Introduction
Gastric cancer has one of the highest incidence
rates and mortality worldwide, especially in East Asia (1,2).
More than 400,000 new patients with gastric cancer are diagnosed in
China every year. The prevalence and mortality in our country are
higher than the world average (3).
Most gastric cancer is adenocarcinoma, especially poorly
differentiated gastric adenocarcinoma, which accounts for
approximately 54%. It features high-degree of malignancy, rapid
metastasis, and poor prognosis, whereas well-differentiated gastric
adenocarcinoma is characterized by slow metastasis and good
prognosis. It is important to understand fully the molecular
mechanism of poorly differentiated gastric cancer and effectively
interfere with such mechanism. The traditional non-target
chemotherapy has severe side-effects, therefore, cancer treatment
and research has focused on molecular target therapy due to its
high selectivity, good efficacy and low incidence of side-effects
(4).
Tea is rich in polyphenols with strong antioxidant
activity (5), and has a high level
of epigallocatechin-3-gallate (EGCG) (6). Research on humans and animal cell
models has shown that EGCG has a variety of pharmacological
effects, such as strong free-radical scavenging, anti-lipid
peroxidation and antiapoptotic, antiviral and antitumor activities
(7). An imbalance between cell
proliferation and apoptosis is one of the primary factors in the
occurrence and development of tumors, therefore, proliferation
inhibition and apoptosis induction of tumor cells are important
methods for prevention and treatment. Many studies have confirmed
that EGCG inhibits proliferation of tumor cells and induces
apoptosis (8,9) such as lung cancer, nasopharyngeal
carcinoma, breast cancer, gastric cancer, colon cancer, prostate
cancer, liver cancer, oral cancer, ovarian cancer and other
malignant tumors (10–12), but its molecular mechanism is
unknown and needs further study.
In the present study, we observed that EGCG induced
apoptosis and inhibited proliferation of poorly differentiated AGS
gastric cancer cells. The search for target genes regulated by EGCG
verified that the target genes could play a role in apoptosis
induction and inhibition of proliferation.
Materials and methods
Materials
AGS cells were purchased from the Cell Resource
Center of Shanghai Institutes for Biological Sciences, Chinese
Academy of Sciences (Shanghai, China), Ham’s F12 medium from
HyClone (Logan, UT, USA), trypsin-EDTA solution and fetal bovine
serum from Invitrogen (Carlsbad, CA, USA), Cell Counting Kit-8
(CCK-8) from Dojindo (Kumamoto, Japan), EGCG from Sigma (St. Louis,
MO, USA), Annexin V-FITC Apoptosis Detection Kit and FACSCalibur
Flow Cytometer from BD Pharmingen (San Diego, CA, USA), BioTek
micro-plate reader from BioTek (Winooski, VT, USA) with primer
designed by Shanghai Sangon Biotech Co. Ltd., Illumina BeadChip
HumanHT-12_V4 from Illumina (San Diego, CA, USA), ON-TARGETplus
SMARTpool siRNA targeting Id1 kit from Dharmacon (Waltham, MA,
USA), Silencer siRNA Transfection II kit from Ambion (Carlsbad, CA,
USA), Agarose I from Amresco (Solon, OH, USA), RNeasy mini kit from
Qiagen (Dusseldorf, Germany), Reverse Transcription System from
Promega (Fitchburg, WI, USA), SYBR Premix Ex Taq from Takara
(Kyoto, Japan), ABI prism 7300 PCR from ABI (Carlsbad, CA, USA),
Amersham ECL plus Western Blotting Detection System from GE
Healthcare (Little Chalfont, UK), Pierce BCA Protein Assay Kit from
Thermo Scientific (Waltham, MA, USA), 8453 UV-visible Spectroscopy
System from Agilent (Santa Clara, CA, USA), inverted fluorescence
microscope IX51 from Olympus (Tokyo, Japan), and Id1 antibody from
Epitomics (Burlingame, CA, USA).
CCK-8 experiment
Poorly differentiated AGS cells were cultured in
Ham’s F12 medium + 10% FBS for 24 h and divided into 7 groups (3
holes in each group). The medium in the holes was substituted with
complete medium with final concentrations of 0, 20, 40, 60, 80, 120
and 240 μg/ml ESPS. The proliferation inhibition rate was
calculated as follows: (control − administration)/(control −
background) × 100%. Control: cells, culture medium, DMSO and CCK-8;
administration: cells, culture medium and different concentrations
of EGCG and CCK-8; background: only medium and CCK-8.
To verify Id1 gene and protein expression in AGS
gastric cancer cells treated with EGCG and siRNA-Id1,
1×106 cells were incubated in 12 100-mm culture dishes
containing 10 ml complete medium. The medium in 6 culture dishes
was substituted with complete medium containing 0, 20, 40, 60, 80
or 100 μg/ml EGCG, and the medium in another 6 culture
dishes was substituted with medium containing 0, 40, 60, 80 or 100
nM siRNA-Id1, and 100 nM control siRNA. The complete medium was
incubated in 5% CO2 at 37°C for 72 h, followed by
addition of CCK-8 solution in the proportion 10 μl/100
μl, which was allowed to stand at 37°C for 1 h. Absorbance
was read at 450 nm wavelength with a microplate reader.
Gene expression microarray
AGS cells (3×106) were incubated in 3
100-mm culture dishes containing 10 ml complete medium. Twenty-four
hours later, when all the cells were normal, the medium was
substituted with complete medium containing 80 μg/ml EGCG.
All treatments were carried out in triplicate. Cells were collected
to separate RNA, and total RNA in all samples were extracted and
inspected for quality in accordance with Qiagen kit instructions.
Qualified total RNA was labeled fluorescently using the Ambion
Illumina RNA amplification kit from Illumina, and cRNA was
hybridized with Illumina BeadChip HumanHT-12_V4 chip after linear
amplification. The film was developed, the hybridization results
were scanned, and the relevant data were analyzed and normalized by
the Average method. The screening criteria for the differentially
expressed genes were as follows: any effective gene either in the
experiment group or control group (P<0.05) with the diffscore
value in experiment group <13 or >13, and the fold-change
>2 of the difference.
