Introduction
Malignant fibrous histiocytoma (MFH), also called
high grade undifferentiated sarcoma, collectively represent the
most common types of sarcoma in the fifth and sixth decades of
life. The overall incidence among adults approximates to 1–2 cases
per 100,000 patients. Most MFH occur in the extremities and deep
soft tissue (1). Approximately 5%
of patients have metastases at presentation and MFH is aggressive
with an overall 5-year survival probability of 50–60% (1). Surgical removal is presently the sole
effective treatment and the goal of surgery is complete resection
with negative margin.
Histopathologically, MFH shows a wide variety of
morphological patterns. MFH commonly presents with marked
cytological and nuclear pleomorphism, often with bizarre tumor
giant cells, admixed with spindle cells and frequently with rounded
histiocyte-like cells in varying proportions (2). MFH shows no evidence of true
monocyte/macrophage/histiocytic differentiation. Several hypotheses
suggest MFH arises from fibroblasts or primitive mesenchymal cells
but current research does not show a definable line of
differentiation. The diagnosis is controversial, but various cases
are eligible for consideration as MFH (high grade undifferentiated
sarcoma). At present, there are no useful immunohistochemical
markers for the diagnosis of MFH; therefore, it is difficult to
search for the origin of MFH.
Our laboratory generated monoclonal antibody FU3
using an MFH cell line as an immunogen. FU3 reacted strongly with
the surface membrane of cultured MFH cells and with perivascular
mesenchymal cells in frozen tissue sections. Accordingly, MFH may
share common antigenicity with perivascular mesenchymal cells
(3). Immuno-electron-microscopic
studies demonstrated FU3-positive reactivity on the surface of cell
membranes, which suggests that FU3 recognizes cell surface antigens
(4–6).
The aim of this study was to identify the antigen
recognized by FU3 antibody. Furthermore, we examined whether the
antigen could be effectively applied to diagnosis and treatment of
MFH.
Materials and methods
Cell culture
The MFH cell line SFT8503 was established in our
laboratory, as described previously (6). The cell line was maintained in growth
medium, Dulbecco’s modified Eagle’s medium/Ham’s F-12 (Wako,
Japan), supplemented with 10% fetal calf serum, streptomycin (50
μg/ml) and penicillin G (50 U/ml).
Protein extraction and western
blotting
The cultured cells were lysed in RIPA lysis buffer
(50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1 mM EDTA, 1% NP-40;
Millipore, Bedford, MA) and the lysed cells were sonicated on ice
for 5 min three times and centrifuged at 15,000 rpm for 20 min at
4°C. The resultant supernatants were subjected to sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). After
electrophoresis, the proteins were transferred electrophoretically
to Immobilon membrane (Millipore). Non-specific sites were blocked
with 5% dry fat milk in Tris-buffered saline (TBS) at 37°C for 1 h
and the membrane was incubated overnight at 4°C with monoclonal FU3
antibody or with commercially available CD13 antibody (Clone 38C12,
ThermoScientific, Cheshire, UK). After washing with TBS-T (TBS
containing 0.05% Tween-20), the membrane was incubated for 1 h with
peroxidase-conjugated anti-mouse IgG. Color was developed with
chemiluminescence reagents according to the instructions supplied
by the manufacturer (DuPont New England Nuclear, Boston, MA).
Immunoaffinity chromatography
Monoclonal antibody FU3, which was generated against
MFH cells using a mouse hybridoma technique as previously described
(3,4), was used to prepare an affinity column
(7). We used cyanogen bromide
CNBr-activated Sepharose 4B (GE Healthcare Ltd., UK) as an
immunoaffinity matrix coupling to mouse FU3 antibody. The column
was washed with phosphate buffered saline (PBS). Antigens bound to
the matrix were then eluted from the column with 0.2 M glycine (pH
2.3). The eluted material was immediately neutralized with PBS and
stored at 4°C. The eluted protein fraction of the column was
purified by SDS-PAGE and immunoblotting. The purified protein was
subjected to N-terminal amino acid sequencing (Takara Bio Inc.
Otsu, Japan).
Tissue samples
Our study included formalin-fixed, paraffin-embedded
sections from 25 MFH [10 men, 15 women; age range, 26–81 (mean, 71)
years], 9 synovial sarcoma [2 men, 7 women; age range, 20–88 (mean,
43) years], 10 liposarcoma [4 men, 6 women; age range, 20–74 (mean,
55) years], 10 leiomyosarcoma (4 men, 6 women; age range, 25–83
(mean, 62) years], 11 chondrosarcoma [7 men, 4 women; age range,
12–63 (mean, 42) years] and 5 osteosarcoma [5 women; age range,
14–57 (mean, 56) years], diagnosed at the Department of Pathology,
Fukuoka University, Japan.
Immunohistochemistry
Immunohistochemical staining was performed using
4-μm thick paraffin-embedded sections, were deparaffinized
and heated in a microwave oven (700 W) for 10 min to expose
antigens in 10 mM Na-citrate buffer (pH 6.0; for CD13) or 1 mM
EDTA/10 mM Tris-HCl buffer (pH 9.0; for FU3). The exposed antigen
was detected using the labeled streptavidin-biotin method. The
reaction was identified with naphthol AS-BI phosphate and
counterstained with Mayer’s hematoxylin.
