Introduction
Macrophage migration inhibitory factor (MIF),
originally named based on its repressive action on the motility of
monocytes/macrophages, belongs to the cytokine family of signaling
molecules. Although its existence as an extracellular regulator had
been postulated since the late 1950s, it was not until 1989 that
MIF was formally identified thanks to cDNA cloning and sequencing
(1). A few years later the three
dimensional structure of MIF was elucidated by structural analysis
of the pure recombinant protein (2–4).
MIF is primarily known as a pro-inflammatory
cytokine released by several cell types of the immune system, in
particular macrophages and T cells, although its production is by
no way restricted to immune cells. In addition to its paracrine
activity, it can also act in endocrine fashion when secreted by
corticotrophic cells of the pituitary (5). Physiologically, MIF has been shown to
counteract the anti-inflammatory activivity of glucocorticoids at
various levels, thus keeping a balance between pro-inflammatory and
anti-inflammatory mechanisms (6).
Expectedly, a dysregulation/exacerbation of MIF activity has been
implicated in pathological conditions as diverse as asthma
(7), chronic colitis (8), rheumatoid arthritis (9), systemic lupus erythematosus (10), psoriasis (11) and non-insulin-dependent diabetes
mellitus (12).
As also seen for other cytokines such as
interleukin-6, evidence is growing that MIF, beside its role in
immune responses, could also be involved in neoplastic
transformation and tumor progression. Among the earliest
experimental observations that MIF might contribute to tumor
development was the negative impact of MIF immunoneutralization on
neoplastic growth in a mouse model of B cell lymphoma (13).
In the context of human cancer, MIF increase
associated with neoplastic diseases was first suggested by ligand
histochemistry using sarcolectin (14–16),
soon followed by histochemical demonstration of MIF overexpression
in prostatic adenocarcinoma metastases (17). More recent studies have reported
MIF overexpression in a variety of human cancers, such as gastric
adenocarcinoma (18), ovarian
cancer (19), colorectal carcinoma
(20) and cervical cancer
(21). In the case of head and
neck squamous cell carcinomas (HNSCC), studies by our group have
disclosed an enhanced MIF expression in oral cavity, laryngeal and
hypopharyngeal squamous cell tumors (22–24).
Of particular interest, stable MIF knockdown in a murine ovarian
cancer cell line has been shown to decrease tumor burden and confer
longer survival in tumor transplanted mice (19). A similar result was obtained after
administration of anti-MIF therapeutics, either MIF-neutralizing
antibodies or the MIF inhibitor ISO-1, to mice grafted with
colorectal carcinoma transplants (20).
MIF exhibits a non-physiologic dopachrome
tautomerase activity which has been extensively exploited to screen
for potential inhibitors, owing to the fact that compounds
antagonizing this enzymatic activity are also likely to disrupt MIF
interactions with cognate receptors such as CD74 and suppress
thereby its effects on target cells (25). Along this line, a new potent
inhibitor of MIF dopachrome tautomerase activity,
4-iodo-6-phenylpyrimidine (4-IPP) has been shown to decrease the
motility and anchorage-independent growth of lung adenocarcinoma
cells (26).
Since MIF has been reported to be overexpressed in
head and neck squamous cell carcinomas (22–24),
the present study was undertaken to examine possible inhibitory
effects of 4-IPP on SCCVII squamous carcinoma cells.
Materials and methods
Inhibitor and antibodies
MIF inhibitor 4-iodo-6-phenylpyrimidine (4-IPP) was
purchased from Tocris Bioscience (Bristol, UK). Stock solutions of
the compound, prepared ≥250-fold concentrated in ethanol, were
stored at −20°C and used within the month. Polyclonal antibody
against purified MIF (14) was
raised in rabbits. Serum IgG fraction was purified as described
previously (24). Rabbit
polyclonal anti-CD74 antibody (FL-296), raised against residues
17-296 of human CD74, was obtained from Santa Cruz Biotechnology
(Santa Cruz, CA). Cell culture medium and supplements were from
Lonza (Verviers, Belgium). Fetal bovine serum (FBS) was from
Hyclone (Logan, UT). Other chemicals were from standard commercial
sources.
Cell line and culture
The squamous cell carcinoma cell line SCCVII
(27) was a kind gift from Dr
Shulin Li (Louisiana State University). Routine cell propagation
and experimental studies were carried out at 37°C in a cell
incubator with humid atmosphere at 5% CO2. Unless
specified otherwise, cells were cultured in T-flasks containing
Dulbecco’s modified essential medium (DMEM) supplemented with
phenol red, 10% FBS, 25 mM
N-2-hydroxyethylpiperazine-N’-2-ethanesulfonic acid (HEPES), 2 mM
L-glutamine and 1X antibiotic/antimycotic mix.
A CD74 knockdown (CD74-KD) cell line derived from
SCCVII, as well as a matched control with normal CD74 expression,
were generated in our laboratory. Knockdown of CD74 expression was
achieved by using anti-CD74 shRNA (mouse) lentiviral particles from
Santa Cruz. Controls were obtained by transduction with shRNA
lentiviral particles encoding a scrambled (sc) shRNA sequence
(CD74sc). Transduced cells were selected by addition of 4
μg/ml puromycin to the culture medium. Preliminary
experiments showed this puromycin concentration to be toxic toward
non-transduced SCCVII cells.
Western blotting
Transduced SCCVII cells (CD74sc and CD74KD) were
plated in Petri dishes (106 cells/dish), cultured for 3
days and then lysed using a detergent cocktail (M-PER Mammalian
Extraction buffer) supplemented with protease (Halt Protease
Inhibitor Cocktail) and phosphatase inhibitors (Halt Phosphatase
Inhibitor Cocktail) (all from Pierce, Rockford, IL, USA). Protein
concentrations were determined by the BCA Protein assay (Pierce)
using bovine serum albumin as standard. Extracted proteins (30
μg) were subjected to 10% SDS-PAGE and electrotransferred
onto nitrocellulose membranes (iBlot® Dry Blotting
system, Life Technologies-Invitrogen, Gent, Belgium).
Immunodetections were performed using anti-CD74 antibody FL-296
(1/200). Peroxidase-labeled anti-rabbit IgG antibody (1/5,000)
(from Amersham Pharmacia Biotech, Roosendaal, The Netherlands) was
used as secondary reagent. Bound peroxidase activity was revealed
using the SuperSignal® West Pico Chemiluminescent
Substrate (Pierce) following the manufacturer’s instructions.
Immunostaining signals were digitalized with a PC-driven LAS-3000
CCD camera (Fujifilm, Tokyo, Japan), using a software specifically
designed for image acquisition (Image Reader, Raytest®,
Straubenhardt, Germany). Immunoreactive band intensities were
quantified using the software AIDA® Image Analyser 3.45
(Raytest).
Measurement of cell culture growth by
cell counting
SCCVII cells were plated at a density of
104 cells/cm2 in 12-well dishes. The next
day, cell cultures were fed fresh medium with or without 4-IPP
(concentrations specified in Results). Measurement of cell culture
density was performed either daily (time-course experiment) or
after 3 days of treatment. Cells were dislodged from the vessel
bottom by treatment with trypsin-EDTA solution (Lonza). After
vigorous pipetting, concentrations of cells in suspension were
determined in an electronic cell counter (model Z1 Coulter counter,
Beckman Coulter, Fullerton CA).