Knockdown of Id1 transcripts using siRNA
transfection
We used ON-TARGETplus SMARTpool siRNA targeting Id1
(50 nM, L-005051-00-0050, Dharmacon) and Silencer siRNA
Transfection II kit (Ambion) in KO-DMEM medium, according to the
manufacturer’s instructions. Non-targeting siRNA (Ambion) was used
as a negative control. The ON-TARGETplus SMARTpool siRNA reagent
and at least 3 of 4 individual siRNAs silenced target gene
expression by at least 75% at the mRNA level when used under
optimal delivery conditions (confirmed using a validated control
siRNA). Silencing was monitored at the mRNA level 24–48 h after
transfection using 100 nM siRNA.
Apoptosis and cell cycle analysis with
flow cytometry
AGS cells were digested with trypsin-EDTA into
single-cell suspensions and then collected. The resuspended cells
(1×105) were centrifuged at 1,000 rpm for 5 min to
remove the supernatant, and the cells were resuspended in 100
μl Annexin V binding solution and transferred into a 5-ml
culture tube. Annexin V-FITC and propidium iodide (PI) (5
μl) was added to the solution, and incubated in the dark at
20–25°C for 15 min, followed by addition of 400 μl Annexin V
binding solution for flow cytometry. Annexin V-FITC has green
fluorescence and PI has red fluorescence. The wavelength of light
excited by the flow cytometer was 488 nm. FITC fluorescence was
detected with a band-pass filter of 515 nm wavelength and PI
fluorescence was detected with a filter of >560 nm. The cell
pellet was added to 1 ml precooled 70% ethanol, fixed at 4°C
overnight, washed with PBS twice, centrifuged at 1,000 rpm for 5
min, resuspended in 0.5 ml PBS containing 50 μg/ml PI and
100 μg/ml RNase A, and incubated in the dark at 37°C for 30
min to determine the cell cycle with a flow cytometer according to
standard procedures. The result was analyzed with a cycle meter and
ModFit software.
Real-time RT-PCR
Real-time RT-PCR was carried out after treatment of
AGS cells. Total RNA was extracted from all samples, quantified,
and reverse transcribed into cDNAs according to the instructions of
the RNeasy mini kit (Qiagen). Real-time RT-PCR was carried out
according to the instructions of the kit of the Reverse
Transcription System (Promega). Target gene primer sequences are
shown in Table II. Reaction
conditions were as follows: 95°C for 30 sec; 95°C for 15 sec, and
62°C for 34 sec (40 cycles). We used the 2−ΔΔCt method
for calculating the relative expression levels of target genes.
 | Table I.Real-time RT-PCR primers. |
Table I.
Real-time RT-PCR primers.
| SN | Gene | Primer sequence (5′
to 3′) |
|---|
| 1 | β-actin | F:
5′TGGAGAAAATCTGGCACCA3′
R: 5′CAGGCGTACAGGGATAGCAC3′ |
| 2 | Id1 | F:
5′ACGACATGAACGGCTGTTACTCAC3′
R: 5′CTCCAACTGAAGGTCCCTGATGTAG3′ |
Western blot analysis
Western blot analysis was carried out after drug
treatment. The cells were incubated with 5% CO2 at 37°C
for 48 h, collected after digestion with pancreatin, washed twice
with PBS, centrifuged at 2,000 rpm for 5 min to remove the
supernatant, and placed on ice for lysis. The proteins were
quantified by the bicinchoninic acid (BCA) method. SDS-PAGE,
membrane transfer, immunoreactivity and gel electrophoresis image
analysis were performed for the target genes.
Statistical analysis
The data were analyzed with SPSS 13.0 statistical
software (SPSS Inc., Chicago, IL, USA), and expressed as the mean ±
SD. Multiple groups were analyzed with one-way analysis of
variance, pairwise comparison was conducted by using the least
significant difference (LSD) t-test, and different groups were
compared using a t-test. P<0.05 was statistically
significant.
Results
Inhibition of AGS cell proliferation and
growth by EGCG
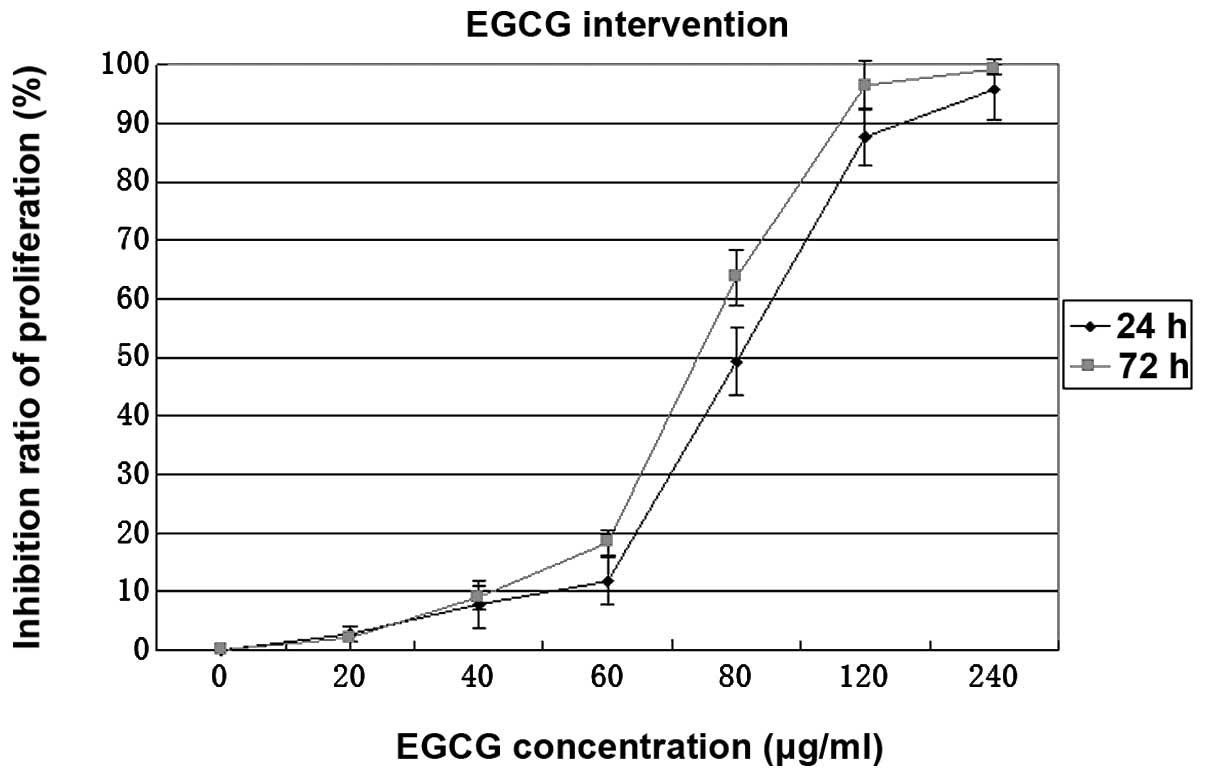
The CCK-8 experiment and cell morphological
observation showed that EGCG inhibited proliferation of human
gastric cancer cells in a concentration-dependent manner (Fig. 1, P<0.01). Proliferation of AGS
cells treated with 80 μg/ml EGCG was significantly inhibited
and the inhibition rate did not differ significantly after 24 and
72 h treatment (Fig. 1,
P>0.05).