The staining results were evaluated
semiquantitatively by two independent observers. Immunostaining was
considered negative if stained tumor cells were <10%. In
specimens considered positive, staining of the tumor was
quantitated on a scale from 1–4 based on the percentage of positive
tumor cells. The scale was structured as follows: 1+, 10–25% of
cells positive; 2+, 25–50% of cells positive; 3+, 50–75% of cells
positive; 4+, >75% of cells positive. In specimens considered
high expression cases, staining of the tumor was quantified on
scales 3+ and 4+.
Small interfering RNA (siRNA)
SFT8503 cells were grown to sub-confluence and
treated with small interfering RNA (siRNA) for CD13 (Smart Pool,
Dharmacon, Chicago, IL) or control siRNA (B-Bridge International,
Sunnyvale, CA) using Lipofeamine 2000 (Invitrogen, Carlsbad, CA)
accroding to the manufacturer’s instructions.
In vitro invasion assay
In vitro Matrigel invasion assay was
performed by using 24-well Chemotaxicell chambers (pore size, 8
μm, Kubota Co., Tokyo, Japan) on 24-well culture plate. The
upper and lower side of each chamber was coated with Matrigel (25
μg/filter, BD Biosciences). Hepatocyte growth factor (HGF;
Peprotech Inc. Rocky Hill, NJ) was used as a chemoattractant. After
incubation of cells for 72 h, the filters were fixed with formalin
and stained with hematoxylin and the total number of cells that had
invaded the Matrigel-coated filter were counted.
Results
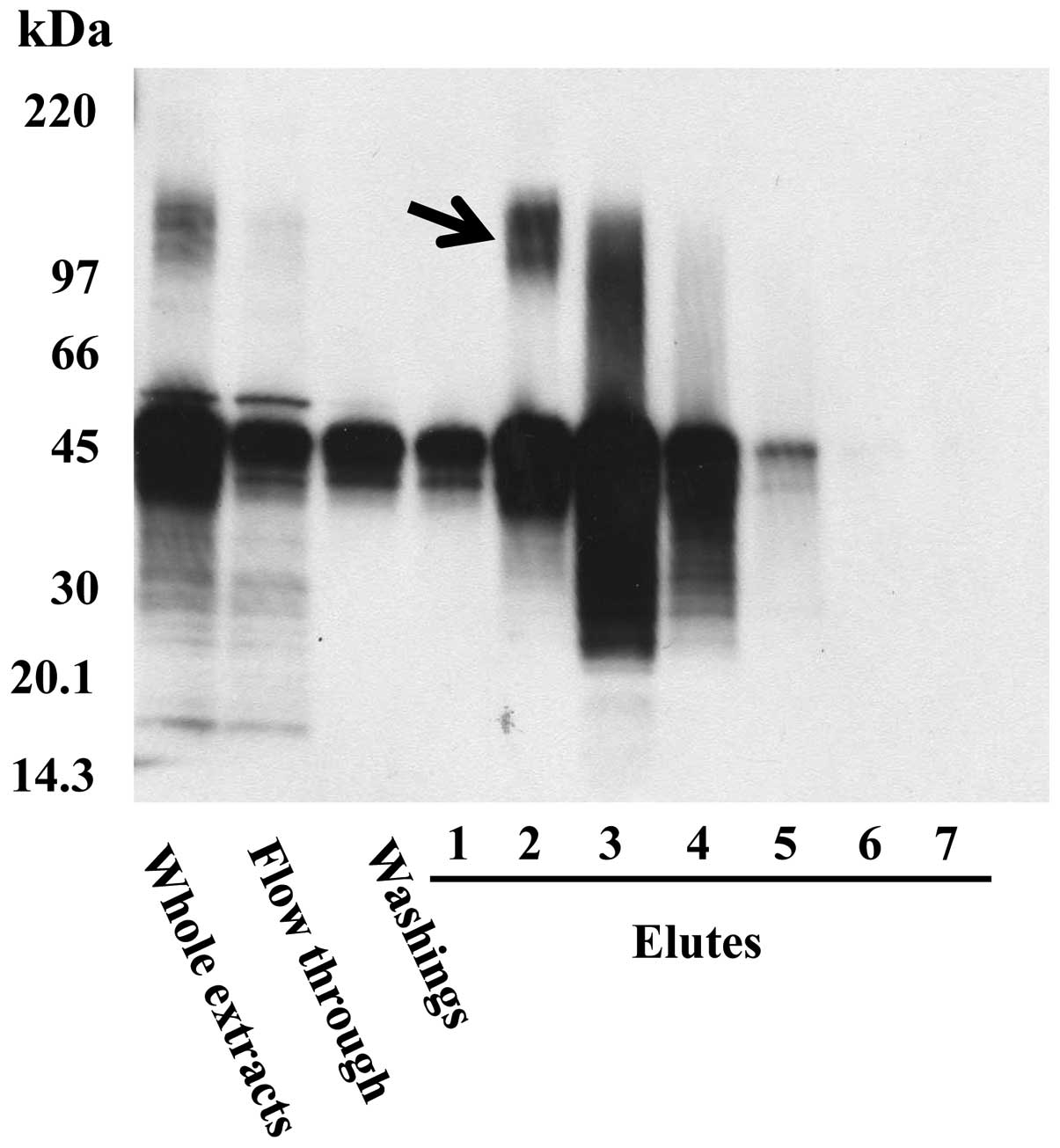
Immunoaffinity chromatography using FU3
antibody
Extracted proteins from SFT8503 MFH cells were
purified using immunoaffinity chromatography with FU3 antibody,
followed by immunoblotting. The N-terminal amino acid sequencing of
the 150-kDa band (Fig. 1, arrow)
revealed A-K-G-F-Y-I-S-K-S-L, which is identical to that of
aminopeptidase N (APN)/CD13, known as an important zinc-dependent
metallo-exopeptidase.
