Introduction
Primary liver cancer, of which hepatocellular
carcinoma (HCC) represents the major subtype accounting for between
85 and 90%, is the sixth most common tumor globally and the third
most common cause of cancer-related death (1). Systemic treatment options for
advanced HCC are limited and most deaths occur within 1 year of
diagnosis (2–4).
Sorafenib is an oral multikinase inhibitor that was
approved by the US Food and Drug Administration in December 2005
for the treatment of advanced renal cell carcinoma (RCC) and in
November 2007 for the treatment of HCC. It has been shown to
inhibit the activity of Raf kinase and several receptor tyrosine
kinases, including vascular endothelial growth factor receptors
(VEGFR)-1, 2 and 3, platelet-derived growth factor receptor
(PDGFR)-α and β, FLT3, Ret and c-Kit. The intracellular signaling
pathway Raf/MEK/ERK and the extracellular receptors VEGFR and PDGFR
have been implicated in the molecular pathogenesis of HCC (5).
The Sorafenib Hepatocellular Carcinoma Assessment
Randomized Protocol (SHARP) trial revealed efficacy of sorafenib in
the treatment of HCC, i.e., both median survival and time to
progression showed 3-month improvements by sorafenib therapy
(6). Cheng et al(7) also reported the efficacy of sorafenib
in patients in the Asia-Pacific region with advanced hepatocellular
carcinoma. Combination therapy with sorafenib has a potential to
improve the outcome of sorafenib monotherapy. Phase II trial of
combination therapy of sorafenib and IFN-α has substantial activity
in patients with metastatic RCC (8,9). The
combination therapy of IFN-α and 5-fluorouracil is partly or
completely effective in about 50% of the patients with advanced HCC
(10). Type I interferon (IFN) has
various effects, including anti-viral effects, antiproliferative
effects and anti-angiogenic effects (11), and our laboratory previously
reported the antiproliferative effect of IFN-α on human liver
cancer cells in vitro and in vivo(12–14).
In addition, type I IFN has suppressive effects on the occurrence
of HCC, and the recurrence of HCC after curative treatment in
patients with chronic hepatitis C virus infection (15–20).
On the basis of above-described background, our current study
examined the growth inhibitory effects of combination treatment of
sorafenib and Pegylated IFN-α2b (PEG-IFN-α2b) on human HCC cell
lines in vitro and in vivo.
Materials and methods
Cell line and cell cultures
This study used two HCC cell lines [KIM-1 (21) and HAK-1B (22)], which were originally established
and characterized in our laboratory and previously confirmed to
retain morphological and functional characteristics of the original
tumor. Both of these two cell lines were established from
surgically resected HCC nodules. KIM-1 is a moderately
differentiated HCC cell line, and HAK-1B is a poorly differentiated
HCC cell line which was derived from a patient with hepatitis C
virus (HCV) infection.
The cells were grown in Dulbecco’s modified Eagle’s
medium (Nissui Seiyaku Co., Tokyo, Japan) supplemented with 2.5%
heat-inactivated (56°C, 30 min) fetal bovine serum (FBS, Bioserum,
Victoria, Australia), 100 U/ml penicillin, 100 mg/ml streptomycin
(Gibco-BRL/Life Technologies Inc., Gaithersburg, MD, USA) and 12
mmol/l sodium bicarbonate, in a humidified atmosphere of 5%
CO2 in air at 37°C.
Sorafenib and pegylated IFN-α2b
Sorafenib, kindly provided by Bayer Pharmaceutical
Corporation (West Haven, CT, USA), was dissolved in dimethyl
sulfoxide (DMSO) to create a 10 mM stock solution and stored at
−20°C for in vitro study. For the in vivo study, we
prepared the solution at time of use.
PEG-IFN-α2b (PEG Intron®) was kindly
provided by MSD K.K. (Tokyo, Japan). The specific activity of
PEG-IFN-α2b was 6.4×107 IU/mg protein.
Effect of sorafenib alone or combination
treatment of sorafenib and PEG-IFN-α2b on the proliferation of HCC
and CHC cell lines in vitro
The effects of sorafenib and/or PEG-IFN-α2b on the
growth of the cultured cells were examined with colorimetry using
3-(4,5-dimethylthiazol-2yl)-2,5-diphenyl tetrazolium bromide (MTT)
assay kits (Chemicon International Inc.) as described (12–14).
Briefly, the cells (1.5–5.5×103 cells per well) were
seeded on 96-well plates (Nunc Inc., Roskilde, Denmark), cultured
for 24 h, and the culture medium was changed to a new one
containing 0.2% DMSO (control) or sorafenib (0.3125, 0.625, 1.25,
2.5, 5, 10 or 20 μM), or both sorafenib (0, 1.25, 2.5 or 5
μM) and PEG-IFN-α2b (0, 2,000, 4,000, 8,000 IU/ml)
(constant-ratio combination). After culturing for 72 h, the number
of viable cells was measured with ImmunoMini NJ-2300 (Nalge Nunc
International, Tokyo, Japan) by setting the test wavelength at 570
nm and the reference wavelength at 630 nm. To keep the optical
density within linear range, all experiments were performed while
the cells were in the logarithmic growth phase.
Combination analysis was performed by using the
method as described by Chou and Talalay (23), and the CalcuSyn software program
(Biosoft, Cambridge, UK) for automated analysis. This program
calculates the combination index (CI). A CI of 0.9–1.1 indicates a
nearly additive effect, a CI of <0.9 a synergistic effect, a CI
of >1.1 an antagonistic effect.
Morphological observation
For morphological observation under a light
microscope, cultured HAK-1B cells were seeded on Lab-Tek tissue
culture chamber slides (Nunc Inc.), cultured with or without 1.25
μM of sorafenib for 72 h, fixed for 10 min in Carnoy’s
solution, and stained with hematoxylin and eosin (H&E).
Quantitative analysis of apoptotic cells
induced by sorafenib and/or PEG-IFN-α2b
HAK-1B and KIM-1 were cultured with the culture
medium containing 0.02% DMSO or 2 μM of sorafenib for 72 h.
For a study of combination therapy, HAK-1B cells were cultured with
sorafenib (1.25 μM) or PEG-IFN-α2b (2,000 IU/ml), or both
sorafenib (1.25 μM) and PEG-IFN-α2b (2,000 IU/ml) for 72 h.
