Introduction
Human urothelial carcinoma (UC) evolves via the
accumulation of numerous genetic alterations, with the loss of p53
and p16INK4a (p16) functions representing important
stages in the development of superficial lesions and their
progression to malignant disease (1). p16 inhibits the activities of
cyclin-dependent kinases (CDKs), which maintain the retinoblastoma
protein (pRb) in its active hypophosphorylated state (2). p16 gene transfection has been shown
to result in a marked decrease in pRb phosphorylation, a decrease
in cell proliferation and the suppression of the tumorigenicity of
bladder tumor cell lines (3,4). It
has been reported that the p16 antitumor peptide dramatically
inhibits the growth of highly aggressive leukemia/lymphoma through
the restoration of p16 function (5).
Current standard drug therapies involve the
intravesical instillation of a chemotherapeutic agent or bacillus
Calmette-Guérin (BCG) for non-muscle-invasive bladder tumors
(6) and cisplatin-based systemic
chemotherapy for locally advanced and metastatic bladder tumors.
BCG instillation may cause severe adverse events, such as
hematuria, fever, irritation or contraction and resistance to BCG
remains an unsolved issue. By contrast, in metastatic tumors, the
treatment of chemorefractory cases and maintenance therapy for
chemosensitive cases are important issues in the development of a
new therapeutic approach, which may be a different procedure from
chemotherapeutic agents.
We hypothesized that a minimum inhibitory sequence
(MIS) peptide of p16 (p16-MIS) may play a role in the anti-tumor
effect in p16-deficient bladder tumor cell lines using the Wr-T
system. The aim of this study was to assess the effect of the
transfer of the p16 peptide on several bladder tumor cell lines
in vitro and the effect of the mouse MIS peptide of p16
(m-p16-MIS) on a mouse bladder tumor cell line (MBT-2) in
vivo.
Materials and methods
Cell lines
The human bladder tumor cell lines, 253J, 575A, J82,
Jon, RT112, RT4, SW1710, SW800, T24 and VMCub1 (kindly provided by
Dr Schalken, Radboud Medical Center, Nijmegen, The Netherlands),
the mouse bladder tumor cell line, MBT-2, and the mouse kidney
tumor cell line, Renca, were cultured in RPMI-1640 containing 10%
inactivated fetal bovine serum (IBL; Gunma, Japan), at 37°C under
an atmosphere of 5% CO2. After the transfer of peptides
to the cells, the viability of the cell line was evaluated using a
WST-8 Cell Counting kit-8 (#347-07621 Dojindo, Kamimashiki,
Japan).
Peptide synthesis
All peptides including Wr-T and r9-p16-MIS for human
and mouse were synthesized at Biogate Co., Ltd. (Yamagata, Japan).
The identity of all peptides was confirmed by mass spectrometry. We
prepared the HCl form of the peptides following high-performance
liquid chromatography purification for in vitro and in
vivo applications. Peptide purity was >95%. The amino acid
sequence of the Wr-T transporter is KETWWETWWTEWWTEWSQGPGrrrrrrrrr
(r, D-enantiomer arginine) (5,7). For
synthesis of the respective p16-MIS for human and mouse, the
10-amino acid sequence FLDTLVVLHR and FLDTLVVLHG, identified as the
MIS of p16 by Fåhraeus et al(8), was defined as the functional core of
the peptide, which is insoluble, as is the entire p16 molecule (MIS
hydrophobicity, 69.2%). We therefore fused r9 into these 10 amino
acids to make the conjugate less hydrophobic (hydrophobicity, 40%),
thus facilitating incorporation into the cells.
Peptide transduction
For the incorporation of the peptide mixture for
in vitro growth suppression, the Wr-T and r9-p16-MIS peptide
were mixed in 10 μl of distilled water at room temperature
for 60 min (final concentrations, 5 μmol/l Wr-T and 8
μmol/l r9-p16-MIS). The solution was then added directly to
190 μl of RPMI-1640 containing 5% fetal bovine serum to
obtain the final concentration indicated. In vivo peptide
delivery to the solid bladder tumor was performed by injecting the
Wr-T/r9-p16-MIS peptide mix (50 nmol Wr-T, 80 nmol r9-p16-MIS) into
the hearts of mice bearing tumors that had grown to a diameter of 3
mm (tumor volume, ∼15 mm3). The control groups were
administered 100 μl of phosphate-buffered saline (PBS)
without peptide, Wr-T, or p16 peptide alone dissolved in 100
μl of PBS and injected as previously described (7).
Reverse transcription-PCR
Total RNA (5 μg) was extracted from each
tumor cell line using TRIzol reagent (#15596-026, Life
Technologies, Tokyo, Japan). Briefly, cells were washed with PBS
and lysed with 1 ml of TRIzol reagent. Chloroform (200 μl)
was added and the mixture was centrifuged at 4°C and 12,000 × g for
15 min. The liquid phase was precipitated with isopropanol. The RNA
pellets were dissolved in Tris-EDTA (TE) buffer. Subsequently, cDNA
was synthesized from the extracted RNA using random primers and a
cDNA synthesis kit (#4368814 High Capacity cDNA Transcription kits;
Applied Biosystems, Tokyo, Japan). Reverse transcription-PCR was
then carried out with a Taq PCR Core kit (#201225; Qiagen, Tokyo,
Japan). Amplification conditions and primer sequences are listed in
Table I. The sense/antisense
primer sequences for CDK4, CDK6 and cyclin D were also described in
a previous publication (9).
 | Table I.PCR primers and conditions. |
Table I.
