Introduction
The family of polyphenolic plant secondary
metabolites known as flavonoids has attracted significant interest
due to their potential health-promoting effects that include cancer
prevention and chemotherapy. Among the flavonoids, the catechins
are found in several land plants; and are abundant in cocoa, red
wine and especially green tea (Camellia sinensis). Green
(but not black) tea is particularly abundant in the flavonoid
epigallocatechin-3-gallate (EGCG), an ester of epigallocatechin and
gallic acid. EGCG has been the subject of extensive research
because there is evidence that it could provide benefits in
treating prostate, breast and other cancers (1–3). As
well as EGCG, other polyphenolics are thought to confer
health-promoting effects, including genistein, quercetin and
curcumin (4).
One of the key mechanisms through which EGCG is
thought to exhibit antitumour effects is through the promotion of
apoptosis. Increased apoptosis caused by EGCG has been demonstrated
in human bladder cancer (5); human
endometrial carcinoma (6);
anaplastic thyroid carcinoma (7);
gastric cancer (8); melanoma
(9); and prostate cancer cell
lines (10). Taken together, the
numerous publications on EGCG suggest that its effects are
pleiotropic. There are several mechanisms through which EGCG could
promote apoptosis including the generation of reactive oxygen
species (ROS) (6); the inhibition
of JAK/STAT3 signaling (11); the
inhibition of NF-κB and Akt activation (12); the downregulation of survivin
expression (8); and the activation
of caspases (13).
EGCG is well known as an antioxidant; but in fact in
some conditions it is a pro-oxidant and can even auto-oxidise in
cell culture, generating ROS (14). A recent study suggests that
EGCG-mediated ROS generation is particularly observed in cancer
cells compared to normal cells (15). In other words EGCG may cause a
selective increase in apoptosis predominantly in cancer cells. Thus
there is a pressing need to understand the concentration of EGCG
required to achieve apoptosis; its ability to synergise with widely
used drugs that promote apoptosis (such as cisplatin); and the
mechanisms through which it promotes apoptosis.
Recent evidence suggests that polyphenolics such as
EGCG might affect alternative splicing. Resveratrol, a polyphenolic
notably abundant in red wine, affects exon inclusion in the
SRSF3 and SMN2 genes by altering the expression of
the splice factors SRSF1, hnRNP A1 and HuR (16). EGCG corrects the aberrant splicing
of IKAP in cells derived from patients with familial dysautonomia
(17) and synergises with
ibuprofen to alter the splicing of Bcl-X and Mcl-1 favouring
pro-apoptotic isoforms (18).
Aberrant alternative splicing is increasingly important in cancer
(19) and many genes involved in
apoptosis are alternatively spliced into pro- or anti-apoptotic
isoforms (20). These include
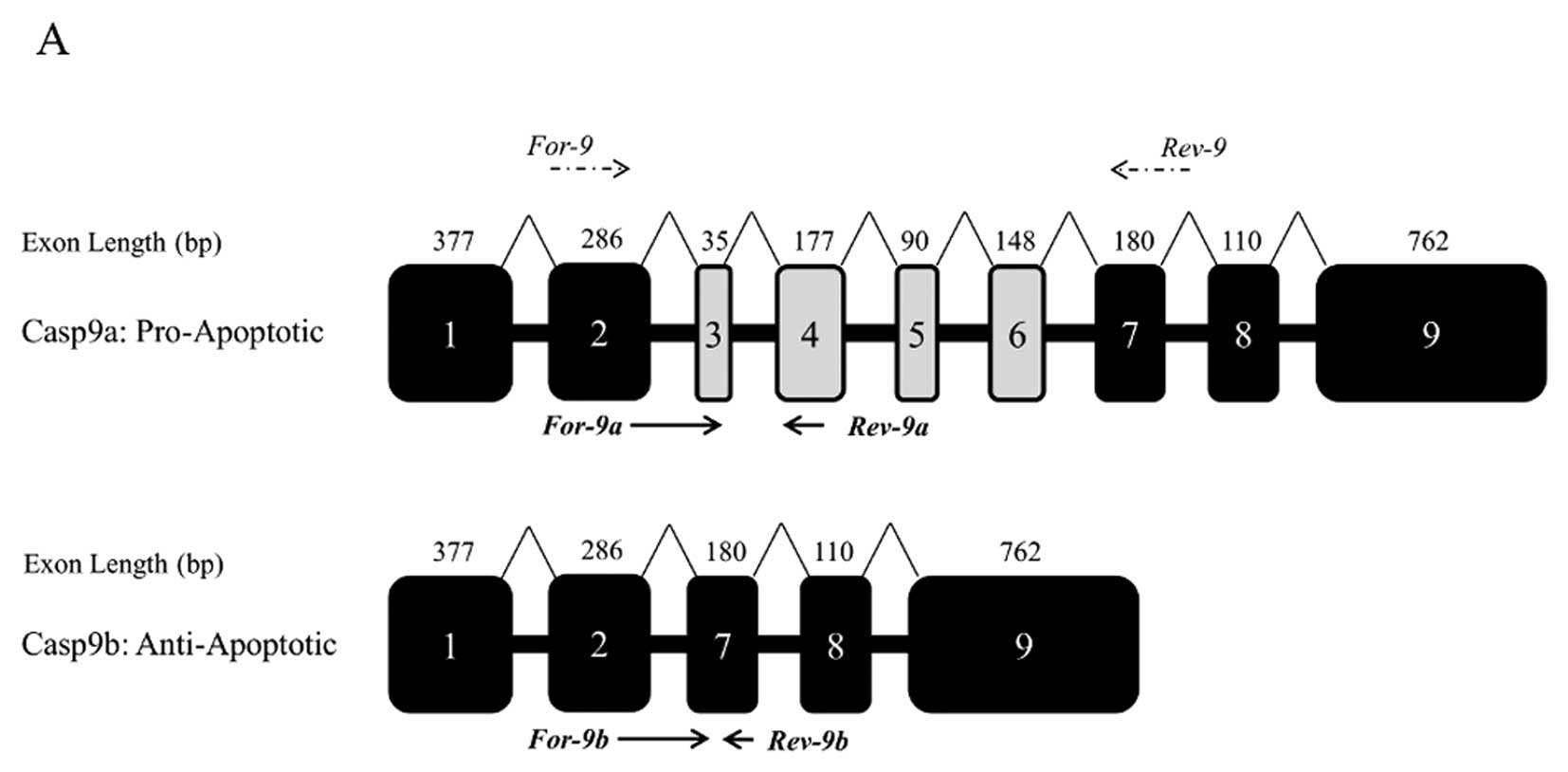
caspase 9, a member of the caspase family of proteases. Caspase 9
is an initiator caspase; its activity increases following DNA
damage and cytochrome c release. The splice isoform caspase
9a is pro-apoptotic (and encodes the full length protease). Caspase
9b results from exclusion of exons 3–6; it lacks catalytic activity
but retains the interaction domains and therefore acts as an
endogenous inhibitor of caspase 9a. The alternative splicing of
caspase 9 is dysregulated in non-small cell lung cancers (21). The reduction of caspase 9b
decreases the IC50 of several chemotherapeutic drugs
(22). It is therefore of interest
to explore new ways in which to modify the alternative splicing of
caspase 9 in vivo.