After incubation, the cells were stained with the Annexin V-EGFP
(enhanced green fluorescent protein) using Apoptosis Detection Kits
(Medical and Biological Laboratories, Nagoya, Japan) according to
the manufacturer’s protocol. After staining, the cells were
analyzed using a FACScan (Becton-Dickinson Immunocytometry Systems,
San Jose, CA, USA), and the rate of Annexin V-EGFP-positive
apoptotic cells was determined.
Effects of sorafenib and/or PEG-IFN-α2b
on HCC cell proliferation in nude mice
This experiment was approved by the institutional
committee for animal experiments and conducted according to the
‘Guide for the Care and Use of Laboratory Animals’ published and
revised by the National Institute of Health in 1985.
Cultured HAK-1B or KIM-1 cells (1.0×106
cells/mouse) were transplanted subcutaneously (s.c.) to 4-week-old
female BALB/c athymic nude mice (Clea Japan Inc., Osaka, Japan). On
the 7th day when tumor size became 5 to 10 mm in diameter (day 0),
the mice were divided into four groups (n=8 each) in a manner to
equalize the mean tumor diameter of every group. Each group was
assigned to one of the four treatments: i) control; ii) PEG-IFN-α2b
alone; iii) sorafenib alone; and iv) sorafenib + PEG-IFN-α2b
(combination).
Sorafenib was diluted with 12.5% Cremophor EL/12.5%
ethanol/75% water for oral dosing in mice. Sorafenib (200
μg/day) was administered by tube feeding once a day for 14
days. PEG-IFN-α2b (1,920 IU) was subcutaneously injected twice a
week for 14 days (days 1, 4, 8 and 11). In the control and the
sorafenib alone groups, 0.1 ml of medium as the replacement of
PEG-IFN-α2b was injected subcutaneously twice a week. In the
control and the PEG-IFN-α2b alone groups, 0.2 ml of Cremophor
EL/ethanol/water (12.5/12.5/75) as the replacement of sorafenib was
administered by tube feeding once a day. The dose of sorafenib (200
μg) in the ratio to the average bodyweight of a mouse (20 g)
was 10 mg/kg and this is almost comparable to a clinical dose (800
mg total daily dose). The clinical dose of PEG-IFN-α2b in chronic
hepatitis C is 96,000 IU/kg per week. Because of species difference
and different target which is not virus, but tumor, we used twice
the dose per week in nude mice.
Tumor size was measured in two directions using
calipers, and tumor volume (mm3) was estimated by using
the equation: length × (width)2 × 0.5. This measurement
was performed every two days. Mouse body weight was measured on
days 0, 7 and 14. Mouse was sacrificed and the tumor was resected
the next day after the completion of the 14-day treatment (day 15).
The resected tumor was fixed in formalin after the weight
measurement, prepared into paraffin sections, and underwent HE
staining and immunohistochemistry.
Immunohistochemistry
Paraffin-embedded tissue samples were cut into
4-μm sections. Anti-mouse CD34 (Rat monoclonal, MEC14.7,
Abcam, Cambridge, UK) (1:50 dilution) and Ki67 (Rabbit monoclonal,
SP6, Abcam, Cambridge, UK) (1:100 dilution) staining were performed
by standard avidin-biotin-peroxidase complex method and
3,3′-diaminobenzidine (DAB) solution was used for color
development. Cleaved caspase-3 (rabbit polyclonal antibody, Cell
Signaling Technologies, Beverly, MA, USA) (1:250 dilution) staining
was performed on the Discovery XT automated staining system
(Ventana Medical Systems, Tucson, AZ, USA) to detect the apoptotic
cells. This automated system uses the streptavidin-biotin complex
method with DAB as a chromogen (Ventana iView DAB detection
kit).
Microvessel density (MVD) was evaluated within the
tumor according to a modified method introduced by Tanigawa et
al(24). Briefly the slides
stained with CD34 were screened at low power field (×40 or ×100)
and the two or three most vascular areas were selected. Microvessel
counts of these areas were performed at high power field (×200,
0.74 mm2). All positive stained cells were counted as
microvessels and every 40 μm length of vessel lumen was
calculated as one point. The average microvessel counts of selected
areas were regarded as MVD, which was expressed as the absolute
number of microvessels per 0.74 mm2.
Immunohistochemically, cleaved caspase-3 was expressed
perinuclearly and Ki67 was on the nuclear. The rate of apoptotic
cells and Ki67 labeling index were evaluated by calculating the
rate of cleaved caspase-3-positive cells and Ki67-positive cells,
respectively.
Statistical analysis
Comparisons of estimated tumor volume and
colorimetric cell growth were performed using two-factor factorial
ANOVA and Student’s t-test, respectively. The other data
comparisons were performed using the Mann-Whitney U test.