PCR primers and conditions.
| Molecule
fragment | Sequence | Annealing temperature
(°C) | Product size
(bp) |
|---|
| p16 | F:
ATAGTTACGGTCGGAGGCC
R: TGGTTACTGCCTCTGGTGC | 60 | 536 |
| Cyclin D | F:
AAAGACAGTTTTTGGGTAATCTTTT
R: CCGGAGCATTTTGATACCAG | 55 | 126 |
| CDK4 | F:
CTTCTGGACACTGAGAGGGC
R: TGGGAGGGGAATGTCATTAA | 61 | 110 |
| CDK6 | F:
CGGAGAACACCCTTGGTG
R: GAGCCTGTCCAGAAGACAGC | 59 | 105 |
| Actin | F:
GTGGGGCGCCCCAGGCACCA
R: CTCCTTAATGTCACGCACGATTTC | 55 | 539 |
Western blot analysis
Cells were promptly lysed with SDS sample lysis
buffer and the extracts were separated by SDS-PAGE using 12.5 to
15% Bis-Tris gradient gels (SuperSepAce, Wako, Osaka, Japan).
Proteins were transferred onto a Hybond-P membrane (#RPN2020F GE
Healthcare Japan, Tokyo, Japan), blocked with 5% dried milk and 1%
normal goat serum-PBS and then sequentially probed with mouse
monoclonal anti-p16INK4 antibody (Clone: F-12, #SC1661;
Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA), mouse
monoclonal anti-p21WAF1 antibody (Clone: EA10; Oncogene
Research Products, Boston, MA, USA), mouse monoclonal
anti-p27Kip1 antibody (Clone: 1B4; Novocastra
Laboratories Ltd, Newcastle, UK), mouse monoclonal anti-pRb
antibody (Clone: LM95.1; Oncogene Research Products), rabbit
polyclonal anti-phospho-Ser780 pRb antibody (#9307S; Cell Signaling
Technology Japan, K.K., Tokyo, Japan), mouse monoclonal anti-actin
antibody (Clone: AC-74; Sigma, St. Louis, MO, USA). Immune
complexes were visualized with ECL Western Blotting Detection
Reagents (#RPN2109; GE Healthcare, Tokyo, Japan) according to the
manufacturer’s instructions and signals were visualized and
digitally captured using an image analyzer (LAS 4000; GE
Healthcare).
Mouse tumor models
Four-week-old female KSN nude mice were obtained
from SLC, Inc. (Hamamatsu, Japan). A total of 100 μl of a
PBS suspension containing 2.0×106 cells of the mouse
bladder tumor cell line, MBT-2, was injected subcutaneously (s.c.)
