Introduction
Neuroblastoma, the most common extracranial
pediatric malignancy, is characterized by a broad spectrum of
clinical behaviors with a low survival rate (1,2). The
clinical prognosis reveals its aggressive behavior, including wide
dissemination, resistance to chemotheraphy, and ability to
metastasize to the bone (3). Since
the tumor is associated with a high fatality rate, various
investigators are working to improve the survival rate, reduce
recurrence, and metastasis of neuroblastoma. Like any other
malignant tumor, the hallmarks of high-risk neuroblastoma are
unregulated cellular proliferation and declined apoptosis.
Apoptosis is the intrinsically programmed cell death that is
necessary to maintain normal homeostasis of the system. Its
diminution violates many of the physiological checkpoints inside
the cell and elicits abnormal behavior in the cellular
microenvironment. The potentially detrimental effects observed with
the absence/reduction of apoptosis can be considered as a function
of the activation of anti-apoptotic signaling cascades (4). Given the important role of apoptosis
regulators in neuroblastoma, a better understanding of the
underlying mechanisms involved in the induction of apoptosis would
be a very important aspect in designing anticancer drugs and
developing efficient therapeutic strategies.
Secreted protein acidic and rich in cysteine (SPARC)
is an evolutionary matricellular glycoprotein composed of three
structural domains with distinct modular functions and plays
critical roles in modulating cell-matrix interactions, cellular
functions and tissue mineralization (5). SPARC plays a significant role in
tissue remodeling, embryogenesis, cellular differentiation and
angiogenesis. As a matricellular protein, SPARC takes part in
multiple biological activities including development and regulation
of matrix remodeling. As such, molecular mechanisms associated with
variation in SPARC levels in the cell would have decisive
consequences in regulating the diverse cellular functions.
The underlying mechanisms involved in SPARC
expression and malignant tumor regression remain elusive; however,
it is known that SPARC expression is highly dependent on various
factors including tumorigenic phenotype, molecular signaling
pathways associated with integrins and growth factors/chemokines
(5). Even though the comprehensive
role of SPARC-mediated tumor regression is not completely
understood, previous studies have clarified the anti-proliferative
and counter adhesive properties of SPARC through specific cytokines
and growth factors (6–8).
The significance of combination therapy arises from
the possibility of achieving a synergistic effect in advanced
treatment modalities. Different combination therapies, like
combination of chemotherapy and radiation therapy, have been
investigated to improve the efficiency (9). Radiation has been recognized as an
efficient mode of therapy for neuroblastoma and provides an
additive pharmacological response when combined with other
treatments (10,11).
Our recent findings showed SPARC overexpression
inhibited cell proliferation, migration and angiogenesis in PNET
cells (12,13). Earlier reports from our group also
proved that SPARC overexpression induced autophagy-mediated
apoptosis in PNET cells (14).
However, the molecular mechanisms associated with SPARC
overexpression leading to autophagy-mediated apoptosis have not
been studied. In the present study, we attempted to elucidate the
efficacy of SPARC overexpression together with radiation therapy as
an efficient way to induce apoptosis in neuroblastoma as well as
investigate associated molecular mechanisms.
This study provides solid evidence describing the
key role of endoplasmic reticulum (ER) stress in invoking the
transcriptional responses as a function of SPARC overexpression. ER
is the sole element responsible for protein synthesis and folding;
an imbalance between the cellular demand and the capacity of ER to
facilitate protein folding eventually activates aberrant cell cycle
regulation, deregulates the intercellular metabolism, and
eventually activates ER stress molecular chaperons. Our study was
also focused on understanding the specific pathways associated with
SPARC overexpression that induce ER stress, which in turn elicit
autophagy-mediated apoptosis in neuroblastoma. The induction of
autophagy as a result of ER stress has been identified by various
investigators (15,16). Autophagy involves sequestration of
autophagosomes that eventually fuse with lysosomes and lead to
cellular degradation; as a result, autophagy has an important role
in eukaryotic cells. Hence, we also investigated the involvement of
autophagy as an event associated with SPARC overexpression and
induction of apoptosis in neuroblastoma.
Materials and methods
Cell culture
SK-N-AS and NB-1691 cells were procured from ATCC
(Manassas, VA) and Dr P. Houghton of St. Jude Children’s Research
Hospital (Memphis, TN), respectively. The cells were maintained in
RPMI-1640 supplemented with 10% FBS (Invitrogen Corp., Carlsbad,
CA), 50 U/ml penicillin and 50 μg/ ml streptomycin (Life
Technologies, Inc., Frederick, MD). Cells were maintained in a 37°C
incubator with a 5% CO2 humidified atmosphere.
Antibodies and reagents
The primary antibodies against SPARC, caspase 3,
LC3, JNK, phospho-JNK, pancreatic ER kinase (PERK), GAPDH (Santa
Cruz Biotechnology, Santa Cruz, CA), PARP, inositol-requiring
enzyme 1α (IRE-1α), BiP, and CHOP (Cell Signaling Technology,
Beverly, MA) were used in this study. HRP- or Alexa Fluor-488/Alexa
Fluor-594-conjugated secondary antibodies, isotype control IgG
(Santa Cruz Biotechnology, Santa Cruz, CA), Vectashield mounting
medium with DAPI (Vector Laboratories, Burlingame, CA), DAB
peroxidase substrate (Sigma, St. Louis, MO), TUNEL (terminal
deoxynucleotidyl transferase-mediated dUTP nick-end-labeling)
detection kit (Roche Molecular Biochemicals, Indianapolis, IN), JNK
Inhibitor II SP600125 (Calbiochem, San Diego, CA), and Salubrinal
(ER stress inhibitor, Fisher) were also used in this study.
Construction of pcDNA3.1-SPARC,
transfection and irradiation of neuroblastoma cells
Human SPARC cDNA was amplified by PCR using
synthetic primers and was cloned into a pcDNA3.1 vector
(Invitrogen, San Diego, CA) in sense orientation as described
previously (17). Neuroblastoma
cells were transfected with plasmid vector containing full-length
cDNA of SPARC (pSPARC) or empty vector (pEV) using FuGene HD
(Roche, Indianapolis, IN) as described earlier (18). After 4–6 h of transfection, the
necessary amount of serum-containing medium was added. After 24 h
of incubation, cells were irradiated with X-ray irradiation at a
dose of 8 Gy using the RS 2000 Biological Irradiator (Rad Source
Technologies, Inc., Boca Raton, FL). Then, the medium replaced, and
cells were incubated for a further 16 h or for the indicated time
period.
Immunocytochemistry
Immunocytochemistry was performed as described
previously (17). Briefly, cells
were cultured on 8-well chamber slides and transfected as above.
