Introduction
Breast cancer is the second leading cause of cancer
related death among women worldwide. It was estimated that there
will be 230,480 new cases of invasive breast cancer and 39,520 new
deaths among US women in 2011 (1).
Among the molecular targets for treatment purpose, epidermal growth
factor receptor (EGFR) and human epidermal growth factor receptor 2
(HER2) were most widely investigated as potential targets for
anticancer drug development since the first report of the
overexpressions in human breast cancers indicates a poor prognosis
(2,3). EGFR overexpression has been found in
15% of unselected, but half of triple-receptor-negative (TRN)
breast cancers (4), and HER2 is
amplified in 20 to 30% of breast cancers. All these changes lead to
enhanced malignant phenotypes and highly aggressive disease
(5,6). HER2-overexpressing breast cancers
also exhibited the capability of resistance to many first-line
chemotherapy and tamoxifen (7–9).
Moreover, many fundamental studies revealed that EGFR and HER2 have
a close relationship with the aberrant activation of Ras proteins,
which is implicated in facilitating virtually all aspects of the
malignant phenotype, including cellular proliferation,
transformation, invasion and metastasis (10).
Growth factor receptor bound protein 2 (Grb2) is one
of the most important proteins participating in EGFR and HER2
signal transduction. It consists of one Src-homology 2 (SH2) domain
and surrounded by two Src-homology 3 (SH3) domains. The SH2 domain
interacts with phosphotyrosines (pTyr) on target proteins, while
the SH3 domains interact with proline-rich sequences (11). Once the membrance receptor is
activated, Grb2 serves as an adaptor protein in many signal
transductions including the mitogen-activated protein kinase (MAPK)
cascade for promoting cell division and/or differentiation
(12). Silencing the Grb2
expression reduced cell growth in vitro indicating the GRB2
protein could be a good target for cancer therapy (13). The SH2 domain is one of the most
prevalent protein-binding modules for protein-protein interaction
which mediate the formation of multiprotein complexes during
signaling. SH2 domains specifically function in protein tyrosine
kinase (PTK) pathways due to the dependence of their binding on
tyrosine phosphorylation (14). In
cells, the specific association of SH2 domains and tyrosine
phosphopeptides is to mediate the reversible relocalization of
proteins, which is important for efficient propagation of PTK
signals (15). The specificity of
SH2 domain has allowed these domains to function as probes to
detect tyrosine phosphorylation in signaling proteins (16,17).
We hypothesized that Grb2-SH2 domain may serve as a negative
inhibitor if it binds to an activated receptor in a living cell,
but lacks SH3 domains to bind those downstream modules.
In order to investigate the possibility of our
hypothesis, we utilized PTD as a tool to bring SH2 domains into
living cells. The most popular PTD is HIV-1 TAT48-60
(GRKKRRQRRRPPQ), which has 12 amino acid arginine-rich peptides
with the ability to rapidly translocate outside proteins into cells
both in vivo and in vitro. Fusion of this PTD with
proteins and peptides was proved to facilitate effective
transduction of the fused cargos into cultured cells and animal
tissues while preserving their biological activity (18,19).
In this study, we constructed, expressed, and purified a fusion
protein containing an SH2 domain derived from Grb2 and a PTD
domain, and investigated its potential functions in breast cancer
cells.
Materials and methods
All chemicals were of analytical grade and purchased
from commercial suppliers.
Bacterial strains, plasmids and cell
lines
E. coli [DH5α and BL21 (DE3)] (Invitrogen,
Carlsbad, CA, USA) were cultured in Luria-Bertani broth medium (LB)
and stored at −70°C. pET-16b was a commercial product (Novagen,
Billerica, MA, USA) and stored at −20°C. The reconstructed plasmid
pET-16b-ptd was a gift from Professor Hua Han, Department of
Medical Genetics and Developmental Biology, Fourth Military Medical
University (20). The breast
cancer cell lines HER2-negative MDA-MB-231 and HER2-positive
SK-BR-3 were preserved in the Department of Molecular Biology of
the Fourth Military Medical University and was routinely cultured
in Dulbecco’s modified Eagle’s medium (DMEM) or RPMI-1640 medium
supplemented with 10% fetal bovine serum (Gibco by Invitrogen,
Carlsbad, CA, USA), 100 U/ml penicillin and 100 μg/ml
streptomycin. Cell cultures maintained at 37°C in a humidified 5%
CO2 atmosphere.
Expression vector construction
The Grb2-SH2 coding DNA sequence was the native
sequence of Grb2-SH2 as reported in Genbank (CCDS11721.1) without
any modification. Grb2-SH2 cDNA was generated by PCR using the
cDNAs of human lymphocytes constructed by the Department of Medical
Genetics and Developmental Biology of Fourth Military Medical
University using the following primers: SH2 sense primer, 5′-GGA
TCC CTA CCG CAG GAA TAT C-3′ and SH2 anti-sense primer, 5′-CTC GAG
AAA CCA CAT CCG TGG-3′. PCR products were purified using QIAquick
Gel Extraction Kit (Qiagen, Hilden, Germany). Resulting PCR
products were digested with BamHI and XhoI into the
PGEM-T-Easy reporting plasmid and subsequently sub-cloned into
digested pET-16b-ptd containing a PTD coding sequence
(5′-TAT GTA TGG TAG GAA GAA ACG TCG ACA GCG TCG TCG-3′) derived
from HIV-1 TAT48–60 (GRKKRRQRRRPPQ) and a
ten-Histidine-tag sequence for easy purification to construct the
expression vector pET-16b-ptd/grb2-sh2. Meanwhile, we also
designed a mutant contrast for Grb2-SH2 sequence with a loss of 60
bases in sequence using a forward primer (5′-GAA GTT CAA TTC TTT
GCG GTA GGG ATC-3′) and a reverse primer (5′-CCG GGG ATC CCT ACC
GCA AAG AAT TG-3′) and inserted into digested
PGEM-T-grb2-sh2 and pET-16b-ptd/grb2-sh2. The
subsequent DNA sequencing and BLAST program (http://www.ncbi.nlm.nih.gov/BLAST/) confirmed all
insertions to be correct.