into the flanks of each mouse to form a solid tumor nodule. Animal
experiments performed in this study were approved by the Laboratory
Animal Resource Center, University of Tsukuba, Tsukuba, Japan. All
mouse procedures, euthanasia and surgery, including bladder tumor
transplantations and peptide injections, were carried out
painlessly or under anesthesia within the strict guidelines of the
Laboratory Animal Resource Center, University of Tsukuba.
Immunohistochemical and TUNEL
staining
Tumors and other organs were fixed in 10%
neutral-buffered formalin overnight and were then processed,
paraffin-embedded, sectioned, mounted onto slides and stained by
the standard hematoxylin and eosin method. Serial paraffin sections
(4 μm thick) were stained using rabbit polyclonal
anti-phospho-Ser780 pRb antibody (diluted 1:100, #9307S; Cell
Signaling Technology), followed by the universal immuno-enzyme
polymer method (#714342 N-Histofine Simple Stain Mouse MAX PO;
Nichirei Biosciences, Tokyo, Japan) according to the manufacturer’s
instructions. The sections were developed with
3,3’-diaminobenzidine tetrahydrochloride, containing 0.03% hydrogen
peroxide, and counterstained with hematoxylin. Apoptotic cells were
detected in tumors harvested from mice 48 h after peptide
administration. Apoptosis in the tumor sections was determined by
the terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick
end-labeling (TUNEL) assay with the use of an ApopTag Peroxidase In
Situ Apoptosis Detection kit (#S7100; Nippon Chemicon, Tokyo,
Japan) following the manufacturer’s instructions.
Results
Expression of p16, phosphorylated pRb and
related molecules in human bladder tumor cell lines
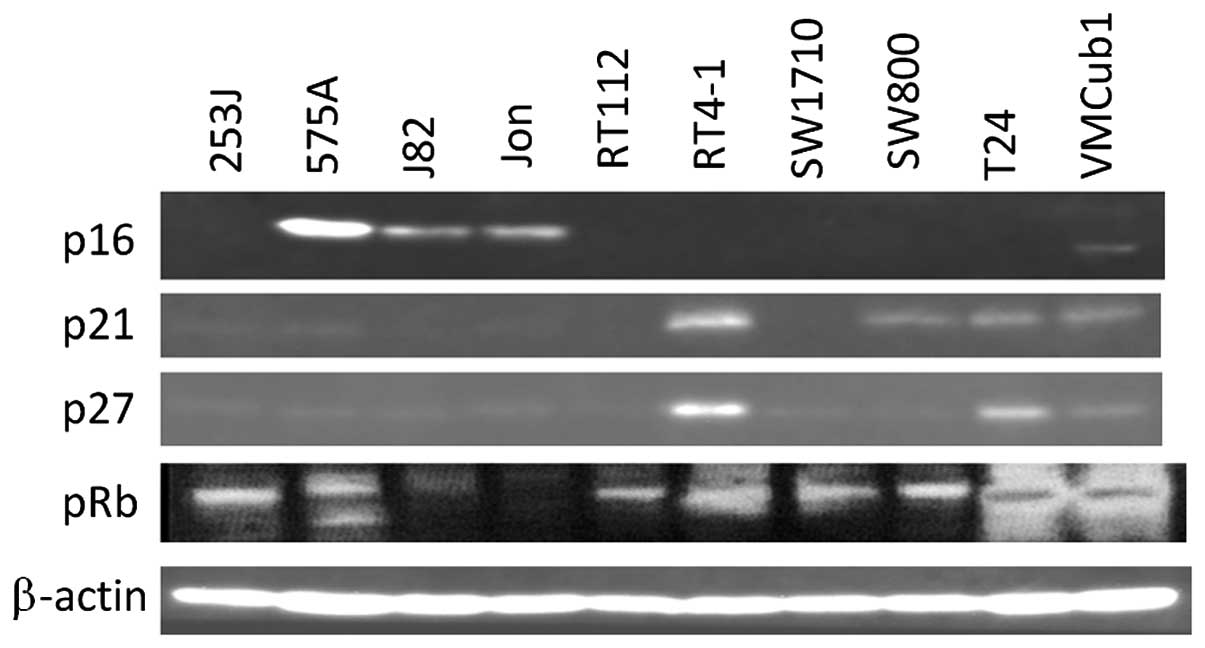
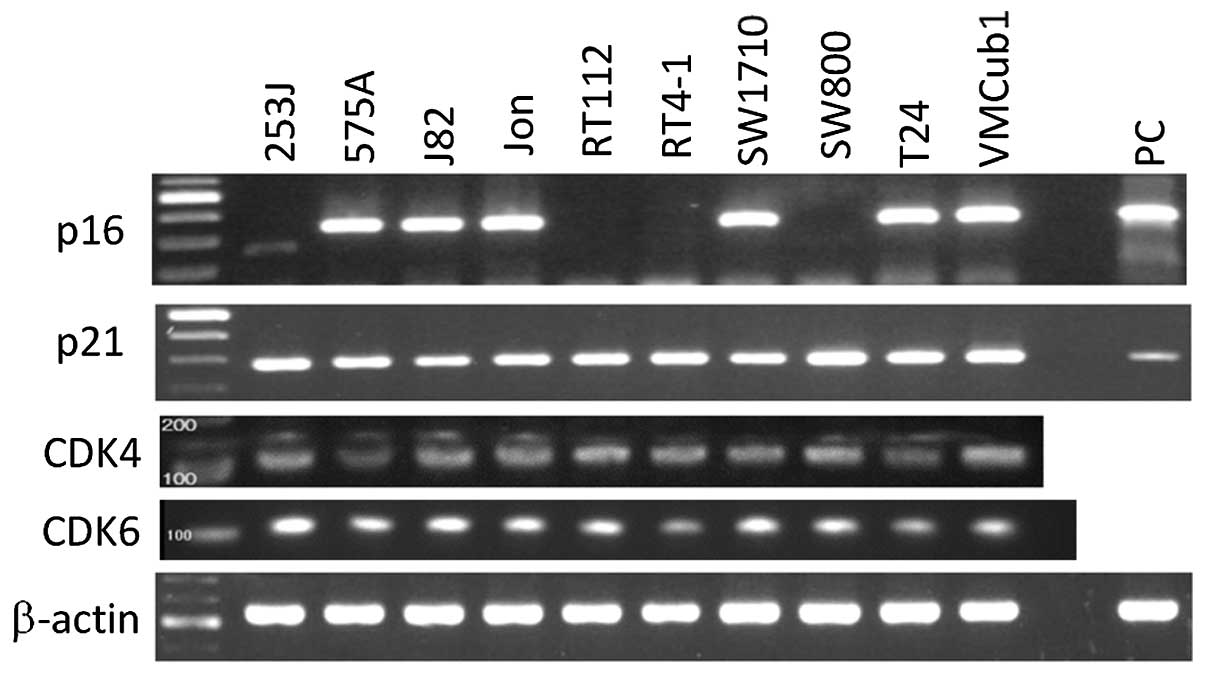
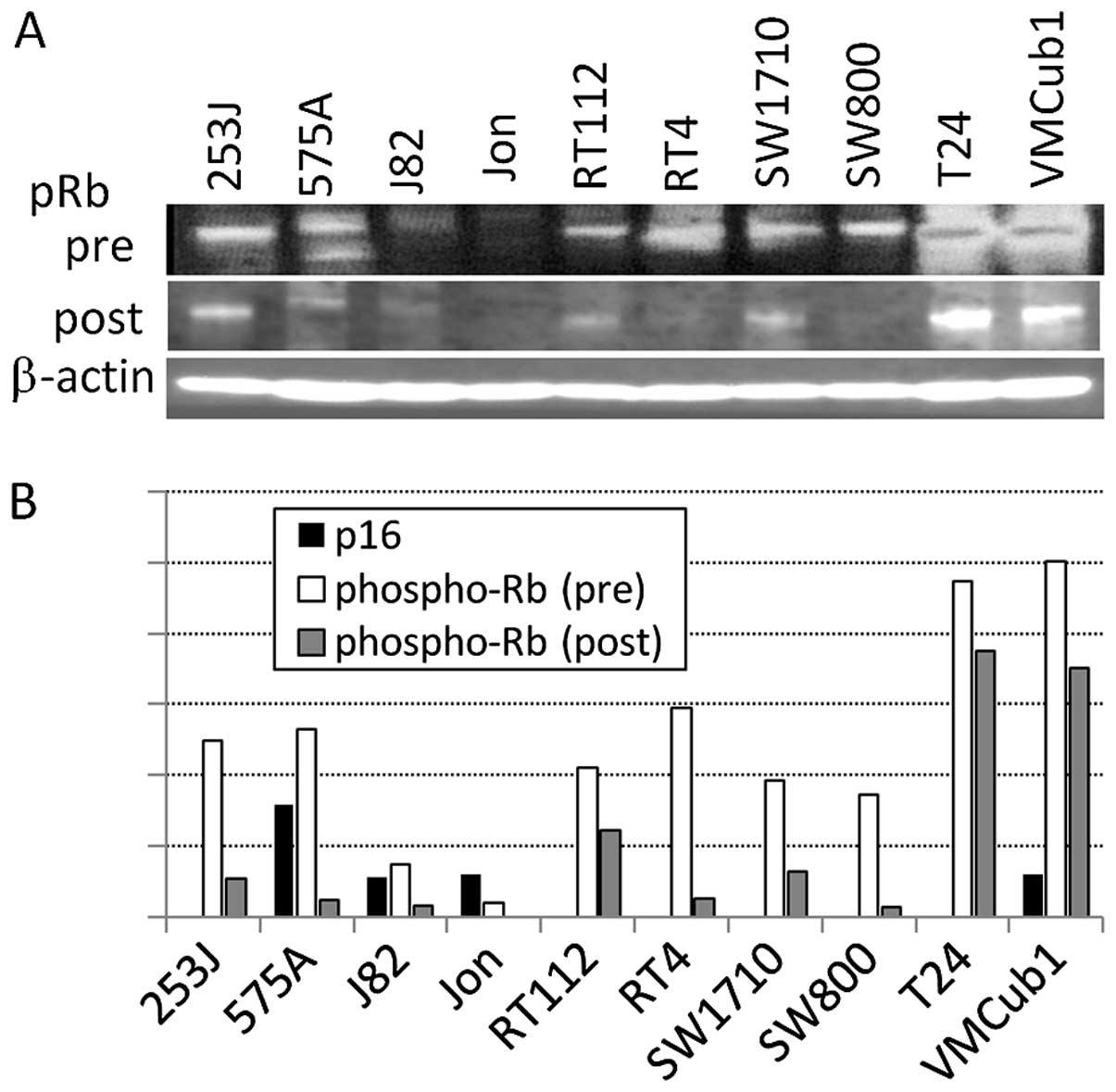
Out of the 10 human bladder tumor cell lines, 7
lines did not express p16 with pRb phosphorylation (Fig. 1) on the mRNA and protein levels.