Forty-eight hours after transfection, cells were fixed with 4%
freshly prepared paraformaldehyde (w/v) in PBS followed by
permeabilization with 0.1% Triton X-100 (w/v) in PBS and blocked
with 1% BSA (w/v) in PBS for 1 h at 4°C. Cells were incubated
overnight at 4°C with anti-SPARC or anti-CHOP antibody followed by
corresponding Alexa Fluor-488- or Alexa Fluor-594-conjugated
secondary antibody for 1 h at room temperature. Slides were mounted
with Vectashield mounting medium with DAPI (Vector Laboratories)
and analyzed under a microscope (Olympus BX61 Fluoview,
Minneapolis, MN). Isotype control IgG served as a negative
control.
Western blotting
Western blot analysis was performed as reported
earlier (19). Briefly, 48 h after
transfection, cells were collected and lysed in RIPA buffer. Equal
amounts of protein were resolved on SDS-PAGE and transferred onto a
PVDF membrane. The blots were blocked with 5% non-fat dry milk and
probed overnight with primary antibodies followed by HRP-conjugated
secondary antibodies. ECL system was used to detect
chemiluminescent signals. All blots were re-probed with GAPDH
antibody to confirm equal loading.
Reverse transcription polymerase chain
reaction (RT-PCR)
Neuroblastoma cells were transfected with mock, pEV
or pSPARC for 36 h. Total RNA was extracted from these cells and
cDNA synthesized using poly-dT primers as described earlier
(20). PCR was performed using the
following primers: SPARC: 5′-GGAAGAAACTGTGGCAGAGG-3′ (sense), and
5′-ATTGCTGCACACCTTCTCAA-3′ (antisense); GAPDH:
5′-TGAAGGTCGGAGTCAACGGATTTGGT-3′ (sense), and
5′-CATGTGGGCCATGAGGTCCACCAC-3′ (antisense).
Flow cytometry
FACS analysis was used to assess cell cycle phases
(FACSCalibur System, BD Bioscience, San Jose, CA) with laser
excitation at 488 nm and an emission at 639 nm band pass filter to
collect red propidium iodide fluorescence. The percentages of cells
in the various phases of the cell cycle (sub-G0/G1, S, and G2/M)
were assessed using Cell Quest software (BD Bioscience, San Jose,
CA).
Terminal deoxy nucleotidyl
transferase-mediated nick labeling (TUNEL) assay and
immunohistochemistry
Apoptosis in neuroblastoma cells after SPARC
transfection alone and in combination was detected using TUNEL
enzyme reagent according to the manufacturer’s instructions and as
described previously (17).
Briefly, 2×103 cells were cultured in 4-well chamber
slides, transfected with pSPARC, irradiated after 24 h of
transfection, fixed in 4% paraformaldehyde in PBS for 1 h at room
temperature, and permeabilized in 0.1% Triton X-100 in 0.1% sodium
citrate in PBS for 10 min on ice. The samples were incubated in
TUNEL reaction mixture in a humidified atmosphere at 37°C for 1 h
in the dark. Slides were mounted and images were captured with an
Olympus BX 60 research fluorescence microscope attached to a CCD
camera, and cells were counted. The apoptotic index was defined as
follows: apoptotic index (%) = 100 × (apoptotic cells/total
cells).
Intra-adrenal tumor model and
immunohistochemistry
The Institutional Animal Care and Use Committee at
the University of Illinois College of Medicine at Peoria approved
all experimental procedures involving the use of animals.
Orthotopic, localized neuroblastoma tumors were established in
C.B-17 SCID mice by injection of 1×106 NB-1691 cells in
100 μl PBS into the retroperitoneal space as described
earlier (21). After 2 weeks of
tumor cell implantation, the mice were separated into six groups
containing 6 animals per group, and each group was injected
intravenously with PBS (mock), pEV or pSPARC (100 μl volume)
and were given three doses on alternate days. Between the first and
the second injections, and the second and the third injections, one
group was irradiated with a dose of 5 Gy each time. Mice were
euthanized when they lost >20% of body weight or had trouble
ambulating, feeding, or grooming. The tumors were removed and
either fixed in 10% phosphate-buffered formaldehyde or snap frozen
and maintained at −70°C until sectioning. Briefly, all tumors were
serially sectioned and tissue sections (5-μm thick) obtained
from the paraffin blocks were stained with hematoxylin and eosin
(H&E) using standard histologic techniques. For
immunohistochemical analysis, sections were incubated with primary
antibody for 2 h at room temperature followed by the HRP-conjugated
secondary antibody. DAB solution was used as the chromogen. Isotype
control IgG was used as a negative control. The nucleus was
counterstained with hematoxylin and sections were mounted and
analyzed.
Statistical analysis
All data are presented as means ± standard error
(SE) of at least three independent experiments, each performed at
least in triplicate. One way analysis of variance (ANOVA) combined
with the Tukey post hoc test of means was used for multiple
comparisons in cell culture experiments. Statistical differences
are presented at probability levels of p<0.05, p<0.01 and
p<0.001.
Results
SPARC overexpression followed by
radiation therapy enhanced apoptosis in neuroblastoma cells
It has been demonstrated that SPARC overexpression
induces apoptosis in PNET cells (14). In this study, initially, the role
of SPARC overexpression to induce apoptosis by itself and in
combination with radiation was investigated in neuroblastoma cells.
