Introduction
Vasohibin-2 (VASH2) belongs to the Vasohibin family
which is composed of Vasohibin-1 (VASH1) and VASH2. The human VASH2
gene, firstly identified by Shibuya et al, is reported to be
located on chromosome lq32.3 and composed of 355 amino acid
residues (1–3). VASH2 is one novel gene homologous to
VASH1 and the overall homology between them is >50% at the amino
acid level (1,3–5).
VEGF is found to induce VASH1 expression in human umbilical vein
endothelial cells (HUVECs) (6)
similarly to fibroblast growth factor 2 (FGF-2) (7,8).
VASH1 was identified to be an intrinsic and specific feedback
inhibitor playing an important role in activating ECs engaged in
angiogenesis (7,9,10),
while exogenous VASH1 hindered sprouting angiogenesis by solid
tumors (11,12). In recent studies, it was found
that, expression of VASH1 is wide-spread rather than confined to
the ECs (13,14). However, unlike VASH1, VASH2 has
been found to promote angiogenesis and is identified as an
extrinsic and VEGF-independent angiogenic factor (1,15).
In recent years, there is increasing research on the interrelation
between VASH2 and carcinoma. VASH2 is highly expressed in
carcinoma, and functions as a tumor growth promoter by angiogenesis
(15,16). The accurate mechanism needs to be
further investigated. Currently, there are few VASH2 antibodies in
the market which can be applied only in western blotting. The exact
intracellular localization of VASH2 is also still unknown, which
makes further research on VASH2 difficult.
In our study, we selected New Zealand rabbit as the
animal for polyclonal antibody production because it has larger
antibody repertoire possessing a higher specificity in recognizing
conformational and modified apitopes than do mouse antibody
(17–19). We immunized the rabbits separately
with a mixture of two specific polypeptides coupled with keyhole
limpet hemocyanin (KLH) and a custom-made prokaryotic recombinant
part-length VASH2 protein. Antibodies purified from the antiserum
were identified by western blotting, immunofluorescence (IF),
immunohistochemisty (IHC) and immunoprecipitate (IP). In order to
further investigate specific recognition capability of the
antibodies, the VASH2 cDNA (encoding for 355 and 311 amino acid
residues) was cloned into the Lv-CMV-EGFP vector, respectively. We
also synthesized a eukaryotic recombinant VASH2 protein (311 amino
acid residues). We found that, VASH2 proteins (355 and 311 amino
acid residues) were successfully recognized by our prepared
antibodies. IF, IHC and WB (post cytoplasmic/nuclear protein
isolation) results showed clear differences in intracellular
localization of VASH2 protein where 355 amino acid residues were
found in the cytoplasm while 311 amino acid residues in the
nucleus. From this study, we also found a new localization of VASH2
protein (311 amino acid residues) which may show us a new research
direction. The preparation of anti-VASH2 polyclonal antibodies will
provide a useful tool and facilitate further study on VASH2.
Materials and methods
Bioinformatics analysis and production of
immunogens
Firstly, the sequence of VASH2 protein (355 amino
acid residues) was analyzed by DNASTAR Protein 5.01 (Madison, WI,
USA). Then we selected two sections of the VASH2 protein with high
antigenicity, low hydrophobicity and favorable surface exposure to
synthesize polypeptides as immunogens. The polypeptides were
synthesized by GL Biochem (Shanghai, China). In addition, a
prokaryotic recombinant part-length VASH2 protein was designed and
produced by Abmart (Shanghai, China).
Preparation and purification of
antibodies
All of the experimental procedures were approved by
the Animal Care and Use Subcommittee at Nanjing Medical University.
Eight New Zealand rabbits (Jiangsu Agricultural Academy of Science,
Nanjing, China), all males, weighing between 2.0 and 2.5 kg, were
used and allowed free access to food and water. Peripheral blood
was extracted before immunization and serum used as antibody
(1:200) to perform western blotting (HepG2 celllysate). After
screening by western blotting, we obtained two rabbits with no
obvious band. These selected two rabbits were immunized
subcutaneously with 100 μg prokaryotic recombinant
part-length VASH2 protein. Another two unscreened rabbits were
immunized subcutaneously with a mixture of two polypeptides (each
peptide 100 μg). Immune solution preparation and procedure
were performed as described (20).
We harvested the antiserum from the rabbit carotid artery 8 weeks
later after 4–5 subcutaneous immunizations (21). The antiserum titer was determined
by indirect ELISA (22–24). The antisera were purified by
immuno-affinity purification or protein-G column purification by
Shanghai GL Biochem when the titer reached 1:25,000.
Cell cultures and tissue sections
Human liver cancer cell line HepG2 was a gift of
Professor Beicheng Sun (Department of General Surgery of The First
Affiliated Hospital of Nanjing Medical University, Nanjing, China).
The cell line was cultured in DMEM (Wisent, Canada) provided with
10% fetal bovine serum (Wisent, Canada) at 37°C with 5%
CO2. Human liver cancer and adjacent normal tissues were
obtained from patients from the First Affiliated Hospital of
Nanjing Medical University. The patients were informed and their
detailed information and written consents were attained.
