Introduction
The sodium/iodine symporter (NIS) is an intrinsic
plasma membrane glycoprotein and responsible for thyroidal,
gastric, salivary, intestinal and mammary iodide transport. The rat
NIS gene was first cloned in 1996 (1), human NIS (hNIS) gene later in the
same year (2) and mouse NIS gene
in 2001 (3). Cloning and
characterization of the NIS gene make it possible to deliver this
gene into non-thyroid cells. NIS can be used with both diagnostic
(125I, 124I, 99mTc) and therapeutic
(131I, 211At, 186Re,
188Re) radioisotopes, and the potential value of NIS
usage has become more important for researchers. There has been
great progress in discovering the biological function of NIS in
thyroid and non-thyroid tissues (4), which results in increasing use of NIS
as a means of achieving imaging or therapeutic goals not only in
thyroid cancer but also in non-thyroidal tumour tissues.
NIS gene has been applied to a series of viral and
non-viral vectors in which it can be used for live animal imaging.
Transient or stable NIS expression in non-thyroidal tissues and
tumour xenografts promoting iodide uptake has led to the use of NIS
as an imaging reporter in many preclinical experiments and some
clinical studies. The three main research areas of NIS reporter
imaging are viral-mediated gene therapy, oncolytic viral therapy
and cell trafficking (e.g., stem cell delivery) (5). NIS is one of the most promising
radionuclide reporters for preclinical and translational
research.
The characteristics of the NIS gene indicate that it
can serve as a good therapeutic gene for cancer therapy. NIS has
been used as a therapeutic gene for therapy of thyroid cancer and
hyperthyroidism for a long time (6). Importantly, NIS has a high bystander
effect, whereby non-transduced tumour cells can be caught in the
electron cross-fire emanating from the transduced cells and still
eradicated by the emission of an electron (β particle) (7). Moreover, because NIS is a normal
human gene and protein, its expression in target cells is unlikely
to be toxic or limit its efficacy in patients. In view of these
advantages, the field of NIS gene therapy has made considerable
strides. Researchers have demonstrated the feasibility of NIS
mediated gene therapy in vitro and/or in vivo for
various tumours such as thyroid, breast, liver, prostate, pancreas,
colorectal, colon, ovarian and uterine cervical carcinomas, and
multiple myeloma (8).
Nasopharyngeal carcinoma (NPC) has a unique
geographical and ethnic predisposition with high incidences in
Southern China, Southeast Asia, the Middle East/North Africa and
the Arctic. P53 protein plays a vital role in regulating the cell
cycle, differentiation and apoptosis, and may also have an
important therapeutic implication (9). Because NPC is highly radiosensitive,
radiotherapy is the primary treatment. In recent years, its
treatment has achieved great progress including gene therapy, such
as a recombinant adenovirus expressing p53 in 2003 (10). However, novel efficient diagnosis
and therapeutic approaches still need to be established in the
management of patients with recurrent, residual or metastatic
disease (11). It is significant
to monitor the in vivo distribution, replication and
elimination of replicating vectors as well as therapeutic gene
expression in the NPC gene therapy. As mentioned above, NIS gene
transfer has the ability of in vivo imaging and therapy for
the radiosensitive tumours. Therefore, it is meaningful to explore
the radiosensitivity of the NPC cells mediated by NIS.
Effective cancer gene therapy depends on efficient
viral infection as well as persistent gene expression in the target
tissue, which needs an ideal gene transfer vector. Due to the
biosafety, huge cloning capacity, low cytotoxicity and
non-replication nature in the target cells and the ease of
manipulation and production, baculovirus has emerged as a novel
vector for in vitro and in vivo gene delivery among a
variety of viral and non-viral vectors (12). However, there have been no report
of NPC cells transducted by baculovirus. Although serum
inactivation of baculovirus is a significant barrier to the
development of this vector for therapeutic gene delivery, NPC has
the advantage of the unique location where baculovirus can be
injected directly, accurately and repeatedly into the target site
of the tumour from the air, which can avoid baculovirus
inactivation in the presence of serum complement to some
extent.
To investigate the applicability of NIS and
baculovirus in NPC therapy and prepare for the next in vivo
gene therapy, we produced the recombinant baculoviruses Bac-GFP and
Bac-NIS, and conducted a series of related experiments on an human
NPC cell line CNE-2Z with the above viruses in vitro.
Materials and methods
Plasmids and cells
Plasmid pFastBac-CMV-GFP was kindly provided by the
Institute of Molecular Biology, The University of Hong Kong;
pFastBac 1 vector was obtained from Invitrogen; pcDNA-hNIS was a
gift of Dr Sissy Jhiang at the Ohio State University; Sf9 cells
from Invitrogen were cultured in Sf900 III medium with 10% fetal
bovine serum (FBS); human NPC line CNE-2Z, purchased from Jiangyin
K.K. Company, were cultured in RPMI-1640 medium supplemented with
10% FBS and 1% penicillin/streptomycin in a humidified environment
with 5% CO2 at 37°C.
Construction of plasmid
pFastBac-CMV-hNIS
Polyhedron (PH) promoter gene in pFastBac 1 vector
was replaced with cytomegalovirus (CMV) promoter gene, and then
plasmid pFastBac-CMV was generated. Human NIS (hNIS) gene was
obtained from pcDNA-NIS with the restriction enzymes SalI
and Xbal. Next, hNIS gene fragment and pFastBac-CMV vector
were digested simultaneously with SalI and Xbal, and
connected by T4 DNA ligase. Plasmid pFastBac-CMV-hNIS was
constructed successfully after verification by agarose gel
electrophoresis and sequencing.
Production of recombinant
baculoviruses
Plasmids pFastBac-CMV-hNIS and pFastBac-CMV-GFP were
transformed into DH10Bac E. coli to generate recombinant
bacmids, which were later transfected into Sf9 cells to generate
recombinant baculoviruses. After amplification, we generated
successfully Bac-NIS (Bac-CMV-hNIS) and Bac-GFP (Bac-CMV-GFP). The
titer of a baculoviral stock was determined through performing a
viral plaque assay. Guidelines and instructions were referred to
Bac-to-Bac Baculovirus Expression System manual.
Infection efficiency and fluorescence
intensity of Bac-GFP and indirect immunofluorescence of
Bac-NIS
CNE-2Z cells were seed in 24-well plates at a
density of 5×104 cells/well and cultured until they were
80% confluent at the time of infection. The initial media were
replaced with PBS including Bac-GFP at MOIs of 0, 50, 100, 200 and
400 with or without 5 mmol/l sodium butyrate just before infection,
which was changed to RPMI with 10% FBS 4 h later. To test the
effect of sodium butyrate, CNE-2Z cells were infected by Bac-GFP at
MOI of 100 and different concentrations of sodium butyrate were
added. The infection efficiency and the mean fluorescence intensity
of Bac-GFP group were determined by flow cytometry. Bac-NIS group
was infected by Bac-NIS at MOI of 200 and stained with indirect
immunofluorescence (anti-NIS goat polyclonal antibody from Santa
Cruz Company, 1:200 and DyLight594 goat anti-rabbit IgG from
MultiSciences Company, 1:200) after 24 h incubation. Then, cells in
Bac-GFP or Bac-NIS group were observed by an inversion fluorescence
microscope. The excitation wavelength of blue laser was 488 nm and
the detection wavelength was 520 nm. All the following infections
of CNE-2Z cells by Bac-NIS or Bac-GFP were treated following the
above process and performed in triplicate in this experiment.
The cytotoxicity of recombinant
baculovirus and sodium butyrate
CNE-2Z cells were seeded in 96-well plates at a
density of 5×103 cells/well and cultured until they were
80–90% confluent before infection. For Bac-GFP group, Bac-GFP at
MOIs of 0, 100, 200, 400, 600 or 800 was added. For sodium butyrate
group, Bac-GFP at a MOI of 400 and sodium butyrate at the
concentrations of 0, 5, 10, 15, 20 or 25 mmol/l were added. In
addition, control group was free of Bac-GFP and sodium butyrate,
and blank group was treated with only RPMI without cells, Bac-GFP
and sodium butyrate. Twenty-four hours later, initial media were
replaced with fresh RPMI, 10 μl of CCK-8 was added into each
well and incubated for another 4 h. The absorption at 450 nm (A450)
was measured with a microplate reader. Cell viability was
calculated with the equation: cell viability (%) = (test well A450
- blank well A450)/(control well A450 - blank well A450) ×
100%.