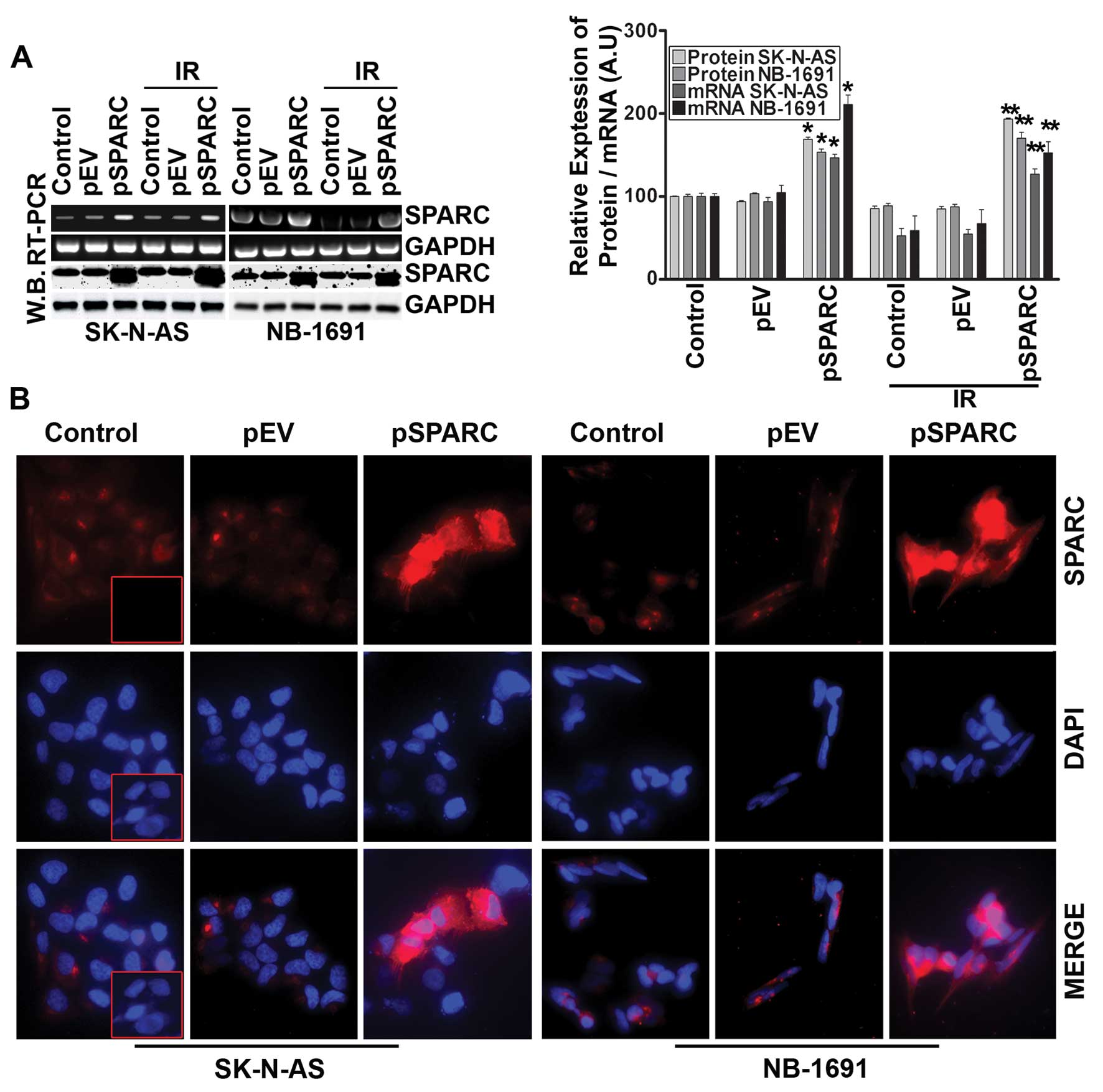
The expression of SPARC significantly increased at both protein and
mRNA levels in cells transfected with pSPARC (Fig. 1A). The expression was increased by
>75% in both cell lines with and without radiation combination
when compared to the respective control or empty vector-treated
counterparts (Fig. 1A). SPARC
expression levels after transfection were also checked by
immunofluorescence analysis, which also demonstrated an apparent
increase in cellular expression of SPARC in pSPARC-transfected
cells (Fig. 1B). Further, flow
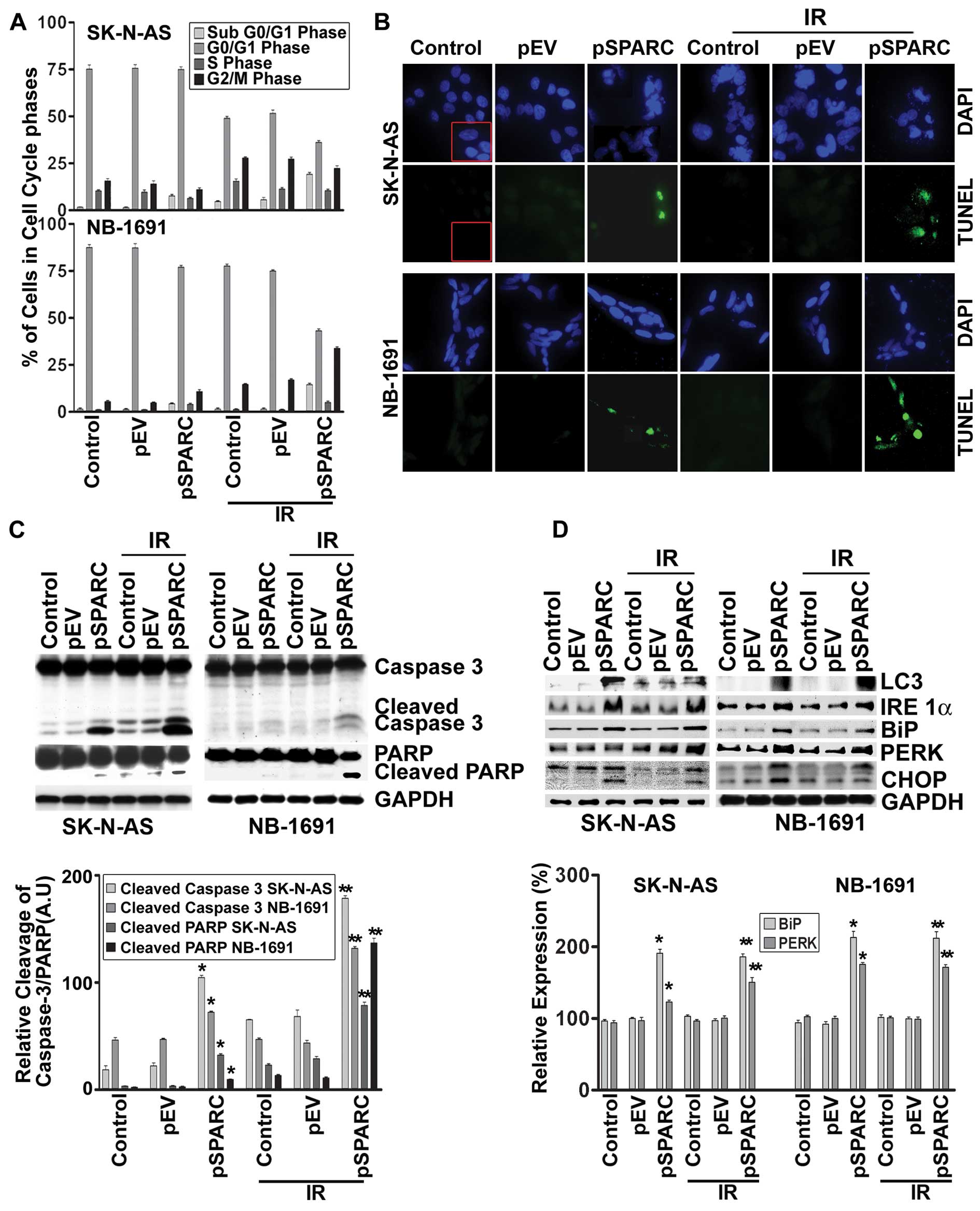
cytometric analysis showed that SPARC transfection alone or in
combination with radiation (IR) dosage of 8 Gy resulted in a
significant increase of the sub-G0/G1 population of cells, which
indicates the induction of apoptosis in the SK-N-AS and NB-1691
neuroblastoma cells (Fig. 2A).