Protein expression and purification
The reconstructed plasmids were transformed into
E. coli BL21 (DE3) and a single colony was picked and grown
overnight in 5 ml LB supplemented with 100 μg/ml ampicillin
at 37°C then diluted 1:100. Protein expression induced by 1 mM
isopropyl β-D-1-thiogalactopyranoside (IPTG) (Sigma-Aldrich, St.
Louis, MO, USA). After induction for 4 h at 33°C, approximately 10
g of wet weight cells from 1-liter culture were harvested by
centrifugation at 6,000 x g for 30 min at 4°C followed by
re-suspension in 60 ml of Tris-HCl (50 mM, pH 8.0) containing 1 mM
phenylmethylsulfonyl fluoride (PMSF) for enzyme stability. The
E. coli cells were then pulse-sonicated for 10×1 m (10 sec
working and 50 sec resting on ice, 300 W). The lysate was
centrifuged at 15,000 x g for 30 min at 4°C. The recombinant
His-tagged PTD-Grb2-SH2 proteins were purified from the cell lysate
using 1 ml of HisTrap™ Ni++ charged columns (Amersham
Pharmacia Biotech, Uppsala, Sweden). The lysate was dialyzed
against binding buffer (20 mM Tris-HCl, pH 7.4, 500 mM NaCl, 5 mM
imidazole) overnight at 4°C. Subsequently, the dialyzed lysate was
injected into the Ni-NTA resin column and binding for 10 min. The
unbound proteins were washed away by using 10 volumes of binding
buffer. The His-tagged proteins were eluted with elution buffer (20
mM Tris-HCl, pH 7.4, 500 mM NaCl, 20 mM imidazole). The eluate was
analyzed by sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE) and western blot analysis. The fractions
containing the purified fusion protein were pooled and dialyzed
against 100 volumes of 20 mM Tris-HCl (pH 7.4) overnight at 4°C.
The protein concentration was determined using a DC protein assay
kit (Bio-Rad, Hercules, CA, USA) with a bovine serum albumin (BSA)
standard. The purified protein was stored at 4°C for use within 1
week or at −70°C for long-term storage.
SDS-PAGE and western blot analysis
SDS-PAGE was followed the procedure described by
Laemmli (21). For western blot
analysis, the proteins were separated by SDS-PAGE on a 15% gel and
transferred onto a polyvinylidene difluoride (PVDF) membrane
(Bio-Rad) in transfer buffer [25 mM Tris, 192 mM glycine, 15%
(vol/vol) methanol] at 4°C at 100 V for 30 min. After blocking with
5% milk in phosphate-buffered saline (PBS, 50 mM phosphate and 0.9%
NaCl; pH 7.2) at room temperature for 1 h or overnight at 4°C, the
membrane was incubated for 1 h with horseradish peroxidase
(HRP)-labeled antibody against the His-tag (Qiagen; 1:2,000 with
2.5% milk in PBS). The membrane was then washed five times with
PBS-T (PBS plus 0.05% Tween-20) and two times with PBS. The target
proteins were visualized with the enhanced chemiluminescence
detection system (ECL, GE Healthcare, Piscataway, NJ, USA).
Immunofluorescence assay
Cells on cover slips were incubated with the target
proteins for 4 h. The samples were fixed in 4% paraformaldehyde,
permeabilized in PBS with 0.1% Triton X-100, blocked in 2% normal
rabbit serum in PBS, and then incubated overnight at 4°C with mouse
anti-His polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz,
CA, USA) at a 1:400 dilution. Cells were extensively washed with
PBS and incubated with the fluorescein-labeled rabbit anti-mouse
secondary antibody (Dako, Glostrup, Denmark) at a 1:100 dilution at
room temperature for 2 h followed by further washing. The results
were assessed under a reflected light fluorescence microscope
(BH2-RFC, Olympus, Tokyo, Japan).
Cell viability assay
The MTT [3-(4, 5-dimethylthiazol-2-yl)-2,
5-diphenyltetrazolium bromide] assay was used to investigate the
cytotoxicity of the fusion protein. Breast cancer cells were plated
in 96-well plates (5×104 cells per well) in
septuplicate. The fusion proteins (including target protein and
mutant contrast) were added into the culture medium (RPMI-1640
medium supplemented with 10% fetal bovine serum) in different
concentrations (0.05 mg/ml, 0.1 mg/ml or 0.2 mg/ml). At the
indicated time, 20 μl aliquots of 5 mg/ml MTT (Sigma) in PBS
were added to each well and incubated for 4 h followed by the
addition of 150 μl of Me2SO. The A490
values were assayed in a Sunrise microplate reader (Tecan, Groedig,
Austria). Proliferation in vitro was also determined by
5-ethynyl-2-deoxyuridine (EdU, Ribobio, Guangzhou, China). EdU
incorporation was determined using a Cell-Light™ EdU Cell
Proliferation Kit (Ribobio), according to the supplier’s
instructions. The electronic microscope was used to observe the
ultrastructure of treated breast cancer cells.
Cell apoptosis analysis
Annexin V-FITC/PI staining was performed using the
Elite ESP flow cytometry (FACSCalibur, Becton-Dickinson
Immunocytometry Systems, San Jose, CA, USA) at 488 nm according to
the manufacturer’s guidelines. Briefly, cells were incubated with
PI and Annexin V-fluorescein isothiocynate in the darkness at room
temperature. Flow cytometric analysis was immediately performed for
apoptosis analysis and the data were analyzed using the Cell Quest
Pro software (BD Biosciences, San Jose, CA, USA). Transmission
electron microscope observation was carried out with the assistance
of the Laboratory Center of Electron Microscope from Fourth
Military Medical University.
Statistical analysis
Statistical analysis was performed using the SPSS
13.0 software package for Windows.
Results
Sequence synthesis, cloning, expression,
purification and identification
Using the recombinant DNA technology, the PCR
amplification fragments consistent with that expected for Grb2-SH2
(310 bp) and Grb2-SH2-Mutant (250 bp) were detected on an agarose
gel and the DNA sequences were confirmed by automatic sequencing.
The successful construction of the expressed recombinant
pET-16b-ptd/grb2-sh2 and pET-16b-ptd/grb2-sh2-Mutant
was confirmed by restriction mapping (Fig. 1A). In the pET system, target genes
are positioned downstream of the bacteriophage T7 late promoter.