125I uptake assay
The assay was performed as described (13). CNE-2Z cells were infected by
Bac-NIS at MOIs of 0, 25, 50, 100, 200 or 400, and washed twice by
Hank’s balanced salt solution (HBSS) 24 h later. To each well was
added 0.1 μCi Na125I and 1 μM NaI in a
total volume of 0.5 ml HBSS and incubated at 37°C for 30 min. Next,
cells were washed 3 times with ice-cold HBSS, and then 0.5 ml 100%
ice-cold dehydrated alcohol was added. Twenty minutes later, the
radioactivity (counts per minute, cpm) in each well was measured by
a γ-counter.
Dynamic 125I uptake
After 24 h of the Bac-NIS or Bac-GFP infection both
at MOI of 200, 125I uptake of CNE-2Z cells was measured
as depicted above at 0, 5, 10, 15, 30, 45, 60, 75, 90, 105 and 120
min after Na125I and NaI incubation, respectively.
125I efflux test
125I efflux was measured as previously
reported (13). Following 45 min
of Na125I and NaI incubation of the CNE-2Z cells
infected with Bac-NIS at MOI of 200, the cell-culture medium was
replaced with fresh HBSS every 5 min and further incubated for in
30 min. The cells were treated with 0.5 ml dehydrated alcohol at 30
min to measure the residual radioactivity in the cells. At the same
time, the buffers replaced at different times were measured.
NIS gene expression time of duration
To test NIS gene expression time, CNE-2Z cells were
infected with Bac-NIS or Bac-GFP at MOI of 400, the 125I
uptake was measured as depicted above at days 1, 2, 4, 8, 12, 16
and 20. During that period of time, cell culture medium was
refreshed every 3 days.
Inhibition of iodine uptake by
NaClO4
Twenty-four hours following infection of CNE-2Z
cells with Bac-NIS at MOI of 200, 0.1 μCi Na125I,
1 μM NaI and NaClO4 at concentrations of 0, 10,
20, 50, 100 or 200 μmol/l in a total volume of 0.5 ml HBSS
were added to each well, 45 min later, CNE-2Z cells were washed,
lysed and counted as described above.
Correlation between radioactivity and
fluorescence intensity in co-infected CNE-2Z cells with Bac-NIS and
Bac-GFP
This experiment was performed to explore the
correlation between 125I uptake and fluorescence
intensity in co-infected CNE-2Z cells. CNE-2Z cells were
co-infected with Bac-NIS and Bac-GFP at total viral MOIs of 0, 50,
100, 200 or 400, and each MOI of each virus for every well was
equal at MOIs of 0, 25, 50, 100 or 200, respectively. Twenty-four
hours after infection, 3 wells of each MOI were tested by flow
cytometry to determine the mean fluorescence intensity. The cells
of 3 wells of each MOI were tested by a γ-counter to measure iodine
uptake.
131I-mediated killing of
CNE-2Z cells in cell colony formation test
To estimate the killing effect of CNE-2Z cells by
131I, a cell colony formation test including six groups
was performed. CNE-2Z cells in all groups were seed in 24-well
plates at a density of 1×105 cells/well and cultured for
24 h. Next, group 1 (Bac-NIS+131I) and group 4 (Bac-NIS)
were infected with Bac-NIS at MOI of 400; group 2
(Bac-GFP+131I) and group 5 (Bac-GFP) were infected with
Bac-GFP at MOI of 400; group 3 (HBSS+131I) and group 6
(HBSS) were treated with HBSS in the corresponding way. Twenty-four
hours later, all the cells were washed twice by HBSS. To each well
in groups 1, 2 and 3 were added 50 μCi Na131I in
a total volume of 0.5 ml HBSS and incubated for 6 h, while groups
4, 5 and 6 were free of Na131I. Then, all the cells were
washed twice with HBSS, trypsinized, counted, plated in a 6-well
plate (300 cells/well), and incubated. During colony growth, the
culture medium was replaced every 3 days. At the 7th day, cells
were washed three times with HBSS and stained with 0.1% crystal
violet solution. The colony was counted only if it contained more
than 30 cells in a well. Colony formation rate (%) = (number of
colonies/number of plated cells) × 100%.
Statistical analysis
Each treatment was carried out in triplicates,
unless noted otherwise. Statistical analysis was performed using
SPSS 21.0 software. All data are presented as the mean ± SD.
Differences were considered significant at P<0.05.
Results
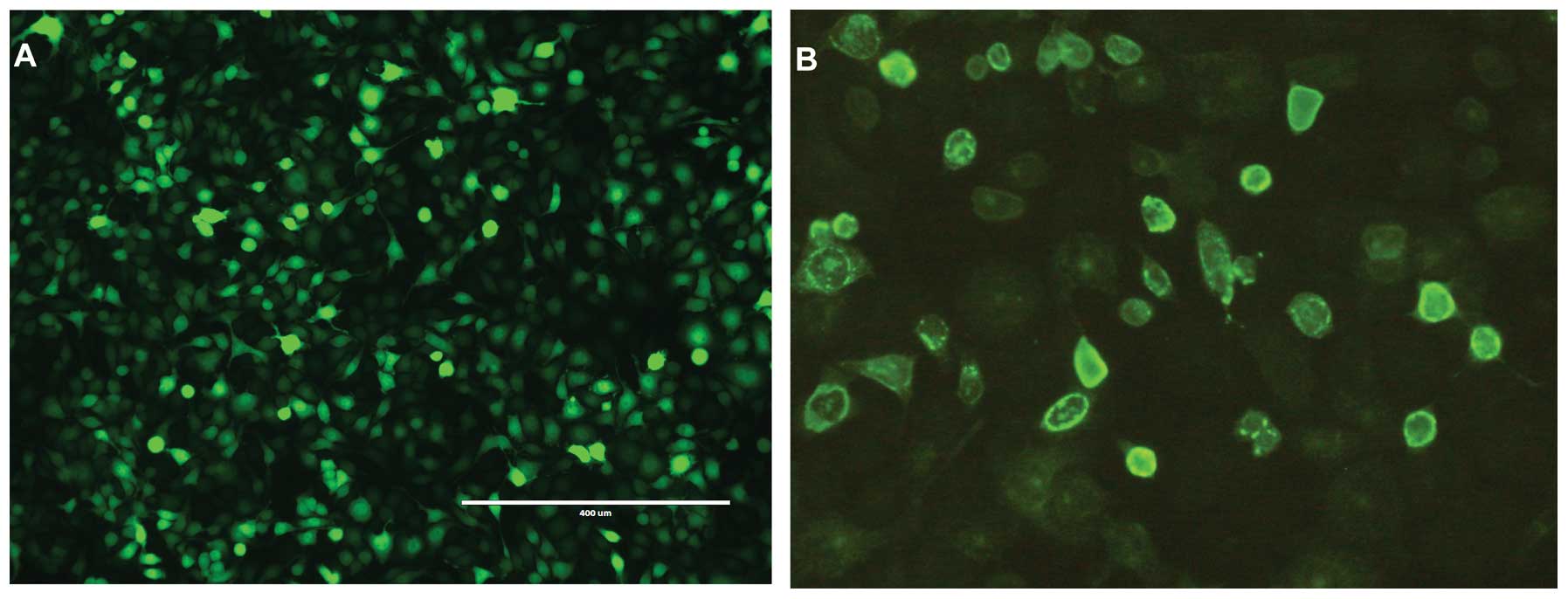
Immunofluorescence of Bac-NIS
The stocks of Bac-NIS and Bac-GFP were
2×109 and 5×109 pfu/ml, respectively. As can
be seen from the Fig. 1B, the NIS
protein was clearly and precisely expressed on CNE-2Z cell
membranes, which indicated that Bac-NIS expressed NIS protein in
infected CNE-2Z cells efficiently. Correspondingly, Fig. 1A depicts abundant GFP expression in
CNE-2Z cells infected with Bac-GFP.