Selection of differentially expressed
genes with gene expression microarray
There were 54 differentially expressed genes when
comparing EGCG-treated and normal control cells, with 37
upregulated genes of diffscore value >13 and 17 downregulated
genes with diffscore value <−13 (Table I). One differentially expressed
gene, Id1, in AGS gastric cancer cells before and after treatment
with EGCG was screened out, and was verified subsequently (Table I). The genes were differentially
expressed before and after the AGS gastric cancer cells were
treated with EGCG.
 | Table I.cDNA microarray expression profiling
of differentially expressed genes. |
Table I.
cDNA microarray expression profiling
of differentially expressed genes.
| Probe ID | Symbol | EGCG. AVG
signal | EGCG. detection
p-value | EGCG.
diffscore | EGCG vs CN
fold-change | CN.AVG signal | CN. detection
p-value | Search key | Definition |
|---|
| ILMN_1689842 | SC4MOL | 469.9426 | 0 | 44.64191 | 2.146925 | 218.891 | 0 | NM_006745.3 | Human
sterol-C4-methyl oxidase-like (SC4MOL), transcript variant 1,
mRNA |
| ILMN_1664861 | ID1 | 1,280.916 | 0 | −40.1308 | 0.483405 | 2649.78 | 0 | NM_181353.1 | Human Id1, dominant
negative HLH protein (ID1), transcript variant 2, mRNA |
| ILMN_1732296 | ID3 | 390.6099 | 0 | −47.3221 | 0.467825 | 834.9481 | 0 | NM_002167.2 | Human Id3, dominant
negative HLH protein (ID3), mRNA |
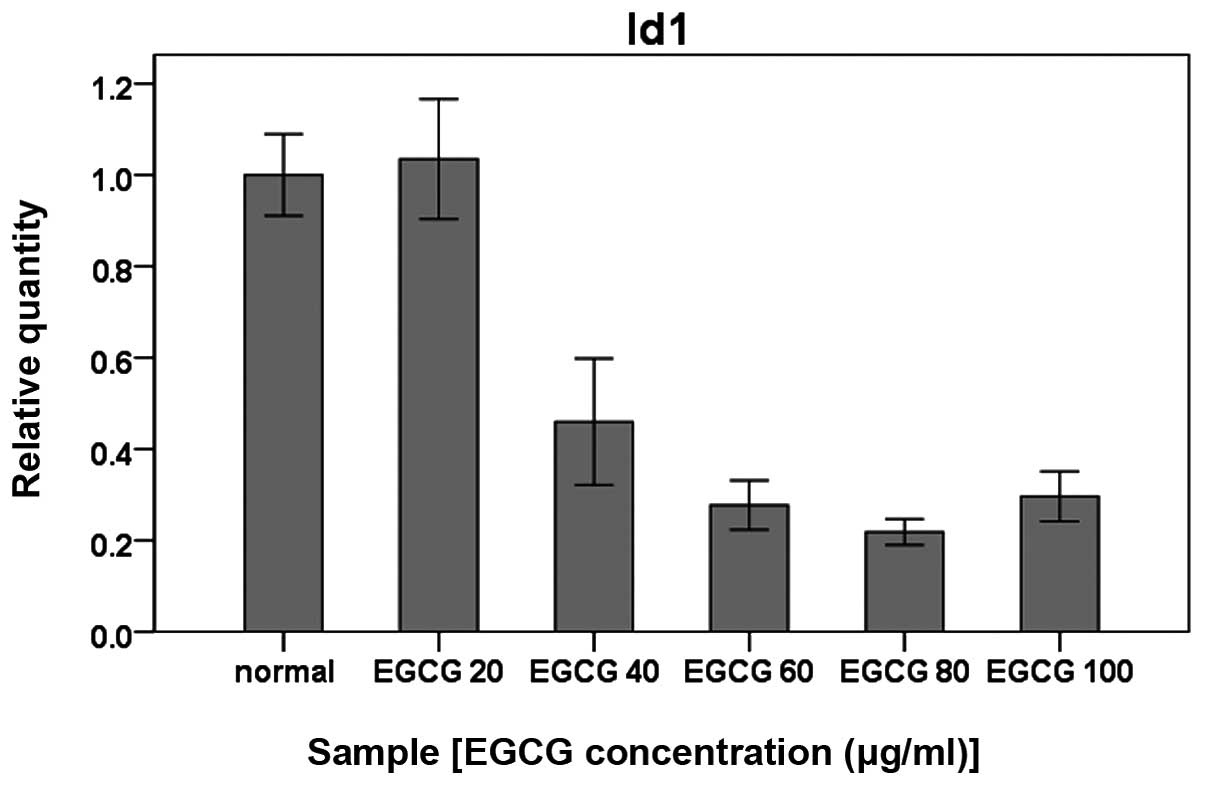
Verification of microarray results with
quantitative real-time RT-PCR and western blot analysis
To verify the results from gene expression
microarray, the differentially expressed gene Id1 was verified by
quantitative real-time RT-PCR, using the primers listed in Table II. Id1 mRNA expression was
significantly reduced in the AGS cells treated with EGCG in a
concentration-dependent manner (Fig.
