Introduction
Ovarian cancer has the highest mortality rate of all
gynecologic tumors and represents the fifth leading cause of cancer
death for women in the United States. More than 70% of patients
present with disease that has spread beyond the ovaries (1). Ovarian cancer is a highly metastatic
disease characterized by intraperitoneal spread (2,3). As
disseminated ovarian cancer is usually confined to the surface
epithelium within the peritoneal cavity, processes such as cell
adhesion, migration, intraperitoneal invasion and proliferation
likely play a predominant role in ovarian cancer pathobiology
(4). Therefore, the development of
more effective treatments for inhibition of invasion/metastasis is
urgently needed in patients with advanced ovarian cancer.
Molecular characterization of ovarian cancer makes
it possible to develop multiple therapeutic approaches targeting
various aspects of the malignancy and to expand the currently
available treatment options (5).
These include inhibitors of poly (ADP-ribose) polymerase (PARP)
(6–8), histone deacetylases (HDACs) (9–11),
ERBB family receptors (12,13),
heat shock proteins (HSPs) (14),
mechanistic target of rapamycin (mTOR)/ hypoxia inducible factor
(HIF) (12,15,16)
and vascular endothelial growth factor (VEGF) (8,11,12).
Some of these approaches might inhibit the migration and invasion
of ovarian cancer cells through direct and/or indirect pathways,
and their application to management of patients with advanced-stage
ovarian cancer is anticipated. However, it has been shown that the
efficacy of each agent is limited to a subset of patients
exhibiting abnormalities specific to each target molecule.
Intensive searches for other candidate molecules involved in the
metastatic process are continuing, with the aim of utilizing them
for personalized therapy of patients with advanced ovarian cancer
(8,12,17).
Small and large non-coding RNAs (ncRNAs) contribute
to acquisition of aggressive tumor behavior in a wide range of
human malignancies. miR-10b is a particularly interesting candidate
given its close correlation with metastatic behavior in human
malignancies including breast (18) and gastric cancers (19), renal cell (20,21)
and urothelial carcinomas (22),
and glioblastoma (23,24). In mammary epithelial cells and
breast cancer cells, miR-10b can directly suppress the translation
of homeobox D10 (HOXD10), an mRNA encoding a transcriptional
repressor that inhibits expression of several genes involved in
cell migration and extracellular matrix remodeling, such as RHOC
and MT1-MMP (MMP14) (18,24,25).
Interestingly, HOXD10 is not only targeted by miR-10b, but also by
a long ncRNA termed HOX transcript antisense RNA (HOTAIR), which
has also been shown to promote breast cancer metastasis. HOTAIR
reprograms the chromatin state, causing increased polycomb
repressive complex-2 (PRC2) occupancy on promoters of genes that
inhibit breast cancer progression, including HOXD10 (26). HOXD10 is a member of the Abd-B
homeobox family encoding a protein with a homeobox DNA-binding
domain, and its expression is reduced in both breast and
endometrial tumors (27).
Overexpression of HOXD10 significantly impairs breast tumor cell
motility and invasiveness, indicating that HOXD10 may serve as a
tumor suppressor (28,29). Although the biological significance
of HOXD10 has not been well defined in ovarian cancer,
over-expression of MMP14 (30) and
RHOC (31), which are negatively
regulated by HOXD10, has been observed during progression of the
disease. If the miR-10b and/or HOTAIR/HOXD10/MMP14 and/or RHOC axis
is involved in the migration/invasion activity of ovarian cancer
cells, these would become good candidate target molecules for the
development of new personalized therapies for patients with ovarian
cancer.
Here, using cell biological methods, we first
investigated whether miR-10b and/or HOTAIR could regulate the
expression of HOXD10 protein, and thus affect the migration and
invasion activities of human ovarian cancer cell lines. We then
examined these abnormalities in samples from primary tumors.
Materials and methods
Cell lines and culture conditions
We used three clear cell adenocarcinoma cell lines
(JHOC-5, JHOC-7 and JHOC8), three serous adenocarcinoma cell lines
(JHOS-2, JHOS-3 and JHOS-4), one mucinous adenocarcinoma cell line
(JHOM-1) and OVCAR-3. They were obtained from Riken Cell Bank
(Tsukuba, Japan). All cell lines except OVCAR3 were maintained in
DMEM/F12 medium supplemented with 10 or 15% (JHOS3) fetal bovine
serum (FBS), 0.1 mM MEM-non-essential amino acid solution (Life
Technologies Inc., Gaithersburg, MD, USA) and 100 μl/ml
penicillin-streptomycin. OVCAR3 was maintained in RPMI-1640 (Life
Technologies) with 10% FBS. All tissue culture reagents were
obtained from Life Technologies.
Tissue samples
Tissue samples from 68 patients with ovarian cancer
and 10 patients with benign ovarian cyst were used for
immunohistochemistry and/or real-time quantitative PCR for miR-10b.
They were obtained from the Department of Obstetrics and
Gynecology, School of Medicine, Iwate Medical University, Morioka,
Japan, between 2005 and 2011. The surgical specimens had been fixed
in 10% buffered formalin solution and embedded in paraffin wax.
Permission for the study was obtained from the Institutional Review
Board (School of Medicine, Iwate Medical University, Iwate, Japan)
and written consent had been obtained from all patients before
surgery.
Transfection with pre-miRNA precursor and
siRNA
Cells were transfected with pre-miR-10b precursor
(50 nM) or pre-miR miRNA precursor negative control using
Lipofectamine™ 2000 (50 nM, Life Technologies) in accordance with
the manufacturer’s protocol. siRNA oligonucleotides (50 nM)
targeting HOTAIR were used as designed by Gupta et al (no. 1
and 2) (26) and predesigned
HOTAIR-specific siRNA (no. 3, n272224, Life Technologies) and
control non-specific human siRNA (Silencer Select Predesigned siRNA
Negative Control no. 2, 4390844, Life Technologies) using
Lipofectamine 2000 in accordance with the manufacturer’s
protocol.
RNA isolation and reverse
transcription
Human Ovarian Surface Epithelial Cell total RNA
(HOSEipC total RNA) was purchased from ScienCell Research
Laboratories (San Diego, CA, USA). Total RNA was extracted from
cells using TRIzol reagent in accordance with the manufacturer’s
protocol (Life Technologies), and transcribed to cDNA with a
SuperScript® III First-Strand Synthesis System (Life
Technologies). Otherwise, total RNA was extracted from 80-μm
sections of the formalin-fixed paraffin-embedded material samples
(FFPE) using a RecoverAll™ Total Nucleic Acid Isolation kit (Life
Technologies), and reverse-transcribed using a TaqMan microRNA
Transcription kit (Life Technologies) and TaqMan Universal PCR
Master mix II w/o UNG (Life Technologies) in accordance with the
manufacturer’s protocol.
Real-time quantitative PCR assay
For quantitative evaluation of the relevant mRNAs,
we used Custom TaqMan Gene Expression Assays (HOXD10,
Hs00157974_m1; MMP14, Hs01037009_g1; RHOC, Hs00747110_s1; HOTAIR,
Hs03296680_s1, Life Technologies) and an ABI PRISM 7500 (Life
Technologies). For normalization of the target,
glyceraldehydede-3-phosphate dehydrogenase (GAPDH, Life
Technologies) was used as an internal control. Triplicate reactions
were run per sample, and average fold differences were calculated
by normalizing the relative expression (ΔΔCt values) to
the User Bulletin no. 2 (Life Technologies).
miRNA detection
The relative expression levels of miR-10b were
measured by a two-step TaqMan assay in accordance with the
manufacturer’s instructions. Reverse transcription of hsa-miR-10b
or the internal control, human U6 were carried out by a TaqMan
microRNA reverse transcription kit (Life Technologies). Then,
real-time PCR reactions were performed using standard
TaqMan® PCR reagents and TaqMan® MicroRNA
assays for hsa-miR-10b and U6 (Life Technologies). The expression
of miR-10b relative to U6 was determined using the ΔΔCt
method.