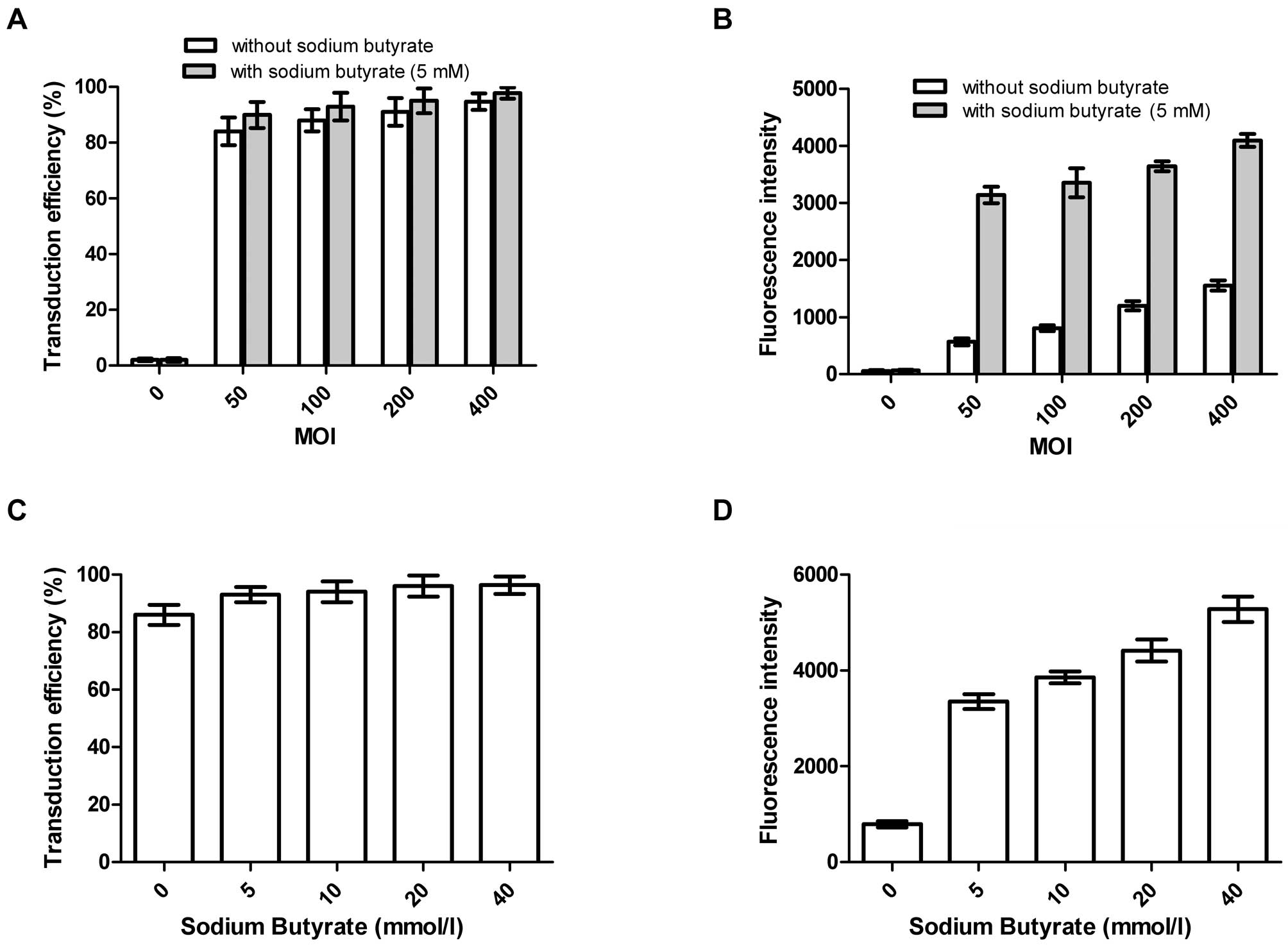
Transduction efficiency and fluorescence
intensity of Bac-GFP with or without sodium butyrate
As can be seen from the Fig. 2, both the transduction efficiency
and fluorescence intensity of Bac-GFP in the CNE-2Z cells became
stronger with increasing MOIs, whilst the former saw a gentle
upward trend and the later surged when 5 mmol/l or different
concentrations of sodium butyrate were added. Additionally, there
were no statistically significant differences for the transduction
efficiency between groups with or without sodium butyrate whether
at different MOIs of Bac-GFP or various concentrations of sodium
butyrate, whereas, the corresponding results of fluorescence
intensity were the opposite, and the differences were statistically
significant. It is noteworthy that the transduction efficiency of
Bac-GFP at the MOI of 400 without or with 5 mmol/l sodium butyrate
reached 94.79 and 97.86%, respectively.
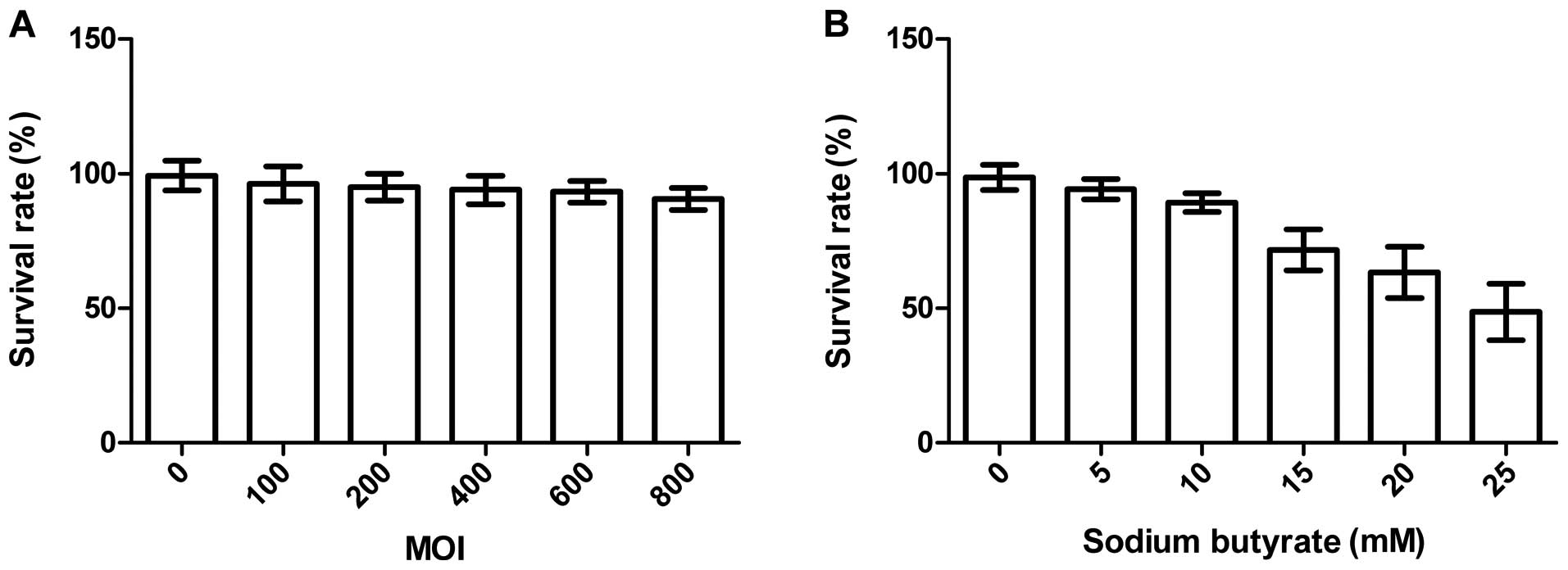
The cytotoxicity of baculovirus and
sodium butyrate
Fig. 3A
demonstrates that the cell survival rate in Bac-GFP group had no
significant decrease with increasing MOIs. Even at MOI of 800,
there was no obvious cell death. Therefore, baculovirus had hardly
any cytotoxic effects on the CNE-2Z cells. As shown in Fig. 3B, the cell survival rate in sodium
butyrate group decreased obviously with increasing concentrations
of sodium butyrate with Bac-GFP at MOI of 400. The cell viability
changed significantly when the concentration of sodium butyrate was
higher than 10 mmol/l. The above suggested that the baculovirus
itself did not decrease the CNE-2Z cell viability significantly,
while relatively high concentration of sodium butyrate generated
cytotoxicity on the cells.
