Introduction
Cancer which performs progressive accumulation of
genetic and epigenetic abnormalities, is a result of very complex
multistep disorder. Six essential properties of cancer distinguish
them from normal cells. Autocrine production of growth signals,
resistance to apoptotic mechanisms, sustained angiogenesis,
limitless replicative potential, tissue invasion and metastasis,
and apoptosis avoidance (1).
Resistance to apoptosis displays the very essential role among
these various properties of cancer cells in tumor development and
progression. Evading apoptosis of cancer cells is related with
their various biochemical properties. particularly, the
upregulation of members of the inhibitor of apoptosis (IAP) family
of proteins (2). Currently, there
are two well-known apoptotic pathways, one initiated through the
engagement of cell surface death receptors by their specific
ligands (3) and the intrinsic
pathway also called the mitochondrial pathway is triggered by
changes in internal cellular integrity, which is induced by several
different stimuli such as antineoplastic drugs, hypoxia,
irradiation, growth factor withdrawal and heat shock (4). Both pathways eventually result in
activation of caspases, cysteine proteases that comprise the
effector arm of the apoptotic process (5). Radiation and other agents induce
caspase activation mainly via the mitochondrial pathway, which
involves mitochondrial integration of apoptotic signals and
subsequent release of cytochrome c and Smac (the second
mitochondria-derived activator of caspase)/DIABLO (direct IAP
binding protein with low pl), Omi/HtrA2 and AIF into the cytosol
(6). This release allows the
assembly of the apoptosome (7).
The apoptosome activates caspase-9, which in turn induces the
activation of caspase-3, -6 and -7 (8). The effector caspases cleave their
cellular specific substrates and generate the typical morphology of
apoptosis.
Inhibitor of apoptosis proteins (IAPs) can inhibit
apoptosis by interacting with and then regulating the ability of
caspase-8 or caspase-9, -3 and -7 (9,10).
c-IAP1 (cellular IAP-1), c-IAP2 (cellular IAP-2) and XIAP (X-linked
IAP) are three important members of IAPs, especially XIAP, which
has many domains interacting with different caspases such as
caspase-3, -7 and -9 (11,12) and its BIR2 domain inhibits
caspase-7 in a non-competitive manner (11). XIAP is highly expressed in many
malignant tumors and has been associated with refractory disease
and poor prognosis (13,14). Because XIAP blocks apoptosis at the
effector phase, a point where multiple signaling pathways converge,
treatments targeting XIAP may prove to be especially effective to
overcome resistance. Smac was identified as a protein which can
antagonize the inhibiting activity of IAPs to apoptosis after
release from mitochondria in response to apoptotic stimulin
(15–19). It has been proven that the domain
of Smac interacts with IAPs is a particular NH2-terminal residue
consisting of four amino acids, Ala-Val-Pro-Ile (18,20).
It has been demonstrated that the AVPI sequence of Smac/DIABLO
interacts with the BIR2 and BIR3 domains of XIAP, allowing the
release of caspase-3 (18) and
caspase-9 (17), respectively.
Through this mechanism, SMAC/ DIABLO prevents the sequestration of
caspases by IAPs, thus facilitating the apoptotic pathway. Since
the AVPI sequence is able to promote apoptosis, many Smac peptides
that are able to mimic this tetrapeptide are synthesize and studied
(21–26). We studied the Tat-SmacN7 protein to
promote apoptosis of tumor cells.
Smac peptides can sensitize glioblastoma cells for
TRAIL-induced apoptosis in vitro and in vivo by
antagonizing XIAP (27). In
addition, the genetic or pharmacologic inactivation of XIAP
increases radiation-induced apoptosis in glioblastoma,
neuroblastoma and pancreatic carcinoma cells (21–23).
SM-164, a potent and well-characterized SMAC mimetic is an
effective radiosensitizer both in vitro and in vivo
of HNSCC cells by eliminating cIAP-1 (24–29).
To translate the concept of improving the activity of caspases for
radiosensitization into a clinically applicable approach to improve
the efficiency of radiotherapy in esophageal carcinoma and the
radioresistant human non-small cell lung cancer (NSCLC), we
investigated in this study the therapeutic potential of Tat-SamcN7
for radiosensitization of Ec109 cells and H460 cells.
Materials and methods
The human esophageal cell line (EC109)
and non-small cell lung cancer (NSCLC) cell line NCI-H460
(H460)
Human esophageal cell line (EC109) was generously
provided by the General Hospital of TianJin Medical University and
human non-small cell lung cancer (NSCLC) NCI-H460 cells were
purchased from Cell Culture Center of Chinese Academy of Medical
Science and Peking Union Medical College. Cells were grown in
25-cm2 flasks containing 3 ml of 1X RPMI-1640 (Hyclone,
China) supplemented with 10% fetal bovine serum (FBS) and 1%
penicillin and 1% streptomycin in a humidified incubator containing
5% CO2 at 37°C. Cell lines were serially passaged
following trypsinization using a trypsin/EDTA solution (Gibco).
Estimation of peptide
internalization
To test the internalization efficiencies of the
synthesized peptides, FITC-SmacN7 or FITC-Tat-SmacN7 at the
concentration of 20 μM, were added into the medium and
continued to incubate for different time periods (2 and 24 h).
Cells were washed three times with PBS and stained with DAPI. The
distribution of fluorescein-labeled peptides was analyzed by a
Leica DMI3000B fluorescence microscope.
WST-1 growth assay
Cells were seeded in 96-well plates and treated with
Tat-SmacN7 peptides at various doses (10, 50, 100 and 500 nM, 1, 5,
10 and 100 μM) for 24 h. WST-1 (Beyotime, China) was added
to the microplates 4 h before measuring the cell viability using
the Multi-Mode Microplate Reader (Synergy HT, BioTek).