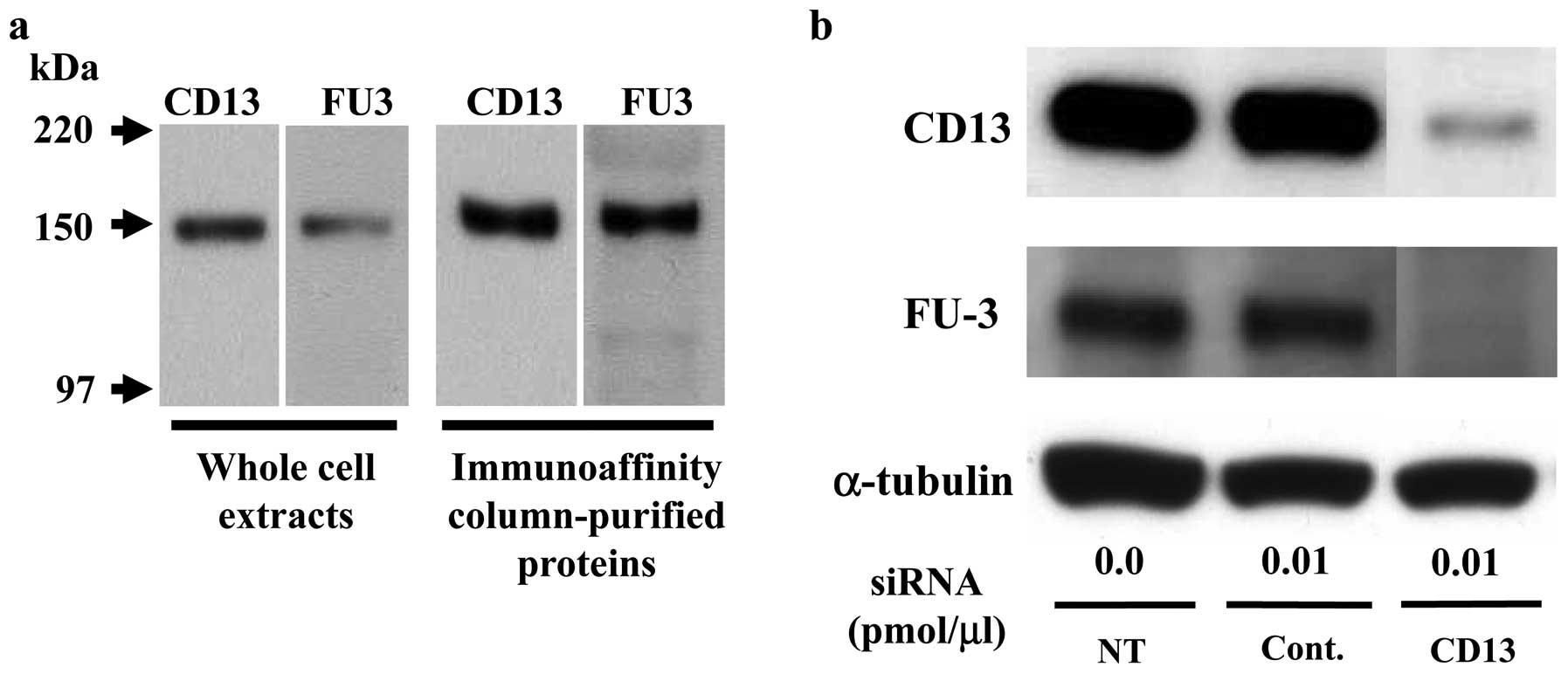
The FU3 recognizing antigen is possibly
aminopeptidase N (CD13)
To confirm the identity of FU3-reactive antigen as
APN/CD13 we used immunoblotting and siRNA methods. First, by
immunoblotting SFT8503 MFH whole cell extracts and an eluted
protein from immunoaffinity chromatography columns, both CD13 and
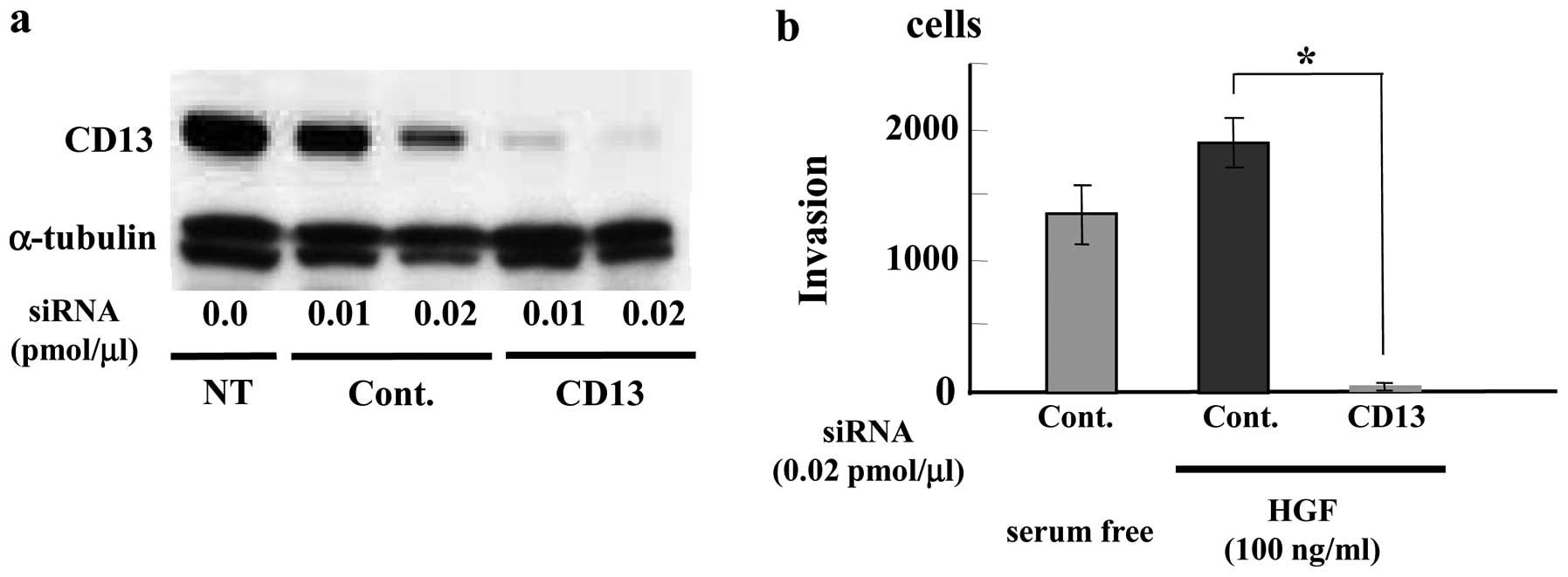
FU3 antibodies recognized an identical 150-kDa band (Fig. 2a).
Second, CD13-specific siRNA treatment (0.01
