculature and lymphatics during
lymphomagenesisAm J
Pathol16322332245200310.1016/S0002-9440(10)63581-X14633598
|
|
7.
|
MM MattilaJK RuoholaT KarpanenDG JacksonK
AlitaloPL HarkonenVEGF-C induced lymphangiogenesis is associated
with lymph node metastasis in orthotopic MCF-7 tumorsInt J
Cancer98946951200210.1002/ijc.1028311948478
|
|
8.
|
M SkobeLM HambergT HawighorstConcurrent
induction of lymphangiogenesis, angiogenesis, and macrophage
recruitment by vascular endothelial growth factor-C in melanomaAm J
Pathol159893903200110.1016/S0002-9440(10)61765-811549582
|
|
9.
|
T KarpanenM EgebladMJ KarkkainenVascular
endothelial growth factor C promotes tumor lymphangiogenesis and
intralymphatic tumor growthCancer Res6117861790200111280723
|
|
10.
|
S HirakawaLF BrownS KodamaK PaavonenK
AlitaloM DetmarVEGF-C-induced lymphangiogenesis in sentinel lymph
nodes promotes tumor metastasis to distant
sitesBlood10910101017200710.1182/blood-2006-05-02175817032920
|
|
11.
|
S HirakawaS KodamaR KunstfeldK KajiyaLF
BrownM DetmarVEGF-A induces tumor and sentinel lymph node
lymphangiogenesis and promotes lymphatic metastasisJ Exp
Med20110891099200510.1084/jem.2004189615809353
|
|
12.
|
MI HarrellBM IritaniA RuddellTumor-induced
sentinel lymph node lymphangiogenesis and increased lymph flow
precede melanoma metastasisAm J
Pathol170774786200710.2353/ajpath.2007.06076117255343
|
|
13.
|
WL McGuireGM ClarkPrognostic factors and
treatment decisions in axillary-node-negative breast cancerN Engl J
Med32617561761199210.1056/NEJM1992062532626071594018
|
|
14.
|
TD ShusterL GirshovichTM WhitneyKS
HughesMulti-disciplinary care for patients with breast cancerSurg
Clin North Am80505533200010.1016/S0039-6109(05)70199-710836005
|
|
15.
|
JK SalamaR HeimannF LinDoes the number of
lymph nodes examined in patients with lymph node-negative breast
carcinoma have prognostic
significance?Cancer103664671200510.1002/cncr.2083015641038
|
|
16.
|
PG MoormanA HamzaJR MarksJA
OlsonPrognostic significance of the number of lymph nodes examined
in patients with lymph node-negative breast
carcinomaCancer9122582262200110.1002/1097-0142(20010615)91:12%3C2258::AID-CNCR1256%3E3.0.CO;2-V
|
|
17.
|
RL CampEB RimmDL RimmA high number of
tumor free axillary lymph nodes from patients with lymph node
negative breast carcinoma is associated with poor
outcomeCancer88108113200010.1002/(SICI)1097-0142(20000101)88:1%3C108::AID-CNCR15%3E3.0.CO;2-B10618612
|
|
18.
|
S OkadaRM AlbrechtS AharinejadDE
SchraufnagelStructural aspects of the lymphocyte traffic in rat
submandibular lymph nodeMicrosc
Microanal8116133200210.1017/S143192760102004912533241
|
|
19.
|
JG HallB MorrisThe origin of the cells in
the efferent lymph from a single lymph nodeJ Exp
Med121901910196510.1084/jem.121.6.90114319406
|
|
20.
|
RN CahillH FrostZ TrnkaThe effects of
antigen on the migration of recirculating lymphocytes through
single lymph nodesJ Exp
Med143870888197610.1084/jem.143.4.8701255114
|
|
21.
|
M LacroixMDA-MB-435 cells are from
melanoma, not from breast cancerCancer Chemother
Pharmacol63567200910.1007/s00280-008-0776-918500520
|
|
22.
|
DT RossU ScherfMB EisenSystematic
variation in gene expression patterns in human cancer cell linesNat
Genet24227235200010.1038/7343210700174
|
|
23.
|
V JoukovK PajusolaA KaipainenA novel
vascular endothelial growth factor, VEGF-C, is a ligand for the
Flt4 (VEGFR-3) and KDR (VEGFR-2) receptor tyrosine kinasesEMBO
J1517511996
|
|
24.
|
Y SatoK MukaiS WatanabeM GotoY
ShimosatoThe AMeX method. A simplified technique of tissue
processing and paraffin embedding with improved preservation of
antigens for immunostainingAm J Pathol12543143519862432790
|
|
25.
|
M StreitAE StephenT HawighorstSystemic
inhibition of tumor growth and angiogenesis by thrombospondin-2
using cell-based antiangiogenic gene therapyCancer
Res6220042012200211929817
|
|
26.
|
YK HongJW ShinM DetmarDevelopment of the
lymphatic vascular system: a mystery unravelsDev
Dyn231462473200410.1002/dvdy.2017915376314
|
|
27.
