Introduction
Glioblastoma multiforme (GBM) is an aggressive,
vascularized brain tumor that is characterized by high
invasiveness. This tumor is capable of gaining resistance to
various therapeutic agents as it utilizes alternative pathways once
its primary signaling cascade is disrupted. Conventional
chemotherapy, radiotherapy and immunotherapy are directed against
tumor cells; in contrast, anti-angiogenic therapy is aimed at the
vasculature of a tumor and will either cause total tumor regression
or keep tumors in a state of dormancy (1). Angiogenesis is an invasive process
involving the extracellular matrix and the proliferation and
migration of endothelial cells (ECs). It is a prerequisite for
tumor growth and metastasis formation (1). Therefore, understanding the cellular
events involved in molecular regulation of the angiogenic events
would provide novel therapeutic targets for the treatment of
cancer.
For many solid tumors, radiation (IR) is the only
treatment tool available that offers a clear survival benefit.
However, even after exposure to IR, a cell population may manage to
survive, either because it receives sub-lethal doses and/or because
it successfully utilizes endogenous repair mechanisms (2). Molecular events in irradiated cells
are altered, leading to upregulation of genes that favor cell
survival and angiogenesis. Overexpression of proteases (e.g., MMPs)
and cytokines and activation of surface receptors protect both
tumor and non-tumor cells from apoptosis and increase their ability
to trigger angiogenesis.
Anti-angiogenic agents may improve radiosensitivity
by directly and/or indirectly inhibiting protective cell survival
signaling pathways in both endothelial and tumor cells, resulting
in increased apoptosis in both endothelial and tumor cells.
Vascular targeting agents may act in an additive fashion to improve
tumor response to radiation by decreasing overall tumor burden
through vascular shutdown and by creating a tumor environment where
maintaining viable, well-oxygenated tumor cells presents an ideal
target for radiation therapy (3).
The matrix metalloproteinases (MMPs) degrade the components of the
extracellular matrix. Among the different factors involved in the
acquisition of invasive capacity and angiogenic properties by tumor
cells, the action of MMPs is critical. Earlier studies in our lab
demonstrated direct evidence for a role of MMP-2 in tumor
angiogenesis in which tumor cells express increased levels of MMP-2
and activate several key molecules leading to rapid cellular
proliferation, increased motility, invasion and angiogenesis in
lung cancer and gliomas (4–6).
Integrins are a family of cell-extracellular matrix
adhesion molecules that play an important role in upregulating
tumor associated blood vessels (7). Integrin αvβ3 has become an attractive
target for drugs suppressing neovascularization from tumors and
biomarker for tumor angiogenesis. Earlier studies have shown that
blocking αvβ3 integrin induced EC death, implying its importance in
pro-angiogenesis (8,9).
The membranous CXC chemokine receptor 4 (CXCR4) and
its ligand, stromal-derived factor-1 (SDF-1), which is also known
as CXCL12, are members of the chemokine family and their critical
and active roles have been demonstrated in tumor growth and
malignancy (10). In many tumors,
metastasis is mainly related to SDF-1/CXCR4 (11). The most important sources of SDF-1
are bone marrow, lymph nodes, muscle and several regions of the
central nervous system (12).
Increased secretion or expression of SDF-1 in various organs in
response to tissue damage such as IR, hypoxia or toxic agents has
also been observed (13). Reports
suggest that the SDF-1α/CXCR4 axis is involved in
neovascularization and has also been detected in ECs (13). This implies that new therapeutic
strategies aimed at blocking the SDF-1/CXCR4 axis could have
important applications. Despite many scientific reports, the role
of MMP-2 and irradiation-induced SDF-1/CXCR4 expression in
regulating angiogenesis has not been completely elucidated.
IR continues to be the main, integral part of GBM
treatment, but it is likely associated with intrinsic cellular
resistance leading to rapid proliferation and high invasiveness.
The role of MMPs in changing the cells to gain resistance to IR is
a major therapeutic target. IR induces an increase in MMP-2 levels
in almost all cancer types (5).
IR-enhanced expression and activation of MMP-2 modifies tumor
progression by altering the availability of various molecules that
promote tumor angiogenesis. Enhanced MMP-2 secretion increases
tumor survival by increasing angiogenic potential. These factors
contribute to the high vascularity as well as radioresistance of
GBM. Since SDF-1/CXCR4 is one of the major signaling axes of
endothelial tubule formation and migration, especially in cancer,
it is essential that we gain a better understanding of this
phenomenon via αvβ3 and the signaling pathway that mediates
radiation-inducible resistance. In this context, we attempted to
study potential drug targets for radiosensitization using pMMP-2
siRNA in 4910 and 5310 human xenograft cell lines.
Materials and methods
Cell culture and reagents
Established human xenograft glioma cell lines 4910
and 5310 (kindly provided by Dr David James, University of
California at San Francisco) are highly invasive in the mouse
brain. These cell lines were generated and maintained in mice
(14). These cells were cultured
in RPMI-1640 medium (Mediatech Inc., Herndon, VA) supplemented with
10% FBS (Invitrogen Corp., Carlsbad, CA), 50 U/ml penicillin and 50
μg/ml streptomycin (Life Technologies, Inc., Frederick, MD)
in a CO2-chamber at 37°C. The human microvascular dermal
endothelial cells (HMEC-1) were cultured in Advanced Dulbecco’s
modified Eagle’s medium (Life Technologies) supplemented with
glutamine, EGF and hydrocortisone (Stem Cell Technologies, British
Columbia, Canada). Specific antibodies against MMP-2, SDF-1, CXCR4,
PI3K, p-PI3K (Tyr 508), AKT, p-AKT (Ser 473), integrin αvβ3, GAPDH
and HRP-conjugated secondary antibodies (Santa Cruz Biotechnology,
Santa Cruz, CA) and Alexa Fluor-conjugated secondary antibodies
(Life Technologies). We also used an αvβ3 integrin blocking
antibody (Millipore Inc., Billerica, MA), recombinant human MMP-2
(rhMMP-2) (EMD Biosciences, San Diego, CA) and recombinant human
SDF-1 (rhSDF-1) proteins (Pro Spec Bio, East Brunswick, NJ) in our
study.
Transient transfection and radiation
The specific MMP-2. siRNA (pM.si) and a scrambled
sequence vectors (pSV) were designed and cloned as described
earlier (15). At 70–80%
confluence, 4910 and 5310 cells were serum-starved for 6 h after
which they were treated with mock (1X PBS), pSV or pM.Si using
X-tremeGENE HP DNA transfection reagent by following the
manufacturer’s instructions (Roche Applied Science, Indianapolis,
IN). The X-ray unit RS 2000 Biological Irradiator (Rad Source
Technologies, Inc., Boca Raton, FL) operating at 150 kV/50 mA and
delivering 0.71 Gy/min was used for radiation treatments. For the
combination treatments, the culture medium was aspirated from
mock-, pSV- or pM.si-treated culture plates and the cells were
further treated with IR (8 Gy) or rhMMP-2 (25 ng/ml) or rhSDF-1 (25
ng/ml) and incubated for additional 12–16 h serum-free medium
DMEMF-12 50/50 (Mediatech, Inc., Manassas, VA).