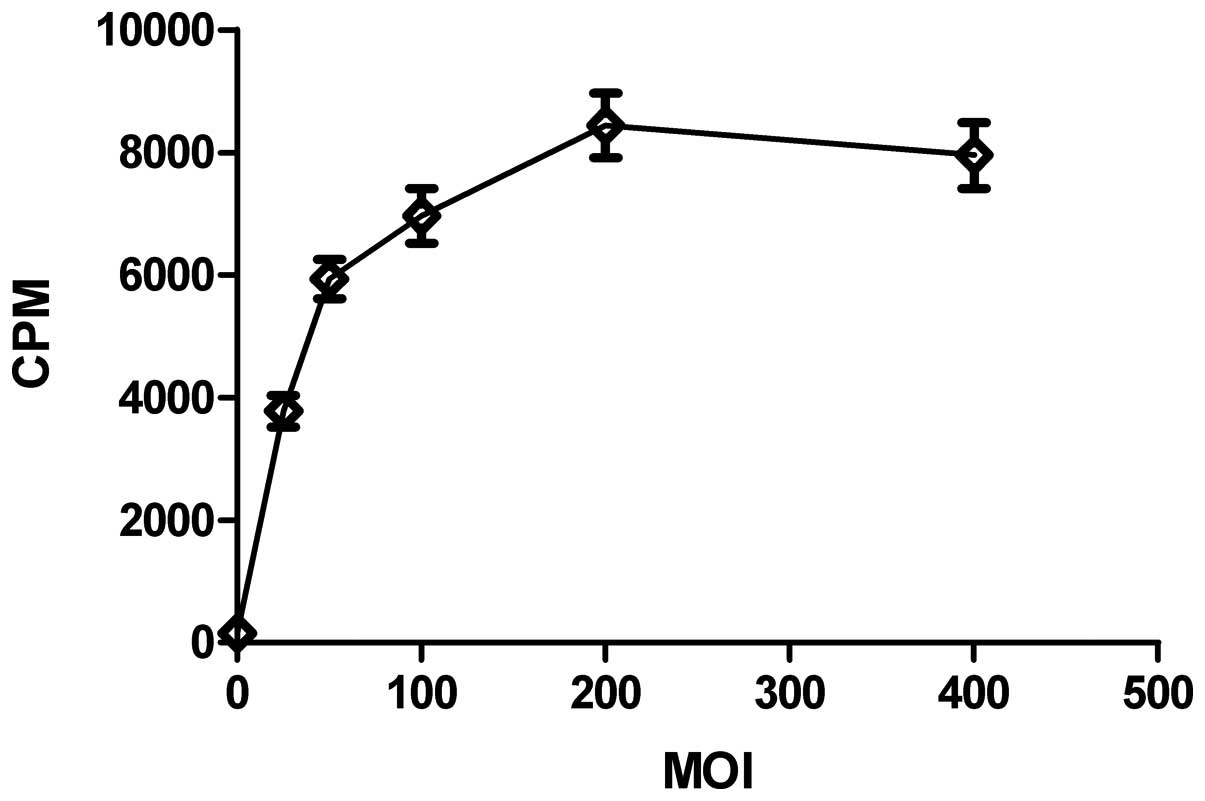
125I uptake at different
MOIs
Fig. 4 shows that
the cpm in the infected CNE-2Z cells changed with the increasing
MOIs of Bac-NIS. It shot up over the first MOI of 100, peaked at
MOI of 200 and then showed a slight downward trend. Therefore,
CNE-2Z cells were infected with Bac-NIS at MOI of 200 in the
following dynamic 125I uptake test.
Dynamic 125I uptake at MOI of
200
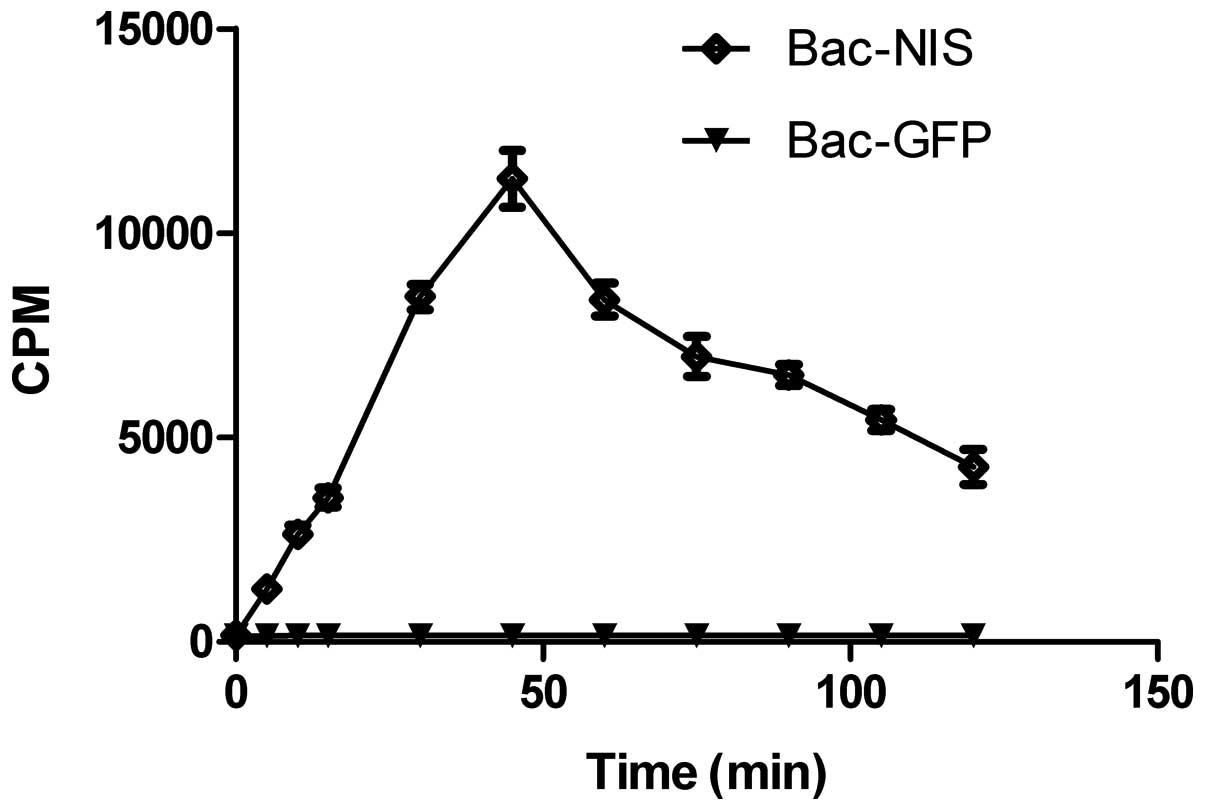
Fig. 5 indicates
CNE-2Z cells absorbed 125I in relation to incubation
time. The first 45 min witnessed a dramatic rise, with cpm peaking
at 11,200 and cpm in the following 75 min saw a fall from the peak
at 45 min to only 4,200 at 120 min. The 125I uptake of
Bac-NIS group was 70-fold higher than that of Bac-GFP group.