Plasmid construction, transient
transfection and lentivirus packaging
The plasmid highly expressed VASH2 (355 amino acid
residues) fused with DDK tag at the c-terminal was constructed as
described (15). Full-length VASH2
cDNA (encoding for 311 amino acid residues) bought from Origene
(USA) was fused with DDK or V5 tag at the c-terminal and cloned
into the Lv-CMV-EGFP vector. All plasmids were verified by
sequencing (Invitrogen, Shanghai, China). Lipofectamine 2000
(Invitrogen) was used as transient transfection reagent according
to the manufacturer’s instructions. Lentivirus packaging method was
the same as described (15). The
primer pair for the VASH2 plasmid (311 amino acid residues) was as
follows: forward, 5′-CGGCTAGCCCCACCATGACCGGCTC-3′ and reverse,
5′-AACTGCAGCTACTTATCGTCGTCATCCTTGTAATCAATTCGGATTTGATAGCCCACTT-3′
(DDK-tag),
5′-AACTGCAGCTACGTAGAATCGAGACCGAGGAGAGGGTTAGGGATAGGCTTACCAATTCGGATTTGATAGCCCACTT-3′
(V5-tag). After transfection with the lentivirus, the VASH2 highly
expressed cells were tested by western blotting and qRT-PCR.
Mouse xenograft models
Female, 3–4-week-old BALB/c nude mice were obtained
from Nanjing Medical University. Aliquots of HepG2, HepG2-VASH2
(355 amino acid residues) and HepG2-VASH2-v5 (311 amino acid
residues fused with V5 tag at the C-terminal) cells (0.1 ml,
1–2×106 cells) were injected into flanks of mice. Four
to six weeks later, xenograft tumors were harvested and fixed in 4%
neutral formalin.
Recombinant protein, nuclear extraction
analysis and western blotting
The VASH2 recombinant protein (311 amino acid
residues) was purchased from Origene (USA). The cytoplasmic and
nuclear samples were isolated using a Nuclear Extraction kit 2900
(Millipore, MA, USA). The experimental procedures were the same as
in the supplier’s manual. Cell lysates were prepared by extracting
protein with radio immunoprecipitation assay buffer. PVDF membranes
(Millipore) were blocked in 5% skim milk and incubated overnight at
4°C with the three rabbit antibodies prepared at a dilution of
1:3,000 (SI), 1:3000 (S2) and 1:200 (S3), respectively. A mouse
anti-human VASH2 antibody, as a verified antibody (2,15),
was a gift from Professor Y. Sato (Department of Vascular Biology,
Institute of Development, Aging and Cancer, Tohoku University,
Sendai, Japan). Both the anti-GAPDH and secondary antibodies were
purchased from Beyotime. The rabbit anti-human H3 antibody was
purchased from Cell Signaling (USA) while the rabbit anti-V5 and
mouse anti-human E-cadherin antibodies were from Abeam (USA).
Immunoprecipitation (IP)
Pierce Co-IP kit (Thermo, USA) was used for
immunoprecipitation analysis according to the supplier’s manual.
The prepared antibodies were coupled with resin (20 μg
antibody/25 μl resin) and then incubated in 3.0 ml HepG2
cell lysate (∼3×107 cells) with gentle mixing overnight
at 4°C. Eluted protein samples were added with SDS-PAGE sample
buffer and boiled at 95°C for 5 min. The normal rabbit IgG
(Millipore) was used as negative control.
Immunofluorescence
HepG2 cell line was arrayed in a 24-well plate
(Corning Inc., USA), and IF was performed as described (25,26).
Three polyclonal antibodies were diluted as follows: SI, 1:200,
S2,1:400, S3,1:50. Mouse-anti-DDK antibody was from Abmart. Second
antibody: goat anti-rabbit IgG-dylight 593 (Invitrogen, USA).
Nucleus dye, 10 μg/ml DAPI (Sigma, St. Louis, MO, USA)
diluted in PBS. Finally, cells were observed under a fluorescence
microscope (Olympus, Japan).
Immunohistochemistry
The immunohistochemical staining procedure was
performed as described (27,28).
Histological sections (5 μm) were analyzed by
immunohistochemistry using the prepared antibodies. The test
dilution of antibodies was 1:50–1:1,000.
Results
Based on the DNASTAR software and comprehensive
analysis, we selected two sections of VASH2 as polypeptides for
synthesizing. The polypeptides had low homology to rabbit and human
protein sequences. The details of the two polypeptides and one
prokaryotic recombinant part-length VASH2 protein are shown in
Table I. We selected two rabbits
with no obvious band after screening by western blotting using the
preimmune serum as antibody (data not shown).
 | Table I.Immunogens for preparation of
anti-VASH2 polyclonal antibodies. |
Table I.
Immunogens for preparation of
anti-VASH2 polyclonal antibodies.
| Name | Location | Amino acid
sequence | Polypeptide
length |
|---|
| SI | aa211–323 | RRAELMD-RQASPP | 113aa |
| S2 | aa317–327 | RRQASPPRRLG | 11aa |
| S3 | aa300–311 | AHSPTQVRSRGK | 12aa |
The antisera were purified using Shanghai GL Biochem
kit and details of the three antibodies are shown in Table II. To identify the specificity of
our antibodies, their reactions (S1–S3) with antigens were tested
by indirect sandwich ELISA. Results indicate that the optimal
concentration for the recombinant antigens was 5μg/ml, the
titer for SI, S2 was 1:64,000 while for S3 was 1:32,000 (Table III).
 | Table II.Detailed summary of the prepared
polyclonal antibodies against VASH2 and their applications. |
Table II.
