Introduction
Osteosarcoma is the most frequent malignant bone
tumor in children and adolescents and the estimated worldwide
incidence ranges between 3 and 4.5 per million (1). Long-term survival in localized
osteosarcoma has increased substantially from 10–20% when surgery
as single treatment was used before the 1980’s up to 20–60% from
1985 onwards. The improvement in survival has been attributed to
the use of intensive multi-agent chemotherapy in combination with
advanced surgery. However, since then no substantial further
improvement of survival has been reported (2). Despite aggressive multimodal therapy,
this devastating tumor often acquires drug resistance and
metastasizes (3). The most
frequent site for metastatic presentation is the lung (3). Death from osteosarcoma is usually the
result of progressive pulmonary metastasis with respiratory failure
due to widespread disease (3).
Hence, there is a real need to develop novel approaches for the
treatment and prevention of osteosarcoma and efficient inhibition
of metastasis, especially to the lung.
The role of carotenoids in reducing the risk of
cancer has been postulated for several decades (4). Fucoxanthin, one of the most abundant
carotenoids found in edible brown algae, has received much
attention over the last few years as cancer chemopreventive and
chemotherapeutic agent (5).
Dietary fucoxanthin is deacetylated into fucoxanthinol in the
intestinal tract by lipase and esterase from the pancreas or in
intestinal cells and incorporated as fucoxanthinol from the
digestive tract into the blood circulation system in mammals
(5). These carotenoids exhibit
antitumor effects in several malignant cell lines without affecting
normal cells (5,6). The main mechanism is suggested to be
the regulatory effects of fucoxanthin and fucoxanthinol on
molecules related to apoptosis and cell cycle (5,6).
Furthermore, it has been shown that fucoxanthin has chemopreventive
activities in a variety of models of cancer (5). However, to date, there is no
information on fucoxanthin- and fucoxanthinol-induced inhibition of
cell growth, migration and invasion of human bone cancer cells. In
this study, we investigated the effects of fucoxanthin and
fucoxanthinol on cell cycle, apoptosis, migration and invasion of
osteosarcoma cells. Furthermore, we also investigated the possible
molecular targets of anti-osteosarcoma activities of fucoxanthin
and fucoxanthinol.
Materials and methods
Cell lines
The human osteosarcoma cell lines, Saos-2, MNNG/HOS
(MNNG) and 143B and mouse osteosarcoma cell line, LM8, were
cultured in Roswell Park Memorial Institute-1640 or Eagle’s minimum
essential medium supplemented with 10% heat-inactivated fetal
bovine serum (FBS), 50 U/ml penicillin and 50 μg/ml
streptomycin at 37°C in a humidified atmosphere with 5%
CO2. Both 143B cell line with high metastatic potential
and MNNG cell line with low metastatic potential, are derived from
TE85 human osteosarcoma cell line (7,8).
Reagents
β-carotene and astaxanthin were purchased from Wako
Pure Chemical Industries (Osaka, Japan). Fucoxanthin was extracted
from brown seaweed Cladosiphon okamuranus Tokida using
acetone as solvent and purified by column chromatography,
liquid-liquid partition and re-crystallization up to >95%
purity. Further purification was performed by RP-HPLC up to >98%
purity, for in vitro assay. Fucoxanthinol was prepared by
enzymatic hydrolysis of purified fucoxanthin using porcine
pancreatic lipase. For this purpose, 195 mg of fucoxanthin, 2 g of
sodium taurocholate and 2 g of porcine pancreatic lipase (Type II;
Sigma-Aldrich, St. Louis, MO, USA) were dissolved in 30 ml of 0.1 M
sodium phosphate buffer (pH 7.0). The reaction buffer was incubated
at 37°C for 3 h. Fucoxanthinol was purified by ODS column
chromatography, liquid-liquid partition and re-crystallization. In
the experiment, we prepared 142 mg of purified fucoxanthinol
(>95% purity, 72% yield). Further purification was achieved by
RP-HPLC up to >98% purity, for in vitro assay. The
identity and purity of the products were confirmed by comparison
with reference fucoxanthin (Wako Pure Chemical Industries) and data
available in the literature. Tumor necrosis factor-α (TNF-α) and
the caspase inhibitor, Z-VAD-fmk, were purchased from PeproTech
(Rocky Hill, NJ, USA) and Promega (Madison, WI, USA), respectively.
Antibodies to Bcl-2, cyclin E, cyclin-dependent kinase (CDK)2,
CDK4, CDK6 and actin were purchased from NeoMarkers (Fremont, CA,
USA). Antibodies to XIAP and cyclin D1 were obtained from Medical
and Biological Laboratories (Nagoya, Japan). Antibodies to IκBα,
phospho-IκBα (Ser32 and Ser36), Akt, phospho-Akt (Thr308),
phospho-Akt (Ser473), phosphoinositide-dependent kinase 1 (PDK1),
phospho-PDK1 (Ser241), phospho-glycogen synthase kinase 3β (GSK3β)
(Ser9), caspase-8, cleaved caspase-3, cleaved caspase-9, cleaved
poly(ADP-ribose) polymerase (PARP), phospho-caspase-9 (Thr125),
survivin, Bak and Bcl-xL were purchased from Cell
Signaling Technology (Beverly, MA, USA). Antibodies to GSK3β and
β-catenin were obtained from BD Transduction Laboratories (San
Jose, CA, USA). Antibodies to cyclin D2, phospho-p130 (Ser952) and
activator protein-1 (AP-1) subunits c-Fos, FosB, Fra-1, Fra-2,
c-Jun, JunB and JunD for super-shift assay were purchased from
Santa Cruz Biotechnology (Santa Cruz, CA, USA). Antibody to matrix
metalloproteinase-1 (MMP-1) was purchased from Daiichi Fine
Chemical (Takaoka, Japan).
Cell culture, viability and apoptosis
assays
For the viability assay, the cell lines were plated
in 96-well culture plates for 24 h in complete culture medium.
Different concentrations of each carotenoid were added and the
cells were incubated for 24 h. Cell viability was evaluated by
measuring the mitochondrial-dependent conversion of the
water-soluble tetrazolium (WST)-8 (Nacalai Tesque, Kyoto, Japan) to
a colored formazan product. WST-8 (5 μl) was added for the
last 4 h of incubation and absorbance at 450 nM was measured using
an automated microplate reader. Apoptotic events in cells were
detected by staining with phycoerythrin-conjugated Apo2.7 antibody
(Beckman Coulter, Marseille, France) (9) and analyzed by flow cytometry (Epics
XL, Beckman Coulter, Fullerton, CA, USA). Since the viability of 20
μM fucoxanthinol-treated Saos-2 cells at 24 h was 0%,
electrophoretic mobility shift and protein expression assays were
carried out at 9–12 h for incubation.
Assessment of caspase activities
After treatment with indicated concentrations of
fucoxanthinol (5, 10 and 20 μM) for 9 h, the activities of
caspases-3/7, -8 and -9 were evaluated, respectively, using
Caspase-Glo 3/7, 8 and 9 assay kits (Promega) according to the
manufacturer’s protocol. The luminescence that is proportional to
caspases-3/7, -8 and -9 activities was determined by a
luminometer.
Cell cycle analysis
Cells were treated with fucoxanthinol at a
concentration of 20 μM. After 9 h of incubation, cell cycle
analysis was performed with the CycleTest Plus DNA reagent kit
(Becton-Dickinson Immunocytometry Systems, San Jose, CA, USA).
Briefly, cells were washed with a buffer solution containing sodium
citrate, sucrose and DMSO, suspended in a solution containing RNase
A and then stained with 125 μg/ml propidium iodide for 10
min. Cell suspensions were analyzed on a Coulter EPICS XL using
EXPO32 software. The population of cells in each cell cycle was
determined with the MultiCycle software.
Reverse transcription-PCR (RT-PCR)
Total cellular RNA from cells was extracted with
TRIzol (Invitrogen, Carlsbad, CA, USA) according to the protocol
provided by the manufacturer. First-strand cDNA was synthesized
from 1 μg total cellular RNA using a PrimeScript RT-PCR kit
(Takara Bio Inc., Otsu, Japan) with random primers. The primers
used were 5′-GGTGCCCAGTGGTTGAAAAAT-3′ (forward) and
5′-CATCACTTCTCCCCGAATCGT-3′ (reverse) for MMP-1 and
5′-GCCAAGGTCATCCATGACAACTTTGG-3′ (forward) and
5′-GCCTGCTTCACCACCTTCTTGATGTC-3′ (reverse) for glyceraldehyde
3-phosphate dehydrogenase (GAPDH). The length of RT-PCR was 40
cycles for MMP-1 and 27 cycles for GAPDH. The PCR products were
fractionated on 2% agarose gels and visualized by ethidium bromide
staining.
Western blot analysis
Cells were lysed in a buffer containing 62.5 mM
Tris-HCl (pH 6.8), 2% sodium dodecyl sulfate, 10% glycerol, 6%
2-mercaptoethanol and 0.01% bromophenol blue. Equal amounts of
protein (20 μg) were subjected to electrophoresis on sodium
dodecyl sulfate-polyacrylamide gels, followed by transfer to a
polyvinylidene difluoride membrane and sequential probing with the
specific antibodies. The bands were visualized with an enhanced
chemiluminescence kit (Amersham Biosciences, Piscataway, NJ,
USA).
Preparation of nuclear extracts and
electrophoretic mobility shift assay (EMSA)
Nuclear extracts were obtained as described by
Antalis and Godbolt (10) with
modifications and EMSA was performed as described previously
(11). Briefly, 5 μg of
nuclear extract was incubated with 32P-labeled probes.
The DNA-protein complex was separated from the free
oligonucleotides on 4% polyacrylamide gel. To examine the
specificity of the probe, we preincubated unlabeled competitor
oligonucleotides with nuclear extracts for 15 min before incubation
with probes. The probes or competitors used were prepared by
annealing the sense and antisense synthetic oligonucleotides as
follows: for the wild-type AP-1 element of the MMP-1 gene,
5′-GATCTTATAAAGCATGAGTCAGACACCTCT-3′; for the
AP-1 element mutant of the MMP-1 gene, 5′-GATCTTAT
AAAGCATGAtggAGACACCTCT-3′ (sites of
mutation are indicated in lowercase); for the AP-1 element of the
interleukin (IL)-8 gene, 5′-GATCGTGATGACTCAGGTT-3′; and for the
nuclear factor-κB (NF-κB) element of the IL-2 receptor α chain
(IL-2Rα) gene, 5′-GATCCGGCAGGGGAATCTCCCTCTC-3′. The
oligonucleotide 5′-GATCTGTCGAATGCAAATCACTAGAA-3′, containing
the consensus sequence of the octamer binding motif, was used to
identify specific binding of the transcription factor, Oct-1. The
underlined sequences above are the AP-1, NF-κB and Oct-1 binding
sites, respectively. To identify transcription factors in the
DNA-protein complex detected by EMSA, we used antibodies specific
for various AP-1 family proteins, including c-Fos, FosB, Fra-1,
Fra-2, c-Jun, JunB and JunD (Santa Cruz Biotechnology), to elicit a
supershift DNA-protein complex formation. These antibodies were
incubated with the nuclear extracts for 45 min at room temperature
before incubation with radiolabeled probe.