|
Y HeScanning electron microscope studies
of the rat mesenteric lymph node with special reference to
high-endothelial venules and hitherto unknown lymphatic
labyrinthArch Histol Jpn48115198510.1679/aohc.48.1
|
|
28.
|
O OhtaniY OhtaniCJ CaratiBJ GannonFluid
and cellular pathways of rat lymph nodes in relation to lymphatic
labyrinths and Aquaporin-1 expressionArch Histol
Cytol66261272200310.1679/aohc.66.26114527167
|
|
29.
|
GT BelzTJ HeathLymph pathways of the
medial retropharyngeal lymph node in dogsJ
Anat18651752619957559125
|
|
30.
|
L NicanderP NafstadT LandsverkRH
EngebretsenA study of modified lymphatics in the deep cortex of
ruminant lymph nodesJ Anat17820321219911810927
|
|
31.
|
MM BlackRE ZachrauAntitumor immunity in
breast cancer patients. Biologic and therapeutic implicationsJ
Reprod Med2321321979226697
|
|
32.
|
V TsakraklidesOT AnastassiadesJH
KerseyPrognostic significance of regional lymph node histology in
uterine cervical
cancerCancer31860868197310.1002/1097-0142(197304)31:4%3C860::AID-CNCR2820310415%3E3.0.CO;2-L4706051
|
|
33.
|
V TsakraklidesP OlsonJH KerseyRA
GoodPrognostic significance of the regional lymph node histology in
cancer of the
breastCancer3412591267197410.1002/1097-0142(197410)34:4%3C1259::AID-CNCR2820340436%3E3.0.CO;2-Y4422558
|
|
34.
|
M OkaS YoshinoS HazamaK ShimodaM SuzukiT
SuzukiPrognostic significance of regional lymph node reaction after
curative resection of advanced gastric cancerBr J
Surg7910911094199210.1002/bjs.18007910341422730
|
|
35.
|
MM BlackC FreemanT MorkS HarveiSJ
CutlerPrognostic significance of microscopic structure of gastric
carcinomas and their regional lymph
nodesCancer27703711197110.1002/1097-0142(197103)27:3%3C703::AID-CNCR2820270329%3E3.0.CO;2-K5549501
|
|
36.
|
SH BennettJW FutrellJA RothRC HoyeAS
KetchamPrognostic significance of histologic host response in
cancer of the larynx or
hypopharynxCancer2812551265197110.1002/1097-0142(1971)28:5%3C1255::AID-CNCR2820280524%3E3.0.CO;2-A5125672
|
|
37.
|
K MalickaAttempt at evaluation of
defensive activity of lymph nodes on the basis of microscopic and
clinical studies in cases of laryngeal cancerPol Med
J101541641971
|
|
38.
|
M CassellaM SkobeLymphatic vessel
activation in cancerAnn NY Acad
Sci979120130200210.1111/j.1749-6632.2002.tb04873.x12543722
|
|
39.
|
C HalinNE ToblerB ViglLF BrownM
DetmarVEGF-A produced by chronically inflamed tissue induces
lymphangiogenesis in draining lymph
nodesBlood11031583167200710.1182/blood-2007-01-06681117625067
|
|
40.
|
KJ Van ZeeDM ManassehJL BevilacquaA
nomogram for predicting the likelihood of additional nodal
metastases in breast cancer patients with a positive sentinel node
biopsyAnn Surg Oncol10114011512003
|
|
41.
|
GG Van den EyndenMK VandenberghePJ van
DamIncreased sentinel lymph node lymphangiogenesis is associated
with nonsentinel axillary lymph node involvement in breast cancer
patients with a positive sentinel nodeClin Cancer
Res1353915397200717875768
|
|
42.
|
UE StuderS ScherzJ ScheideggerEnlargement
of regional lymph nodes in renal cell carcinoma is often not due to
metastasesJ Urol14424324519902374186
|
|
43.
|
I Van der AuweraSJ Van LaereGG Van den
EyndenIncreased angiogenesis and lymphangiogenesis in inflammatory
versus noninflammatory breast cancer by real-time reverse
transcriptase-PCR gene expression quantificationClin Cancer
Res10796579712004
|
|
44.
|
K ShirakawaM ShibuyaY
HeikeTumor-infiltrating endothelial cells and endothelial precursor
cells in inflammatory breast cancerInt J
Cancer99344351200210.1002/ijc.1033611992402
|
|
45.
|
TP PaderaA KadambiE di TomasoLymphatic
metastasis in the absence of functional intratumor
lymphaticsScience29618831886200210.1126/science.107142011976409
|
|
46.
|
SF SchoppmannP BirnerJ
StocklTumor-associated macrophages express lymphatic endothelial
growth factors and are related to peritumoral lymphangiogenesisAm J
Pathol161947956200210.1016/S0002-9440(10)64255-1
|
|
47.
|
H HattoriCaution should be taken in using
CD31 for distinguishing the vasculature of lymph nodesJ Clin
Pathol56638639200310.1136/jcp.56.8.638-a12890824
|