Results
Effect of sorafenib alone or combination
treatment of sorafenib and PEG-IFN-α2b on the proliferation of
HAK-1B or KIM-1 HCC cells in vitro
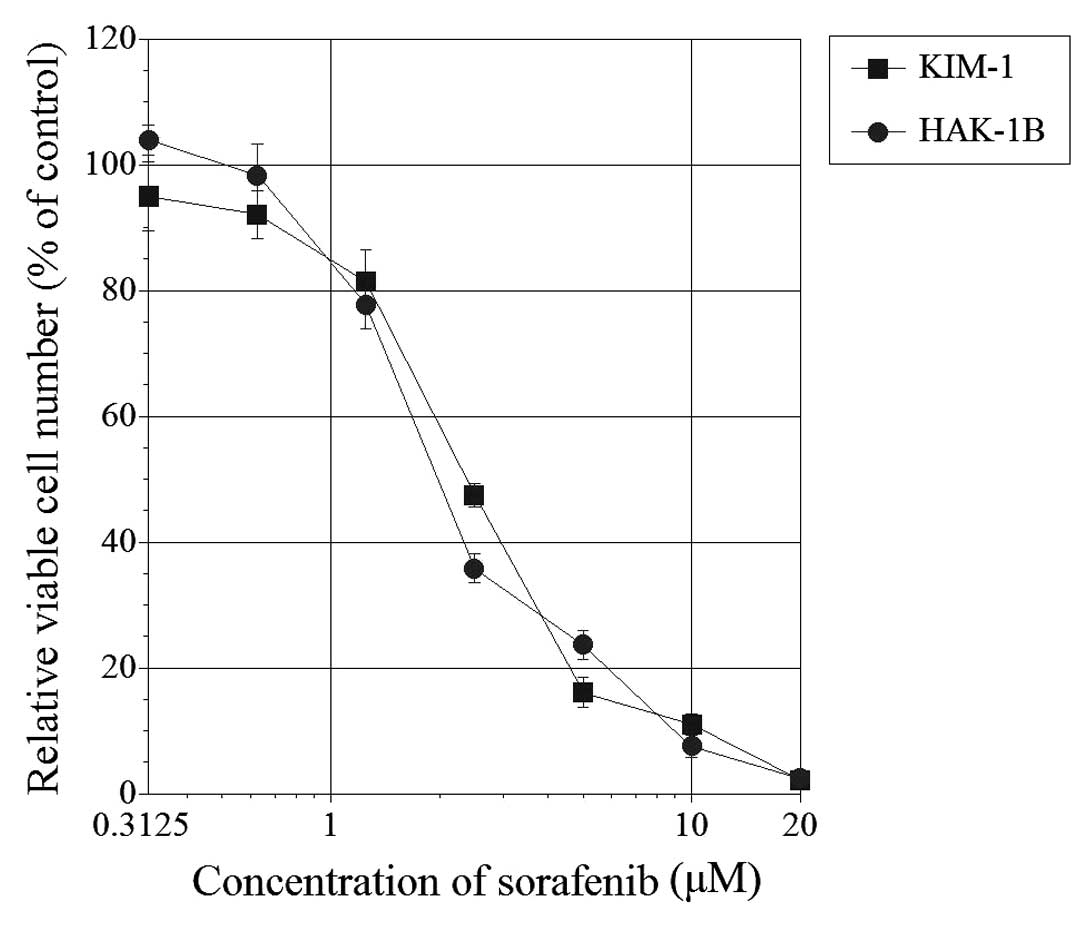
Seventy-two hours after the addition of sorafenib,
the relative viable cell number was suppressed in both HAK-1B and
KIM-1 cell lines in a dose-dependent manner (Fig. 1). The 50% inhibitory concentration
(IC50) was 2.1 μM for HAK-1B and 2.5 μM
for KIM-1.
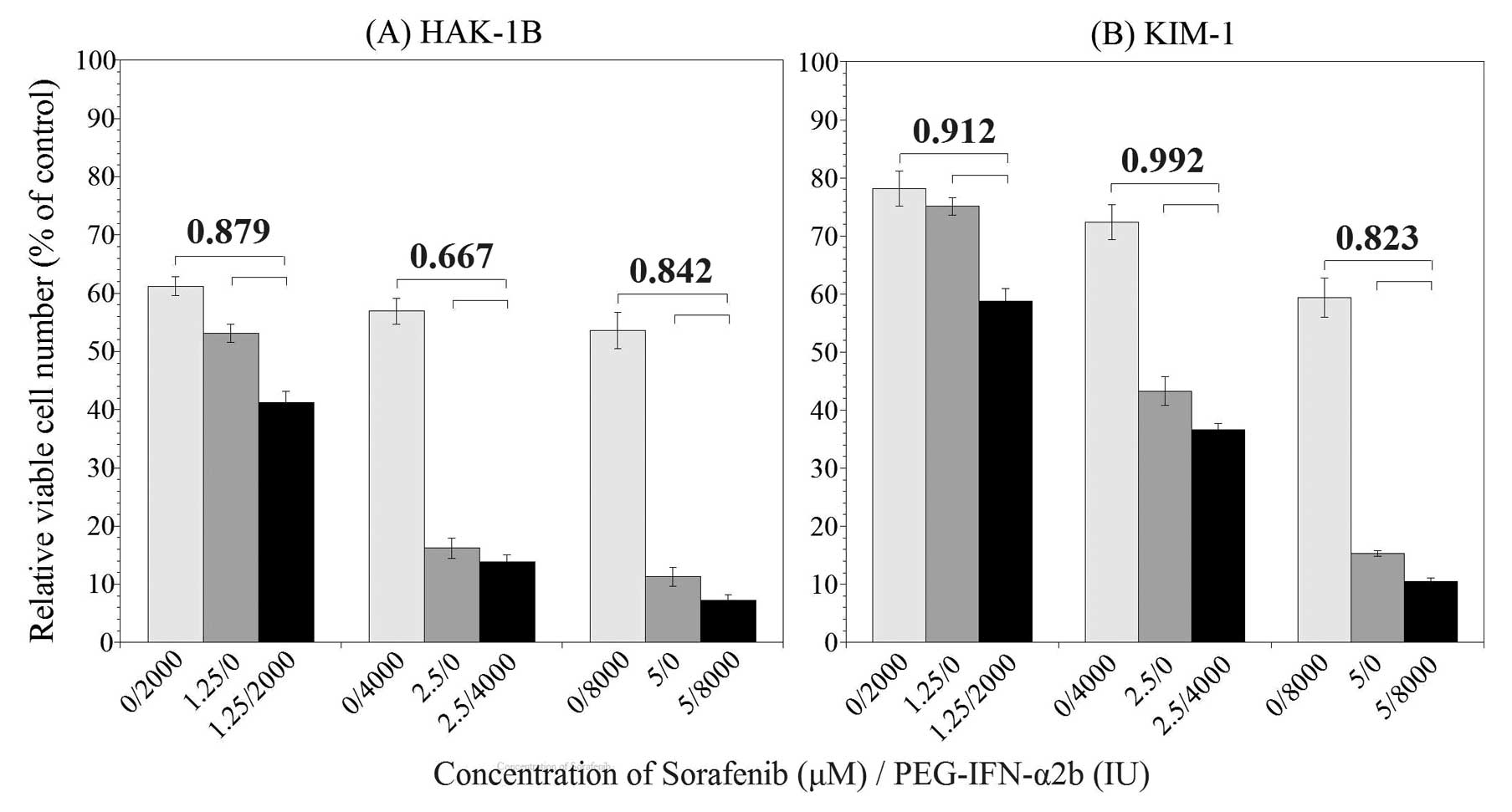
Seventy-two hours after the addition of PEG-IFN-α2b
and sorafenib, the relative viable cell number was suppressed to
various degrees. The results are shown in Fig. 2. In HAK-1B cell line (Fig. 2A), significant difference in the
relative viable cell number was observed between combination group
and sorafenib or PEG-IFN-α2b alone groups, additionally, CI in all
combination of PEG-IFN-α2b and sorafenib was <0.9. The CI was
0.879 in the combination of 2,000 IU/ml of PEG-IFN-α2b and 1.25
μM of sorafenib, 0.667 in 4,000 IU/ml of PEG-IFN-α2b and 2.5
μM of sorafenib, and 0.842 in 8,000 IU/ml of PEG-IFN-α2b and
5.0 μM of sorafenib. According to the definition of the CI,
these results indicate that a combination of PEG-IFN-α2b and
sorafenib may produce a synergistic growth inhibitory effect in
HAK-1B cell line. In KIM-1 cell line (Fig. 2B), there was also a significant
difference in the relative viable cell numbers between combination
group and monotherapy groups. The CI was 0.912 in the combination
of 2,000 IU/ml of PEG-IFN-α2b and 1.25 μM of sorafenib,
0.992 in 4,000 IU/ml of PEG-IFN-α2b and 2.5 μM of sorafenib,
and 0.823 in 8,000 IU/ml of PEG-IFN-α2b and 5.0 M of sorafenib.