Preparation of tumor conditioned media
and gelatin zymography
Following transfection, the cells were cultured in
RPMI-1640 serum medium for 24 h. At the end of experiment, the
culture medium from mock-, pSV- or pM.si-treated culture plates was
aspirated and serum-free DMEM-F-12 50/50 medium was added and
incubated for another 12–16 h as above. The tumor conditioned media
(CM) were collected from different treatments and quantified
(6). Gelatin zymographic analyses
were performed to determine the MMP-2 gelatinolytic activity using
CM after different treatments as described previously (5). Further, angiogenic experiments were
performed by culturing the endothelial cells (ECs) in CM obtained
from various treatments as described above (4). After culturing in CM for 12–16 h,
whole endothelial cell lysates were prepared and subjected to
western blotting.
Western blotting and
immunoprecipitation
Western blotting and immunoprecipitation experiments
were performed as described earlier (16). At the end of different treatments,
the 4910, 5310 and ECs were gently washed with pre-chilled 1X PBS
and whole cell lysates were prepared using RIPA buffer. Equal
amounts of protein were fractionated on SDS-PAGE and immunoblotted
with primary antibodies followed by incubation with
species-specific, HRP-conjugated secondary antibodies. Signals were
detected using ECL-enhanced Western blotting detection system
(Amersham Pharmacia, Piscataway, NJ). For immunoprecipitation,
equal amounts of protein (200 μg) from ECs were
immunoprecipitated with antibodies against SDF-1, αvβ3 and Nsp-IgG
using μMACS protein G microbeads and MACS separation columns
following the manufacturer’s protocol. The immunoprecipitates were
subjected to Western blotting.
In vitro angiogenic assay
Angiogenesis was performed as described earlier
(17). Briefly, after transfection
of 4910 and 5310 cells with mock, pSV or pM.Si, the cells were
washed and incubated in serum-free medium for 16 h. For in
vitro angiogenesis assay, the conditioned medium was collected
and centrifuged to clear cellular debris. Approximately
4×104 ECs were allowed to grow overnight in CM from 4910
and 5310 human xenograft cells in 96-well plates coated with
Matrigel. After the incubation period, the formation of
capillary-like structures was captured using a microscope attached
to a CCD camera.
Immunocytochemical and
immunohistochemical analysis
Immunocytochemical and immunohistochemical analyses
were performed as described previously (18). ECs were incubated in chamber slides
for 16 h with the CM of 4910 and 5310 xenograft cells treated with
mock or pSV or pM.Si with or without IR. The ECs were washed in PBS
and fixed in 4% paraformaldehyde and permeabilized in 0.1% Triton
X-100. Non-specific binding was blocked by BSA in PBS, followed by
incubation with respective primary antibodies for 2 h at room
temperature. The cells were washed and incubated with respective
Alexa Fluor-conjugated secondary antibodies, subsequently mounted.
Nuclei were counterstained with 4′,6-diamidino-2-phenylindole
(DAPI). For immunohistochemical analysis, tissue sections (4–5 mm)
(pSV or pM.Si with or without IR), were de-paraffinized in xylene,
rehydrated in graded ethanol solutions, permeabilized in 0.1%
Triton X-100 and incubated overnight at 4°C with anti-SDF-1
antibody. Slides were washed twice in PBS and incubated in
HRP-conjugated secondary antibodies for 1 h at room temperature.
The HRP-conjugated secondary antibody-incubated sections were
washed and further incubated with DAB (3,39-diaminobenzidine)
solution for 5–10 min while hematoxylin was used for nuclear
counterstaining, mounted and photographed under a microscope.
In vivo angiogenesis assay
In vivo angiogenesis assay was performed
using the dorsal air sac model in athymic nude mice (nu/nu;
5–7-week old) as previously described (5). Initially, the mice were anesthetized
by intraperitoneal injection of ketamine (50 mg/kg) and xylazine
(10 mg/kg). Dorsal airsac was made by injecting 10 ml of air in the
completely anesthetized mice. A 1.5–2.0-cm superficial incision was
made horizontally along the edge of the dorsal air sac with the
help of forceps and sterile diffusion chambers (Fisher, Hampton,
NH) containing 4910 and 5310 cells (1.5×106 cells)
transfected with mock, pSV or pM.Si with or without IR were placed
underneath the skin and carefully sutured. After 14 days, the
animals were anesthetized with ketamine/xylazine and sacrificed by
intracardial perfusion with saline (10 ml) and followed by 10 ml of
10% formalin/0.1 M phosphate solution. The tissue surrounding the
implanted chambers was carefully resected and the chambers were
removed from the subcutaneous air fascia. The air sac covering the
chambers was photographed under visible light. The number of blood
vessels within the chamber in the area of the air sac was counted
and their lengths were measured. The Institutional Animal Care and
Use Committee of the University of Illinois College of Medicine at
Peoria (Peoria, IL) approved all surgical interventions and
post-operative animal care. The animal protocol number is 858, May
27, 2009 and renewed on April 27, 2010.
Statistical analysis
Data from at least three independent experiments
were statistically analyzed using one way ANOVA and significant
difference among various treatments were presented as mean ± SE at
p<0.05 and p<0.01. Densitometric analyses was performed using
ImageJ 1.42 (NIH, Bethesda, MD).
Results
pM.Si-CM of human xenograft cell lines
downregulated IR-induced angiogenesis
Our previous study (5) suggests that the infiltrative behavior
and the survival mechanisms of glioma have a definite relationship
to MMP-2 expression and IR exposure. Therefore, we investigated the
ability of pM.Si to prevent this characteristic aggressive
angiogenesis following radiation. The results of in vitro
angiogenesis assay (Fig. 1A)
indicated that there was a marked rise in the number of
capillary-like endothelial tube structures when treated with IR (8
Gy)- of 4910 and 5310 cells as compared to control-CM.