p21 and p27, which are downstream of p53, are expressed on a
protein level in most p16-deficient lines. The mRNA expression of
CDK4 and CDK6, which are further downstream of p16, was observed in
all the 10 lines (Fig. 2).
Growth inhibition of bladder tumor cells
after r9-p16-MIS transduction assessed by cell proliferation
assay
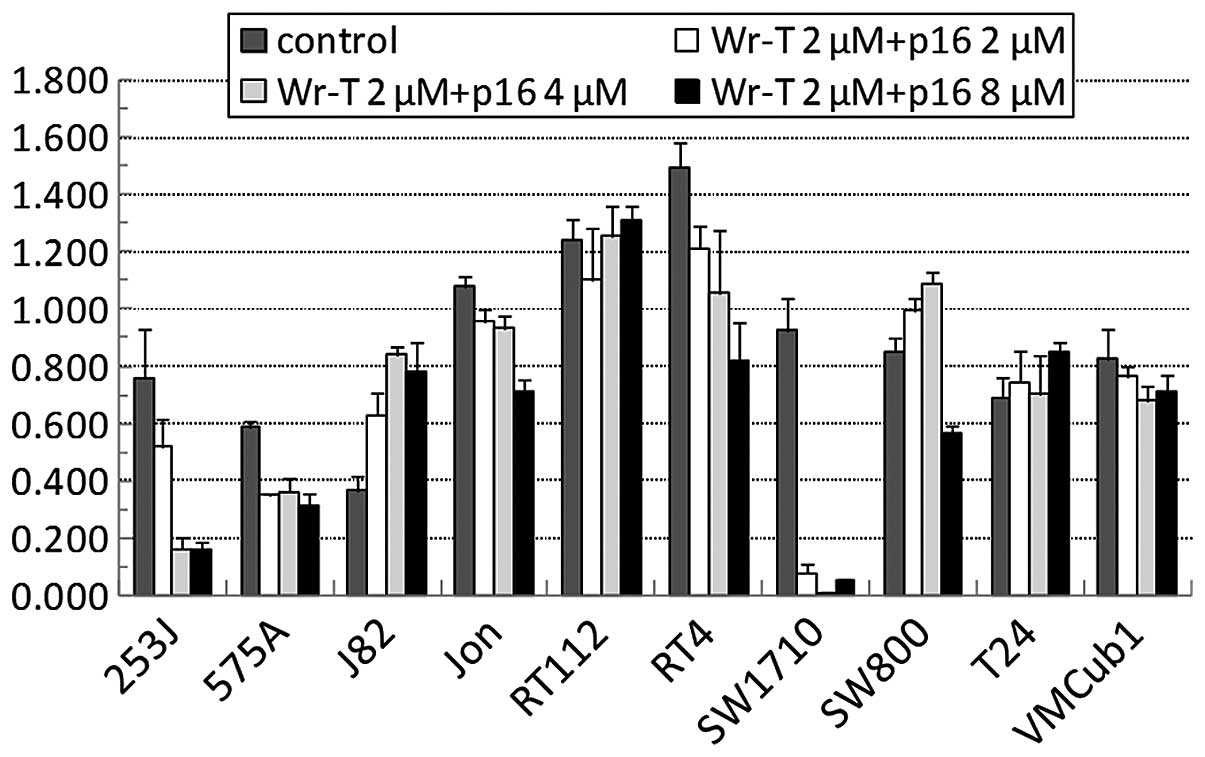
We then suppressed the growth of the bladder tumor
cells using the Wr-T-transported r9-p16-MIS. The r9-p16-MIS was
introduced into each bladder tumor cell line beginning with
1.0×105 cells per incubation with Wr-T (final
concentrations, 5 μmol/l Wr-T and 2–8 μmol/l
r9-p16-MIS) (Fig. 3) and cell
growth was monitored by the WST-8 cell proliferation assay. The
administration of r9-p16-MIS produced some growth suppression in 7
out of the 10 cell lines. In 4 cell lines, >50% inhibition was
observed at concentrations of at least 8 μmol/l of
r9-p16-MIS, e.g., 80, 50, 50 and 95% in the 253J, 575A, RT4 and
SW1710 cells, respectively (Fig.
4). These cell lines demonstrated a significant decrease in the
expression of phosphorylated pRb following r9-p16-MIS transduction
(Fig. 5).