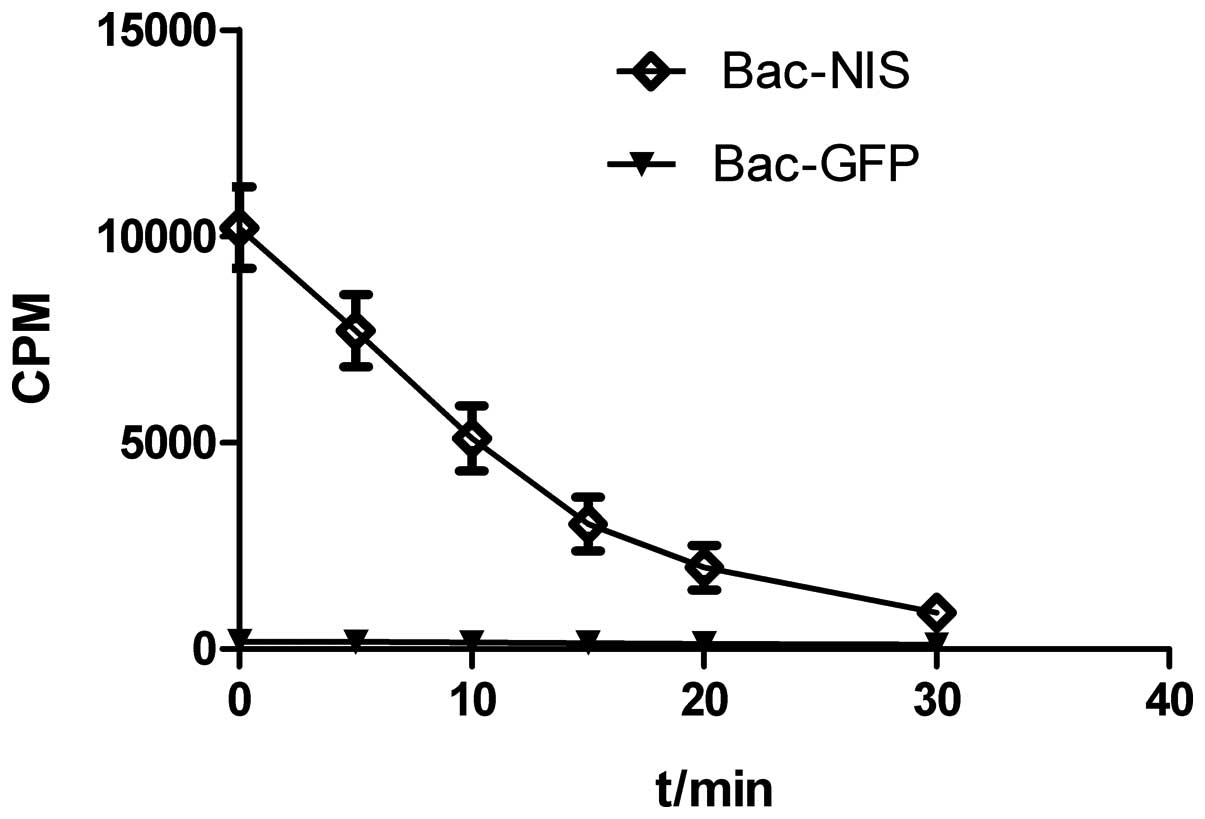
125I efflux test
Fig. 6 shows the
continuous outflow of 125I into the medium as time went
on. Nearly half of the total cellular iodine was released in the
initial 10 min and only less than 10% remained in infected cells at
the time of 30 min.
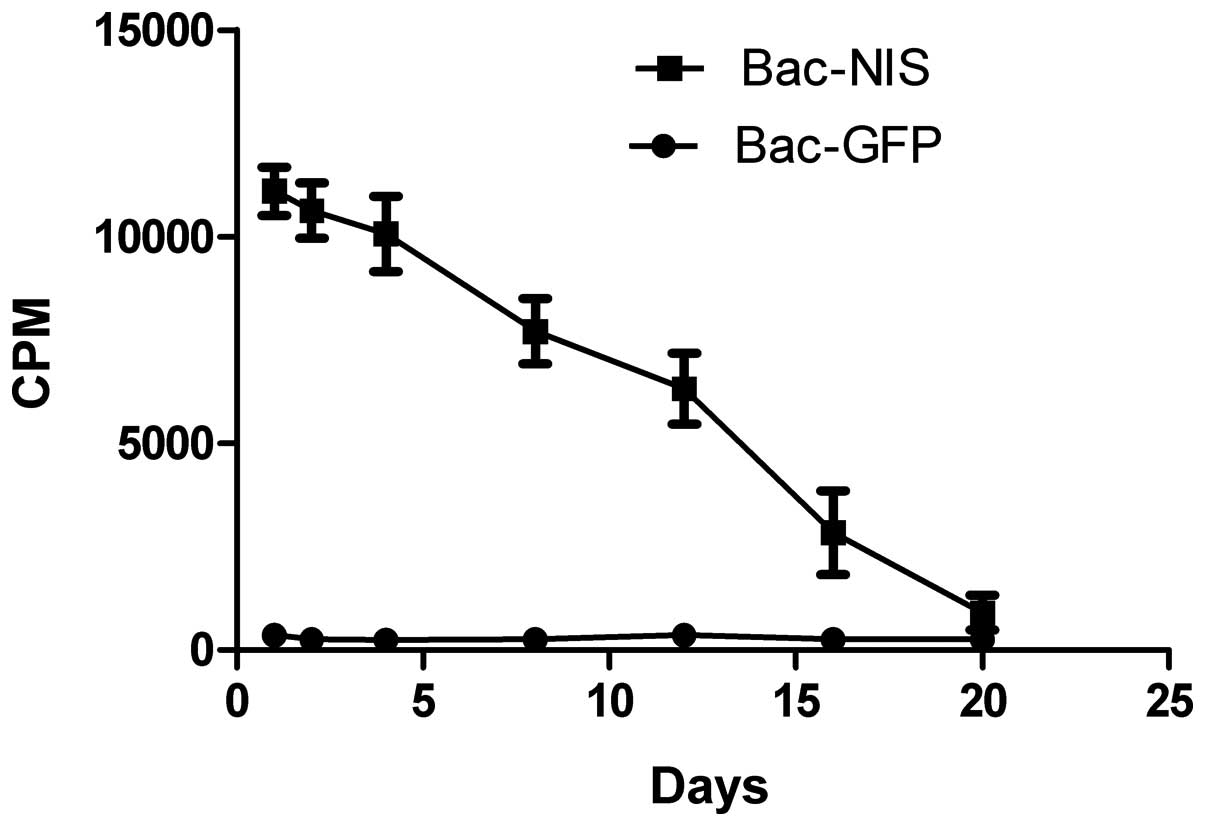
NIS gene expression time of duration
After CNE-2Z cells were infected with Bac-NIS at MOI
of 400, the 125I uptake was measured at different days.
The results in Fig. 7 describe
that the cpm leveled off relatively over the first 4 days,
descended gently between the 4th and 8th day, and reached the
bottom at day 20, which indicated that the expression of NIS was
stable within 8 days after infection.