pmol/μl) of SFT8503 MFH cells caused significant
downregulation of both CD13 and FU3-reactive 150 kDa proteins
(Fig. 2b.). In view of the
evidence, we considered that the FU3 reactive antigen was identical
to APN/CD13.
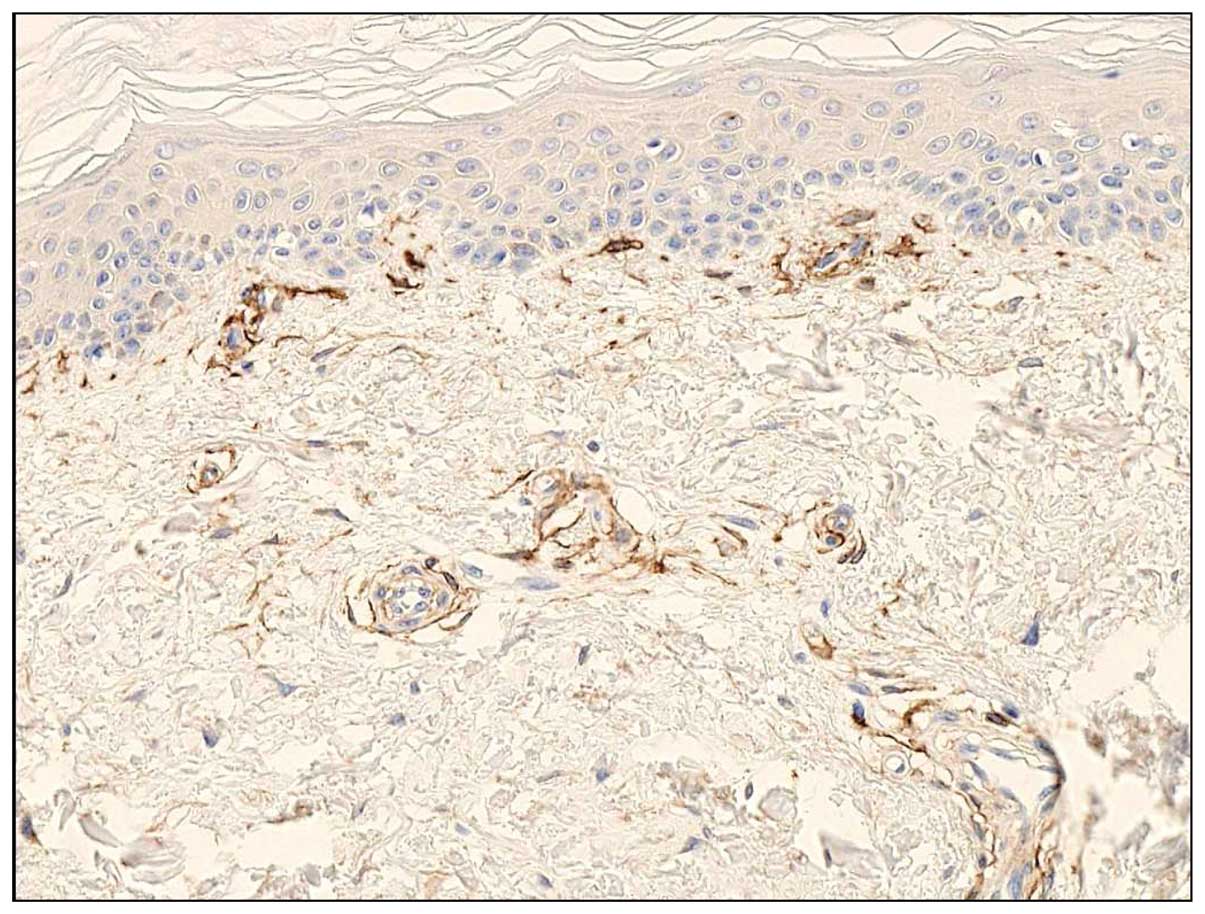
CD13 expression in normal skin
Immunohistochemically, CD13 antibody stained
perivascular mesenchymal cells, represented by small spindle or
polygonal cells around small blood vessels, in normal skin
(Fig. 3). A small number of