Cell invasion assay
ACEA electrosensing ×16 microtiter plates were
coated with 215 μg/ml rat tail type I collagen (BD
Biosciences, San Jose, CA, USA). Saos-2 cells were seeded at
3×105 cells/ml in 100 μl of serum-free medium
without or with fucoxanthinol (50 or 100 nM) in the upper chamber
with 8-μm pore size and the lower chamber contained 10%
serum. The ACEA plate was connected to the ACEA Device Station at
37°C and the cells that invaded through type I collagen were
monitored every 1 h in real-time by an ACEA Sensor Analyzer for 24
h and quantitated using ACEA RT-CES Integrated software.
Cell migration assay
Saos-2 cell migration was also assessed using ACEA
electrosensing ×16 microtiter plates. Saos-2 cells were seeded at
3×105 cells/ml in 100 μl of serum-free medium
without or with fucoxanthinol (625 or 1,250 nM) in the upper
chamber with 8-μm pore size and the lower chamber contained
serum-free medium supplemented with 50 ng/ml recombinant human
stromal cell-derived factor-1α (SDF-1α) (PeproTech Inc.). The ACEA
plate was connected to the ACEA Device Station at 37°C and migrated
cells were monitored every 1 h in real time by the ACEA Sensor
Analyzer for 6 h and quantitated using ACEA RT-CES Integrated
software.
Transplant metastasis of LM8 tumor cells
in mice
A mouse osteosarcoma cell line, LM8 cells
(5×106 cells/mouse) in 0.1 ml phosphate buffered saline
was injected subcutaneously into the back of 5-week-old female C3H
mice (Japan SLC, Hamamatsu, Japan) on day 0. Treatment was
initiated on the day after cell injection. Fucoxanthin was
dissolved in soybean oil and 200 mg/kg of fucoxanthin or vehicle
only was given by oral gavage every day for 35 days. The mice were
weighed once a week and the size of the primary tumor was measured
weekly. Mice were also monitored for evidence of morbidity
including anorexia, dehydration, dyspnea, decreased activity and
grooming behavior. On day 35, all mice were euthanized and
autopsied to confirm metastatic lung disease. Lung sections were
prepared and the area of lung metastasis was measured. Primary
tumors were dissected out for measurement of weight and staining
with hematoxylin and eosin (H&E) and terminal deoxynucleotidyl
transferase-mediated dUTP nick-end labeling (TUNEL) using a
commercial kit (Roche Applied Science, Mannheim, Germany). This
experiment was performed according to the Guidelines for Animal
Experimentation of the University of the Ryukyus and was approved
by the Animal Care and Use Committee of the University of the
Ryukyus (permit mumbers: 5150, 5274 and 5338).
Statistical analysis
Data were expressed as mean ± SD. Differences
between groups were examined for statistical significance using the
unpaired Student’s t-test. A P<0.05 denoted the presence of a
statistically significant difference.
Results
Effect of fucoxanthin and fucoxanthinol
on osteosarcoma cell viability
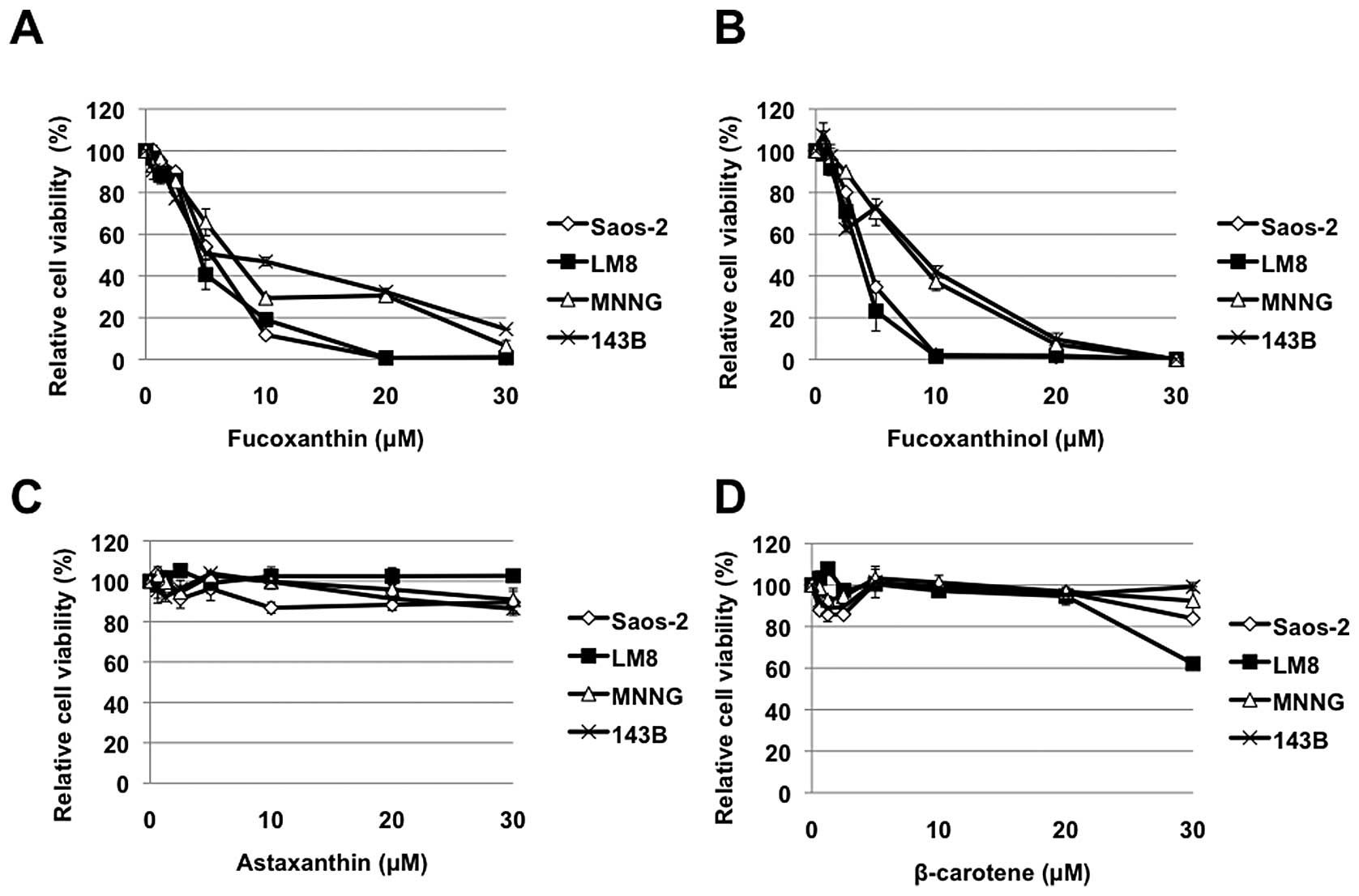
We first examined the effects of carotenoids on the
cell viability of osteosarcoma cell lines. Fucoxanthin and
fucoxanthinol used in this study reduced cell viability of all 4
osteosarcoma cell lines in a dose-dependent manner (Fig. 1A and B). In contrast, the effects
of other carotenoids, β-carotene and astaxanthin, were less
significant, although β-carotene reduced LM8 cell viability at 30
μM concentration (Fig. 1C and
D).
Caspase-dependent induction of apoptosis
by fucoxanthinol
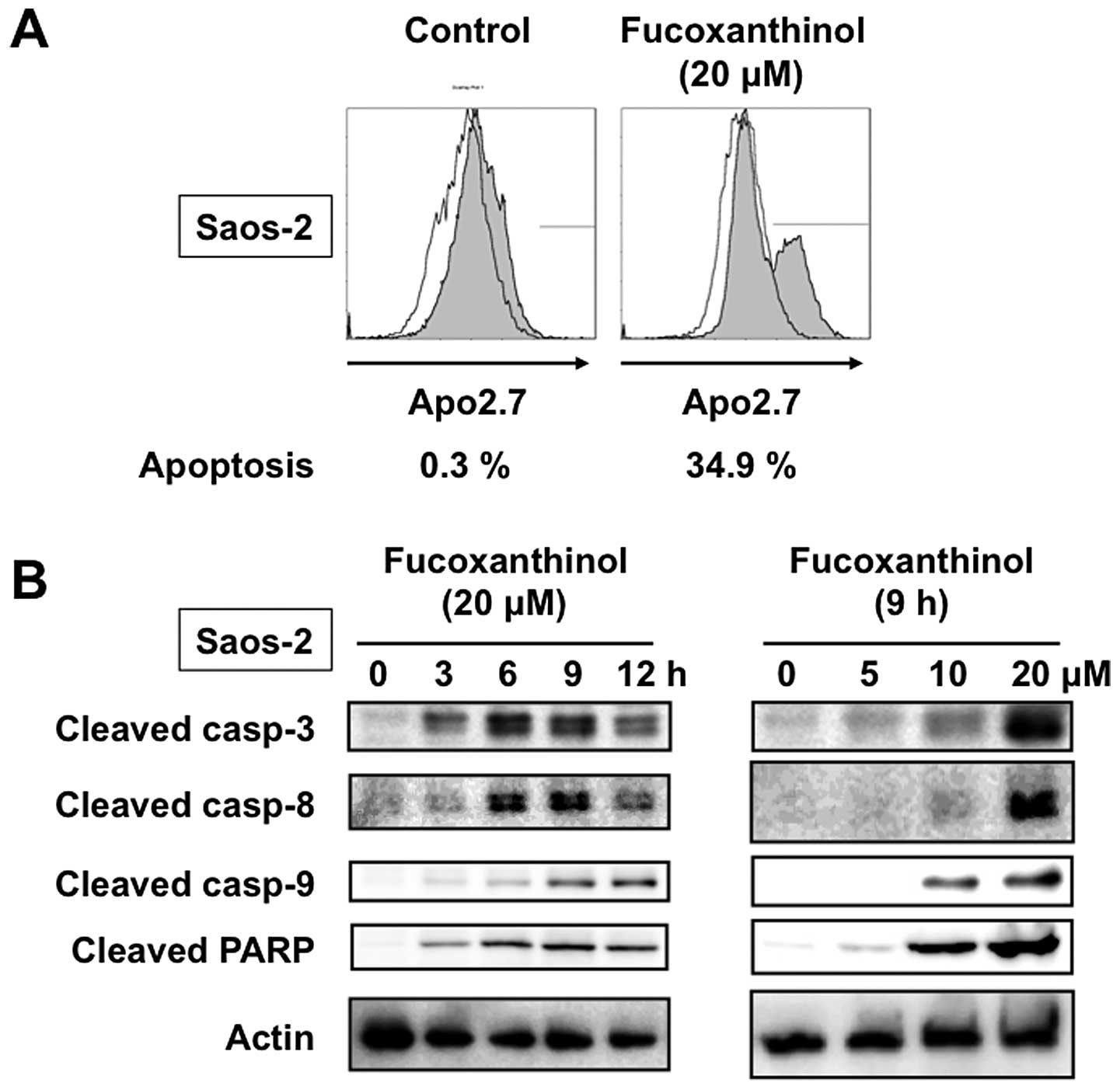
The apoptosis-inducing activity of fucoxanthinol was
analyzed by immunostaining with Apo2.7, which specifically detects
the 38-kDa mitochondrial membrane antigen, 7A6, expressed on the
mitochondrial outer membrane during apoptosis (9). The proportion of 7A6-positive cells
among Saos-2 cells incubated for 9 h without fucoxanthinol was
0.3%, but increased to 34.9% when the cells were treated with 20
μM fucoxanthinol (Fig. 2A).