Western blot analysis
Seventy-two hours after transfection, nucleic and
cytoplasmic proteins were collected using NE-PER™ Nuclear and
Cytoplasmic Extraction Reagent (Pierce, Thermo Fisher Scientific
Inc., Rockford, IL, USA). Equal amounts of protein sample were
separated by 4–12% Nu-PAGE and transferred to PVDF membranes by
electroblotting. The primary antibodies used were anti-HOXD10
(ABE128; Millipore, Billerica, MA) and anti-MMP14 (ab3644; Abcam,
Cambridge, MA, USA), both diluted in immunoreaction enhancer
solution (Can Get Signal Solution1, Toyobo, Osaka, Japan). We also
used primary antibodies against RhoC (#3430; Cell Signaling,
Beverly, MA, USA), GAPDH (Clone 1D4; Covance, Princeton, NJ, USA)
and lamin B (sc-6217; Santa Cruz Biotechnology, Santa Cruz, CA,
USA). Signals were detected using an ECL Prime Western Blotting
Detection kit (GE Healthcare, Buckinghamshire, UK) and ChemidDoc
XRS (Bio-Rad Laboratories, Hercules, CA, USA). The intensity of the
detected signals was measured by using Image J (freely available
java-based public-domain image processing and analysis program
developed at the National Institutes of Health).
In vitro migration and invasion
assays
For Transwell migration assays, 2.5–5×104
cells were placed in the top chamber on a non-coated membrane
(24-well insert; pore size, 8 μm; BD Bioscience, San Jose,
CA, USA). For invasion assays, 2.5–5×104 cells were
placed in the top chamber on a Matrigel-coated membrane (24-well
insert; pore size, 8 μm; BD Bioscience). In both assays, the
cells were placed in medium containing 1% FBS, and medium
supplemented with 20% FBS was used as a chemoattractant in the
lower chamber. The cells were incubated for 72 h, and those that
did not migrate or invade through the pores were removed with a
cotton swab. Cells were fixed with methanol, stained with DAPI
(Dojindo Laboratories, Kumamoto, Japan) and counted. Individual
experiments had triplicate inserts, and five randomly selected
fields were counted per insert.
Immunohistochemistry
Four-micrometer-thick sections were cut from
formalin-fixed, paraffin-embedded samples, and stained with
hematoxylin and eosin. Serial sections were stained using the
avidin-biotin system and antigen retrieval methods on a Ventana
automated immunostainer with the Ventana immunohistochemistry
detection system (Ventana Medical Systems, Tucson, AZ, USA), in
accordance with the manufacturer’s manual. The primary antibody
used for MMP14 immunostaining was a rabbit polyclonal anti-MMP14
antibody (Abcam), and for HOXD10 immunostaining a mouse monoclonal
anti-HOXD10 antibody (Santa Cruz Biotechnology). Quantitative
comparative analysis of immunohistochemical staining was carried
out in each case. Two independent pathologists performed
quantitative assessment of immunohistochemical staining. For
HOXD10, staining in nuclei was graded as follows: 0, no
immunoreactive cells evident; 1, proportion of immunoreactive cells
<20%; 2, 20–70%; 3, >70%. For the final estimation of HOXD10
immunoreactivity, patients with a score of 0/1 were considered
negative, and those with a score of 2/3 as positive. MMP14
imunoreactivity was graded according to the number of
immunoreactive cells (proportion score) and staining intensity
(intensity score). In brief, proportion scores were graded as
follows: 0, no immunoreactive cells evident; 1, <20%; 2, 20–70%;
3, >70%. Intensity scores were graded as follows: 0, no
immunoreactivity; 1, staining intensity weak; 2, intermediate; and
3, strong. The finally estimated immunoreactivity was considered
negative if the sum of the proportion and intensity scores ranged
from 0 to 3 and positive if the sum ranged from 4 to 6.
Statistical analysis
Data were analyzed by the Mann-Whitney U test for
non-parametric samples or Fisher’s exact test. Differences at
p<0.05 were considered to be statistically significant.
Results
Expression of miR-10b, HOTAIR and HOXD10
protein in ovarian cancer cell lines
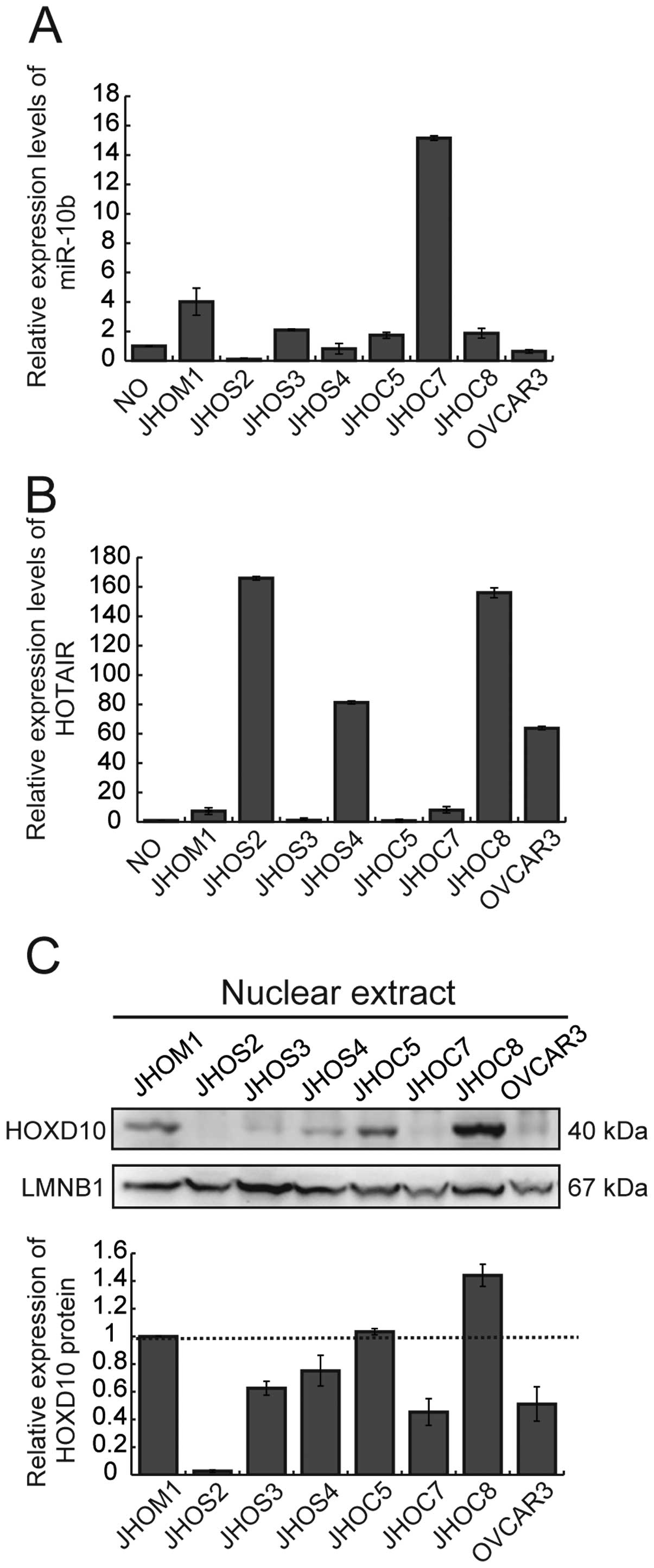
We first examined the expression of miR-10b and
HOTAIR in 8 ovarian cancer cell lines and RNAs extracted from human
normal ovary (Fig. 1A and B). The
expression level of both ncRNAs varied among the cell lines. Gain
of miR-10b was observed in 2 cell lines (JHOM1 and JHOC7) in
comparison with normal ovary (Fig.