In this report we show that EGCG promotes apoptosis
in the prostate cancer cell line PC3 even at a concentration of 1
μM; that it synergises with cisplatin to promote apoptosis
and can do so at low concentrations in cell culture; and that it
modifies the alternative splicing of caspase 9 favouring the
pro-apoptotic isoform. The latter finding suggests that EGCG, and
other polyphenolic compounds, may affect the alternative splicing
of cancer-associated genes in vivo.
Materials and methods
Cell treatments
All materials were purchased from Sigma-Aldrich
unless otherwise stated. EGCG was prepared in the solvent
dimethylsulfoxide (DMSO). Cisplatin (cis-diaminodichloroplatinum
(II)) was prepared in PBS containing 0.9% (w/v) NaCl. The prostate
cancer cell line, PC3 was obtained from ECACC - an
androgen-independent cell line established from a bone metastasis
of grade IV prostate cancer. PC3 cells were cultured in DMEM (5 mM
glucose) containing 10% FBS, L-glutamine and
penicillin-streptomycin. Prior to treatment with EGCG, cells were
serum-starved overnight. EGCG was diluted in fresh DMEM (5 mM
glucose) media to give final concentrations stated and added to
cells for the indicated amounts of time. Control cells with no EGCG
treatment had equal amounts of DMSO added to media to control for
any DMSO-mediated effects. For cisplatin treatment, cells were
serum-starved overnight and then incubated with the stated
concentrations of cisplatin and EGCG. After 24 h media was removed
and cells washed with fresh DMEM media to remove traces of
cisplatin. Media containing EGCG was then added for a further 48 h
before cells were analysed.
DNA fragmentation assay
For DNA fragmentation analysis cells were lysed in
buffer (10 mM Tris-HCl, 10 mM EDTA, 0.5% (v/v) Triton X-100, pH
8.0). RNA was digested using RNase (0.1 mg/ml at 37°C for 1 h)
followed by proteinase K treatment for 2 h at 50°C. DNA was
extracted and precipitated out using phenol, chloroform and isoamyl
alcohol. One microgram of DNA was then electrophoretically
separated on a 1.8% agarose gel containing ethidium bromide (10
μg/ml) and visualized using a UV illuminator (Minibis, DNR
Bio-Imaging Systems).
Cell proliferation analysis
Cell proliferation was measured using trypan blue.
Cells were seeded in 12-well plates (70,000 cells per well) and
treated with EGCG/cisplatin as stated. Cells were then trypsinised,
pelleted and re-suspended in an appropriate volume of media
containing equal amounts of 0.4% (w/v) trypan blue. Cells were then
counted using a haemocytometer.
Acridine orange staining
Cells were seeded and grown on coverslips in 6-well
plates and then treated with EGCG for 48 h as described previously.
Cells were then fixed using 90% methanol for 15 min and air dried.
For staining, slides were dipped in fresh phosphate buffer [0.66%
(w/v) potassium phosphate mono-basic + 0.32% (w/v) sodium phosphate
dibasic, pH 6.4–6.5) and stained in a solution of acridine orange
(0.12 mg/ml in phosphate buffer) for 2 min. Slides were washed
three times in phosphate buffer, then placed on microscope slides
and the morphology of the nuclei analysed and scored immediately
using a fluorescence microscope (×40 objective lens, Nikon Eclipse
80i). Nuclei and micronuclei (DNA) were stained yellow/green and
the cytoplasm was stained red. Relative proportions of normal,
apoptotic nuclei and micronuclei were determined and recorded in a
total of at least 2,000 cells per treatment. The experiment was
repeated three times.