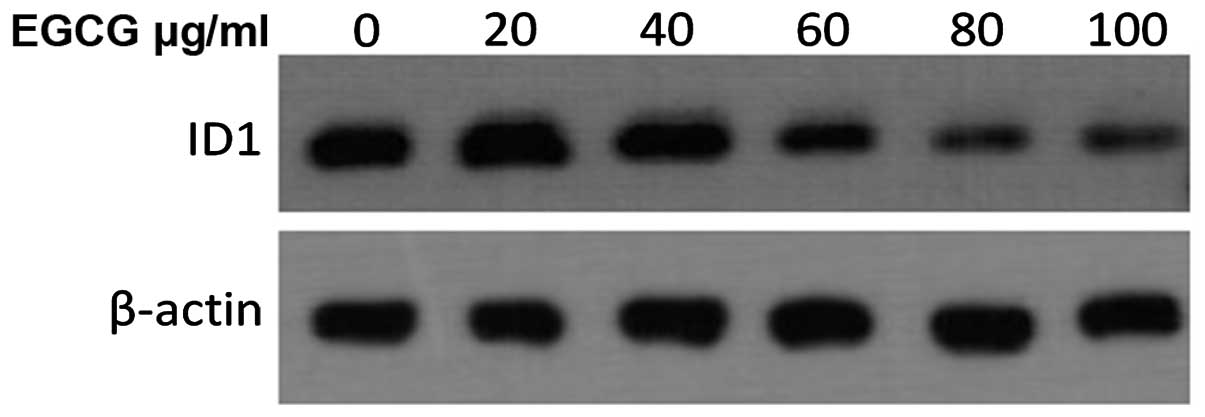
2, P<0.01). Western blot analysis further showed that Id1
protein expression in AGS cells was consistent with the mRNA
expression (Fig. 3).
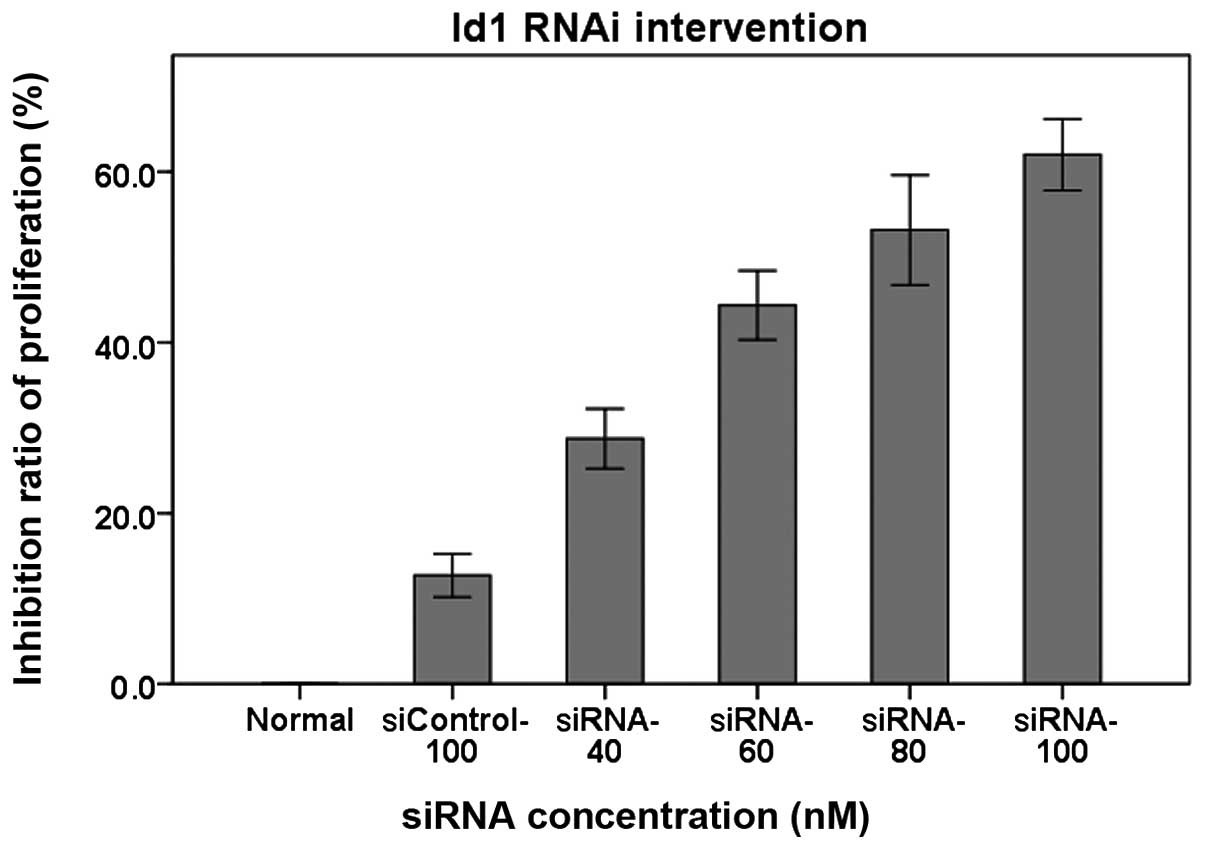
Detection of Id1 with CCK-8 to observe
influence of RNAi on AGS cell proliferation
The CCK-8 experiment and cell morphological
observation showed that siRNA-Id1 inhibited proliferation of AGS
cells in a concentration-dependent manner (Fig. 4, P<0.01). Proliferation of AGS
cells was significantly inhibited by 80 nM siRNA-Id1.