These results indicate that combination therapy may produce an
additive or synergistic growth inhibitory effect in KIM-1 cell
line.
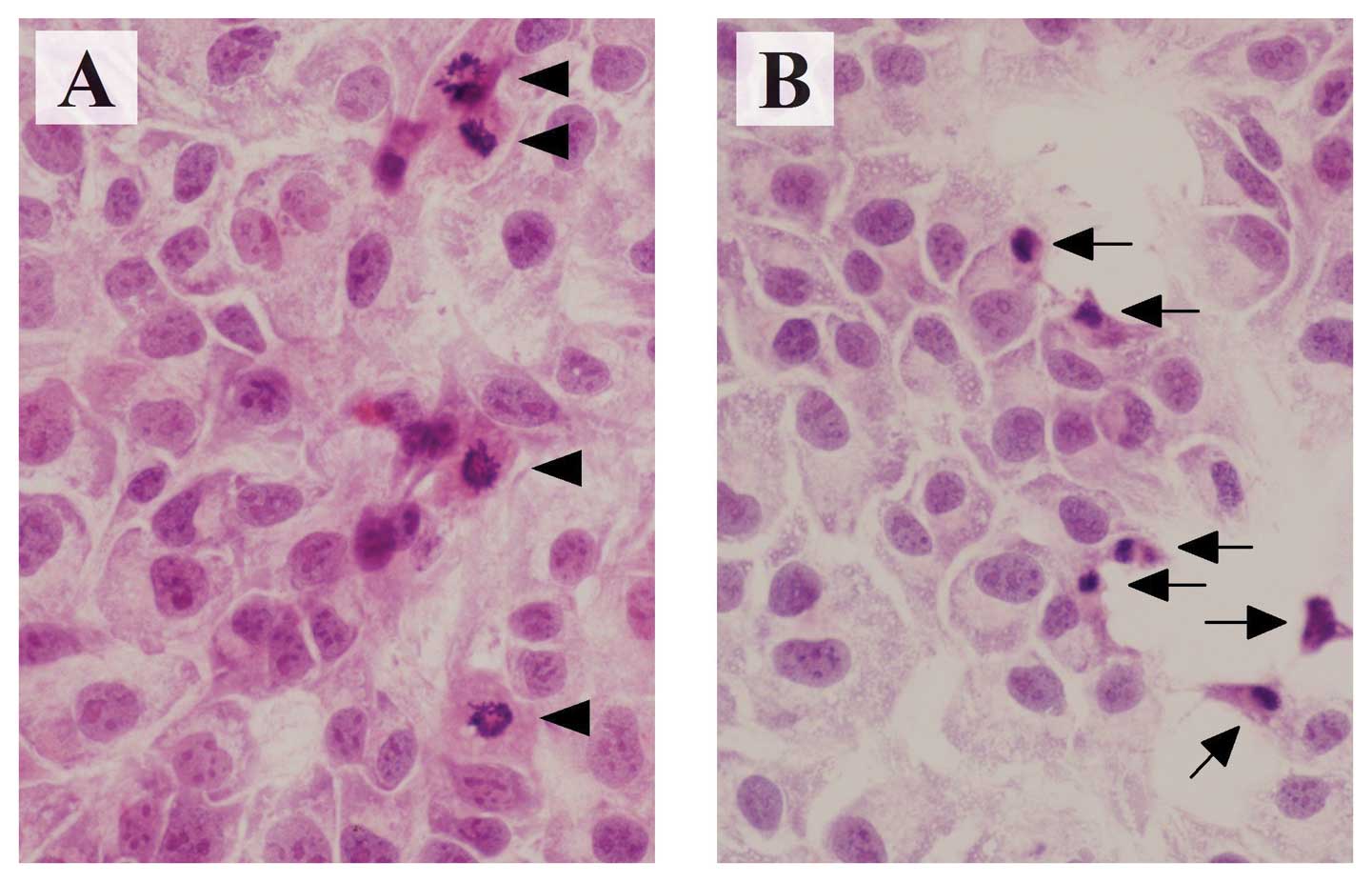
Morphologically, HAK-1B cells showed characteristic
features of apoptosis, such as cytoplasmic shrinkage and nuclear
chromatin condensation at 72 h after adding 1.25 μM of
sorafenib (Fig. 3).