Radiation exposure and clonogenic
assay
The clonogenic colony formation assay was done on
single-cell suspension. Briefly, cells were seeded in RPMI-1640
medium into 6-well plates (Corning, China), after adherence to the
flask the next day, cells were treated with Tat-SmacN7 peptides at
the concentration of 20 μM, 4 h later, cells were irradiated
at room temperature in different doses of radiation (0, 2, 4 and 6
Gy) with Cr137r-ray (Atomic Energy of Canada) at a dose
rate of 0.71 116 Gy/min. After 10–14 days, colonies were stained
with gentian violet solution for 3 min and counted. Calculation of
survival fractions (SF) was done using the equation SF = colonies
counted/cells seeded × (PE/100), taking into consideration the
individual plating efficiency (PE). All experiments were repeated
at least 2 times.
Annexin V-FITC/PI staining and flow
cytometry for determination of apoptosis
Cells from single and combination treatments were
harvested and processed with a FITC Annexin V Apoptosis Detection
kit 1 (BD Biosciences) for flow cytometric analysis for
quantitative determination of apoptosis.
Western blot analysis
Western blot analysis was done using the following
antibodies: rabbit anti-XIAP from Abcam (AB21278), mouse
anti-β-actin (CW0623A, Shanghai, China), goat anti-rabbit IgG and
goat anti-mouse IgG conjugated to horseradish peroxidase (ZSGB,
Biotechnology).
RNA extraction, cDNA synthesis and
quantitative real-time PCR
Total RNA was purified and carried out as described
previously (30). Equal amounts of
total RNA was reverse-transcribed using PrimeScript RT reagent kit
(Takara Co., Japan) according to the manufacturer’s instructions.
cDNA samples were mixed with primers and SYBR Master Mix (Life
Technologies) in a total volume of 25 μl. All samples were
analyzed in triplicate using an ABI PRISM 7500 Sequence Detection
system (Applied Biosystems-Life Technologies). The threshold cycle
(CT) values for each reaction were determined and
averaged using the TaqMan SDS analysis software (Applied
Biosystems-Life Technologies). The changes in the expression of a
target gene were calculated by the comparative CT method
(fold changes = 2[−ΔΔCT]). PCR primers for the
caspase-3, -8 and -9 and the housekeeping gene GAPDH were obtained
from Sangon Biotech (Shanghai, China). The specific primer pairs
used were: CASP3, 5′-ATCACAGCAAAAG GAGCAGTTT-3′ (forward) and
5′-ACACCACTGTCTGTC TCAATGC-3′ (reverse); CAPS8, 5′-TAGGGACAGGAATGG
AACACA-3′ (forward) and 5′-TGGGAGAGGATACAGCAG ATG-3′ (reverse);
CASP9, 5′-TCTGGAGGATTTGGTGAT GTC-3′ (forward) and
5′-CATTTTCTTGGCAGTCAGGTC-3′ (reverse); GAPDH,
5′-ATGACATCAAGAAGGTGGTG-3′ (forward) and 5′-CATACCAGGAAATGAGCTTG-3′
(reverse).
Caspase activation assay
The activity of caspase-3, -8 and -9, was analyzed
using a fluorogenic caspase assay with Ac-DEVD-AFC, Ac-IETD-AFC,
Ac-LEHD-AFC (BD Pharmingen) as a substrate, respectively (31). The results are expressed as fold
change compared to the control.
Human esophageal ectopic xenografts in
athymic nude mice
Six-week-old athymic nude mice were purchased from
Vital River (Beijing, China) and housed in the certified animal
facility in the Institute of Radiation Medicine, Chinese Academy of
Medical Sciences. All experimental procedures were performed
according to the Guide for the Care and Use of Laboratory Animals
published by the US National Institutes of Health (NIH Publication
No. 85-23, revised 1996). For developing ectopic human esophageal
cancer xenografts, Ec109 cells (5×106) in 100 μl
of PBS (1:1) were implanted by subcutaneous (s.c.) injection on the
right leg of each mouse. Tumors were measured using an external
caliper and volume was calculated by the formula: π/6 × length ×
width × height. The mice were randomly divided into four groups:
control (cont), Tat-SmacN7, radiation, Tat-SmacN7 combined
radiation. The treatment started when the tumor size reached 100
mm3 at day 10 after tumor inoculation. Animals in
control group did not receive any therapy. Tat-SmacN7 peptides (1
mg/kg) were administered locally twice at day 10 and 12 after tumor
inculation, radiation (2 Gy) were given once a day, 10 days in all.
The same nude mice were anaesthetized using chloral hydrate
solution (0.35 g/kg) intraperitoneally (i.p.) before radiation was
delivered directly to the tumor with the rest of the animal
shielded. For combination treatment, Tat-SmacN7 was given 30 min
prior to radiation exposure. The tumor growth was measured once
every other day, from at least 6 mice in each group.