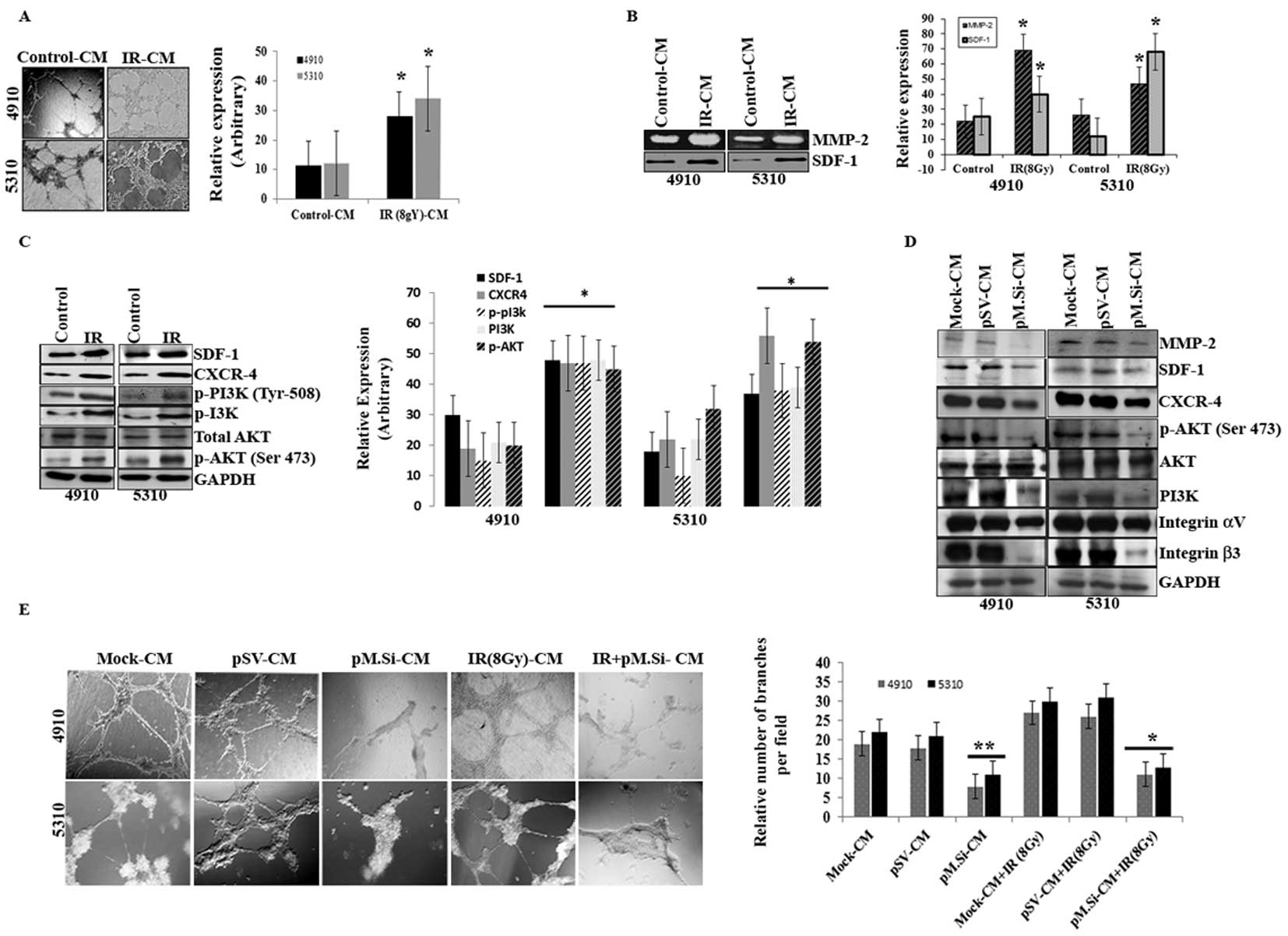 | Figure 1pM.Si-CM downregulates IR-induced
angiogenesis in human xenograft cell lines 4910 and 5310. Cells
(4910 and 5310) were treated with mock, pSV or pM.Si alone or in
combination with IR (8 Gy) as described in Materials and methods.
(A) In vitro angiogenesis. The conditioned media was added
to 96-well plates that were coated with Matrigel and pre-seeded
with human dermal microvascular endothelial cells (ECs)
(2×104 cells/well). After overnight incubation at 37°C,
cells were observed under the bright field microscope for the
formation of capillary-like structures. The degree of angiogenic
induction by mock- and IR-CM were quantified for the numerical
value of the product of the relative capillary length and number of
branch points per field and indicated in a bar diagram. The data
are presented as the mean ± SE of three independent replicates with
significance denoted by *p<0.01. (B) Gelatin
zymography (MMP-2) and western blot analysis (SDF-1) were
performed. The experiments were carried out thrice and the data are
presented as the mean ± SE of three independent replicates with
significance denoted by *p<0.01. (C) Seventy-two
hours post-transfection, xenograft cells were harvested and whole
cell lysates were prepared using RIPA buffer. Whole cell lysates
were subjected to western blotting for SDF-1, CXCR4, p-PI3K (Tyr
508), PI3K, AKT and p-AKT (Ser 473). GAPDH was used to confirm
equal loading. The data are presented as the mean ± SE of three
independent replicates with significance denoted by
*p<0.01. (D) ECs were grown on the mock-, pSV- and
pM.Si-CM for 16 h. Cells were then collected and whole cell lysates
were subjected to western blotting for MMP-2, SDF-1, CXCR4, p-AKT
(Ser 473), AKT, PI3K and integrin αvβ3. The blots were stripped and
re-probed with GAPDH antibody as an internal control for the
respective proteins. (E) In vitro angiogenesis was performed
under similar conditions as described for (A). The degree of
angiogenic induction was quantified for the numerical value of the
product of the relative capillary length and number of branch
points per field. The data are presented as the mean ± SE of three
independent replicates with significance denoted by
*p<0.05 and **p<0.01. |
Because MMP-2 activity was thought to be necessary
for endothelial tubule formation, we next examined whether IR
altered MMP-2 activity. This effect was measured by gelatin
zymography and determined to function in a dose-dependent manner
(data not shown). At a dose of 8 Gy, MMP-2 activity was upregulated
in IR-treated 4910 and 5310 cells compared to control-CM (Fig. 1B). We next examined the relative
expression of SDF-1 with IR in the CM of both cell lines. SDF-1 was
significantly upregulated in IR treatment as compared to control in
4910 and 5310 cells (Fig. 1B).
When whole cell lysates were subjected to western blotting, there
was remarkable increase in the expression levels of SDF-1, CXCR-4,
p-PI3K (Tyr 508), PI3K, AKT, p-AKT (Ser 473) in IR treated cells,
compared to controls (Fig.
1C).
As SDF-1 is known to regulate the morphogenesis of
ECs (19), we next proceeded to
verify the effect of pM.Si-CM in downregulating SDF-1 expression as
well as CXCR4 and downstream signaling molecules in ECs. We
previously demonstrated that transcriptional knockdown of MMP-2 in
tumor cells inhibits secretion of SDF-1 (20). pM.Si-CM abrogated the expression of
SDF-1 and CXCR4 when incubated with ECs for 16 h (Fig. 1D) and this reduction correlated
with reduced expression of MMP-2 in ECs. Because several studies
indicate that PI3K/AKT mediates the activation of angiogenesis, we
determined the effect of MMP-2 suppression on PI3K/AKT. As shown in
Fig. 1D, pM.Si-CM inhibited the
expression of PI3K and phosphorylation of AKT (Ser 473) as compared
to mock- and pSV-CM in ECs.
Studies indicate direct interaction between MMP-2
and integrin αvβ3 and their coordinated interplay is essential to
endothelial tubule formation (17,21,22).
Based on this information, we investigated the role of pM.Si-CM on
MMP-2 and integrin αvβ3 on ECs in MMP-2 regulated SDF-1 expression
in 4910 and 5310 cells. Western blot analysis revealed (Fig. 1D) αvβ3 suppression by pM.Si-CM,
thereby indicating the critical role of MMP-2 in angiogenesis.
Since IR is a vasculogenic stimulus, we investigated the effect of
radiation on endothelial cell capillary-like network formation.
MMP-2 promotes tumor vascularization and, in turn, renders the
tumor cells resistant to radiotherapy. pM.Si-CM with or without IR
from 4910 and 5310 cells failed to induce capillary network
formation when added to ECs. On the other hand, mock- and pSV-CM
triggered the angiogenic process, which is evident from the
increase in the percentage of branching when compared to
non-irradiated counterparts (Fig.
1E).
pM.Si-CM inhibited IR-induced PI3K/AKT
expression and angiogenesis in ECs and supplementation of
rhMMP-2/rhSDF-1 reverses inhibition
Our results show that pM.Si-CM with or without IR
diminished the expression of protein tyrosine kinase PI3K and
phosphorylation of PI3K and phosphorylation of AKT (Ser 473) in ECs
compared to mock- and pSV-CM (Fig.