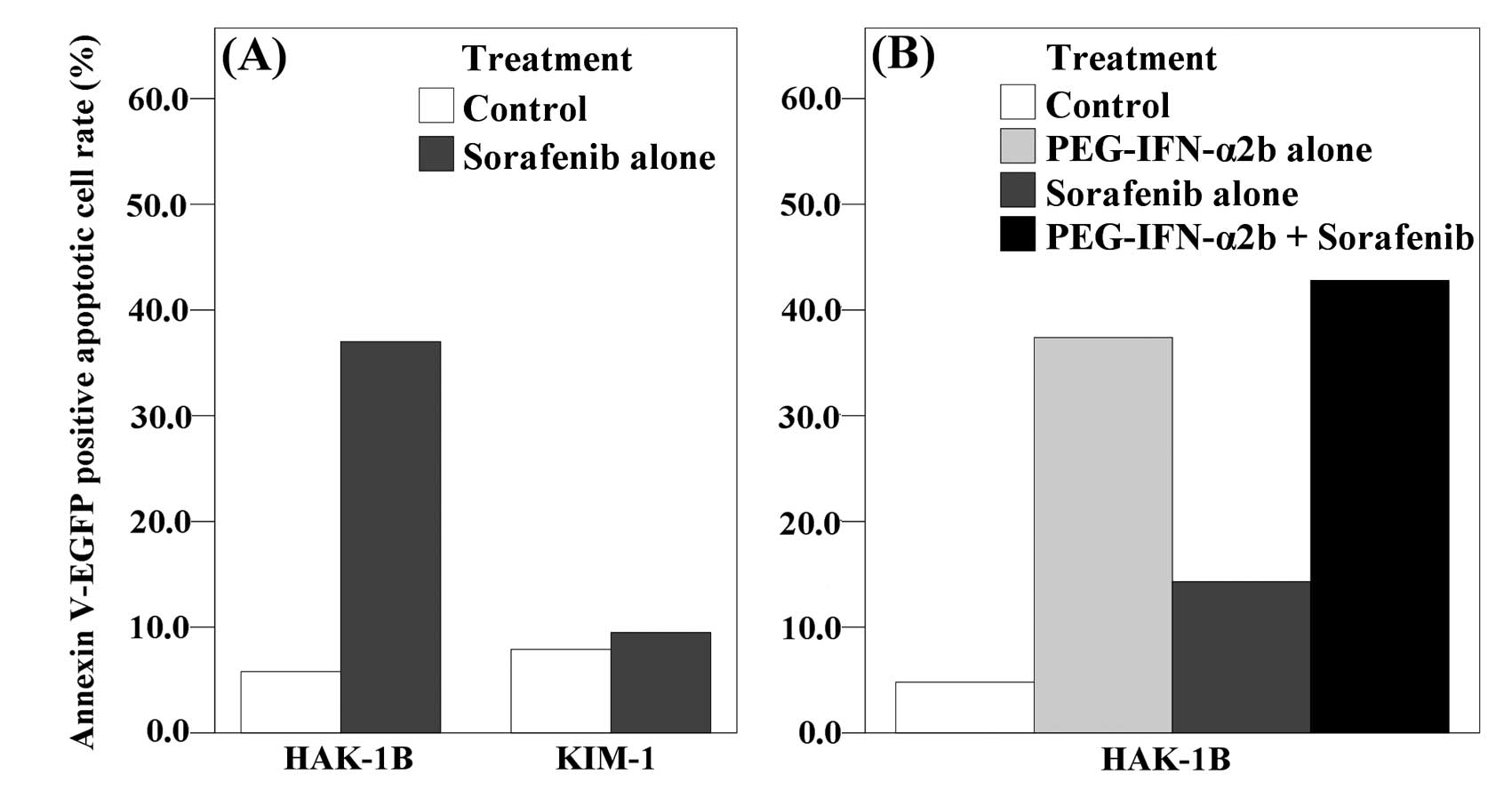
The rate of Annexin V-EGFP positive apoptotic cells
was increased by adding 2 μM of sorafenib in HAK-1B cells
(5.8% of the control and 37.8% of the sorafenib). In KIM-1 cells,
however, the increase was relatively small (7.9% of the control and
9.5% of the sorafenib) (Fig. 4A).
In another setting, the combination group with PEG-IFN-α2b showed
higher rate of apoptosis than control or monotherapy groups in
HAK-1B (4.8% of control, 37.4% of the PEG-IFN-α2b, 14.3% of the
sorafenib, 42.8% of the combination) (Fig. 4B).
Effects of sorafenib and/or PEG-IFN-α2b
on HAK-1B or KIM-1 cell proliferation in nude mice
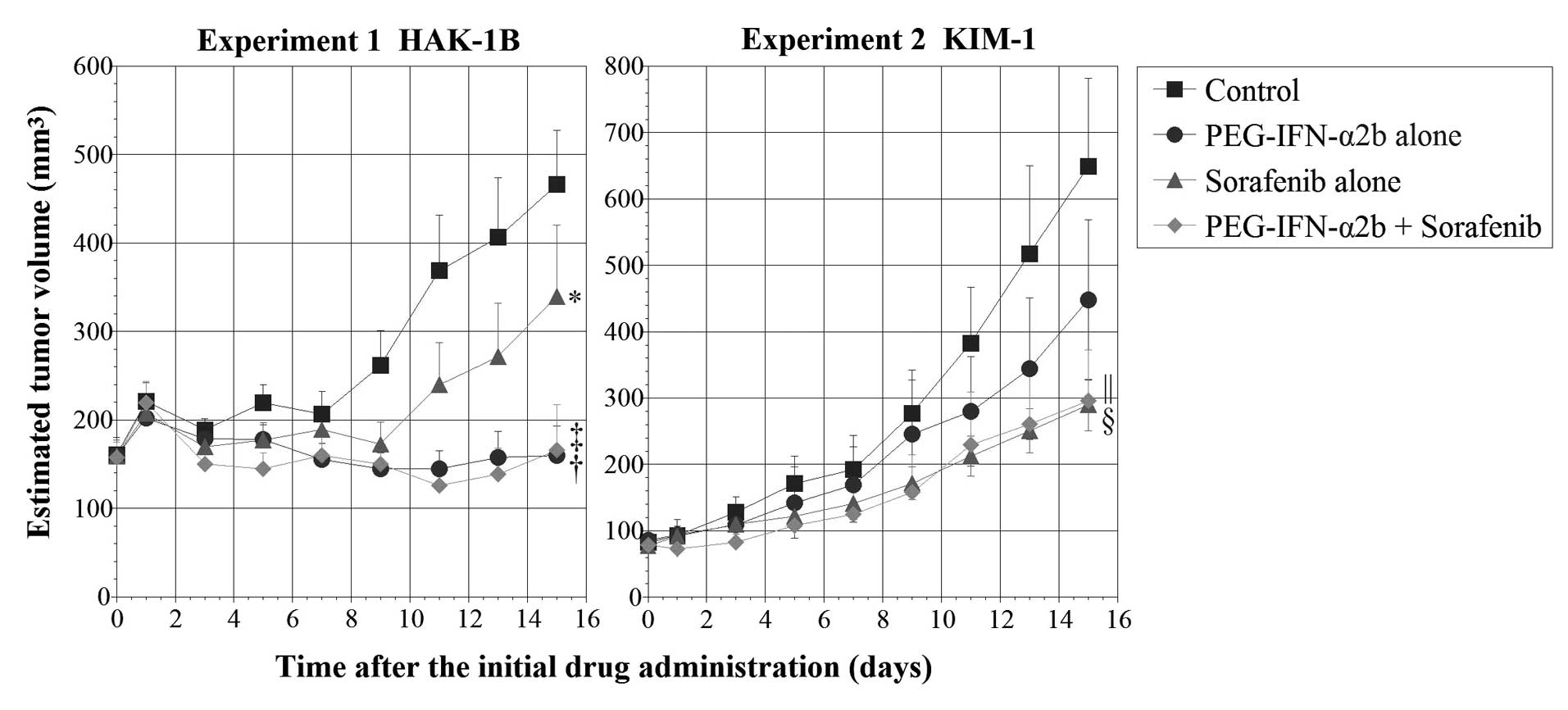
Chronological changes in estimated tumor volume
after subcutaneous injection of cultured HAK-1B cells or KIM-1
cells to nude mice are summarized in Fig. 5. The actual tumor weights at the
time of sacrifice are shown in Table
I. In the experiment of HAK-1B tumors, the tumor volume of mice
receiving PEG-IFN-α2b, sorafenib, and sorafenib+PEG-IFN-α2b was 34,
73 and 36%, respectively, of the control volume and the tumor
weight was 23, 71 and 34%, respectively, of the control weight.