1A). Overexpression of HOTAIR was observed in 4 cell lines
(JHOS2, JHOS4, JHOC8 and OVCAR3) in comparison with normal ovary
(Fig. 1B). We examined the
correlation between the expression level of miR-10b and/or HOTAIR,
and HOXD10 protein (Fig. 1C) in
these cell lines, but no significant relationship between the
expression of ncRNAs and HOXD10 protein was evident.
Next, using a cell biological approach, we
investigated whether ovarian cancers showed an inverse correlation
between the expression of miR-10b and/or HOTAIR, and HOXD10
protein, which has been proven previously in other tumors.
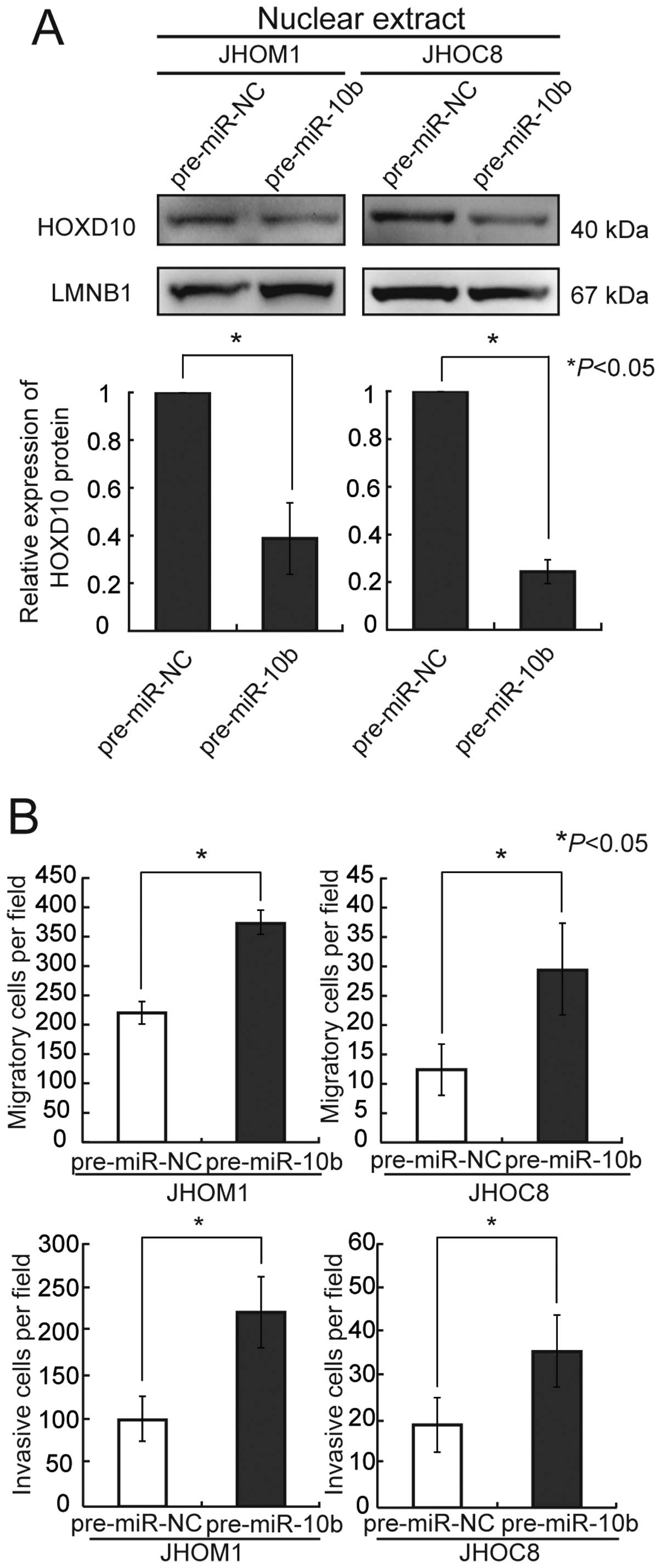
Overexpression of precursor miR-10b in
ovarian cancer cell lines
Overexpression of precursor miR-10b induced a
decrease of HOXD10 protein in the ovarian cancer cell lines JHOM1
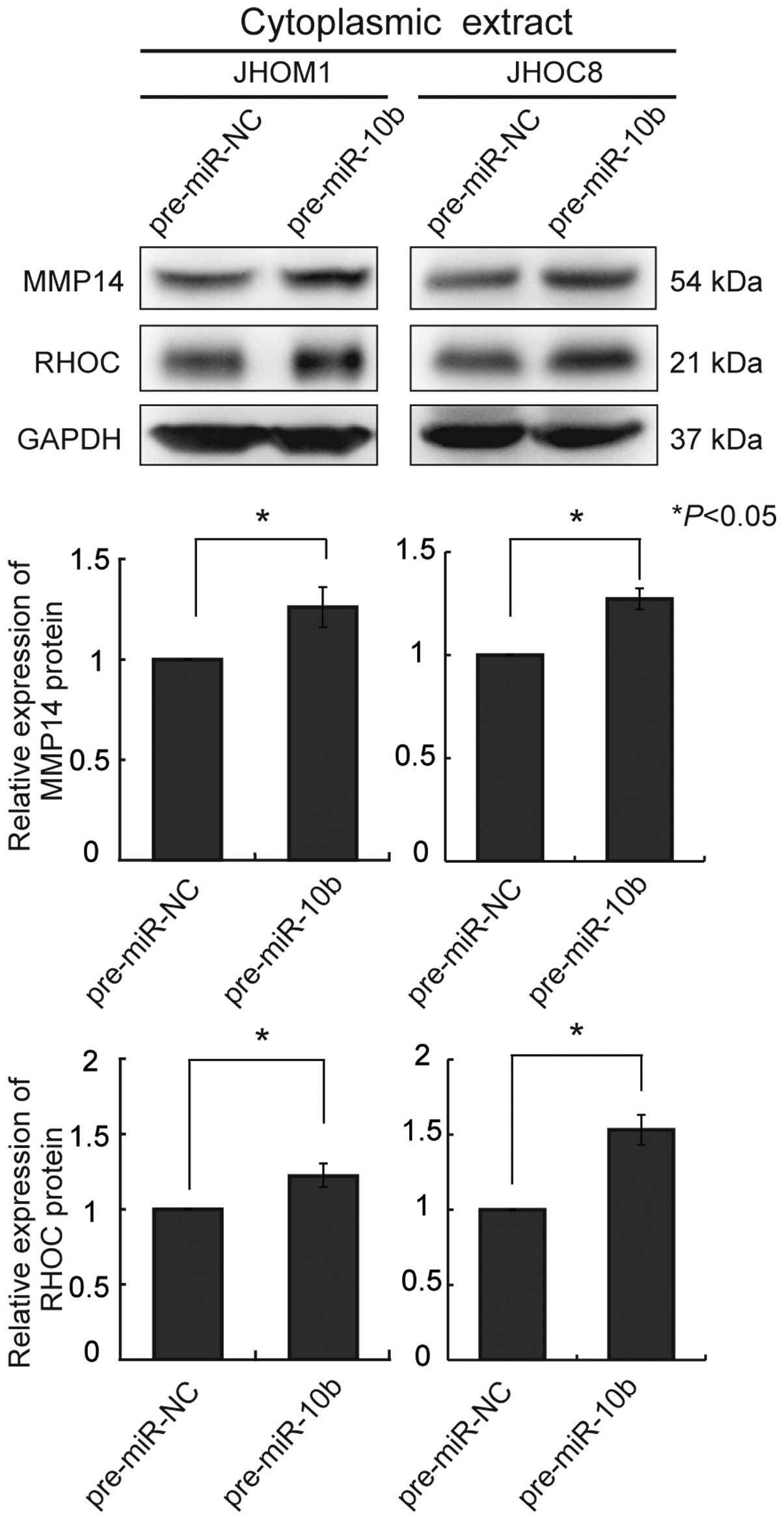
and JHOC8 (Fig. 2A). We also
examined cytoplasmic extracts for expression of MMP14 and RHOC
protein. A significant increase of both proteins was observed in
each cell line (Fig. 3). In these
cells, both migration and invasion activities were markedly
upregulated 72 h after transfection, in comparison with the
controls (Fig. 2B).