2A). A recent study suggested that IR upregulates αvβ3
expression in ECs and consecutively phosphorylates AKT (Ser 473),
which may provide a tumor escape mechanism from radiation injury
mediated by integrin survival signaling (23). In view of this, we investigated the
role of pM.Si-CM on αvβ3 expression in ECs. We demonstrated that
αvβ3 was downregulated by IR-induced pM.Si-CM as compared to
mock-CM- or pSV-CM-treated ECs (Fig.
2A). Further, our results show that pM.Si-CM combined with IR
potentially reduced endothelial network formation, which indicates
a strong relationship between IR-induced MMP-2 and αvβ3
downregulation with respect to angiogenesis in ECs. Fig. 2B shows an angiogenic graph
indicating the efficacy of pM.Si-CM obtained from 4910 and 5310
cells, in downregulating the number of capillary network branches
per field and reveals reductions ≤80 and 73%, respectively,
compared to mock- and pSV-CM. In contrast, pM.Si-CM combined with
IR demonstrated ≤40 and 45% reduction in the number of capillary
formation in 4910 and 5310 cells, respectively, which shows the
efficacy of pM.Si-CM in combination therapy. To further assess the
role of rhMMP-2 in SDF-1 expression, rhMMP-2 recombinant protein
was added to the CM of pM.Si and pSV and incubated for 12–16 h in
ECs. There was remarkable elevation of SDF-1 and CXCR4 expression
in ECs observed in pM.Si-CM-treated cells as shown in Fig. 2C. These findings suggest that MMP-2
upregulates the expression of SDF-1 and CXCR4 in ECs as also shown
in the graph (Fig. 2C). To assess
further, the pM.si-CM inhibited SDF-1 expression, we elevated the
SDF-1 by rhSDF-1 in pSV-and pM.Si-CM of both the cell lines in ECs.
The addition of rhSDF-1 to pM.Si-CM-treated ECs reversed
pM.Si-CM-mediated inhibition of capillary network formation by ECs
(Fig. 2D). These results suggest
that MMP-2 downregulation inhibits SDF-1 expression and secretion,
which results in impaired endothelial tubule formation.
Additionally, western blot analysis established that the
supplementation of rhSDF-1 restored SDF-1 mediated elevation of AKT
phosphorylation, which was inhibited by pM.Si-CM from both 4910 and
5310 cells and indicates AKT-mediated angiogenesis (Fig. 2E). Thus, rhSDF-1 elicited a rapid
and robust increase in the expression of pM.Si-CM-abrogated
pro-angiogenic molecules and endothelial capillary network
formation.
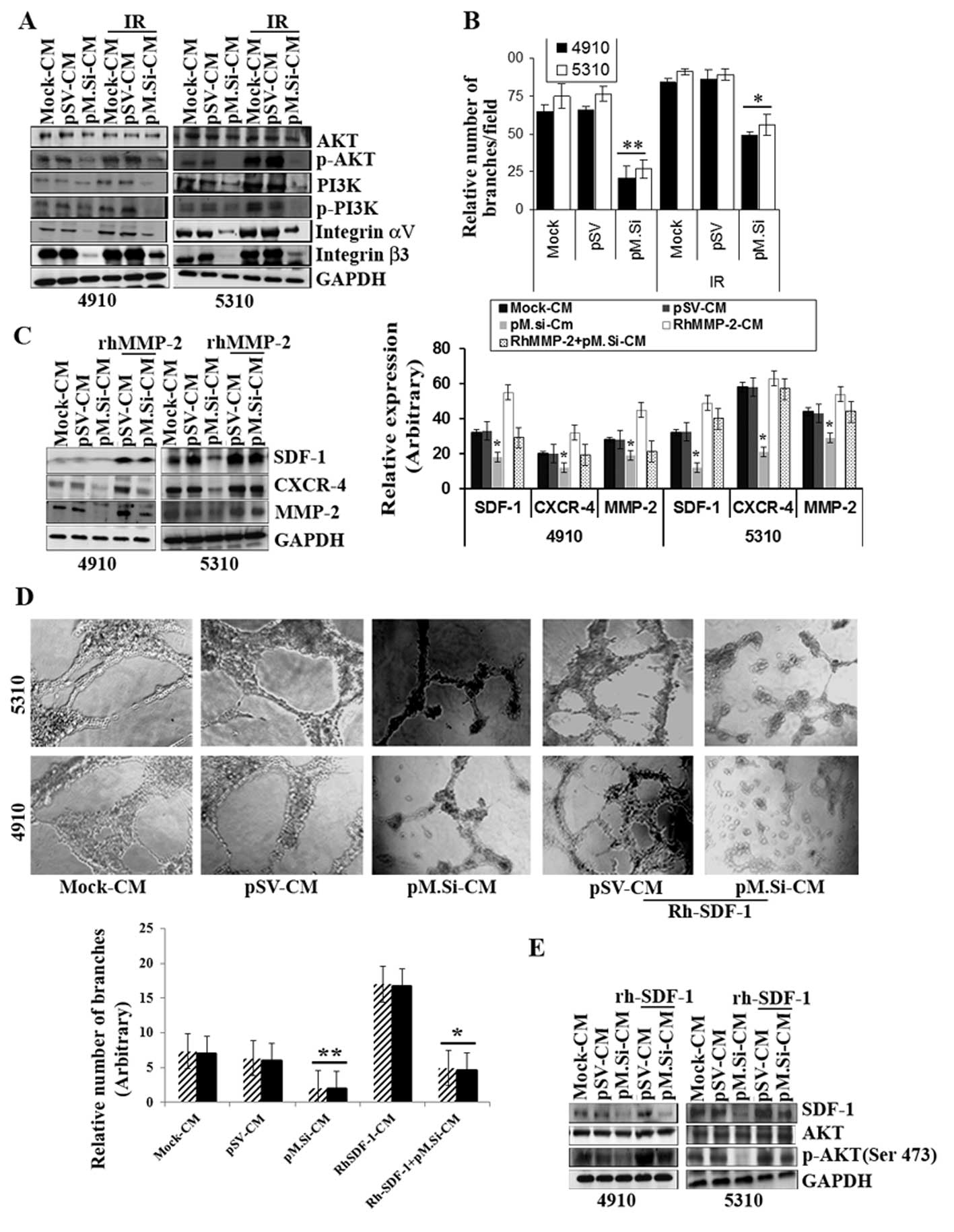 | Figure 2pM.Si-CM inhibits IR-induced PI3K/AKT
expression and angiogenesis in ECs and supplementation of
rhMMP-/rhSDF-1 reverses inhibition. (A) ECs were grown in mock-,
pSV- and pM.Si-CM with or without IR. The whole cell lysates were
subjected to western blotting to check the expression levels of
AKT, p-AKT, PI3K, p-PI3K, integrin αv and integrin β3 using
specific antibodies. The blot was restriped and GAPDH was used as a
loading control. (B) In vitro angiogenesis was done with
overnight incubation of ECs at 37°C with mock-, pSV-, IR (8 Gy)-CM
or pM.Si alone or in combination with IR (8 Gy). The cells were
observed under a bright field microscope for the formation of
capillary-like structures. The degree of angiogenic induction was
quantified for the relative capillary length and number of branch
points per field. The data are presented as the mean ± SE of three
independent replicates with significance denoted by
*p<0.05 and **p<0.01. (C) ECs from 4910
and 5310 cells were grown in the presence of mock-, pSV- or
pM.Si-CM with or without IR (8 Gy) for 16 h. pSV- and pM.Si-CM were
treated with 25 ng/ml rhMMP-2. Whole cell lysates of ECs were
prepared at the end of 16-h treatment and subjected to western
blotting to check the expression levels of SDF-1, CXCR4 and MMP-2
using specific antibodies. The blot was restriped and GAPDH was
used as a loading control. The data are presented as the mean ± SE
of three independent replicates with significance denoted by
*p<0.05 and **p<0.01. (D) In
vitro angiogenesis was carried out under similar conditions as
noted in Fig. 1A and is
representative of at least three independent repetitions. RhSDF-1
(25 ng/ml) was added to pSV- and pM.Si-CM and incubated for 16 h.