Results
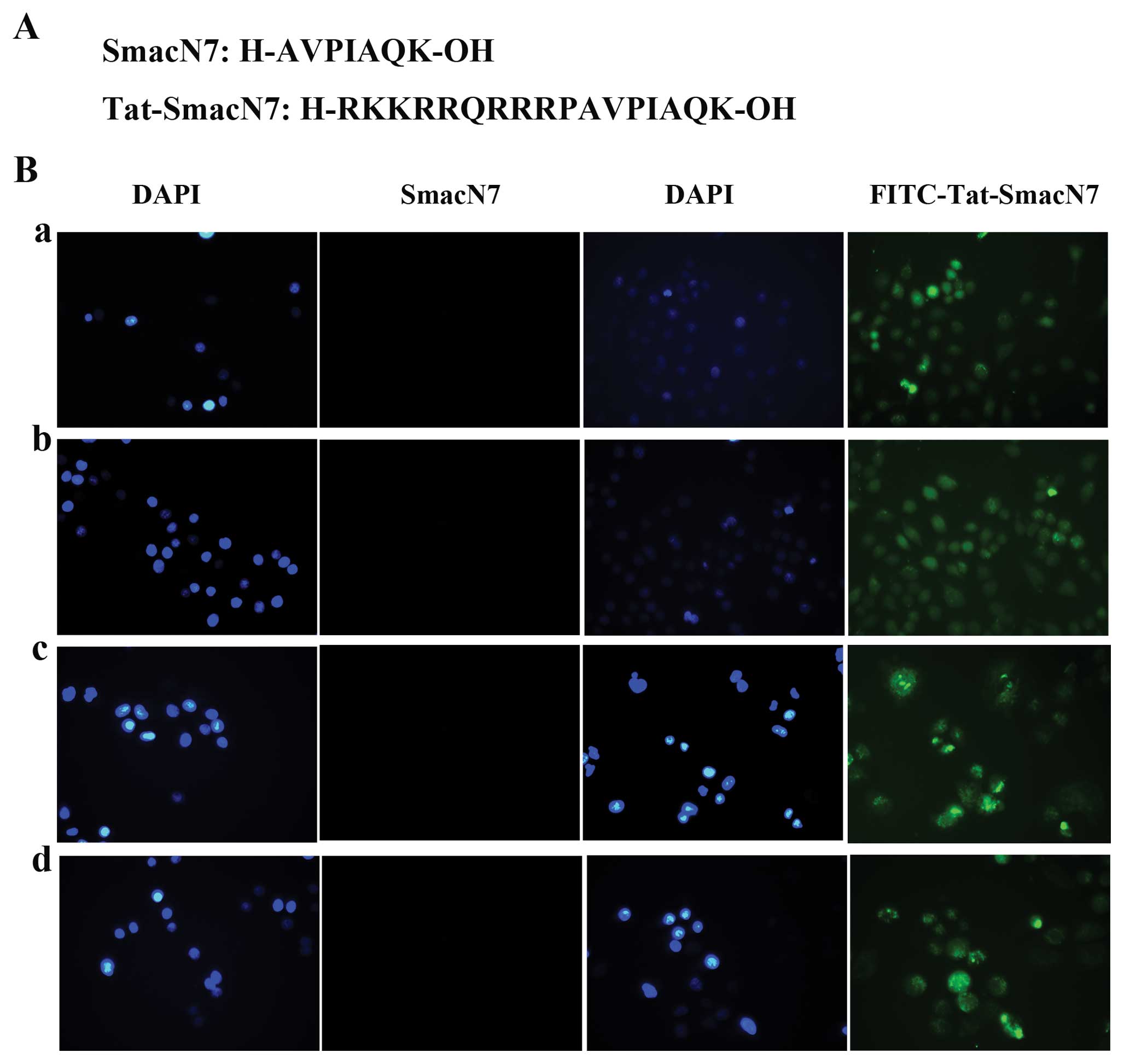
Tat domain-conjugated NH2 terminus of
Smac acts as a cell-permeable compound
Before our investigation into whether synthetic Smac
peptides containing the four N-terminal residues are essential for
XIAP inactivation and would induce apoptosis in tumor cells in a
manner similar to the cytosolic active form of Smac (25), we conducted the NH2-terminal Smac
peptide into cells. To address this question, we used the
Tat-SmacN7 which linked the seven N-terminal residues of the Smac
protein to the protein-transduction domain of the TAT protein to
facilitate intracellular delivery. Cellular uptake of Smac peptides
was estimated by fluorescence microscope (Leica DMI3000B). We saw
that without the Tat residues, SmacN7 did not accumulate in cells,
but Tat-SmacN7 showed sufficient amount of accumulation in cells
after being added at 2 h and Tat-SmacN7 is accumulated in cells
still after being added at 24 h (Fig.
1B).
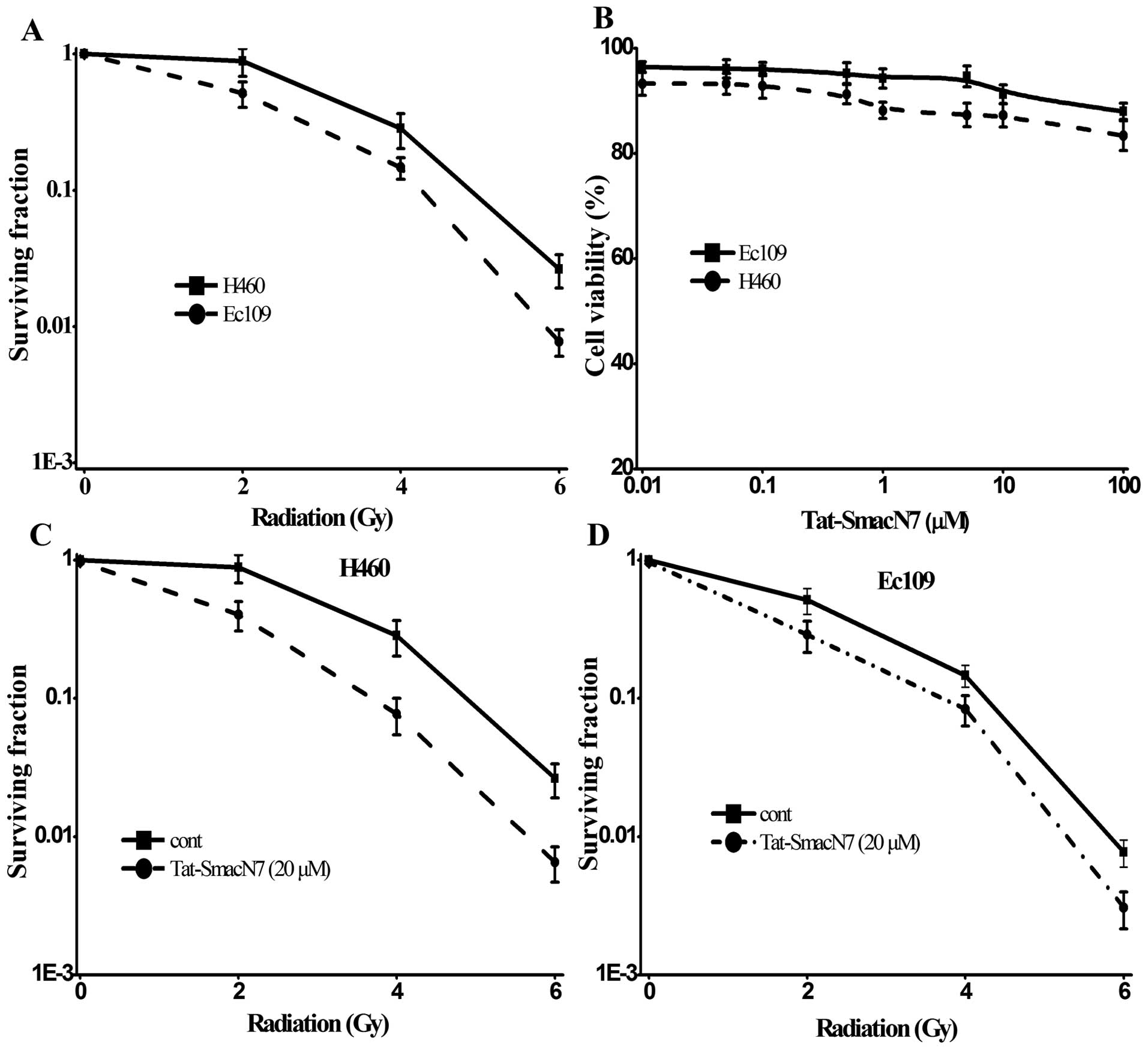
Sensitivity of H460 and Ec109 cell lines
to radiation or Tat-SmacN7 as a single agent or combination
We first determined radiosensitivity among the two
cell lines by classic clonogenic assay after exposure to various
doses of radiation (0, 2, 4 and 6 Gy). As shown in Fig. 2A, Ec109 cell lines were relatively
sensitive, whereas the H460 were relatively resistant to radiation.