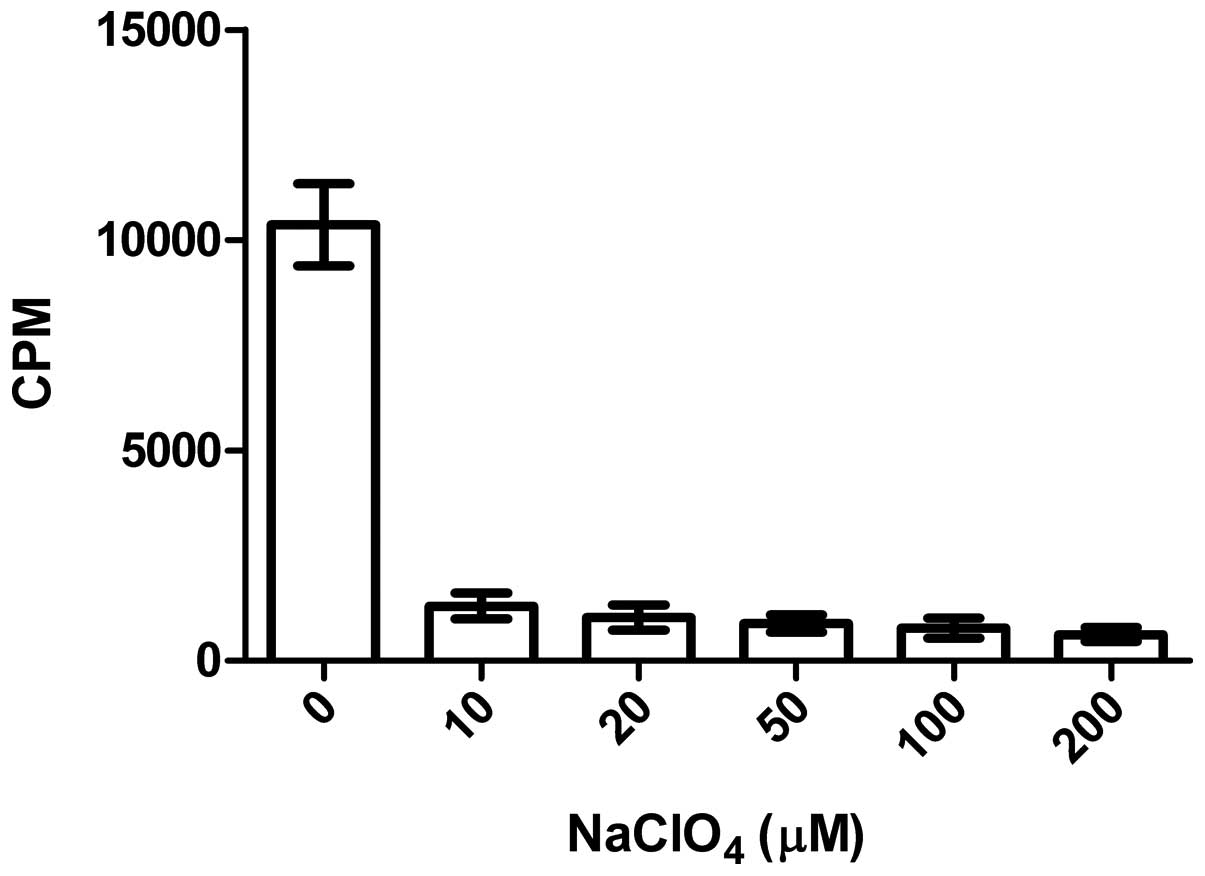
Inhibition of NaClO4
Fig. 8 illustrates
that the impact of NaClO4 on 125I uptake of
CNE-2Z cells was obvious, with 87.5, 90.1, 91.5, 92.6 and 94.0% of
125I uptake inhibited along with increasing
concentrations of NaClO4, which demonstrated that the
function of the NIS protein in the CNE-2Z cells was also inhibited
by the presence of NaClO4 as in thyroid tissue.
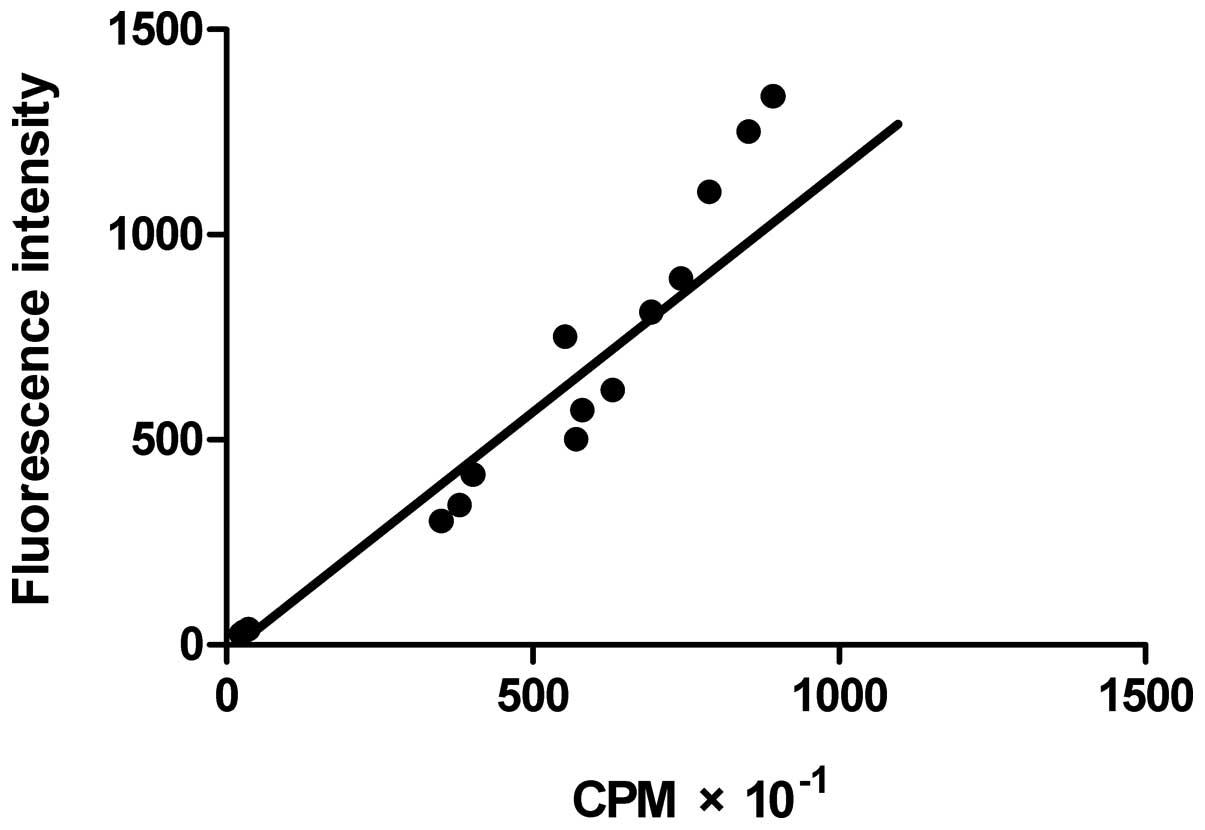
Correlation between 125I
uptake and GFP fluorescence intensity in co-infected CNE-2Z
cells
The correlation between cpm and GFP fluorescence
intensity is depicted in Fig. 9,
which displays a definite trend, and the correlation coefficiency
between them was 0.917, a high positive correlation. The above data
show that the GFP expression level is highly correlated to that of
NIS, so the level of radioactivity mirrored the GFP expression
level.