Sub-G1 cell cycle analysis
To identify and quantify the sub-G1 cell population
of cells that would correspond with cells undergoing apoptosis
cells were analysed using flow cytometry. Cells were trypsinized,
pelleted and resuspended in PBS. Cells were then fixed in 70%
ice-cold ethanol and stored at −20°C overnight. Ethanol was then
removed and cells washed with PBS before being resuspended in
S-phase buffer (4 mM sodium citrate, 0.1% Triton X-100, 50
μg/ml propidium iodide, 500 μg/ml RNase A) and
incubated in the dark for 20 min before being analysed on the flow
cytometer (C6, Accuri). Control cells with no treatment were used
to initially optimise and model collection gating conditions used
to detect the sub-G1 cell population. All gates were then constant
throughout subsequent analyses. Additionally during analysis the
sub-G1 marker (M1) was placed based on control cells (n=16) and was
kept constant for all other treated cells (n=4). Raw data were
analysed and histograms constructed using Cyflogic v.1.2.1
software. The relative proportion of cells in the sub-G1 population
was then recorded. Data from four independent experiments were
recorded.
Reverse transcription and standard
PCR
RNA was isolated using TRI reagent per
manufacturer’s instructions. RNA was then quantified using Nanodrop
spectrophotometer (Thermo Fischer Scientific, Newark, DE, USA). One
microgram of PC3 total RNA was reverse-transcribed using MMLV
transcriptase (Promega) and random primers as the priming agent.
After 50 min of incubation at 42°C, the reactions were stopped by
heating at 70°C for 15 min. To evaluate the expression of
endogenous caspase 9 splice variants, an upstream 5′ forward primer
in exon 2 of caspase 9 (5′-GCTCTTCCTTTGTTCATC-3′) and a 3′ reverse
primer in exon 7 (5′-CATCTGGCTCGGGGTTA-3′) were used. PCR was
carried out using GoTaq HotStart Polymerase (Promega) as per
manufacturer’s recommendations and 5 ng/μl cDNA (thermal
cycling conditions: 95°C for 2 min, followed by 33 cycles of 95°C
for 30 sec, 57°C for 30 sec and 72°C for 30 sec, and a final
extension step of 72°C for 5 min. The PCR product was examined by
electrophoresis using 2% (w/v) agarose gel electrophoresis. The
agarose gel was then analysed using a UV illuminator (Minibis, DNR
Bio-Imaging Systems). To control for potential loading differences
between samples and to assess whether there was a shift in
alternative splicing of caspase 9 the ratio of caspase 9a/9b was
assessed by measuring the area of each PCR peak using ImageJ
software. The ratio between bands was then determined and expressed
for each sample.
Real-time PCR
Quantitative PCR was performed using 2× SensiFAST
SYBR Hi-ROX master mix (Bioline) using primers at 300 nM
concentration on StepOne Plus Real-Time PCR System (Applied
Biosystems, Warrington, UK). Primers were designed to span at least
one exon boundary using the Primer Express 2.0 software (Applied
Biosystems) and were purchased from Sigma-Genosys (Haverhill, UK).
For caspase 9a analysis a forward primer located across exon 2/3
boundary (5′-AGTGG ACATTGGTTCTGGAG) and a reverse primer located in
exon 4 (5′-CTTCTCACAGTCGATGTTGG-3′) were used. For caspase 9b
analysis a forward primer located across exon 2/7 boundary
(5′-TGGTGATGTCGAGCAGAAAG) and a reverse primer located in exon 4
(5′-CTGGTCGAAGGTCCTCA AAC-3′) were used. To evaluate 18S rRNA
(accession no. NR_003286.2) expression a forward primer
(5′-ACCCGTTGA ACCCCATTCGTGA-3′) and reverse primer (5-GCCTCACTA
AACCATCCAATCGG’-3′) were used. PCR reaction conditions were 20 sec
at 95°C then 40 cycles of; 3 sec at 95°C and 30 sec at 60°C. In
addition a melting curve analysis was performed to check for
multiple products and primer-dimer amplification. Melting curve
analyses were conducted by a stepwise decrease in temperature from
95 to 60°C over a 35-min period with measurement of total
SYBR-Green fluorescence every 1°C. Fold changes in expression were
calculated by using a standard curve method according to
established methods (23,24). Gene expression was normalised to
the corresponding 18S value for each sample.
Statistical analysis
Data from experiments are presented as mean ±
standard error mean (SEM), with number of replicates stated in
figure legends. Statistical significance was tested using a paired
two-tailed Student’s t-test assuming equal variance.