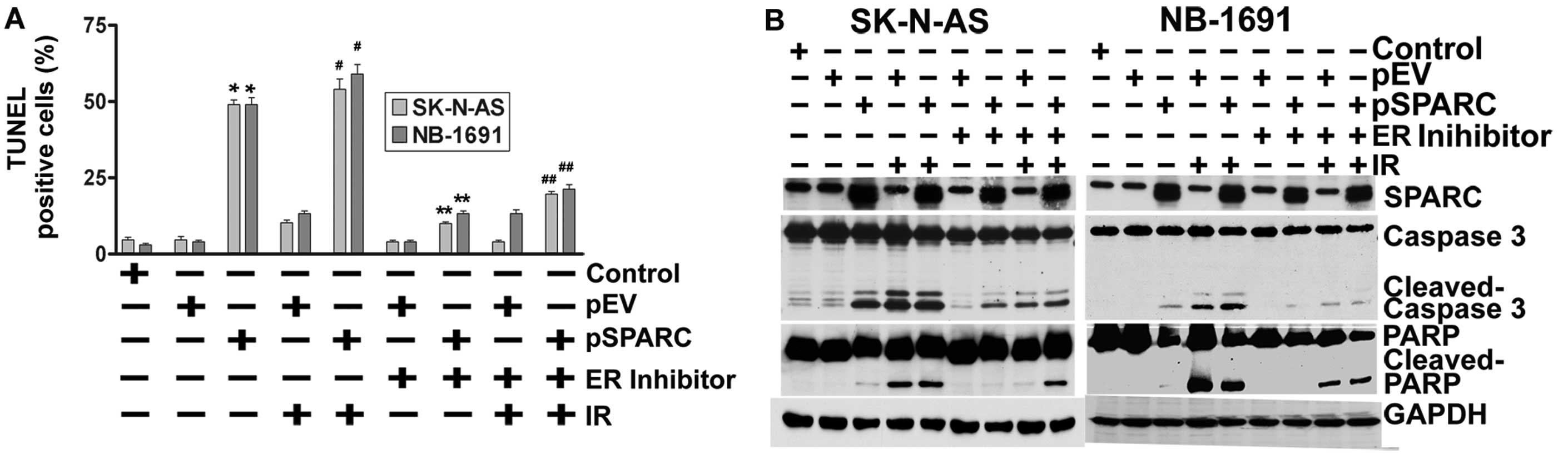
SPARC and IR-induced apoptosis was further confirmed by TUNEL assay
(Fig. 2B) and cleavage of caspase
3 and PARP (Fig. 2C). These
results demonstrate that SPARC overexpression increased the
sensitivity of neuroblastoma cells to radiation.
 | Figure 2.SPARC overexpression sensitizes cells
to radiation in neuroblastoma cells. SK-N-AS and NB-1691
neuroblastoma cells were seeded in dishes and left overnight. Cells
were transfected with pEV or pSPARC and cultured. After 24 h, cells
were irradiated with 8 Gy and incubated for another 16 h. (A) Cells
were collected and subjected to FACS analysis with propidium iodide
staining for DNA content analysis and are represented in a
graphical manner. Columns: mean of three experiments; bars, ± SD.
*p<0.01 vs. pEV; **p<0.01 vs. IR + pEV.
(B) Neuroblastoma cells were cultured in 8-well chamber slides,
transfected and irradiated as described above. Cells were fixed
with paraformaldehyde, permeabilized and blocked. Cells were
incubated with TUNEL reaction mixture for 1 h. Nuclei were
counterstained with DAPI and slides were mounted and photographed.
Inset, negative control. (C) Western blot analysis was performed
for caspase 3 and PARP from total cell lysates using specific
antibodies. GAPDH served as a loading control. Densitometric
analysis showing levels of cleaved caspase 3 and PARP. Columns,
mean of three experiments; bars, ± SD. *p<0.01 vs.
pEV; **p<0.01 vs. IR+pEV. (D) Total cell lysates were
used for western blot analysis to detect protein levels for LC3,
IRE 1α, BiP, PERK and CHOP using specific antibodies. GAPDH served
as a loading control. Densitometric analysis showing levels of BiP
and PERK. Columns: mean of three experiments; bars, ± SD.
*p<0.01 vs. pEV; **p<0.01 vs. IR +
pEV. |
SPARC overexpression induces
autophagy
Our earlier studies showed that SPARC overexpression
led to autophagy-mediated apoptosis in PNET cells (14). To understand the molecular pathways
associated with SPARC overexpression leading to autophagy,
expression of autophagy marker protein microtubule-associated
protein light chain-3 II (LC3-II), which is formed as a result of
phosphoatidylethanolamine conjugation of LC3-I was chosen (22). Increased expression of LC3 was
observed for SPARC-overexpressed neuroblastoma cell lines, which
confirms autophagy as a part of the molecular events leading to
apoptosis (Fig. 2D). To better
understand the cellular pathways associated with SPARC
overexpression leading to autophagy-mediated apoptosis, the role of
endoplasmic reticulum (ER) stress (22) was investigated. IRE 1, a type 1 ER
transmembrane bifunctional glycoprotein having serine/threonine
kinase and endoribonuclease activities in its cytosolic domain, was
found to be upregulated with SPARC overexpression (Fig. 2D). In addition, other ER molecular
chaperons, BiP and PERK, were also found to be activated with
increased SPARC expression at an early time period of 24 h in
NB-1691 and SK-N-AS cell lines (Fig.
2D). The prolonged stress led to the upregulation of the
proapoptotic transcription factor, CHOP, which was induced as a
result of DNA damage and/or other stress conditions (Fig. 2D). A significant increase of these
molecules in pSPARC treated cells indicated a direct involvement of
autophagy in SPARC induced apoptosis in neuroblastoma.
Endoplasmic reticulum stress activates
the c-Jun N-terminal kinase (JNK) pathway
There is a wealth of knowledge pointing out the
profound role of c-Jun N-terminal kinase (JNK) in apoptosis
(23). In addition, it was shown
that IRE1α can also initiate cell death through the activation of
the JNK pathway (24). Hence, we
sought to determine the effect of SPARC overexpression on
phosphorylation of JNK. The results show that phospho-JNK levels
increased ∼2-fold as a result of SPARC overexpression (Fig. 3A). To understand the involvement of
JNK pathway and ER stress molecules in the apoptosis signaling
cascade, SPARC-transfected neuroblastoma cells were treated with a
JNK inhibitor after radiation. When phosphorylation of JNK was
inhibited by the pharmacological inhibitor, the expression levels
of ER stress molecules BiP and PERK were downregulated; these
results illustrate that ER stress activation was initiated by the
phosphorylation of JNK (Fig. 3B).