Typically, products contain a prophage (λDE3) encoding the
highly processive T7 RNA polymerase under control of the
IPTG-inducible lacUV5 promoter that ensures tight control of
recombinant gene basal expression. SDS-PAGE showed the expression
of soluble proteins with a molecular mass value consistent with
that expected for PTD-Grb2-SH2 (17.6 kDa) and PTD-Grb2-SH2-Mutant
(16 kDa). Recombinants PTD-Grb2-SH2 and PTD-Grb2-SH2-Mutant were
expressed in E. coli after induction of IPTG. In order to
obtain maximum soluble protein expression, we adjusted conditions,
including temperature and time. Finally, we determined that a
temperature of 33°C and induction time of 4 h was optimal for
obtaining maximum amount of soluble protein (Fig. 1B). HisTrap™ Ni++ charged
columns (Amersham Pharmacia, Piscataway, NJ, USA) were used to
purify the His-tagged proteins. Purification products were analyzed
by 15% SDS-PAGE and identified by western blot analysis (Fig. 1C).
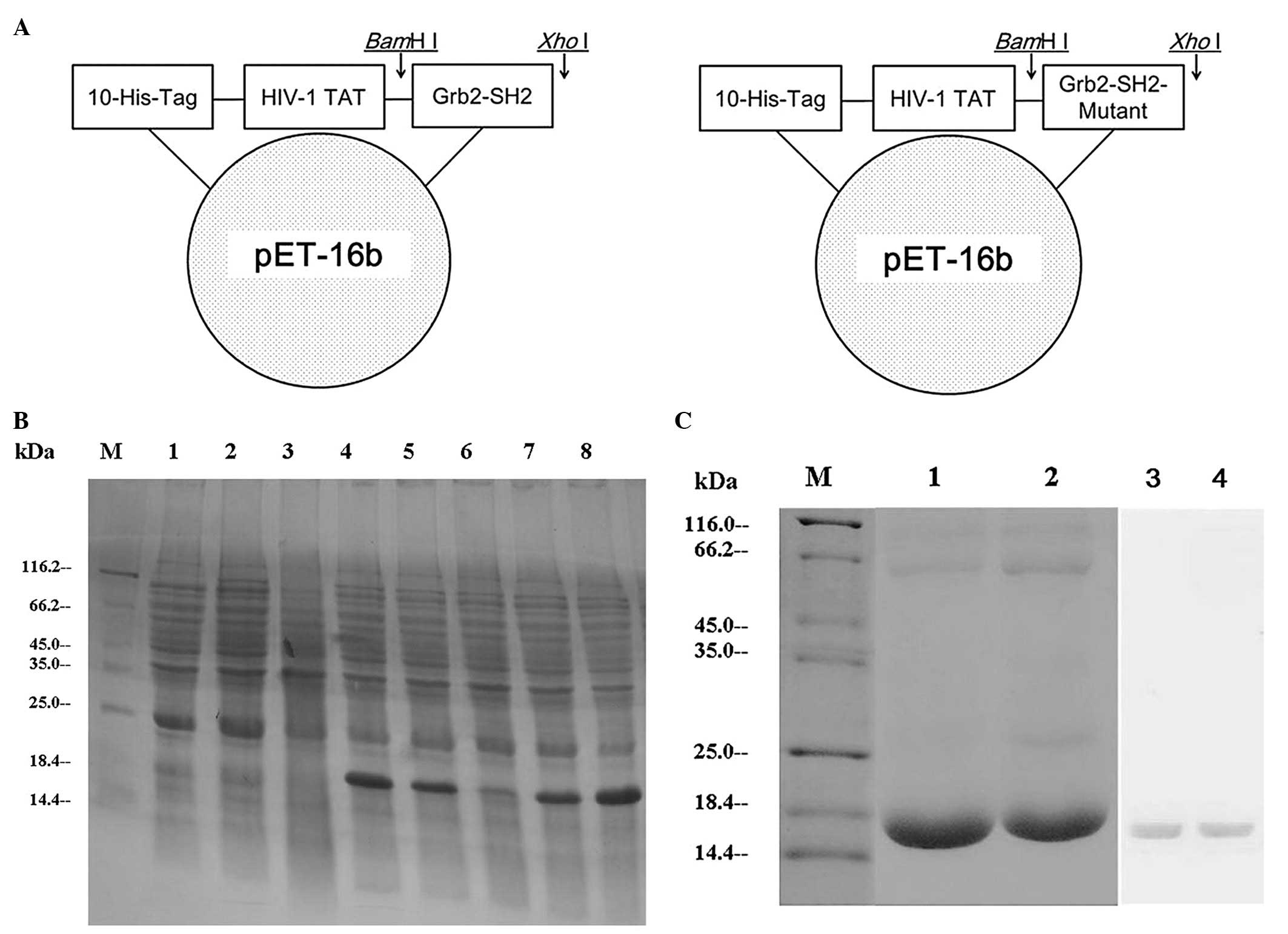 | Figure 1Construction, expression and
purification of PTD-Grb2-SH2 and PTD-Grb2-SH2-Mutant. (A) The
Grb2-SH2 coding frame is presented with 10 histidines and the HIV-1
TAT domain. (B) The expression of the recombinants. Lane M, protein
molecular weight marker; lane 1, E. coli BL21(DE3) lysates
after IPTG; lane 2, pET-16b plasmid after IPTG; lane 3,
pET-16b-ptd/grb2-sh2 before IPTG; lane 4,
pET-16b-ptd/grb2-sh2 recombinant after IPTG; lane 5, the
crude lysate supernatant of induced pET-16b-ptd/grb2-sh2
after IPTG; lane 6, pET-16b-ptd/grb2-sh2-Mutant before IPTG;
lane 7, pET-16b-ptd/grb2-sh2-Mutant after IPTG; lane 8, the
crude lysate supernatant of induced
pET-16b-ptd/grb2-sh2-Mutant after IPTG. (C) The purification
and identification of the recombinants. Lane M, protein molecular
weight marker; lane 1, the purified PTD-Grb2-SH2-Mutant fusion
protein; lane 2, the purified PTD-Grb2-SH2 fusion protein; lane 3,
the purified PTD-Grb2-SH2-Mutant fusion protein was identified by
western blot analysis; lane 4, the purified PTD-Grb2-SH2 fusion
protein was identified by western blot analysis. |
Transduction of the recombinants into
breast cancer cell line MDA-MB-231
An immunofluorescence assay with an anti-His-tag
antibody was used to investigate whether the PTD-Grb2-SH2 and
PTD-Grb2-SH2-Mutant could be transduced into living cells as
designed. Purified PTD-Grb2-SH2 and PTD-Grb2-SH2-Mutant were added
to cultured MDA-MB-231 cells for 2 h of incubation. Then, both
recombinants were observed in the cells under a fluorescent
microscope (Fig. 2). As shown in
Fig. 2B and D, the recombinants
including PTD-Grb2-SH2 and PTD-Grb2-SH2-Mutant were dispersed
throughout the cytoplasm and mainly located in the nucleus,
indicating HIV-1 TAT48-60 (GRKKRRQRRRPPQ) helped the target
peptides to pass through both the cellular and nuclear membranes in
living cells as reported (22).