We then determined the sensitivity of these lines to Tat-SmacN7 at
various drug concentrations ≤100 μM for 24-h treatment,
using the WST-1 cell viability assay. Unlike human breast cancer
MDA-MB-231 and ovarian cancer SK-OV3 cell lines, in which SM-164,
another Smac mimetic compound was highly potent at low nanomolar
concentrations (24,30,31),
the two lines were highly resistant to Tat-SmacN7 as a single agent
(21) as shown in Fig. 2B, two cell lines are resistant to
Tat-SmacN7 as a single agent with only 15–20% growth inhibition at
100 μM.
We next determined the potential radiosensitizing
effect of Tat-SmacN7 using drug concentrations at 20 μM in
combination with different doses of radiation. Tat-SmacN7
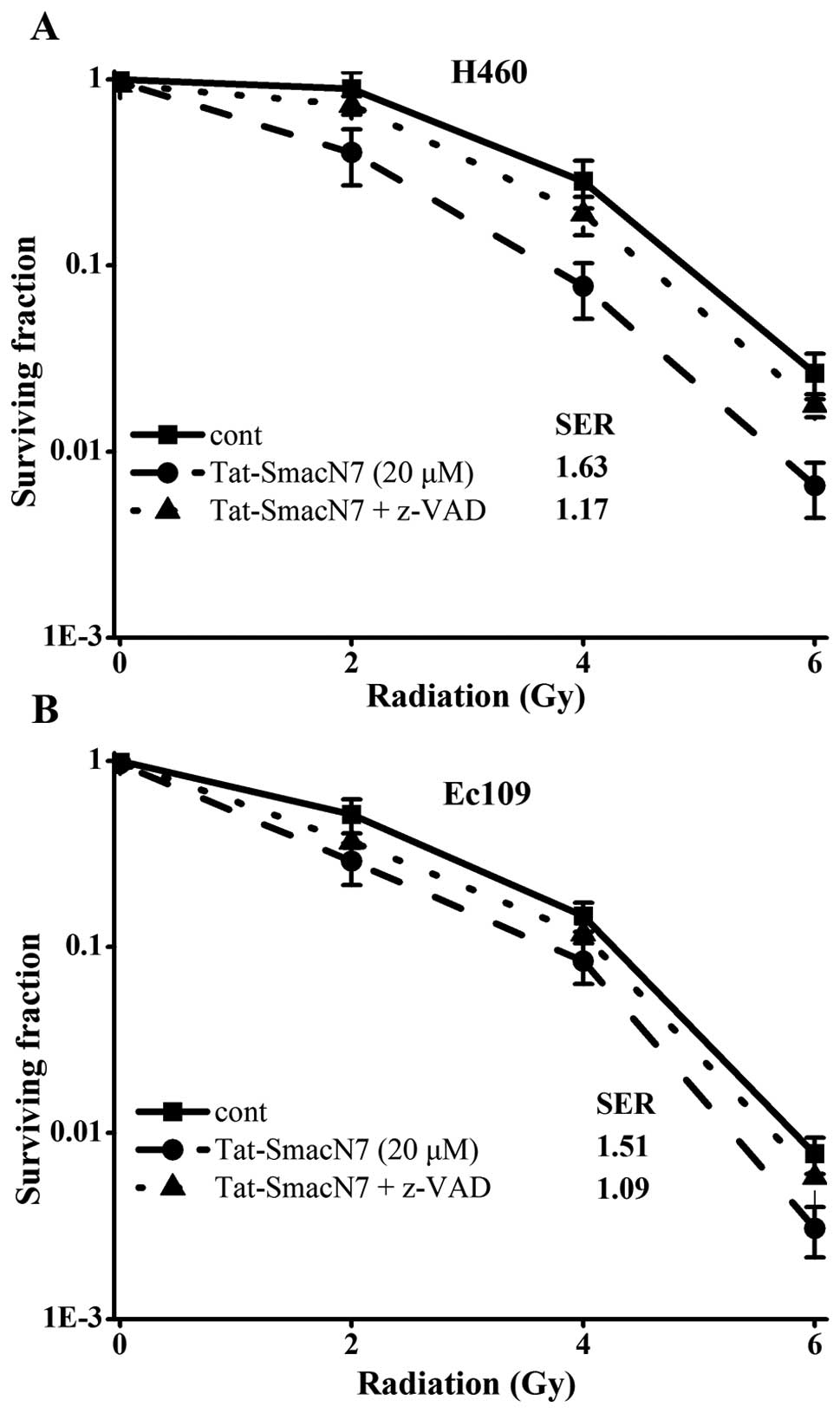
dose-dependent radiosensitization was observed in H460 and Ec109
cells with a SER (sensitivity enhancement ratio) of 1.63 or 1.51,
respectively, as shown in Fig. 2C and
D.