Measurement of cell culture growth by
crystal violet stain assay
Cell density was also assessed by colorimetry after
crystal violet staining, as described previously (28). Cells were seeded in 96-well plates
(1,000–2,000 cells/well). The following day, they were fed fresh
medium (DMEM, 10% FBS) with or without 4-IPP. After a 3-day
exposure, the culture medium was discarded and cells were fixed
with 1% glutaraldehyde. Following fixation, cells were stained with
1% crystal violet. Destaining was achieved under gently running tap
water and cell monolayers were lysed in 0.2% Triton X-100. The
absorbance of cell lysates was measured at 570 nm using a
Labsystems Multiskan MS microplate reader.
Measurement of cell culture growth by
bromodeoxyuridine incorporation
DNA synthesis in SCCVII cells was evaluated on the
basis of bromodeoxyuridine (BrdU) incorporation measured using an
enzyme immunoassay kit developed by Roche Diagnostics (Mannheim,
Germany). The assay was performed following the manufacturer’s
instructions, with minor modifications. Briefly, SCCVII cells were
plated in 96-well plates (2,000 cells/well) and treated the
following day with 4-IPP. For the assessment of BrdU incorporation
into DNA, the cell cultures were exposed for 50 min to BrdU
labelling solution, followed by fixation and denaturation with an
ethanol-based mixture. Thereafter cells were incubated for 30 min
in a blocking solution (Animal-Free Blocker, Vector Laboratories,
Burlingame, CA). The next step consisted in incubating cells in the
presence of an anti-BrdU antibody conjugated with horseradish
peroxidase. Immunocomplexes were detected by using a mixture of
H2O2/tetramethylbenzidine as substrate. After
addition of the stop solution (1 M H2SO4),
the absorbance of the samples was measured at 450 nm (reference
wavelength, 690 nm) in a microplate reader.
Cell cycle analysis by flow
cytometry
The effect of 4-IPP on SCCVII cell cycle was
examined using the Cell Cycle Phase Determination kit from Cayman
Chemical Co. (Ann Arbor, MI). Cells were seeded in 6-well plates
(10,000 cells/well) and cultured for 24 h prior to exposure to 10,
20 or 40 μM 4-IPP or vehicle for 48 h. After treatment,
cells were collected (trypsin-EDTA solution), centrifuged (500 × g,
5 min) and washed twice with assay buffer (Cayman Chemical Co.)
before incubation in fixative solution (Cayman Chemical Co.) for at
least 2 h. Fixed cells were centrifuged, suspended in 0.5 ml
staining solution (propidium iodide and RNase solution) (Cayman
Chemical Co.) and incubated for 30 min at room temperature in the
dark. Finally, the samples were analyzed in the FL2 channel of a
flow cytometer (FACSCalibur, Becton-Dickinson, Franklin Lakes, NJ)
equipped with a 488-nm excitation laser.
Evaluation of MIF immunoreactivity by
enzyme-linked immunosorbent assay
The immunoreactivity of MIF protein was assessed by
a sandwich enzyme-linked immunosorbent assay (ELISA) using a
commercial kit (DuoSet ELISA Development kit, R&D Systems,
Minneapolis, MN). Ninety-six well ELISA plates were coated
overnight with 100 μl/well of capture antibody (mouse
anti-human MIF). After 3 rinses of the wells with washing buffer
(PBS, 0.05% Tween-20), non-specific binding was prevented by adding
the reagent diluent containing 1% BSA and incubating for 1 h. The
above mentioned rinsing procedure was applied between all
subsequent steps. The blocking step was followed by the addition of
increasing concentrations of human recombinant MIF in reagent
diluent. After an incubation period of 2 h, 100 μl of
detection antibody (biotinylated goat anti-human MIF) were added to
each well. A 2-h exposure to the detection antibody was followed by
a 20-min incubation with 100 μl of horseradish
peroxidaseconjugated streptavidin. Immunocomplexes were revealed by
the addition of 100 μl substrate solution
(H2O2/tetramethylbenzidine). After stopping
the reaction with H2SO4, the absorbance of
the samples was measured at 450 nm (reference wavelength, 570 nm)
as described above.
Immunofluorescence microscopy
SCCVII cells were plated at a density of 5,000
cells/cm2 on sterile round glass coverslips in 12-well
dishes. The following day, they were fed fresh medium with or
without 20 μM 4-IPP. After 3 days of drug exposure, cell
monolayers were fixed with 4% paraformaldehyde in Dulbecco’s PBS
(DPBS). Following fixation, paraformaldehyde was changed for DPBS
where cell cultures were stored at 4°C until immunostaining. Before
application of antibodies, cell monolayers were rinsed several
times with PBS (0.04 M Na2HPO4, 0.01 M
KH2PO4, 0.12 M NaCl, pH 7.2) containing 0.1%
Triton X-100 (the same detergent-containing buffer was used for
subsequent incubations and rinsing steps). Prior to exposure to
primary antibodies, cells were preincubated for 20 min in PBS
containing 0.05% casein and 0.05 M NH4Cl to prevent
non-specific adsorption of immunoglobulins. Cells were exposed for
60 min to the primary antibody (either anti-MIF or anti-CD74)
diluted 1:50 in PBS containing 0.05% casein. This was followed by a
30-min exposure to biotinylated goat anti-rabbit IgG (Vector
Laboratories). Thereafter, the cell preparations were incubated for
30 min in the presence of Texas Red-conjugated streptavidin (Vector
Laboratories). After final rinses in PBS, the coverslips were
mounted on glass slides using commercial anti-fading medium
(Vectashield®, Vector Laboratories). Negative controls
were produced by omitting the incubation step with primary
antibodies. This modification resulted in a decrease of the
fluorescence signal to background level.
The cell preparations were examined on a Leitz
Orthoplan microscope equipped with a Ploem system for
epi-illumination. Excitation wavelength of 560 nm and emission
wavelength of 590 nm were used for the observation of Texas Red
fluorescence. Image capture was achieved using a PC-driven digital
camera (Leica DC 300F, Leica Microsystems AG, Heerbrugg,
Switzerland) operated with Leica IM50 software. Quantitative
analysis of fluorescence signals was performed on digitalized
images thanks to Image J™ (a public domain image software developed
by W. Rasband at the Research Services Branch of the National
Institute of Mental Health, NIH). Images were analyzed in the red
channel after RGB split. Gray levels (on a scale of 0–225),
corresponding to fluorescence intensity were determined in 65 cells
in each control or treated cultures. Distribution histograms were
plotted using Sigma Plot (Systat Software, Inc., Hounslow, UK).
Confocal microscopy observations were carried out using an Olympus
FV1000D laser scanning inverted microscope equipped with a red
laser diode (LD559).
Phase contrast microscopy
Appearance of live cells in cultures was documented
by phase-contrast microscopy using a Wilovert inverted microscope
(Leitz, Wetzlar, Germany) equipped with a Leica DC 300F digital
camera (see above).
Cell migration assay
Cell invasiveness was assessed by a Boyden chamber
assay consisting of 24-well plates (lower chambers) with cell
culture inserts (upper chambers), both chambers being separated by
a polycarbonate membrane (8-μm size) coated with an
artificial extracellular matrix (Chemicon Cell Invasion Assay kit,
Millipore, Billerica, MA). The kit was used according to the
manufacturer’s instructions. SCCVII cells were seeded in cell
culture inserts (1.5×105 cells/insert) in serum-free
medium (DMEM). The lower chambers were filled with complete medium
(DMEM, 10% FBS) with or without 40 μM 4-IPP (n=4). After 96
h, cells were wiped off the upper surface of cell culture inserts
with a cotton-tipped swab and the migrating cells were stained with
crystal violet and counted in 6 microscopic fields (magnification
×10) using a Zeiss Axio scope A1 microscope (Carl Zeiss,
Germany).