fibroblasts also reacted with CD13 antibody; however, the
stratified squamous epithelium and endothelial cells were not CD13
antibody-reactive. These findings were similar to those of FU3
reactive cells (3–6), supporting of the identification of
FU3 antigen as APN/CD13.
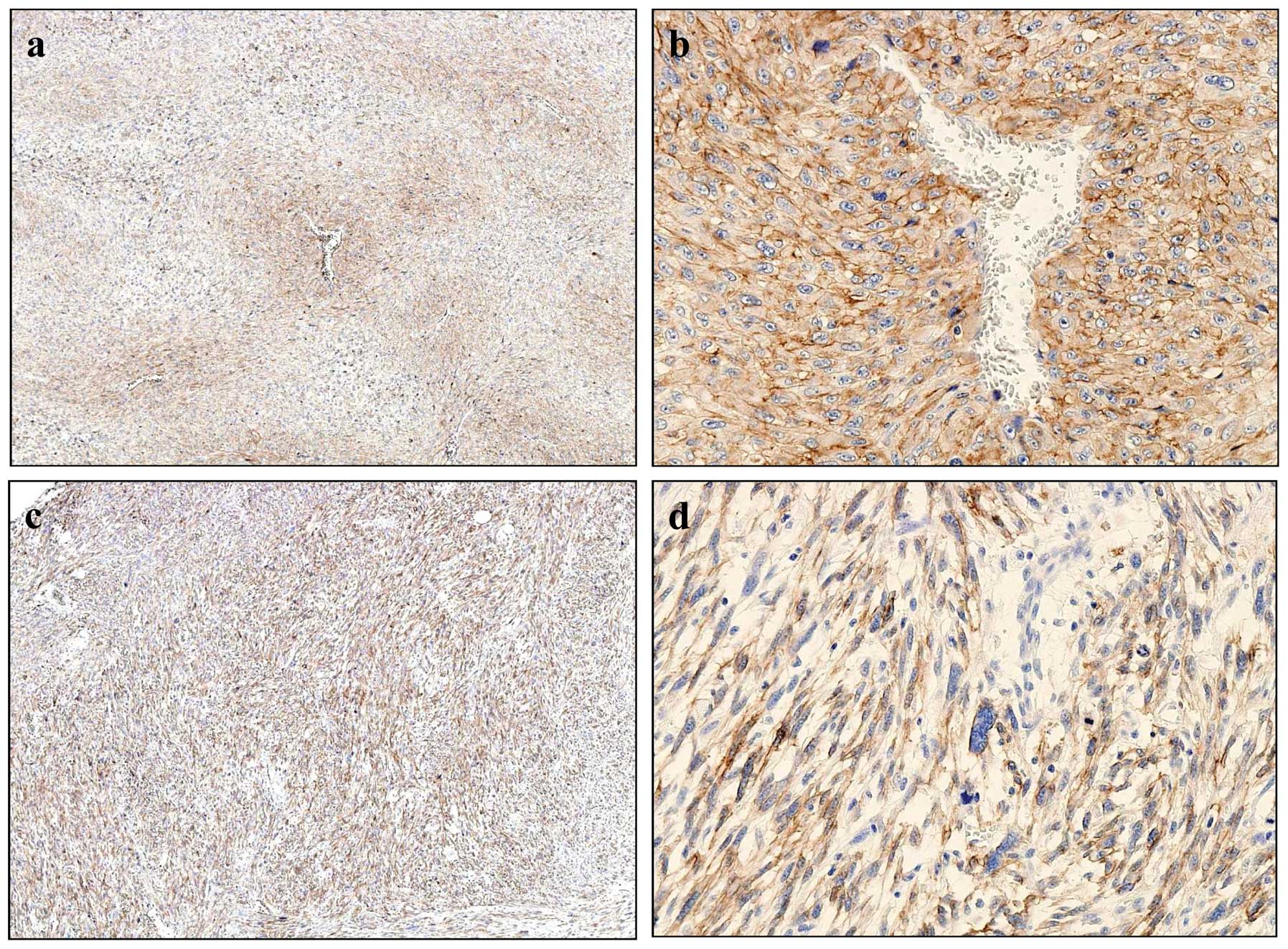
CD13 expression in MFH and soft tissue
tumors
Expression of CD13 was examined
immunohistochemically in 70 soft tissue tumors wich included 25 MFH
and 45 other soft tissue tumors (Fig.