Statistically, there were significant differences both in tumor
volume and weight between the control group and the PEG-IFN-α2b
alone group (P<0.0001 vs. control in tumor volume, P<0.0001
vs. control in tumor weight) or the combination group (P<0.0001
vs. control in tumor volume, P<0.001 vs. control in tumor
weight) and between the sorafenib alone group and the PEG-IFN-α2b
alone group (P<0.005 vs. sorafenib alone in tumor volume,
P<0.05 vs. sorafenib alone in tumor weight). Although there was
a significant difference between the sorafenib alone group and the
combination group in tumor volume (P<0.001), this was not the
case in the actual tumor weight (P=0.099). In the experiment of
KIM-1 tumors, the tumor volume of mice receiving PEG-IFN-α2b,
sorafenib, and sorafenib+PEG-IFN-α2b was 69, 45 and 46%,
respectively, of the control volume and the tumor weight was 75, 41
and 37%, respectively, of the control weight. Statistically, there
were significant differences in both tumor volume and weight
between the control and the sorafenib alone group (P<0.0001 vs.
control in tumor volume, P<0.05 vs. control in tumor weight) or
the combination group (P<0.001 vs. control in tumor volume,
P<0.05 vs. control in tumor weight).
 | Table IThe weight of subcutaneous tumors of
HAK-1B cells or KIM-1 cells in nude mice at sacrifice. |
Table I
The weight of subcutaneous tumors of
HAK-1B cells or KIM-1 cells in nude mice at sacrifice.
| Tumor weight (g)
|
|---|
| Treatment group | HAK-1B | KIM-1 |
|---|
| Control | 0.333±0.03 | 0.504±0.17 |
| PEG-IFN-α2b
alone | 0.078±0.02a | 0.379±0.18 |
| Sorafenib alone | 0.236±0.06 | 0.206±0.04c |
| PEG-IFN-α2b +
sorafenib | 0.113±0.04b | 0.185±0.12c |
The results of immunohistochemical examination are
summarized in Table II. The
significant decrease of MVD and increase of apoptotic cells were
observed in the sorafenib group (P<0.0005 and 0.05 respectively
vs. control in HAK-1B, P<0.05 and 0.05 respectively vs. control
in KIM-1) and the combination group (P<0.05 and 0.05
respectively vs. control in HAK-1B, P<0.05 and 0.05,
respectively, vs. control in KIM-1) compared to the control group
in both HAK-1B and KIM-1 tumors, although there was no significant
difference between the combination group and monotherapy groups.
Ki67 labeling index was significantly lower in the combination
group (P<0.005 vs. control, P<0.05 vs. PEG-IFN-α2b group)
than in the control group or the PEG-IFN-α2b group only in
KIM-1.
 | Table IIMVD and the ratio of apoptotic cells
and Ki67 positive cells in human HCC tumors subcutaneously
transplanted in nude mice. |
Table II
MVD and the ratio of apoptotic cells
and Ki67 positive cells in human HCC tumors subcutaneously
transplanted in nude mice.
| Cell line | Treatment group | MVD | Apoptotic
cells | Ki67 positive
cells |
|---|
| HAK-1B | Control | 100.8±7.7 | 3.8±0.4 | 36.8±2.0 |
| Peg-IFN-α2b
alone | 114.9±16.7 | 4.4±0.4 | 37.5±4.6 |
| Sorafenib
alone | 53.8±4.3a | 6.7±1.3b | 38.3±2.0 |
| Peg-IFN-α2b +
soragfenib | 69.4±10.1b | 5.6±1.3b | 35.3±2.2 |
| KIM-1 | Control | 125.9±16.2 | 4.6±0.4 | 6.7±0.2 |
| Peg-IFN-α2b
alone | 97.4±10.4 | 5.1±0.4 | 7.5±0.8 |
| Sorafenib
alone | 85.1±6.6b | 6.5±0.7b | 5.7±0.4 |
| Peg-IFN-α2b +
soragfenib | 79.0±7.2b | 6.3±0.6b | 4.6±0.5c |
Discussion
In this study, we showed the synergistic effect of
sorafenib and PEG-IFN-α2b on HAK-1B cells in vitro. We
previou sly reported that PEG-IFN-α2b induced apoptosis on both
HAK-1B and KIM-1 cells in vitro(14). We found that sorafenib also induced
apoptosis on HAK-1B in vitro. On the other hand, the
increase of apoptotic cells was not clearly observed on KIM-1 cells
in spite of the fact that the proliferation of KIM-1 cells was
inhibited by sorafenib in MTT assay. A possible explanation is that
cell proliferation might be inhibited by other antiproliferative
mechanisms. The blockade of Raf signaling which is the main effect
of sorafenib can lead to the repression of transforming growth
factor α-epidermal growth factor receptor autocrine loops of tumor
cells (5). Such a mechanism could
have inhibited the growth of KIM-1 cells. In addition, a limitation
of in vitro study is that we are not able to assess the
indirect anti-angiogenic effect against endothelial cells.
In the in vivo study, there was a significant
reduction of tumor volume and weight in the combination group on
both HAK-1B and KIM-1 tumors compared with the control group.
However, there was no significant difference between the
combination and the monotherapy groups, and it seemed that HAK-1B
tumors were sensitive to PEG-IFN-α2b and KIM-1 tumors to sorafenib.