Results
Several studies have used very high concentrations
of EGCG, these are arguably unrealistic in terms of what could be
administered to a patient. With this in mind, we decided to examine
the effects of lower concentrations, 1 and 25 μM EGCG on
cell survival and apoptosis in PC3 cells, a widely studied
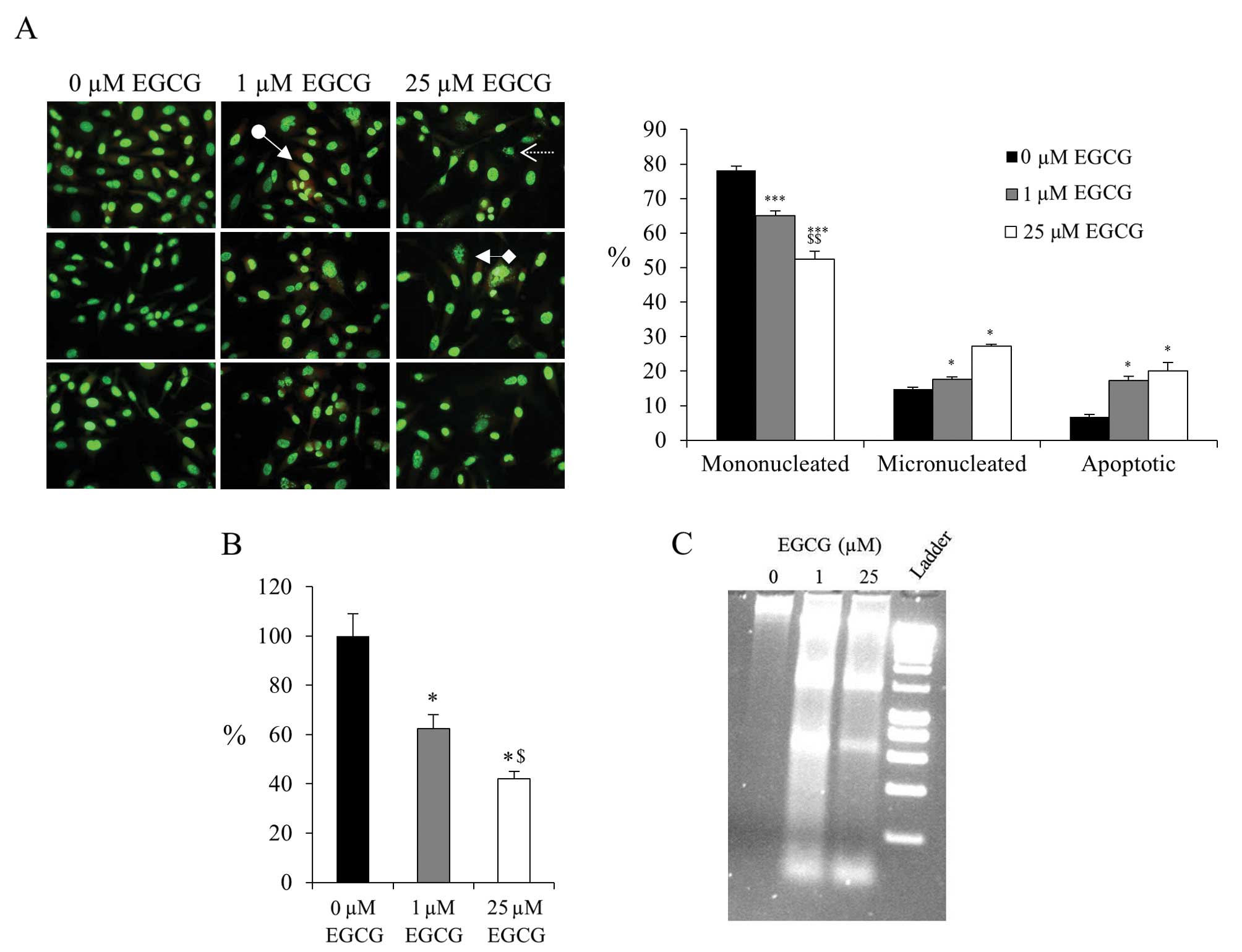
androgen-independent prostate cancer cell line. Acridine orange
staining of cells treated for 48 h revealed a significant increase
in the proportion of cells with micronuclei (indicative of DNA
fragmentation) and of apoptotic bodies (Fig. 1A). A total of 1 μM EGCG was
sufficient to cause a significant increase in the proportion of
apoptotic bodies and reduced cell survival (Fig. 1B). The presence of increased DNA
fragmentation, a characteristic of apoptosis, was confirmed by
agarose gel electrophoresis (Fig.
1C).
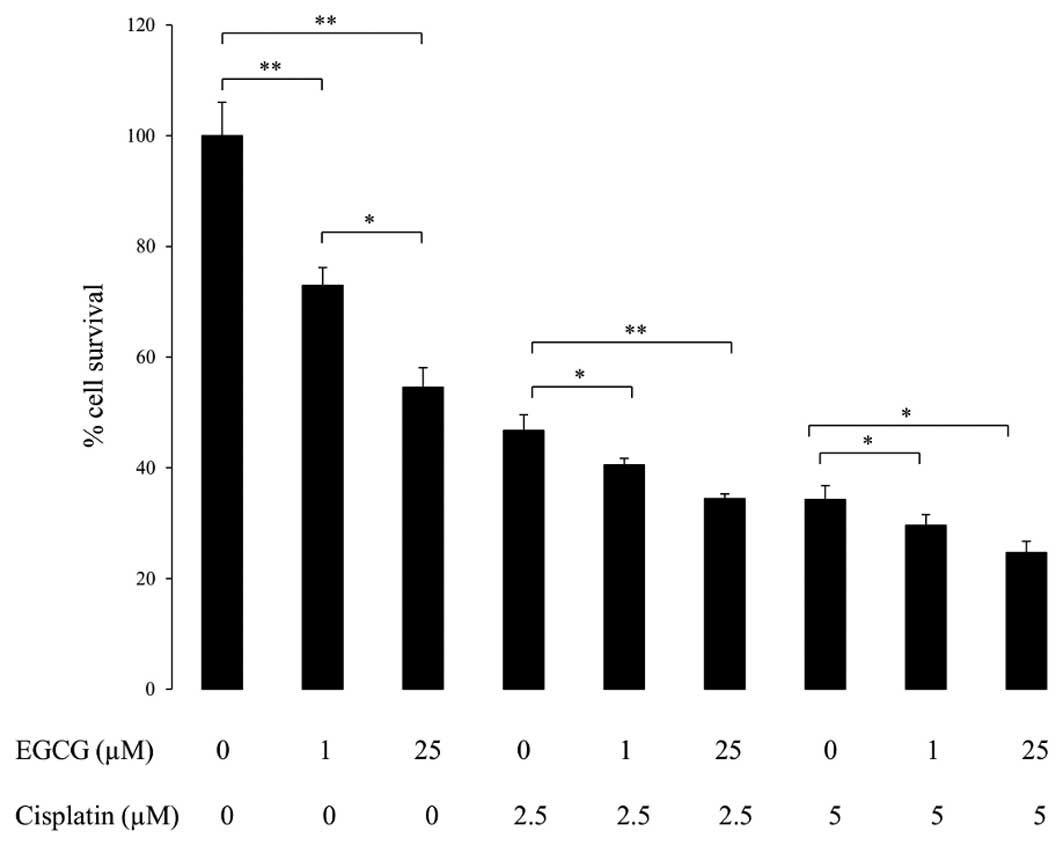
It is important to assess the ability of EGCG to
promote apoptosis in combination with a drug such as cisplatin,
known as the ‘penicillin of cancer’ due to its widespread use.
