Introduction
Hepatocellular carcinoma (HCC) is a highly malignant
disease with extremely poor prognosis. Due to its difficult early
diagnosis, high malignancy, and most importantly the
ineffectiveness of treatments using radiotherapy and chemotherapy,
HCC is the third leading cause of cancer deaths worldwide, with
more than 600,000 deaths each year (1–3).
Conventional surgical resection is still the major treatment
strategy for HCC (4,5), however, the 5-year overall survival
rate after hepatic resection remains low. A single treatment method
cannot satisfy clinical needs (6).
Therefore, understanding the molecular mechanisms underlying liver
tumor formation, cancer progression, recurrence, and metastasis may
contribute to the discovery of more effective intervention methods
and liver cancer treatment targets.
The opioid receptor family members are all G
protein-coupled receptors (7). The
activation of opioid receptors can promote intracellular signal
transduction via different pathways to regulate a variety of
physiological body functions. Among the three classic opioid
receptors, δ opioid receptor (DOR) was the first to be
cloned (8,9). The human DOR gene is located
on chromosome 6q24-25. The coding region of DOR is 1,119 bp
and encodes for 372 amino acid residues (10,11).
DOR protein is widely distributed throughout the human body.
According to the literature, DOR is present in various human
cancers (12–15). Moreover, DOR is involved in
malignant transformation or tumor progression. It was shown that
the activation of the RTK/PI3K/Akt signaling pathway through DOR
can increase the survival rate of NG108-15 cells, which is a
neuroblastoma-glioma hybrid cell line (16). The direct activation of DOR
affects the invasive ability of the HCT-8/E11 colon cancer cell
line (17).
Functional studies of DOR further demonstrated that
DOR plays important roles in the formation and progression of other
liver diseases, including hepatitis, hepatic fibrosis, liver
damage, and cholestatic liver disease (18–20).
Our previous study (21)
determined that the specific DOR agonist, DADLE, via the activation
of DOR on the liver cell membrane, plays a role in protecting liver
cells from apoptosis through the mitochondrial apoptotic
pathway.
This study aims to investigate DOR expression in HCC
and its involvement in tumor progression. Our data suggest that DOR
is widely expressed in human HCC tissues and cells and may promote
cancer cell growth. The downregulation of DOR can significantly
inhibit HCC progression. Our long-term research goal is to use DOR
as a diagnostic, prognostic, and treatment response marker for
HCC.
Materials and methods
Samples
The surgical specimens from 41 patients who had
primary surgical treatment of HCC at Guilin Medical University
Affiliated Hospital between 2009 and 2010 were studied. These
surgical specimens included the primary HCC lesion and its
corresponding adjacent tissue (2 cm away from the edge of the tumor
lesion). All cases were pathologically confirmed, and the patients
had no prior treatment before surgery. Forty cases of normal
adjacent liver tissues from liver trauma and hepatic hemangioma
were used as the control tissues. All of the samples were collected
with approval from the Medical Ethics Committee at Guilin Medical
University Affiliated Hospital.
Cell culture
LO2, HepG2, and Hep3B cells (American Type Culture
Collection, USA) were cultured in RPMI-1640 medium (Gibco-BRL, NY,
USA) supplemented with 10% fetal bovine serum (Hyclone
Laboratories, Inc., UT, USA) and 100 U/ml of penicillin plus 100
U/ml of streptomycin in a 37°C incubator with 5% CO2 and
95% air. MHCC97-H and MHCC97-L cells (American Type Culture
Collection) were cultured in DMEM high glucose medium supplemented
with 10% fetal bovine serum (Hyclone Laboratories) and 100 U/ml of
penicillin plus 100 U/ml of streptomycin in a 37°C incubator with
5% CO2.
Immunohistochemistry staining
The paraffin sections were dewaxed with xylene and
rehydrated in descending concentrations of ethanol. The endogenous
peroxidase was inhibited, and the slides were incubated with
antibodies against DOR (1:200; Santa Cruz Biotechnology, Santa
Cruz, CA, USA) and incubated at 4°C overnight in a humidified
container. After washing with PBS three times, the tissue slides
were treated with a non-biotin horseradish peroxidase detection
system according to the manufacturer’s instructions (Dako).
Immunofluorescence
Cells in the logarithmic growth phase were digested
with 0.25% trypsin. The cells were seeded on coverslips inside the
wells at a concentration of 1×105 cells/ml and incubated
for 24 h. After the incubation, the old medium was discarded, and
the cells were washed three times in PBS. Paraformaldehyde (4%) was
used to fix the cells for 30 min, followed by three washes in PBS
to remove the excess fixative. Cells were then incubated with 0.5%
Triton X-100 for 20 min at room temperature (RT), washed three
times with PBS, and blocked using 5% goat serum at RT for 30 min.