Growth inhibition of PTD-Grb2-SH2 in the
breast cancer cell lines MDA-MB-231 and SK-BR-3
The fresh recombinants of PTD-Grb2-SH2 and
PTD-Grb2-SH2-Mutant were incubated with the breast cancer cell
lines MDA-MB-231 and SK-BR-3 to investigate whether the new
proteins could affect the proliferation of breast cancer cells
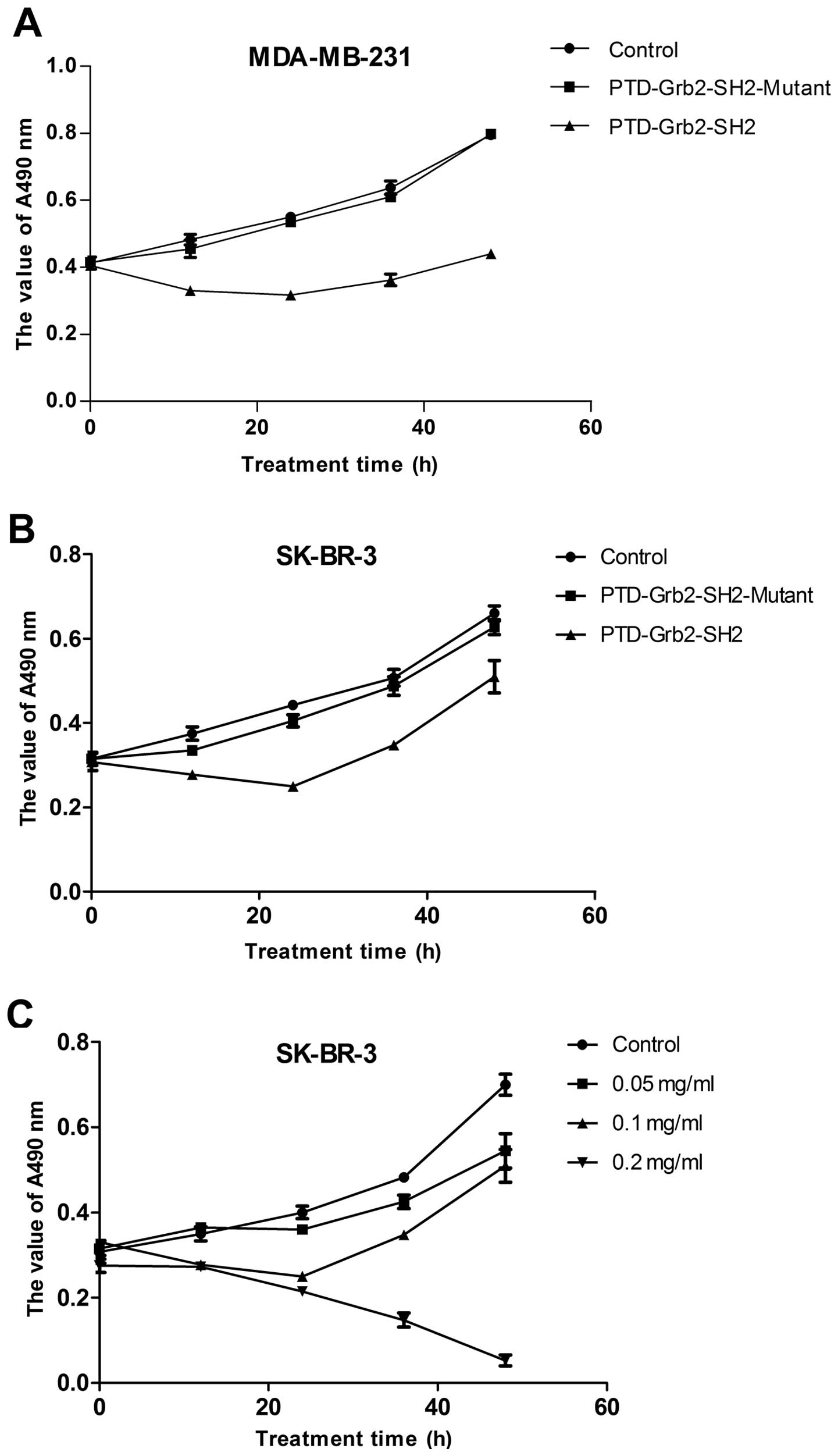
in vitro. Fig. 3A and B
show that the proliferation of MDA-MB-231 was inhibited by
PTD-Grb2-SH2 in a time-dependent manner, but not by the mutant
protein. Due to the similar inhibition effects of PTD-Grb2-SH2 in
two different breast cancer cell lines, we chose the SK-BR-3 cells
to look for the optimal concentration. Fig. 3C reveals that the recombinant
PTD-Grb2-SH2 exhibited significant toxicity to breast cancer cells
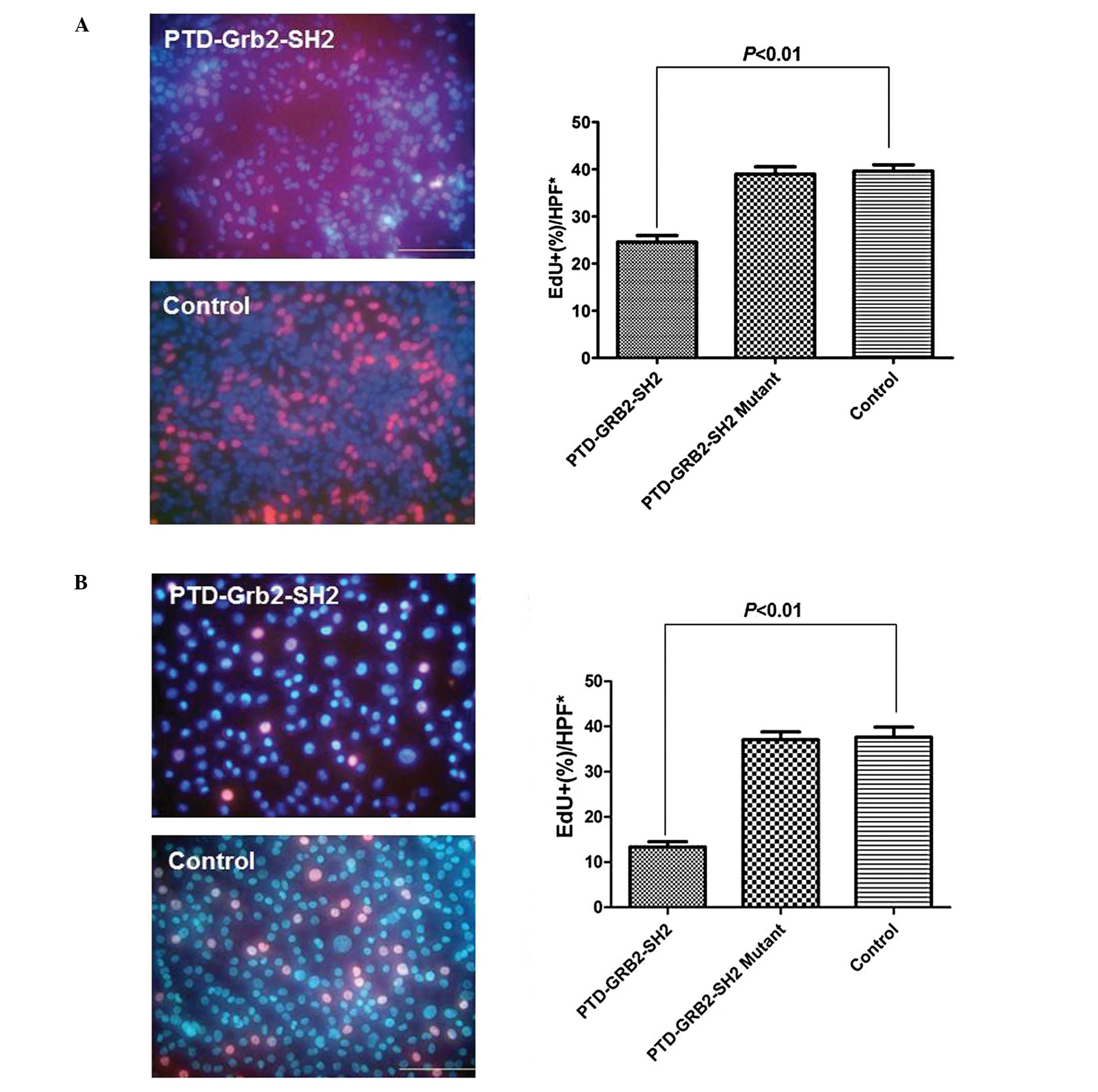
in a dose- and time-dependent manner in vitro. EdU
identified the proliferation rates of MDA-MB-231 and SK-BR-3 cells.
After incubation with PTD-Grb2-SH2 (0.1 mg/ml) for 12 h, a
decreased rate of cell proliferation was detected in MDA-MB-231
cells, compared to the untreated group (24.6±1.4 vs. 39.6±1.3%,
P<0.01). The proliferation rate of SK-BR-3 cells was also found
to be decreased in the treated group, compared to the control group