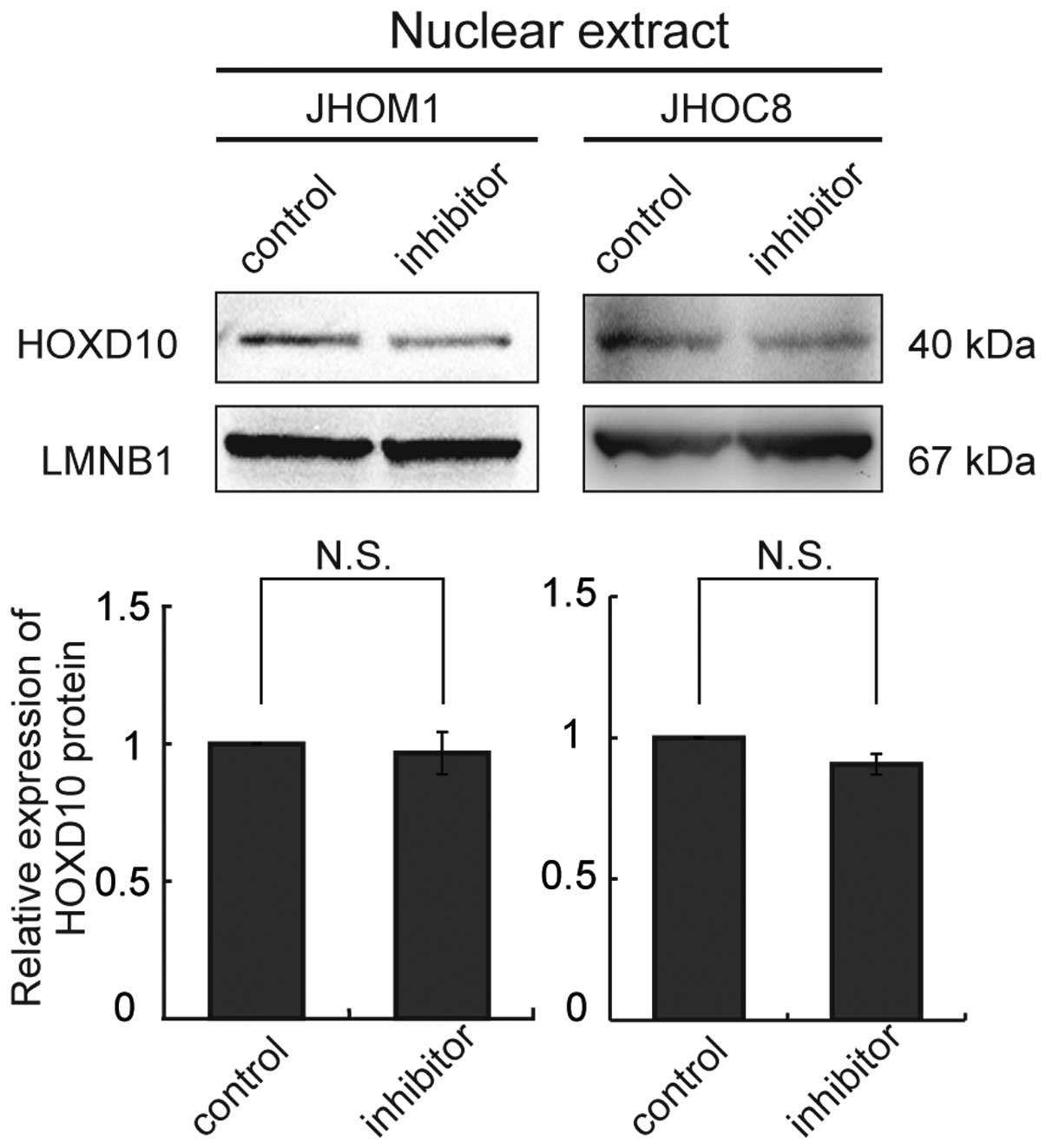
We used a locked nucleic acid (LNA; Exiqon, Vedbaek,
Denmark) approach to knockdown miR-10b in cells (JHOM1 and JHOC8)
showing relative overexpression of miR-10b, but this failed to
upregulate HOXD10 protein expression in these cell lines (Fig. 4).
Knockdown of HOTAIR in ovarian cancer
cell lines
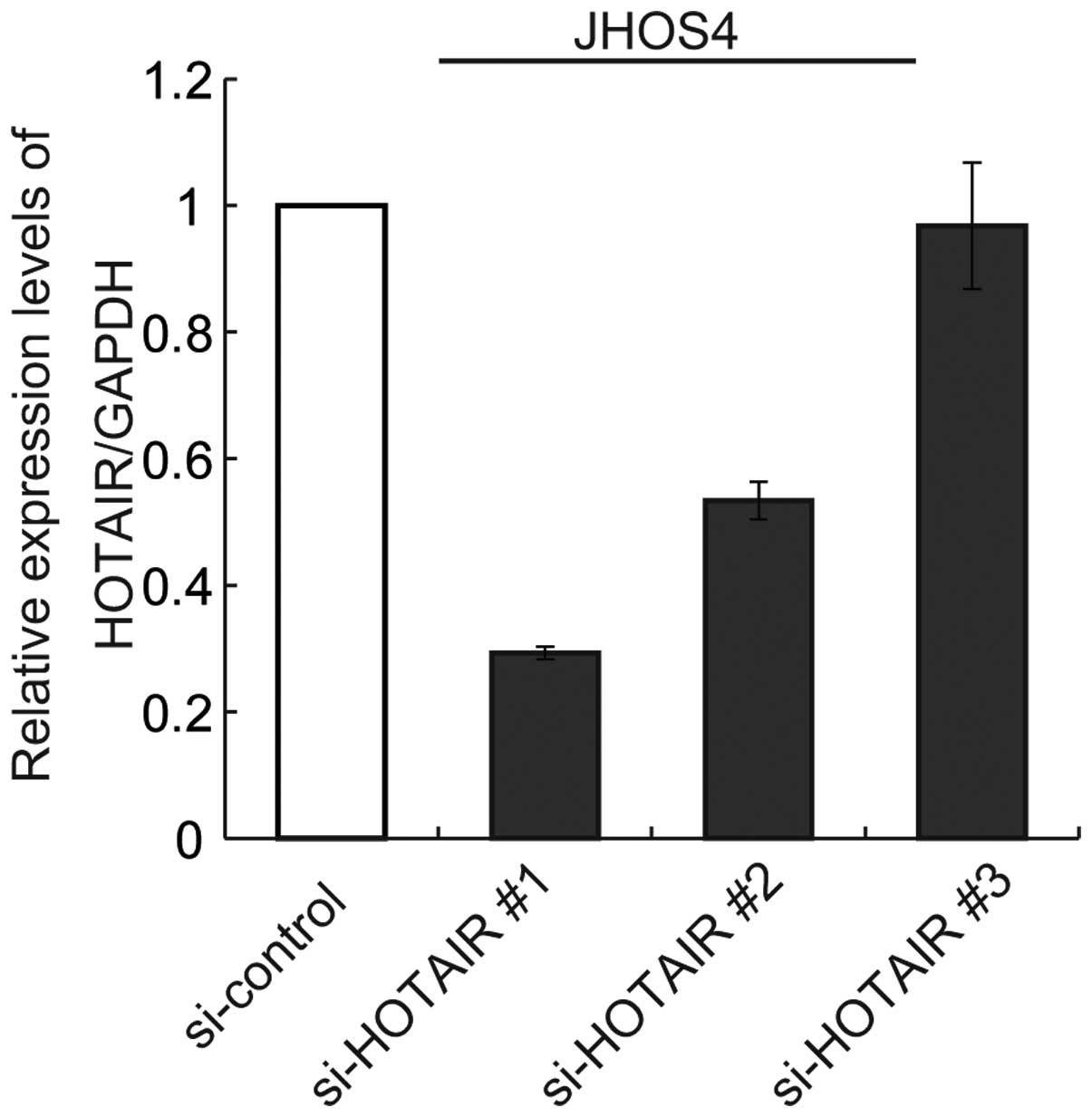
Next, we tested the knockdown efficiency of HOTAIR
expression using siRNAs (no. 1, 2 and 3). Only si-HOTAIR no. 1 was
able to achieve significant downregulation of HOTAIR expression by
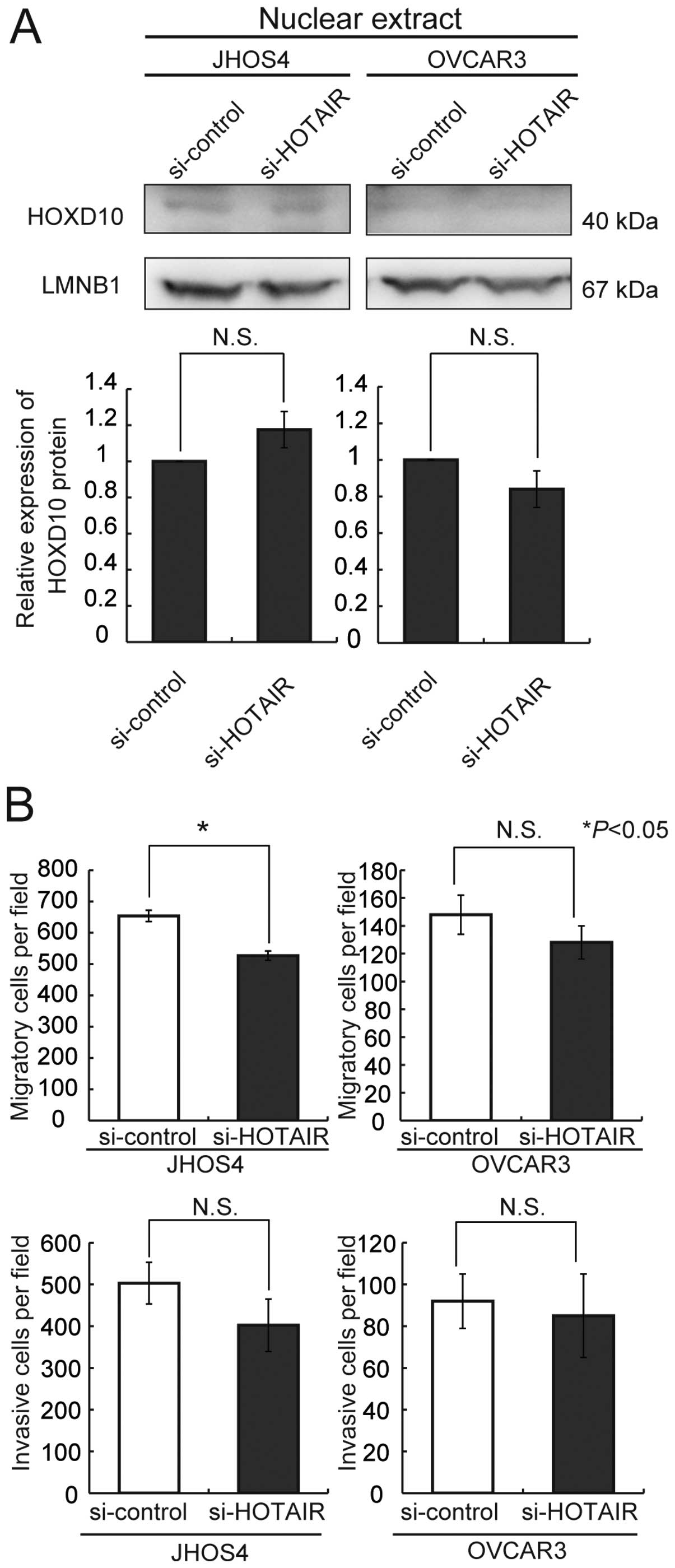
80% in comparison with the control siRNAin JHOS4 cells (Fig. 5). We then treated JHOS4 and OVCAR3,
which express high levels of HOTAIR, with si-HOTAIR no. 1. No
significant upregulation of HOXD10 protein expression was observed
in either of the cell lines after treatment with HOTAIR siRNA
(Fig. 6A). But, knockdown of
HOTAIR was significantly induced decreasing of only cell migration
activity of JHOS4 (Fig. 6B).
Although both cell lines showed a tendency for decreased migration
and/or invasion activities, the difference was not significant
relative to that achieved with control siRNA (Fig. 6B). Therefore, we concluded that the
decrease of the migration and invasion activities resulting from
treatment with HOTAIR siRNA might not be attributable to
alterations of HOXD10 protein.
Immunohistochemistry for HOXD10 and MMP14
proteins and its relationship with miR-10b expression
We next investigated miR-10b expression in primary
ovarian cancers. As in vitro data had suggested that
upregulation of miR-10b expression had induced a decrease of HOXD10
protein expression followed by overexpression of MMP14, we
immunohistochemically examined the expressions of HOXD10 and MMP14,
and also miR-10b, using real-time quantitative PCR in 68 patients
with primary ovarian cancer.
