Introduction
Nano-scale particle research has recently become a
very important field in materials science. Nanoparticles (1 to 100
nm) usually have physical properties different from those of large
particles (1–10 μm). It has been found that nanoparticles
exhibit a variety of novel properties, depending on particle size,
including magnetic, optical, and other physical properties as well
as surface reactivity (1). Hard
metals consisting of tungsten carbide (WC) and metallic cobalt (Co)
particles are important industrial materials (2). WC-Co nanoparticle composites have the
potential to replace standard materials for tools and wear parts
because of their increased hardness and toughness. By means of
particle size reduction, the fracture toughness and wear resistance
of WC-Co can be increased significantly. Research evidence
indicated that WC-Co fine particle mixture was carcinogenic in
exposed workers (2). Based on the
fact that nanoparticles of metallic nickel and titanium dioxide
(TiO2) cause more pronounced toxicity than fine
particles (3–7), the pathogenic effects of WC-Co
particles may well vary with their size.
Among the possible mechanisms of metal toxicity,
apoptosis plays an important role in carcinogenesis (8). While a number of known or suspected
human carcinogenic metallic compounds have been shown to induce
apoptosis, the relevance of these observations for the carcinogenic
process is, however, still not completely understood (9). Apoptosis induced by WC-Co fine
particles has been reported in previous in vitro studies
(2,9); however, the signal pathways induced
by WC-Co particles still remain elusive.
Apoptosis is a highly regulated process that is
involved in physiological as well as pathological conditions
(10–13). Deregulation of apoptosis has been
implicated in carcinogenesis, tumor progression and resistance of
tumor cells to radio- and chemotherapy (14,15).
Malfunction of apoptosis plays an important role in many disease
processes. An inefficient elimination of mutated cells may favor
carcinogenesis or tumorigenesis; failure to clear inflammatory
cells may prolong the inflammation because of the release of
damaging histotoxins (9). However,
excessive apoptosis has been shown to contribute to pulmonary
fibrosis in mice (16).
Furthermore, enhanced apoptosis may indirectly trigger compensatory
cell proliferation to ensure tissue homeostasis and promote the
fixation of mutagenic events or multiplication of mutated cells.
Studies have demonstrated that apoptosis is also involved in
pulmonary disorders, such as acute lung injury, diffuse alveolar
damage, idiopathic pulmonary fibrosis, and other lung disorders
caused by bleomycin, silica, endotoxins, and the deposition of
immune complexes (16–19). Inhibition of apoptosis by gene
deletion strategies or by caspase inhibitors abrogate the
pathologic effects of these agents (16–20),
supporting the potential role of apoptosis in the inflammatory,
pulmonary fibrosis, and immunopathologic disorders. Therefore, the
apoptotic properties may be important for elucidating the
mechanisms of adverse health effects induced by WC-Co
particles.
Accordingly, the objectives of this study are to
compare the difference in cytotoxicity and apoptosis induced by
WC-Co nano- and fine particles, and to elucidate the mechanisms of
cell death induced by WC-Co particles. This study provides insight
into the role of apoptosis in the possible pathogenicity and
carcinogenicity induced by WC-Co nanoparticles.
Materials and methods
Materials
WC-Co nanoparticles (99.9% pure, molecularly mixed
at the ratio of 85:15, agglomerated powder) were purchased from
Inframat® Advanced Materials™ LLC (Farmington, CT, USA).
WC-Co fine particles were purchased from Alfa ASAR. Eagle’s minimal
essential medium (EMEM) was obtained from Lonza (Walkersville, MD).
Fetal bovine serum (FBS), trypsin, pencillin/streptomycin and
L-glutamine were purchased from Life Technologies Inc.
(Gaithersburg, MD). YO-PRO-1 [YP, 1 mM solution in dimethyl
sulfoxide (DMSO)] and propidium iodide (PI, 1.0 mg/ml in water)
were purchased from Invitrogen (Carlsbad, CA). Hoechst 33342, 2′,
7′-dichlorodihydrofluorescein diacetate (H2DCFDA) and
dihydroethidium (DHE) were purchased from Molecular Probes (Eugene,
OR). Anti-h/m caspase-8 antibody was obtained from R&D Systems
(Minneapolis, MN). BID and cleaved caspase 3 antibodies were
purchased from Cell Signaling Technology (Beverley, MA). All other
antibodies were obtained from Santa Cruz Biotechnology Co. (Santa
Cruz, CA). Cell proliferation kit I (MTT assay kit) was obtained
from Roche Applied Science (Penzberg, Germany). Mitochondria
Staining Kit and catalase were purchased from Sigma-Aldrich (Saint
Louis, MO).
Preparation of WC-Co particles
Stock solutions of WC-Co nano- or fine particles
were prepared by sonification on ice using a Branson Sonifier 450
(Branson Ultrasonics Corp., Danbury, CT) in sterile PBS (10 mg/ml)
for 30 sec, then kept on ice for 15 sec and sonicated again for a
total of 3 min at a power of 400 W. Before use, these particles
were diluted to a designed concentration in fresh culture medium.
All samples were prepared under sterile conditions.
Surface area and size distribution
measurements
Surface area of WC-Co particles was measured using
the Gemini 2360 Surface Area Analyzer (Mircomeritics, Norcross, GA)
with a flowing gas technique according to the manufacturer’s
instructions. The size distribution of WC-Co particles was detected
using scanning electron microscopy (SEM). Briefly, WC-Co particles
were prepared by sonification. Then, the samples were diluted in
double-distilled water and air dried onto a carbon planchet. Images
were collected on a scanning electron microscope (Hitachi S-4800,
Japan) according to the manufacturer’s instructions. Optimas 6.5
image analysis software (Media Cybernetics, Bethesda, MD) was used
to measure the diameter of WC-Co particles.
Cell culture
Mouse epidermal JB6 cells were maintained in 5% FBS
EMEM containing 2 mM L-glutamine and 1% penicillin-streptomycin
(10,000 U/ml penicillin and 10 mg/ml streptomycin) at standard
culture conditions (37°C, 80% humidified air and 5%
CO2). For all treatments, cells were grown to 80%
confluence.
Determination of free radical
formation
All ESR measurements were conducted using a Varian
E9 ESR spectrometer and a flat cell assembly. Hyperfine couplings
were measured (to 0.1 G) directly from magnetic field separation
using potassium tetraperoxochromate (K3CrO8)
and 1,1-diphenyl-2-picrylhydrazyl as reference standards. An EPR
DAP 2.0 program was used for data acquisition and analyses.
Reactants were mixed in test tubes in a total final volume of 450
μl. The reaction mixture was then transferred to a flat cell
for ESR measurement. The concentrations given in the figure legends
are final concentrations and measurements were made at room
temperature and under ambient air, except those specifically
indicated otherwise. The receiver gain, time constant, sweep time,
sweep width, modulation frequency, modulation amplitude and
microwave power were set constant to allow relative intensity
comparisons of spectra. All spectra shown are the accumulation of
five scans. Hyperfine couplings constants were determined using the
WinSim program of the NIEHS public EPR software tools, available
over the internet (http://epr.niehs.nih.gov). The relative radical
concentration was estimated by measuring the peak-to-peak height
(mm) of the observed spectra.
H2DCFDA and DHE are used for staining general ROS or
oxygen radicals (•O2−) produced in intact
cells, respectively. Hoechst 33342 is a nucleic acid stain. JB6
cells were seeded into a 24-well plate. Cells were grown 24 h and
then starved in 0.1% FBS EMEM overnight. Cells were then treated
with/without various concentrations of WC-Co nano- or fine
particles in the presence of H2DCFDA (5 μM), DHE (2
μM) and Hoechst 33342 (3 μM) for 1 h. The cells were
washed 3 times with PBS, followed by addition of fresh 0.1% FBS
EMEM. The images were captured with a fluorescence microscope
(Axiovert 100M, Zeiss, Germany).