After removing the serum, a rabbit anti-human DOR monoclonal
antibody (1:500 dilution) was added and incubated at 37°C for 2 h.
The cells were washed three times in PBS for 5 min each before
incubating with fluorescein isothiocyanate (FITC)-labeled goat
anti-rabbit secondary antibody (1:200 dilution) at 37°C for 30 min.
After three washes in PBS for 5 min each, all of the coverslips
were collected and mounted onto glass slides using glycerol. All of
the samples were analyzed using fluorescent microscopy. The primary
antibody was omitted in the negative control.
Cell viability
Cells during the logarithmic growth stage were
digested using 0.25% trypsin. Cells were seeded into a 96-well
plate at a concentration of 1×104 cells/ml and incubated
at RT for 24 h. After the cells attached, different doses of
naltrindole (Sigma, USA) were added to 6-wells for each dose. The
negative control group had no naltrindole treatment. The plate was
incubated in a 5% CO2 incubator for 48 h and then 20
μl of MTT (5 mg/ml) was added and incubated in a
CO2 incubator for 4 h at 37°C. After the incubation, the
excess liquid was discarded and the cells were incubated with 150
μl of DMSO at RT for 10 min on a shaker. The
OD570 value was measured using a microplate reader.
Cell cycle analysis
Cells were digested in trypsin and centrifuged to
collect the cell pellets. The cell pellets were washed three times
in PBS, fixed in pre-chilled 70% ethanol and then stored at 4°C
overnight. The next day, the cells were washed three times with PBS
and resuspend into 100 μl of PBS at a final concentration of
1×106 cells/ml. Comprehensive DNA Stain (500 μl)
(RNase 50 mg/l, propidium iodide (PI) 100 mg/l and Triton X-100 1
ml/l) was added to the cell solution followed by light protected
incubation at RT for 15 min. The labeled cells were analyzed using
flow cytometry.
Apoptosis detection
During the early stage of apoptosis, the loss of
cell membrane symmetry causes phosphatidylserine (PS) to be exposed
on the outside of the cell membrane. Annexin V-FITC can bind
specifically with PS on the intact cell membrane. Therefore,
Annexin V-FITC staining can be used to detect early stage apoptotic
cells in a faster and more sensitive way. The cells were digested
in 0.25% trypsin and then incubated at a concentration of
1×106 cells/ml with 5 μl of Annexin V-FITC and
PI. The cells were further incubated protected from light at 37°C
for 15 min and analyzed using flow cytometry.
Hoechst 33342 staining
The cells were cultured for 24 h in a 6-well plate
with poly-L-lysine pre-coated coverslips. After apoptosis
induction, the medium from the cell culture was removed. The cells
were fixed in 4% paraformaldehyde for 30 min. The fixing solution
was then removed and the cells were washed with PBS twice for 3 min
each. After washing, 5 μg/ml of Hoechst 33342 dye was added
to each sample. The plate was incubated at 37°C for 10 min. The
staining solution was removed followed by two washes in PBS for 3
min each. One drop of fluorescence quencher was added to the slide.
The coverslip with the cells attached was exposed to the
fluorescence quencher and mounted onto the slide carefully to avoid
bubbles. The slides were observed and documented using fluorescent
microscopy.
Total RNA extraction and real-time PCR
(RT-PCR) analysis
Total RNA was extracted as previously described
(22) and the total RNA
concentration was measured. The primer sequences for DOR and
β-actin are listed in Table I.
RT-PCR was performed according to the RT-PCR kit manual (Takara Bio
Inc.). The PCR products were analyzed using 1.0% agarose gel
electrophoresis and a gel imaging system.
 | Table I.Primers sequences for DOR and
β-actin. |
Table I.
Primers sequences for DOR and
β-actin.
| Primers | DOR | β-actin |
|---|
| Forward |
5′-ACCAAGATCTGCGTGTTCCT-3′ |
5′-AAGGAAGGCTGGAAGAGTGC-3′ |
| Reverse |
5′-CGATGACGAAGATGTGGATG-3′ |
5′-CTGGGACGACATGGAGAAAA-3′ |
RNA interference (RNAi)
Cells at a concentration of 5×104
cells/ml were seeded into a 6-well plate. After reaching 70%
confluence, the cells were transfected with lentiviral vectors
according to the transfection agent manual. The short interfering
RNA (siRNA) sequences targeting DOR (synthesized by Invitrogen) are
listed in Table II. The RNA
interference efficiency was tested using RT-PCR and western
blotting.
 | Table II.The RNAi sequences targeting DOR. |
Table II.