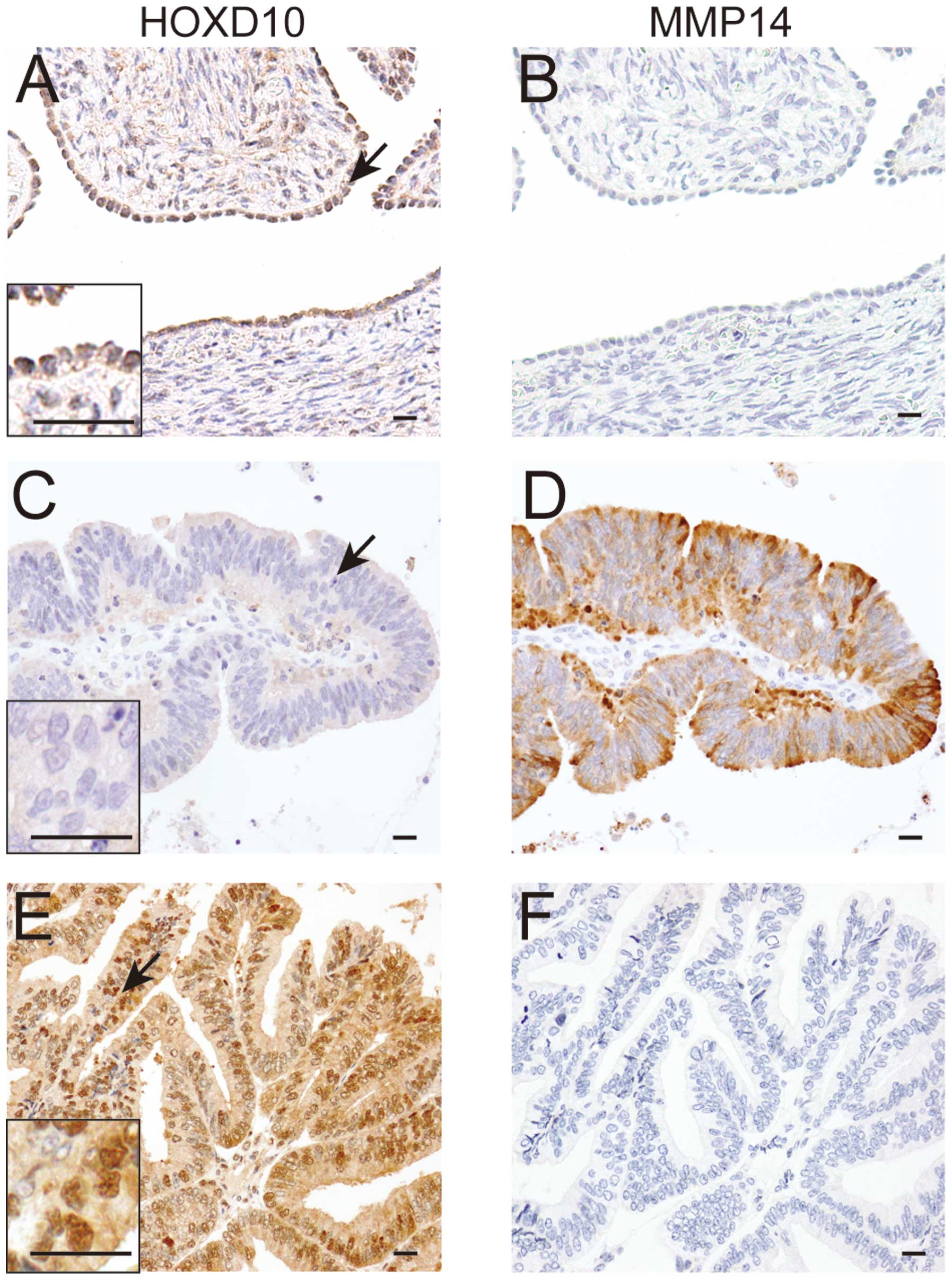
In normal ovaries, positive immunoreactivity for
HOXD10 protein was observed in the nuclei of mesothelial surface
lining cells, while MMP14 was negative (Fig. 7A and B). Positive signals for
HOXD10 and MMP14 proteins were observed in 47 (69%) and 25 (37%) of
68 patients. A significant inverse correlation between
immunoreactivity of HOXD10 and MMP14 was observed (Fig. 7C–F, Table I). We also examined the
correlations between HOXD10 and/or MMP14 immunoreactivity and
clinicopathological parameters in the patients, but no significant
relationship was found (Table II).
However, as 48 (70%) of the 68 studied patients had stage I/II
cancer, and various histological subtypes of ovarian cancer were
included, some bias was probably present, making them unsuitable
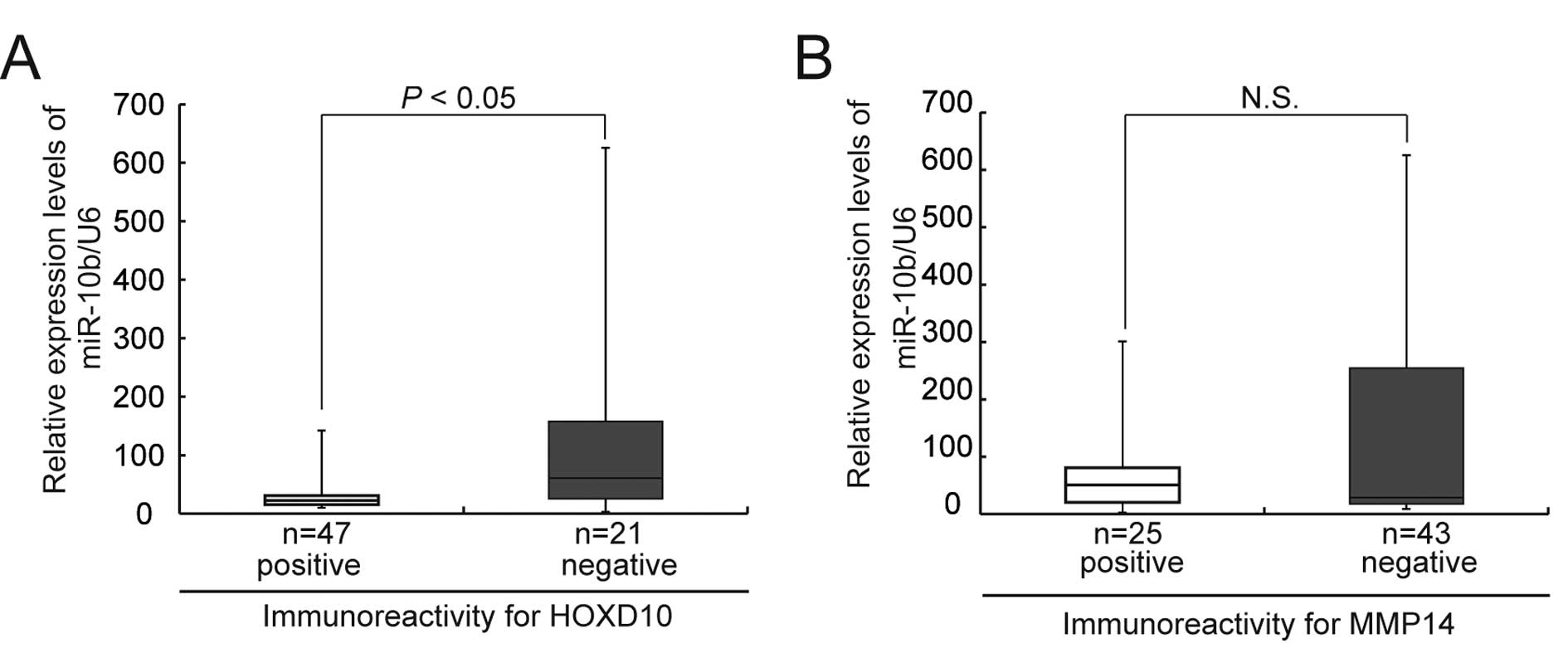
for statistical evaluation. The expression level of miR-10b was
inversely correlated with HOXD10 protein expression (Fig. 8A), but not with MMP14 expression
(Fig. 8B).
 | Table I.Immunohistochemistry for HOXD10 and
MMP14 in 68 ovarian cancers. |
Table I.
Immunohistochemistry for HOXD10 and
MMP14 in 68 ovarian cancers.
| MMP14
| P-value |
|---|
| Positive | Negative |
|---|
| HOXD10 | | | |
| Positive | 12 | 35 | 0.005 |
| Negative | 13 | 8 | |
 | Table II.Correlation between HOXD10 or MMP14
immunoreactivity and clinicopathological factors in 68 patients
with ovarian cancer. |
Table II.