Cytotoxicity assay
Cytotoxicity of WC-Co particles to JB6 cells was
assessed by an MTT assay kit following the manufacturer’s
instructions. Briefly, cells were plated in 100 ml EMEM at a
density of 104 cells/well in a 96-well plate. The cells
were grown for 24 h and then, treated with various concentrations
of WC-Co particles. After 24-h incubation, 10 ml MTT labeling
reagent was added in each well and the plates were further
incubated for 4 h. Afterward, 100 ml solubilization solution was
added to each well and the plate was incubated overnight at 37°C.
The optical density (OD) of the wells was measured at a wavelength
of 575 nm with reference of 690 nm using an ELISA plate reader.
Results were calculated using the OD measured without cells.
Detection of apoptosis
YP staining was used to detect cell apoptosis in JB6
cells. Briefly, cells were seeded onto a 24-well plate overnight.
Then, cells were treated with/without various concentrations of
WC-Co nano- or fine particles for 24 h. Before microscopy, YP dye
was added into the cultures (10 μg/ml) and cells were
further incubated for 1 h. Then, cells were washed two times with
EMEM medium. Apoptotic cells were monitored using a fluorescent
microscope (Axiovert 100M). Percentage of cells exhibiting
apoptosis was calculated.
Sub-cellular fractionation and western
blot analysis
Briefly, cells were plated onto a 100×20 mm cell
culture dish. The cultures were grown for 24 h and then starved in
0.1% FBS EMEM overnight. Cells were treated with/without WC-Co
nano- or fine particles. After treatment, the cells were extracted
with 1X SDS sample buffer supplemented with protease inhibitor
cocktail (Sigma-Aldrich). Protein concentrations were determined
using the bicinchoninic acid method (Pierce, Rockford, IL). Equal
amounts of proteins were separated by 4–12% Tris glycine gels.
Immunoblots for expression of Fas, FADD, caspase 3, caspase 8,
caspase 9, BID, cleaved BID, BAX, Bcl-2, cytochrome c, AIF,
lamin A/C, β-actin and β-tubulin were detected. Experiments were
performed three or more times, and equal loading of protein was
ensured by measuring β-actin and β-tubulin expression.
To prepare the subcellular fractionation, after
treatment cells were washed twice with cold PBS. Then, cells were
resuspended in 50 μl 1X Cytosol Extraction Buffer Mix and
incubated on ice for 10 min. Cells were homogenized by passing
through 22-gauge needles 30 times. The lysate was centrifuged at
700 × g for 10 min at 4°C to remove unbroken cells and nuclei. The
supernatant was then re-centrifuged at 10,000 × g for 30 min at
4°C. The resulting supernatant was the cytosolic fraction while the
pellet contained mitochondria. The cytosolic fraction was diluted
using 50 μl of 2X SDS sample buffer. The mitochondrial
pellet was resuspended in 20 μl Mitochondria Extraction
Buffer Mix plus 20 μl of 2X SDS sample buffer. These two
different fractions were used for western blot analysis.
Detection of mitochondrial membrane
permeability
JB6 cells were seeded onto a 24-well plate
overnight. Cells were treated with/without WC-Co nano- or fine
particles supplemented with or without catalase (10,000 U/ml) for
24 h. Changes in mitochondrial membrane permeability were evaluated
using a mitochondrial staining kit (JC1 staining) according to the
manufacturer’s instructions. Briefly, a staining mixture was
prepared by mixing the staining solution with an equal volume of
the EMEM medium. Cells were incubated in the staining mixture (0.4
ml/well) for 30 min at 37°C in a humidified atmosphere containing
5% CO2. Thereafter, cells were washed two times in
medium, followed by addition of fresh medium. Mitochondrial
membrane permeability was monitored on a fluorescence microscope
(Axiovert 100M).
Isolation of rat lung macrophages
Brown Norway rats were obtained from Charles River
Laboratories (Wilmington, MA). The animals were housed in
AAALA-accredited facility, specific-pathogen-free, environmentally
controlled facility located at National Institute for Occupational
Safety and Health (Morgantown, WV). The rats were monitored to be
free of endogenous viral pathogens, parasites, mycoplasma,
helicobacter and CAR Bacillus. Rats were acclimated
for at least 5 days before use, and were housed in ventilated cages
that were provided with HEPA-filtered air, with α-Dri virgin
cellulose chips and hardwood β-chips used as bedding. The rats were
maintained on ProLab 3500 diet and tap water, both of which were
provided ad libitum. Rats were anesthetized using brevital
sodium (40 mg/kg), and exposed to 100 μl/rat of 1% WC-Co
nanoparticles suspended in sterile saline (100 μl of saline
for control rat) by intranasal droplet application 4 times (totally
4 g/rat) on day 0, 7, 14 and 21, respectively. At 24 h after the
last treatment, rats were sacrificed by intraperitoneal injection
of 0.2 g/kg pentobarbital, and then the descending aorta was cut.
The trachea was exposed, and a cannula was inserted.
Bronchoalveolar lavage fluid (BALF) was collected using saline.
After centrifugation, cells in BALF were seeded into a 6-well plate
in 5% FBS EMEM containing 2 mM L-glutamine and 1%
penicillin-streptomycin (10,000 U/ml penicillin and 10 mg/ml
streptomycin) at standard culture conditions (37°C, 80% humidified
air and 5% CO2). After 4-h incubation, the unattached
cells were washed out by using 5% FBS EMEM for 3 times. Attached
cells (rat lung macrophages) were used for mitochondrial membrane
permeability or apoptotic detections. Mitochondrial membrane
permeability of rat lung macrophages was evaluated using a
mitochondrial staining kit according to the manufacturer’s
instructions as stated above.
Apoptotic detection of rat lung
macrophages
Dual staining using YP and PI were used to
distinguish between apoptosis and necrosis (or late apoptosis) as
described previously (21,22) with some modifications. Rat lung
macrophages were obtained as stated above. YP and PI were added
into the cultures with a final concentration of 10 μg/ml and
1 μM, respectively. Thereafter, rat lung macrophages were
incubated in 5% FBS EMEM medium containing YP and PI dyes at 37°C
in a humidified atmosphere containing 5% CO2 for 1 h.
Then, cells were washed two times in medium, followed by addition
of fresh medium. Apoptotic cells were monitored using a
fluorescence microscope (Axiovert 100M). YP stained cells were
detected with a blue excitation filter. PI stained cells were
measured using a green excitation filter.
Statistical analysis
Data are presented as means ± standard errors (SE)
of the number of experiments/samples. Data were analyzed using
one-way analysis of variance (ANOVA). Significance was set at
p≤0.05.
Results
Surface area and size distribution of
WC-Co particles
Gemini 2360 Surface Area Analyzer and scanning
electron microscopy were used to measure the surface area and size
distribution of WC-Co particles, respectively. The average surface
area of WC-Co nano and fine particles were published previously
(23). The average surface area of
WC-Co nanoparticles was 2.73 m2/g compared to 0.16
m2/g for fine particles. The average size distribution
of WC-Co nano- and fine particles is 95.53 nm and 39.00 μm,
respectively.