4 and Table I). Twenty cases
of MFH (80%) were positive for CD13 and of these, there were 17
(68%) high expression cases. In MFH, CD13 expression pattern were
classified into two types; perivascular (Fig. 4a and b) and diffuse types (Fig. 4c and d). Tissues of the
perivascular type exhibited intense cytoplasmic and membrane
staining of CD13 (Fig. 4b). In the
diffuse type, almost all MFH cells had positively stained
cytoplasms (Fig. 4d). Positive
reactivity with CD13 was observed in several synovial sarcoma,
leiomyosarcoma and osteosarcoma, but high-expression was found only
in two cases of leiomyosarcoma (Table
I).
 | Table I.CD13 expression in soft tissue
tumors. |
Table I.
CD13 expression in soft tissue
tumors.
| No. of cases | Positive cases
(%) | High expression cases
(>50% of cells) |
|---|
| MFH | 25 | 20 (80) | 17 (68%) |
| Synovial sarcoma | 9 | 5 (56) | 0 (0%) |
| Liposarcoma | 10 | 0 (0) | 0 (0%) |
| Leiomyosarcoma | 10 | 3 (30) | 2 (20%) |
| Chondrosarcoma | 11 | 0 (0) | 0 (0%) |
| Osteosarcoma | 5 | 5 (100) | 0 (0%) |
Inhibition of MFH (SFT8503) cell invasion
by CD13 siRNA
To investigate a biological role for APN/CD13 in
MFH, we examined effect of CD13 siRNA treatment on MFH cell
invasion. We used HGF as a chemoattractant factor because
overexpression of HGF has been reported in MFH (8,9).
Treatment of SFT8503 MFH cells with CD13 siRNA (0.01 and 0.02
pmol/μl) downregulated CD13 expression in a dose-dependent
manner at 48 h (data not shown) and at 72 h (Fig. 5a) after transfection. HGF (100
ng/ml) induced greater SFT8503 cell invasion than serum-free
medium. In the presence of CD13-targeted siRNA, HGF-stimulated MFH
cell invasion was significantly attenuated as compared with that
observed with control siRNA (Fig.
5b).
Discussion
Development of an MFH recognizing FU3 antibody
provided some important findings, especially on the cellular origin
of MFH (4–6). The antibody, however, has not been
widely used, one of the reasons may be that FU3 antibody is
available only on frozen tissue sections but not on
paraffin-embedded specimens. In this study, we demonstrated that
the FU3 antibody recognizies an antigen identical to APN/CD13 using
immunoaffinity chromatography and direct N-terminal amino acid
sequencing. Immunohistochemically, greater amounts of APN/CD13 were
observed more frequently in MFH tissues as compared with that
observed in other sarcomas in paraffin-embedded specimens.
Moreover, MFH cell invasion was significantly suppressed by
transfection of APN/CD13 siRNA. The results from this study may
point toward the use of APN/CD13, the FU3 antigen, as an important
biomarker in the diagnosis and treatment of patients with MFH.
APN/CD13, a 150-kDa metalloproteinase, is a
multifunctional cell surface aminopeptidase. The human APN gene has
been mapped to chromosome 15q25-26. APN/CD13 expression has been
reported in hematopoietic cells of myeloid origin, fibroblasts,
synaptic membranes in the central nervous system and epithelial
cells of liver, kidney and intestine (10,11).
High expression levels of APN/CD13 has been detected in various
epithelial tumors and its expression correlates with increased
clinical malignant behavior in pancreatic carcinoma and in colon
and non-small lung cancer (12–14).
There is little information on the expression of
APN/CD13 and its role in MFH. An immunohistochemical study of MFH
demonstrated a positive reaction to APN/CD13 in six of ten cases
(15). Another report showed four
cell lines derived from an MFH expressed APN/CD13, using flow
cytometric analysis (16).