Id1 expression in AGS cells treated with
Id1 RNAi
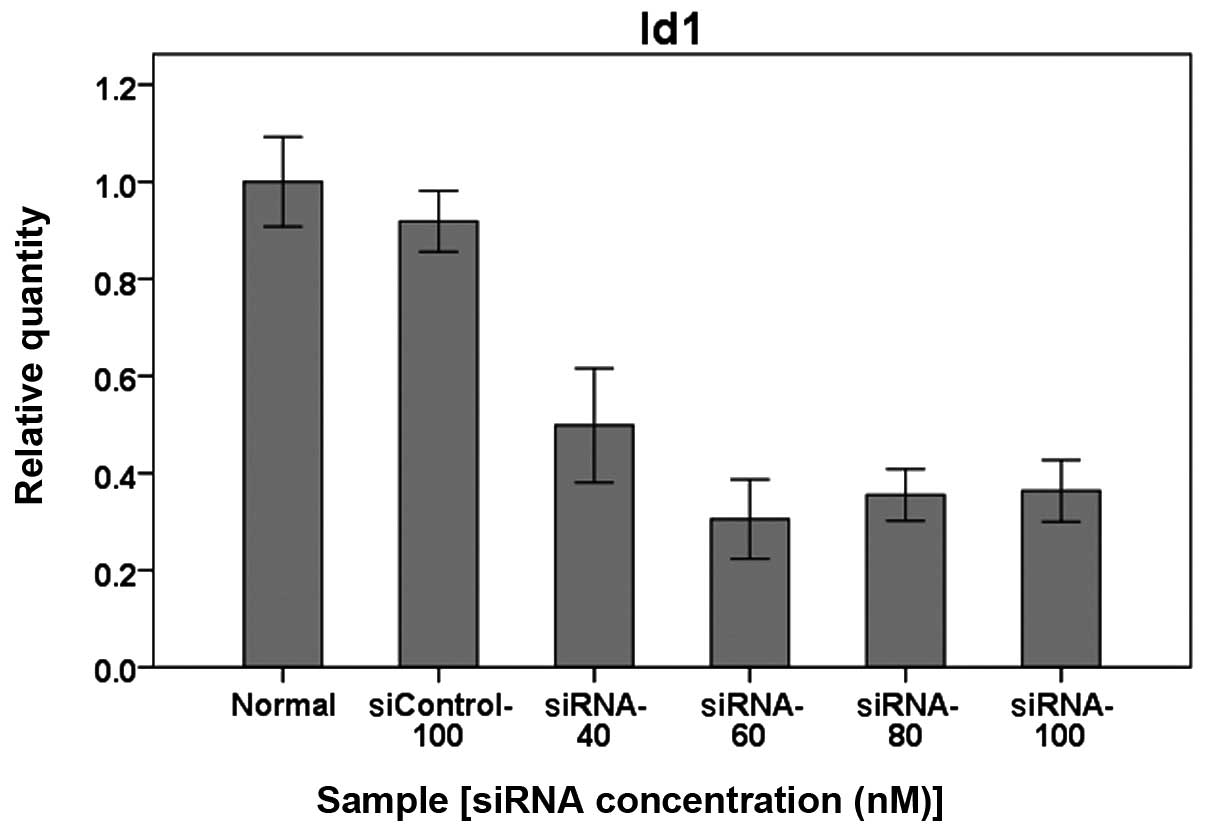
Real-time RT-PCR showed that mRNA expression of Id1
was significantly downregulated in AGS cells treated with siRNA-Id1
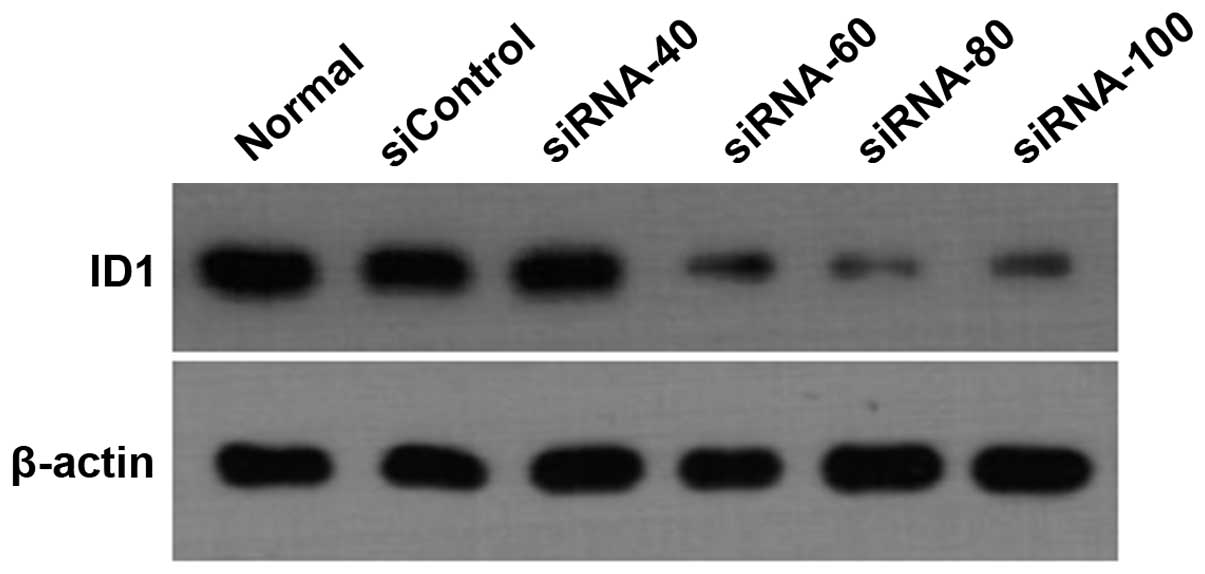
in a concentration-dependent manner (Fig. 5, P<0.01). Western blot analysis
further showed that Id1 protein expression in AGS cells was
consistent with mRNA expression (Fig.
6).
Apoptosis and cell cycle of AGS gastric
cancer cells treated with EGCG and Id1 RNAi
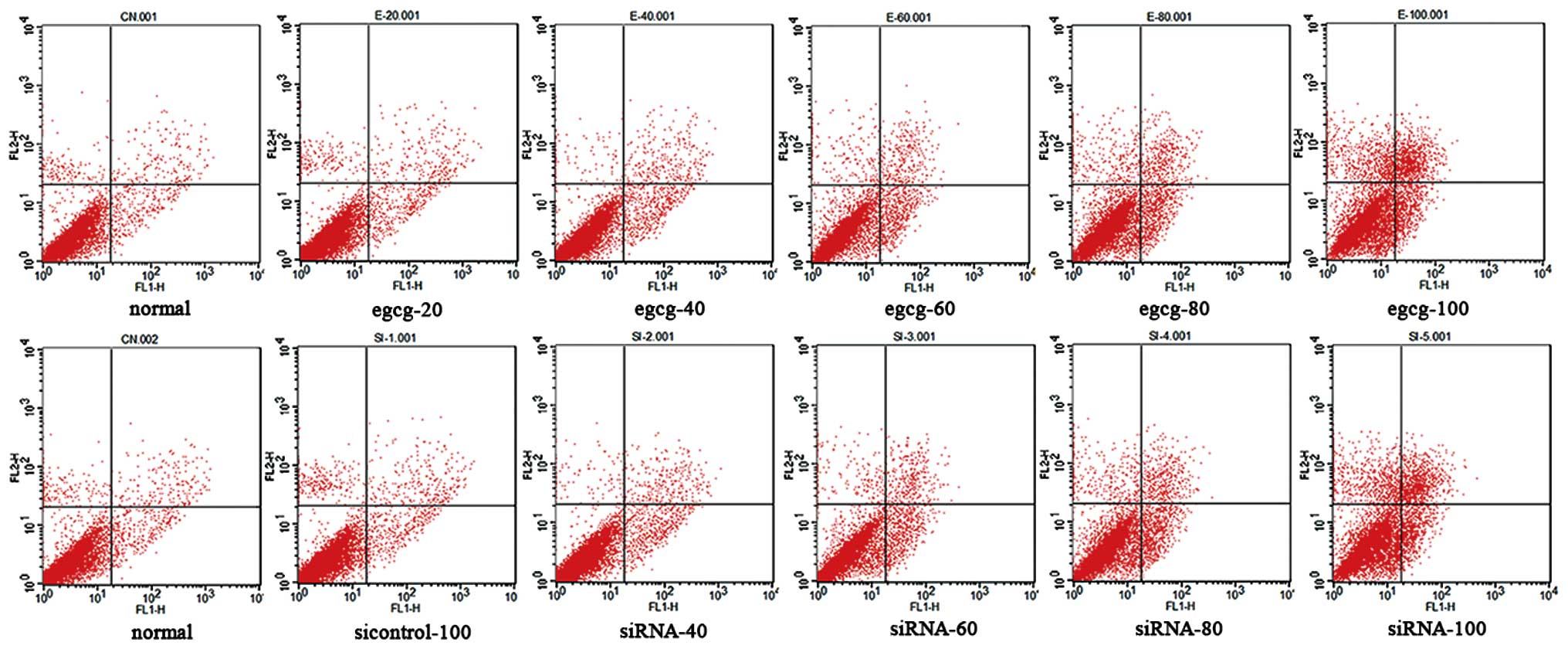
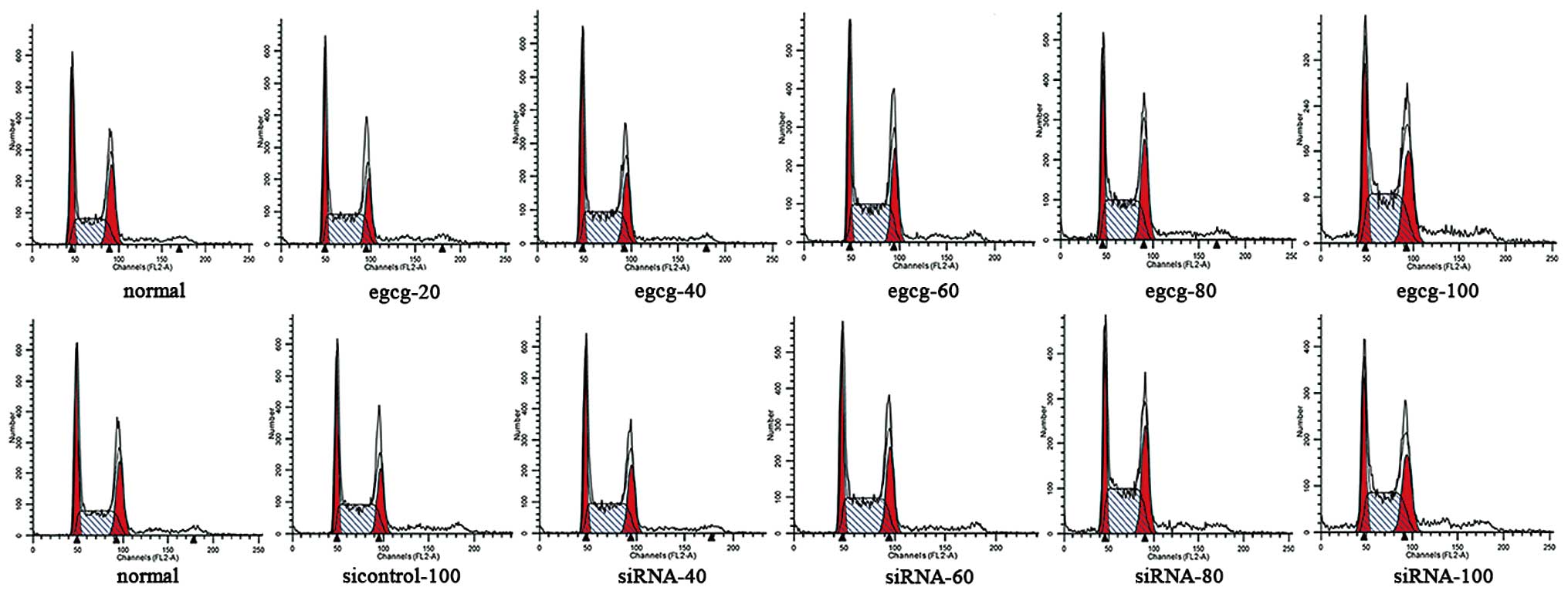
Flow cytometry showed that EGCG and siRNA-Id1
induced apoptosis of AGS cells in a concentration-dependent manner
(Fig. 7). After treatment with
Id1-RNAi, changes in AGS cell proliferation and apoptosis were
similar to those in cells treated with EGCG. AGS cells were
arrested at S phase after treatment with EGCG and siRNA-ID1
(Fig. 8, P<0.05).
Discussion
An increasing number of studies have shown that EGCG
regulates transduction of signaling molecules related to tumor cell
metastasis and migration, and inhibits tumor cell proliferation and
induces apoptosis and cell cycle arrest (13,14).
In the present study, CCK-8 experiments and morphological
observation showed that EGCG inhibited proliferation of AGS gastric
cancer cells in a concentration-dependent manner. Flow cytometry
showed that EGCG concentration-dependently induced apoptosis of AGS
cells and cell cycle arrest at S phase. We further used gene
expression microarray analysis for initial screening of
differentially expressed genes. There were 54 differentially
expressed genes when comparing EGCG-treated and normal control
cells, with 37 upregulated and 17 down-regulated genes. Han et
al have demonstrated that Id1 expression is often high in
gastric cancer tissues and cell lines and its expression level is
related to the degree of malignancy (15). Therefore, high Id1 expression is
directly related to the malignant potential of gastric cancer
cells. Tsuchiya et al have also found that gastric cancer
cells with high Id1 expression have strong metastatic ability
(16). As far as we know, the role
of Id1 in EGCG-induced tumor inhibition has not been reported so
far. Thus, we determined the differentially expressed Id1 gene by
screening with gene expression microarray.
The Id protein family is a helix-loop-helix (HLH)
family of transcription factors, including Id1-Id4. Id protein
family members have a highly conserved HLH area, which can be
combined with a basic HLH protein (bHLH) to form a heterodimer,
thereby inhibiting the bHLH binding to target genes, reducing bHLH
transcription factor activity, inhibiting cell differentiation and
promoting cell proliferation (17,18).