In vivo bladder tumor suppression by Wr-T
and r9-p16 transduction system
In view of the therapeutic potential of the Wr-T and
r9-p16-MIS delivery system, we investigated the efficacy of this
system for the treatment of the mouse bladder tumor cell line,
MBT-2, using allografts transplanted s.c. into KSN nude mice. The
expression of p16 was not observed in the MBT-2 cells (Fig. 6A) and that of phosphorylated pRb
was downregulated following mouse r9-p16-MIS delivery in a
concentration-dependent manner (Fig.
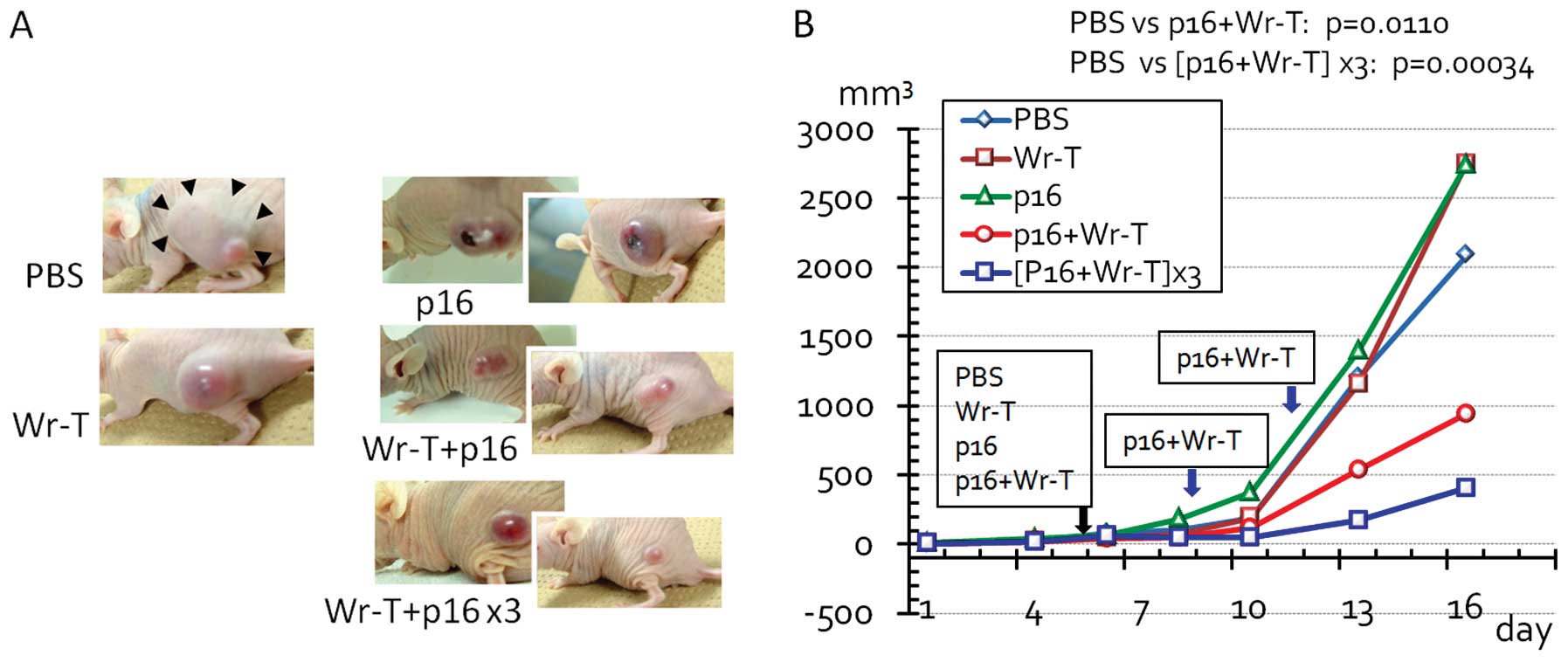
6B). When the tumors had reached 3 mm in diameter, we
administered the Wr-T and mouse r9-p16-MIS mixture to the mice via
cardiac delivery. A significant decrease in tumor size was observed
following Wr-T and mouse r9-p16-MIS transduction compared with the
peptide-free tumors (PBS vs. single and triple doses of Wr-T and
mouse r9-p16-MIS, p=0.011 and 0.00034, respectively) (Fig. 7). By the 5th day after peptide
transduction, the diameter of the peptide-free tumor was 33% larger
than that of the tumors treated with Wr-T and mouse r9-p16-MIS.