Cisplatin is a platinum-based alkylating-like drug that crosslinks
DNA, causing apoptosis. PC3 cells were treated initially for 24 h
with combinations of EGCG and cisplatin, and then for a further 24
h with EGCG alone (Fig. 2). A
significant drop in cell survival was observed in response to the
lower concentration, 1 μM EGCG. As expected, cisplatin
promoted apoptosis at 2.5 and 5 μM. The effect of cisplatin
was augmented when combined with EGCG treatment suggesting that
EGCG synergises with cisplatin to promote apoptosis.
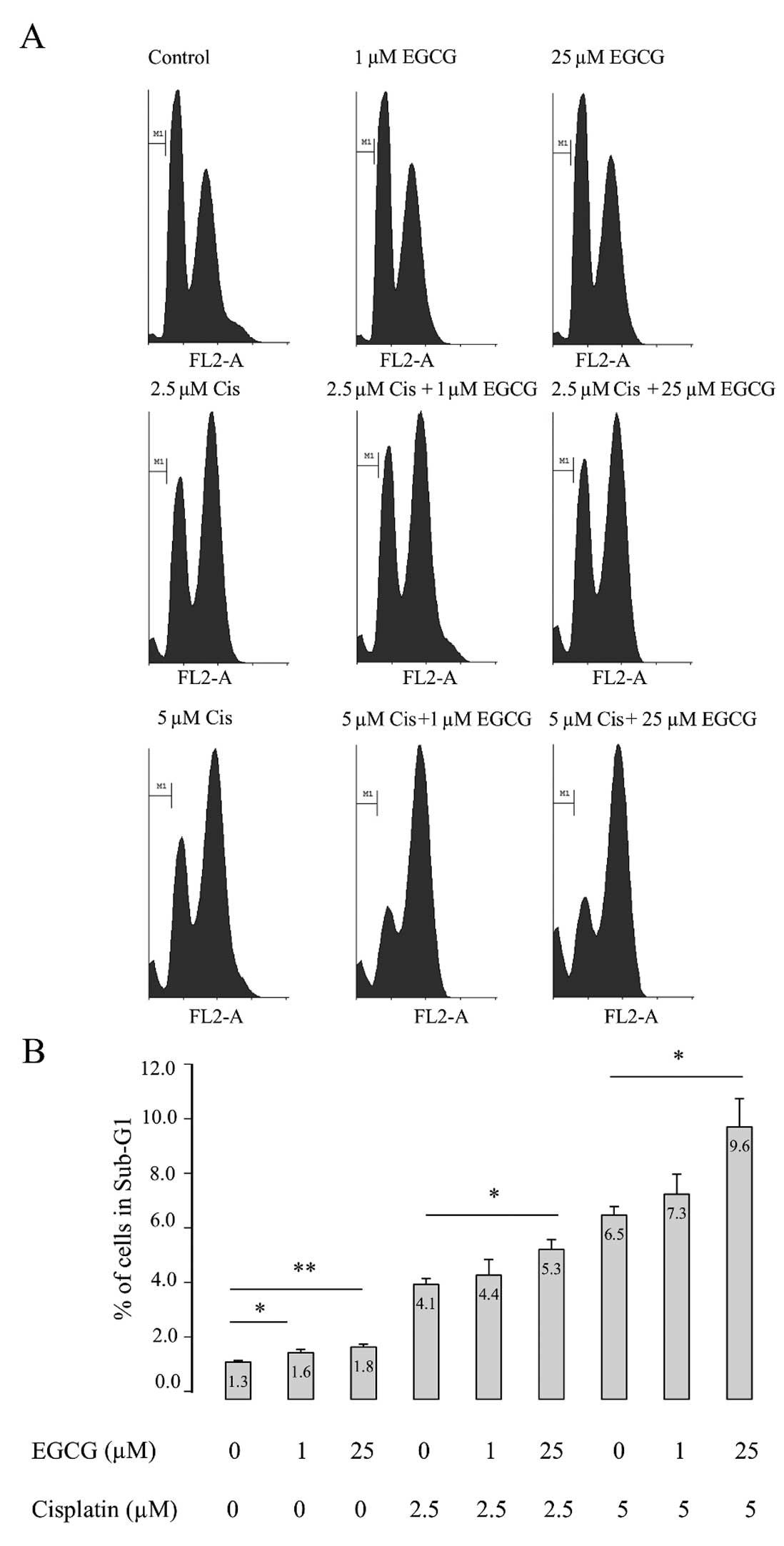
In order to confirm that EGCG might enhance the
apoptosis promoting effects of cisplatin, flow cytometry was used
in order to analyse the sub-G1 region. This method exploits the
fact that after fixation, fragmented DNA leaks out of apoptotic
cells. The dye propidium iodide was used to determine the
proportion of cells in the sub-G1 region (Fig. 3A). The raw data were imported into
the Cyflogic analysis software tool and histograms produced showing
the distribution of the cells within the cell cycle. Control
untreated PC3 cells were used to model gating parameters and the
sub-G1 marker was set-up for all further analyses. Using the
statistics tool the number and percentage of cells within the
sub-G1 region was recorded for all treatments. EGCG treatment alone
resulted in significantly increased percentage of cells in the
sub-G1 region. As expected cisplatin treatment further increased
the percentage of cells within the sub-G1 region. The combination
of cisplatin and 25 μM EGCG further increased the percentage
of cells within the sub-G1 region confirming that EGCG potentiates
apoptosis in combination with cisplatin.