We next investigated the role of caspases in fucoxanthinol-induced
apoptosis by measuring the cleavage of known caspase substrates by
immunoblot analysis. Fucoxanthinol cleaved the caspase-3-specific
substrate, PARP, in Saos-2 cells in a time- and dose-dependent
manner. In addition, fucoxanthinol processed the initiator
caspases-8 and -9 and the executioner caspase-3 in Saos-2 cells in
a time- and dose-dependent manner (Fig. 2B). We also investigated whether
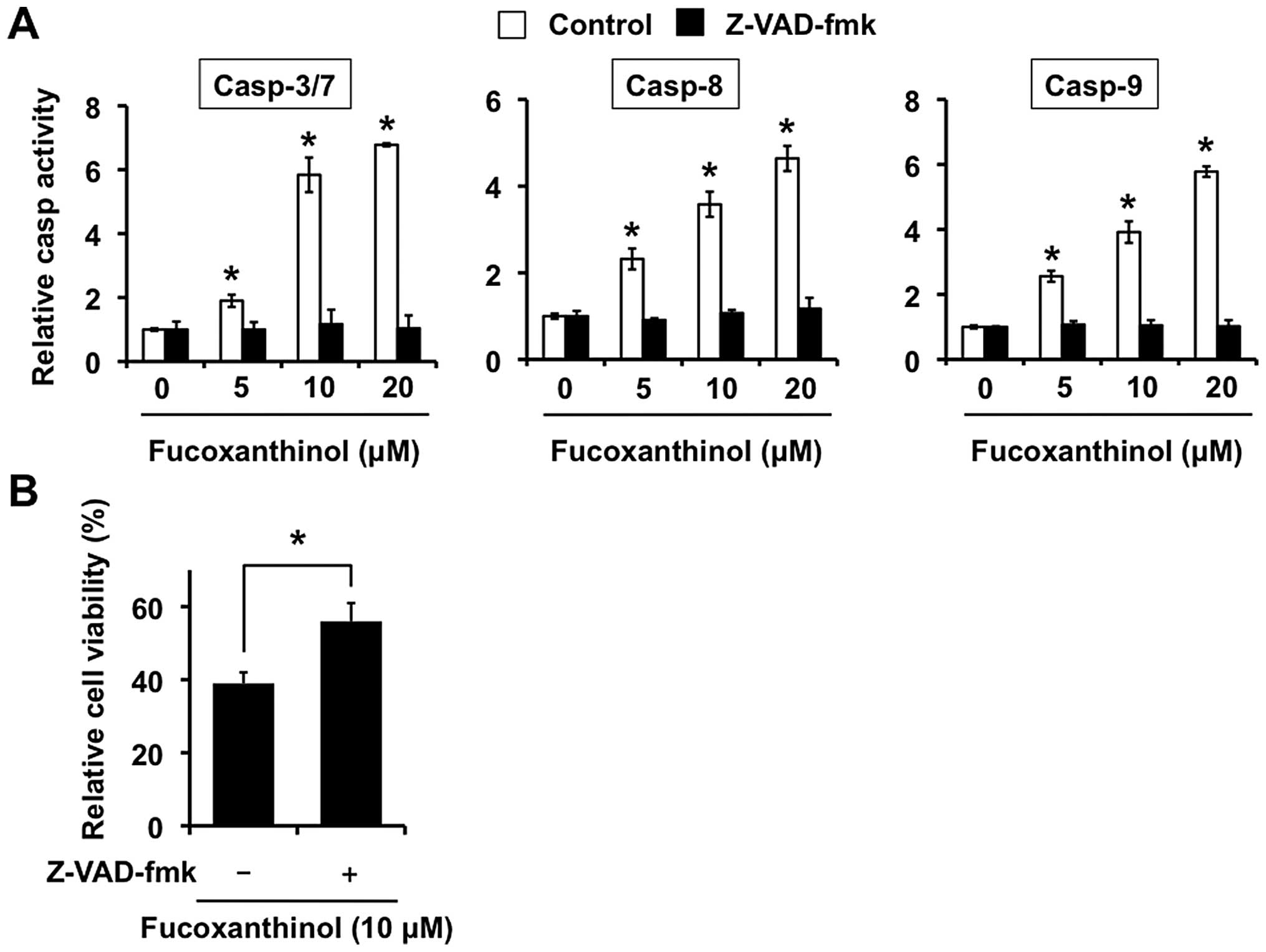
fucoxanthinol can activate the caspases by examining protease
activity using fluorescence substrates specific for caspases-3/7,
-8 and -9. As shown in Fig. 3A,
after treatment with fucoxanthinol for 9 h, the activities of
caspases-3/7, -8 and -9 were all obviously increased in a
dose-dependent manner compared with controls.
To investigate further the involvement of the
caspase pathway, Saos-2 cells were treated with a broad spectrum
caspase inhibitor, Z-VAD-fmk, together with fucoxanthinol. Saos-2
cell treatment with fucoxanthinol decreased the activities of
caspases-3/7, -8 and -9 after adding Z-VAD-fmk (Fig. 3A). As shown in Fig. 3B, reduced cell viability induced by
fucoxanthinol was significantly diminished by Z-VAD-fmk. These
results indicate that fucoxanthinol-induced apoptosis of Saos-2
cells is mediated through caspase activation.
Fucoxanthinol causes G1 cell
cycle arrest
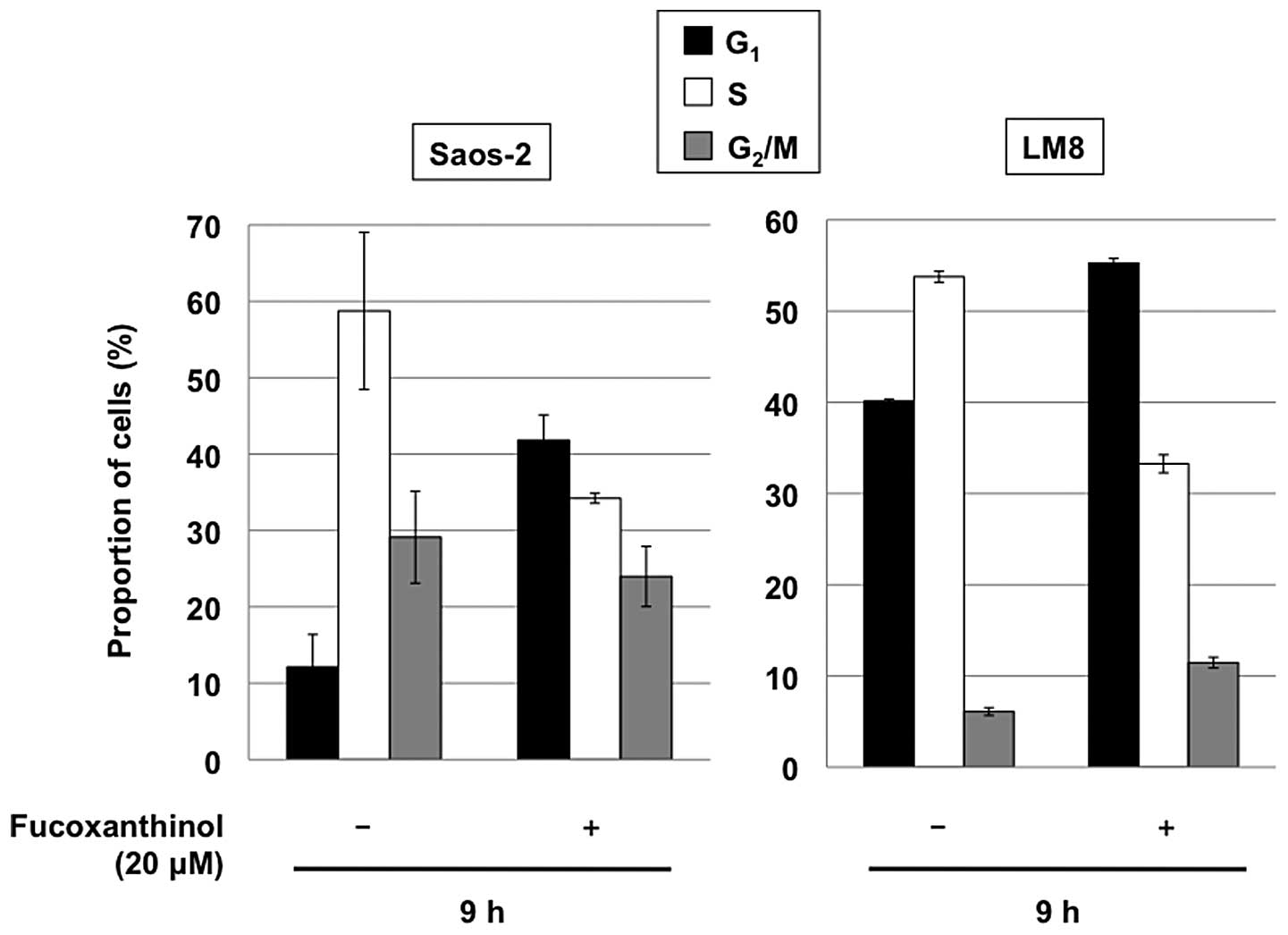
We also examined the distribution of cellular DNA
contents by flow cytometric analysis. Cultivation of Saos-2 and LM8
cells with 20 μM fucoxanthinol for 9 h increased the
population of the cells in the G1 phase, with marked
reduction of the S phase, relative to untreated cells (Fig. 4). These results indicate that,
together with induction of apoptosis, fucoxanthinol induces
G1 cell cycle arrest in osteosarcoma cells.