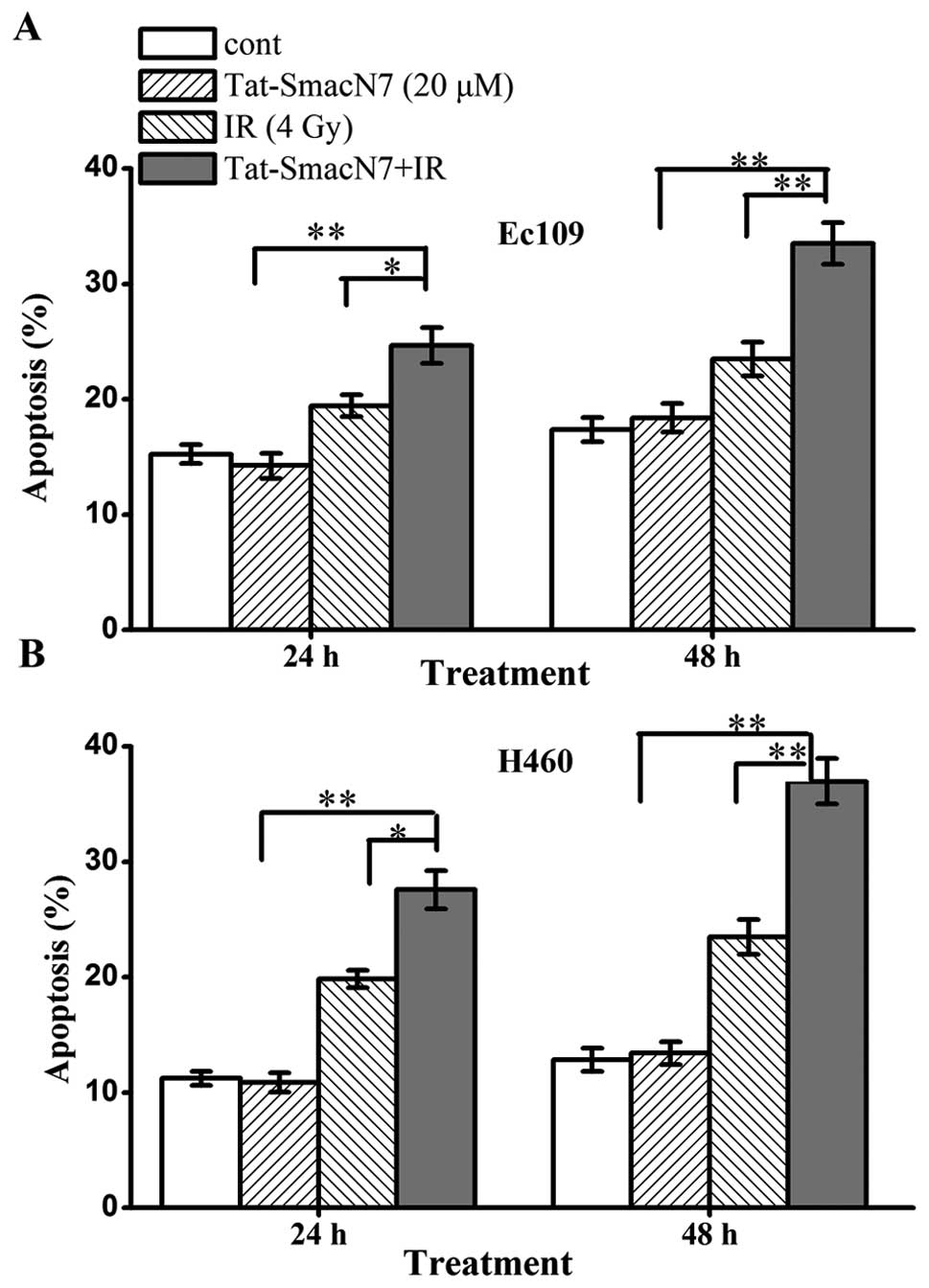
Radiosensitization by Tat-SmacN7 is
attributable to enhancement of induction of apoptosis
To determine the nature of Tat-SmacN7
radiosensitization, we performed FACS analysis of the two lines
treated with Tat-SmacN7 or radiation, alone or in combination.
Exposure to 20 μM Tat-SmacN7 or radiation with 4 Gy induced
a moderate level of apoptosis. The combination of radiation and
Tat-SmacN7, significantly enhanced radiation-induced apoptosis
(Fig. 3, p<0.05). Tat-SmacN7
alone caused a time-dependent induction of apoptosis, which was
enhanced by radiation (from 24.6 to 33.5% at 48 h in Ec109 cells
and from 27.6 to 37% in H460 cells), although radiation alone had a
minimal effect on apoptosis induction. Taken together, these
results suggest that Tat-SmacN7-mediated radiosensitization in the
two cell lines is associated with enhanced apoptosis.