Statistical analyses
SigmaPlot® 11 software was used for
statistical analyses. Parametric analysis was performed by ANOVA
(>2 groups, pairwise comparisons achieved by the Holm-Sidak
method) or Student’s t-test (2 groups). Non-parametric analysis
used the Mann-Whitney rank sum test.
Results
SCCVII cells express both MIF and
CD74
Demonstration of MIF in SCCVII cells by
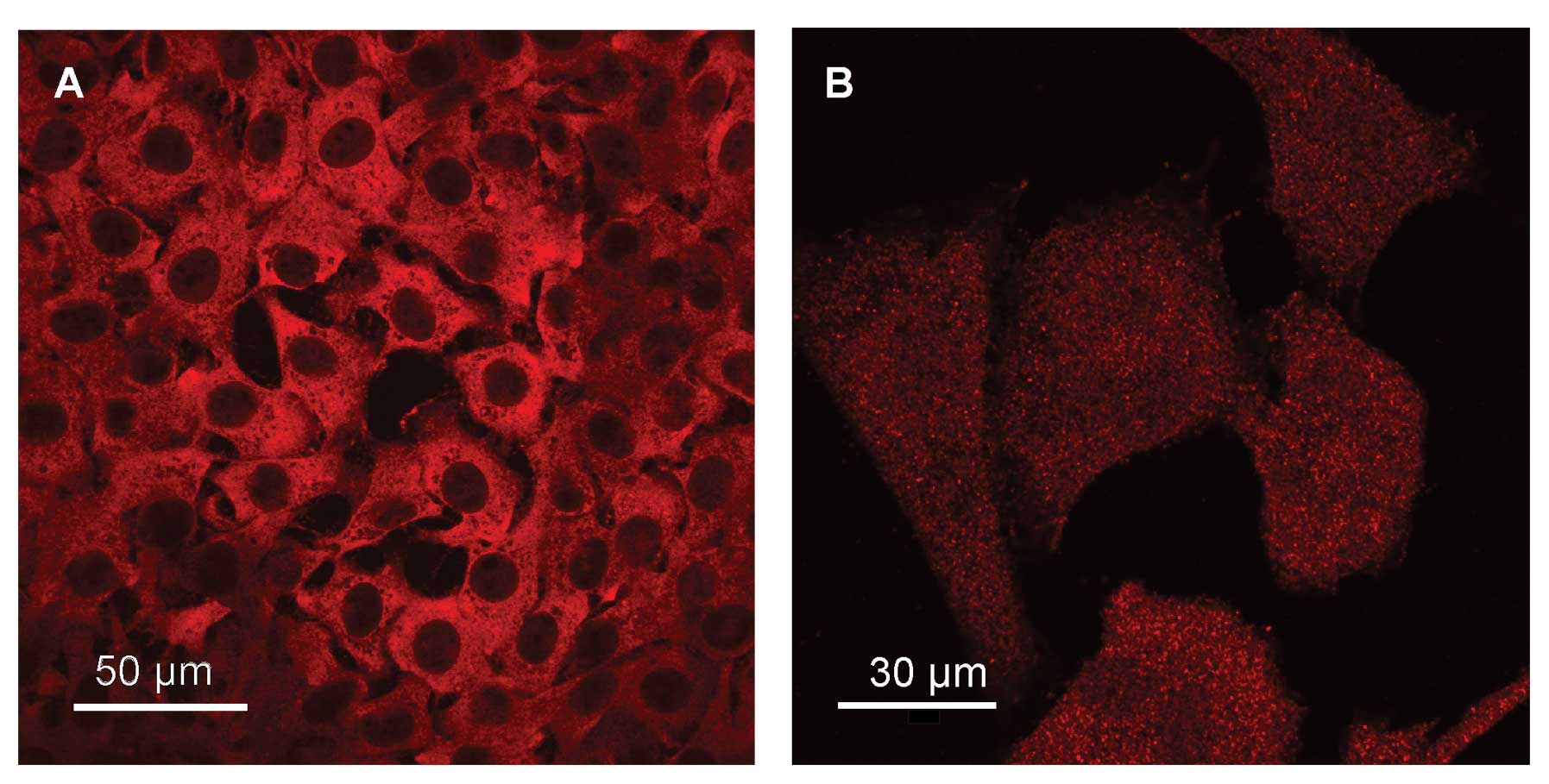
immunofluorescence microscopy is illustrated in Fig. 1A. MIF immunoreactivity is
extranuclear and appears distributed throughout the cytoplasm of
cells. Unlike that recently reported for MIF distribution in
glioblastoma cells (29), the
current data give no evidence of a prominent perinuclear
localization. Similar to MIF, CD74 seems to be expressed by most,
if not all SCCVII cells. Immunoreactive CD74 exhibits a punctuated
pattern on the whole cell surface (Fig. 1B), a distribution consistent with
the membrane localization of the receptor.
4-IPP alters MIF immunoreactivity
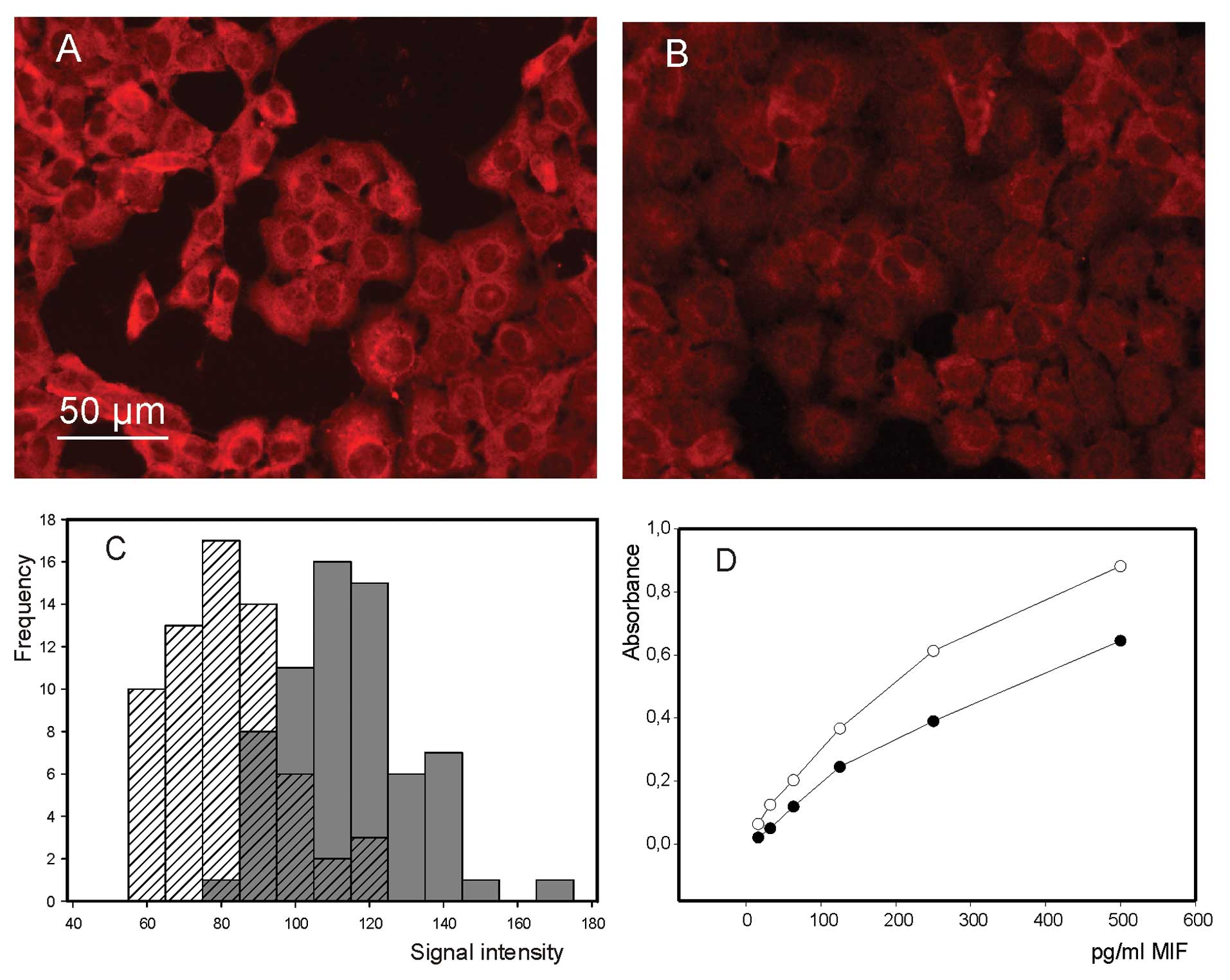
Fluorescence micro scopy examination of MIF in
4-IPP-treated cells revealed a decrease in the intensity of
immunofluorescence signal (Fig. 2A and
B), further confirmed by image analysis (Fig. 2C). Even though this observation is
suggestive of a decrease in MIF expression, the inhibitory action
of 4-IPP should not be expected to affect cell MIF content. Yet, it
is well known that 4-IPP, like other irreversible MIF inhibitors,
reacts covalently with the N-terminal proline of MIF (26), inducing thereby a conformational
change of the protein that could modify its immunoreactivity. Thus,
we checked MIF immunoreactivity by enzyme-linked immunoassay in
presence and absence of 4-IPP. As revealed by data presented in
Fig. 2D, the addition of 4-IPP
resulted in a marked decrease of absorbance associated with the
formation of immunocomplexes, clearly showing that 4-IPP
drastically alters MIF immunoreactivity.