It was also found that when activation of JNK was inhibited, the
TUNEL positivity of pSPARC-transfected cells either alone or in
combination with radiation was significantly diminished (Fig. 3C). Further, inhibition of JNK
activity resulted in a marked decrease of cleavage of caspase 3 and
PARP among pSPARC-transfected cells, thereby confirming the active
involvement of JNK in regulating apoptosis in these cells (Fig. 3D).
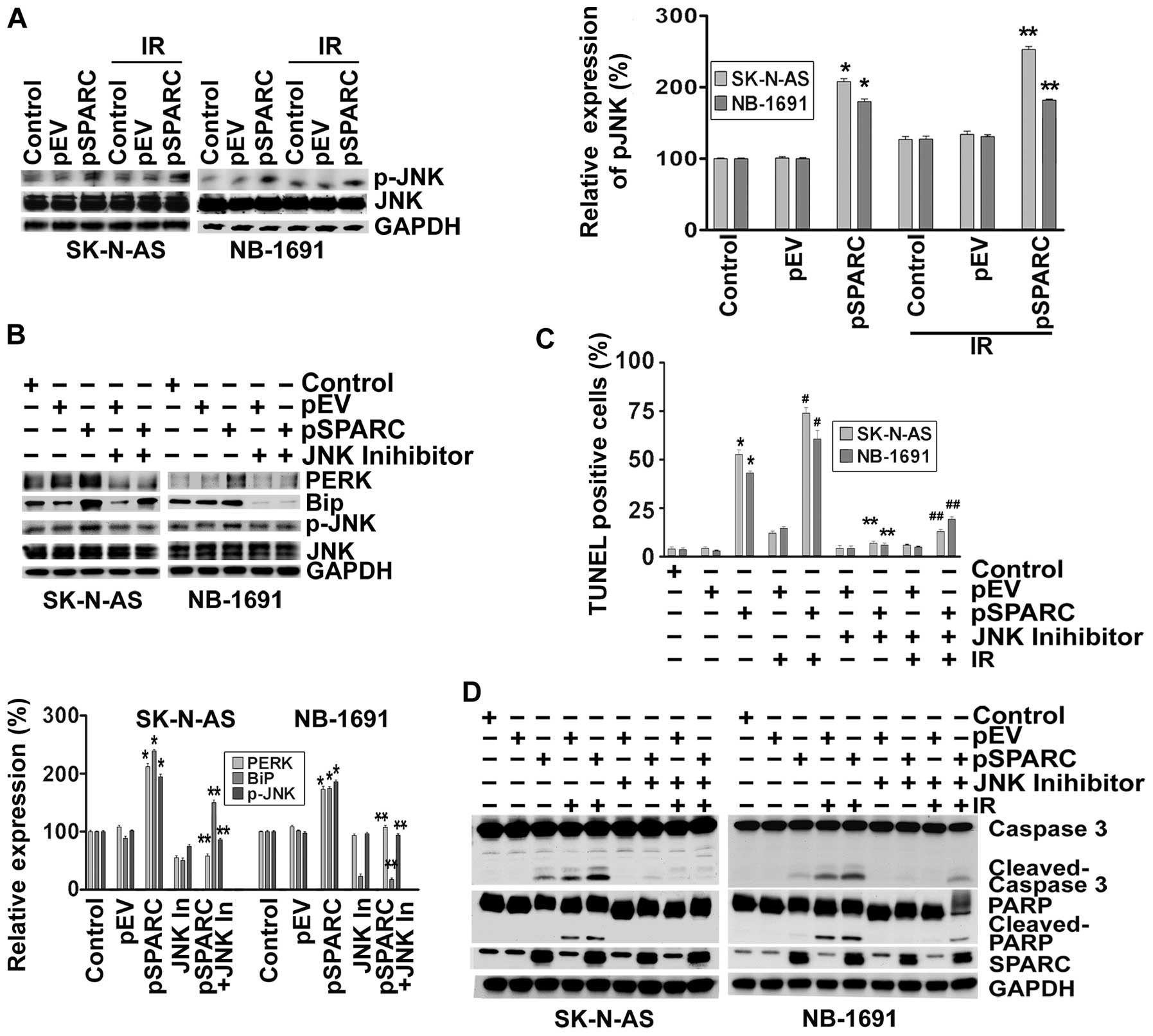 | Figure 3.SPARC overexpression induces
phosphorylation of JNK through endoplasmic reticulum (ER) stress.
(A) SK-N-AS and NB-1691 neuroblastoma cells were seeded in dishes
and left overnight. Cells were transfected with pEV or pSPARC and
cultured. After 24 h, cells were irradiated with 8 Gy and incubated
for another 16 h. Cells were collected and the cell lysates were
subjected to western blotting for phospho-JNK (p-JNK) and JNK.
GAPDH served as a loading control. Results are representative of
three independent experiments. Phospho-JNK band intensities were
quantified by densitometry using ImageJ (NIH) software and shown as
the bar graph. Columns, mean of triplicate experiments; bars, ± SD.
*p<0.01 vs. pEV; **p<0.01 vs. IR + pEV.
(B) Neuroblastoma cells were transfected with pEV or pSPARC and
cultured. After 24 h, cells were treated with JNK inhibitor for 16
h. Cells were collected and the lysates were subjected to western
blotting for PERK, BiP, phospho-JNK (p-JNK) and JNK. GAPDH served
as a loading control. Results are representative of three
independent experiments. Densitometric analysis for PERK, BiP and
p-JNK was performed using ImageJ (NIH) software and shown as the
bar graph. Columns, mean of triplicate experiments; bars, ± SD.
*p<0.01 vs. pEV; **p<0.01 vs. pSPARC.
(C) Neuroblastoma cells were cultured in 8-well chamber slides and
transfected and irradiated as described above. After irradiation,
cells were treated with JNK inhibitor for 16 h. TUNEL assay was
performed as described in Fig. 2B.
The TUNEL-positive cell population was quantified and shown as the
bar graph. Columns, mean of triplicate experiments; bars, ± SD.