Detailed summary of the prepared
polyclonal antibodies against VASH2 and their applications.
| Name | Serum titer | Purification
methods | Concentration
(mg/ml) | Applications |
|---|
| SI |
1.0×105 | Protein-G
column | 2.00 | WB, IF |
| S2 |
5.0×104 | Immunoaffinity
purification | 1.60 | WB, IP, IF,
IHC |
| S3 |
2.5×104 | Immunoaffinity
purification | 0.14 | WB, IF |
 | Table III.Indirect sandwich ELISA analysis
results of anti-VASH2 antibodies |
Table III.
Indirect sandwich ELISA analysis
results of anti-VASH2 antibodies
| Antibody ID | 2k | 4k | 8k | 16k | 32k | 64k | 128k | 256k | PC | NC |
|---|
| SI | 2.137 | 1.426 | 0.824 | 0.464 | 0.370 | 0.274 | 0.194 | 0.122 | 2.475 | 0.131 |
| S2 | 2.118 | 1.284 | 0.778 | 0.585 | 0.414 | 0.276 | 0.177 | 0.155 | 2.472 | 0.121 |
| S3 | 2.167 | 1.438 | 0.856 | 0.519 | 0.329 | 0.234 | 0.176 | 0.152 | 2.516 | 0.119 |
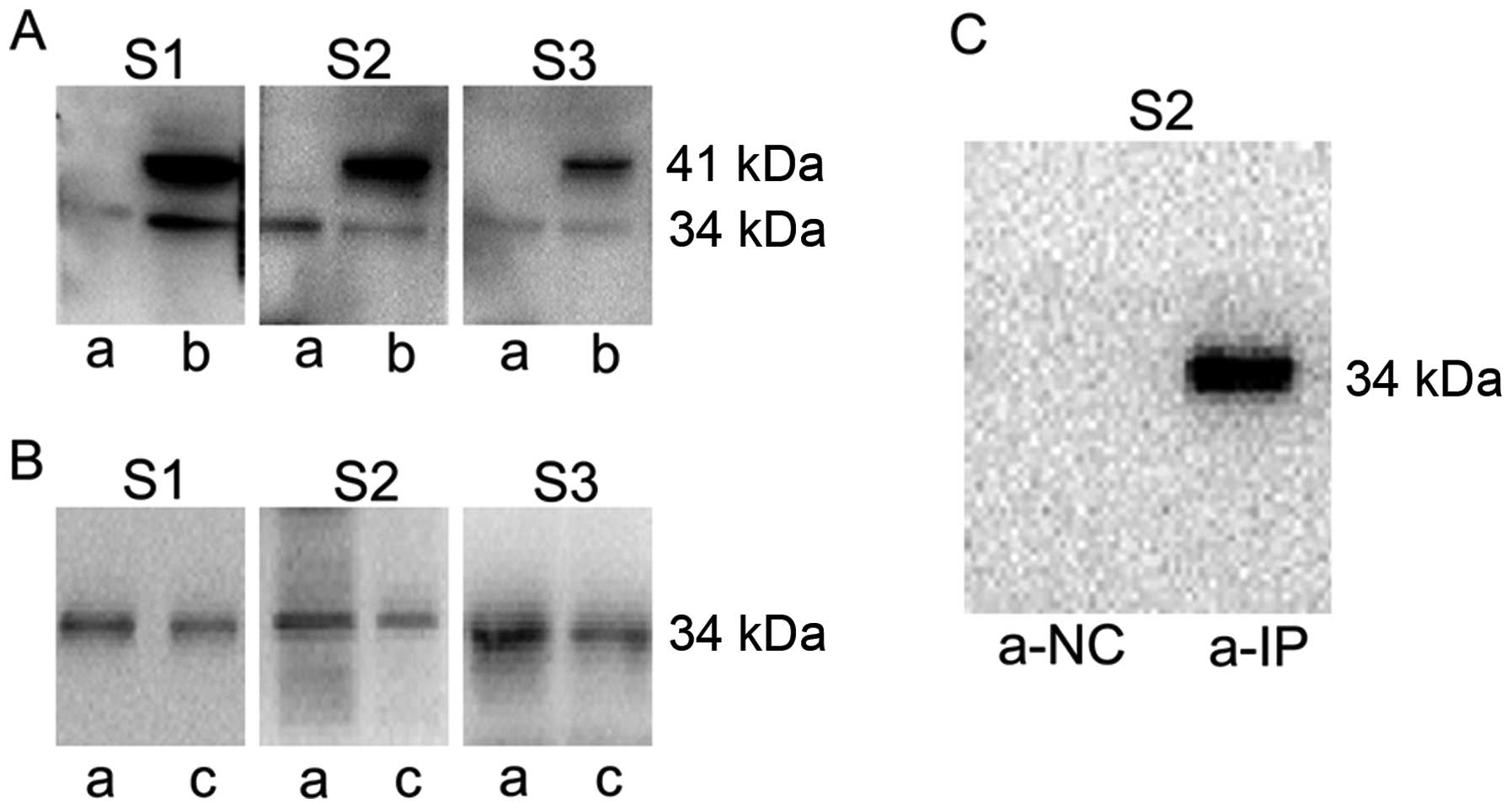
In order to identify the specificity of the
antibodies against VASH2, western blotting was performed. In HepG2,
we observed a single band at 34 kDa, while two bands at 41 and 34
kDa were detected in HepG2-VASH2 (355 amino acid residues)
(Fig. 1A). To further demonstrate
recognition capability to 34 kDa VASH2 protein, the VASH2
recombinant protein (311 amino acid residues) purchased from
Origene was tested (Fig. 1B). The
results indicate that all the prepared polyclonal antibodies have
high specificity to VASH2 protein (311 amino acid residues).