Effects of fucoxanthinol on the
expression of cell cycle regulatory proteins
G1-S progression is influenced by diverse
growth signaling pathways that converge on the control of CDK,
including CDK4 or CDK6 in conjunction with D type cyclins and CDK2
in conjunction with cyclin E (12). The best characterized substrates of
G1 CDKs are the retinoblastoma protein (pRB) and the
pRB-related proteins, p107 and p130 (12). Each of these pRB family members can
block the progression from G1 into S and is thought to
do so, at least in part, by binding to E2F transcription factors
and repressing genes that contribute to S phase entry (12). These pRB family proteins are
phosphorylated and released from E2Fs in the late G1
phase of the cell cycle (12). In
pRB (−) Saos-2 cells, p130 but not p107 was phosphorylated and
released from E2F-4 in a CDK2-dependent process in late
G1 and S phase cells (13).
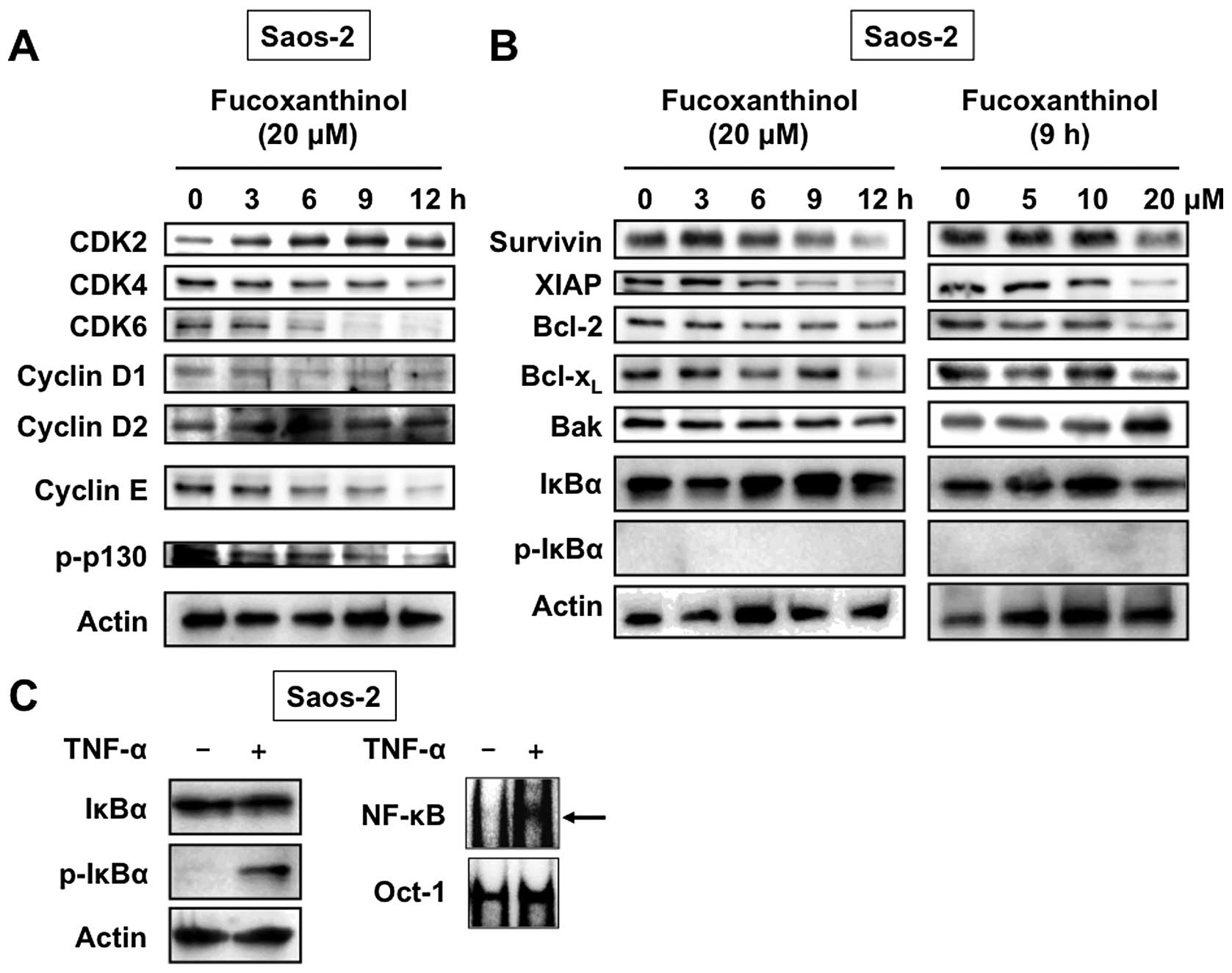
To clarify the molecular mechanism of
fucoxanthinol-induced G1 cell cycle arrest, we
investigated its effects on the expression of several intracellular
regulators of cell cycle by western blot analysis. As shown in
Fig. 5A, fucoxanthinol
significantly reduced the expression of CDK4, CDK6 and cyclin E in
Saos-2 cells in a time-dependent manner, but had no effect on CDK2,
cyclin D1 and cyclin D2 expression levels. Furthermore,
fucoxanthinol inhibited phosphorylation of p130. Comparable loading
of protein was confirmed with a specific antibody for the
housekeeping gene product actin (Fig.
5A). These results indicate that fucoxanthinol dephosphorylates
p130 by inhibiting the expression of CDK4, CDK6 and cyclin E,
resulting in G1 cell cycle arrest.
Effects of fucoxanthinol on the
expression of apoptosis regulatory proteins
To elucidate the possible molecular targets of
fucoxanthinol-induced apoptosis of Saos-2 cells, we examined the
expression of important apoptosis regulators. As shown in Fig. 5B, fucoxanthinol potently reduced
the expression of anti-apoptotic proteins survivin, XIAP, Bcl-2 and
Bcl-xL in a time- and dose-dependent manner, but had no
effect on proapoptotic protein Bak.
Inhibitory effects of fucoxanthinol on
Akt activation
Members of the NF-κB family control the expression
of several genes that regulate cell survival, proliferation and
apoptosis (14). NF-κB is inactive
in the cytosol because it is bound to IκBα and becomes active after
IκBα has been phosphorylated and subsequently degraded (15). Because CDK4, CDK6, cyclin E,
survivin, XIAP, Bcl-2 and Bcl-xL are controlled by NF-κB
(16–22), we determined the effects of
fucoxanthinol on the phosphorylation and degradation of IκBα. As
shown in Fig. 5B, treatment of
Saos-2 cells with fucoxanthinol did not affect the level of total
IκBα. In addition, phosphorylated IκBα could not be detected in
untreated or fucoxanthinol-treated Saos-2 cells (Fig. 5B). The activation of NF-κB in
Saos-2 cells in response to TNF-α stimulus was assessed by western
blotting and EMSA. EMSA detects nuclear factor binding to a
specific consensus NF-κB sequence. As shown in Fig. 5C, treatment of Saos-2 cells with
TNF-α resulted in increases in IκBα phosphorylation and NF-κB-DNA
binding, while basal levels of phosphorylated IκBα as well as
NF-κB-DNA binding were not observed in Saos-2 cells. These results
show that fucoxanthinol does not affect NF-κB activation.
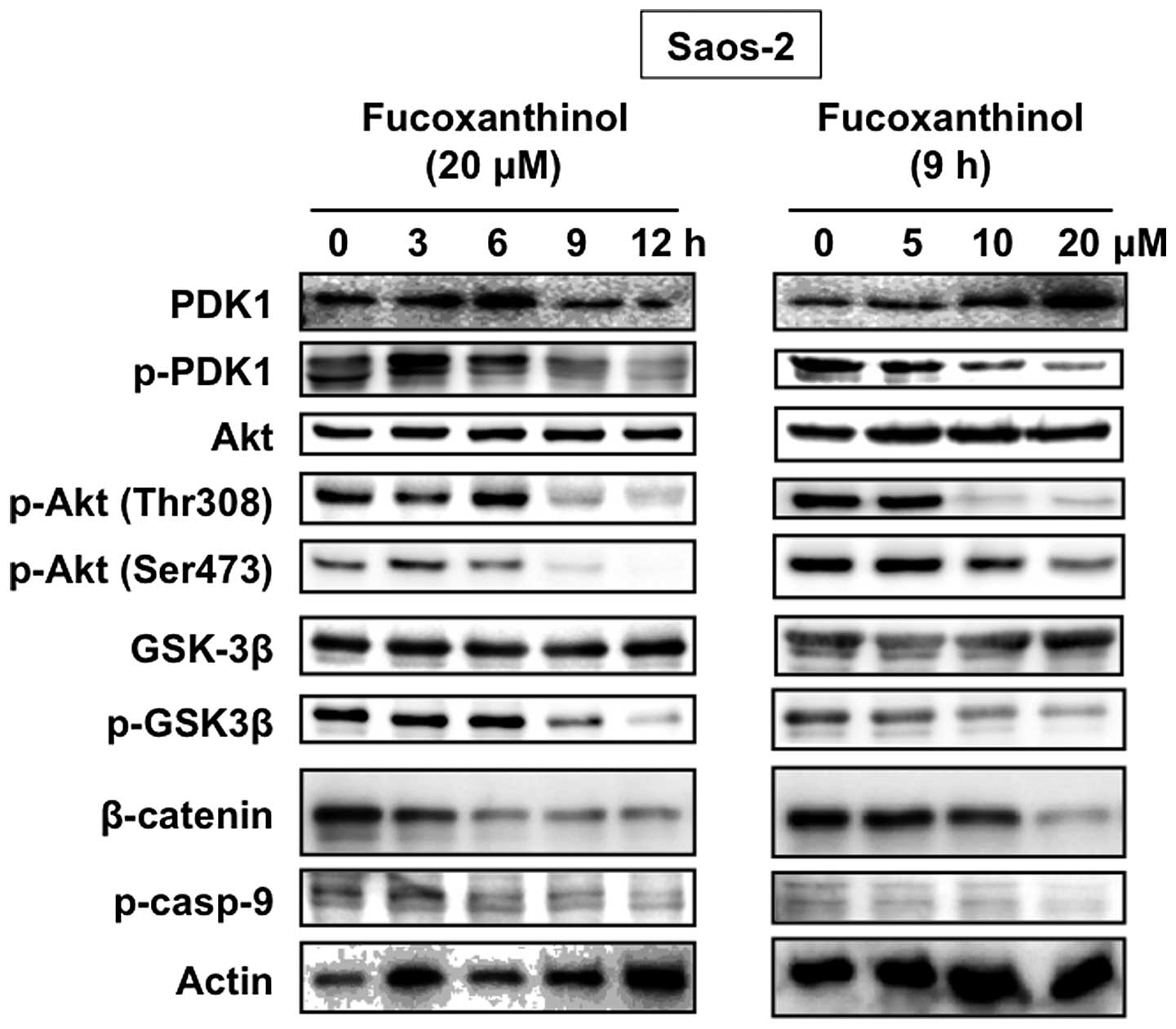
The phosphatidylinositol 3-kinase (PI3K)-Akt pathway
also plays an important role in various cellular processes
including cell growth and survival in osteosarcoma cells (23). Akt prevents apoptosis by activating
anti-apoptotic signals by phosphorylating GSK3β and caspase-9 and
by activating NF-κB (24). To
determine whether the Akt activity is associated with the apoptotic
effects of fucoxanthinol, we examined the protein expression and
phosphorylation level of Akt after fucoxanthinol treatment. Akt was
constitutively activated in Saos-2 cells and fucoxanthinol
decreased the phosphorylation level of Akt at both Thr308 and
Ser473 sites (Fig. 6).