*p<0.01 vs. pEV; **p<0.01 vs. pSPARC;
#p<0.01 vs. IR + pEV; ##p<0.01 vs. IR +
pSPARC. (D) Neuroblastoma cells were plated, transfected,
irradiated and treated with JNK inhibitor as described above. Cells
were collected and the cell lysates were subjected to western
blotting for SPARC, caspase 3 and PARP. GAPDH served as a loading
control. Results are representative of three independent
experiments. |
Endoplasmic reticulum stress regulates
apoptosis
Next, the contributory role of ER stress to induce
apoptosis as a result of JNK activation by SPARC overexpression was
tested using an ER stress inhibitor in pSPARC-transfected and
irradiated neuroblastoma cells. As anticipated, inhibition of ER by
a pharmacological inhibitor also significantly reduced the TUNEL
positivity of SK-N-AS and NB-1691 neuroblastoma cells (Fig. 4A). Further, we also noted a sharp
decrease in the activation of caspase 3 and cleavage of PARP among
the pSPARC-transfected and ER inhibitor-treated cells (Fig. 4B).
SPARC overexpression in combination with
irradiation in an orthotopic neuroblastoma model suppresses tumor
growth in vivo
Based on the in vitro results, it could be
proposed that SPARC overexpression invokes ER stress, which in turn
enhances autophagy-mediated apoptosis in neuroblastoma. This
hypothesis was tested by orthotopically implanting NB-1691
neuroblastoma cells in mice and treating with pSPARC alone and in
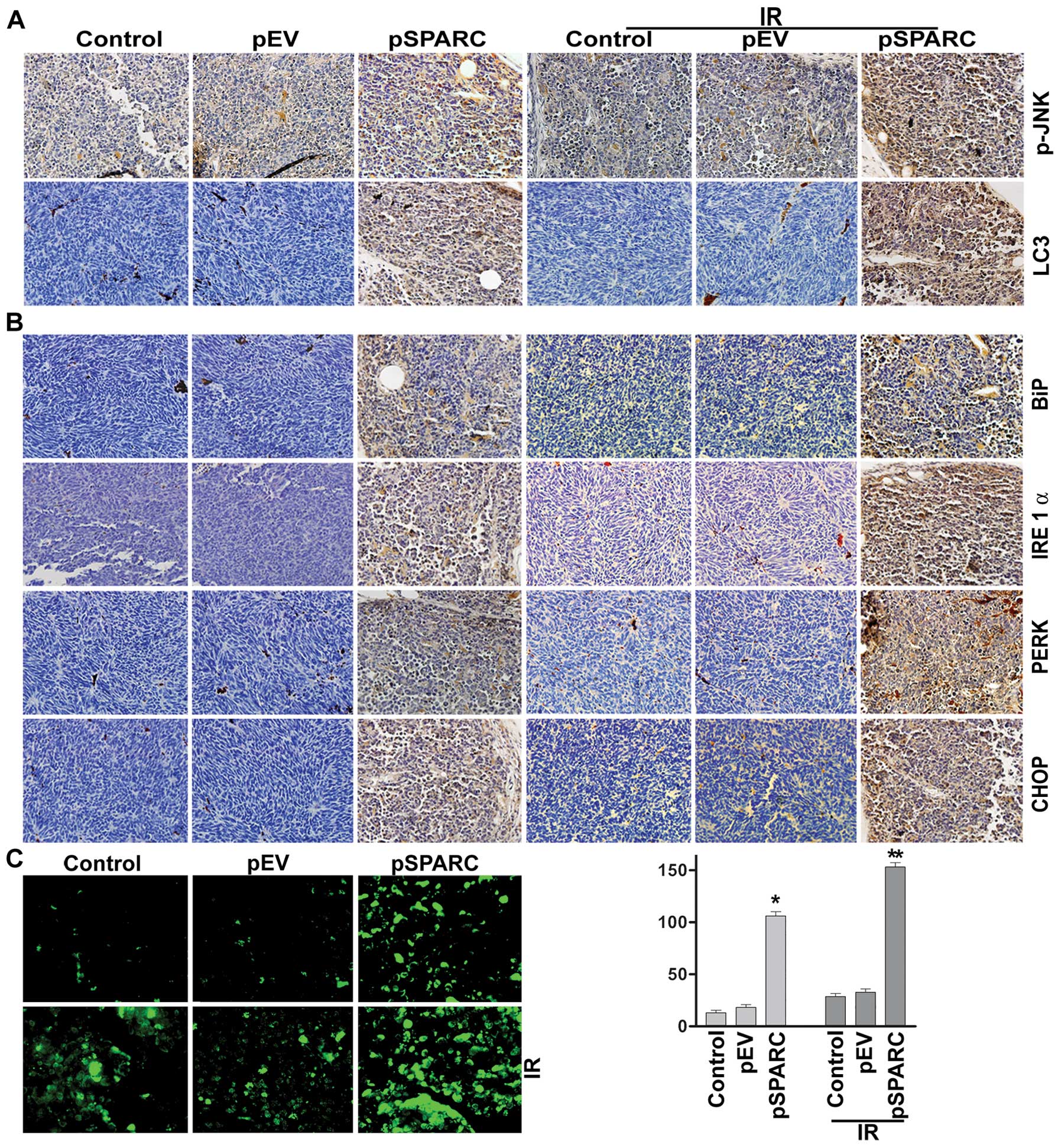
combination with radiation. Increased SPARC expression levels were
observed in pSPARC-treated tumors as compared to mock or
pEV-treated tumors. The expression levels of phospho-JNK and LC3
were found to increase in tumors treated with pSPARC alone and in
combination with radiation (Fig.
5A). Further, the ER stress molecules IRE 1α, BiP, PERK and
CHOP were also expressed in elevated levels in pSPARC-treated
tumors (Fig. 5B). TUNEL analysis
confirmed pSPARC-induced apoptosis in vivo and a remarkable
increase in apoptosis was observed with the combination treatment
of pSPARC and radiation (Fig. 5C).
The in vivo results thus corroborate the in vitro
findings and support the hypothesis that ER stress plays a key role
in regulating the induction of apoptosis in SPARC-overexpressed
neuroblastoma cells. In addition, these results demonstrate the
involvement of LC3 and active JNK as part of the signal pathway
leading to apoptosis in the presence of SPARC overexpression and
irradiation.
Discussion
It is well established that the induction of
apoptosis is critical to prevent the progression of any cancer.
SPARC, through its inherent involvement in directing ECM
deposition, cell-ECM interactions and growth factor signaling,
plays numerous roles in regulating the multiple hallmarks of cancer
including angiogenesis, migration, proliferation and survival
(5). The results of the present
study demonstrate that the combination treatment of SPARC
overexpression and irradiation induces apoptosis in a synergistic
manner in neuroblastoma cells. However, the tumor suppressive
properties of SPARC are highly dependent on various aspects,
especially cell phenotype and the tumor microenvironment (25,26).
Hence, elucidation of the mechanisms underlying SPARC-mediated
tumor suppression and the numerous confounding factors have been
investigated in order to develop therapeutic strategies to confront
cancer growth and metastasis. Activation of caspases is recognized
as a critical event in most of the anticancer signaling pathways
(27). SPARC overexpression alone
or in combination with radiation treatment has been shown to
enhance cleavage of caspase 3 and PARP in neuroblastoma cells.