The RNAi sequences targeting DOR.
| Primers | Forward | Reverse |
|---|
| DOR siRNA-1 |
GCCAAGCUGAUCAACAUCUTT |
AGAUGUUGAUCAGCUUGGCTT |
| DOR siRNA-2 |
GUCCGGUACACUAAGAUGATT |
UCAUCUUAGUGUACCGGACTT |
| DOR siRNA-3 |
CCAUCGACUACUACAAUAUTT |
AUAUUGUAGUAGUCGAUGGTT |
Western blot analysis
Cells were harvested and lysed in 2 ml of lysis
buffer [50 mM Tris-HCl, 137 mM NaCl, 10% glycerol, 100 mM sodium
orthovanadate, 1 mM PMSF, 10 mg/ml aprotinin, 10 mg/ml leupeptin,
1% NP-40 and 5 mM cocktail solution (pH 7.4)] to extract the
proteins. The extracted proteins were quantified using the BCA
method and analyzed using SDS-PAGE. The extracted proteins were
transferred onto a PVDF membrane by semi-dry transfer. The membrane
was blocked in 5% non-fat milk overnight at 4°C. The membrane was
washed using TBST and incubated with primary antibody at 37°C for 1
h, washed with TBST and then incubated with secondary antibody at
37°C for 1 h. The membrane was washed with TBST and developed for 5
min using autoradiography. Quantity One software was used to
analyze the optical density. The results are displayed as the ratio
of the optical density value/internal reference optical density
value.
Scratch test
Cells were incubated in a 6-well plate to 100%
confluence. A sterilized tip was then used to scratch a line in the
center of the monolayer cells. The dead cells were removed by
washing and cell growth was observed and documented at different
time-points (0, 24, 48 and 72 h) under a microscope. Image-pro Plus
software was used to measure the distance between the scratch edges
at different time-points (0, 24, 48 and 72 h). The cell migration
distance at different time-points was measured based on the
following equation: Distance at 0 h / Distance at 24, 48, or 72 h.
The results were analyzed using statistical software.
Cell invasion test
Transwell migration chambers (Corning, NY, USA) were
used for the cell invasion test. Different groups of MHCC97-H cells
were digested using trypsin. Cell counting was performed and the
cells were then resuspended to a final concentration of
l×105 cells/ml in DMEM media. Resuspended cells (200
μl) were added to the upper chamber in each Transwell
(8-μm diameter pore size) and 500 μl of medium
supplemented with serum was added to the lower chamber. Transwell
migration chambers were incubated in a 5% CO2 incubator
for 24 h, washed three times with PBS and then stained with crystal
violet. Five fields were randomly picked and the cell numbers were
counted in every field using a light microscope at ×200
magnification. The mean value of the cell numbers in the five
fields was used as the number of cells that passed through the
artificial human basal membrane.
Inoculation of nude mice
The animal experiments were approved by the Medical
Ethics Committee at Guilin Medical University. Ten nude mice (male,
6–8-week-old and weighing ∼20 g) were purchased from the Animal
Center at Guilin Medical University. The mice were randomly divided
into two groups with five mice in each group. All of the mice were
spleen inoculated with cells (0.5l×105 cells per mouse)
transfected with control oligonucleotides (N-control) or
DOR-siRNAs. Animals were sacrificed after four weeks using cervical
dislocation and the xenograft tumor was harvested aseptically for
testing.
Statistical analyses
SPSS 16.0 statistical software was used to perform
all of the statistical analyses. The data are the mean ± standard
deviation. Both a one-way ANOVA and an LSD-t test were used to
compare the differences among the different groups. The difference
between two groups was considered to be statistically significant
at p<0.05.