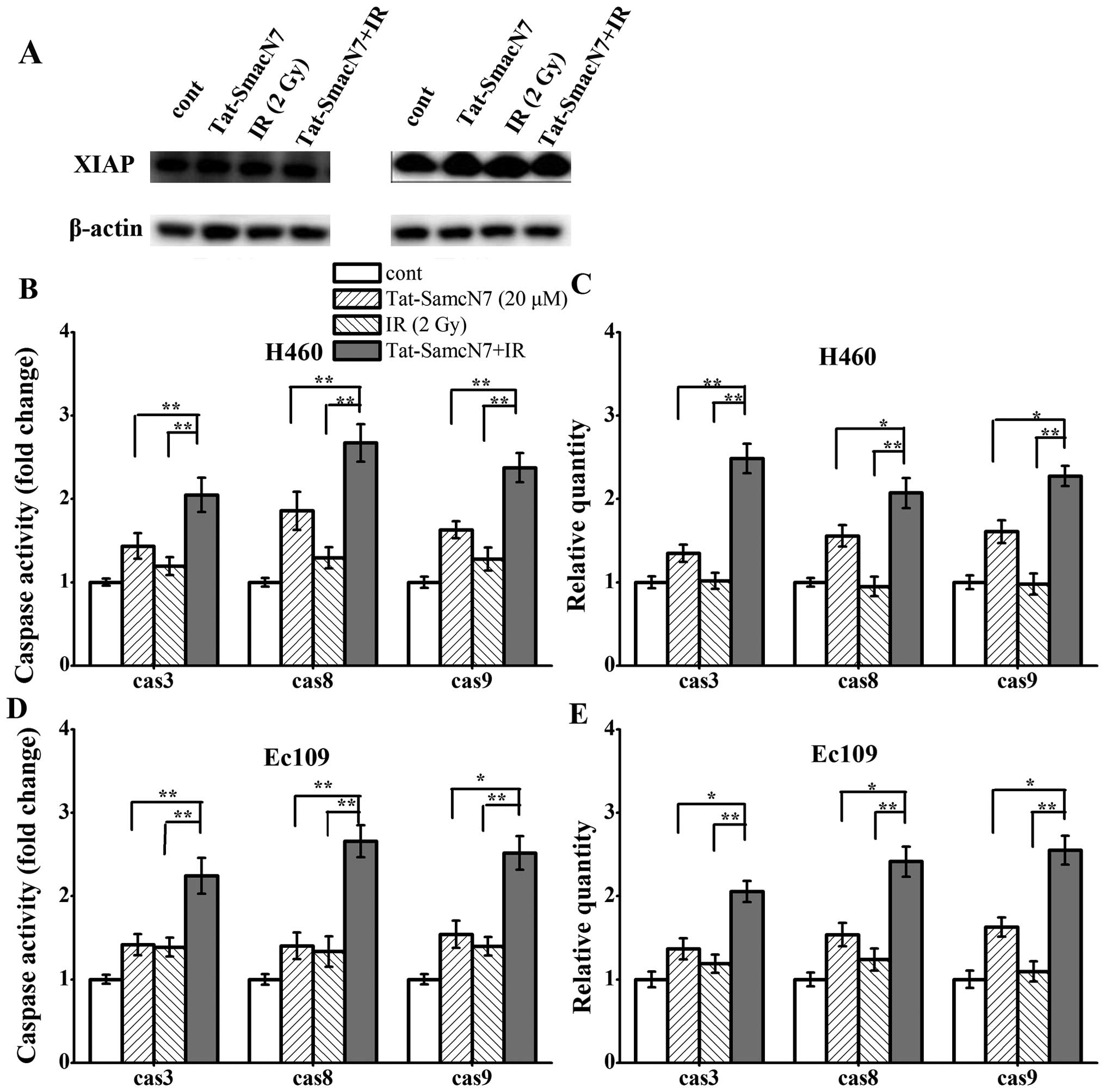
Tat-SmacN7 radiosensitization is
dependent on caspase expression and activity
As shown in Fig.
4A, the expression of XIAP protein level varied slightly among
the two cell lines with different treatment. Furthermore, in both
lines, consistent with previous reports (17,20–22),
Smac mimetic compound had no effect on XIAP levels (Fig. 4A). We investigated whether the two
cell lines are sensitive to Tat-SmacN7 radiosensitization. Cells
were treated with Tat-SmacN7, radiation alone, or the combination
and the mRNA expression of caspase-3, -8 and -9 was measured. As
shown in Fig. 4B and C, in H460
cells, Tat-SmacN7 alone treatment had a 1.34-, 1.55- and 1.60-fold
RNA expression of caspase-3, -8 or -9 effect and had a 1.43-, 1.85-
and 1.62-fold activity of the three caspases respectively, whereas
radiation alone had a minimal effect on caspase expression and
activation. Combinational treatment caused a significant increase
in the caspase RNA expression with a 2.48-, 2.07- and 2.27-fold and
activity with a 2.04-, 2.67- and 2.37-fold for caspase-3, -8 and
-9, respectively.
Similar results were observed in another sensitive
line of Ec109 cells with combination treatment causing maximal
activation of caspases (Fig. 4D and
E). Tat-SmacN7 alone treatment had a 1.36-, 1.53- and 1.62-fold
RNA expression of caspase-3, -8 or -9 effect and had a 1.41-, 1.40-
and 1.53-fold activity of these three caspases respectively, the
radiation alone had a 1.38-, 1.33- and 1.39-fold effect on caspases
expression and activation. Combinational treatment caused a
significant increase in caspases mRNA expression with a 2.05-,
2.41-and 2.54-fold and activity with a 2.24-, 2.65- and 2.51-fold
increases for caspase-3, -8 and -9.
Blockage of caspase activation largely
abrogated Tat-SmacN7 radiosensitization to enhance apoptosis
Finally, we tested if caspase activation, which
leads to apoptosis induction, is the main cause for Tat-SmacN7
radiosensitization. We used the pan-caspase inhibitor -z-VAD-fmk to
inactivate caspases. As shown in Fig.
5, z-VAD-fmk treatment completely blocked apoptotic combination
of Tat-SmacN7 and radiation in both Ec109 and H460 cells.
Consequently, z-VAD-fmk largely abrogated Tat-SmacN7
radiosensitization with SER reduction from 1.63 to 1.17 in H460
cells and 1.51 to 1.09 in Ec109 cells, both are statistically
significant (p<0.05).
Tat-SmacN7 sensitizes treatment with
radiation in vivo
Finally, we investigated the effects of Tat-SmacN7
in malignant esophageal carcinoma xenograft model in vivo.
Human Ec109 malignant cells were implanted into the right leg of
athymic mice and Tat-SmacN7 peptides, radiation or combinations
were locally administered from day 10 after tumor cell inoculation,
Tat-smacN7 was given at day 10 and 12 after tumor inoculation,
radiation was given every day for 10 days. Treatment with
Tat-smacN7 peptides significantly sensitized esophageal carcinoma
cells for apoptosis induced by radiation, we next determined the
radiosensitizing activity of Tat-SmacN7 in vivo. As shown in
Fig. 6, administration of
Tat-SmacN7 alone at a dose of 1 mg/kg/day twice had no effect on
tumor growth in nude mice. Radiation treatment at the clinically
relevant dose of 2 Gy/day for 10 days had a moderate antitumor
activity. In contrast, the combination of Tat-SmacN7 and radiation
caused a remarkable suppression of tumor growth, which is
statistically significantly greater than either treatment alone
(Fig. 6C). The combination
treatment was well-tolerated by the animals with a minimal loss of
body weight (Fig. 6D). Radiation
is the reason for the decreased body weight. Taken together, our
results indicate that Tat-SmacN7 sensitizes Ec109 cells to
radiation, as assayed in both in vitro cell culture and
in vivo tumor xenograft models and acts as a novel class of
radiosensitizer.