Cell apoptosis and proliferation play an important role in the
occurrence and development of malignant tumors (19). Id molecules can regulate
cyclin-dependent kinase (cdk) inhibitors to shorten the cell cycle.
Idl is directly involved in regulating the cell cycle by antisense
oligonucleotides (20,21) or microinjection of Id1 antibody
(22) to inhibit partially Id1
protein expression, thus delaying cell entry into S phase. Idl
promotes cdk4 and cdk2 to combine with retinoblastoma protein
(pRb), by inhibiting transcriptional level of p16ink4a
and p21WAF1, which catalyzes phosphorylation of pRb. E2F
and other types of protein can escape from pRb to activate more
cancer gene transcription, so the cells enter into S phase and
become cancerous (23) and
proliferate (24). In fibroblasts,
Id1 activates the Ras-Raf-MEK pathway to promote cell proliferation
(25). Ling et al (26) found that Id1 induces proliferation
of prostate cancer cells by activating the mitogen-activated
protein kinase pathway, which has a positive correlation with the
degree of tumor cell malignancy (27). Tumor necrosis factor-α-induced Id1
increases the activity of nuclear factor (NF)-κB and the
anti-apoptotic effectors Bcl-xL and intercellular adhesion
molecule-1, by inactivating the Bax and caspase-3 pathways, thus
inhibiting apoptosis (28). Id-1
can also promote Bcl-2 expression and decrease expression of Bax
and caspase-3 by inhibiting the p53 signaling pathway and
activating the NF-κB signaling pathway, thus preventing tumor cell
apoptosis (29). Tsuchiya et
al (16) reported that tumor
cell proliferation and migration are significantly reduced in the
Id1/Id3 double knockout gastric cancer cell line MKN 45. The number
of peritoneal metastases is significantly reduced, and the size of
single metastases is also decreased, which proves that Id1 and Id3
knockout can significantly inhibit peritoneal metastasis of gastric
cancer cells. Therefore, Id might be a good target for treatment
and prevention of gastric cancer.
After poorly differentiated AGS gastric cancer cells
were treated with siRNA-Id1, proliferation slowed significantly,
thus inhibiting Id1 mRNA and protein expression. Flow cytometry
showed that apoptosis was increased and cells were arrested at S
phase. Id1 RNAi affected the phenotypic changes in AGS gastric
cancer cells in a dose-dependent manner. The above results suggest
that Id1 plays its oncogenic role through promoting cancer cell
proliferation and inhibiting apoptosis. Id1 participated in
proliferation, apoptosis and other biological behavior of poorly
differentiated AGS gastric cancer cells.
Previous studies have shown that EGCG can induce
apoptosis through mitochondria (30,31),
p53 (32–34), Bcl-2 (35) cell signaling pathways (36,37),
reactive oxygen species (38) and
telomerase (39). In different
cell lines, EGCG can induce apoptosis through different mechanisms.
We found that EGCG lowered Id1 expression to induce apoptosis and
inhibit proliferation of poorly differentiated AGS gastric cancer
cells, but it is necessary to investigate further how to achieve
the above through a certain mechanism and whether the activation of
signal transduction pathways is affected.
Molecular target therapy has become the trend in
cancer treatment and research due to its high selectivity, good
efficacy and few side-effects. Our experiments proved that EGCG
could play a role in inhibiting proliferation, promoting apoptosis,
and affecting cell cycle of poorly differentiated AGS gastric
cancer cells, which is closely related to down-regulation of Id1
expression. Therefore, Id1 may be one of the targets regulated by
EGCG for tumor inhibition.
Abbreviations:
|
EGCG
|
epigallocatechin-3-gallate;
|
|
CCK-8
|
cell counting kit-8;
|
|
PI
|
propidium iodide
|
Acknowledgements
Shanghai Municipal Health Bureau Key
Disciplines Grant, no. ZK2012A05; National Natural Science
Foundation, no. 81070344.
References
|
1.
|
Krejs GJ: Gastric cancer: epidemiology and
risk factors. Dig Dis. 28:600–603. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
3.
|
Yang L: Incidence and mortality of gastric
cancer in China. World J Gastroenterol. 12:17–20. 2006.
|
|
4.
|
Hemalswarya S and Doble M: Potential
synergism of natural products in the treatment of cancer. Phytother
Res. 20:239–249. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Cao G, Chen M, Song Q, et al: EGCG
protects against UVB-induced apoptosis via oxidative stress and the
JNK1/c-Jun pathway in ARPE19 cells. Mol Med Rep. 5:54–59.
2012.PubMed/NCBI
|
|
6.
|
Ziaedini A, Jafari A and Zakeri A:
Extraction of antioxidants and caffeine from green tea (Camelia
sinensis) leaves: kinetics and modeling. Food Sci Technol.
16:505–510. 2010.PubMed/NCBI
|
|
7.
|
Chen D, Wan SB, Yang H, Yuan J, Chan TH
and Dou QP: EGCG, green tea polyphenols and their synthetic analogs
and prodrugs for human cancer prevention and treatment. Adv Clin
Chem. 53:155–177. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Rao SD and Pagidas K:
Epigallocatechin-3-gallate, a natural polyphenol, inhibits cell
proliferation and induces apoptosis in human ovarian cancer cells.
Anticancer Res. 30:2519–2523. 2010.PubMed/NCBI
|
|
9.
|
Patra SK, Rizzi F, Silva A, Rugina DO and
Bettuzzi S: Molecular targets of (−)-epigallocatechin-3-gallate
(EGCG): specificity and interaction with membrane lipid rafts. J
Physiol Pharmacol. 59(Suppl 9): 217–235. 2008.
|
|
10.
|
Johnson JJ, Bailey HH and Mukhtar H: Green
tea polyphenols for prostate cancer chemoprevention: a
translational perspective. Phytomedicine. 17:3–13. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Mandel SA, Amit T, Kalfon L, Reznichenko
L, Weinreb O and Youdim MB: Cell signaling pathways and iron
chelation in the neurorestorative activity of green tea
polyphenols: special reference to epigallocatechin gallate (EGCG).
J Alzheimers Dis. 15:211–222. 2008.PubMed/NCBI
|
|
12.
|
Yang H, Zonder JA and Dou QP: Clinical
development of novel proteasome inhibitors for cancer treatment.