Only the S2 antibody performed successfully in IP
analysis. No band in a-NC (normal rabbit IgG), while a clear band
at 34 kDa is observed in a-IP (Fig.
1C).
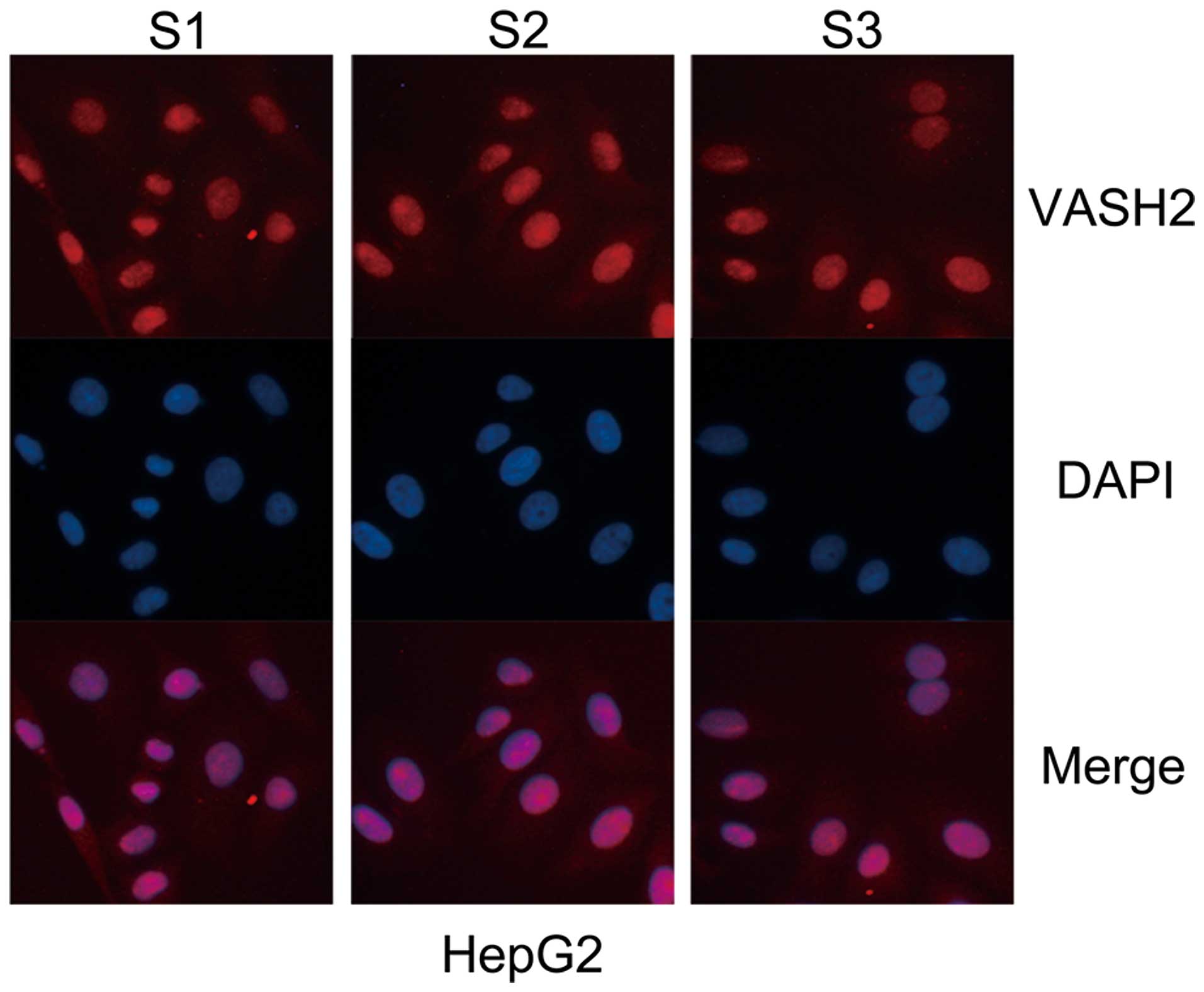
IF analysis was carried out using the HepG2 cell
line (Fig. 2). The results
demonstrate that the three antibodies can be used for IF. Moreover,
we found that human VASH2 protein was mainly localized in the
nucleus and only slight in the cytoplasm.