4-IPP inhibits the proliferation of
SCCVII cells
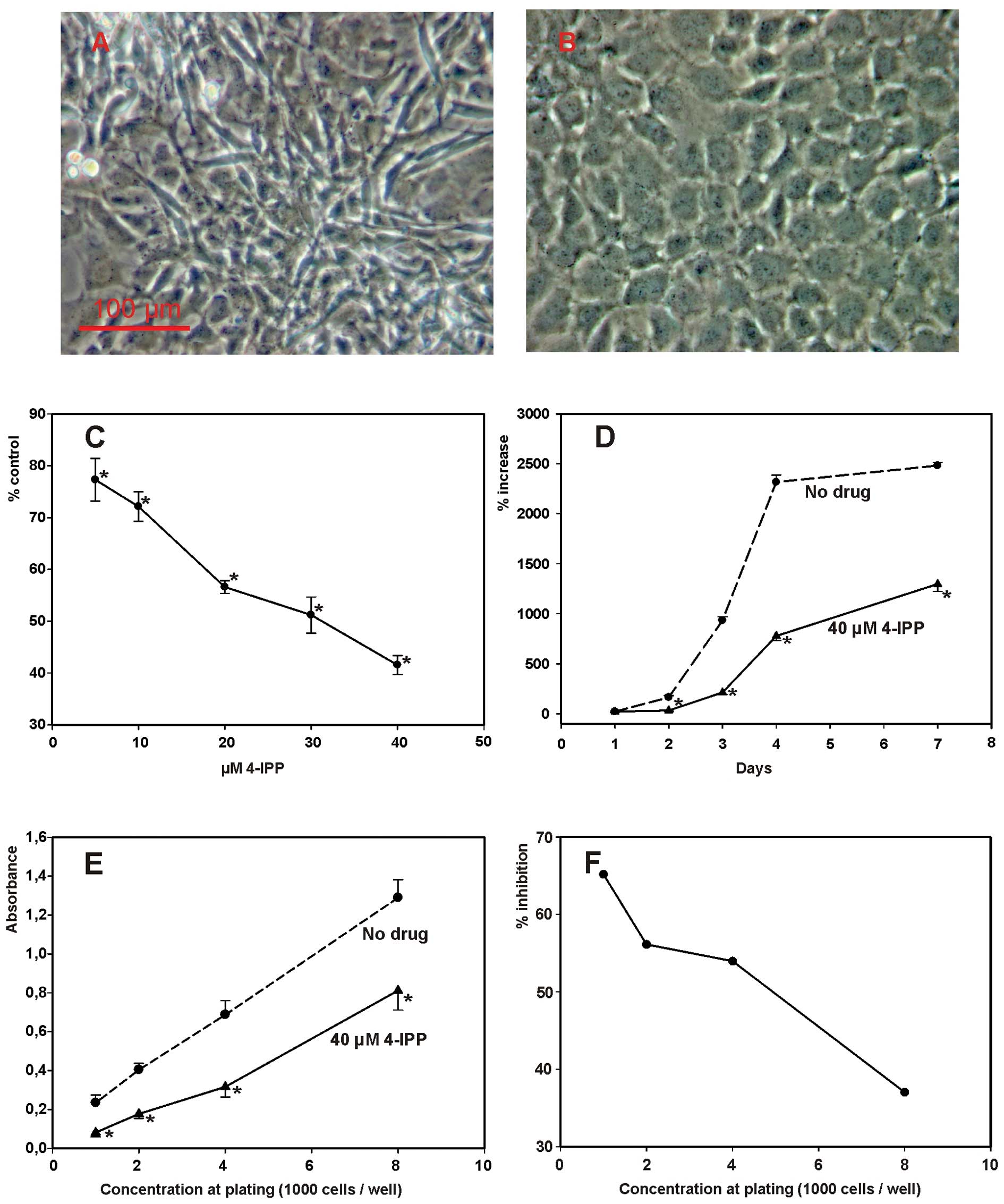
Routine examination of SCCVII cell cultures exposed
to 4-IPP (Fig. 3A and B) suggested
that the MIF inhibitor might exert an inhibitory effect on cell
proliferation. Thus, cell culture growth studies were conducted in
order to disclose a possible effect of MIF inhibition on cell
proliferation. As illustrated in Fig.
3C, 4-IPP provoked a
dose-dependent decrease of cell culture growth with an
IC50 ∼30 μM. At 40 μM 4-IPP, its
inhibitory action on cell proliferation was already apparent after
1 day of exposure (Fig. 3D).