We next examined the effect of fucoxanthinol on the
phosphorylation levels of two Akt downstream targets: GSK3β and
caspase-9, in Saos-2 cells. As shown in Fig. 6, constitutive phosphorylation of
GSK3β and caspase-9 was seen in Saos-2 cells and fucoxanthinol
reduced the phosphorylation level. A key downstream target of GSK3β
is the proto-oncogene, β-catenin. Fucoxanthinol inhibited GSK3β
phosphorylation, thereby maintaining GSK3β in its active form.
Active GSK3β induced β-catenin phosphorylation, resulting in
increased ubiquitin-mediated proteolysis and decreased levels of
signaling-competent β-catenin. Fucoxanthinol reduced the levels of
β-catenin in Saos-2 cells (Fig.
6). Akt is phosphorylated at Thr308 in the kinase activation
loop mediated by PDK1. Fucoxanthinol inhibited the phosphorylation
of PDK1, but not the total PDK1 (Fig.
6).
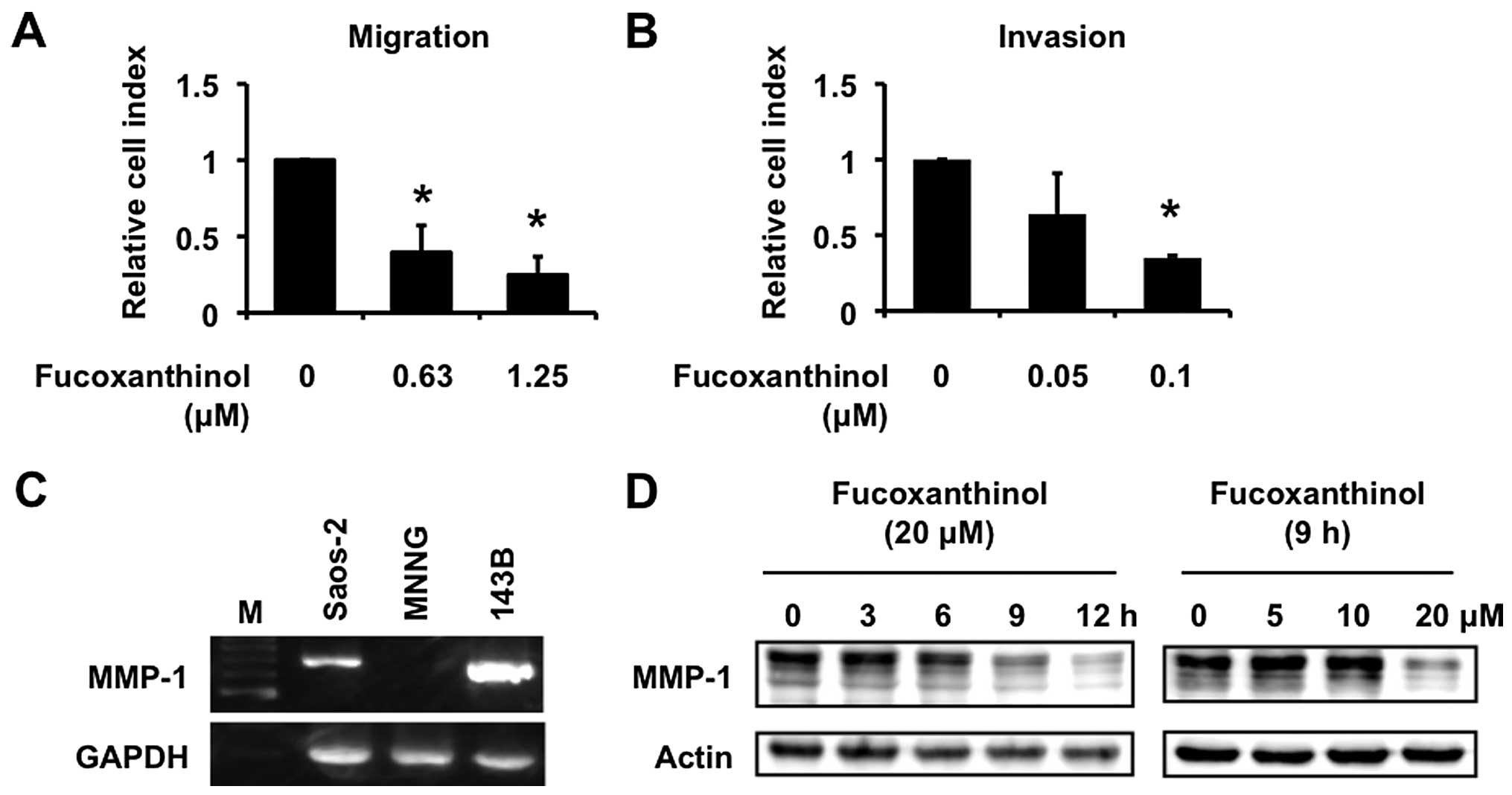
Fucoxanthinol inhibits cell migration and
invasion
Osteosarcoma is characterized by a high metastatic
potential. The chemokine SDF-1α and its receptor CXCR4, play a
crucial role in adhesion and migration of osteosarcoma cells
(25). Fucoxanthinol inhibited
SDF-1α-induced migration of Saos-2 cells in a dose-dependent manner
(Fig. 7A). The effect of
fucoxanthinol on invasion of Saos-2 cells was examined by using a
type I collagen-coated transwell cell culture chambers. Saos-2
cells moved from the top chamber to the bottom chamber in the
absence of fucoxanthinol, but the penetration of type I
collagen-coated filter by Saos-2 cells was inhibited in the
presence of fucoxanthinol (Fig.
7B).
We have recently reported that MMP-1, otherwise
known as collagenase-1, plays important roles in invasion of
osteosarcoma cells (26). MMP-1
was highly expressed in the high-frequent pulmonary metastatic
human osteosarcoma cell line 143B compared with MNNG cells
(26). Saos-2 cells also expressed
MMP-1 mRNA (Fig. 7C) and
fucoxanthinol inhibited such expression in a time- and
dose-dependent manner (Fig.
7D).
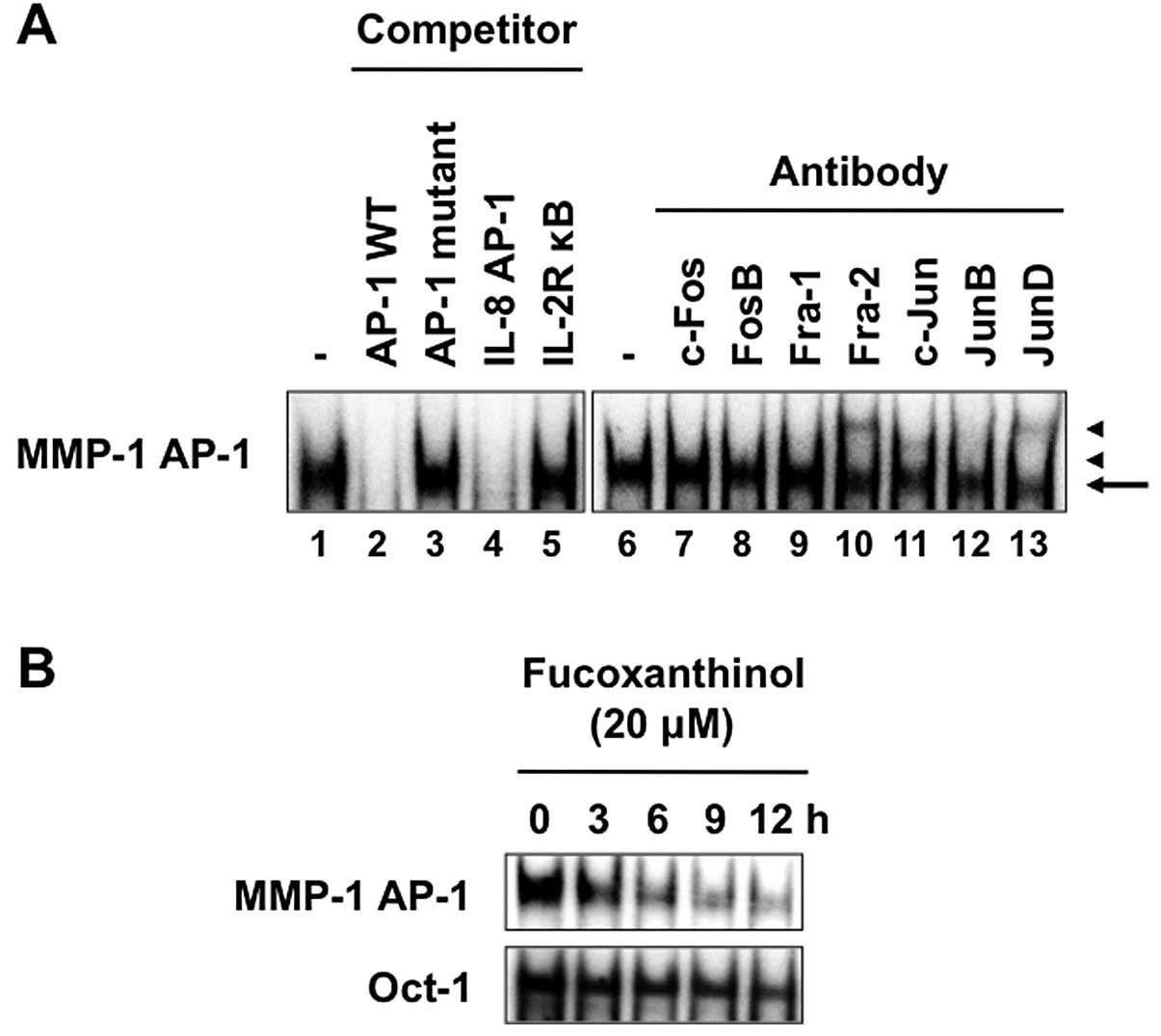
Fucoxanthinol inhibits AP-1
activation
We reported previously that AP-1 signaling pathway
upregulates the expression of MMP-1 (26). EMSA was performed with a
double-stranded oligonucleotide representing the AP-1 element in
the MMP-1 promoter. A protein complex bound to the AP-1 site was
detected in nuclear extracts from Saos-2 cells (Fig. 8A, lane 1). The specificity of
DNA-protein complex formation was assessed by competition studies
with unlabeled competitors. As expected, a ‘cold’ MMP-1 wild-type
AP-1 oligonucleotide or an AP-1 site from the IL-8 promoter
effectively competed with the labeled MMP-1 AP-1 probe and
eliminated the binding of nuclear extracts from Saos-2 cells
(Fig. 8A, lanes 2 and 4). In
contrast, the unlabeled MMP-1 AP-1 mutant or consensus NF-κB site
from the IL-2Rα promoter could not compete with the labeled AP-1
probe (Fig. 8A, lanes 3 and 5).