Having established that EGCG and cisplatin together
promote apoptosis in PC3 cells, the alternative splicing of caspase
9 was examined using RT-PCR, initially using a forward primer in
exon 2 and a reverse primer in exon 7 (Fig. 4A). Caspase 9a (full length caspase
9) expression was significantly increased with EGCG alone (even
with 1 μM). Cisplatin alone also significantly increased
caspase 9a; and a combination of 2.5 μM cisplatin and 25
μM EGCG increased the ratio of caspase 9a to 9b 5-fold
(Fig. 4B). As standard PCR can be
notoriously unreliable in terms of the quantification of splice
variants, we developed qPCR primers that are specific to the
caspase 9 splice isoforms. The qPCR data confirmed the findings
(Fig. 4C), indicating that EGCG
alone results in a shift of the alternative splicing of caspase 9
towards the pro-apoptotic isoform. The combination of 25 μM
EGCG and cisplatin (2.5 and 5 μM) further increased the
ratio of caspase 9a to 9b.
Discussion
The flavonoids are a class of natural compounds that
have attracted considerable interest. Nutritionists estimate that
the average intake of flavonoids by humans in a normal diet is 1–2
grams per day (25). Flavonoids
are absorbed from the gastrointestinal tract (26,27),
whereas medical flavonoids are administered directly to the
diseased tissue if accessible; for example in the skin or the
throat, or along a route leading immediately to the target, such as
the nasal or the vascular systems (25). Therefore a compound such as EGCG is
worth considering in terms of potential use in adjuvant
therapy.
We find that relatively low concentrations of EGCG
(as little as 1 μM) are able to induce apoptosis in the PC3
prostate cancer cell line. Other studies have demonstrated that
EGCG inhibits proliferation with IC50 values of 39–100
μM (28–30). In this study we show that EGCG
concentrations of as little as 1 μM can inhibit
proliferation and that 25 μM EGCG resulted in 60% reduction
in survival of PC3 cells. These concentration discrepancies may be
the result of different treatment times used and general cell
culture conditions, but suggest that even low doses of EGCG may
have a substantial negative impact on prostate cancer cell
survival. These results were further confirmed using acridine
orange staining (for the detection of micronuclei and apoptotic
bodies), DNA fragmentation and flow cytometry. EGCG also appeared
to potentiate the apoptosis-promoting effects of cisplatin. It has
been reported that plasma concentrations of cisplatin in patients
that have undergone a drip infusion range from 3.0–10.3 μM
(or 0.9–3.1 μg/ml) (31).
Therefore, the range of cisplatin concentrations used in this study
is comparable to therapeutic ranges experienced by patients.
Interestingly, previous study has shown that EGCG and cisplatin
have the potential to work together to enhance apoptosis and
increase cisplatin toxicity in ovarian cancer cells (32). The drug oxaliplatin, a third
generation platinum drug, has also been shown to synergise with
EGCG, curcumin and other phytochemicals in the context of ovarian
cancer cell lines (33). In this
study we show that EGCG may work synergistically with cisplatin to
increase apoptosis and reduce the proportion of the anti-apoptotic
splice variant caspase 9b.
As is the case with all phytochemicals, any
beneficial effects of EGCG are likely to depend on its
bioavailability, metabolism and dose. A recent human study
demonstrated that after intake of brewed green tea EGCG was found
in prostate tissue suggesting that there is bioavailability of EGCG
and potential for EGCG to be active in the prostate after oral
consumption (34). However it
should be noted that an excessive intake of EGCG may be toxic
(35). Furthermore, EGCG has been
shown to interact directly with specific drugs such as sunitinib,
reducing their bioavailability (36). With these caveats in mind, a recent
study has shown that nanoparticle-mediated delivery of a compound
such as EGCG could enhance its bioavailability while limiting any
unwanted toxicity (37). These
findings suggest that the administration of EGCG together with
cisplatin to patients might prove to be an effective adjuvant
therapy for prostate cancer.
Previous studies have indicated that polyphenolics
including EGCG might cause changes in alternative splicing of
several genes (16–18). In this study we confirm these
observations by showing that EGCG can cause a significant
alteration in caspase 9 alternative splicing, favouring the
expression of the active protease. There could be several
mechanisms through which EGCG might achieve this effect. One is by
altering the expression of specific splice factors (16). Alternatively, EGCG might affect the
expression and activity of protein kinases (such as SRPK1, Clk/Sty)
and phosphatases (such as PP1) that modify the localisation and
activity of splice factors (18).
It is also possible that EGCG does not directly modify the
machinery of alternative splicing; but rather, alternative splicing
changes as a result of the initiation of apoptosis. However, the
fact that EGCG also modifies the alternative splicing of genes that
are not directly involved in apoptosis such as IKAP (17) would argue in favour of a more
generalised effect on alternative splicing.
Further research is required to determine the
precise mechanism through which EGCG modifies alternative splicing.
The possibility that EGCG might modify alternative splicing
directly is of interest to the alternative splicing field and
warrants further research. Many other pre-mRNAs are differentially
spliced in cancer and therefore EGCG could cause, in specific
contexts, additional beneficial changes in splicing patterns of
apoptotic and other cancer-associated genes. In the future, it is
likely that highly sophisticated approaches will be used to target
and manipulate the use of specific splice sites in caspase 9 and
other apoptotic genes using modified antisense oligonucleotides;
and small molecule inhibitors will target splice factors and splice
factor kinases such as SRPK1 that are involved in the regulation of
alternative splicing (38).