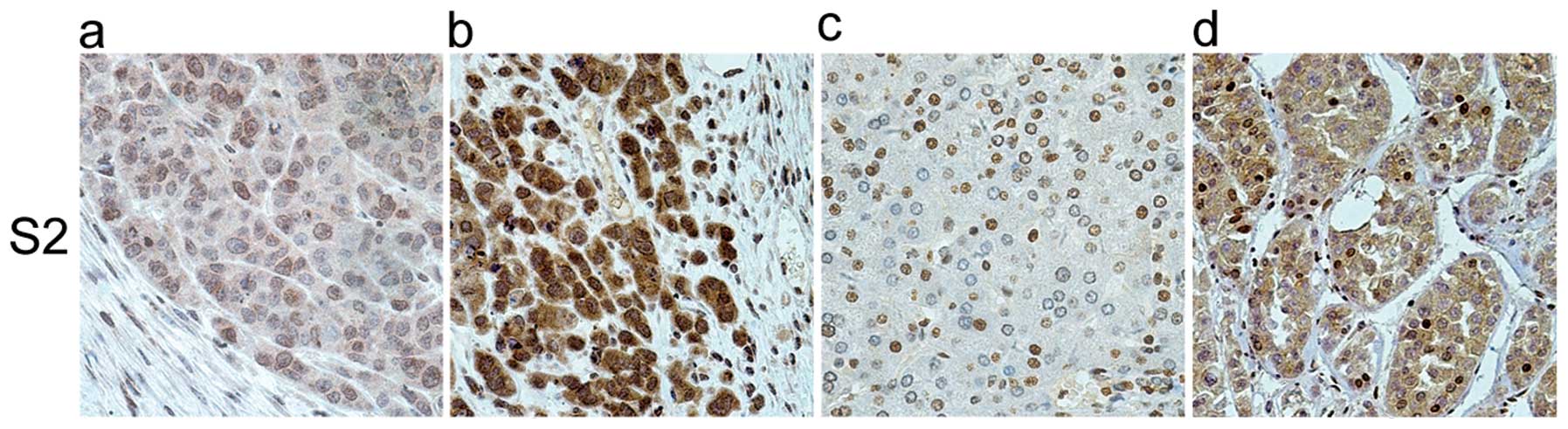
Human liver cancer, adjacent normal tissues and the
harvested xenograft tumor tissues were tested by IHC. The results
revealed that only S2 possessed the specificity and sensitivity to
recognize VASH2 protein in IHC (Fig.
3). Fig. 3 shows that the
VASH2 protein is mainly localized in the nucleus. In the
HepG2-VASH2 (355 amino acid residues), we observed that the
cytoplasm was deeply stained compared to HepG2, meaning that the
overexpressed VASH2 (355 amino acid residues) was mainly in the
cytoplasm. In the human liver cancer adjacent normal tissue, we
observed that the VASH2 protein was mainly localized in the nucleus
while only slight in the cytoplasm. A question arose as to whether
the two VASH2 proteins (355 amino acid residues and 311 amino acid
residues) had different intracellular localizations.
In order to further confirm the intracellular
localization of the two VASH2 proteins, the cytoplasmic and nuclear
cellular extracts were prepared and then analyzed by western
blotting. We tested localization effects of nuclear/cytoplasmic
proteins using E-cadherin, H3 and GAPDH (Fig. 4A). The E-cadherin band was observed
only in the cytoplasmic extracts while the H3 band was in the
nucleus. The GAPDH band was observed mainly in the cytoplasmic
extracts while only slightly in the nuclear extracts. These results
demonstrate that the nuclear and cytoplasmic proteins used to show
localization effects were successfully isolated. Using the verified
mouse anti-human VASH2 antibody (11,15)
and the prepared rabbit polyclonal antibodies (S2, S3), we observed
that, exogenous VASH2 (41 kDa, 355 amino acid residues) was mainly
located in the cytoplasm while endogenous VASH2 (34 kDa, 311 amino
acid residues) was located in the nucleus (Fig. 4A). A slight band at 41 kDa in the
HepG2-VASH2 nucleus (Fig. 4A) was
considered to be a result of negligible contamination of
cytoplasmic protein. To further verify the nuclear localization of
VASH2, IF analysis using the anti-DDK antibody was performed for
the HepG2-VASH2 (transient overexpressed c-terminal DDK-tagged 311
and 355 amino acid residues VASH2). The HepG2-VASH2 (311 amino acid
residues) nucleus was stained red while the cytoplasm was red in
the HepG2-VASH2 (355 amino acid residues) under the fluorescence
microscope (Fig. 4B). IHC results
from the mouse xenograft (HepG2-VASH2-V5, 311 amino acid residues)
show that V5 was localized both in the cytoplasm and nucleus
(Fig. 4C). In Figs. 1A and 4A, no band at 41 kDa was observed in the
HepG2 lysate while in the HepG2 IF (Fig. 2) and HepG2 IHC (Fig. 3a), slight stain in the cytoplasm
was observed. These results indicate that the endogenous VASH2
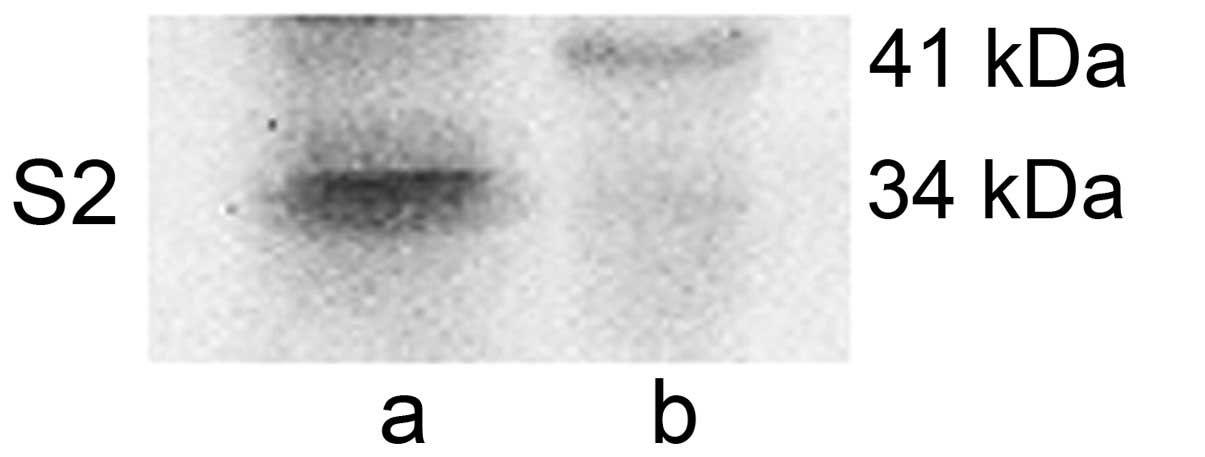
protein exists in the cytoplasm. When double dose of HepG2
cytoplasmic lysates were loaded as western blotting samples, a
slight band at 41 kDa in the HepG2 cytoplasm was observed (Fig. 5).