Discussion
Because of intrinsic resistance of many tumors to
established therapies, current attempts to improve the survival of
cancer patients largely depend on methods to target tumor cell
resistance and to identify novel anticancer agents (32). The key mechanism is to promote
apoptosis of target cells for most antitumor therapies, including
chemotherapy, γ-irradiation, immunotherapy and cytokines, but
defects in apoptosis programs may cause resistance (30,31),
whereas the mechanism of the defects have not been fully determined
to find a way to increase the sensitivity of the tumor cells to the
therapies. In this study, we determined the radiosensitizing
activity of Tat-SmacN7, a small molecule protein that contain an
apoptosis promoting AVPI sequence, which can disrupts inhibitory
binding of XIAP to caspase-9 and -3 (24). We found that Tat-SmacN7, at
non-toxic concentrations, significantly sensitized the tumor cells
to radiation both in vitro cell culture and in vivo
xenograft tumor models.
Our study revealed that the two cell lines are
resistant to Tat-SmacN7 as a single agent, a potent inhibitor of
IAPs (24) and H460 cell lines are
more resistant to radiation than Ec109 cell lines. On the other
hand, Tat-SmacN7 can act as a radiosensitizing agent equally
effectively in both cell types, suggesting that Tat-SmacN7 can be
studied as a potent radiosensitizer in some cancer cells,
especially for the radioresistant cancer, which is consistent with
recent studies showing that overexpression of SMAC protein itself
enhanced radiation induced apoptosis in several cancer cell lines,
including neuroblastoma, glioblastoma and pancreatic carcinoma and
HNSCC cells (21,27). It is well proven that in vertebrate
cells, apoptosis proceeds through either the signaling cascade
known as the intrinsic mitochondrial or the extrinsic death
receptor pathway, both of which converge on activating the
executioner caspase-3 and -7 (33,34).
Our study revealed mechanistically that cellular sensitivity to
Tat-SmacN7 as a single agent is attributable to its intrinsic
sensitivity to caspase-8 activation. On the other hand, Tat-SmacN7
radiosensitization in resistant cells is mainly mediated by
activation of intrinsic apoptosis pathway because of disruption of
XIAP-casapse-9 binding, leading to full activation of caspase-9. We
elucidated that the underlying mechanism of Tat-SmacN7
radiosensitization is mainly through increase in the RNA expression
of caspases and improve activation of caspases to induce apoptosis.
This conclusion was strongly supported by the functional rescue
experiments in which decrease of apoptosis through inactivation of
caspases using pharmaceutical (z-VAD) approaches largely abrogates
Tat-SmacN7 radiosensitization. Thus, induction of apoptosis through
caspase activation plays a key role in radiosensitization by
Tat-SmacN7 in the two cell types. Compared with the two cell lines,
we found that the sensitivity to Tat-SmacN7 radisensitization is
not dependent on the expression of XIAP which can explain the
radioresistance to but on the activity of caspases to
Tat-SmacN7.
In conclusion, we report here that Tat-SmacN7 is a
potent and novel class of radiosensitizer, regardless of the
sensitivity to Tat-SmacN7 as a single agent. Tat-SmacN7
radiosensitization is mainly mediated by caspase activation and the
RNA expression through removal of negative blockers cIAP-1 (through
degradation) and XIAP (through disrupting its inhibitory binding to
active caspase-9), leading to induction of apoptosis. Our study
suggests that cancer patients might benefit from
Tat-SmacN7-radiation combinational therapy and provides the
proof-of-concept for future development of Tat-SmacN7 or other SMAC
mimetic compounds as a class of radiosensitizing drugs against
cancer cells.
Acknowledgements
This study was supported by National
Natural Science Foundation of China (no. 31170804, 31240052), the
Natural Science Foundation of Tianjin (no. 10JCZDJC16900,
11ZCGYSY02400, 12JCYBJC15300), the Science Research Foundation for
Doctor-Subject of High School of the National Education Department
(no. 20101106110046, 20121106120044) and the Development Foundation
of Chinese Academy of Medical Sciences and Peking Union Medical
College (no. 1137, 1247).
References
|
1
|
Hanahan D and Weinberg RA: The hallmarks
of cancer. Cell. 100:57–70. 2000. View Article : Google Scholar
|
|
2
|
Hotchkiss RS, Strasser A, McDunn JE and
Swanson PE: Cell death. N Engl J Med. 361:1570–1583. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ashkenazi A and Dixit VM: Death receptors:
signaling and modulation. Science. 281:1305–1308. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Budihardjo I, Oliver H, Lutter M, Luo X
and Wang X: Biochemical pathways of caspase activation during
apoptosis. Annu Rev Cell Dev Biol. 15:269–290. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vaux DL and Korsmeyer SJ: Cell death in
development. Cell. 96:245–254. 1999. View Article : Google Scholar
|
|
6
|
Van Loo G, Saelens X, van Gurp M,
MacFarlane M, Martin SJ and Vandenabeele P: The role of
mitochondrial factors in apoptosis: a Russian roulette with more
than one bullet. Cell Death Differ. 9:1031–1042. 2002.PubMed/NCBI
|
|
7
|
Zou H, Li Y, Liu X and Wang X: An APAF-1.
cytochrome c multi meric complex is a functional apoptosome that
activates procaspase-9. J Biol Chem. 274:11549–11556. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kuida K, Haydar TF, Kuan CY, Gu Y, Taya C,
Karasuyama H, Su MS, Rakic P and Flavell RA: Reduced apoptosis and
cytochrome c-mediated caspase activation in mice lacking caspase 9.