(13.4±1.1 vs. 37.6±2.2%, P<0.01). However, there is no
difference between the cells treated with PTD-Grb2-SH2-Mutant (0.1
mg/ml) and control in either breast cancer cell line (Fig. 4).
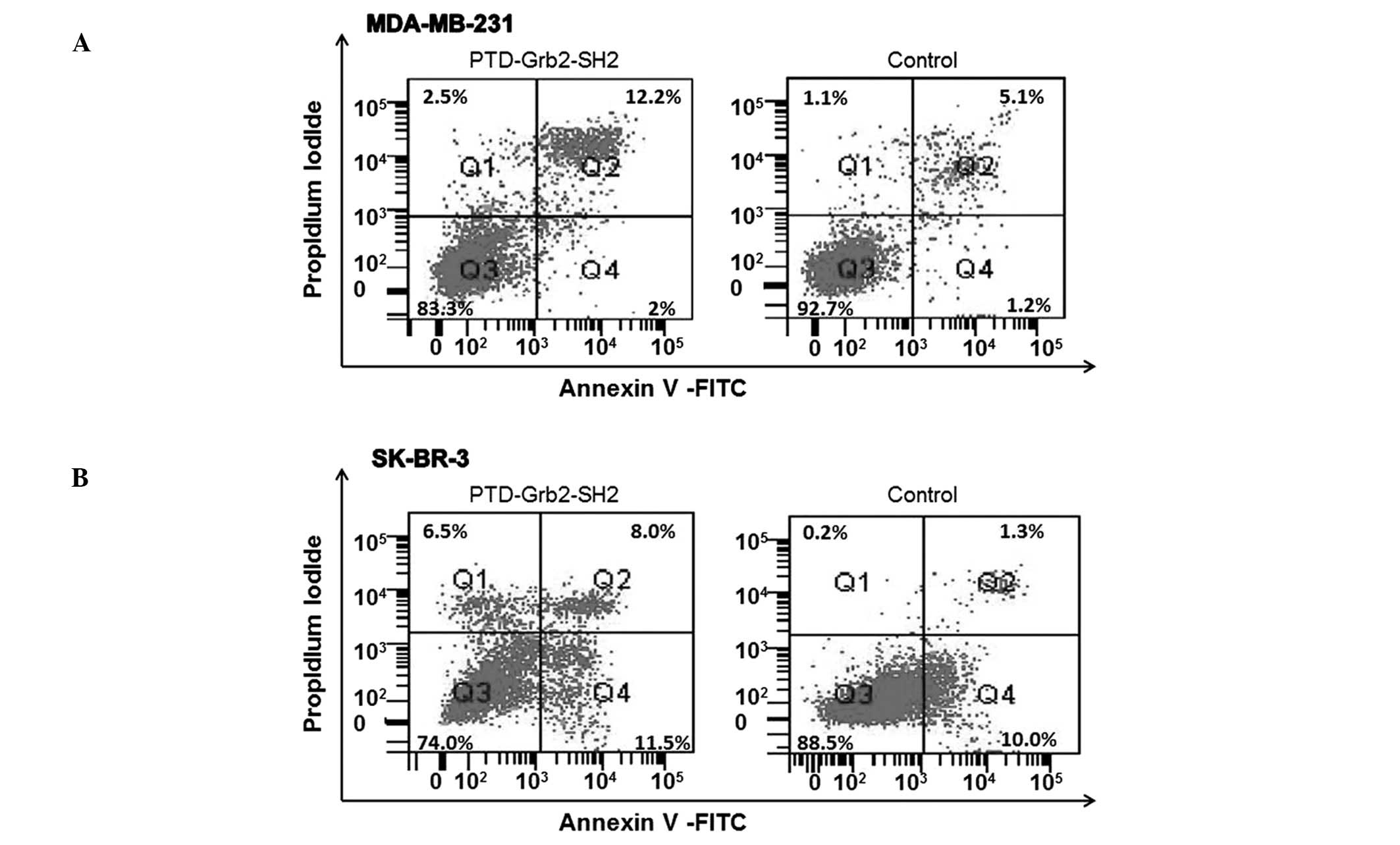
PTD-Grb2-SH2 induces apoptosis in
MDA-MB-231 and SK-BR-3 cells
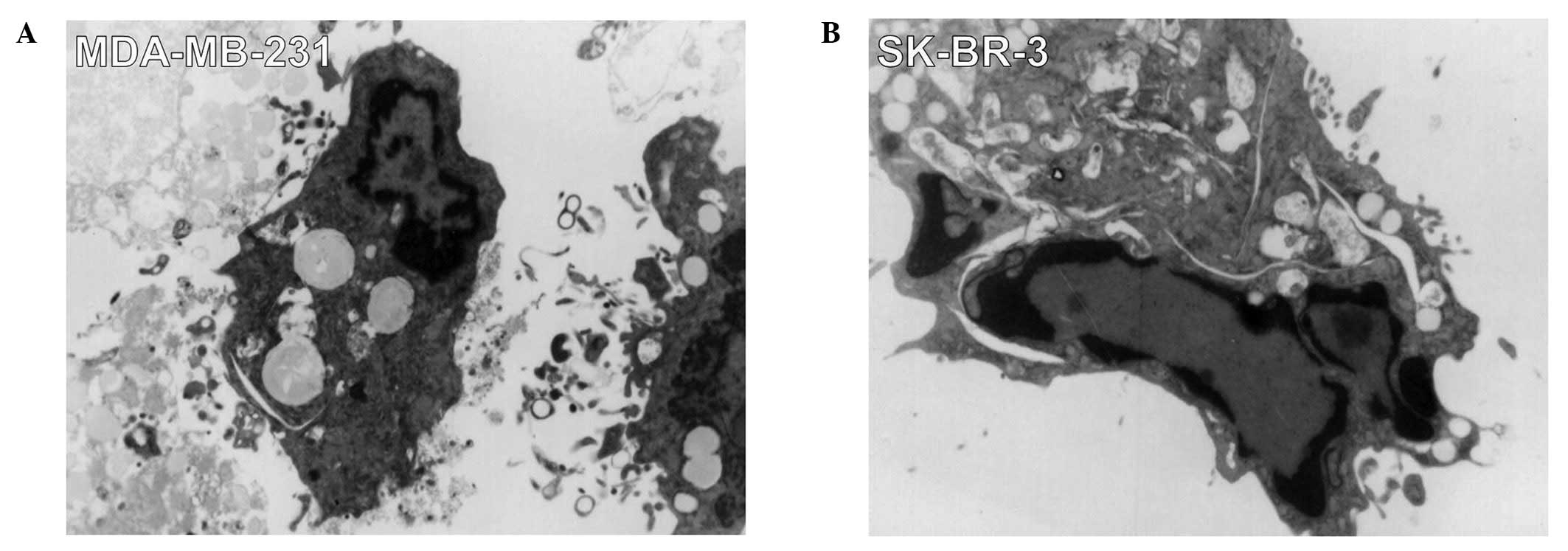
By light microscopy, after 12 h incubation with the
fusion protein (0.1 mg/ml), many breast cancer cells began to
shrink and lose their normal fibroblast-like shape compared with
the untreated cells. We gathered the treated cells to be observed
under an electron microscope. Characteristic forms associated with
cellular apoptosis could be observed, including the shrinkage of
cellular and nuclear membranes and the appearance of many
high-density structures and vesicles in MDA-MB-231 and SK-BR-3
cells were observed. Many vesicles appeared in the cytoplasm and
the chromosome condensed into armillary shapes and concentrated
beneath karyotheca, resulting in crescent or ring shapes (Fig. 5). We identified these changes as
apoptotic phenomena, suggesting that PTD-Grb2-SH2 may induce
apoptosis in breast cancer cells. To further determine the
cytotoxicity of PTD-Grb2-SH2 on cell proliferation, MDA-MB-231 and
SK-BR-3 cells were exposed to PTD-Grb2-SH2 (0.1 mg/ml) for 12 h. In
order to differentiate this from necrosis and to confirm it as
apoptosis, we performed fluorescein-conjugated Annexin V (Annexin
V-FITC) flow cytometry. We quantitated the number of cells
undergoing apoptosis. Our results showed that PTD-Grb2-SH2 induced
apoptosis in 14.2% of MDA-MB-231 cells and 19.5% of SK-BR-3 cells,
compared to the controls (6.3 and 11.3%, respectively) (Fig. 6).
Discussion
Although the discovery and characterization of HER2
and herceptin have resulted in great progress in breast cancer
treatment, many patients still eventually relapse. Consequently,
there is an urgent need for additional therapeutic strategy other
than HER2 signaling pathway. Grb2 is an important adapter protein
and the first trigger for many cellular signal pathways involved in
the processes of cell proliferation and mitogenesis (23). Blocking the interaction between
pTyr-containing activated receptors and the SH2 domain of Grb2 is
considered to be an effective and non-cytotoxic strategy in the
development of new anti-proliferate agents due to its potential to
shut down the Ras activation pathway (24). This makes the Grb2-SH2 domain an
ideal target for breast cancer treatment.