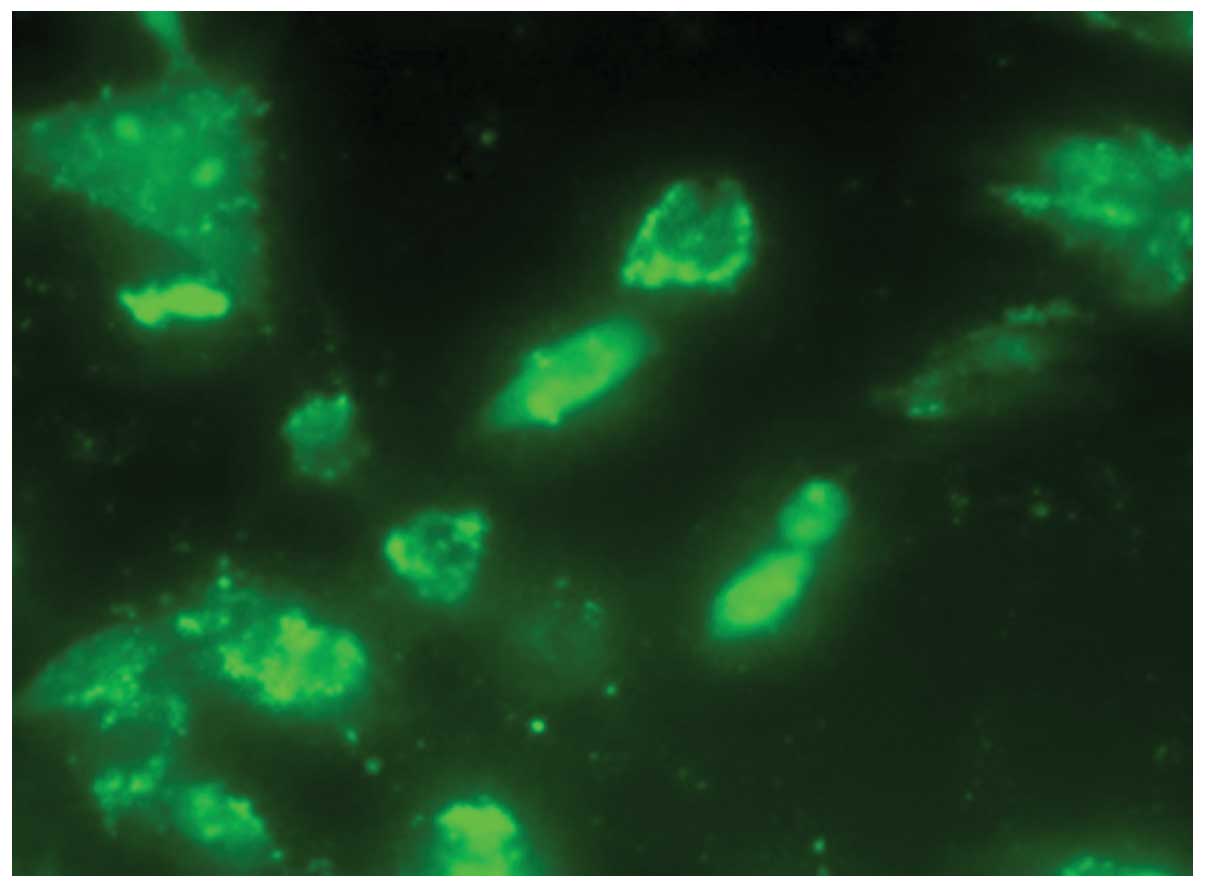
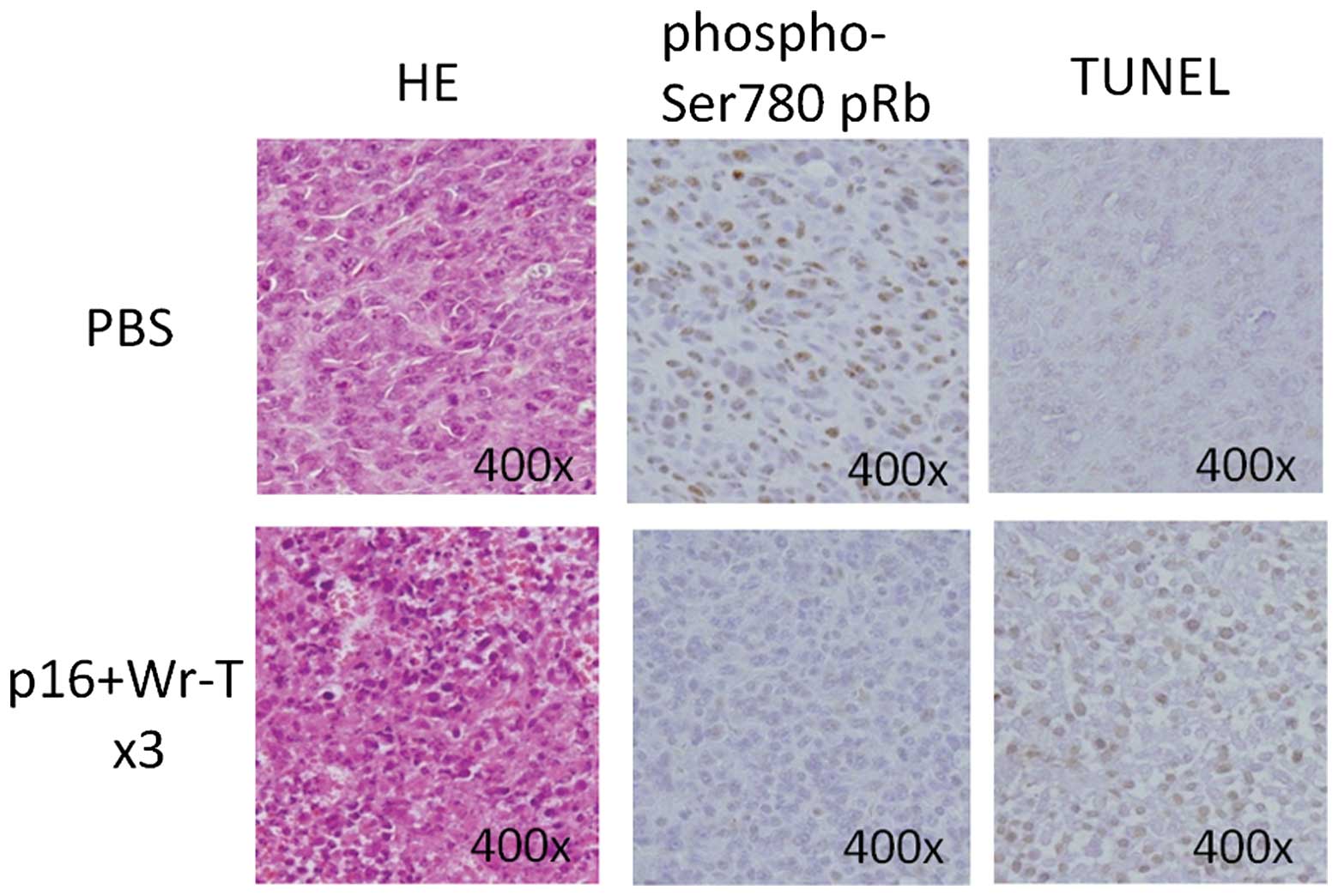
At 72 h post-transduction, the expression of
phospho-Ser780 pRb in the tumors treated with Wr-T and mouse
r9-p16-MIS mixture was decreased, as assessed by
immunohistochemistry (Fig. 8). By
contrast, TUNEL analysis showed an increase in the presence of
positively-stained apoptotic bodies in the tumors treated with the
Wr-T and mouse r9-p16-MIS mixture (Fig. 8).