Immunohistochemically, APN/CD13 antibody showed strong reactivity
with perivascular mesenchymal cells in the normal skin tissue and
with MFH cells, to a similar extent seen with FU3. These findings
lend support to our result that the FU3 recognizing antigen is
identical to APN/CD13. Although we can not directly compare our
results with those of previous results of APN/CD13 expression
(15), owing to the differences in
assessment methods, our findings were roughly consistent with
previous studies on other soft tissues sarcomas. All our five cases
of osteosarcoma were positive for APN/CD13, but no high expression
cases were observed. Only two cases of leiomyosarcoma showed high
expression. APN/CD13 immunostaining may be applicable to narrow the
differential diagnosis of soft tissue sarcomas to MFH.
Our immunohistochemical study in normal skin
demonstrated APN/CD13 expression in the perivascular cells and in
some dermal fibroblasts. APN/CD13 expression in perivascular cells
has also been reported in lung tissue; the majority of these
CD13-positive cells were slender perivascular fibroblastic cells
(17). Dermal fibroblasts express
APN/CD13 to a relatively great degree in vitro (18,19).
On the basis of immunoreactivity, MFH cells may have intimate
relationship with perivascular cells and fibroblasts.
APN/CD13 might participate in tumor progression by
regulating processes such as tumor invasion and angiogenesis
(12,20–24).
Our study, however, is the first to show that downregulation of
APN/CD13 expression leads to marked suppression of invasion by MFH
cells, although this is based on only in vitro data. In a
previous study with osteosarcoma cell lines, CD13 siRNA treatment
caused reduced cellular attachment to and increased proteolytic
degeneration of the extracellular matrix (25). Anti-APN/CD13 antibody reduced the
migratory activity of human dermal fibroblasts (19). These reduced activities may also
occur in MFH cells and these possibilities are now under
investigation in our laboratory.
Since the discovery in 1976 of the first APN
enzymatic inhibitor bestatin, many APN inhibitors have been
developed (26). Bestatin is
already used clinically for the treatment of adult acute
non-lymphocytic leukemia via peroral administration.
Bestatin-mediated suppression of APN/CD13 activity in an
APN/CD13-expressing ovarian carcinoma cells led to reduced
migration, proliferation and peritoneal dissemination of tumor
cells in a mouse model, which resulted in prolonged survival
(27). Bestatin may also represent
a new approach for improving the therapeutic efficacy of
radiotherapy for uterine cervical carcinoma (28) and for enhancing paclitaxel
chemosensitivity in ovarian carcinoma (29). Recently, some novel potent APN/CD13
inhibitors have also been reported, several of which show better
inhibitory activity than bestatin against APN on human carcinoma
cells (30,31).
In conclusion, our study indicates that APN/CD13 may
be useful for diagnosing MFH and importantly, might serve as a new
molecular target for therapy for patients with MFH.
Acknowledgements
We acknowledge the expert technical
assistance of Ms. M. Onitsuka, M. Ishiguro and C. Fujita in
immunohisto-chemical staining and in vitro studies.
References
|
1.
|
Gustafson P: Soft tissue sarcoma.
Epidemiology and prognosis in 508 patients. Acta Orthop Scand
(Suppl). 259:1–31. 1994.PubMed/NCBI
|
|
2.
|
Fletcher CD: Pleomorphic malignant fibrous
histiocytoma: fact or fiction? A critical reappraisal based on 159
tumors diagnosed as pleomorphic sarcoma. Am J Surg Pathol.
16:213–228. 1992. View Article : Google Scholar
|
|
3.
|
Isayama T, Iwasaki H and Kikuchi M: The
origin of malignant fibrous histiocytoma immunohistochemical
analysis with monoclonal antibodies. Medical Bulltein of Fukuoka
University. 14:191–203. 1987.
|
|
4.
|
Iwasaki H, Isayama T, Johzaki H and
Kikuchi M: Malignant fibrous histiocytoma. Evidence of perivascular
mesenchymal cell origin immunocytochemical studies with monoclonal
anti-MFH antibodies. Am J Pathol. 128:528–537. 1987.
|
|
5.
|
Iwasaki H, Yoshitake K, Ohjimi Y, et al:
Malignant fibrous histiocytoma. Proliferative compartment and
heterogeneity of ‘histiocytic’ cells. Am J Surg Pathol. 16:735–745.
1992.PubMed/NCBI
|
|
6.
|
Iwasaki H, Isayama T, Ohjimi Y, et al:
Malignant fibrous histiocytoma. A tumor of facultative histiocytes
showing mesenchymal differentiation in cultured cell lines Cancer.
69:437–447. 1992.
|
|
7.
|
Ellis SM, Nabeshima K and Biswas C:
Monoclonal antibody preparation and purification of a tumor cell
collagenase-stimulatory factor. Cancer Res. 49:3385–3391.
1989.PubMed/NCBI
|
|
8.
|
Yamamoto T, Marui T, Akisue T, et al:
Coexpression of hepatocyte growth factor and its receptor c-Met
correlates with high MIB-1 proliferative index in malignant fibrous
histiocytoma. Pathol Res Pract. 200:397–402. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Wallenius V, Hisaoka M, Helou K, et al:
Overexpression of the hepatocyte growth factor (HGF) receptor (Met)
and presence of a truncated and activated intracellular HGF
receptor fragment in locally aggressive/malignant human
musculoskeletal tumors. Am J Pathol. 156:821–829. 2000. View Article : Google Scholar
|
|
10.
|
Luan Y and Xu W: The structure and main
functions of aminopeptidase N. Curr Med Chem. 14:639–647. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Zhang X and Xu W: Aminopeptidase N
(APN/CD13) as a target for anti-cancer agent design. Curr Med Chem.