Observations based on cell counting were confirmed by crystal
violet stain assay (Fig. 3E). In
the latter assay, the extent of the cytostatic effect produced by
4-IPP was inversely related to cell plating concentration (Fig. 3F). IPP-4-induced decline in cell
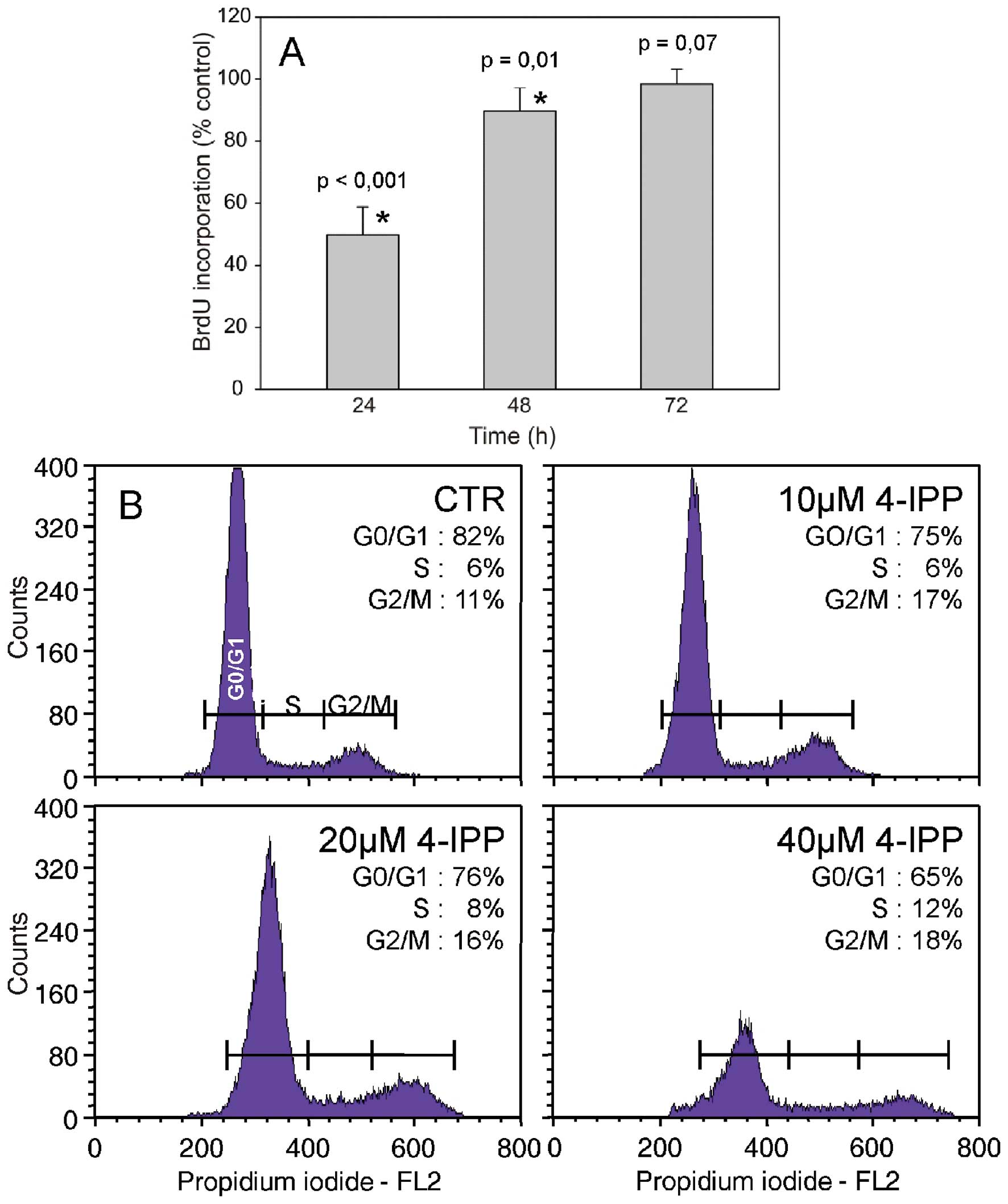
proliferation prompted us to examine the effect of the MIF
inhibitor on DNA synthesis and the cell division cycle. Cell
treatment with 4-IPP resulted in a transient reduction of BrdU
incorporation into DNA, which lasted 2 days after drug addition
(Fig. 4A). As shown by flow
cytometry analysis of the cell cycle, a 2-day exposure to 4-IPP led
to a substantial cell accumulation in the G2/M phase of the cell
cycle.
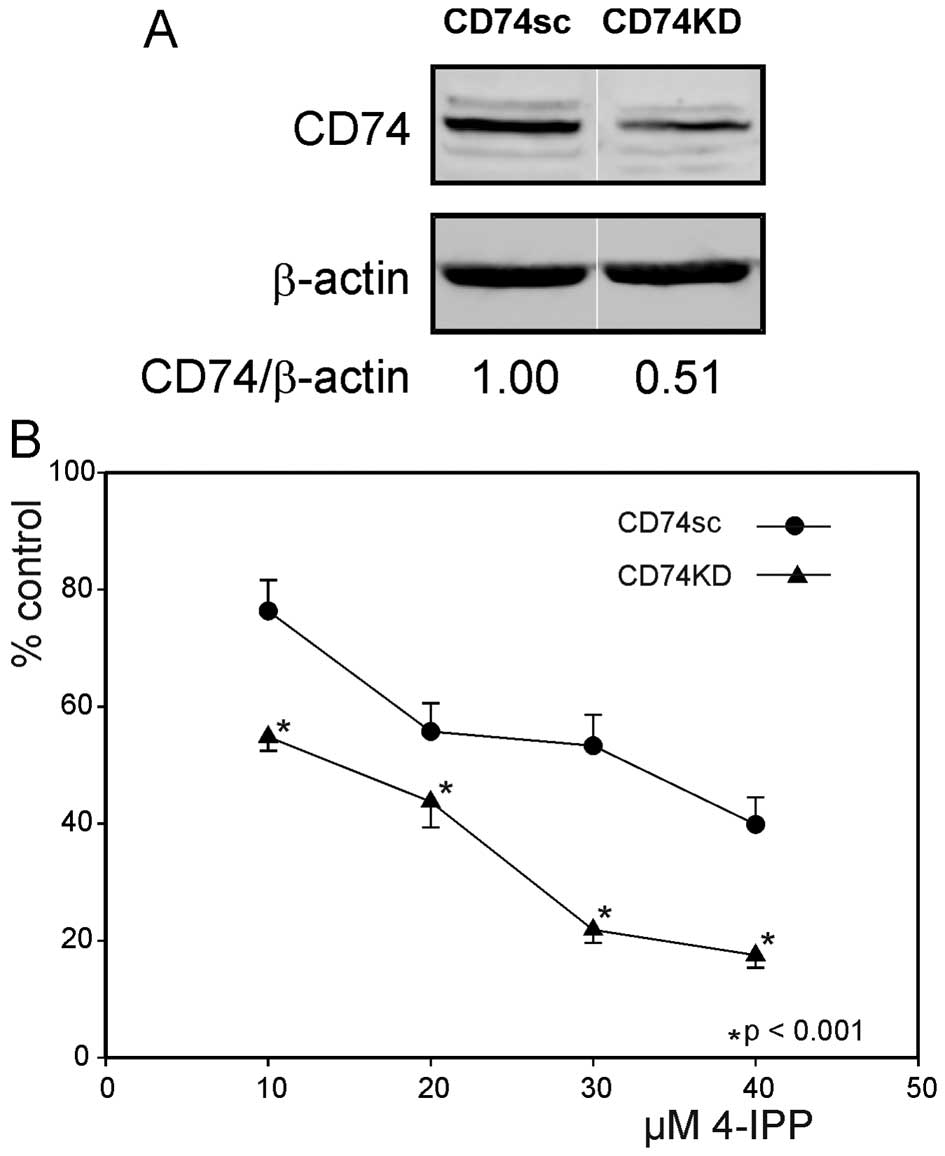
Expression of CD74 modulates SCCVII cell
sensitivity to 4-IPP
MIF action on target cells notably depends on
binding of its cognate receptor CD74 and the ensuing activation of
downstream transduction cascade(s). Thus, we wondered whether a
change of CD74 expression in SCCVII might influence their
sensitivity to 4-IPP. The expression of CD74 was partially
abrogated by lentiviral transduction of shRNA targeting the
transcript (Fig. 5A). Fig. 5B illustrates the inhibition of
growth induced by 4-IPP in CD74KD cells, as compared to control
CD74sc cells. Blunting of CD74 expression clearly sensitizes cells
to the cytostatic action of 4-IPP.