The degree of capillary network formation is indicated in a graph.
The data are presented as the mean ± SE of three independent
replicates with significance denoted by *p<0.05 and
**p<0.01. (E) Endothelial cells were treated with
pSV-CM or pM.Si-CM supplemented with rhSDF-1 (25 ng/ml) for 16 h.
The whole cell lysates were subjected to western blot analysis for
the expression of SDF-1, AKT and p-AKT with their respective
antibodies and GAPDH was used as the loading control. |
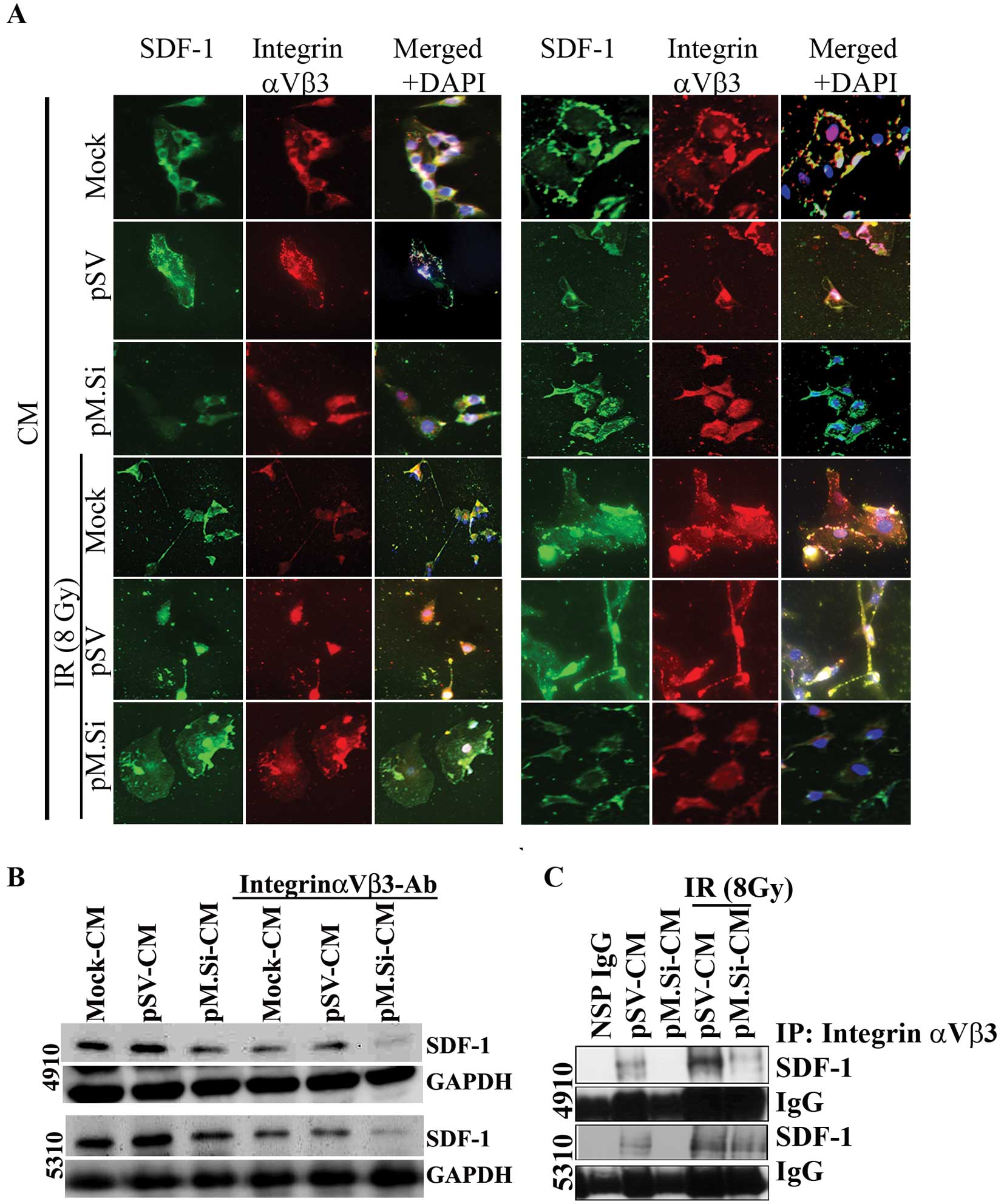
Knockdown of MMP-2 by pM.Si-CM inhibits
IR-induced SDF-1 expression via integrin αvβ3 in ECs
Understanding how endothelial MMP activity is
involved in the angiogenic phenotype has enormous implications on
cancer therapy since angiogenesis is necessary for tumor growth and
metastasis. Immunofluorescence analysis of ECs revealed that
co-localization of SDF-1 and integrin αvβ3 decreased in pM.Si- or
IR-induced pM.Si-CM compared to mock- and pSV-CM from 4910 and 5310
cells (Fig. 3A). The essential
functional role of SDF-1/αvβ3 interaction with pM.Si-CM of 4910 and
5310 cells was further verified by treating CM with a
function-blocking anti-αvβ3 integrin antibody. The supplementation
substantially inhibited SDF-1 expression in ECs treated with mock-
and pSV-CM. pM.Si-CM along with blocking anti-αvβ3 integrin
antibody further decreased SDF-1 expression compared to mock- and
pSV-CM anti-αvβ3 integrin-treated controls as shown in Fig. 3B. We next examined their expression
levels and their interaction using co-immunoprecipitation. We
observed a significant decrease in the expression levels of SDF-1
in ECs treated with pM.Si-CM of both cell lines. The αvβ3 binds to
SDF-1 in ECs treated with mock- and pSV-CM from 4910 and 5310 cells
and facilitates the activation of pI3K/AKT signaling. On the other
hand, ECs treated with pM.Si showed decreased binding of αvβ3 to
SDF-1 (Fig. 3C), implying the
critical role of MMP-2 in recruiting SDF-1 to αvβ3 and promoting
angiogenesis.