15:2850–2865. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Ishii K, Usui S, Sugimura Y, et al:
Aminopeptidase N regulated by zinc in human prostate participates
in tumor cell invasion. Int J Cancer. 92:49–54. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Ikeda N, Nakajima Y, Tokuhara T, et al:
Clinical significance of aminopeptidase N/CD13 expression in human
pancreatic carcinoma. Clin Cancer Res. 9:1503–1508. 2003.PubMed/NCBI
|
|
14.
|
Tokuhara T, Hattori N, Ishida H, et al:
Clinical significance of aminopeptidase N in non-small cell lung
cancer. Clin Cancer Res. 12:3971–3978. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Mechtersheimer G and Moller P: Expression
of aminopeptidase N (CD13) in mesenchymal tumors. Am J Pathol.
137:1215–1222. 1990.PubMed/NCBI
|
|
16.
|
Mori A, Tagawa T, Kamei T, Murata T, Inui
M and Ohse S: Characterization of four cell lines derived from a
human malignant fibrous histiocytoma of the maxillary sinus. Oral
Oncol. 37:527–536. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Ichimura E, Yamada M, Nishikawa K, Abe F
and Nakajima T: Immunohistochemical expression of aminopeptidase N
(CD13) in human lung squamous cell carcinomas, with special
reference to Bestatin adjuvant therapy. Pathol Int. 56:296–300.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Gabrilovac J, Cupic B, Breljak D, Zekusic
M and Boranic M: Expression of CD13/aminopeptidase N and
CD10/neutral endopeptidase on cultured human keratinocytes. Immunol
Lett. 91:39–47. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Lai A, Ghaffari A and Ghahary A:
Inhibitory effect of anti-aminopeptidase N/CD13 antibodies on
fibroblast migration. Mol Cell Biochem. 343:191–199. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Mishima Y, Terui Y, Sugimura N, et al:
Continuous treatment of bestatin induces anti-angiogenic property
in endothelial cells. Cancer Sci. 98:364–372. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Pasqualini R, Koivunen E, Kain R, et al:
Aminopeptidase N is a receptor for tumor-homing peptides and a
target for inhibiting angiogenesis. Cancer Res. 60:722–727.
2000.PubMed/NCBI
|
|
22.
|
Saiki I, Fujii H, Yoneda J, et al: Role of
aminopeptidase N (CD13) in tumor-cell invasion and extracellular
matrix degradation. Int J Cancer. 54:137–143. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Fujii H, Nakajima M, Saiki I, Yoneda J,
Azuma I and Tsuruo T: Human melanoma invasion and metastasis
enhancement by high expression of aminopeptidase N/CD13. Clin Exp
Metastasis. 13:337–344. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Wulfanger J, Schneider H, Wild P, et al:
Promoter methylation of aminopeptidase N/CD13 in malignant
melanoma. Carcinogenesis. 33:781–790. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Kido A, Krueger S, Haeckel C and Roessner
A: Inhibitory effect of antisense aminopeptidase N (APN/CD13) cDNA
transfection on the invasive potential of osteosarcoma cells. Clin
Exp Metastasis. 20:585–592. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Umezawa H, Aoyagi T, Suda H, Hamada M and
Takeuchi T: Bestatin, an inhibitor of aminopeptidase B, produced by
actinomycetes. J Antibiot (Tokyo). 29:97–99. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Terauchi M, Kajiyama H, Shibata K, et al:
Inhibition of APN/CD13 leads to suppressed progressive potential in
ovarian carcinoma cells. BMC Cancer. 7:1402007. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Tsukamoto H, Shibata K, Kajiyama H,
Terauchi M, Nawa A and Kikkawa F: Aminopeptidase N (APN)/CD13
inhibitor, Ubenimex, enhances radiation sensitivity in human
cervical cancer. BMC Cancer. 8:742008. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Yamashita M, Kajiyama H, Terauchi M, et
al: Involvement of aminopeptidase N in enhanced chemosensitivity to
paclitaxel in ovarian carcinoma in vitro and in vivo. Int J Cancer.
120:2243–2250. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Zhang X, Zhang L, Zhang J, et al: Design,
synthesis and preliminary activity evaluation of novel
3-amino-2-hydroxyl-3-phenylpropanoic acid derivatives as
aminopeptidase N/CD13 inhibitors. J Enzyme Inhib Med Chem.
28:545–551. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Su L, Jia Y, Zhang L, Xu Y, Fang H and Xu
W: Design, synthesis and biological evaluation of novel amino acid
ureido derivatives as aminopeptidase N/CD13 inhibitors. Bioorg Med
Chem. 20:3807–3815. 2012. View Article : Google Scholar : PubMed/NCBI
|