Free radical generation induced by WC-Co
particles in JB6 cells
The ESR spin trapping technique and fluorescent
staining were used to detect free radical generation during
incubation of WC-Co nano- or fine particles with JB6 cells
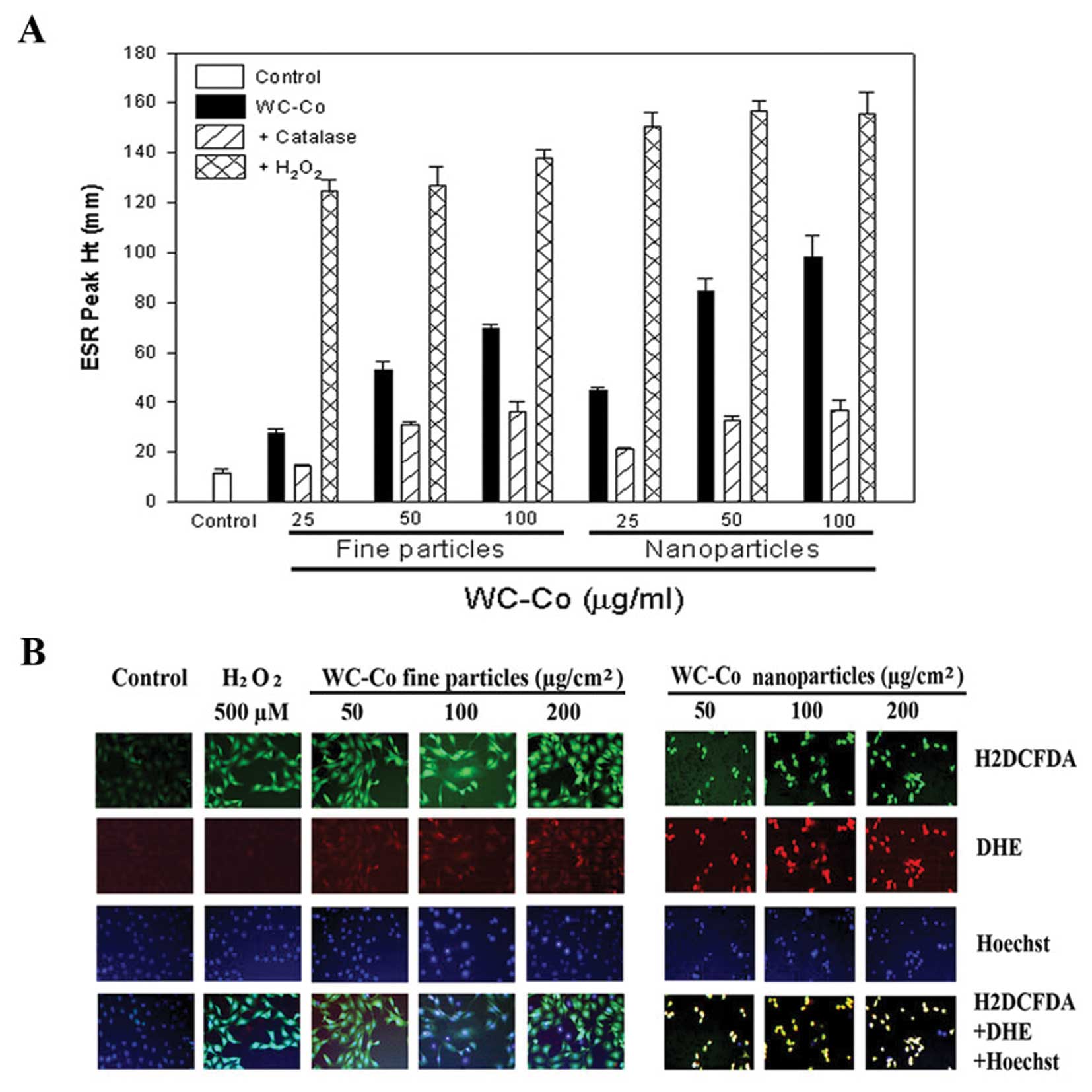
(Fig. 1). Using the ESR spin
trapping technique, we observed the formation of free radicals in
JB6 cells exposed to WC-Co nano- or fine particles. After 5 min
exposure of JB6 cells to WC-Co particles, DMPO radical adducts were
recorded. The ESR signal from WC-Co nanoparticles was much stronger
than fine particles (Fig. 1A).
Addition of catalase, an H2O2 scavenger
decreased the radical generation, while addition of
H2O2 dramatically increased the ROS adduct
signal (Fig. 1A). These results
suggest that ROS generated during exposure of WC-Co particles to
JB6 cells was formed via a metal-dependent Fenton reaction.
H2DCFDA, a general ROS sensitive dye, and DHE, an
•O2− specific dye, were used to monitor ROS
generation induced by WC-Co particles in intact cells. Hoechst
33342 was used for nucleic localization. Results showed that WC-Co
nanoparticles induced stronger ROS generation, indicated by DHE
staining, compared to fine particles (Fig. 1B).
Effects of WC-Co particles on cell
viability and apoptotic induction
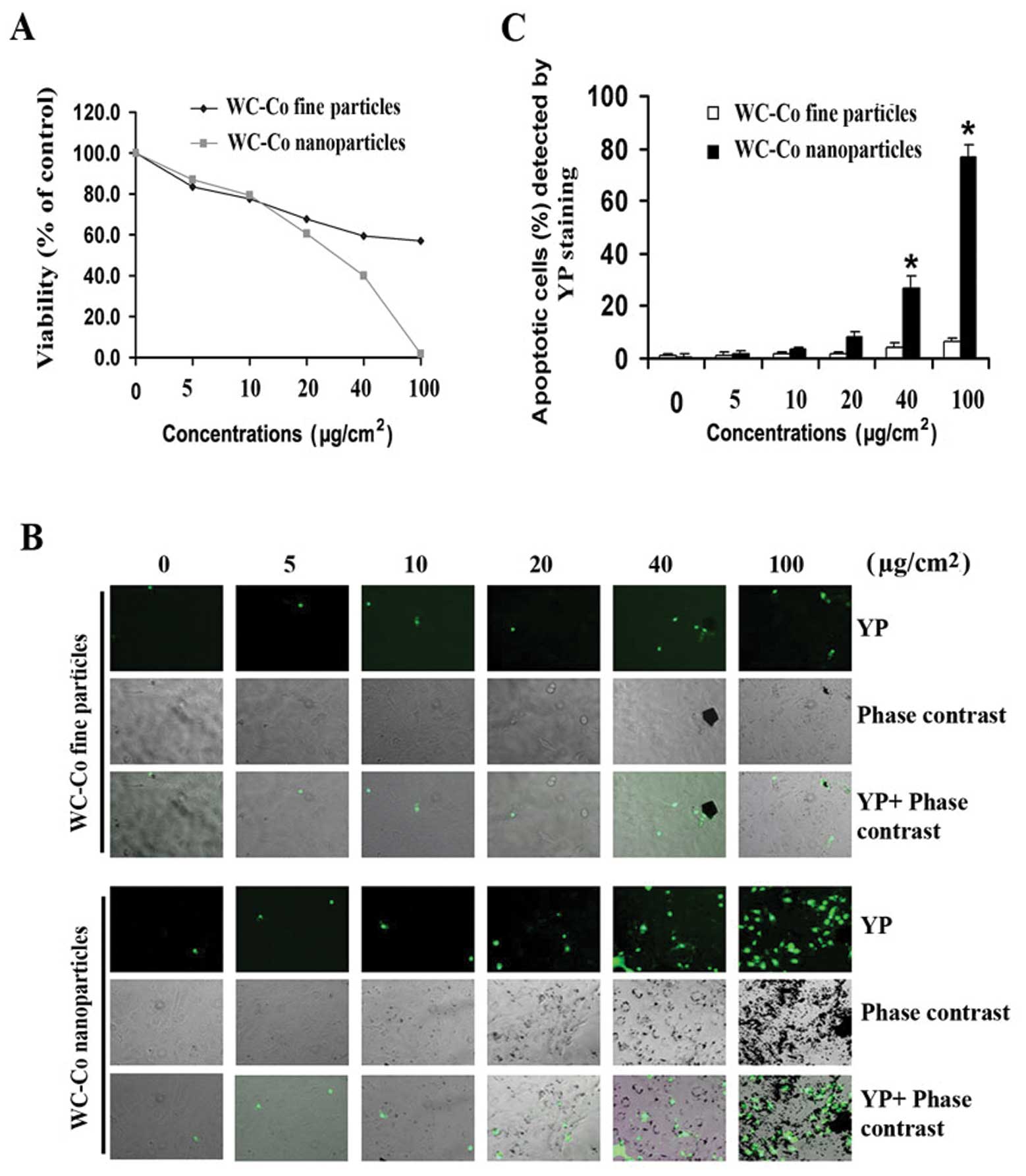
To determine whether there is a difference in the
cytotoxicity induced by different sizes of WC-Co particles, various
concentrations (5–100 μg/cm2) of WC-Co nano- or
fine particles were used to study the effects on cell viability in
JB6 cells using the MTT assay (Fig.