The exact composition of the transcription factor DNA-protein
complex in Saos-2 cells was analyzed by supershift assay using
specific antibodies. These experiments identified Fra-2, c-Jun and
JunD as the predominant components of the AP-1 complex on the MMP-1
AP-1 site in Saos-2 cells (Fig. 8A,
lanes 10, 11 and 13). Fucoxanthinol decreased the protein
complex bound to the AP-1 site in nuclear extracts derived from
Saos-2 cells and such effect was time-dependent (Fig. 8B), suggesting that fucoxanthinol
suppressed the invasion of Saos-2 cells by suppressing the AP-1
signaling pathway. The inhibitory effect also appeared specific to
AP-1 and not related to cell death, because no significant change
in binding of Oct-1 was observed after treatment with
fucoxanthinol.
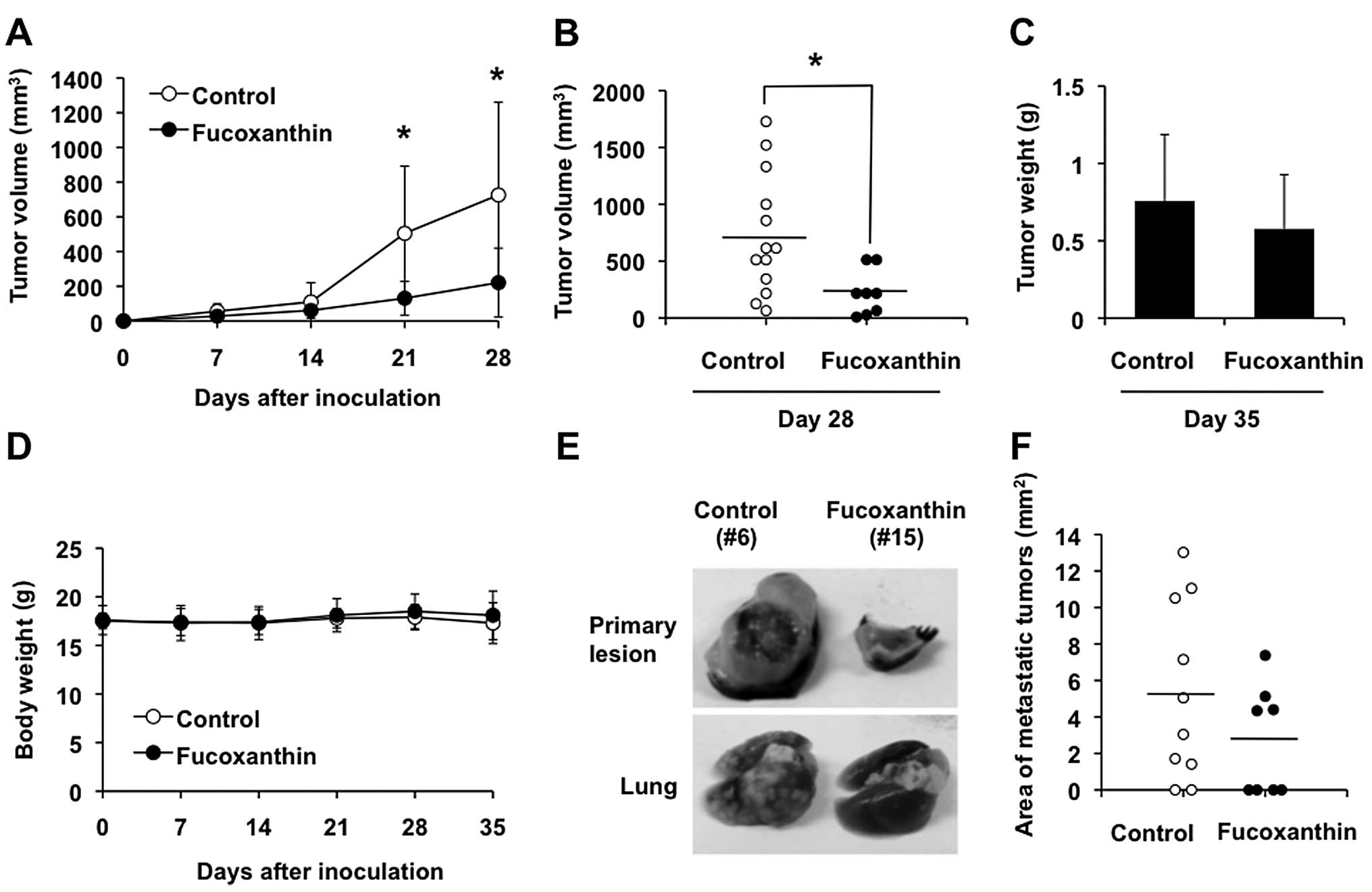
Fucoxanthin reduces primary tumor size
and pulmonary metastasis in mice
Inoculation of the murine osteosarcoma cell line,
LM8, into the skin of C3H mice results in the formation of tumors
with high metastatic potential to the lung (27). We assessed the antitumor activity
of fucoxanthin using this model. Subcutaneous inoculation of LM8
cells was followed by the appearance of tumors that exhibited rapid
growth, reaching 726 mm3 in size within 4 weeks
(Fig. 9A and B). Three of 13 mice
inoculated with LM8 cells died between 4 and 5 weeks after
inoculation (Table I). Multiple
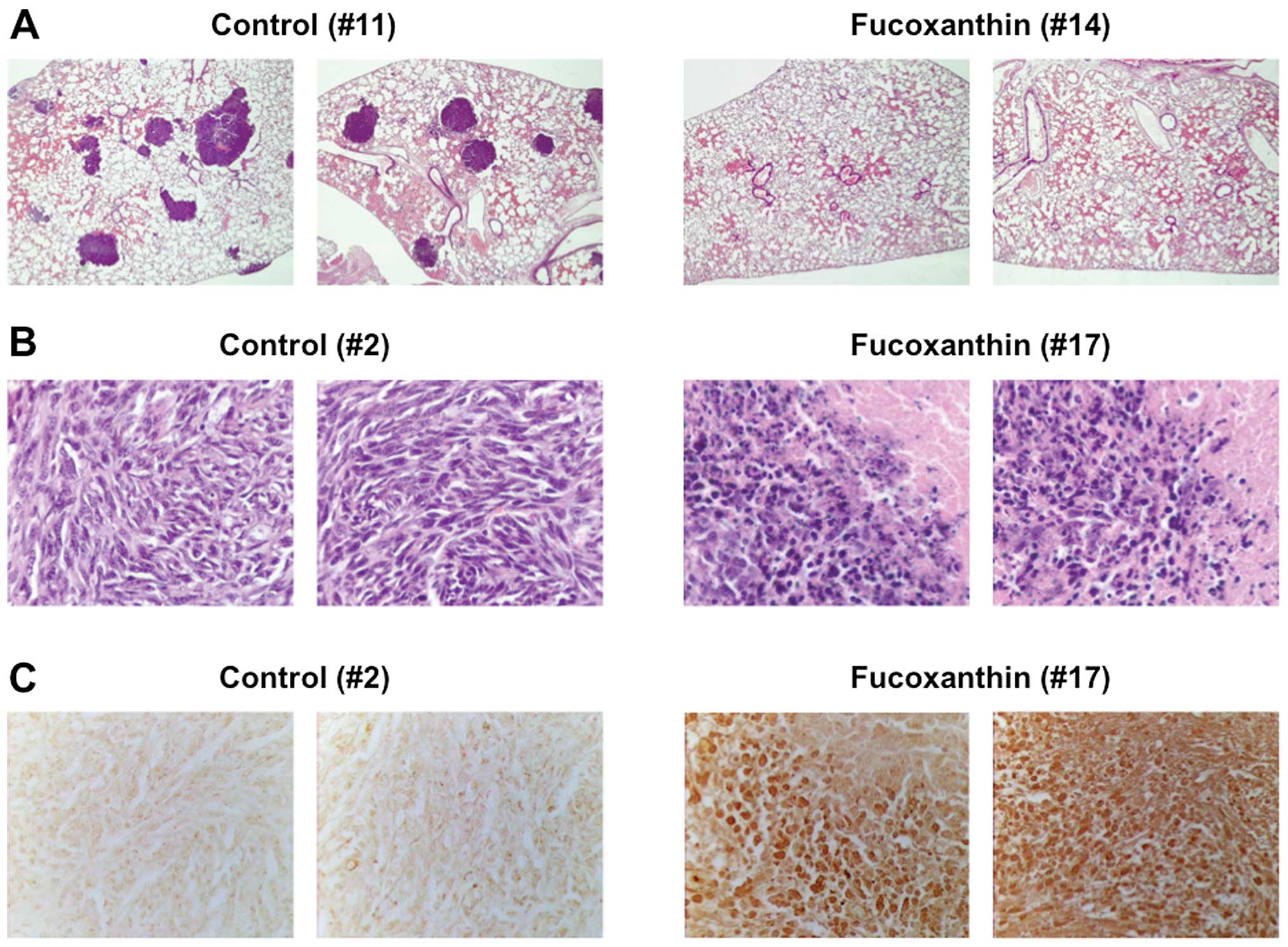
metastatic nodules were found macroscopically and confirmed
histopathologically in the lungs of 8 of 10 LM8-inoculated mice
(80%) at 5 weeks after inoculation (Table I, Fig.