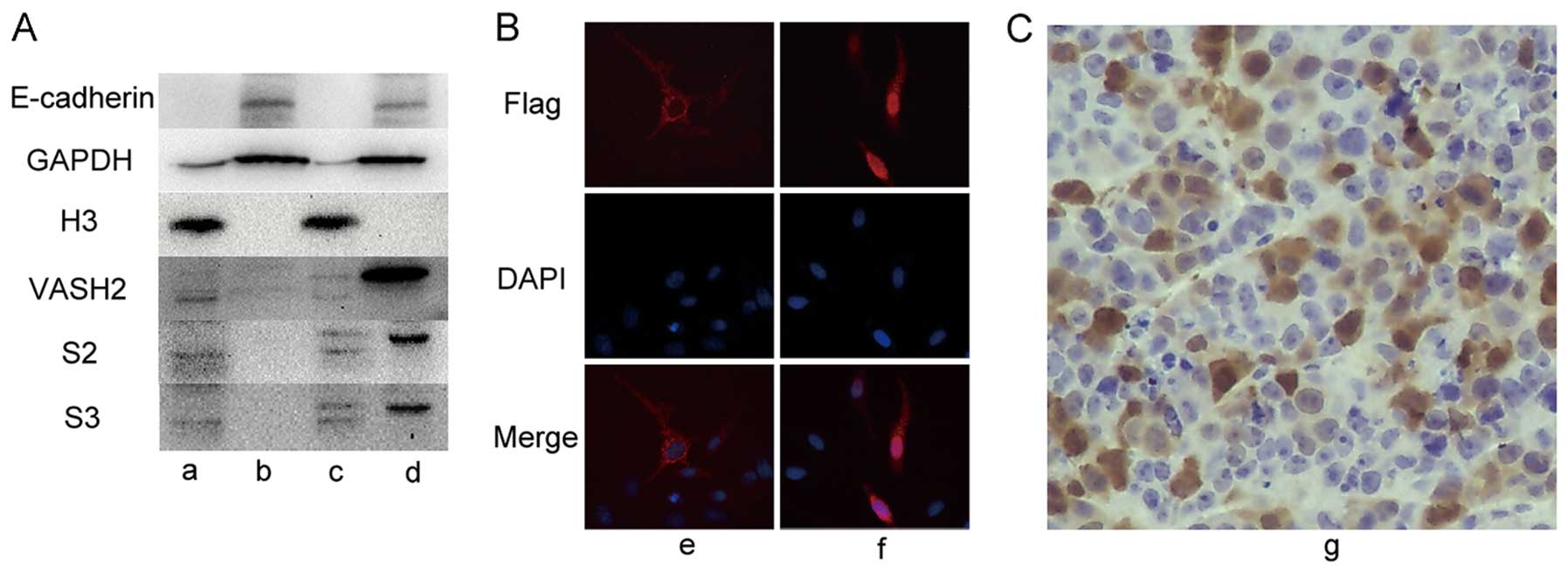 | Figure 4.Intracellular localization of the
VASH2 protein, a, HepG2-nucleus; b, HepG2-cytoplasm; c,
HepG2-VASH2-nucleus (355 amino acid residues); d,
HepG2-VASH2-cytoplasm (355 amino acid residues); e,
HepG2-VASH2-Flag (355 amino acid residues) magnification, ×400; f,
HepG2-VASH2-Flag (311 amino acid residues) magnification, ×400; g,
mouse xenograft tumor tissue (HepG2-VASH2-V5, 311 amino acid
residues) magnification, ×200. (A) The cytoplasmic and nuclear
cellular extracts prepared and analyzed by western blotting. (B) IF
analysis with the anti-DDK antibody performed in HepG2-VASH2
(transient overexpressed c-terminal DDK-tagged 311 and 355 amino
acid residues VASH2). (C) IHC analysis performed in the mouse
xenograft tumor tissue (HepG2-VASH2-V5, 311 amino acid
residues). |
Discussion
VASH2, a novel gene homologous to VASH1, is
considered as an angiogenesis promoter (2). Recently, it has been found that VASH2
plays an important role in carcinoma angiogenesis and malignant
transformation (15). VASH2 is
also reported to be expressed in gastric cancer cells (4). The available data demonstrate that
VASH2 is associated with carcinoma and angiogenesis. Although the
accurate mechanism involving VASH2 and angiogenesis in carcinoma is
unknown, VASH2 investigation may provide a novel target for tumor
therapy. We have been studying VASH2 function and molecular
mechanisms for several years. However, the supply of VASH2
antibodies in the market is very limited (available for western
blotting only). This shortage has sometimes hindered further
research on VASH2. Hence, effective VASH2 antibodies should be
exploited for future research use. Herein, we have prepared and
evaluated VASH2 polyclonal antibodies by western blotting, IF, IHC
and IP. The detailed applications of these three antibodies are
summarized in Table II. All the
applications listed were achieved successfully in this study.