2). After 24-h treatment, both WC-Co nano- and fine particles
caused a dose-dependent cytotoxicity in JB6 cells (Fig. 2A). Cytotoxicity induced by WC-Co
nanoparticles was significantly higher than fine particles between
the concentration of 40 and 100 μg/cm2. To detect
apoptosis induced by WC-Co particles, YP staining was used. Results
indicated that both WC-Co nano- and fine particles induced JB6 cell
apoptosis (Fig. 2B). The
percentage of apoptotic cells was significantly higher in cells
treated with nanoparticles than fine particles between the
concentration of 40 and 100 μg/cm2. At the
concentration of 100 μg/cm2, there was an 8-fold
increase in apoptosis induced by nanoparticles compared to fine
particles (Fig. 2C).
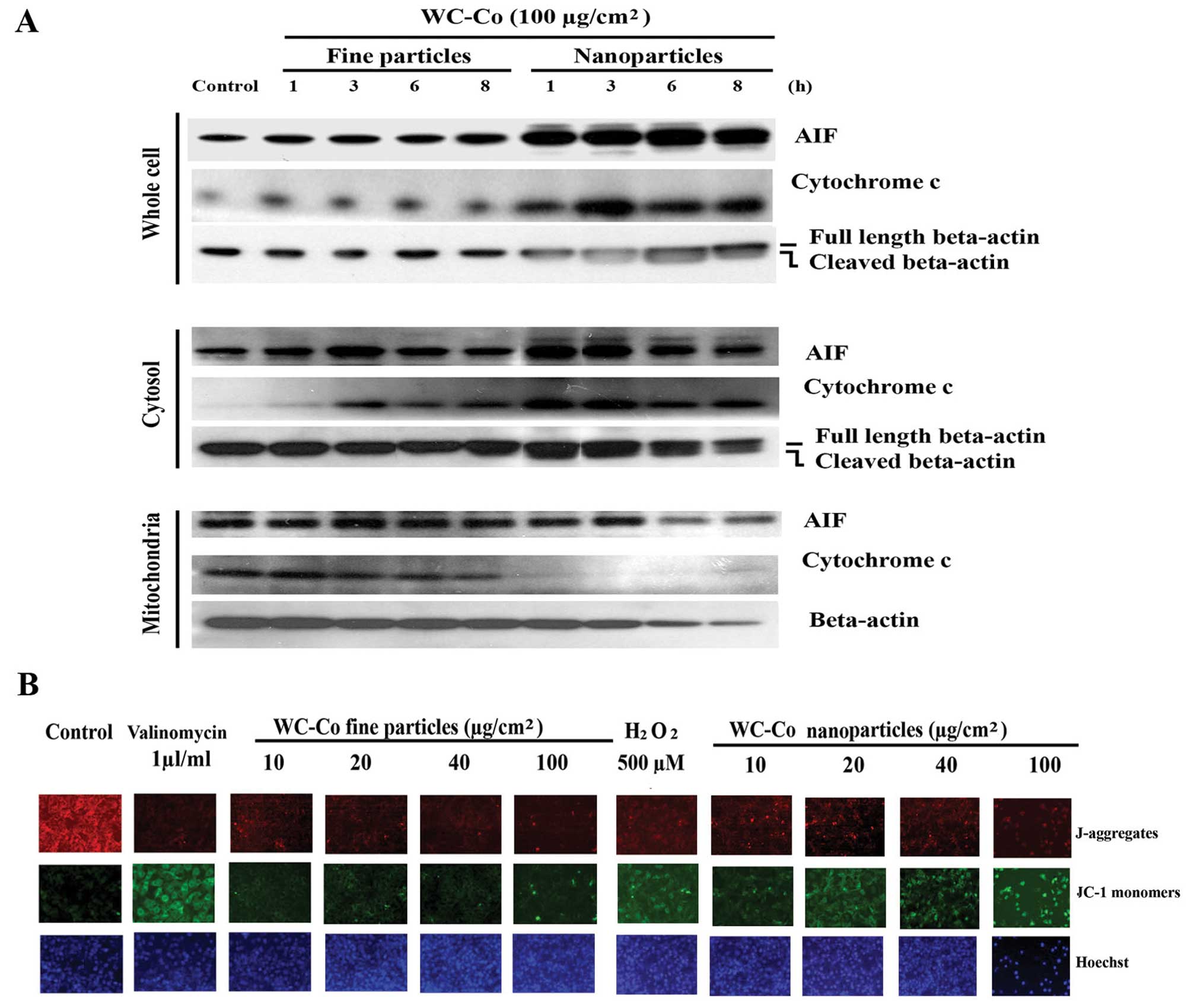
Effects of WC-Co particles on Fas, FADD,
caspase 3, 8 and 9, lamin A/C, BID, BAX and Bcl-2
To investigate the involvement of extrinsic signals
in the apoptotic process induced by WC-Co particles, expression of
Fas and FADD was examined. JB6 cells were treated with WC-Co nano-
or fine particles (100 μg/cm2) for 1, 3, 6 and 8
h, respectively, and then the protein expression patterns were
detected by western blot analysis (Fig. 3). Results demonstrated that WC-Co
nano- and fine particles, especially nanoparticles, stimulated Fas
and FADD expression.
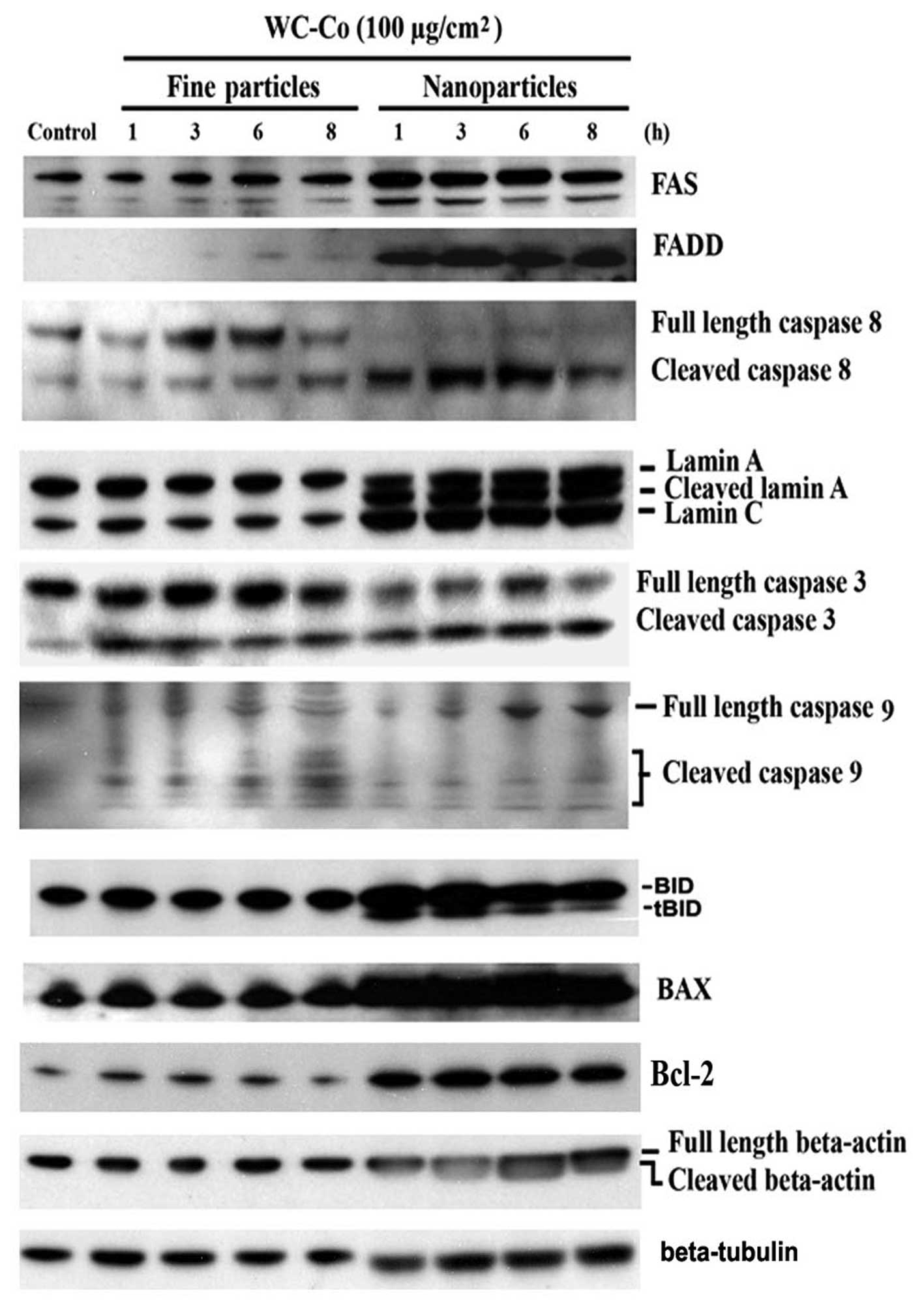 | Figure 3Effects of WC-Co particles on Fas,
FADD, caspase 3, 8 and 9, lamin A/C, BAX, BID and Bcl-2. JB6 cells
were seeded onto a culture dish and incubated overnight. Cells were
treated with 100 μg/cm2 WC-Co nanoor fine
particles for 1, 3, 6 and 8 h. Expressions of Fas, FADD, caspase 3,
8, and 9, lamin A/C, BAX, BID and Bcl-2 were analyzed by western
blot analysis. Expression of β-tubulin was set as a protein loading
control. |
Caspases are a family of cysteine proteases which
play essential roles in apoptosis, necrosis and inflammation
(24). Eleven caspases have been
identified in humans. There are two types of apoptotic caspases:
initiator caspases and effector caspases. Initiator caspases (e.g.