Expert Opin Investig Drugs. 18:957–971. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Khan N and Mukhtar H: Multitargeted
therapy of cancer by green tea polyphenols. Cancer Lett.
269:269–280. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Lin RW, Chen CH, Wang YH, et al:
(−)-Epigallocatechin gallate inhibition of osteoclastic
differentiation via NF-kappaB. Biochem Biophys Res Commun.
379:1033–1037. 2009.
|
|
15.
|
Han S, Gou C, Hong L, et al: Expression
and significances of Id1 helix-loop-helix protein overexpression in
gastric cancer. Cancer Lett. 216:63–71. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Tsuchiya T, Okaji Y, Tsuno NH, et al:
Targeting Id1 and Id3 inhibits peritoneal metastasis of gastric
cancer. Cancer Sci. 96:784–790. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Aranha MM, Sola S, Low WC, Steer CJ and
Rodrigues CM: Caspases and p53 modulate FOXO3A/Id1 signaling during
mouse neural stem cell differentiation. J Cell Biochem.
107:748–758. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Benezra R, Davis RL, Lockshon D, Turner DL
and Weintraub H: The protein Id: a negative regulator of
helix-loop-helix DNA binding proteins. Cell. 61:49–59. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Evan G and Littlewood T: A matter of life
and cell death. Science. 281:1317–1322. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Barone MV, Pepperkok R, Peverali FA and
Philipson L: Id proteins control growth induction in mammalian
cells. Proc Natl Acad Sci USA. 91:4985–4988. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Hara E, Yamaguchi T, Nojima H, et al:
Id-related genes encoding helix-loop-helix proteins are required
for G1 progression and are repressed in senescent human
fibroblasts. J Biol Chem. 269:2139–2145. 1994.PubMed/NCBI
|
|
22.
|
Peverali FA, Ramqvist T, Saffrich R,
Pepperkok R, Barone MV and Philipson L: Regulation of G1
progression by E2A and Id helix-loop-helix proteins. EMBO J.
13:4291–4301. 1994.PubMed/NCBI
|
|
23.
|
Alani RM, Young AZ and Shifflett CB: Id1
regulation of cellular senescence through transcriptional
repression of p16/Ink4a. Proc Natl Acad Sci USA. 98:7812–7816.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Lee TK, Man K, Ling MT, et al:
Over-expression of Id-1 induces cell proliferation in
hepatocellular carcinoma through inactivation of p16INK4a/RB
pathway. Carcinogenesis. 24:1729–1736. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Ohtani N, Zebedee Z, Huot TJ, et al:
Opposing effects of Ets and Id proteins on p16INK4a expression
during cellular senescence. Nature. 409:1067–1070. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Ling MT, Wang X, Ouyang XS, et al:
Activation of MAPK signaling pathway is essential for Id-1 induced
serum independent prostate cancer cell growth. Oncogene.
21:8498–8505. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Cheung HW, Ling MT, Tsao SW, Wong YC and
Wang X: Id-1-induced Raf/MEK pathway activation is essential for
its protective role against taxol-induced apoptosis in
nasopharyngeal carcinoma cells. Carcinogenesis. 25:881–887. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Ling MT, Wang X, Ouyang XS, Xu K, Tsao SW
and Wong YC: Id-1 expression promotes cell survival through
activation of NF-kappaB signalling pathway in prostate cancer
cells. Oncogene. 22:4498–4508. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Kim H, Chung H, Kim HJ, et al: Id-1
regulates Bcl-2 and Bax expression through p53 and NF-kappaB in
MCF-7 breast cancer cells. Breast Cancer Res Treat. 112:287–296.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Chen C, Shen G, Hebbar V, Hu R, Owuor ED
and Kong AN: Epigallocatechin-3-gallate-induced stress signals in
HT-29 human colon adenocarcinoma cells. Carcinogenesis.
24:1369–1378. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Qanungo S, Das M, Haldar S and Basu A:
Epigallocatechin-3-gallate induces mitochondrial membrane
depolarization and caspase-dependent apoptosis in pancreatic cancer
cells. Carcinogenesis. 26:958–967. 2005. View Article : Google Scholar
|
|
32.
|
Gupta S, Ahmad N, Nieminen AL and Mukhtar
H: Growth inhibition, cell-cycle dysregulation, and induction of
apoptosis by green tea constituent (−)-epigallocatechin-3-gallate
in androgen-sensitive and androgen-insensitive human prostate
carcinoma cells. Toxicol Appl Pharmacol. 164:82–90. 2000.
|
|
33.
|
Hofmann CS and Sonenshein GE: Green tea
polyphenol epigallocatechin-3 gallate induces apoptosis of
proliferating vascular smooth muscle cells via activation of p53.
FASEB J. 17:702–704. 2003.PubMed/NCBI
|
|
34.
|
Roy AM, Baliga MS and Katiyar SK:
Epigallocatechin-3-gallate induces apoptosis in estrogen
receptor-negative human breast carcinoma cells via modulation in
protein expression of p53 and Bax and caspase-3 activation. Mol
Cancer Ther. 4:81–90. 2005.PubMed/NCBI
|
|
35.
|
Nihal M, Ahmad N, Mukhtar H and Wood GS:
Anti-proliferative and proapoptotic effects of
(−)-epigallocatechin-3-gallate on human melanoma: possible
implications for the chemoprevention of melanoma. Int J Cancer.
114:513–521. 2005.
|
|
36.
|
Ahmad N, Gupta S and Mukhtar H: Green tea
polyphenol epigallocatechin-3-gallate differentially modulates
nuclear factor kappaB in cancer cells versus normal cells. Arch
Biochem Biophys. 376:338–346. 2000. View Article : Google Scholar
|
|
37.
|
Gupta S, Hastak K, Afaq F, Ahmad N and
Mukhtar H: Essential role of caspases in
epigallocatechin-3-gallate-mediated inhibition of nuclear factor
kappa B and induction of apoptosis. Oncogene. 23:2507–2522. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Vittal R, Selvanayagam ZE, Sun Y, et al:
Gene expression changes induced by green tea polyphenol
(−)-epigallocatechin-3-gallate in human bronchial epithelial 21BES
cells analyzed by DNA microarray. Mol Cancer Ther. 3:1091–1099.
2004.
|
|
39.
|
Naasani I, Seimiya H and Tsuruo T:
Telomerase inhibition, telomere shortening, and senescence of
cancer cells by tea catechins. Biochem Biophys Res Commun.
249:391–396. 1998. View Article : Google Scholar : PubMed/NCBI
|