Correlation between HOXD10 or MMP14
immunoreactivity and clinicopathological factors in 68 patients
with ovarian cancer.
| HOXD10
| MMP14
|
|---|
| Factor | Positive
(n=47) | Negative
(n=21) | P-value | Positive
(n=25) | Negative
(n=43) | P-value |
|---|
| Age | 53.6 (25–80) | 55 (34–80) | | 52.32 (34–80) | 55.18 (25–80) | |
| Stage | | | | | | |
| I | 32 | 12 | 0.353 | 18 | 26 | 0.244 |
| Other | 15 | 9 | | 7 | 19 | |
| Histology | | | | | | |
| Serous | 12 | 9 | 0.955 | 9 | 12 | 0.592 |
| Other | 35 | 12 | | 16 | 31 | |
| Node status | | | | | | |
| Positive | 6 | 3 | 0.557 | 3 | 6 | 0.647 |
| Negative | 36 | 17 | | 21 | 32 | |
| Not done | 5 | 1 | | 1 | 5 | |
Discussion
Many studies have investigated the association
between miRNA alterations and the biological characteristics of
ovarian cancer, and several candidate miRNAs have been nominated
(32–39). These miRNAs hold promise for the
detection of early-stage ovarian cancer, evaluation of
prognosis/drug resistance, and development of targeted cancer
treatment. In vitro and in vivo studies have
suggested that miR-7 (34,38), miR-21 (38), miR-34 (40,41),
miR-22 (39), and miR-429 (members
of the miR-200 family) (37) may
directly and/or indirectly control the expression of
metastasis-associated genes related to invasion/ migration and
epithelial-mesenchymal transition (EMT). The present study newly
highlighted miR-10b as a novel inducer of metastasis in ovarian
cancers.
As mentioned in the introduction, miR-10b is a
strong inducer of metastasis in breast cancer cells (18), and is also one of the most
upregulated miRNAs in human pancreatic adenocarcinomas (42,43)
and glioblastomas (24,44–46),
which are both highly metastatic and/or invasive cancers. miR-10b
suppresses the synthesis of the HOXD10 protein, permitting
expression of the pro-metastatic gene products RHOC urokinase
plasminogen activator receptor (uPAR), α3-integrin, and MMP14
(18,25). In addition, TWIST, a wel-known
transcriptional factor related to EMT, activates the transcription
of miR-10b by binding directly to an E-box sequence proximal to its
putative promoter (18). Although
miR-10b does not trigger EMT by itself, it might be required for
TWIST-induced cell motility and invasiveness in ovarian cancer
cells (18).
Evidence for a significant role of HOTAIR in
metastasis of several malignancies has been increasing. Since Gupta
et al (26) first
documented that loss of HOTAIR can inhibit the invasiveness of
cancer cells, particularly those possessing excessive PRC2
activity, similar evidence has been detected not only in breast
cancer (47) but also
gastrointestinal stromal tumors (48), and colorectal (49) and hepatocellular carcinomas
(50). HOTAIR reprograms the
chromatin state, causing increased PRC2 occupancy on promoters of
genes, including HOXD10 (26),
inhibiting breast cancer progression. In the present study,
although we failed to demonstrate that HOTAIR can repress HOXD10
expression in ovarian cancer, and did not examine the promoter
status of HOXD10, knockdown of HOTAIR appeared to decrease the
migration/invasion activity of ovarian cancer cells (Fig. 6B). The effect of HOTAIR on invasion
and migration might be exerted via pathways other than HOXD10, such
as MMP9 and VEGF (50).
Targeting of metastasis-promoting miRNAs is emerging
as a novel therapeutic strategy for cancer treatment. The effect of
the miR-10b antagomir has been reported in a 4T1 mouse mammary
tumor metastasis model (51,52).
Administration of miR-10b antagomirs to mice bearing highly
metastatic cells does not reduce primary mammary tumor growth, but
blocks dissemination of cancer cells from the primary tumor
(52).
Because the miR-10b antagomir would prevent
metastatic intraperitoneal dissemination, it would be worth
investigating whether it can be added as a prophylactic therapy for
patients with early-stage ovarian cancer. Moreover, further studies
using clinical samples would be warranted to clarify whether
quantification of miR-10b in intraperitoneal fluid and/or
peripheral blood would be applicable as a biomarker of
intraperitoneal dissemination of ovarian cancers.
Acknowledgements
This study was supported in part by
Grants-in-Aid for Scientific Research (22390071), the MIAST
project, and a Grant-in-Aid for Strategic Medical Science Research
from the Ministry of Education, Culture, Sports, Science and
Technology of Japan.
References
|
1.
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar
|
|
2.
|
Hu X, Li D, Zhang W, Zhou J, Tang B and Li
L: Matrix metalloproteinase-9 expression correlates with prognosis
and involved in ovarian cancer cell invasion. Arch Gynecol Obstet.
286:1537–1543. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Fidler IJ: The pathogenesis of cancer
metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev
Cancer. 3:453–458. 2003.
|
|
4.
|
Fishman DA, Liu Y, Ellerbroek SM and Stack
MS: Lysophosphatidic acid promotes matrix metalloproteinase (MMP)
activation and MMP-dependent invasion in ovarian cancer cells.
Cancer Res. 61:3194–3199. 2001.PubMed/NCBI
|
|
5.
|
Sourbier C: Ovarian cancer: emerging
molecular-targeted therapies. Biologics. 6:147–154. 2012.PubMed/NCBI
|
|
6.
|
Ledermann J, Harter P, Gourley C, et al:
Olaparib maintenance therapy in platinum-sensitive relapsed ovarian
cancer. N Engl J Med. 366:1382–1392. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Yamamoto N, Nokihara H, Yamada Y, et al: A
phase I, dose-finding and pharmacokinetic study of olaparib
(AZD2281) in Japanese patients with advanced solid tumors. Cancer
Sci. 103:504–509. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Campos SM and Ghosh S: A current review of
targeted therapeutics for ovarian cancer. J Oncol. 2010:1493622010.
View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Kumagai T, Wakimoto N, Yin D, et al:
Histone deacetylase inhibitor, suberoylanilide hydroxamic acid
(Vorinostat, SAHA) profoundly inhibits the growth of human
pancreatic cancer cells. Int J Cancer. 121:656–665. 2007.
View Article : Google Scholar
|
|
10.
|
Ramalingam SS, Parise RA, Ramanathan RK,
et al: Phase I and pharmacokinetic study of vorinostat, a histone
deacetylase inhibitor, in combination with carboplatin and
paclitaxel for advanced solid malignancies. Clin Cancer Res.
13:3605–3610. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Itamochi H: Targeted therapies in
epithelial ovarian cancer: molecular mechanisms of action. World J
Biol Chem. 1:209–220. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Yap TA, Carden CP and Kaye SB: Beyond
chemotherapy: targeted therapies in ovarian cancer. Nature reviews
Cancer. 9:167–181. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Pautier P, Joly F, Kerbrat P, et al: Phase
II study of gefitinib in combination with paclitaxel (P) and
carboplatin (C) as second-line therapy for ovarian, tubal or
peritoneal adenocarcinoma (1839IL/0074). Gynecol Oncol.
116:157–162. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Cohen M, Dromard M and Petignat P: Heat
shock proteins in ovarian cancer: a potential target for therapy.
Gynecol Oncol. 119:164–166. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Behbakht K, Sill MW, Darcy KM, et al:
Phase II trial of the mTOR inhibitor, temsirolimus and evaluation
of circulating tumor cells and tumor biomarkers in persistent and
recurrent epithelial ovarian and primary peritoneal malignancies: a
Gynecologic Oncology Group study. Gynecol Oncol. 123:19–26. 2011.
View Article : Google Scholar
|
|
16.
|
Huynh H, Teo CC and Soo KC: Bevacizumab
and rapamycin inhibit tumor growth in peritoneal model of human
ovarian cancer. Mol Cancer Ther. 6:2959–2966. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Lord CJ and Ashworth A: Targeted therapy
for cancer using PARP inhibitors. Curr Opin Pharmacol. 8:363–369.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Ma L, Teruya-Feldstein J and Weinberg RA:
Tumour invasion and metastasis initiated by microRNA-10b in breast
cancer. Nature. 449:682–688. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Liu Z, Zhu J, Cao H, Ren H and Fang X:
miR-10b promotes cell invasion through RhoC-AKT signaling pathway
by targeting HOXD10 in gastric cancer. Int J Oncol. 40:1553–1560.