caspase 8) cleave inactive pro-forms of effector caspases, thereby
activating them. Effecter caspases (e.g. caspase 3) in turn cleave
other protein substrates resulting in the apoptotic process. Our
results indicated that caspase 3 and 8 were significantly cleaved
and a slight activation of caspase 9 was observed. BAX and BID, two
proapoptotic members of Bcl-2 family, were upregulated by WC-Co
particles. Bcl-2, an anti-apoptotic factor, was also upregulated.
The cellular morphology associated with the apoptotic process has
been well characterized by membrane blebbing, formation of
apoptotic bodies, and chromosome condensation. These apoptotic
changes are the result of the cleavage of cellular proteins, such
as lamin and actin (25,26). In this study, cleavage of lamin A
and β-actin were observed, especially in cells treated with WC-Co
nanoparticles. The cleavage of lamin A and β-actin were detected as
early as 1 h post-exposure of WC-Co nanoparticles. Interestingly,
WC-Co nanoparticles induced lamin C upregulation without
cleavage.
Effects of WC-Co particles on cytochrome
c and AIF
The intrinsic pathway of apoptosis is initiated in
the mitochondria. Its activation involves release of cytochrome
c and other proapoptotic factors from the mitochondrial
intermembrane space (27). AIF is
a proapoptotic factor, a mitochondrial protein (28). It is normally confined to the
mitochondrial intermembrane space (29). After release from mitochondria into
the cytoplasm, both cytochrome c and AIF can stimulate cell
apoptosis by initiating the intrinsic pathway. To test the effects
of WC-Co particles on the intrinsic pathway of apoptosis, JB6 cells
were treated with nano- or fine particles (100
μg/cm2) for 1, 3, 6 and 8 h, respectively.
Western blots revealed that both nano- and fine particles induced
cytochrome c and AIF upregulation as well as release from
mitochondria to the cytoplasm after 1 h treatment, with nano-WC-Co
exhibiting greater potency (Fig.
4A).
Effects of WC-Co particles on
mitochondrial membrane permeability
Mitochondrial membrane permeability change is a
hallmark for apoptosis (30). JB6
cells were treated with or without various concentrations of WC-Co
particles for 24 h. Mitochondrial membrane permeability was
evaluated using a JC-1 staining kit according to the manufacturer’s
instructions. The results indicated that both WC-Co nano- and fine
particles induced a significant change in the mitochondrial
membrane permeability compared to control, with nano WC-Co
exhibiting greater potency. Valinomycin (1 μl/ml culture
medium) and H2O2 (500 μM) for 1 h were
used as positive controls and Hoechst 33342 was used for nucleic
localization (Fig. 4B).
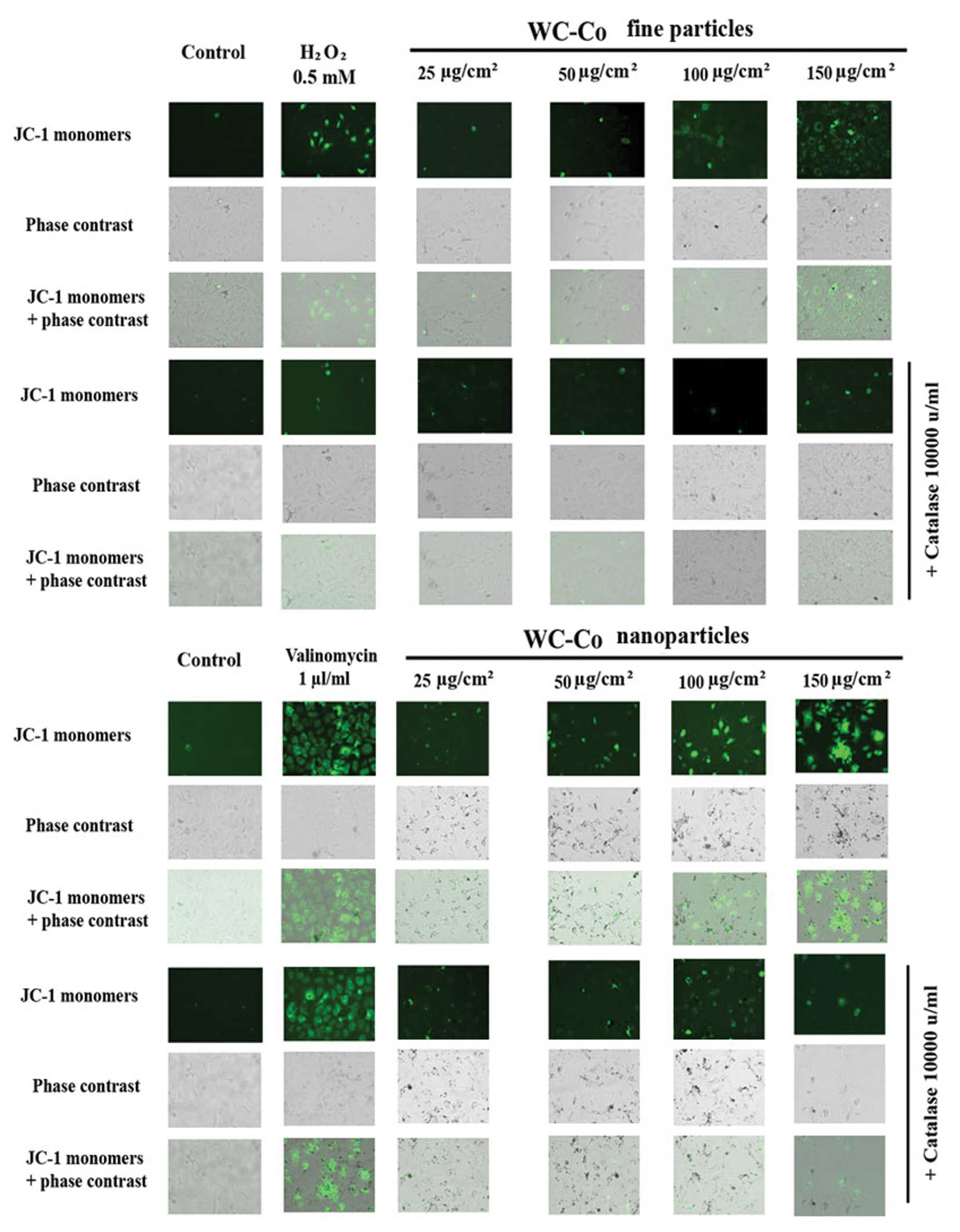
Effect of catalase on WC-Co
particle-induced mitochondrial membrane permeability
alteration
According to the results obtained above as well as
our previous studies (23), ROS
may play an important role in WC-Co particle-induced mitochondrial
membrane permeability alteration. To verify this hypothesis, the
inhibitory effect of catalase on WC-Co particle-induced
mitochondrial membrane permeability alteration was examined by
using a mitochondrial staining kit (Fig. 5). The results showed that catalase
could slightly inhibit any WC-Co particle-induced mitochondrial
membrane permeability damage. In addition, catalase significantly
inhibited mitochondrial membrane permeability damage induced by
H2O2 (a free radical-apoptotic reagent), but
not by valinomycin (a non-free radical-apoptotic reagent).