However, at the same time, it is clearly worth exploring the
potential of readily available phytochemical compounds such as ECGG
to achieve therapeutically desirable changes in splice isoform
expression.
Acknowledgements
Dr Rachel Hagen was supported by a
QR-funded grant (University of the West of England, Bristol, UK);
Dr Veronica Chedea was supported by a Royal Society International
Travel Grant (reference TG092176). We wish to thank David Corry for
technical assistance.
References
|
1.
|
Bettuzzi S, Brausi M, Rizzi F, Peracchia G
and Corti A: Chemoprevention of human prostate cancer by oral
administration of green tea catechins in volunteers with high-grade
prostate intraepithelial neoplasia: a preliminary report from a
one-year proof-of-principle study. Am Assoc Cancer Res.
66:1234–1240. 2006.PubMed/NCBI
|
|
2.
|
Hsieh TC and Wu JM: Targeting CWR22Rv1
prostate cancer cell proliferation and gene expression by
combinations of the phytochemicals EGCG, genistein, and quercetin.
Anticancer Res. 29:4025–4032. 2009.PubMed/NCBI
|
|
3.
|
Stuart Ec, Scandlyn MJ and Rosengren RJ:
Role of epigallocatechin gallate (EGCG) in the treatment of breast
and prostate cancer. Life Sci. 79:2329–2336. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Chen D and Dou QP: Tea polyphenols and
their roles in cancer prevention and chemotherapy. Int J Mol Sci.
9:1196–1206. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Philips BJ, Coyle CH, Morrisroe SN,
Chancellor MB and Yoshimura N: Induction of apoptosis in human
bladder cancer cells by green tea catechins. Biomed Res.
30:207–215. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Manoharan M, Fatima I, Saxena R, Chandra
V, Sankhwar PL and Dwivedi A: (-)-Epigallocatechin-3-gallate
induces apoptosis in human endometrial adenocarcinoma cells via ROS
generation and p38 MAP kinase activation. J Nutr Biochem. Sep
5–2012.(Epub ahead of print).
|
|
7.
|
Lim YC and Cha YY:
Epigallocatechin-3-gallate induces growth inhibition and apoptosis
of human anaplastic thyroid carcinoma cells through suppression of
EGFR/ERK pathway and cyclin B1/CDK1 complex. J Surg Oncol.
104:776–780. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Onoda C, Kuribayashi K, Nirasawa S, Tsuji
N, Tanaka M, Kobayashi D and Watanabe N:
(-)-Epigallocatechin-3-gallate induces apoptosis in gastric cancer
cell lines by downregulating survivin expression. Int J Oncol.
38:1403–1408. 2011.PubMed/NCBI
|
|
9.
|
Shen Q, Tian F, Jiang P, Li Y, Zhang L, Lu
J and Li J: EGCG enhances TRAIL-mediated apoptosis in human
melanoma A375 cell line. J Huazhong Univ Sci Technolog Med Sci.
29:771–775. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Siddiqui IA, Malik A, Adhami VM, Asim M,
Hafeez BB, Sarfaraz S and Mukhtar H: Green tea polyphenol EGCG
sensitizes human prostate carcinoma LNCaP cells to TRAIL-mediated
apoptosis and synergistically inhibits biomarkers associated with
angiogenesis and metastasis. Oncogene. 27:2055–2063. 2007.
View Article : Google Scholar
|
|
11.
|
Lin HY, Hou SC, Chen SC, Kao MC, Yu CC,
Funayama S, Ho CT and Way TD: (-)-Epigallocatechin gallate induces
Fas/CD95-mediated apoptosis through inhibiting constitutive and
IL-6-induced JAK/STAT3 signaling in head and neck squamous cell
carcinoma cells. J Agric Food Chem. 60:2480–2489. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Singh M, Singh R, Bhui K, Tyagi S, Mahmood
Z and Shukla Y: Tea polyphenols induce apoptosis through
mitochondrial pathway and by inhibiting nuclear factor-kappaB and
Akt activation in human cervical cancer cells. Oncol Res.
19:245–257. 2011. View Article : Google Scholar
|
|
13.
|
Das A, Banik NL and Ray SK: Flavonoids
activated caspases for apoptosis in human glioblastoma T98G and
U87MG cells but not in human normal astrocytes. Cancer.
116:164–176. 2009.PubMed/NCBI
|
|
14.
|
Li GX, Chen YK, Hou Z, Xiao H, Jin H, Lu
G, Lee MJ, Liu B, Guan F, Yang Z, Yu A and Yang CS: Pro-oxidative
activities and dose-response relationship of
(-)-epigallocatechin-3-gal-late in the inhibition of lung cancer
cell growth: a comparative study in vivo and in vitro.
Carcinogenesis. 31:902–910. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Min NY, Kim JH, Choi JH, Liang W, Ko YJ,
Rhee S, Bang H, Ham SW, Park AJ and Lee KH: Selective death of
cancer cells by preferential induction of reactive oxygen species
in response to (-)-epigallocatechin-3-gallate. Biomed Biophys Res
Commun. 421:91–97. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Markus MA, Marques FZ and Morris BJ:
Resveratrol, by modulating RNA processing factor levels, can
influence the alternative splicing of pre-mRNAs. PLoS One.
6:e289262011. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Anderson SL, Qiu J and Rubin BY: EGCG
corrects aberrant splicing of IKAP mRNA in cells from patients with
familial dysautonomia. Biochem Biophys Res Commun. 310:627–633.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Kim MH: Protein phosphatase 1 activation
and alternative splicing of Bcl-X and Mcl-1 by EGCG + ibuprofen. J
Cell Biochem. 104:1491–1499. 2008.PubMed/NCBI
|
|
19.
|
David CJ and Manley JL: Alternative
pre-mRNA splicing regulation in cancer: pathways and programs
unhinged. Genes Dev. 24:2343–2364. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Miura K, Fujibuchi W and Unno M: Splice
variants in apoptotic pathway. Exp Oncol. 34:212–217.