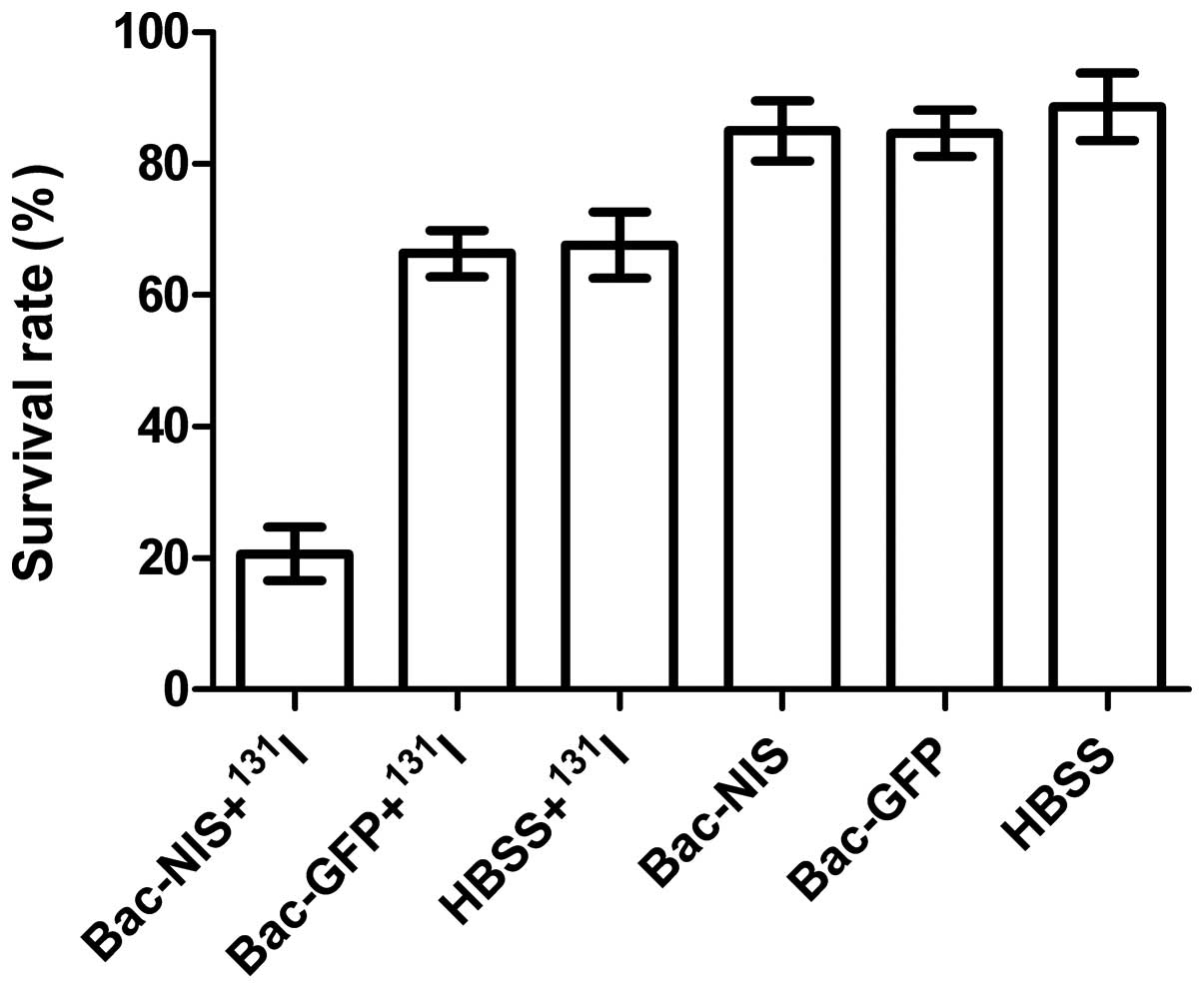
The cell colony formation test
A cell colony formation test can evaluate the
killing effect of 131I in infected tumour cells and the
results of the colony-forming assay show the survival rate. The
results in Fig. 10 indicate that
the cell survival rate of the group Bac-NIS with 131I
was 20.7%, which was substantially lower than that of the other
five groups (P<0.01). The cell viability in group 2 (66.3%) was
similar to that of the group 3 (67.6%). Cell survival rates of the
three groups without 131I were virtually equal, which
also indicated baculovirus had scarcely any impact on cell
survival. Therefore, the treatment of Bac-NIS with 131I
can kill infected CNE-2Z cells efficiently in vitro.
Discussion
Baculovirus can transduce a broad range of mammalian
and avian cells (14), and mediate
efficient gene expression in a wide range of vertebrate cells
(15). In this study, we
constructed Bac-GFP and Bac-NIS with high titre successfully and
the transduction rate of baculovirus on CNE-2Z cells reached 91.16%
at MOI of 200. The radioiodine uptake of the infected CNE-2Z cells
by Bac-NIS at the MOI of 200 reached the peak at 45 min and was
significantly reduced with escalating doses of NaClO4,
which indicated that the NIS protein expressed in NPC cells could
perform its normal function in iodine uptake just as in thyroid
cells. High infection efficiency, low cytotoxicity, durable NIS
expression, a physiological protein without immunogenicity
theoretically, and easiness to obtain radioactive iodine
(131I, 125I, 124I, etc.) and
technetium are favorable factors for NIS gene imaging and therapy
of NPC.
For quantitative use in visualization and
analyzation by PET and SPECT, the NIS gene has gained a broad
application in the non-invasive imaging approach monitoring cell
migration, localization, proliferation and other biological
processes or observing the real-time level and duration of target
gene expression (16). It is
extremely advantageous if gene therapy vectors can achieve the
reliable non-invasive monitoring of vector biodistribution and
population together with therapeutic gene expression, which is
critical for the evaluation of the success or failure of gene
therapy approaches. However, most therapeutic genes cannot be
quantified directly by using molecular imaging just as NIS gene, so
the NIS reporter gene and a therapeutic gene can be combined to
perform indirect imaging (17). In
this study, we adopted co-infection approach in which CNE-2Z cells
were co-infected with Bac-GFP and Bac-NIS at the same MOIs, and the
result showed correlation coefficiency of 0.917 between the
fluorescence intensity and radioactivity. A similar way has been
reported (18). Consequently,
there is a correlation between GFP and NIS expression in CNE-2Z
cells and we can use the changes in the level of radioactivity to
assess changes in GFP expression level. Furthermore, the way of
co-infection is an effective approach to fulfil multi-gene
co-expression in the target cells when GFP gene is replaced by a
desired gene.
The effective 131I therapy of most
differentiated thyroid cancer has been conformed more than 60 years
and the field of radiotherapy by NIS gene transfer has made huge
leaps in recent years (19). The
cell colony formation test demonstrated that NIS-mediated CNE-2Z
cells could be effectively and specifically killed by
131I, which laid a good foundation for the application
of NIS in NPC. Furthermore, 188Re (Rhenium) radiating
β-rays (20) and 211At
(Astatine) radiating α-rays (21)
mediated by NIS have even better radiotherapeutic effects than
131I for their higher radiation energy, which are
stronger weapons for NIS-mediated cancer gene therapy suggesting
that NIS gene is a promising candidate for NPC radiotherapy.
Since the discovery that baculovirus could enter
mammalian cells and mediate transgene expression under the control
of mammalian promoters (22),
recombinant baculovirus vectors have become an efficient tool for
gene transfer into mammalian cells (12). Of particular concern is serum
inactivation of gene transduction in vivo mediated by
baculovirus (23). However, the
locus of NPC is another favorable factor for gene therapy. Bac-NIS
can be injected directly, repeatedly and accurately into the local
region of the tumour when needed, which can avoid circulation in
the blood and have a good targeting property. Therefore, this can
escape serum inactivation to a certain extent. In addition, sodium
butyrate as a histone deacetylase inhibitor can obviously enhance
infection efficiency of baculovirus, while it has some cytotoxicity
(24), we also confirmed this
conclusion above. The results above indicated that sodium butyrate
improved the efficiency of baculovirus infection in CNE-2Z cells
but that this effect was not quite statistically significant.
Having high infection rate without sodium butyrate and the
convenience of treatment, recombinant baculovirus is an appropriate
gene delivery vector for use in NPC.
Radiotherapy is the standard treatment for NPC, and
it can produce a variety of complications for the location of the
tumour at the base of skull and in close proximity to radiation
dose-limiting organs, giving brachytherapy an advantage in this
aspect (15). NIS can mediate
active 131I transport and 131I has been used
successfully to treat thyroid disorders for a long time. Given this
and the unique feature of 131I, the ability of Bac-NIS
to accumulate 131I may be applied in NPC brachytherapy
with its unique advantages: the amount and time of 131I
injection can be adjusted; accurate application is still feasible
even in the irregular contour of the nasopharynx; NIS can serve as
an imaging reporter at the same time. All of the above data show
Bac-NIS has a wide application prospect in NPC brachytherapy.