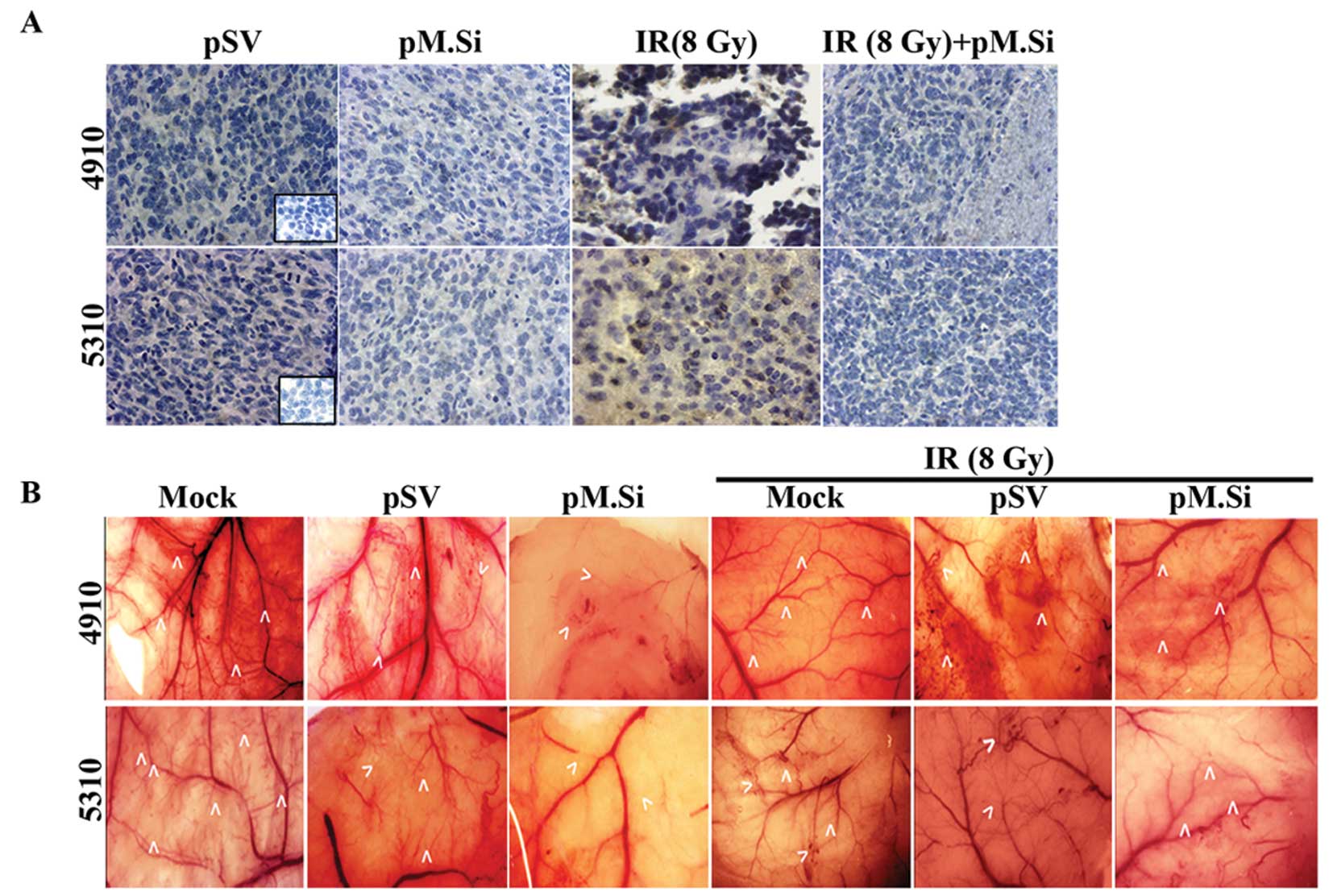
Immunocytochemical and
immunohistochemical analyses
We next sought to determine the effect of pM.si
alone or in combination with IR on SDF-1 and angiogenesis in
vivo. The brain tumor sections were prepared as described
earlier (5). pSV and pM.Si alone
or in combination with IR were evaluated histologically for
expression levels of SDF-1 (Fig.
4A). In pSV treated tumor section a high expression of SDF-1
was noted compared to pM.Si alone or combined with IR treatment.
These in vivo results corroborate with our in vitro
results.
pM.Si treatment suppresses angiogenesis
in vivo
To confirm our in vitro findings, we examined
whether suppression of MMP-2 could inhibit tumor angiogenesis using
the dorsal air sac assay. Implantation of a chamber containing 4910
and 5310 cells as described in Materials and methods resulted in
the formation and development of new blood vessels (Fig. 4B) with curled, thin structures and
many tiny bleeding spots in addition to the pre-existing vessels.
However, a marked reduction in the development of such
tumor-induced microvasculature was observed in the pM.Si treatments
(with or without IR) in 4910 and 5310 cells as compared to mock and
pSV treatments.
Discussion
After radiation treatment of GBM primary tumors,
patients are at high risk of metastatic invasion due to extensive
angiogenic process (24). These
patients could benefit from the addition of MMP-2 inhibitors to
standard radiotherapy. Our study was designed to examine the
effects of combining MMP-2 inhibition and radiation on human
xenograft cells and their conditioned media (CM) on microtube
formation of endothelial cells (ECs) in vitro and in
vivo. Our results demonstrate that MMP-2-depleted-CM abrogated
IR-induced SDF-1/CXCR4 expression and PI3/AKT-mediated angiogenesis
in glioma xenograft cells. We also showed MMP-2 downregulation led
to weakened interaction of MMP-2 with integrin αvβ3, thereby
enhancing endothelial inhibition of microtubule formation.
Altered proteinase activity was observed in several
studies following irradiation of tumor cells and tissues.
Upregulation of MMP-2 subsequent to different irradiation
conditions was observed in different tumor types (e.g., pancreatic
cancer, glioblastoma, colorectal cancer and fibrosarcoma), leading
to enhanced cell invasion (25–31).
Gelatinases have been identified as major factors for high-grade
gliomas and their expression is directly correlated to the type of
malignancies (28). However, the
cellular and molecular events that promote GBM are not completely
understood. Since GBM cells are capable of switching their
dependency from one signaling pathway to an alternative pathway, it
is necessary to impede the gelatinase activity of MMP-2, which is
the main cause of diverse mechanisms for GBM survival. Several
studies have indicated the angiogenic process is mostly related to
alterations in the gelatinolytic activity of MMPs (28). Even though tumors invariably recur
within the radiation field, radiotherapy is a mainstay in the
treatment of GBM. Any method that improves local control of the
tumor by radiotherapy would improve the curability of patients with
GBM (32). Here, we propose that
the cure rates for this malignancy might be improved by targeting
MMP-2-mediated SDF-1/CXCR4 expression and pathway, thereby
preventing reconstitution of tumor vasculature following
radiation.
As an initial step of our study, we observed that
tumor-conditioned medium from irradiated 4910 and 5310 cells
enhanced capillary tube formation in ECs as compared to control-CM,
which correlated with high MMP-2 and SDF-1 expression levels. MMP-2
upregulation led to increase in CXCR4, PI3K and phosphorylation of
AKT (Ser 473) as compared to mock and pSV in 4910 and 5310 cells.
We investigated whether MMP-2-downregulated CM by pM.Si. plays any
role in curbing the molecular and morphological events in ECs.
pM.Si-CM alone or in combination with IR was capable of inhibiting
the upregulation of angiogenic molecules to a significant extent.
This result correlated with the results of our in vitro
angiogenesis assay; Matrigel assay revealed that CM of pM.Si with
or without IR showed impaired growth and enhanced branching
distortion of ECs compared to a highly branched network observed in
mock-and pSV-CM with or without IR. Upregulation of MMP-2 by IR
paves the way for degrading the underlying basement membrane and
provides a route for sprouting ECs. Similarly, IR-CM has generated
extensive tube formation on ECs, which was accompanied by increased
secretion/expression of SDF-1. The new microvessels formed in tumor
angiogenesis are abnormal and remain leaky and demonstrate other
morphologic abnormalities as they lack a properly formed basement
membrane (33,34). In contrast, IR in combination with
pM.Si effectively suppressed endothelial tube formation as compared
to mock- and pSV- CM. This substantiates the role of pM.Si-CM on
MMP-2-generated angiogenesis.