Cell. 94:325–337. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gyrd-Hansen M and Meier P: IAPs: from
caspase inhibitors to modulators of NF-κB, inflammation and cancer.
Nat Rev Cancer. 10:561–574. 2010.
|
|
10
|
Altieri DC: Survivin and IAP proteins in
cell-death mechanisms. Biochem J. 430:199–205. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Suzuki Y, Nakabayashi Y, Nakata K, Reed JC
and Takahashi R: X-linked inhibitor of apoptosis protein (XIAP)
inhibits caspase-3 and -7 in distinct modes. J Biol Chem.
276:27058–27063. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shiozaki EN, Chai J, Rigotti DJ, Riedl SJ,
Li P, Srinivasula SM, Alnemri ES, Fairman R and Shi Y: Mechanism of
XIAP-mediated inhibition of caspase-9. Mol Cell. 11:519–527. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Deveraux QL and Reed JC: IAP family
proteins-suppressors of apoptosis. Genes Dev. 13:239–252. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wagenknecht B, Glaser T, Naumann U, Kügler
S, Isenmann S, Bähr M, Korneluk R, Liston P and Weller M:
Expression and biological activity of X-linked inhibitor of
apoptosis (XIAP) in human malignant glioma. Cell Death Differ.
6:370–376. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Du C, Fang M, Li Y, Li L and Wang X: Smac,
a mitochondrial protein that promotes cytochrome c-dependent
caspase activation by eliminating IAP inhibition. Cell. 102:33–42.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Verhagen AM, Ekert PG, Pakusch M, Silke J,
Connolly LM, Reid GE, Moritz RL, Simpson RJ and Vaux DL:
Identification of DIABLO, a mammalian protein that promotes
apoptosis by binding to and antagonizing IAP proteins. Cell.
102:43–53. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Srinivasula SM, Hegde R, Saleh A, Datta P,
Shiozaki E, Chai J, Lee RA, Robbins PD, Fernandes-Alnemri T, Shi Y
and Alnemri ES: A conserved XIAP-interaction motif in caspase-9 and
Smac/DIABLO regulates caspase activity and apoptosis. Nature.
410:112–116. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chai J, Du C, Wu JW, Kyin S, Wang X and
Shi Y: Structural and biochemical basis of apoptotic activation by
Smac/DIABLO. Nature. 406:855–862. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Green DR: Apoptotic pathways: paper wraps
stone blunts scissors. Cell. 102:1–4. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wu G, Chai J, Suber TL, Wu JW, Du C, Wang
X and Shi Y: Structural basis of IAP recognition by Smac/DIABLO.
Nature. 408:1008–1012. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Giagkousiklidis S, Vogler M, Westhoff MA,
Kasperczyk H, Debatin KM and Fulda S: Sensitization for
gamma-irradiation-induced apoptosis by second mitochondria-derived
activator of caspase. Cancer Res. 65:10502–10513. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Giagkousiklidis S, Vellanki SH, Debatin KM
and Fulda S: Sensitization of pancreatic carcinoma cells for
gamma-irradiation-induced apoptosis by XIAP inhibition. Oncogene.
26:7006–7016. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Vellanki SH, Grabrucker A, Liebau S,
Proepper C, Eramo A, Braun V, Boeckers T, Debatin KM and Fulda S:
Small-molecule XIAP inhibitors enhance gamma-irradiation-induced
apoptosis in glioblastoma. Neoplasia. 11:743–752. 2009.PubMed/NCBI
|
|
24
|
Lu J, Bai L, Sun H, Nikolovska-Coleska Z,
McEachern D, Qiu S, Miller RS, Yi H, Shangary S, Sun Y, Meagher JL,
Stuckey JA and Wang S: SM-164: a novel, bivalent Smac mimetic that
induces apoptosis and tumor regression by concurrent removal of the
blockade of cIAP-1/2 and XIAP. Cancer Res. 68:9384–9393. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sun H, Nikolovska-Coleska Z, Lu J, Meagher
JL, Yang CY, Qiu S, et al: Design, synthesis and characterization
of a potent, nonpeptide, cell-permeable, bivalent Smac mimetic that
concurrently targets both the BIR2 and BIR3 domains in XIAP. J Am
Chem Soc. 129:15279–15294. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang J, McEachern D, Li W, Davis MA, Li H,
Morgan MA, Bai L, Sebolt JT, Sun H, Lawrence TS, Wang S and Sun Y:
Radiosensitization of head and neck squamous cell carcinoma by a
SMAC-mimetic compound, SM-164, requires activation of caspases. Mol
Cancer Ther. 10:658–669. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fulda S, Wick W, Weller M and Debatin KM:
Smac agonists sensitize for Apo2L/TRAIL- or anticancer drug-induced
apoptosis and induce regression of malignant glioma in vivo. Nat
Med. 8:808–815. 2002.
|
|
28
|
Qin L, Tong T, Song Y, Xue L, Fan F and
Zhan Q: Aurora-A interacts with Cyclin B1 and enhances its
stability. Cancer Lett. 275:77–85. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bockbrader KM, Tan M and Sun Y: A small
molecule Smac-mimic compound induces apoptosis and sensitizes
TRAIL-and etoposide-induced apoptosis in breast cancer cells.
Oncogene. 24:7381–7388. 2005. View Article : Google Scholar
|
|
30
|
Herr I and Debatin KM: Cellular stress
response and apoptosis in cancer therapy. Blood. 98:2603–2614.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mow BM, Blajeski AL, Chandra J and
Kaufmann SH: Apoptosis and the response to anticancer therapy. Curr
Opin Oncol. 13:453–462. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lowe SW and Lin AW: Apoptosis in cancer.
Carcinogenesis. 21:485–495. 2000. View Article : Google Scholar
|
|
33
|
Hengartner MO: The biochemistry of
apoptosis. Nature. 407:770–776. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tait SW and Green DR: Mitochondria and
cell death: outer membrane permeabilization and beyond. Nat Rev Mol
Cell Biol. 11:621–632. 2010. View
Article : Google Scholar : PubMed/NCBI
|