Although radiation and chemotherapy in the treatment
of NPC are effective, local failure or regional failure presenting
as persistent or recurrent tumour is still inevitable. Early
detection is of great concern for any form of salvage therapy. As
is well known that it is difficult to confirm residual or recurrent
tumour in the cervical lymph nodes after radiotherapy (25). In addition, durable responses are
rarely encountered in metastatic disease and the recurrent tumours
are more resistant to chemotherapy after initial responses in
cytotoxic chemotherapy. However, the combination Bac-NIS and
mesenchymal stem cells (MSC) has the potential to solve these
problems because the adoptively transferred MSCs can migrate and
engraft into the stroma of established tumour lesions (26). MSCs as a cellular delivery vehicle
with specific tropisms has been exploited in some tumours (27). Importantly, the infection
efficiency of recombinant baculovirus is quite high in MSCs
(28). In addition, not only would
MSCs protect baculovirus from serum complement attack, but also
they would allow the amplification of the initial viral load with
the possibility of increasing viral spread through cell-to-cell
contact specifically at tumour sites (27). Therefore, MSCs as a vehicle for
targeted delivery of NIS to tumours may be applied in NPC, and a
relative study reported the image-guided and tumour stroma-targeted
131I therapy of hepatocellular cancer after systemic
MSCs-mediated NIS gene delivery (29). Therefore, this approach is not
limited to radiologically and reliably detectable tumours, but can
also be used to target minimal residual, recurrent and
micrometastatic tumours, which demonstrates a better prospect of
application in NPC for the above problems. In this context, the
approach of MSCs-mediated therapeutic gene with easy mode of
administration (intravenous injection) may in the future provide
the means for therapy of NPC and our study establishes a solid
foundation for this.
In this study, CNE-2Z cells were first infected with
Bac-GFP and Bac-NIS. We found that baculovirus was able to
penetrate and deliver target genes into CNE-2Z cells and the
transduction rate reached as high as 94.79% at MOI of 400, which
not only expanded the transduction range of baculovirus, but also
proved the feasibility of gene delivery mediated by baculovirus in
NPC. The high related coefficient between 125I uptake
and GFP fluorescence intensity in co-infected CEZ-2Z cells and
131I-mediated killing of tumour cells demonstrated that
Bac-NIS could be applied in molecular imaging and radioactive
therapy in NPC. Therefore, we believe the combination Bac-NIS has
bright prospects in diagnosis and treatment of NPC.
Acknowledgements
This study was supported with research
funds (114119a6400) from the Science and Technology Commission of
Shanghai Municipality, China.
References
|
1.
|
Dai G, Levy O and Carrasco N: Cloning and
characterization of the thyroid iodide transporter. Nature.
379:458–460. 1996. View
Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Smanik P, Liu Q, Furminger T, et al:
Cloning of the human sodium iodide symporter. Biochem Biophys Res
Commun. 226:339–345. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Pinke L, Dean D, Bergert E, Spitzweg C,
Dutton C and Morris J: Cloning of the mouse sodium iodide
symporter. Thyroid. 11:935–939. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Dohán O, De la Vieja A, Paroder V, et al:
The sodium/iodide symporter (NIS): characterization, regulation,
and medical significance. Endocr Rev. 24:48–77. 2003.PubMed/NCBI
|
|
5.
|
Penheiter AR, Russell SJ and Carlson SK:
The sodium iodide symporter (NIS) as an imaging reporter for gene,
viral, and cell-based therapies. Curr Gene Ther. 12:33–47. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Carvalho DP and Ferreira AC: The
importance of sodium/iodide symporter (NIS) for thyroid cancer
management. Arq Bras Endocrinol Metabol. 51:672–682. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Ferlini C, Amelio RD and Scambia G:
Apoptosis induced by ionizing radiation. The biological basis of
radiosensitivity. Subcell Biochem. 36:171–186. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Kogai T and Brent GA: The sodium iodide
symporter (NIS): Regulation and approaches to targeting for cancer
therapeutics. Pharmacol Ther. 135:355–370. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Sun Y, Yi H, Yang Y, et al: Functional
characterization of p53 in nasopharyngeal carcinoma by stable shRNA
expression. Int J Oncol. 34:1017–1027. 2009.PubMed/NCBI
|
|
10.
|
Peng Z: Current status of gendicine in
China: recombinant human Ad-p53 agent for treatment of cancers. Hum
Gene Ther. 16:1016–1027. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Li X, Liu X, Li C, et al: Recombinant
adeno-associated virus mediated RNA interference inhibits
metastasis of nasopharyngeal cancer cells in vivo and in
vitro by suppression of Epstein-Barr virus encoded LMP-1. Int J
Oncol. 29:595–603. 2006.PubMed/NCBI
|
|
12.
|
Airenne KJ, Mahonen AJ, Laitinen OH and
Yla-Herttuala S: Baculovirus-mediated gene transfer: an emerging
universal concept. Gene and Cell Therapy: Therapeutic Mechanisms
and Strategies. Smith Templeton N: Chapter 11.3rd edition. CRC
Press; Boca Raton, FL: pp. 263–281. 2008
|
|
13.
|
Weiss SJ, Philp NJ and Grollman EF: Iodide
transport in a continuous line of cultured cells from rat thyroid.
Endocrinology. 114:1090–1098. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Chen CY, Lin CY, Chen GY and Hu YC:
Baculovirus as a gene delivery vector: recent understandings of
molecular alterations in transduced cells and latest applications.
Biotechnol Adv. 29:618–631. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Wang C, Busse J and Gitterman M: A simple
afterloading applicator for intracavitary irradiation of carcinoma
of the nasopharynx. Radiology. 115:737–738. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Dingli D, Russell SJ and Morris JC III: In
vivo imaging and tumor therapy with the sodium iodide symporter. J
Cell Biochem. 90:1079–1086. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Blasberg RG: Molecular imaging and cancer.
Mol Cancer Ther. 2:335–343. 2003.
|
|
18.
|
Yaghoubi S, Wu L, Liang Q, et al: Direct
correlation between positron emission tomographic images of two
reporter genes delivered by two distinct adenoviral vectors. Gene
Ther. 8:1072–1080. 2001. View Article : Google Scholar
|
|
19.
|
Hingorani M, Spitzweg C, Vassaux G, et al:
The biology of the sodium iodide symporter and its potential for
targeted gene delivery. Curr Cancer Drug Targets. 10:242–267. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Shen D, Marsee D, Schaap J, et al: Effects
of dose, intervention time, and radionuclide on sodium iodide
symporter (NIS)-targeted radionuclide therapy. Gene Ther.
11:161–169. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Willhauck MJ, Samani B-RS, Wolf I, et al:
The potential of 211 Astatine for NIS-mediated radionuclide therapy
in prostate cancer. Eur J Nucl Med Mol Imaging. 35:1272–1281. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Hofmann C, Sandig V, Jennings G, Rudolph
M, Schlag P and Strauss M: Efficient gene transfer into human
hepatocytes by baculovirus vectors. Proc Natl Acad Sci.
92:10099–10103. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Kaikkonen MU, Ylä-Herttuala S and Airenne
KJ: How to avoid complement attack in baculovirus-mediated gene
delivery. J Invertebr Pathol. 107:S71–S79. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Hu YC, Tsai CT, Chang YJ and Huang JH:
Enhancement and prolongation of baculovirus mediated expression in
mammalian cells: focuses on strategic infection and feeding.
Biotechnol Prog. 19:373–379. 2008.PubMed/NCBI
|
|
25.
|
Wei WI, Ho CM, Wong MP, Fung Ng W, Lau SK
and Lam KH: Pathological basis of surgery in the management of
postradiotherapy cervical metastasis in nasopharyngeal carcinoma.
Arch Otolaryngol Head Neck Surg. 118:923–930. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Studeny M, Marini FC, Champlin RE,
Zompetta C, Fidler IJ and Andreeff M: Bone marrow-derived
mesenchymal stem cells as vehicles for interferon-β delivery into
tumors. Cancer Res. 62:3603–3608. 2002.
|
|
27.
|
Hall B, Andreeff M and Marini F: The
participation of mesenchymal stem cells in tumor stroma formation
and their application as targeted-gene delivery vehicles. Handb Exp
Pharmacol. 263–283. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Ho YC, Chung YC, Hwang SM, Wang KC and Hu
YC: Transgene expression and differentiation of baculovirus
transduced human mesenchymal stem cells. J Gene Med. 7:860–868.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Knoop K, Kolokythas M, Klutz K, et al:
Image-guided, tumor stroma-targeted 131I therapy of
hepatocellular cancer after systemic mesenchymal stem cell-mediated
NIS gene delivery. Mol Ther. 19:1704–1713. 2011.PubMed/NCBI
|