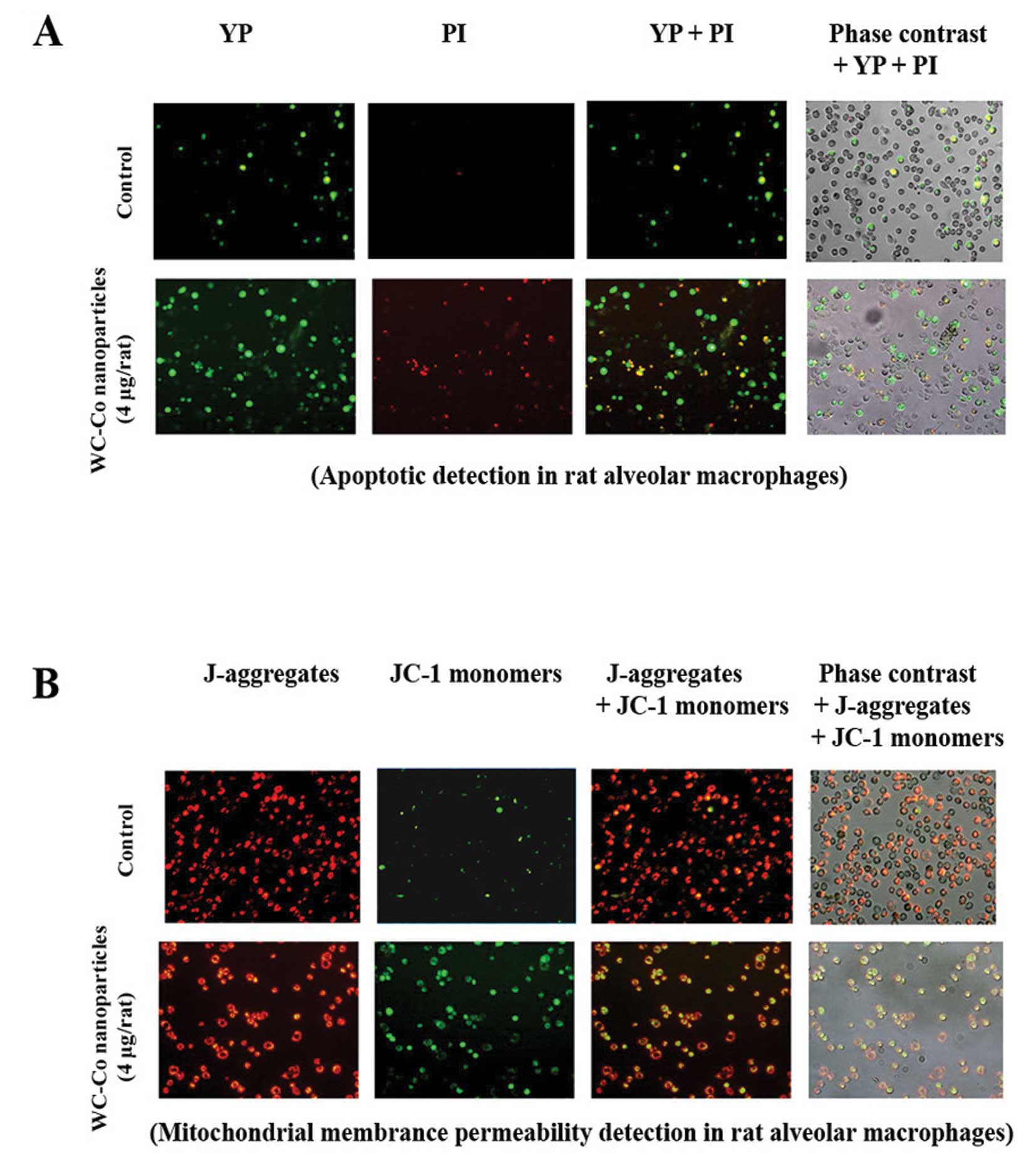
Apoptosis and mitochondrial membrane
permeability alteration induced by WC-Co nanoparticles in rat lung
macrophages
Apoptosis and mitochondrial membrane permeability
alteration induced by WC-Co nanoparticles in rat lung macrophages
were investigated as described in Materials and methods. Results
indicated that lung macrophages from WC-Co nanoparticle-treated
rats exhibited more apoptosis and mitochondrial membrane
permeability damage compared to control (Fig. 6).
Discussion
With the increased use of nanoparticles in modern
industries, inhaled nanoparticles are increasingly being recognized
as a potential health threat (31). Hard metal, a mixture of WC-Co, is
an important industrial material. Occupational exposure of workers
to the WC-Co particle mixture has been shown to be carcinogenic,
and Co itself can also induce occupational asthma (32). WC-Co nanoparticles were introduced
in industry (33) to improve
hardness and toughness compared to standard WC-Co materials. The
increasing use of WC-Co nanoparticles may lead to an increased
human exposure and adverse health effects (34). The International Agency for
Research on Cancer (IARC) has classified Co and its compounds as
possibly carcinogenic to humans (35). However, the molecular mechanisms
involved in hard metal-induced carcinogenesis are unclear. In
addition, the health effects of occupational exposure to WC-Co
nanoparticles are unknown. The molecular events mediating cellular
responses to WC-Co particles also remain to be elucidated.
It is well known that the toxicity of particles to
the lung in both occupational and environmental settings is not
only related to exposure but also to the particle size. We have
previously shown that nanoparticles of TiO2 and metallic
nickel produce higher cytotoxicity than their conventional fine
particles, respectively (6,7).
Accordingly, WC-Co nanoparticles may be more toxic than WC-Co fine
particles.
In this study, we observed that both WC-Co nano- and
fine particles induced ROS generation in a dose-dependent manner.
This result is in agreement with our previous study which indicated
that compared to fine particle WC-Co nanoparticles generated a
higher level of free radicals and induced greater oxidative stress,
as evidenced by a decrease of GSH levels (23). Further studies indicated that
catalase inhibits WC-Co particle-induced mitochondrial membrane
permeability damage in JB6 cells. These results demonstrate that
hydrogen peroxide may play an important role in the pathogenicity
induced by WC-Co particles.
Using MTT and apoptotic assays, we observed that
both WC-Co nano- and fine particles were cytotoxic in JB6 cells,
while nanoparticles showed higher cytotoxicity and apoptotic
induction than fine particles. In an inhalation study in rats,
Oberdoerster et al demonstrated TiO2
nanoparticles to be more inflammatory than fine TiO2
particles (4). However, when
normalized to surface area, they found that the dose-response
curves for the nano- and fine particles were similar, suggesting
that the pulmonary toxicity might be mediated by surface effects.
In the present study, surface area of WC-Co nanoparticles was
17-fold greater than fine particles. However, WC-Co nanoparticles
exhibited potency for cytotoxicity and apoptosis induction which
was somewhat less than 17-fold greater than fine particles.
Therefore, in agreement with our previous study with
TiO2 particles (7),
specific surface area measured by gas absorption, tends to over
correct for the greater cytotoxicity probably because it over
estimates the surface area of agglomerates.
Apoptosis and necrosis are two forms of cell death
that have been defined on the basis of distinguishable,
morphological criteria (36).