CXCR4 is recognized as the most commonly expressed
receptor on many types of cancer cells. Its upregulation has been
associated with metastasis and its suppression is accompanied by
inhibition of SDF-1-induced invasion of tumor cells (35). It has been demonstrated that low
doses of IR increase the expression of chemokines along with CXCR4
in human ECs (36). SDF-1/CXCR4
signaling is one of the major GBM survival factors because it
enhances endothelial tubule formation. Our results indicate that CM
of both cell lines treated with pM.Si abrogate IR-induced
SDF-1/CXCR4 expression on ECs compared to mock-CM and pSV-CM.
In our study, knockdown of MMP-2 at the
transcriptional level also prevented IR-induced phosphorylation of
AKT and PI3K as supported by distorted microtububle formation in
vitro. Moreover, supplementation of rhMMP-2 or rhSDF-1 reversed
the effect of pM.Si. The downstream molecules PI3K and AKT reversed
their expression levels after rhMMP-2 or rhSDF-1 supplementation in
pM.Si-treated CM, thereby confirming that molecular modulations are
due to the critical role of the pM.Si-mediated effect.
In the present study, we have established that
SDF-1/CXCR4 induced downstream signaling of PI3K and AKT. This
event might have generated more secretion of SDF-1, which in turn,
activated more MMP-2 exogenously. The PI3K/AKT pathway is involved
in prototypical endothelial functions including the regulation of
angiogenesis. In ECs, phosphoinositide 3-kinases are activated
downstream of several receptors, including G-protein-coupled
receptors (e.g., chemokine receptors), tyrosine kinases (e.g.,
vascular endothelial growth factor receptors), integrins and death
receptors (e.g., TNFα receptor). In turn, phosphoinositide 3-kinase
signaling promotes nitric oxide release (through endothelial nitric
oxide synthase phosphorylation), angiogenesis (through RhoA),
endothelial progenitor cell (EPC) recruitment and cell viability
and increasing evidence suggests that these angiogenic stimuli are
modulated by ionizing radiation (37–40).
Blood vessel growth is regulated by angiogenic CXC
chemokines and radiation is a vasculogenic stimulus. IR induces DNA
damage in the nucleus, triggering a large network of intracellular
signaling events. These include transient activation of
pro-survival pathways and PI3K/AKT signaling as well as
upregulation of the expression of the chemokine receptor CXCR4. Low
doses of radiation increase the expression of chemokines, including
CXCR4, in human ECs (36). We
investigated the effect of IR on endothelial cell chemokine
signaling, receptor expression and endothelial network formation.
The downstream signaling networks play an important role in tumor
radiosensitivity by eliciting pro-survival and pro-inflammatory
responses. The PI3K/AKT pathway, which is constitutively activated
in various cancers, plays a critical role in promoting endothelial
cell growth.
Integrins are extracellular matrix receptors
involved in angiogenesis. In order for angiogenesis to occur in
IR-treated or untreated xenograft cells, the binding of SDF-1 to
its receptor CXCR4 must occur. This leads to activation of ECs and
results in the upregulation of specific integrin receptors on the
cell surface, such as integrin αvβ3, as compared to mock and pSV
xenograft cells. Many studies have indicated that expression of
integrin αvβ3 is upregulated on the surface of proliferating ECs in
angiogenic microvessels, including those in glioblastoma (grade IV
malignant astrocytoma) (41–43).
We found that IR induced expression of αvβ3 in ECs. We analyzed the
combinational in vitro effects of IR (8 Gy) and pM.Si-CM by
measuring endothelial αvβ3 integrin expression. First,
immunocytochemical analysis revealed diminished integrin signaling
with SDF-1 when treated with pM.Si-CM with or without IR in ECs in
comparison with mock- and pSV-CM. IR upregulated αvβ3 expression
and activated AKT in ECs, thus forming a defense mechanism and
survival signal against IR damage (23). However, this effect was
counteracted by pM.Si-CM when combined with IR as compared to mock-
or pSV-CM. Second, treatment with pM.Si-CM resulted in the
inhibition of IR-induced AKT phosphorylation and enhancement of
IR-induced endothelial tubule formation in vitro. Third,
pM.Si with or without IR-CM treatment further impaired vascular
morphogenesis significantly. Finally, our in vivo
angiogenesis assay results substantiated the role of pM.Si in
preventing neovascularization.
The expression of MMP-2-mediated SDF-1/CXCR4 in ECs
was markedly attenuated in the presence of pM.Si-CM alone or
pM.Si-CM with IR (8 Gy). Based on the results of the present study,
we conclude it is conceivable that αvβ3 integrin-mediated
angiogenesis mechanisms after radiation toxicity can effectively be
interrupted by co-administration of pMMP-2 siRNA.
Acknowledgements
This study was supported by award
NS64535-01A2 (to J.S.R.) from the National Institute of
Neurological Disorders and Stroke. We thank Noorjehan Ali for
technical assistance. We also thank Susan Renner for manuscript
preparation, Diana Meister and Sushma Jasti for manuscript
review.
References
|
1
|
Ribatti D and Djonov V: Angiogenesis in
development and cancer today. Int J Dev Biol. 55:343–344. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kargiotis O, Geka A, Rao JS and Kyritsis
AP: Effects of irradiation on tumor cell survival, invasion and
angiogenesis. J Neurooncol. 100:323–338. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wachsberger P, Burd R and Dicker AP: Tumor
response to ionizing radiation combined with antiangiogenesis or
vascular targeting agents: exploring mechanisms of interaction.
Clin Cancer Res. 9:1957–1971. 2003.
|
|
4
|
Chetty C, Lakka SS, Bhoopathi P, Kunigal
S, Geiss R and Rao JS: Tissue inhibitor of metalloproteinase 3
suppresses tumor angiogenesis in matrix metalloproteinase
2-down-regulated lung cancer. Cancer Res. 68:4736–4745. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Badiga AV, Chetty C, Kesanakurti D, et al:
MMP-2 siRNA inhibits radiation-enhanced invasiveness in glioma
cells. PLoS One. 6:e206142011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kesanakurti D, Chetty C, Bhoopathi P,
Lakka SS, Gorantla B, Tsung AJ and Rao JS: Suppression of MMP-2
attenuates TNF-α induced NF-κB activation and leads to JNK mediated
cell death in glioma. PLoS One. 6:e193412011.
|
|
7
|
Robinson SD and Hodivala-Dilke KM: The
role of beta3-integrins in tumor angiogenesis: context is
everything. Curr Opin Cell Biol. 23:630–637. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Stupack DG, Puente XS, Boutsaboualoy S,
Storgard CM and Cheresh DA: Apoptosis of adherent cells by
recruitment of caspase-8 to unligated integrins. J Cell Biol.
155:459–470. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Brooks PC, Montgomery AM, Rosenfeld M,
Reisfeld RA, Hu T, Klier G and Cheresh DA: Integrin alpha v beta 3
antagonists promote tumor regression by inducing apoptosis of
angiogenic blood vessels. Cell. 79:1157–1164. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Barbero S, Bonavia R, Bajetto A, et al:
Stromal cell-derived factor 1alpha stimulates human glioblastoma
cell growth through the activation of both extracellular
signal-regulated kinases 1/2 and Akt. Cancer Res. 63:1969–1974.