Further, our study demonstrates that SPARC overexpression directed
ER stress as a function of apoptosis via activation of ER stress
transducers (e.g., PERK, IRE 1α and BiP).
The ER performs diverse functions including protein
folding and also plays a major role in calcium homeostasis
(28). It is well known that
protein folding occurs in the ER prior to transport to various
extracellular surface or intracellular organelles. There is
significant evidence suggesting the essential role of ER in the
regulation of apoptosis as well as autophagy (16,29).
Several types of cellular stress conditions can affect the protein
folding process. Since unfolded or misfolded protein presents a
threat to the cell, the ER lumen triggers the unfolded protein
response (UPR) to circumvent cellular damage. The response to this
ER stress induces activation of inositol-requiring endoplasmic
reticulum-to-nucleus signal kinase (IRE)-1α (30). In addition, RNA-dependent protein
kinase (PKR)-like ER kinase (PERK) is a ubiquitous short-term
perturbation to ER stress that leads to transduction of luminal
signals across the ER membrane to its cytosolic kinase domain
(31). This, in turn, changes the
reserve ER chaperone leading to expression of molecules like BiP.
Given that BiP overexpression is known to suppress the UPR,
enhanced expression of BiP contributes to effective ER stress
response (32). Further, PERK
amplification also leads to the phosphorylation of the α subunit of
the translation factor, eIF2α, that ultimately inhibits protein
synthesis by impeding the assembly of the 80s ribosome (30). This scenario presents pertinent
evidence for the involvement of ER stress in the signaling cascade.
We observed the upregulation of PERK, IRE 1α and BiP in both
SK-N-AS and NB-1691 cells at an earlier time-point after SPARC
transfection. These ER stress transducers in turn induced
activation of the transcription factor CHOP (Fig. 2D).
Likewise, multiple pathways seem to be involved in
ER stress-initiated apoptosis, including the formation of
autophagosomes and activation of JNK as illustrated by the
expression of autophagy markers and active participation of kinases
in regulating apoptosis. The diverse signaling activities
associated with SPARC overexpression also highlight the ER protein
flux. Even though the entire mechanism associated with ER
stress-mediated apoptosis is unclear, the downstream ER stress
signaling could be correlated with activation of CHOP (33). Further, it has been previously
shown that activated IRE recruits the scaffolding protein TRAF2 to
the ER membrane, triggering the mitogen-activated protein (MAP)
kinase cascade and leading to c-Jun N-terminal kinase (JNK)
activation (22).
It is further apparent from the results that when
active JNK was inhibited after SPARC overexpression, the ER stress
transducers were downregulated; this result exemplifies the
involvement of ER molecular chaperones in eliciting c-Jun
N-terminal kinase as part of the signaling cascade. It was found
that when phosphorylation of JNK was inhibited, apoptosis was
inhibited in both cell lines, which confirms the active involvement
of JNK in regulating apoptosis. In addition, when phosphorylation
of JNK was inhibited, the expressions of ER stress molecules PERK,
BiP and IRE were also downregulated, thereby demonstrating that ER
stress activation induces phosphorylation of JNK. Further, the role
of ER stress in directing apoptosis was tested using an ER stress
inhibitor. We found that when ER stress was inhibited, apoptosis
was significantly reduced (Fig.
4), which further confirms the role of ER stress in the
signaling cascade associated with SPARC overexpression leading to
autophagy-mediated apoptosis in neuroblastoma cells.
Autophagy involves sequestration of autophagosomes
that eventually fuse with lysosomes leading to cellular degradation
and has an important role in eukaryotic cells. Autophagy is
regulated by a set of evolutionarily conserved autophagy-related
(Atg) proteins. It was found that microtubule-associated protein
light chain 3 II (LC3-II) is formed as a result of
phosphoatidylethanolamine conjugation of LC3-I (also known as Atg8)
indicate the formation of autophagosomes (34). Increased LC3 levels emphasize the
coupling of UPR to autophagy in neuroblastoma. It has been
previously demonstrated by the authors that SPARC overexpression
leads to autophagy-mediated apoptosis in medulloblastoma (12). Even though the cross-talk between
ER stress-induced autophagy and apoptosis is not completely
understood, recent reports propose that PERK-eIF2α pathway or
IRE-TRAF2-JNK pathway could be the crucial mediator of ER
stress-induced autophagy (15).
This study also provides strong evidence that
integrating radiation as part of the combination treatment
intensified the degree of apoptosis by sensitizing the cells in a
synergistic manner as indicated by flow cytometry analysis, TUNEL
assay, and activation of apoptotic molecules like caspase 3 and
cleavage of PARP. Given the complexity of events initiated as part
of ER stress induction, it could be proposed that there is an
interplay of multiple intracellular pathways during SPARC-induced
autophagy and apoptosis in neuroblastoma cells. The efficacy of the
proposed hypothesis when tested in vivo showed concomitant
results in the expression of ER stress molecular chaperons as a
function of increased SPARC levels. These results confirm that ER
stress has a key role in regulating the induction of apoptosis in
SPARC-overexpressed neuroblastoma cells. The immunohistochemical
analysis of phospho-JNK and LC3 expression levels in response to
increased SPARC levels and the combination treatment further
corroborates the in vitro results. In conclusion the results
impart new insights regarding ER stress-mediated apoptosis in
SPARC-overexpressed cells that should be explored further as a
potential therapeutic option for neuroblastoma.
Acknowledgements
The authors thank Debbie McCollum for
assistance in manuscript preparation, and Diana Meister and Sushma
Jasti for manuscript review. We also thank Dr P. Houghton (St. Jude
Children’s Research Hospital, Memphis, TN) for providing the
NB-1691 neuroblastoma cell line. This study was funded by NCI
CA147792 to Jasti S. Rao from the National Institutes of Health
(NIH).
References
|
1.
|
Maris JM and Matthay KK: Molecular biology
of neuroblastoma. J Clin Oncol. 17:2264–2279. 1999.PubMed/NCBI
|
|
2.
|
Smith MA, Seibel NL, Altekruse SF, et al:
Outcomes for children and adolescents with cancer: challenges for
the twenty-first century. J Clin Oncol. 28:2625–2634. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Jennings RW, LaQuaglia MP, Leong K,
Hendren WH and Adzick NS: Fetal neuroblastoma: prenatal diagnosis
and natural history. J Pediatr Surg. 28:1168–1174. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Fulda S and Debatin KM: Apoptosis pathways
in neuroblastoma therapy. Cancer Lett. 197:131–135. 2003.