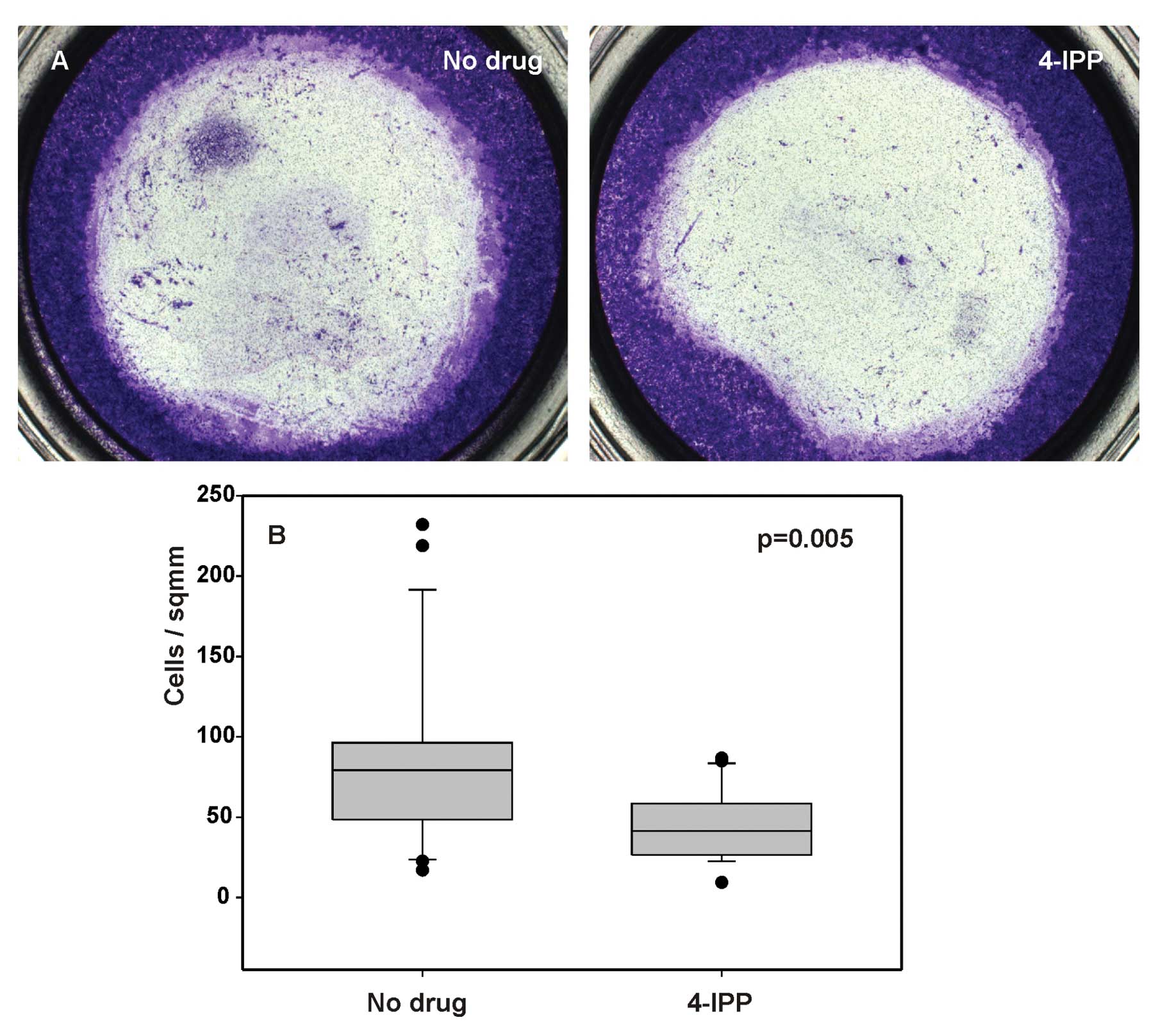
4-IPP reduces cell invasiveness
To evaluate whether MIF activity correlates with the
invasiveness of SCCVII cells, we performed an in vitro cell
invasion assay to determine the effect of 4-IPP on cell migration
in Boyden chambers. 4-IPP at a concentration of 40 μM
reduced the number of cells that migrated through the membrane as
compared to untreated cells (Fig.
6A). The decrease of cell migration as a result of 4-IPP action
was quantified by cell counting (Fig.
6B, p= 0.005). This result suggests that MIF promotes cell
migration through extracellular matrix and could contribute to
tumor invasiveness in vivo.
Discussion
The prominent role of MIF as a proinflammatory
cytokine makes it an attractive therapeutic target for the
management of autoinflammatory disorders. Thus, there has been an
intensive search for pharmacological compounds capable of blocking
MIF activity. The design of new molecules and the high throughput
screening for potential MIF inhibitors have been facilitated by the
fact that MIF possesses an enzymatic activity of D-dopachrome
tautomerase. Albeit of unknown physiological significance, this
tautomerase activity has been exploited as a validation tool for
the identification of MIF inhibitors, since substances blocking
this enzymatic activity would also be likely to interfere with MIF
binding to its cognate receptor CD74. The first published report of
a pharmacologically active MIF inhibitor described the properties
of ISO-1 ((S,R)-3-(4-hydroxyphenyl)-4,5-dihydro-5-isoxazole acetic
acid methyl ester). In vitro, ISO-1 was shown to abrogate
MIF-mediated macrophage response to bacterial endotoxin, whereas
in vivo it improved animal survival in a murine model of
peritonitis-induced sepsis (30).
There is converging evidence that MIF contributes to
tumor progression in various human cancers. In particular, recent
observations of our group suggest an involvement of MIF in HNSCC.
Indeed, immunohistochemistry reveals an increase of MIF
immunostaining intensity during progression to neoplasia in
hypopharyngeal (24), oral cavity
(22) and laryngeal (23) squamous cell carcinomas. Higher
serum levels were also found in patients with HNSCC, as compared to
healthy individuals (23). These
clinical observations were complemented by in vitro and
in vivo studies on squamous cell carcinoma cell line SCCVII,
where we showed that MIF knockdown decreases cell proliferation and
motility and increases cell sensitivity to the anticancer drugs
cisplatin and 5-fluorouracil (23).
Insofar as MIF seems to contribute to tumor
progression, MIF inhibitors might be effective as anticancer drugs.
Lending support to this assertion, ISO-1 has been reported to
reduce the proliferation of glioblastoma multiforme cells in
vitro (29) and inhibit the
growth of tumor xenografts produced by the inoculation of prostate
cancer cells (31) or colon
carcinoma cells (20). In the
present study, we investigated the impact of pharmacological MIF
inhibition on the behaviour of SCCVII squamous carcinoma cells,
since previous observations show that they are partially dependent
on endogenous MIF for growth (23). As a test compound, we used 4-IPP
(4-iodo-6-phenylpyrimidine), a suicide substrate which has been
recently shown to exhibit 5–10 times more inhibitory potency than
ISO-1 (26).
In the present study, preliminary experiments based
on immunofluorescence staining revealed that SCCVII cells exhibit
both MIF and CD74 immunoreactivities. Although similar studies have
rarely been performed on tumor cells, MIF and CD74 have been
previously shown to be co-expressed in non-small cell lung cancer
(32). The intracellular pattern
of MIF immunofluorescence in SCCVII cells was reminiscent of that
reported for macrophages (33). As
could be expected for a cell surface receptor, the distribution of
immunoreactive CD74 markedly differed from that of MIF. Assuming
that MIF can be secreted by SCCVII cells, the simultaneous
expression of the cytokine and its receptor implies the possibility
of auto-crine stimulation.