2003.
|
|
11
|
Shen XY, Wang SH, Liang ML, Wang HB, Xiao
L and Wang ZH: The role and mechanism of CXCR4 and its ligand SDF-1
in the development of cervical cancer metastasis. Ai Zheng.
27:1044–1049. 2008.(In Chinese).
|
|
12
|
Zou YR, Kottmann AH, Kuroda M, Taniuchi I
and Littman DR: Function of the chemokine receptor CXCR4 in
haematopoiesis and in cerebellar development. Nature. 393:595–599.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kucia M, Jankowski K, Reca R, et al:
CXCR4-SDF-1 signalling, locomotion, chemotaxis and adhesion. J Mol
Histol. 35:233–245. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Giannini C, Sarkaria JN, Saito A, et al:
Patient tumor EGFR and PDGFRA gene amplifications retained in an
invasive intracranial xenograft model of glioblastoma multiforme.
Neurooncology. 7:164–176. 2005.PubMed/NCBI
|
|
15
|
Kesanakurti D, Chetty C, Dinh DH, Gujrati
M and Rao JS: Role of MMP-2 in the regulation of IL-6/Stat3
survival signaling via interaction with alpha5beta1 integrin in
glioma. Oncogene. 32:327–340. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kesanakurti D, Chetty C, Maddirella DR,
Gujrati M and Rao JS: Essential role of cooperative NF-κB and Stat3
recruitment to ICAM-1 intronic consensus elements in the regulation
of radiation-induced invasion and migration in glioma. Oncogene.
Nov 26–2012.(Epub ahead of print). View Article : Google Scholar
|
|
17
|
Chetty C, Lakka SS, Bhoopathi P and Rao
JS: MMP-2 alters VEGF expression via aVB3 integrin-mediated PIK/AKT
signaling in A549 lung cancer cells. Int J Cancer. 127:1081–1095.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kesanakurti D, Chetty C, Maddirela DR,
Gujrati M and Rao JS: Functional cooperativity by direct
interaction between PAK4 and MMP-2 in the regulation of
anoikis-resistance, migration and invasion in glioma. Cell Death
Dis. (In Press).
|
|
19
|
Salvucci O, Yao L, Villalba S, Sajewicz A,
Pittaluga S and Tosato G: Regulation of endothelial cell branching
morphogenesis by endogenous chemokine stromal-derived factor-1.
Blood. 99:2703–2711. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bhoopathi P, Chetty C, Gogineni VR,
Gujrati M, Dinh DH, Rao JS and Lakka SS: MMP-2 mediates mesenchymal
stem cell tropism towards medulloblastoma tumors. Gene Ther.
18:692–701. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Trisciuoglio D, Iervolino A, Zupi G and
Del Bufalo D: Involvement of PI3K and MAPK signaling in
bcl-2-induced vascular endothelial growth factor expression in
melanoma cells. Mol Biol Cell. 16:4153–4162. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Berglin L, Sarman S, van der Plloeg I, et
al: Reduced choroidal neovascular membrane formation in matrix
metallo proteinase-2-deficient mice. Invest Ophthalmol Vis Sci.
44:403–408. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Abdollahi A, Griggs DW, Zieher H, et al:
Inhibition of alpha(v) beta3 integrin survival signaling enhances
antiangiogenic and antitumor effects of radiotherapy. Clin Cancer
Res. 11:6270–6279. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Prise KM, Schettino G, Folkard M and Held
KD: New insights on cell death from radiation exposure. Lancet
Oncol. 6:520–528. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Albert JM, Cao C, Geng L, Leavitt L,
Hallahan DE and Lu B: Integrin alpha v beta 3 antagonist
Cilengitide enhances efficacy of radiotherapy in endothelial cell
and non-small-cell lung cancer models. Int J Radiat Oncol Biol
Phys. 65:1536–1543. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Monferran S, Skuli N, Delmas C, Favre G,
Bonnet J, Cohen-Jonathan-Moyal E and Toulas C: Alphavbeta3 and
alphavbeta5 integrins control glioma cell response to ionising
radiation through ILK and RhoB. Int J Cancer. 123:357–364. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Monnier Y, Farmer P, Bieler G, et al:
CYR61 and alphaVbeta5 integrin cooperate to promote invasion and
metastasis of tumors growing in preirradiated stroma. Cancer Res.
68:7323–7331. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rao JS: Molecular mechanisms of glioma
invasiveness: the role of proteases. Nat Rev Cancer. 3:489–501.
2003. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Qian LW, Mizumoto K, Urashima T, et al:
Radiation-induced increase in invasive potential of human
pancreatic cancer cells and its blockade by a matrix
metalloproteinase inhibitor, CGS27023. Clin Cancer Res.
8:1223–1227. 2002.
|
|
30
|
Trog D, Yeghiazaryan K, Fountoulakis M, et
al: Pro-invasive gene regulating effect of irradiation and combined
temozolomide-radiation treatment on surviving human malignant
glioma cells. Eur J Pharmacol. 542:8–15. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Speake WJ, Dean RA, Kumar A, Morris TM,
Scholefield JH and Watson SA: Radiation induced MMP expression from
rectal cancer is short lived but contributes to in vitro invasion.
Eur J Surg Oncol. 31:869–874. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tseng D, Vasquez-Medrano DA and Brown JM:
Targeting SDF-1/CXCR4 to inhibit tumour vasculature for treatment
of glioblastomas. Br J Cancer. 104:1805–1809. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hanahan D and Folkman J: Patterns and
emerging mechanisms of the angiogenic switch during tumorigenesis.
Cell. 86:353–364. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liekens S, De Clercq E and Neyts J:
Angiogenesis: regulators and clinical applications. Biochem
Pharmacol. 61:253–270. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Balkwill F: The significance of cancer
cell expression of the chemokine receptor CXCR4. Semin Cancer Biol.
14:171–179. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chang CC, Lerman OZ, Thanik VD, et al:
Dose-dependent effect of radiation on angiogenic and angiostatic
CXC chemokine expression in human endothelial cells. Cytokine.
48:295–302. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Heissig B, Rafii S, Akiyama H, et al:
Low-dose irradiation promotes tissue revascularization through VEGF
release from mast cells and MMP-9-mediated progenitor cell
mobilization. J Exp Med. 202:739–750. 2005. View Article : Google Scholar
|
|
38
|
Moeller BJ, Cao Y, Li CY and Dewhirst MW:
Radiation activates HIF-1 to regulate vascular radiosensitivity in
tumors: role of reoxygenation, free radicals and stress granules.
Cancer Cell. 5:429–441. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Garcia-Moruja C, Alonso-Lobo JM, Rueda P,
et al: Functional characterization of SDF-1 proximal promoter. J
Mol Biol. 348:43–62. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Fujimori A, Okayasu R, Ishihara H, et al:
Extremely low dose ionizing radiation up-regulates CXC chemokines
in normal human fibroblasts. Cancer Res. 65:10159–10163. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Brooks PC, Clark RA and Cheresh DA:
Requirement of vascular integrin alpha v beta 3 for angiogenesis.
Science. 264:569–571. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Brooks PC: Role of integrins in
angiogenesis. Eur J Cancer. 32A:2423–2429. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Gladson CL: Expression of integrin alpha v
beta 3 in small blood vessels of glioblastoma tumors. J Neuropathol
Exp Neurol. 55:1143–1149. 1996. View Article : Google Scholar : PubMed/NCBI
|