9E, bottom panel, left and 10A, left panels). Treatment of
these mice with 200 mg/kg/day of fucoxanthin after inoculation of
LM8 showed significant reduction of the primary tumor volume
associated with increased apoptotic cells in the tumor as judged by
H&E staining and TUNEL assay (Fig.
9A, B and E, top panel, right, 10B and C, right panels), although the
difference in tumor weight after 5 weeks of treatment, compared
with control, was less conspicuous (Fig. 9C). The mean tumor volume was lower
in mice treated with fucoxanthin than with control, albeit
statistically insignificant after 5 weeks of treatment, because the
3 untreated mice with huge tumors died between 4 and 5 weeks after
inoculation (data not shown). Although 4 of 8 fucoxanthin-treated
mice (50%) had visible gross lung nodules, all mice were still
alive at 5 weeks after inoculation (Table I). The area of lung metastasis in
fucoxanthin-treated mice tended to be smaller than the
vehicle-treated control mice but the difference was not significant
(Fig. 9F). We found little
difference in body weight of the fucoxanthin-treated and untreated
groups (Fig. 9D) and no obvious
abnormalities in the treated mice at this dosage.
 | Table I.C3H mice inoculated with LM8
cells. |
Table I.
C3H mice inoculated with LM8
cells.
| Mouse no. | Treatment | Lung metastases at
35 days | Survival |
|---|
| 1 | Control | + | Alive |
| 2 | Control | ++ | Alive |
| 3 | Control | + | Alive |
| 4 | Control | NE | Deada |
| 5 | Control | ++ | Alive |
| 6 | Control | +++ | Alive |
| 7 | Control | − | Alive |
| 8 | Control | ++ | Alive |
| 9 | Control | NE | Deada |
| 10 | Control | NE | Deada |
| 11 | Control | ++ | Alive |
| 12 | Control | ++ | Alive |
| 13 | Control | − | Alive |
| Total | | 8/10 (80%) | 10/13 (77%) |
| 14 | Fucoxanthin | − | Alive |
| 15 | Fucoxanthin | − | Alive |
| 16 | Fucoxanthin | + | Alive |
| 17 | Fucoxanthin | ++ | Alive |
| 18 | Fucoxanthin | − | Alive |
| 19 | Fucoxanthin | − | Alive |
| 20 | Fucoxanthin | ++ | Alive |
| 21 | Fucoxanthin | + | Alive |
| Total | | 4/8 (50%) | 8/8 (100%) |
Discussion
The main issue addressed by this study is whether
carotenoids have any effects on osteosarcoma cells and the possible
molecular mechanisms of any such effect. The results showed that
fucoxanthin isolated from C. okamuranus Tokida and
fucoxanthinol prepared by enzymatic hydrolysis of purified
fucoxanthin exhibited anti-osteosarcoma properties; these
properties appear to be at least in part attributable to the
inhibition of Akt and AP-1 signal pathways in osteosarcoma
cells.
In this study, we demonstrated that fucoxanthin and
fucoxanthinol decreased cell viability in the 4 tested osteosarcoma
cell lines in a concentration-dependent manner. The inhibitory
effects of fucoxanthin and fucoxanthinol on the cell viability were
remarkable compared with other carotenoids. Further, we studied the
effect of fucoxanthinol on the induction of apoptosis and the
results demonstrated that fucoxanthinol induced apoptosis through
activation of caspases-3, -8 and -9 in Saos-2 cells. Inhibition of
cell viability in Saos-2 cells treated with fucoxanthinol was
significantly diminished by the general caspase inhibitor
Z-VAD-fmk. However, since Z-VAD-fmk partially diminished a decrease
in cell viability, it is suggested that the apoptosis signaling in
Saos-2 cells by fucoxanthinol is mediated by both caspase-dependent
and -independent pathways. The apoptotic effect of fucoxanthinol
was associated with suppression of expression of survivin, XIAP,
Bcl-2 and Bcl-xL. Dephosphorylation of p130 through the
downregulation of CDK4, CDK6 and cyclin E also seems to contribute
to the activation of G1 cell cycle arrest.
The Akt signaling pathway is important for cell
survival and apoptosis (24).
Increased Akt phosphorylation has been reported in osteosarcoma
cell lines U2OS and MG63 (23).
The present results showed that Akt is constitutively
phosphorylated at both Ser473 and Thr308 in Saos-2 cells.
Fucoxanthinol inhibited Akt phosphorylation at these two sites. As
the downstream targets of Akt, GSK3β and caspase-9 have been
reported to be involved in the regulation of cell survival. Akt
promotes cell survival by phosphorylating GSK3β and caspase-9,
which inactivates them and prevents apoptosis (24). The results showed constitutive
phosphorylation of GSK3β and caspase-9 in Saos-2 cells and
fucoxanthinol suppressed the phosphorylation levels. To further
confirm the role of GSK3β in fucoxanthinol-induced apoptosis, we
examined the expression of β-catenin, a downstream target of GSK3β.
The results showed that fucoxanthinol reduced the level of
β-catenin. NF-κB is activated by stimulation of the IκB kinase
complex, which phosphorylates IκBα. IκB kinase phosphorylation by
Akt is essential for NF-κB activation (28). However, we could not observe
constitutive activation of NF-κB in Saos-2 cells. Basal levels of
phosphorylated IκBα and NF-κB RelA as well as NF-κB-DNA binding
were not observed in Saos-2 cells (Fig. 5C and data not shown) (29). In addition, fucoxanthinol did not
alter the levels of total and phosphorylated IκBα. Thus,
fucoxanthinol-induced apoptosis is independent of NF-κB. We
investigated the effect of fucoxanthinol on the upstream event of
Akt, PDK1. The results demonstrated that fucoxanthinol inhibited
the expression of phosphorylated PDK1, but not the total level of
PDK1. Akt promoted cell survival by inhibiting apoptosis through
its ability to phosphorylate and inactivate several targets, which
are components of the intrinsic cell death machinery, including the
Bcl-2 and IAP family members. Akt can be an upstream regulator of
XIAP, which possess an Akt phosphorylation site (30). Furthermore, survivin and Bcl-2 have
been shown to be downstream targets of Akt signaling (31,32).
Thus, inhibition of Akt pathway mediates, at least in part, the
induction of apoptosis and cell cycle arrest in G1 phase
induced by fucoxanthinol.
The mean 5-year survival rate of patients with
metasta-sizing osteosarcoma is only 20–30% (33). Therefore, many investigators have
focused on the development of new agents for blocking cancer cell
metastasis. MMPs play an important role in tumor angiogenesis,
metastasis and stimulation of growth factor release from the
extracellular matrices (34). We
investigated the anti-metastatic functions of fucoxanthinol on the
migration and invasion of Saos-2 cells and the results indicated
that fucoxanthinol can inhibit in vitro migration and
invasion ability of Saos-2 cells.
Our results also showed that fucoxanthinol inhibited
MMP-1 expression. MMP-1 is involved in the invasive metastatic
potential of osteosarcoma cells (26). Recent studies showed that MMP-1
silencing inhibits osteosarcoma pulmonary metastases in vivo
(35). Thus, inhibition of MMP-1
expression or enzyme activity can be an early target to prevent
cancer metastasis. We have reported that AP-1 signaling pathway
plays a crucial role in constitutive transactivation of MMP-1 in
osteosarcoma cells (26).
Fucoxanthinol inhibited AP-1-DNA binding activity. Taken together,
our findings suggest that fucoxanthinol has anti-metastatic
activities by blocking AP-1 resulting in inhibition of MMP-1.
The concentration of fucoxanthin or fucoxanthinol
used in this study to exhibit anti-osteosarcoma effects may not be
pharmacologically achievable in humans. Because the concentrations
that induce antitumor effects in vitro often differ from
those in vivo, future in vivo efficacy studies with
fucoxanthin or fucoxanthinol should be conducted in animal models.
In the present study, we described the antitumor properties of
fucoxanthin in mice.