Apoptosis is a programmed cell death which is now widely recognized
as being of critical importance in health and disease. Although
studies have demonstrated that WC-Co fine particles induce cell
apoptosis (2,9), the molecular pathways have not been
well investigated. Our results suggest that, after 24-h treatment,
both WC-Co nano- and fine particles induce JB6 apoptosis in a dose
response manner within a certain dose range. Further studies
revealed that treatment with WC-Co nanoparticles by intranasal
droplet application induced both apoptosis and necrosis (or late
apoptosis) in rat lung macrophages. Tozawa et al reported
with a single intratracheal administration, WC-Co dusts induced
marked fibrotic changes in rat pulmonary tissues after 6 months
(37). Combined with our results,
apoptosis or necrosis in rat lung macrophages induced by WC-Co
nanoparticles may play an important role in the formation of the
experimental pneumoconiosis (37).
In mammals, signaling cascades culminating in
apoptotic cell death can be divided into intrinsic or extrinsic
pathways. The extrinsic pathway is initiated upon activation of
death receptors. The intrinsic pathway can be initiated by cellular
stresses, such as cytochrome c release from mitochondria
into the cytoplasm. In this study, WC-Co particles induced Fas,
FADD upregulation and caspase 8 activation. These results imply
that in the apoptotic process induced by WC-Co particles, the
extrinsic signal pathway is initiated. To investigate the
involvement of intrinsic pathways in the apoptotic process induced
by WC-Co particles, AIF and cytochrome c release from
mitochondria to the cytoplasm were examined. Our results show that
WC-Co particles induced upregulation and release of AIF and
cytochrome c from mitochondria to the cytoplasm. In
addition, the mitochondrial permeability assay showed that WC-Co
particles induced significant changes in the mitochondrial membrane
permeability, which was in parallel with western blot results.
These results indicate that the apoptotic process induced by WC-Co
particles involves both intrinsic and extrinsic pathways.
Lamins are nuclear membrane structural components,
which are important for maintaining normal cell functions.
Proteolysis of lamins has been observed in different cells
undergoing apoptosis (38).
Degradation of lamina proteins can be triggered by both the
extrinsic and the intrinsic pathways (39). Our results show that lamin A, but
not C, was cleaved in cells treated with WC-Co nanoparticles,
suggesting involvement of lamin A in the apoptotic process induced
by WC-Co nanoparticles. The actin cytoskeleton is capable of
responding to complex signaling cascades. The major cytoskeletal
filament, actin, can be degraded during the execution phase of
apoptosis (40). Reports indicate
that disruption of actin filament integrity promptly induces
apoptosis in adherent epithelial cells (41). In addition, the dynamic state of
actin is important in the regulation of ion channels (42). In the present study, WC-Co
particles induced β-actin cleavage after 1-h treatment in JB6
cells. These results, combined with our previous study with
metallic nickel particles (7) and
a report by White et al(41), suggest that the actin cytoskeletal
network may act as a target for apoptosis and an early signaling
component toward apoptotic commitment in the apoptotic process
induced by metallic particles.
The Bcl-2 proteins are a family of proteins involved
in the response to apoptosis. Some of these proteins, such as BAX
and BID, are proapoptotic, while others, such as Bcl-2, are
anti-apoptotic. In the present study, WC-Co particles induced both
proapoptotic factors of BAX and BID and anti-apoptotic factor of
Bcl-2, especially in nanoparticle-treated cells. Increased Bcl-2
may antagonize the effects of activated BID on the translocation of
cytochrome c(7). In
addition, Bcl-2 activation may provide the necessary conditions for
the selection of cells that have become resistant to apoptosis,
which may also be important in the WC-Co-induced carcinogenic
process. Therefore, further research is needed to elucidate the
role of activation of Bcl-2 in the carcinogenicity of WC-Co
particles.
Mitochondrial dysfunction has been shown to
participate in the induction of apoptosis. Changes in the
mitochondrial membrane permeability are considered early events in
apoptosis (43). Many proapoptotic
proteins, including cytochrome c, AIF, heat shock proteins,
Smac/Diablo, and endonuclease G, can be released from the
mitochondria into the cytoplasm following the formation of a pore
in the mitochondrial membrane (44). AIF is involved in initiating a
caspase-independent pathway of apoptosis by causing DNA
fragmentation and chromatin condensation (45). However, cytochrome c induces
apoptosis in a caspase-dependent pathway (46). In this study, WC-Co particles,
especially nanoparticles, induced a significant increase in the
mitochondrial membrane permeability in JB6 cells after 24-h
treatment and release of cytochrome c and AIF from
mitochondria into cytoplasm. In addition, lung macrophages from
rats exposed to WC-Co nanoparticles by intranasal droplet
application (4 g/rat) for 21 days exibited significant
mitochondrial membrane permeability alteration in rat lung
macrophages. These results indicate that the intrinsic
mitochondrial pathway is involved in WC-Co particle-induced
apoptosis.
Caspases, a family of aspartic acid-specific
proteases, are the major effectors of apoptosis. The execution of
apoptosis comprises both caspase-dependent and caspase-independent
processes. In the present study, WC-Co particles significantly
activated caspase 3 and 8. Caspase 9 was slightly activated. Taken
together, our results suggest that apoptosis induced by WC-Co
particles in JB6 cells involves both caspase-dependent and
caspase-independent pathways.
In conclusion, the major findings of the present
study show that both WC-Co nano- and fine particles induced JB6
cell and rat lung macrophage apoptosis. WC-Co nanoparticles
elicited higher cytotoxicity and apoptotic induction than fine
particles. WC-Co particles stimulated ROS generation in JB6 cells
and rat lung macrophages. Catalase exhibited partly inhibitory
effects on WC-Co particle-induced ROS generation and mitochondrial
membrane permeability damage. To our knowledge, this is the first
study showing that WC-Co particles activated both extrinsic and
intrinsic apoptotic pathways in JB6 cells, which include
upregulation of Fas, FADD, caspase 3, 8 and 9, BAX and BID, as well
as release of AIF and cytochrome c from mitochondria into
the cytoplasm. Lamin A and β-actin were cleaved. Furthermore, an
increase of mitochondrial membrane permeability was observed in
both WC-Co particle-treated JB6 cells and rat lung macrophages. On
a mass basis, WC-Co nanoparticles exhibited higher cytotoxicity and
apoptotic induction than fine particles. Particle size may play a
critical role in the toxicity of this hard metal. Specific surface
area tends to overcorrect for the greater toxicity when comparing
the cytotoxicity of WC-Co nano- with fine particles. Apoptosis
induced by WC-Co nano- and fine particles in JB6 cells may be
mediated through ROS generation.
Acknowledgements
This study was partly supported by the
National Nature Science Foundation of China (Grant no. 81273111),
the Foundations of Innovative Research Team of Educational
Commission of Zhejiang Province (T200907), the Nature Science
Foundation of Ningbo city (Grant no. 2012A610185), the Ningbo
Scientific Project (SZX11073), the Scientific Innovation Team
Project of Ningbo (no. 2011B82014), Innovative Research Team of
Ningbo (2009B21002) and K.C. Wong Magna Fund in Ningbo
University.
References
|
1
|
Dagani R: Nanostructured materials promise
to advance range of technologies. Chem Engineer News. 23:18–24.
1992. View Article : Google Scholar
|
|
2
|
Lombaert N, Lison D, Van Hummelen P and
Kirsch-Volders M: In vitro expression of hard metal dust (WC-Co) -
responsive genes in human peripheral blood mononucleated cells.
Toxicol Appl Pharmacol. 227:299–312. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Driscoll KE, Maurer JK, Lindenschmidt RC,
Romberger D, Rennard SI and Crosby L: Respiratory tract responses
to dust: relationships between dust burden, lung injury, alveolar
macrophage fibronectin release, and the development of pulmonary
fibrosis. Toxicol Appl Pharmacol. 106:88–101. 1990. View Article : Google Scholar
|
|
4
|
Oberdorster G, Ferin J and Lehnert BE:
Correlation between particle size, in vivo particle persistence,
and lung injury. Environ Health Perspect. 102(Suppl 5): 173–179.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Oberdorster G: Pulmonary effects of
inhaled ultrafine particles. Int Arch Occup Environ Health. 74:1–8.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhao J, Bowman L, Zhang X, et al: Titanium
dioxide (TiO2) nanoparticles induce JB6 cell apoptosis through
activation of the caspase-8/Bid and mitochondrial pathways. J
Toxicol Environ Health A. 72:1141–1149. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhao J, Bowman L, Zhang X, et al: Metallic
nickel nano- and fine particles induce JB6 cell apoptosis through a
caspase-8/AIF mediated cytochrome c-independent pathway. J
Nanobiotechnol. 7:22009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pulido MD and Parrish AR: Metal-induced
apoptosis: mechanisms. Mutat Res. 533:227–241. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lombaert N, De Boeck M, Decordier I,
Cundari E, Lison D and Kirsch-Volders M: Evaluation of the
apoptogenic potential of hard metal dust (WC-Co), tungsten carbide
and metallic cobalt. Toxicol Lett. 154:23–34. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Raff MC: Social controls on cell survival
and cell death. Nature. 356:397–400. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Steller H: Mechanisms and genes of
cellular suicide. Science. 267:1445–1449. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
White E: Life, death, and the pursuit of
apoptosis. Genes Dev. 10:1–15. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wyllie AH, Kerr JF and Currie AR: Cell
death: the significance of apoptosis. Int Rev Cytol. 68:251–306.