2012.PubMed/NCBI
|
|
21.
|
Shultz JC, Goehe RW, Wijesinghe DS,
Murudkar C, Hawkins AJ, Shay JW, Minna JD and Chalfant CE:
Alternative splicing of caspase 9 is modulated by the
phosphoinositide 3-kinase/Akt pathway via phosphorylation of
SRp30a. Cancer Res. 70:9185–9196. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Shultz JC, Goehe RW, Murudkar CS,
Wijesinghe DS, Mayton EK, Massiello A, Hawkins AJ, Mukerjee P,
Pinkerman RL, Park MA and Chalfant CE: SRSF1 regulates the
alternative splicing of caspase 9 via a novel intronic splicing
enhancer affecting the chemotherapeutic sensitivity of non-small
cell lung cancer cells. Mol Cancer Res. 9:889–900. 2011. View Article : Google Scholar
|
|
23.
|
Rasmussen R: Quantification on the
LightCycler. Rapid Cycle Real-Time PCR, Methods and Applications.
Meuer S, Wittwer C and Nakagawara K: Springer Press; Heidelberg:
pp. 2001
|
|
24.
|
Applied Biosystems: Guide to Performing
Relative Quantitation of Gene Expression Using Real-Time
Quantitative PCR, 2008.
|
|
25.
|
Havsteen BH: The biochemistry and medical
significance of the flavonoids. Pharmacol Ther. 96:67–202. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Crespy V, Morand C, Manach C, Besson C,
Demigne C and Remesy C: Part of quercetin absorbed in the small
intestine is conjugated and further secreted in the intestinal
lumen. Am J Physiol. 277:G120–G126. 1999.PubMed/NCBI
|
|
27.
|
Pforte H, Hempel J and Jacobasch G:
Distribution pattern of a flavonoid extract in the gastrointestinal
lumen and wall of rats. Nahrung. 43:205–208. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Albrecht DS, Clubbs EA, Ferruzzi M and
Bomser JA: Epigallocatechin-3-gallate (EGCG) inhibits PC-3 prostate
cancer cell proliferation via MEK-independent ERK1/2 activation.
Chem Biol Interact. 171:89–95. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Yu HN, Shen SR and Yin JJ: Effects of
interactions of EGCG and cd(2+) on the growth of PC-3 cells and
their mechanisms. Food Chem Toxicol. 45:244–249. 2007.
|
|
30.
|
Caporali A, Davalli P, Astancolle S,
D’Arca D, Brausi M, Bettuzzi S and Corti A: The chemopreventive
action of catechins in the TRAMP mouse model of prostate
carcinogenesis is accompanied by clusterin over-expression.
Carcinogenesis. 25:2217–2224. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Kurihara N, Kubota T, Hoshiya Y, Otani Y,
Ando N, Kumai K and Kitajima M: Pharmacokinetics of
cis-diamminedichloro-platinum (II) given as low-dose and high-dose
infusions. J Surg Oncol. 62:135–138. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Chan MM, Soprano KJ, Weinstein K and Fong
D: Epigallocatechin-3-gallate delivers hydrogen peroxide to induce
death of ovarian cancer cells and enhances their cisplatin
susceptibility. J Cell Physiol. 207:389–396. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Yunos NM, Beale P, Yu JQ and Huq F:
Synergism from the combination of oxaliplatin with selected
phytochemicals in human ovarian cancer cell lines. Anticancer Res.
31:4283–4289. 2011.PubMed/NCBI
|
|
34.
|
Henning SM, Aronson W, Niu Y, Conde F, Lee
NH, Seeram NP, Lee RP, Lu J, Harris DM, Moro A, Hong J, Pak-Shan L,
Barnard RJ, Ziaee HG, Csathy G, Go VL, Wang H and Heber D: Tea
polyphenols and theaflavins are present in prostate tissue of
humans and mice after green and black tea consumption. J Nutr.
136:1839–1843. 2006.PubMed/NCBI
|
|
35.
|
Rohde J, Jacobsen C and Kromann-Andersen
H: Toxic hepatitis triggered by green tea. Ugeskr Laeger.
173:205–206. 2011.PubMed/NCBI
|
|
36.
|
Singh M, Bhatnagar P, Srivastava AK, Kumar
P, Shukla Y and Gupta KC: Enhancement of cancer chemosensitization
potential of cisplatin by tea polyphenols
poly(lactide-co-glycolide) nanoparticles. J Biomed Nanotechnol.
7:2022011. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Ge J, Tan BX, Chen Y, Yang L, Peng XC, Li
HZ, Lin HJ, Zhao Y, Wei M, Cheng K, Li LH, Dong H, Gao F, He JP, Wu
Y, Qiu M, Zhao YL, Su JM, Hou JM and Liu JY: Interaction of green
tea polyphenol epigallocatechin-3-gallate with sunitinib: potential
risk of diminished sunitinib bioavailability. J Mol Med (Berl).
89:595–602. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Amin EM, Oltean S, Hua J, Gammons MV,
Hamdollah-Zadeh M, Welsh GI, Cheung MK, Ni L, Kase S, Rennel ES,
Symonds KE, Nowak DG, Royer-Pokora B, Saleem MA, Hagiwara M,
Schumacher VA, Harper SJ, Hinton DR, Bates DO and Ladomery MR: WT1
mutants reveal SRPK1 to be a downstream angiogenesis target by
altering VEGF splicing. Cancer Cell. 20:768–780. 2011. View Article : Google Scholar : PubMed/NCBI
|