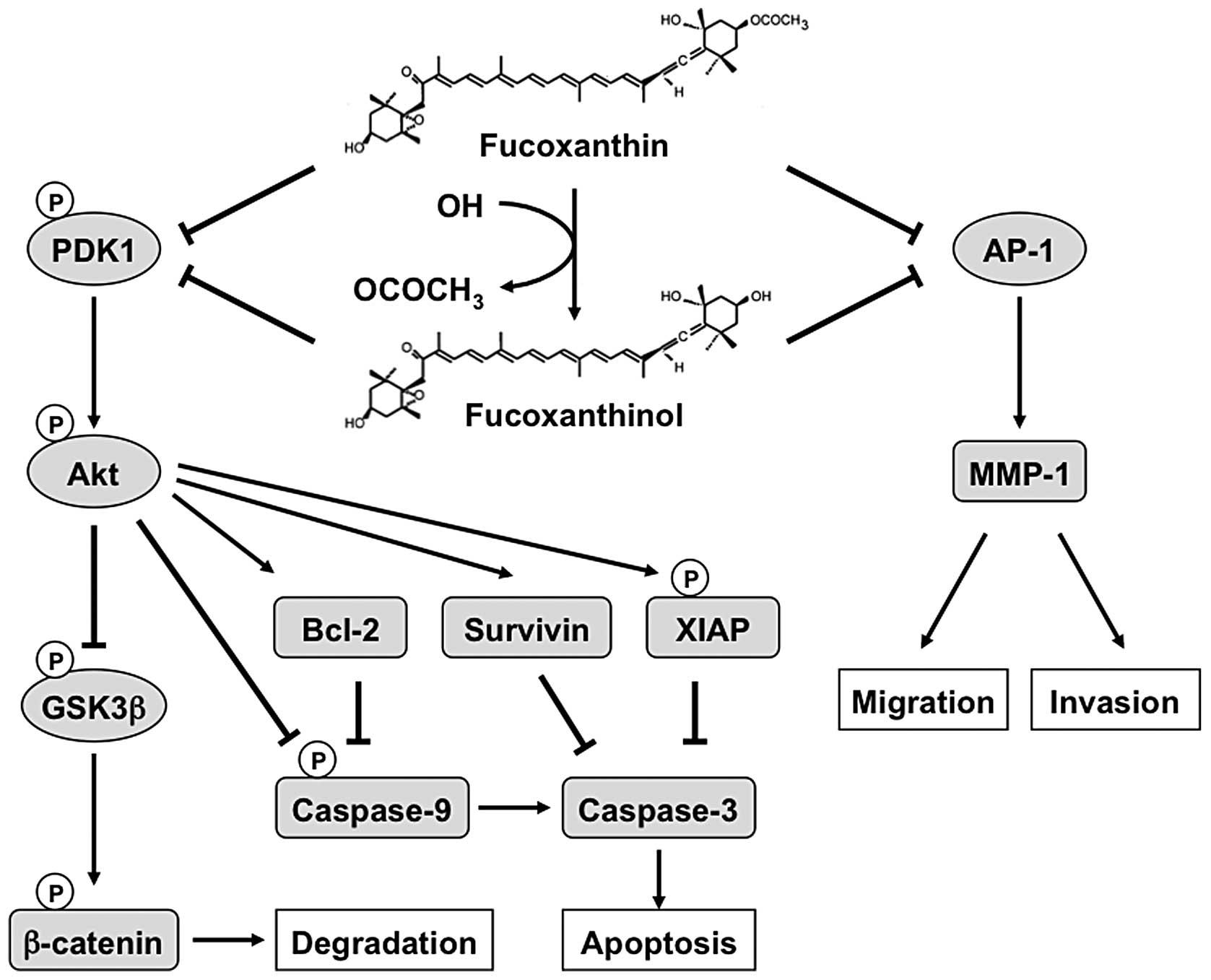
The novel findings of this study are that
fucoxanthin and fucoxanthinol are potent inducers of apoptosis and
cell cycle arrest, with anti-metastatic properties in osteosarcoma
cells at least in part via inhibition of Akt and AP-1. A
hypothetical model for the actions of fucoxanthin and fucoxanthinol
is shown in Fig. 11. Based on
these findings, it is tempting to speculate that fucoxanthin or
fucoxanthinol alone or in combination with other conventional
chemotherapeutics may be potentially useful in the treatment of
osteosarcoma.
References
|
1.
|
Savage S and Mirabello L: Using
epidemiology and genomics to understand osteosarcoma etiology.
Sarcoma. 2011:5481512011. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Anninga JK, Gelderblom H, Fiocco M, Kroep
JR, Taminiau AHM, Hogendoorn PCW and Egeler RM: Chemotherapeutic
adjuvant treatment for osteosarcoma: where do we stand? Eur J
Cancer. 47:2431–2445. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Marina N, Gebhardt M, Teot L and Gorlick
R: Biology and therapeutic advances for pediatric osteosarcoma.
Oncologist. 9:422–441. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Musa-Veloso K, Card JW, Wong AW and Cooper
DA: Influence of observational study design on the interpretation
of cancer risk reduction by carotenoids. Nutr Rev. 67:527–545.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Peng J, Yuan J-P, Wu C-F and Wang J-H:
Fucoxanthin, a marine carotenoid present in brown seaweeds and
diatoms: metabolism and bioactivities relevant to human health. Mar
Drugs. 9:1806–1828. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Yamamoto K, Ishikawa C, Katano H, Yasumoto
T and Mori N: Fucoxanthin and its deacetylated product,
fucoxanthinol, induce apoptosis of primary effusion lymphomas.
Cancer Lett. 300:225–234. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Rhim JS, Putman DL, Arnstein P, Huebner RJ
and McAllister RM: Characterization of human cells transformed in
vitro by N-methyl-N′-nitro-N-nitrosoguanidine. Int J Cancer.
19:505–510. 1977.
|
|
8.
|
Hensler PJ, Annab LA, Barrett JC and
Pereira-Smith OM: A gene involved in control of human cellular
senescence on human chromosome 1q. Mol Cell Biol. 14:2291–2297.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Zhang C, Ao Z, Seth A and Schlossman SF: A
mitochondrial membrane protein defined by a novel monoclonal
antibody is preferentially detected in apoptotic cells. J Immunol.
157:3980–3987. 1996.PubMed/NCBI
|
|
10.
|
Antalis TM and Godbolt D: Isolation of
intact nuclei from hematopoietic cell types. Nucleic Acids Res.
19:43011991. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Mori N and Prager D: Transactivation of
the interleukin-1α promoter by human T-cell leukemia virus type I
and type II Tax proteins. Blood. 87:3410–3417. 1996.
|
|
12.
|
Sherr CJ and Roberts JM: Living with or
without cyclins and cyclin-dependent kinases. Genes Dev.
18:2699–2711. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Cheng L, Rossi F, Fang W, Mori T and
Cobrinik D: Cdk2-dependent phosphorylation and functional
inactivation of the pRB-related p130 protein in pRB(−),
p16INK4A(+) tumor cells. J Biol Chem. 275:30317–30325.
2000.PubMed/NCBI
|
|
14.
|
Perkins ND: The diverse and complex roles
of NF-κB subunits in cancer. Nat Rev Cancer. 12:121–132. 2012.
|
|
15.
|
Hayden MS and Ghosh S: Shared principles
in NF-κB signaling. Cell. 132:344–362. 2008.
|
|
16.
|
Iwanaga R, Ohtani K, Hayashi T and
Nakamura M: Molecular mechanism of cell cycle progression induced
by the oncogene product Tax of human T-cell leukemia virus type I.
Oncogene. 20:2055–2067. 2001. View Article : Google Scholar
|
|
17.
|
Zahradka P, Werner JP, Buhay S, Litchie B,
Helwer G and Thomas S: NF-κB activation is essential for
angiotensin II-dependent proliferation and migration of vascular
smooth muscle cells. J Mol Cell Cardiol. 34:1609–1621. 2002.
|
|
18.
|
Zhu L, Fukuda S, Cordis G, Das DK and
Maulik N: Anti-apoptotic protein survivin plays a significant role
in tubular morphogenesis of human coronary arteriolar endothelial
cells by hypoxic preconditioning. FEBS Lett. 508:369–374. 2001.
View Article : Google Scholar
|
|
19.
|
Stehlik C, de Martin R, Kumabashiri I,
Schmid JA, Binder BR and Lipp J: Nuclear factor (NF)-κB-regulated
X-chromosome-linked iap gene expression protects endothelial cells
from tumor necrosis factor α-induced apoptosis. J Exp Med.
188:211–216. 1998.
|
|
20.
|
Pahl HL: Activators and target genes of
Rel/NF-κB transcription factors. Oncogene. 18:6853–6866. 1999.
|
|
21.
|
Grossmann M, O’Reilly LA, Gugasyan R,
Strasser A, Adams JM and Gerondakis S: The anti-apoptotic
activities of Rel and RelA required during B-cell maturation
involve the regulation of Bcl-2 expression. EMBO J. 19:6351–6360.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Nicot C, Mahieux R, Takemoto S and
Franchini G: Bcl-XL is up-regulated by HTLV-I and
HTLV-II in vitro and in ex vivo ATLL samples. Blood. 96:275–281.
2000.PubMed/NCBI
|
|
23.
|
Jin S, Pang R-P, Shen J-N, Huang G, Wang J
and Zhou J-G: Grifolin induces apoptosis via inhibition of PI3K/AKT
signalling pathway in human osteosarcoma cells. Apoptosis.
12:1317–1326. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Manning BD and Cantley LC: AKT/PKB
signaling: navigating downstream. Cell. 129:1261–1274. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Perissinotto E, Cavalloni G, Leone F,
Fonsato V, Mitola S, Grignani G, Surrenti N, Sangiolo D, Bussolino
F, Piacibello W and Aglietta M: Involvement of chemokine receptor
4/stromal cell-derived factor 1 system during osteosarcoma tumor
progression. Clin Cancer Res. 11:490–497. 2005.PubMed/NCBI
|
|
26.
|
Kimura R, Ishikawa C, Rokkaku T, Janknecht
R and Mori N: Phosphorylated c-Jun and Fra-1 induce matrix
metalloproteinase-1 and thereby regulate invasion activity of 143B
osteosarcoma cells. Biochim Biophys Acta. 1813:1543–1553. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Asai T, Ueda T, Itoh K, Yoshioka K, Aoki
Y, Mori S and Yoshikawa H: Establishment and characterization of a
murine osteosarcoma cell line (LM8) with high metastatic potential
to the lung. Int J Cancer. 76:418–422. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Ozes ON, Mayo LD, Gustin JA, Pfeffer SR,
Pfeffer LM and Donner DB: NF-κB activation by tumour necrosis
factor requires the Akt serine-threonine kinase. Nature. 401:82–85.
1999.
|
|
29.
|
Ali NN, Gilston V and Winyard PG:
Activation of NF-κB in human osteoblasts by stimulators of bone
resorption. FEBS Lett. 460:315–320. 1999.
|
|
30.
|
Dan HC, Sun M, Kaneko S, Feldman RI,
Nicosia SV, Wang H-G, Tsang BK and Cheng JQ: Akt phosphorylation
and stabilization of X-linked inhibitor of apoptosis protein
(XIAP). J Biol Chem. 279:5405–5412. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Liang Y-L, Wang L-Y, Wu H, Ma D-Z, Xu Z
and Zha X-L: PKB phosphorylation and survivin expression are
cooperatively regulated by disruption of microfilament
cytoskeleton. Mol Cell Biochem. 254:257–263. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Pugazhenthi S, Nesterova A, Sable C,
Heidenreich KA, Boxer LM, Heasley LE and Reusch JE-B: Akt/protein
kinase B up-regulates Bcl-2 expression through cAMP-response
element-binding protein. J Biol Chem. 275:10761–10766. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Longhi A, Errani C, De Paolis M, Mercuri M
and Bacci G: Primary bone osteosarcoma in the pediatric age: state
of the art. Cancer Treat Rev. 32:423–436. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Gialeli G, Theocharis AD and Karamanos NK:
Roles of matrix metalloproteinases in cancer progression and their
pharmacological targeting. FEBS J. 278:16–27. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Jawad MU, Garamszegi N, Garamszegi SP,
Correa-Medina M, Diez JA, Wen R and Scully SP: Matrix
metalloproteinase 1: role in sarcoma biology. PLoS One.
5:e142502010. View Article : Google Scholar : PubMed/NCBI
|