In this study, we designed, constructed, expressed
and purified a fusion protein that contained a PTD domain and a
Grb2-SH2 domain. The PTD domain is considered to deliver proteins
of more than 120 kDa into living cells and tissues (25,26).
Fusion proteins containing HIV-1 TAT have been reported to be
successfully transduced into tumor cells and applied for anticancer
therapy (27–29). We chose TAT48–60
(GRKKRRQRRRPPQ) as the PTD domain for transduction because this
motif is the smallest one carrying PTD function without interfering
with the target protein’s function (22). Our data confirmed that both the
fusion protein and mutant protein successfully passed through the
cellular and nuclear membranes of living breast cancer cells with
the help of PTD domain. TAT48-60 delivery is a convenient research
tool to study the function of small target peptides or small
proteins in living cells. In previous studies we used this PTD
domain to successfully deliver SH3 domains into the leukemia cell
line K562 and the hepatocarcinoma cell line HepG-2 (30,31).
The Grb2-SH2 domain is a highly preserved domain in both prokaryote
and eukaryote, and the fusion proteins we designed are small
proteins with molecular weight less than 20 kDa. Although the
prokaryotic expression system we chose is not a theoretically
proper expression system for a eukaryotic protein, we confirmed the
right construction by automatic sequencing and identified the
target proteins by western blot analysis to prevent
miss-construction and expression.
In function assay, we directly incubated the
recombinants with HER2-negative MDA-MB-231 and HER2-positive
SK-BR-3 to test the effects on the proliferation of breast cancer
cells. Our data exhibited that PTD-Grb2-SH2 inhibited the
proliferation of both breast cancer cell lines, but the
PTD-Grb2-SH2-Mutant did not exert any inhibition to these cell
lines, indicating the loss in basic sequence of Grb2-SH2 will
result in dysfunction of the target protein. Our results also
revealed that PTD-Grb2-SH2 exhibited significant toxicity to breast
cancer cells in a dose- and time-dependent manner in vitro,
which is an appropriate characteristic for anticancer drug
developing. To date, many strategies have been used to inhibit the
function of Grb2 in order to block crucial intracellular signals.
Some studies have focused on designing and synthesizing
phosphotyrosines or their mimics based upon space structures of
Grb2 (32). Grb2 is made up of one
SH2 domain surrounded by two SH3 domains. While both SH2 and SH3
domains possess a strong ability to recognize and specifically bind
to their ligands, SH2 domains are the major known binding modules
for tyrosine-phosphorylated proteins and are the prototype for
protein-protein interactions that mediate the formation of
multi-proteins complexes during signaling (33). Integrating these basic results with
our data, we think that the strength and specificity of Grb2-SH2
for its ligands give this domain the ability of inhibiting Grb2
related signaling in living breast cancer cells.
We chose HER2-negative MDA-MB-231 and HER2-positive
SK-BR-3 in the function assay as Grb2 intermediates the
mitogen-activated protein kinase (MAPK) pathway and also regulates
receptor trafficking (34–36). It works with various RTKs, with
EGFR being its major binding partner (37,38),
which regulates many cell functions. Our data showed PTD-Grb2-SH2
inhibited HER2-negative and -positive breast cancer cells,
indicating this fusion protein possesses non-specificity for single
pathway. This is also the reason why we did not test the downstream
molecules or pathways for this protein. There are too many
molecules and pathways associated with Grb2 that remain to be
elucidated in further experiments. Nevertheless, we still used
electron microscopy and flow cytometry assay to identify the
apoptotic phenomena induced by PTD-Grb2-SH2.
In conclusion, our study successfully constructed a
target fusion protein expressing both PTD and Grb2-SH2 domains and
showed that expression of the fusion protein resulted in growth
inhibition and cell death in breast cancer cells regardless of
HER2-phenotype. We proved that the TAT48–60 is a useful
tool and could be capable of delivering outside proteins through
the plasma membrane of living cells, and even delivering a protein
directly to the cell nucleus. This technique will help us to
demonstrate protein function in living cells. We also demonstrated
that the SH2 domain is a highly conserved protein functional domain
and can maintain its biological activity even when expressed in
bacteria. The extent of inhibition depend on the concentration of
the protein and the length of the time, moreover, it suggested that
the protein might induce apoptosis in breast cancer cells. These
results indicate that the PTD-Grb2-SH2 protein has the potential to
be developed for treatment of breast cancer.
Acknowledgements
This study was supported by grant no.
30901457 from National Natural Science Foundation of China.
References
|
1
|
DeSantis C, Siegel R, Bandi P and Jemal A:
Breast cancer statistics, 2011. CA Cancer J Clin. 61:409–418. 2011.
View Article : Google Scholar
|
|
2
|
Harris AL, Nicholson S, Sainsbury JR,
Farndon J and Wright C: Epidermal growth factor receptors in breast
cancer: association with early relapse and death, poor response to
hormones and interactions with neu. J Steroid Biochem. 34:123–131.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sainsbury JR, Malcolm AJ, Appleton DR,
Farndon JR and Harris AL: Presence of epidermal growth factor
receptor as an indicator of poor prognosis in patients with breast
cancer. J Clin Pathol. 38:1225–1228. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nielsen TO, Hsu FD, Jensen K, et al:
Immunohistochemical and clinical characterization of the basal-like
subtype of invasive breast carcinoma. Clin Cancer Res.
10:5367–5374. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Slamon DJ, Godolphin W, Jones LA, et al:
Studies of the HER-2/neu proto-oncogene in human breast and ovarian
cancer. Science. 244:707–712. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Foster CS, Gosden CM and Ke Y: HER2/neu
expression in cancer: the pathologist as diagnostician or prophet?
Hum Pathol. 34:635–638. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rosen PP, Lesser ML, Arroyo CD, Cranor M,
Borgen P and Norton L: Immunohistochemical detection of HER2/neu in
patients with axillary lymph node negative breast carcinoma. A
study of epidemiologic risk factors, histologic features, and
prognosis. Cancer. 75:1320–1326. 1995. View Article : Google Scholar
|
|
8
|
Carlomagno C, Perrone F, Gallo C, et al:
c-erb B2 overexpression decreases the benefit of adjuvant tamoxifen
in early-stage breast cancer without axillary lymph node
metastases. J Clin Oncol. 14:2702–2708. 1996.
|
|
9
|
Gusterson BA, Gelber RD, Goldhirsch A, et
al: Prognostic importance of c-erbB-2 expression in breast cancer.