View Article : Google Scholar
|
|
5.
|
Arnold SA and Brekken RA: SPARC: a
matricellular regulator of tumorigenesis. J Cell Commun Signal.
3:255–273. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Mok SC, Chan WY, Wong KK, Muto MG and
Berkowitz RS: SPARC, an extracellular matrix protein with
tumor-suppressing activity in human ovarian epithelial cells.
Oncogene. 12:1895–1901. 1996.PubMed/NCBI
|
|
7.
|
Sato N, Fukushima N, Maehara N, et al:
SPARC/osteonectin is a frequent target for aberrant methylation in
pancreatic adenocarcinoma and a mediator of tumor-stromal
interactions. Oncogene. 22:5021–5030. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Sova P, Feng Q, Geiss G, et al: Discovery
of novel methylation biomarkers in cervical carcinoma by global
demethylation and microarray analysis. Cancer Epidemiol Biomarkers
Prev. 15:114–123. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Chen Y, Chen JC and Tseng SH: Effects of
tetrandrine plus radiation on neuroblastoma cells. Anticancer Res.
29:3163–3171. 2009.PubMed/NCBI
|
|
10.
|
Chen M, Hough AM and Lawrence TS: The role
of p53 in gemcitabine-mediated cytotoxicity and radiosensitization.
Cancer Chemother Pharmacol. 45:369–374. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Steel GG and Peckham MJ: Exploitable
mechanisms in combined radiotherapy-chemotherapy: the concept of
additivity. Int J Radiat Oncol Biol Phys. 5:85–91. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Heck A: [Endoscopic operations spare the
patient. Rapid rehabilitation, dispensing with large incisions,
removal of appendix, gallbladder and esophagus]. Krankenpfl J.
29:148–149. 1991.(In German).
|
|
13.
|
Bhoopathi P, Gondi CS, Gujrati M, Dinh DH
and Lakka SS: SPARC mediates Src-induced disruption of actin
cytoskeleton via inactivation of small GTPases Rho-Rac-Cdc42. Cell
Signal. 23:1978–1987. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Bhoopathi P, Chetty C, Gujrati M, Dinh DH,
Rao JS and Lakka SS: Cathepsin B facilitates autophagy mediated
apoptosis in SPARC overexpressed primitive neuroectodermal tumor
cells. Cell Death Differ. 17:1529–1539. 2010. View Article : Google Scholar
|
|
15.
|
Hoyer-Hansen M and Jaattela M: Connecting
endoplasmic reticulum stress to autophagy by unfolded protein
response and calcium. Cell Death Differ. 14:1576–1582. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Ding WX, Ni HM, Gao W, et al: Differential
effects of endoplasmic reticulum stress-induced autophagy on cell
survival. J Biol Chem. 282:4702–4710. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Bhoopathi P, Chetty C, Gujrati M, Dinh DH,
Rao JS and Lakka SS: The role of MMP-9 in the anti-angiogenic
effect of secreted protein acidic and rich in cysteine. Br J
Cancer. 102:530–540. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Mohanam S, Jasti SL, Kondraganti SR, et
al: Stable transfection of urokinase-type plasminogen activator
antisense construct modulates invasion of human glioblastoma cells.
Clin Cancer Res. 7:2519–2526. 2001.PubMed/NCBI
|
|
19.
|
Bhoopathi P, Chetty C, Kunigal S, Vanamala
SK, Rao JS and Lakka SS: Blockade of tumor growth due to matrix
metalloproteinase-9 inhibition is mediated by sequential activation
of beta1-integrin, ERK, and NF-kappaB. J Biol Chem. 283:1545–1552.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Chetty C, Lakka SS, Bhoopathi P, Kunigal
S, Geiss R and Rao JS: Tissue inhibitor of metalloproteinase 3
suppresses tumor angiogenesis in matrix metalloproteinase
2-down-regulated lung cancer. Cancer Res. 68:4736–4745. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Tivnan A, Tracey L, Buckley PG, Alcock LC,
Davidoff AM and Stallings RL: MicroRNA-34a is a potent tumor
suppressor molecule in vivo in neuroblastoma. BMC Cancer.
11:332011. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Ogata M, Hino S, Saito A, et al: Autophagy
is activated for cell survival after endoplasmic reticulum stress.
Mol Cell Biol. 26:9220–9231. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Liu J and Lin A: Role of JNK activation in
apoptosis: a double-edged sword. Cell Res. 15:36–42. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Hetz C: The unfolded protein response:
controlling cell fate decisions under ER stress and beyond. Nat Rev
Mol Cell Biol. 13:89–102. 2012.PubMed/NCBI
|
|
25.
|
Clark CJ and Sage EH: A prototypic
matricellular protein in the tumor microenvironment - where there’s
SPARC, there’s fire. J Cell Biochem. 104:721–732. 2008.PubMed/NCBI
|
|
26.
|
Podhajcer OL, Benedetti L, Girotti MR,
Prada F, Salvatierra E and Llera AS: The role of the matricellular
protein SPARC in the dynamic interaction between the tumor and the
host. Cancer Metastasis Rev. 27:691–705. 2008. View Article : Google Scholar
|
|
27.
|
Fulda S and Debatin KM: IFNgamma
sensitizes for apoptosis by upregulating caspase-8 expression
through the Stat1 pathway. Oncogene. 21:2295–2308. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Deniaud A, Sharaf el Dein O, Maillier E,
Poncet D, Kroemer G, Lemaire C and Brenner C: Endoplasmic reticulum
stress induces calcium-dependent permeability transition,
mitochondrial outer membrane permeabilization and apoptosis.
Oncogene. 27:285–299. 2008. View Article : Google Scholar
|
|
29.
|
Kuma A, Hatano M, Matsui M, et al: The
role of autophagy during the early neonatal starvation period.
Nature. 432:1032–1036. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Urano F, Wang X, Bertolotti A, Zhang Y,
Chung P, Harding HP and Ron D: Coupling of stress in the ER to
activation of JNK protein kinases by transmembrane protein kinase
IRE1. Science. 287:664–666. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Lin JH, Li H, Zhang Y, Ron D and Walter P:
Divergent effects of PERK and IRE1 signaling on cell viability.
PLoS One. 4:e41702009. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Bertolotti A, Zhang Y, Hendershot LM,
Harding HP and Ron D: Dynamic interaction of BiP and ER stress
transducers in the unfolded-protein response. Nat Cell Biol.
2:326–332. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Zinszner H, Kuroda M, Wang X, et al: CHOP
is implicated in programmed cell death in response to impaired
function of the endoplasmic reticulum. Genes Dev. 12:982–995. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Mah LY and Ryan KM: Autophagy and cancer.
Cold Spring Harb Perspect Biol. 4:a0088212012.PubMed/NCBI
|