Formerly, VASH2 was reported as a secreted protein
(1,2,16).
We analyzed both the 311 and 355 amino acid residues of VASH2
protein sequence using the protein subcellular localization
predicting software Hum-mPLoc 2.0 (http://www.csbio.sjtu.edu.cn/bioinf/hum-multi-2/).
The prediction result showed extracellular localization for both.
This result was not consistent with our present data. Then, we used
the Nakai Server software (http://psort.hgc.jp/form.html) and the result showed
nuclear localization while the peptide sequence ‘RRRQASPPRRLGRREKS’
was predicted as nuclear location signal. Our present data showed
that, full length VASH2 composed of 355 amino acid residues was
located in the cytoplasm while the 311 amino acid residues VASH2 in
the nucleus. This was verified by western blot analysis of
cyto-plasmic/nuclear cellular extracts using the verified mouse
anti-human VASH2 antibody (11,15)
and the prepared rabbit polyclonal antibodies (S2, S3). From these
results we observed that, exogenous VASH2 (41 kDa, 355 amino acid
residues) was mainly located in the cytoplasm while endogenous
VASH2 (34 kDa, 311 amino acid residues) was mainly located in the
nucleus (Fig. 4A). Results from
this study also indicate that endogenous VASH2 protein also exists
in the cytoplasm. This was observed in the HepG2 IF (Fig. 2) and HepG2 IHC (Fig. 3a) where slight dyeing in the
cytoplasm was observed. This was confirmed when double doses of
HepG2 cytoplasmic lysates was analyzed by western blotting, giving
a slight band at 41 kDa in the HepG2 cytoplasm (Fig. 5). We thus also conclude that, both,
the 311 and 355 amino acid residues of VASH2 protein have
intracellular expression, but the latter is of low abundance. In
human liver cancer tissue IHC (Fig.
3d), we observed that both the cytoplasm and nucleus were
stained. Compared to human liver cancer adjacent tissue, the
cytoplasm VASH2 protein was highly expressed. Because of inadequate
number of cases, the result needs to be further demonstrated by
expanding the number of cases.
VASH2 is closely related with carcinoma, but the
specific downstream signaling pathway has not been found so far. In
the past, VASH2 was considered as a single cytoplasm localized
protein. Here, our study confirms that the different VASH2 protein
isoforms have different intracellular localization, thus VASH2
proteins need to be divided into two types: karyo and cytoplasmic
type.
In conclusion, we have successfully generated three
rabbit anti-human VASH2 polyclonal antibodies which can be applied
for western blotting, IF, IP and IHC. We also confirmed that the
VASH2 protein has different intracellular localizations, 355 amino
acid residues being mostly in the cytoplasm while 311 amino acid
residues in the nucleus. The results from this study show us a new
direction for further study on VASH2.
Acknowledgements
This study was supported by grants
from NSFC (nos. 81172267, 81170336 and 30972912) and the
translational research of early diagnosis and comprehensive
treatment in pancreatic cancer (201202007).
References
|
1.
|
Kimura H, Miyashita H, Suzuki Y, Kobayashi
M, Watanabe K, Sonoda H, Ohta H, Fujiwara T, Shimosegawa T and Sato
Y: Distinctive localization and opposed roles of vasohibin-1 and
vasohibin-2 in the regulation of angiogenesis. Blood.
113:4810–4818. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Shibuya T, Watanabe K, Yamashita H,
Shimizu K, Miyashita H, Abe M, Moriya T, Ohta H, Sonoda H,
Shimosegawa T, Tabayashi K and Sato Y: Isolation and
characterization of vasohibin-2 as a homologue of VEGF-inducible
endothelium-derived angiogenesis inhibitor vasohibin. Arterioscler
Thromb Vase Biol. 26:1051–1057. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Sato Y and Sonoda H: The vasohibin family:
a negative regulatory system of angiogenesis genetically programmed
in endothelial cells. Arterioscler Thromb Vase Biol. 27:37–41.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Shen Z, Kauttu T, Seppanen H, Vainionpaa
S, Ye Y, Wang S, Mustonen H and Puolakkainen P: Vasohibin-1 and
vasohibin-2 expression in gastric cancer cells and TAMs. Med Oncol.
29:2718–2726. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Sato Y: The vasohibin family: novel
regulators of angiogenesis. Vascul Pharmacol. 56:262–266. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Abe M and Sato Y: cDNA microarray analysis
of the gene expression profile of VEGF-activated human umbilical
vein endothelial cells. Angiogenesis. 4:289–298. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Watanabe K, Hasegawa Y, Yamashita H,
Shimizu K, Ding Y, Abe M, Ohta H, Imagawa K, Hojo K, Maki H, Sonoda
H and Sato Y: Vasohibin as an endothelium-derived negative feedback
regulator of angiogenesis. J Clin Invest. 114:898–907. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Shimizu K, Watanabe K, Yamashita H, Abe M,
Yoshimatsu H, Ohta H, Sonoda H and Sato Y: Gene regulation of a
novel angiogenesis inhibitor, vasohibin, in endothelial cells.