Discussion
The p16 gene is located on the short arm of
chromosome 9 at the p21 locus. p16 is a kinase inhibitor that
inhibits the activity of CDK4 and CDK6 to phosphorylate pRb. In
bladder tumors, the abnormality of chromosome 9 has been reported
as an early genetic event in both non-muscle- and muscle-invasive
UC (10). Thus, the p16-pRb
pathway could be considered a therapeutic target based on the
molecular mechanism of bladder tumor carcinogenesis.
During investigations of the introduction of the p16
gene into cell lines, growth arrest and suppression of
tumorigenicity were observed in ovarian (11) and bladder (3) cancers. We hypothesized that peptide
transfer is more acceptable than gene transfection for clinical
application; therefore, we designed a protocol for systemic and
local administration to inhibit the growth of transplanted and
orthotopic bladder tumors. We synthesized the p16-MIS amino acid
sequence, which represented the minimal function of p16, and
delivered it into bladder tumor cell lines using a peptide delivery
system as described by Kondo et al(5). As shown in Fig. 7, an in vitro analysis
demonstrated that p16-MIS inhibited tumor growth; this effect was
not only dependent on the loss of p16 but also on the degree of pRb
phosphorylation.
In the present study, the overexpression of p16 was
observed in some of the bladder tumor cell lines irrespective of
the pRb phosphorylation status. As shown in Fig. 1, p16 was overexpressed rather than
normally expressed in some of the cell lines, e.g., 575A. Recently,
Nakazawa et al(12)
reported that p16 overexpression was observed in 16 to 50% of
cytology samples of bladder tumors. A possible mechanism of p16
overexpression was explained as the self-regulation that
accompanies abnormally high levels of cell proliferation.
Alternatively, whether or not the expression of p16 is functional
should be clarified. Asamoto et al(13) suggested that the overexpression of
p16 mRNA indicates the deregulation of pathways involving the p16
gene in mice. Since p16-MIS transduction is most likely useful to
the p16-expressing 575A bladder tumor cells when accompanying
phosphorylated pRb accumulation, another explanation may be that
the p16 protein in these cell lines may not function properly
through the p16-pRb molecular pathway.
Due to the delivery of p16-MIS, pRb phosphorylation
was shown to be reduced by western blot analysis, irrespective of
the previous expression/phosphorylation of pRb, as indicated in
Fig. 5. Our histological analysis
of tissues post-p16 delivery showed that pRb phosphorylation was
inhibited and that the delivery peptide seemed to function in place
of the normal p16 molecule. In addition, apoptosis was deemed to be
induced after the p16-MIS transfer as apoptotic bodies were
increased in the transplanted tumors treated with the p16 peptide,
as shown by TUNEL staining. These findings demonstrated that the
present peptide delivery system induced tumor cell apoptosis as
well as cell growth inhibition.
A comparison of a single dose with 3 doses indicated
that multiple administrations were more effective in suppressing
tumor growth. However, tumors treated with 3 doses were still
histologically active, suggesting that this delivery system is
limited. Since muscle-invasive or metastatic UC frequently contains
several genetic alterations, including p53 abnormality (14), a combination of either the
transduction of another peptide, e.g., p53, or chemotherapeutic
agents may be more effective for the systemic treatment of
metastatic UC.
Systemic administration, as shown in the animal
model, demonstrated that a p16-MIS transfer system may be available
for clinical use in patients with systemic disease, such as distant
metastases of UC. Current standard systemic chemotherapy for
metastatic UC is a combination of gemcitabine and cisplatin, i.e.,
GC therapy (15). Although GC
therapy is expected to produce a 60–70% response rate, refractory
cases or severe adverse events are often experienced. Thus,
patients with refractory tumors or severe adverse events may be
potential candidates for p16-MIS transduction therapy. Sequential
and maintenance therapy using p16-MIS may be a treatment option for
a well-controlled case following systemic chemotherapy.
A limitation of this study was that we were unable
to evaluate the efficacy of the p16 peptide against
non-muscle-invasive bladder tumors by intravesical instillation.
Intravesical BCG instillation is the current standard treatment for
moderate- to high-risk UC of the bladder. Low-grade
non-muscle-invasive UC has a limited gene alteration and the p53
mutation is not very frequent (14). The findings described above support
the application of the intravesical instillation of a p16 peptide
to prevent the implantation of floating tumor cells after
transurethral resection, since its instillation is similar to an
in vitro peptide transfer. Of note, Sato et
al(16) reported that the
overexpression and phosphorylation of pRb in bladder tumor cells
predicts a poor response to BCG therapy. Thus, combined therapy by
p16 peptide transfer with BCG instillation may be a promising
treatment for non-muscle-invasive bladder tumors, since restoring
pRb function by p16 peptide transduction may be effective in
treating BCG-refractory bladder tumors. The following step after
the animal model is the application of p16-MIS delivery alone or in
combination with BCG instillation using a mouse intravesical
implantation model of the MBT-2 cell line (17) to examine the toxicity of p16
peptide transfer to normal organs, particularly to normal
urothelial cells of urinary bladder mucosa.
In conclusion, the delivery of p16-MIS using a novel
peptide transduction system may be a new therapeutic option for
metastatic UC; however, additional experiments are required to
investigate the efficacy of the local administration and toxicity
of the p16 peptide.