1980. View Article : Google Scholar
|
|
14
|
Lu X, Lee M, Tran T and Block T: High
level expression of apoptosis inhibitor in hepatoma cell line
expressing Hepatitis B virus. Int J Med Sci. 2:30–35. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ramp U, Krieg T, Caliskan E, et al: XIAP
expression is an independent prognostic marker in clear-cell renal
carcinomas. Hum Pathol. 35:1022–1028. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kuwano K, Miyazaki H, Hagimoto N, et al:
The involvement of Fas-Fas ligand pathway in fibrosing lung
diseases. Am J Respir Cell Mol Biol. 20:53–60. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bardales RH, Xie SS, Schaefer RF and Hsu
SM: Apoptosis is a major pathway responsible for the resolution of
type II pneumocytes in acute lung injury. Am J Pathol. 149:845–852.
1996.PubMed/NCBI
|
|
18
|
Levine AJ: p53, the cellular gatekeeper
for growth and division. Cell. 88:323–331. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Matute-Bello G, Winn RK, Jonas M, Chi EY,
Martin TR and Liles WC: Fas (CD95) induces alveolar epithelial cell
apoptosis in vivo: implications for acute pulmonary inflammation.
Am J Pathol. 158:153–161. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
McCabe MJ Jr: Mechanisms and consequences
of silica-induced apoptosis. Toxicol Sci. 76:1–2. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gawlitta D, Oomens CW, Baaijens FP and
Bouten CV: Evaluation of a continuous quantification method of
apoptosis and necrosis in tissue cultures. Cytotechnology.
46:139–150. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Boffa DJ, Waka J, Thomas D, et al:
Measurement of apoptosis of intact human islets by confocal optical
sectioning and stereologic analysis of YO-PRO-1-stained islets.
Transplantation. 79:842–845. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ding M, Kisin ER, Zhao J, et al:
Size-dependent effects of tungsten carbide-cobalt particles on
oxygen radical production and activation of cell signaling pathways
in murine epidermal cells. Toxicol Appl Pharmacol. 241:260–268.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Grossmann J, Mohr S, Lapentina EG, Fiocchi
C and Levine AD: Sequential and rapid activation of select caspases
during apoptosis of normal intestinal epithelial cells. Am J
Physiol. 274:G1117–G1124. 1998.PubMed/NCBI
|
|
25
|
Okinaga T, Kasai H, Tsujisawa T and
Nishihara T: Role of caspases in cleavage of lamin A/C and PARP
during apoptosis in macrophages infected with a periodontopathic
bacterium. J Med Microbiol. 56:1399–1404. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mashima T, Naito M and Tsuruo T:
Caspase-mediated cleavage of cytoskeletal actin plays a positive
role in the process of morphological apoptosis. Oncogene.
18:2423–2430. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Caroppi P, Sinibaldi F, Fiorucci L and
Santucci R: Apoptosis and human diseases: mitochondrion damage and
lethal role of released cytochrome C as proapoptotic protein. Curr
Med Chem. 16:4058–4065. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Susin SA, Lorenzo HK, Zamzami N, et al:
Molecular characterization of mitochondrial apoptosis-inducing
factor. Nature. 397:441–446. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Susin SA, Daugas E, Ravagnan L, et al: Two
distinct pathways leading to nuclear apoptosis. J Exp Med.
192:571–580. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Piacenza L, Irigoin F, Alvarez MN, et al:
Mitochondrial super-oxide radicals mediate programmed cell death in
Trypanosoma cruzi: cytoprotective action of mitochondrial iron
superoxide dismutase overexpression. Biochem J. 403:323–334. 2007.
View Article : Google Scholar
|
|
31
|
Long TC, Tajuba J, Sama P, et al: Nanosize
titanium dioxide stimulates reactive oxygen species in brain
microglia and damages neurons in vitro. Environ Health Perspect.
115:1631–1637. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lison D: Human toxicity of
cobalt-containing dust and experimental studies on the mechanism of
interstitial lung disease (hard metal disease). Crit Rev Toxicol.
26:585–616. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kear BH, Skandan G and Sadangi RK: Factors
controlling decarburization in HVOF sprayed nano-WC/Co
hardcoatings. Scripta Materialia. 44:1703–1707. 2001. View Article : Google Scholar
|
|
34
|
Busch W, Kuhnel D, Schirmer K and Scholz
S: Tungsten carbide cobalt nanoparticles exert hypoxia-like effects
on the gene expression level in human keratinocytes. BMC Genomics.
11:652010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
IARC Working Group on the Evaluation of
Carcinogenic Risks to Humans: Cobalt in hard metals and cobalt
sulfate, gallium arsenide, indium phosphide and vanadium pentoxide.
IARC Monogr Eval Carcinog Risks Hum. 86:1–294. 2006.PubMed/NCBI
|
|
36
|
Helewski KJ, Kowalczyk-Ziomek GI and
Konecki J: Apoptosis and necrosis - two different ways leading to
the same target. Wiad Lek. 59:679–684. 2006.(In Polish).
|
|
37
|
Tozawa T, Kitamura H, Koshi K, Ikemi Y and
Ambe K: Experimental pneumoconiosis induced by cemented tungsten
and sequential concentrations of cobalt and tungsten in the lungs
of the rat. Sangyo Igaku. 23:216–226. 1981.(In Japanese).
|
|
38
|
Goldberg M, Harel A and Gruenbaum Y: The
nuclear lamina: molecular organization and interaction with
chromatin. Crit Rev Eukaryot Gene Expr. 9:285–293. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Broers JL, Bronnenberg NM, Kuijpers HJ,
Schutte B, Hutchison CJ and Ramaekers FC: Partial cleavage of
A-type lamins concurs with their total disintegration from the
nuclear lamina during apoptosis. Eur J Cell Biol. 81:677–691. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Janmey PA: The cytoskeleton and cell
signaling: component localization and mechanical coupling. Physiol
Rev. 78:763–781. 1998.PubMed/NCBI
|
|
41
|
White SR, Williams P, Wojcik KR, et al:
Initiation of apoptosis by actin cytoskeletal derangement in human
airway epithelial cells. Am J Respir Cell Mol Biol. 24:282–294.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Gourlay CW and Ayscough KR: The actin
cytoskeleton: a key regulator of apoptosis and ageing? Nat Rev Mol
Cell Biol. 6:583–589. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ly JD, Grubb DR and Lawen A: The
mitochondrial membrane potential (deltapsi(m)) in apoptosis; an
update. Apoptosis. 8:115–128. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Siskind LJ, Kolesnick RN and Colombini M:
Ceramide forms channels in mitochondrial outer membranes at
physiologically relevant concentrations. Mitochondrion. 6:118–125.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Landshamer S, Hoehn M, Barth N, et al:
Bid-induced release of AIF from mitochondria causes immediate
neuronal cell death. Cell Death Differ. 15:1553–1563. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Garland JM and Rudin C: Cytochrome c
induces caspase-dependent apoptosis in intact hematopoietic cells
and overrides apoptosis suppression mediated by bcl-2, growth
factor signaling, MAP-kinase-kinase, and malignant change. Blood.
92:1235–1246. 1998.
|