Only in KIM-1 tumors that might be sensitive to sorafenib, Ki67
labeling index was lower in the combination group than in the
control group. Recently Wang et al(25) reported that combination therapy of
sorafenib with recombinant human INF-α2a was effective in
vitro and in vivo on two HCC cell lines, Huh-7 and
Sk-Hep-1. In their study, the significant differences between
combination and monotherapy groups were clearly observed. This
partial difference might be due to the different experimental
settings, such as different cell lines and different dose of drugs.
One of the greatest differences, we surmise, is the site of IFN
administration. They injected IFN directly into subcutaneous
tumors, whereas we did subcutaneously but not into the tumors.
Since sorafenib is a multikinase inhibitor, it is
considered that sorafenib has both direct antiproliferative effect
due to the blockade of Raf kinase on tumor cells themselves and
indirect effect due to the blockade of receptor tyrosine kinases,
such as VEGFR-2, on endothelial cells followed by the inhibition of
angiogenesis (5). Therefore we
also evaluated MVD of xenografts and confirmed the significant
decrease of MVD in the sorafenib alone and the combination group in
both HAK-1B and KIM-1 tumors. It has been repeatedly shown that IFN
suppresses the growth of various types of human tumors that were
transplanted into mice through the anti-angiogenic effect.
Tedjarati et al(26)
reported that the subcutaneous injection of 7,000 IU per week of
PEG-IFN-α2b into nude mice bearing human ovarian cancer cells
induced a significant decrease of CD31-positive endothelial cells
and Huang et al(27) showed
similar results with the subcutaneous injection of 70,000 IU per
week of PEG-IFN-α2b on human prostate cancer cells. PEG-IFN-α2b
administered at higher or lower doses was less effective. In our
current study, however, there was no significant decrease of MVD in
the PEG-IFN-α2b group compared with the control group. Moreover, in
our previous report, the decrease of artery-like blood vessels was
not observed in the same HAK-1B tumors by the administration of
PEG-IFN-α2b at either higher or lower doses (14).
Another notable finding regarding the MVD in this
study is the discrepancy between MVD and tumor weight or size.
Interestingly, the reduction of tumor weight and size was not so
much in sorafenib monotherapy group in HAK-1B tumors despite the
most prominent decrease of MVD was observed in this group. On the
other hand, there was a significant reduction of tumor weight and
size in PEG-IFN-α2b alone group in HAK-1B, although this group did
not show any significant decrease of MVD. This result supports our
previous findings in which we showed there was no relationship
between tumor shrinkage and the number of artery-like blood vessels
in HAK-1B tumors after the administration of the various
concentration of PEG-IFN-α2b (14). Hlatky et al(28) mentioned in their review article
that the efficacy of anti-angiogenic agents cannot be simply
visualized by alterations in microvessel density during treatment
because of the difference of the tightness of the coupling between
vessel drop-out and tumor-cell drop-out after the treatment. In
addition, Yao et al(29)
recently reported that the expression of VEGFR-1 in tumor cells
which is normally expressed specifically in endothelial cells were
strongly associated with anti-PlGF antibody efficacy, but not with
anti-angiogenesis. More studies are needed to investigate new
approaches to assess the efficacy of anti-angiogenic drugs in
vivo and molecular mechanisms of their action of
‘anti-angiogenic’ drugs.
In conclusion, we demonstrated the synergistic
antiproliferative effect of combination therapy on HAK-1B cells
in vitro. Although, in vivo the synergistic effects
of the combination therapy were not clearly observed, the
combination therapy induced nearly maximal antitumor effects,
independent of the HCC cell sensitivity to antitumor effects of
single therapy with either PEG-IFN-α2b or sorafenib. These findings
suggest that PEG-IFN-α2b might be a promising candidate for use in
combination therapy with sorafenib and warrant further
investigation.
Acknowledgements
We are grateful to Ms. Akemi Fujiyoshi
and Ms. Sachiyo Maeda for their excellent technical assistance.
This study was supported in part by grant-in-aid from Ministry of
Health, Labor and Welfare of Japan.
References
|
1
|
Ferlay J, Shin H-R, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Llovet JM, Burroughs A and Bruix J:
Hepatocellular carcinoma. Lancet. 362:1907–1917. 2003. View Article : Google Scholar
|
|
3
|
Bruix J and Sherman M: Management of
hepatocellular carcinoma. Hepatology. 42:1208–1236. 2005.
View Article : Google Scholar
|
|
4
|
Llovet JM, Bustamante J, Castells A,
Vilana R, Ayuso Mdel C, Sala M, Brú C, Rodés J and Bruix J: Natural
history of untreated nonsurgical hepatocellular carcinoma:
rationale for the design and evaluation of therapeutic trials.
Hepatology. 29:62–67. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wilhelm SM, Adnane L, Newell P, Villanueva
A, Llovet JM and Lynch M: Preclinical overview of sorafenib, a
multikinase inhibitor that targets both Raf and VEGF and PDGF
receptor tyrosine kinase signaling. Mol Cancer Ther. 7:3129–3140.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Llovet JM, Ricci S, Mazzaferro V, Hilgard
P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A,
Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR,
Seitz JF, Borbath I, Häussinger D, Giannaris T, Shan M, Moscovici
M, Voliotis D and Bruix J; SHARP Investigators Study Group:
Sorafenib in advanced hepatocellular carcinoma. N Engl J Med.
359:378–390. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S,
Kim JS, Luo R, Feng J, Ye S, Yang TS, Xu J, Sun Y, Liang H, Liu J,
Wang J, Tak WY, Pan H, Burock K, Zou J, Voliotis D and Guan Z:
Efficacy and safety of sorafenib in patients in the Asia-Pacific
region with advanced hepatocellular carcinoma: a phase III
randomised, double-blind, placebo-controlled trial. Lancet Oncol.
10:25–34. 2009. View Article : Google Scholar
|
|
8
|
Gollob JA, Rathmell WK, Richmond TM,
Marino CB, Miller EK, Grigson G, Watkins C, Gu L, Peterson BL and
Wright JJ: Phase II trial of sorafenib plus interferon alfa-2b as
first- or second-line therapy in patients with metastatic renal
cell cancer. J Clin Oncol. 25:3288–3295. 2007. View Article : Google Scholar
|
|
9
|
Escudier B, Szczylik C, Hutson TE, Demkow
T, Staehler M, Rolland F, Negrier S, Laferriere N, Scheuring UJ,
Cella D, Shah S and Bukowski RM: Randomized phase II trial of
first-line treatment with sorafenib versus interferon alfa-2a in
patients with metastatic renal cell carcinoma. J Clin Oncol.