Acknowledgements
This study was supported by a
Grant-in-Aid from the Japan Society for the Promotion of Science
(JSPS), Japan (no. 21592031). We thank Dr Eisaku Kondo for his
helpful advice and also thank Mrs. Noriko Kunita and Mrs. Taeko
Asano for their technical support.
References
|
1.
|
Shaw NJ, Georgopoulos NT, Southqate J and
Trejdosiewicz LK: Effect of loss of p53 and p16 function on life
span and survival of human urothelial cells. Int J Cancer.
116:634–639. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Le Frère-Belda MA, Gil Diez de Medina S,
Daher A, Martin N, Albaud B, Heudes D, Abbou CC, Thiery JP, Zafrani
ES, Radvanyi F and Chopin D: Profiles of the 2 INK4a gene products,
p16 and p14ARF, in human reference urothelium and bladder
carcinomas, according to pRb and p53 protein status. Hum Pathol.
35:817–824. 2004.PubMed/NCBI
|
|
3.
|
Wu Q, Possati L, Montesi M, Gualandi F,
Rimessi P, Morelli C, Trabanelli C and Barbanti-Brodano G: Growth
arrest and suppression of tumorigenicity of bladder-carcinoma cell
lines induced by the P16/CDKN2 (p16INK4A, MTS1) gene and other loci
on human chromosome 9. Int J Cancer. 65:840–846. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Chatterjee SJ, George B, Goebell PJ,
Alavi-Tafreshi M, Shi SR, Fung YK, Jones PA, Cordon-Cardo C, Datar
RH and Cote RJ: Hyperphosphorylation of pRb: a mechanism for RB
tumour suppressor pathway inactivation in bladder cancer. J Pathol.
203:762–770. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Kondo E, Seto M, Yoshikawa K and Yoshino
T: Highly efficient delivery of p16 antitumor peptide into
aggressive leukemia/lymphoma cells using a novel transporter
system. Mol Cancer Ther. 3:1623–1630. 2004.PubMed/NCBI
|
|
6.
|
Sylvester RJ, van der Meijden AP and Lamm
DL: Intravesical bacillus Calmette-Guerin reduces the risk of
progression in patients with superficial bladder cancer: a
meta-analysis of the published results of randomized clinical
trials. J Urol. 168:1964–1970. 2002. View Article : Google Scholar
|
|
7.
|
Kondo E, Tanaka T, Miyake T, Ichikawa T,
Hirai M, Adachi M, Yoshikawa K, Ichimura K, Ohara N, Moriwaki A,
Date I, Ueda R and Yoshino T: Potent synergy of dual antitumor
peptides for growth suppression of human glioblastoma cell lines.
Mol Cancer Ther. 7:1461–1471. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Fåhraeus R, Laín S, Ball KL and Lane DP:
Characterization of the cyclin-dependent kinase inhibitory domain
of the INK4 family as a model for a synthetic tumour suppressor
molecule. Oncogene. 16:587–596. 1998.PubMed/NCBI
|
|
9.
|
Arvanitis DA and Spandidos DA:
Deregulation of the G1/S phase transition in cancer and squamous
intraepithelial lesions of the uterine cervix: A case control
study. Oncol Rep. 20:751–760. 2008.PubMed/NCBI
|
|
10.
|
Simoneau AR, Spruck CH III,
Gonzalez-Zulueta M, Gonzalgo ML, Chan MF, Tsai YC, Dean M, Steven
K, Horn T and Jones PA: Evidence for two tumor suppressor loci
associated with proximal chromosome 9p to q and distal chromosome
9q in bladder cancer and the initial screening for GAS1 and PTC
mutations. Cancer Res. 56:5039–5043. 1996.
|
|
11.
|
Wolf JK, Kim TE, Fightmaster D, Bodurka D,
Gershenson DM, Mills G and Wharton JT: Growth suppression of human
ovarian cancer cell lines by the introduction of a p16 gene via a
recombinant adenovirus. Gynecol Oncol. 73:27–34. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Nakazawa K, Murata S, Yuminamochi T, Ishii
Y, Ohno S, Nakazawa T, Kondo T and Katoh R: p16(INK4a) expression
analysis as an ancillary tool for cytologic diagnosis of urothelial
carcinoma. Am J Clin Pathol. 132:776–784. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Asamoto M, Hori T, Baba-Toriyama H, Sano
M, Takahashi S, Tsuda H and Shirai T: p16 gene overexpression in
mouse bladder carcinomas. Cancer Lett. 127:9–13. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Knowles MA: Molecular pathogenesis of
bladder cancer. Int J Clin Oncol. 13:287–297. 2008. View Article : Google Scholar
|
|
15.
|
von der Maase H, Hansen SW, Roberts JT,
Dogliotti L, Oliver T, Moore MJ, Bodrogi I, Albers P, Knuth A,
Lippert CM, Kerbrat P, Sanchez Rovira P, Wersall P, Cleall SP,
Roychowdhury DF, Tomlin I, Visseren-Grul CM and Conte PF:
Gemcitabine and cisplatin versus methotrexate, vinblastine,
doxorubicin, and cisplatin in advanced or metastatic bladder
cancer: results of a large, randomized, multinational, multicenter,
phase III study. J Clin Oncol. 18:3068–3077. 2000.
|
|
16.
|
Sato M, Yanai H, Morito T, Oda W, Shin-no
Y, Yamadori I, Tshushima T and Yoshino T: Association between the
expression pattern of p16, pRb and p53 and the response to
intravesical bacillus Calmette-Guerin therapy in patients with
urothelial carcinoma in situ of the urinary bladder. Pathol Int.
61:456–460. 2011. View Article : Google Scholar
|
|
17.
|
Horiguchi Y, Kikuchi E, Ozu C, Nishiyama
T, Oyama M, Horinaga M, Yoshioka K and Tachibana M: Establishment
of orthotopic mouse superficial bladder tumor model for studies on
intravesical treatments. Hum Cell. 21:57–63. 2008. View Article : Google Scholar : PubMed/NCBI
|