The finding that 4-IPP treatment resulted in a
decrease of MIF immunofluorescence intensity in SCCVII cells was at
first sightly surprising since MIF inhibition has not been reported
to decrease the expression of the cytokine in treated cells. As a
matter of fact, ISO-1 has been observed to enhance rather than
reduce MIF expression in glioblastoma multiforme cells (29). Further investigations relying on
ELISA showed that 4-IPP actually alters MIF immunoreactivity, a
finding consistent with a covalent modification of the latter.
Similar observations on 4-IPP-induced alteration of MIF
immunoreactivity have been reported previously (33).
Assessment of cell proliferation in presence of
4-IPP showed that the drug exerts a dose-dependent cytostatic
effect on SCCVII cells, without evidence of apoptotic cells in
treated cultures. CD74 knock-down definitely resulted in an
increase of SCCVII cell sensitivity to 4-IPP. The additive effect
of CD74 knock-down and 4-IPP suggests that MIF promotes SCCVII cell
proliferation by interacting with CD74/CD44 and triggering
downstream signalling events. Although the identity of the
signalling pathway was not specifically investigated in the present
study, MIF-induced enhancement of cell proliferation is generally
assumed to occur via the activation of the ERK1/2 transduction
cascade and the ensuing expression cell cycle proteins required for
progression through G1/S (34,35).
As shown recently with renal carcinoma cell lines, MIF also
promotes cell proliferation by activating Src, which in turn
phosphorylates and destabilizes cdk inhibitor
p27Kip1(36). Insofar
as MIF contributes to cell progression through G1/S phase, its
inhibition would be expected to result in cell accumulation in
G0/G1 phase. Such arrest in G0/G1 has indeed been demonstrated in
HEK293 cells after MIF knockdown (37). Yet, cell cycle analysis by flow
cytometry revealed that 4-IPP-treated SCCVII cells accumulate in
G2/M. This observation raises the intriguing possibility that MIF
might be involved in the control of mitosis by acting on G2/M
regulators such as cdc25.
In the original report describing 4-IPP as a newly
designed MIF inhibitor, the latter was shown to abrogate the
migration of A549 lung carcinoma cells through collagen-coated
membranes. Using a similar assay system, we noted that 4-IPP
interferes with SCCVII cell migration across ECM-coated membranes.
In a variety of neoplasms, MIF expression has been shown to
correlate with tumor invasiveness/aggressivity. The mechanisms
underlying MIF effects on tumor cell motility/invasiveness still
remain a matter of debate and might depend on cell context.
Observations on A549 cells point to involvement of the Rho GTPase
Rac1 as an effector mediating MIF action on cell
migration/invasiveness (38). A
more recent study on chondrosarcoma cells suggests that MIF
enhances cell migration via activation of the PI3K/Akt/NFκB signal
transduction pathway and an increase of αvβ3 integrin expression
(39).
The present study showed that pharmacological
inhibition of MIF in squamous carcinoma cells resulted in impaired
proliferation and invasiveness. HNSCC is not only a
life-threatening disease, but its treatment can also be
particularly debilitating. As revealed by a recent study of our
group (23), MIF expression in
HNSCC correlates with an unfavourable prognosis. Thus, the use of
MIF inhibitors, particularly in conjunction with CD74 inhibition,
might open new opportunities for target-directed treatment of these
neoplasms.
Acknowledgements
N.K. is the recipient of a fellowship
from the Fondation Rose and Jean Hoguet. G.L. is Senior Research
Associate of the National Fund for Scientific Research (Belgium)
and the recipient of a grant from the Belgian Fund for Medical
Scientific Research.
References
|
1.
|
Weiser WY, Temple PA, Witek-Giannotti JS,
Remold HG, Clark SC and David JR: Molecular cloning of a cDNA
encoding a human macrophage migration inhibitory factor. Proc Natl
Acad Sci USA. 86:7522–7526. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Sugimoto H, Suzuki M, Nakagawa A, Tanaka I
and Nishihira J: Crystal structure of macrophage migration
inhibitory factor from human lymphocyte at 2.1 A resolution. FEBS
Lett. 389:145–148. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Sun HW, Bernhagen J, Bucala R and Lolis E:
Crystal structure at 2.6-A resolution of human macrophage migration
inhibitory factor. Proc Natl Acad Sci USA. 93:5191–5196. 1996.
View Article : Google Scholar
|
|
4.
|
Kato Y, Muto T, Tomura T, Tsumura H,
Watarai H, Mikayama T, Ishizaka K and Kuroki R: The crystal
structure of human glycosylation-inhibiting factor is a trimeric
barrel with three 6-stranded beta-sheets. Proc Natl Acad Sci USA.
93:3007–3010. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Nishino T, Bernhagen J, Shiiki H, Calandra
T, Dohi K and Bucala R: Localization of macrophage migration
inhibitory factor (MIF) to secretory granules within the
corticotrophic and thyrotrophic cells of the pituitary gland. Mol
Med. 1:781–788. 1995.PubMed/NCBI
|
|
6.
|
Flaster H, Bernhagen J, Calandra T and
Bucala R: The macrophage migration inhibitory factor-glucocorticoid
dyad: regulation of inflammation and immunity. Mol Endocrinol.
21:1267–1280. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Rossi AG, Haslett C, Hirani N, Greening
AP, Rahman I, Metz CN, Bucala R and Donnelly SC: Human circulating
eosinophils secrete macrophage migration inhibitory factor (MIF).
Potential role in asthma. J Clin Invest. 101:2869–2874. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
de Jong YP, Abadia-Molina AC, Satoskar AR,
Clarke K, Rietdijk ST, Faubion WA, Mizoguchi E, Metz CN, Alsahli M,
ten Hove T, Keates AC, Lubetsky JB, Farrell RJ, Michetti P, van
Deventer SJ, Lolis E, David JR, Bhan AK and Terhorst C: Development
of chronic colitis is dependent on the cytokine MIF. Nat Immunol.
2:1061–1066. 2001.PubMed/NCBI
|
|
9.
|
Morand EF, Leech M, Weedon H, Metz C,
Bucala R and Smith MD: Macrophage migration inhibitory factor in
rheumatoid arthritis: clinical correlations. Rheumatology (Oxford).
41:558–562. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Foote A, Briganti EM, Kipen Y, Santos L,
Leech M and Morand EF: Macrophage migration inhibitory factor in
systemic lupus erythematosus. J Rheumatol. 31:268–273.