2012.PubMed/NCBI
|
|
20.
|
Wotschofsky Z, Liep J, Meyer HA, et al:
Identification of metastamirs as metastasis-associated microRNAs in
clear cell renal cell carcinomas. Int J Biol Sci. 8:1363–1374.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
White NM, Khella HW, Grigull J, et al:
miRNA profiling in metastatic renal cell carcinoma reveals a
tumour-suppressor effect for miR-215. Br J Cancer. 105:1741–1749.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Zaravinos A, Radojicic J, Lambrou GI, et
al: Expression of miRNAs involved in angiogenesis, tumor cell
proliferation, tumor suppressor inhibition, epithelial-mesenchymal
transition and activation of metastasis in bladder cancer. J Urol.
188:615–623. 2012. View Article : Google Scholar
|
|
23.
|
Karsy M, Arslan E and Moy F: Current
progress on understanding microRNAs in glioblastoma multiforme.
Genes Cancer. 3:3–15. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Sasayama T, Nishihara M, Kondoh T, Hosoda
K and Kohmura E: MicroRNA-10b is overexpressed in malignant glioma
and associated with tumor invasive factors, uPAR and RhoC. Int J
Cancer. 125:1407–1413. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Myers C, Charboneau A, Cheung I, Hanks D
and Boudreau N: Sustained expression of homeobox D10 inhibits
angiogenesis. Am J Pathol. 161:2099–2109. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Gupta RA, Shah N, Wang KC, et al: Long
non-coding RNA HOTAIR reprograms chromatin state to promote cancer
metastasis. Nature. 464:1071–1076. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Osborne J, Hu C, Hawley C, Underwood LJ,
O’Brien TJ and Baker VV: Expression of HOXD10 gene in normal
endometrium and endometrial adenocarcinoma. J Soc Gynecol Investig.
5:277–280. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Reddy SD, Ohshiro K, Rayala SK and Kumar
R: MicroRNA-7, a homeobox D10 target, inhibits p21-activated kinase
1 and regulates its functions. Cancer Res. 68:8195–8200. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Carrio M, Arderiu G, Myers C and Boudreau
NJ: Homeobox D10 induces phenotypic reversion of breast tumor cells
in a three-dimensional culture model. Cancer Res. 65:7177–7185.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Roy R, Yang J and Moses MA: Matrix
metalloproteinases as novel biomarkers and potential therapeutic
targets in human cancer. J Clin Oncol. 27:5287–5297. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Horiuchi A, Imai T, Wang C, et al:
Up-regulation of small GTPases, RhoA and RhoC, is associated with
tumor progression in ovarian carcinoma. Lab Invest. 83:861–870.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Zaman MS, Maher DM, Khan S, Jaggi M and
Chauhan SC: Current status and implications of microRNAs in ovarian
cancer diagnosis and therapy. J Ovarian Res. 5:442012. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Chang S and Sharan SK: BRCA1 and
MicroRNAs: emerging networks and potential therapeutic targets. Mol
Cells. 34:425–432. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Bovicelli A, D’Andrilli G and Giordano A:
New players in ovarian cancer. J Cell Physiol. 226:2500–2504. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Asadollahi R, Hyde CA and Zhong XY:
Epigenetics of ovarian cancer: from the lab to the clinic. Gynecol
Oncol. 118:81–87. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Shahab SW, Matyunina LV, Hill CG, et al:
The effects of MicroRNA transfections on global patterns of gene
expression in ovarian cancer cells are functionally coordinated.
BMC Med Genomics. 5:332012. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Chen J, Wang L, Matyunina LV, Hill CG and
McDonald JF: Overexpression of miR-429 induces
mesenchymal-to-epithelial transition (MET) in metastatic ovarian
cancer cells. Gynecol Oncol. 121:200–205. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Lou Y, Yang X, Wang F, Cui Z and Huang Y:
MicroRNA-21 promotes the cell proliferation, invasion and migration
abilities in ovarian epithelial carcinomas through inhibiting the
expression of PTEN protein. Int J Mol Med. 26:819–827.
2010.PubMed/NCBI
|
|
39.
|
Li J, Liang S, Yu H, Zhang J, Ma D and Lu
X: An inhibitory effect of miR-22 on cell migration and invasion in
ovarian cancer. Gynecol Oncol. 119:543–548. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40.
|
Siemens H, Jackstadt R, Hunten S, et al:
miR-34 and SNAIL form a double-negative feedback loop to regulate
epithelial-mesenchymal transitions. Cell Cycle. 10:4256–4271. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
41.
|
Kim NH, Kim HS, Li XY, et al: A
p53/miRNA-34 axis regulates Snail1-dependent cancer cell
epithelial-mesenchymal transition. J Cell Biol. 195:417–433. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
42.
|
Preis M, Gardner TB, Gordon SR, et al:
MicroRNA-10b expression correlates with response to neoadjuvant
therapy and survival in pancreatic ductal adenocarcinoma. Clin
Cancer Res. 17:5812–5821. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43.
|
Bloomston M, Frankel WL, Petrocca F, et
al: MicroRNA expression patterns to differentiate pancreatic
adenocarcinoma from normal pancreas and chronic pancreatitis. JAMA.
297:1901–1908. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44.
|
Teplyuk NM, Mollenhauer B, Gabriely G, et
al: MicroRNAs in cerebrospinal fluid identify glioblastoma and
metastatic brain cancers and reflect disease activity. Neuro Oncol.
14:689–700. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45.
|
Huse JT, Brennan C, Hambardzumyan D, et
al: The PTEN-regulating microRNA miR-26a is amplified in high-grade
glioma and facilitates gliomagenesis in vivo. Genes Dev.
23:1327–1337. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46.
|
Ciafre SA, Galardi S, Mangiola A, et al:
Extensive modulation of a set of microRNAs in primary glioblastoma.
Biochem Biophys Res Commun. 334:1351–1358. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
47.
|
Chisholm KM, Wan Y, Li R, Montgomery KD,
Chang HY and West RB: Detection of long non-coding RNA in archival
tissue: correlation with polycomb protein expression in primary and
metastatic breast carcinoma. PLoS One. 7:e479982012. View Article : Google Scholar : PubMed/NCBI
|
|
48.
|
Niinuma T, Suzuki H, Nojima M, et al:
Upregulation of miR-196a and HOTAIR drive malignant character in
gastrointestinal stromal tumors. Cancer Res. 72:1126–1136. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
49.
|
Kogo R, Shimamura T, Mimori K, et al: Long
noncoding RNA HOTAIR regulates polycomb-dependent chromatin
modification and is associated with poor prognosis in colorectal
cancers. Cancer Res. 71:6320–6326. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
50.
|
Geng YJ, Xie SL, Li Q, Ma J and Wang GY:
Large intervening non-coding RNA HOTAIR is associated with
hepatocellular carcinoma progression. J Int Med Res. 39:2119–2128.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
51.
|
Yang J, Mani SA, Donaher JL, et al: Twist,
a master regulator of morphogenesis, plays an essential role in
tumor metastasis. Cell. 117:927–939. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
52.
|
Ma L, Reinhardt F, Pan E, et al:
Therapeutic silencing of miR-10b inhibits metastasis in a mouse
mammary tumor model. Nat Biotechnol. 28:341–347. 2010. View Article : Google Scholar : PubMed/NCBI
|