Biochem Biophys Res Commun. 327:700–706. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Nasu T, Maeshima Y, Kinomura M,
Hirokoshi-Kawahara K, Tanabe K, Sugiyama H, Sonoda H, Sato Y and
Makino H: Vasohibin-1, a negative feedback regulator of
angiogenesis, ameliorates renal alterations in a mouse model of
diabetic nephropathy. Diabetes. 58:2365–2375. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Shirasuna K, Kobayashi A, Nitta A, Nibuno
S, Sasahara K, Shimizu T, Bollwein H and Miyamoto A: Possible
action of vasohibin-1 as an inhibitor in the regulation of
vascularization of the bovine corpus luteum. Reproduction.
143:491–500. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Tamaki K, Moriya T, Sato Y, Ishida T,
Maruo Y, Yoshinaga K, Ohuchi N and Sasano H: Vasohibin-1 in human
breast carcinoma: a potential negative feedback regulator of
angiogenesis. Cancer Sci. 100:88–94. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Yoshinaga K, Ito K, Moriya T, Nagase S,
Takano T, Niikura H, Sasano H, Yaegashi N and Sato Y: Roles of
intrinsic angiogenesis inhibitor, vasohibin, in cervical
carcinomas. Cancer Sci. 102:446–451. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Nimmagadda S, Geetha-Loganathan P, Prols
F, Scaal M, Christ B and Huang R: Expression pattern of Vasohibin
during chick development. Dev Dyn. 236:1358–1362. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Naito H, Kidoya H, Sato Y and Takakura N:
Induction and expression of anti-angiogenic vasohibins in the
hematopoietic stem/progenitor cell population. J Biochem.
145:653–659. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Xue X, Gao W, Sun B, Xu Y, Han B, Wang F,
Zhang Y, Sun J, Wei J, Lu Z, Zhu Y, Sato Y, Sekido Y, Miao Y and
Kondo Y: Vasohibin 2 is transcriptionally activated and promotes
angiogenesis in hepatocellular carcinoma. Oncogene. May
21–2012.(Epub ahead of print),. View Article : Google Scholar
|
|
16.
|
Takahashi Y, Koyanagi T, Suzuki Y, Saga Y,
Kanomata N, Moriya T, Suzuki M and Sato Y: Vasohibin-2 expressed in
human serous ovarian adenocarcinoma accelerates tumor growth by
promoting angiogenesis. Mol Cancer Res. 10:1135–1146. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Huang Y, Gu B, Wu R, Zhang J, Li Y and
Zhang M: Development of a rabbit monoclonal antibody group against
Smads and immunocytochemical study of human and mouse embryonic
stem cells. Hybridoma (Larchmt). 26:387–391. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Spieker-Polet H, Sethupathi P, Yam PC and
Knight KL: Rabbit monoclonal antibodies: generating a fusion
partner to produce rabbit-rabbit hybridomas. Proc Natl Acad Sci
USA. 92:9348–9352. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Tan Z, Zhang J, Su Z, Gu B, Jiang X, Luo
J, Ji H, Wang G, Tao B, Zhao X, Chen L, Yu G, Zhu W and Zhang M:
Production of rabbit monoclonal antibodies against mouse embryonic
stem cells and identification of pluripotency-associated surface
antigens. J Immunol Methods. 365:149–157. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Saradhi M, Krishna B, Mukhopadhyay G and
Tyagi RK: Purification of full-length human pregnane and xenobiotic
receptor: polyclonal antibody preparation for immunological
characterization. Cell Res. 15:785–795. 2005. View Article : Google Scholar
|
|
21.
|
Chen TF, Zhang YL, Xu WL, Li ZQ, Hou B,
Wang CL, Fan M, Qian LJ, Zhou RP and Zhang CG: Prokaryotic
expression, polyclonal antibody preparation, and sub-cellular
localization analysis of Na+, K+-ATPase beta2
subunit. Protein Expr Purif. 37:47–52. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Crowther JR: ELISA. Theory and practice.
Methods Mol Biol. 42:1–218. 1995.PubMed/NCBI
|
|
23.
|
Crowther JR: The ELISA guidebook. Methods
Mol Biol. 149:III–IV. 1–413. 2000.PubMed/NCBI
|
|
24.
|
Tsumuraya T, Takeuchi K, Yamashita S,
Fujii I and Hirama M: Development of a monoclonal antibody against
the left wing of ciguatoxin CTX1B: thiol strategy and detection
using a sandwich ELISA. Toxicon. 60:348–357. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Tang S, Wang Y, Zhang D, Gao Y, Ma Y, Yin
B, Sun J, Liu J and Zhang Y: Reprogramming donor cells with oocyte
extracts improves in vitro development of nuclear transfer embryos.
Anim ReprodSci. 115:1–9. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Lin J, Lin X, Yang GH, Wang Y, Peng BW and
Lin JY: Toxoplasma gondii: expression of GRA1 gene in endoplasmic
reticulum promotes both growth and adherence and modulates
intracellular calcium release in macrophages. Exp Parasitol.
125:165–171. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Tan Z, Zhang J, Su Z, Gu B, Jiang X, Luo
J, Ji H, Wang G, Tao B, Zhao X, Chen L, Yu G, Zhu W and Zhang M:
Production of rabbit monoclonal antibodies against mouse embryonic
stem cells and identification of pluripotency-associated surface
antigens. J Immunol Methods. 365:149–157. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Hama Y, Chano T, Inui T, Matsumoto K and
Okabe H: Preparation of mouse monoclonal antibody for RB1CC1 and
its clinical application. PLoS One. 7:e320522012. View Article : Google Scholar : PubMed/NCBI
|