2004.PubMed/NCBI
|
|
11.
|
Donn RP, Plant D, Jury F, Richards HL,
Worthington J, Ray DW and Griffiths CE: Macrophage migration
inhibitory factor gene polymorphism is associated with psoriasis. J
Invest Dermatol. 123:484–487. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Sanchez-Zamora Y, Terrazas LI,
Vilches-Flores A, Leal E, Juárez I, Whitacre C, Kithcart A, Pruitt
J, Sielecki T, Satoskar AR and Rodriguez-Sosa M: Macrophage
migration inhibitory factor is a therapeutic target in treatment of
non-insulin-dependent diabetes mellitus. FASEB J. 24:2583–2590.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Chesney J, Metz C, Bacher M, Peng T,
Meinhardt A and Bucala R: An essential role for macrophage
migration inhibitory factor (MIF) in angiogenesis and the growth of
a murine lymphoma. Mol Med. 5:181–191. 1999.PubMed/NCBI
|
|
14.
|
Zeng F-Y, Weiser WY, Kratzin H, Stahl B,
Karas M and Gabius HJ: The major binding protein of the interferon
antagonist sarcolectin in human placenta is a macrophage migration
inhibitory factor? Arch Biochem Biophys. 303:74–80. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Zeng FY, Gerke V and Gabius HJ:
Characterization of the macrophage migration inhibitory
factor-binding site of sarcolectin and its relationship to human
serum albumin. Biochem Biophys Res Commun. 200:89–94. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Kayser K, Bovin NV, Korchagina EY,
Zeilinger C, Zeng FY and Gabius H-J: Correlation of expression of
binding sites for synthetic blood group A-, B- and H-trisaccharides
and for sarcolectin with survival of patients with bronchial
carcinoma. Eur J Cancer. 30A:653–657. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Meyer-Siegler K and Hudson PB: Enhanced
expression of macrophage migration inhibitory factor in prostatic
adenocarcinoma metastases. Urology. 48:448–452. 1996. View Article : Google Scholar
|
|
18.
|
He XX, Yang J, Ding YW, Liu W, Shen QY and
Xia HH: Increased epithelial and serum expression of macrophage
migration inhibitory factor (MIF) in gastric cancer: potential role
of MIF in gastric carcinogenesis. Gut. 55:797–802. 2006. View Article : Google Scholar
|
|
19.
|
Hagemann T, Robinson SC, Thompson RG,
Charles K, Kulbe H and Balkwill FR: Ovarian cancer cell-derived
migration inhibitory factor enhances tumor growth, progression, and
angiogenesis. Mol Cell Ther. 6:1993–2002. 2007.PubMed/NCBI
|
|
20.
|
He XX, Chen K, Yang J, Li XY, Gan HY, Liu
CY, Coleman TR and Al-Abed Y: Macrophage migration inhibitory
factor promotes colorectal cancer. Mol Med. 15:1–10.
2009.PubMed/NCBI
|
|
21.
|
Krockenberger M, Engel JB, Kolb J,
Dombrowsky Y, Häusler SF, Kohrenhagen N, Dietl J, Wischhusen J and
Honig A: Macrophage migration inhibitory factor expression in
cervical cancer. J Cancer Res Clin Oncol. 136:651–657. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Kindt N, Lechien J, Decaestecker C,
Rodriguez A, Chantrain G, Remmelink M, Laurent G, Gabius H-J and
Saussez S: Expression of macrophage migration-inhibitory factor is
correlated with progression in oral cavity carcinomas. Anticancer
Res. 32:4499–4505. 2012.PubMed/NCBI
|
|
23.
|
Kindt N, Preillon J, Kaltner H, Gabius
H-J, Chevalier D, Rodriguez A, Johnson BD, Megalizzi V,
Decaestecker C, Laurent G and Saussez S: Macrophage migration
inhibitory factor in head and neck squamous cell carcinoma:
clinical and experimental studies. J Cancer Res Clin Oncol.
139:727–737. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Cludts S, Decaestecker C, Johnson B,
Lechien J, Leroy X, Kindt N, Kaltner H, André S, Gabius HJ and
Saussez S: Increased expression of macrophage migration inhibitory
factor during progression to hypopharyngeal squamous cell
carcinoma. Anticancer Res. 30:3313–3319. 2010.PubMed/NCBI
|
|
25.
|
Cournia Z, Leng L, Gandavadi S, Du X,
Bucala R and Jorgensen WL: Discovery of human macrophage migration
inhibitory factor (MIF)-CD74 antagonists via virtual screening. J
Med Chem. 52:416–424. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Winner M, Meier J, Zierow S, Rendon BE,
Crichlow GV, Riggs R, Bucala R, Leng L, Smith N, Lolis E, Trent JO
and Mitchell RA: A novel, macrophage migration inhibitory factor
suicide substrate inhibits motility and growth of lung cancer
cells. Cancer Res. 68:7253–7257. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Khurana D, Martin EA, Kasperbauer JL,
O’Malley BW Jr, Salomao DR, Chen L and Strome SE: Characterization
of a spontaneously arising murine squamous cell carcinoma (SCC VII)
as a prerequisite for head and neck cancer immunotherapy. Head
Neck. 23:899–906. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Journe F, Chaboteaux C, Dumon JC, Leclercq
G, Laurent G and Body JJ: Steroid-free medium discloses oestrogenic
effects of the bisphosphonate clodronate on breast cancer cells. Br
J Cancer. 91:1703–1710. 2004.PubMed/NCBI
|
|
29.
|
Baron N, Deuster O, Noelker C, Stüer C,
Strik H, Schaller C, Dodel R, Meyer B and Bacher M: Role of
macrophage migration inhibitory factor in primary glioblastoma
multiforme cells. J Neurosci Res. 89:711–717. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Al-Abed Y, Dabideen D, Aljabari B, Valster
A, Messmer D, Ochani M, Tanovic M, Ochani K, Bacher M, Nicoletti F,
Metz C, Pavlov VA, Miller EJ and Tracey KJ: ISO-1 binding to the
tautomerase active site of MIF inhibits its pro-inflammatory
activity and increases survival in severe sepsis. J Biol Chem.
280:36541–36544. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Meyer-Siegler KL, Iczkowski KA, Leng L,
Bucala R and Vera PL: Inhibition of macrophage migration inhibitory
factor or its receptor (CD74) attenuates growth and invasion of
DU-145 prostate cancer cells. J Immunol. 177:8730–8739. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
McClelland M, Zhao L, Carskadon S and
Arenberg D: Expression of CD74, the receptor for macrophage
migration inhibitory factor, in non-small cell lung cancer. Am J
Pathol. 174:638–646. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Merk M, Baugh J, Zierow S, Leng L, Pal U,
Lee SJ, Ebert AD, Mizue Y, Trent JO, Mitchell R, Nickel W, Kavathas
PB, Bernhagen J and Bucala R: The Golgi-associated protein p115
mediates the secretion of macrophage migration inhibitory factor. J
Immunol. 182:6896–6906. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Lue H, Kapurniotu A, Fingerle-Rowson G,
Roger T, Leng L, Thiele M, Calandra T, Bucala R and Bernhagen J:
Rapid and transient activation of the ERK MAPK signalling pathway
by macrophage migration inhibitory factor (MIF) and dependence on
JAB1/CSN5 and Src kinase activity. Cell Signal. 18:688–703. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Bifulco C, McDaniel K, Leng L and Bucala
R: Tumor growth-promoting properties of macrophage migration
inhibitory factor. Curr Pharm Des. 14:3790–3801. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Du W, Wright BM, Li X, Finke J, Rini BI,
Zhou M, He H, Lal P and Welford SM: Tumor-derived macrophage
migration inhibitory factor promotes an autocrine loop that
enhances renal cell carcinoma. Oncogene. 32:1469–1474. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Liu L, Ji C, Chen J, Li Y, Fu X, Xie Y, Gu
S and Mao Y: A global genomic view of MIF knockdown-mediated cell
cycle arrest. Cell Cycle. 7:1678–1692. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Rendon BE, Roger T, Teneng I, Zhao M,
Al-Abed Y, Calandra T and Mitchell RA: Regulation of human lung
adenocarcinoma cell migration and invasion by macrophage migration
inhibitory factor. J Biol Chem. 282:29910–29918. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39.
|
Lee CY, Su MJ, Huang CY, Chen MY, Hsu HC,
Lin CY and Tang CH: Macrophage migration inhibitory factor
increases cell motility and up-regulates αvβ3 integrin in human
chondrosarcoma cells. J Cell Biochem. 113:1590–1598.
2012.PubMed/NCBI
|