International (Ludwig) Breast Cancer Study Group. J Clin Oncol.
10:1049–1056. 1992.PubMed/NCBI
|
|
10
|
Bianco R, Melisi D, Ciardiello F and
Tortora G: Key cancer cell signal transduction pathways as
therapeutic targets. Eur J Cancer. 42:290–294. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chardin P, Cussac D, Maignan S and Ducruix
A: The Grb2 adaptor. FEBS Lett. 369:47–51. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Seger R and Krebs EG: The MAPK signaling
cascade. FASEB J. 9:726–735. 1995.PubMed/NCBI
|
|
13
|
Di Fulvio M, Henkels KM and
Gomez-Cambronero J: Short-hairpin RNA-mediated stable silencing of
Grb2 impairs cell growth and DNA synthesis. Biochem Biophys Res
Commun. 357:737–742. 2007.PubMed/NCBI
|
|
14
|
Machida K and Mayer BJ: The SH2 domain:
versatile signaling module and pharmaceutical target. Biochim
Biophys Acta. 1747:1–25. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pawson T: Protein modules and signalling
networks. Nature. 373:573–580. 1995. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mayer BJ, Jackson PK and Baltimore D: The
noncatalytic src homology region 2 segment of abl tyrosine kinase
binds to tyrosine-phosphorylated cellular proteins with high
affinity. Proc Natl Acad Sci USA. 88:627–631. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mayer BJ, Jackson PK, Van Etten RA and
Baltimore D: Point mutations in the abl SH2 domain coordinately
impair phosphotyrosine binding in vitro and transforming activity
in vivo. Mol Cell Biol. 12:609–618. 1992.PubMed/NCBI
|
|
18
|
Schwarze SR, Hruska KA and Dowdy SF:
Protein transduction: unrestricted delivery into all cells? Trends
Cell Biol. 10:290–295. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lindgren M, Hallbrink M, Prochiantz A and
Langel U: Cell-penetrating peptides. Trends Pharmacol Sci.
21:99–103. 2000. View Article : Google Scholar
|
|
20
|
Liang Y, Sun Q, Jiang S, et al:
Construction and expression of a vector containing protein
transduction domain and bcr/abl fusion gene. Zhonghua Xue Ye Xue Za
Zhi. 23:5–8. 2002.(In Chinese).
|
|
21
|
Laemmli UK: Cleavage of structural
proteins during the assembly of the head of bacteriophage T4.
Nature. 227:680–685. 1970. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sangadala S, Okada M, Liu Y, Viggeswarapu
M, Titus L and Boden SD: Engineering, cloning, and functional
characterization of recombinant LIM mineralization protein-1
containing an N-terminal HIV-derived membrane transduction domain.
Protein Expr Purif. 65:165–173. 2009. View Article : Google Scholar
|
|
23
|
Yarden Y and Sliwkowski MX: Untangling the
ErbB signalling network. Nat Rev Mol Cell Biol. 2:127–137. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lung FD and Tsai JY: Grb2 SH2
domain-binding peptide analogs as potential anticancer agents.
Biopolymers. 71:132–140. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim D, Jeon C, Kim JH, et al: Cytoplasmic
transduction peptide (CTP): new approach for the delivery of
biomolecules into cytoplasm in vitro and in vivo. Exp Cell Res.
312:1277–1288. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Schwarze SR, Ho A, Vocero-Akbani A and
Dowdy SF: In vivo protein transduction: delivery of a biologically
active protein into the mouse. Science. 285:1569–1572. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tan M, Lan KH, Yao J, et al: Selective
inhibition of ErbB2-overexpressing breast cancer in vivo by a novel
TAT-based ErbB2-targeting signal transducers and activators of
transcription 3-blocking peptide. Cancer Res. 66:3764–3772. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Harada H, Kizaka-Kondoh S and Hiraoka M:
Antitumor protein therapy; application of the protein transduction
domain to the development of a protein drug for cancer treatment.
Breast Cancer. 13:16–26. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sorriento D, Campanile A, Santulli G, et
al: A new synthetic protein, TAT-RH, inhibits tumor growth through
the regulation of NFkappaB activity. Mol Cancer. 8:972009.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liang YJS, Han H, Liu L, Sun Q, Chen R, Wu
R, Du J and Li Q: TAT PTD-BCR/ABL SH3 fusion protein induces the
apoptosis of K562 leukemic cell line. J Fourth Mil Med Univ.
23:42002.
|
|
31
|
Yin JK, Liang YM, He XL, et al: Fusion
protein containing SH3 domain of c-Abl induces hepatocarcinoma
cells to apoptosis. Hepatol Res. 37:454–463. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ogura K, Shiga T, Yokochi M, Yuzawa S,
Burke TR Jr and Inagaki F: Solution structure of the Grb2 SH2
domain complexed with a high-affinity inhibitor. J Biomol NMR.
42:197–207. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dierck K, Machida K, Mayer BJ and Nollau
P: Profiling the tyrosine phosphorylation state using SH2 domains.
Methods Mol Biol. 527:131–155. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jiang X, Huang F, Marusyk A and Sorkin A:
Grb2 regulates internalization of EGF receptors through
clathrin-coated pits. Mol Biol Cell. 14:858–870. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yamazaki T, Zaal K, Hailey D, Presley J,
Lippincott-Schwartz J and Samelson LE: Role of Grb2 in
EGF-stimulated EGFR internalization. J Cell Sci. 115:1791–1802.
2002.PubMed/NCBI
|
|
36
|
Burke P, Schooler K and Wiley HS:
Regulation of epidermal growth factor receptor signaling by
endocytosis and intracellular trafficking. Mol Biol Cell.
12:1897–1910. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Schulze WX, Deng L and Mann M:
Phosphotyrosine inter-actome of the ErbB-receptor kinase family.
Mol Syst Biol. 1:2005.0008. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Seiden-Long I, Navab R, Shih W, et al:
Gab1 but not Grb2 mediates tumor progression in Met overexpressing
colorectal cancer cells. Carcinogenesis. 29:647–655.
2008.PubMed/NCBI
|