27:1280–1289. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sakon M, Nagano H, Dono K, Nakamori S,
Umeshita K, Yamada A, Kawata S, Imai Y, Iijima S and Monden M:
Combined intraarterial 5-fluorouracil and subcutaneous interferon-α
therapy for advanced hepatocellular carcinoma with tumor thrombi in
the major portal branches. Cancer. 94:435–442. 2002.
|
|
11
|
Pestka S, Langer JA, Zoon KC and Samuel
CE: Interferons and their actions. Annu Rev Biochem. 56:727–777.
1987. View Article : Google Scholar
|
|
12
|
Yano H, Iemura A, Haramaki M, Ogasawara S,
Takayama A, Akiba J and Kojiro M: Interferon alfa receptor
expression and growth inhibition by interferon alfa in human liver
cancer cell lines. Hepatology. 29:1708–1717. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hisaka T, Yano H, Ogasawara S, Momosaki S,
Nishida N, Takemoto Y, Kojiro S, Katafuchi Y and Kojiro M:
Interferon-αCon1 suppresses proliferation of liver cancer cell
lines in vitro and in vivo. J Hepatol. 41:782–789. 2004.
|
|
14
|
Yano H, Ogasawara S, Momosaki S, Akiba J,
Kojiro S, Fukahori S, Ishizaki H, Kuratomi K, Basaki Y, Oie S,
Kuwano M and Kojiro M: Growth inhibitory effects of pegylated IFN
alpha-2b on human liver cancer cells in vitro and in vivo. Liver
Int. 26:964–975. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ikeda K, Saitoh S, Arase Y, Chayama K,
Suzuki Y, Kobayashi M, Tsubota A, Nakamura I, Murashima N, Kumada H
and Kawanishi M: Effect of interferon therapy on hepatocellular
carcinogenesis in patients with chronic hepatitis type C: a
long-term observation study of 1643 patients using statistical bias
correction with proportional hazard analysis. Hepatology.
29:1124–1130. 1999. View Article : Google Scholar
|
|
16
|
Ikeda K, Arase Y, Saitoh S, Kobayashi M,
Suzuki Y, Suzuki F, Tsubota A, Chayama K, Murashima N and Kumada H:
Interferon beta prevents recurrence of hepatocellular carcinoma
after complete resection or ablation of the primary tumor-A
prospective randomized study of hepatitis C virus-related liver
cancer. Hepatology. 32:228–232. 2000. View Article : Google Scholar
|
|
17
|
Mazzella G, Accogli E, Sottili S, Festi D,
Orsini M, Salzetta A, Novelli V, Cipolla A, Fabbri C, Pezzoli A and
Roda E: Alpha interferon treatment may prevent hepatocellular
carcinoma in HCV-related liver cirrhosis. J Hepatol. 24:141–147.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nishiguchi S, Kuroki T, Nakatani S,
Morimoto H, Takeda T, Nakajima S, Shiomi S, Seki S, Kobayashi K and
Otani S: Randomised trial of effects of interferon-α on incidence
of hepatocellular carcinoma in chronic active hepatitis C with
cirrhosis. Lancet. 346:1051–1055. 1995.
|
|
19
|
Nishiguchi S, Tamori A and Kubo S: Effect
of long-term postoperative interferon therapy on intrahepatic
recurrence and survival rate after resection of hepatitis C
virus-related hepatocellular carcinoma. Intervirology. 48:71–75.
2005. View Article : Google Scholar
|
|
20
|
Sakaguchi Y, Kudo M, Fukunaga T, Minami Y,
Chung H and Kawasaki T: Low-dose, long-term, intermittent
interferon-alpha-2b therapy after radical treatment by
radiofrequency ablation delays clinical recurrence in patients with
hepatitis C virus-related hepatocellular carcinoma. Intervirology.
48:64–70. 2005. View Article : Google Scholar
|
|
21
|
Murakami T: Establishment and
characterization of human hepatoma cell line (KIM-1). Acta Hepatol
Jpn. 25:532–539. 1984. View Article : Google Scholar
|
|
22
|
Yano H, Iemura A, Fukuda K, Mizoguchi A,
Haramaki M and Kojiro M: Establishment of two distinct human
hepatocellular carcinoma cell lines from a single nodule showing
clonal dedifferentiation of cancer cells. Hepatology. 18:320–327.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chou TC and Talalay P: Quantitative
analysis of dose-effect relationships: the combined effects of
multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 22:27–55.
1984. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tanigawa N, Lu C, Mitsui T and Miura S:
Quantification of sinusoid-like vessels in hepatocellular
carcinoma: its clinical and prognostic significance. Hepatology.
26:1216–1223. 1997.PubMed/NCBI
|
|
25
|
Wang L, Jia D, Duan F, Sun Z, Liu X, Zhou
L, Sun L, Ren S, Ruan Y and Gu J: Combined anti-tumor effects of
IFN-α and sorafenib on hepatocellular carcinoma in vitro and in
vivo. Biochem Biophys Res Commun. 422:687–692. 2012.
|
|
26
|
Tedjarati S, Baker CH, Apte S, Huang S,
Wolf JK, Killion JJ and Fidler IJ: Synergistic therapy of human
ovarian carcinoma implanted orthotopically in nude mice by optimal
biological dose of pegylated interferon alpha combined with
paclitaxel. Clin Cancer Res. 8:2413–2422. 2002.
|
|
27
|
Huang SF, Kim SJ, Lee AT, Karashima T,
Bucana C, Kedar D, Sweeney P, Mian B, Fan D, Shepherd D, Fidler IJ,
Dinney CP and Killion JJ: Inhibition of growth and metastasis of
orthotopic human prostate cancer in athymic mice by combination
therapy with pegylated interferon-alpha-2b and docetaxel. Cancer
Res. 62:5720–5726. 2002.PubMed/NCBI
|
|
28
|
Hlatky L, Hahnfeldt P and Folkman J:
Clinical application of antiangiogenic therapy: microvessel
density, what it does and doesn’t tell us. J Natl Cancer Inst.
94:883–893. 2002.PubMed/NCBI
|
|
29
|
Yao J, Wu X, Zhuang G, Kasman IM, Vogt T,
Phan V, Shibuya M, Ferrara N and Bais C: Expression of a functional
VEGFR-1 in tumor cells is a major determinant of anti-PlGF
antibodies efficacy. Proc Natl Acad Sci USA. 108:11590–11595. 2011.
View Article : Google Scholar : PubMed/NCBI
|