Results
High expression of DOR in HCC tissues and
cells
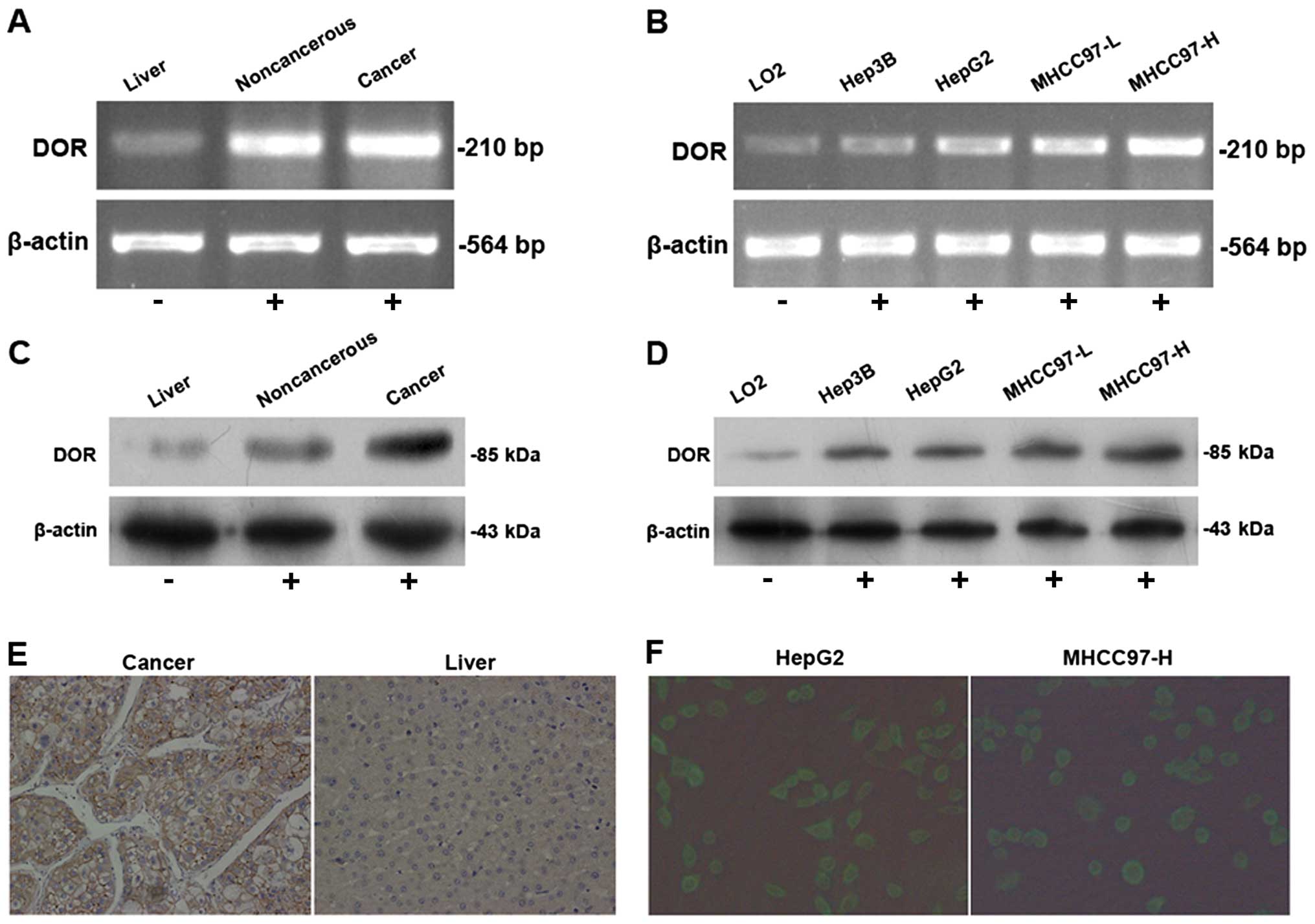
The RT-PCR results demonstrated variable levels of
DOR expression in 41 cases of HCC samples. The DOR mRNA levels in
HCC lesions were higher than in the adjacent tumor tissues and
normal liver tissues (p<0.05) (Fig.
1A). The DOR mRNA was expressed in different types of
HCC cells at levels that were significantly higher than in normal
liver cells (p<0.05) (Fig. 1B).
Western blot analysis determined that DOR protein expression in HCC
tissue was higher than in the adjacent tumor tissues and normal
liver tissues (p<0.05) (Fig.
1C). Moreover, DOR protein expression in different types of HCC
cells was higher than in normal liver cells (p<0.05) (Fig. 1D).
Immunohistochemical detection of DOR expression and
distribution of human hepatocellular carcinoma were carried out.
The results show that the DOR positive staining in HCC are mainly
located in the cell membrane, a small amount of staining in the
cytoplasm and none in the nucleus (Fig. 1E). Immunofluorescence using
FITC-conjugated antibodies was used to detect DOR expression and
its distribution in HCC and normal cells. The results from the HCC
cells showed that the green fluorescence FITC signal was
distributed evenly on the cancer cell membrane, was weak in the
cytoplasm and was absent in the nucleus (Fig. 1F), indicating that DOR was mainly
expressed on the HCC cell membrane.
Activation of DOR promotes HCC cell
proliferation
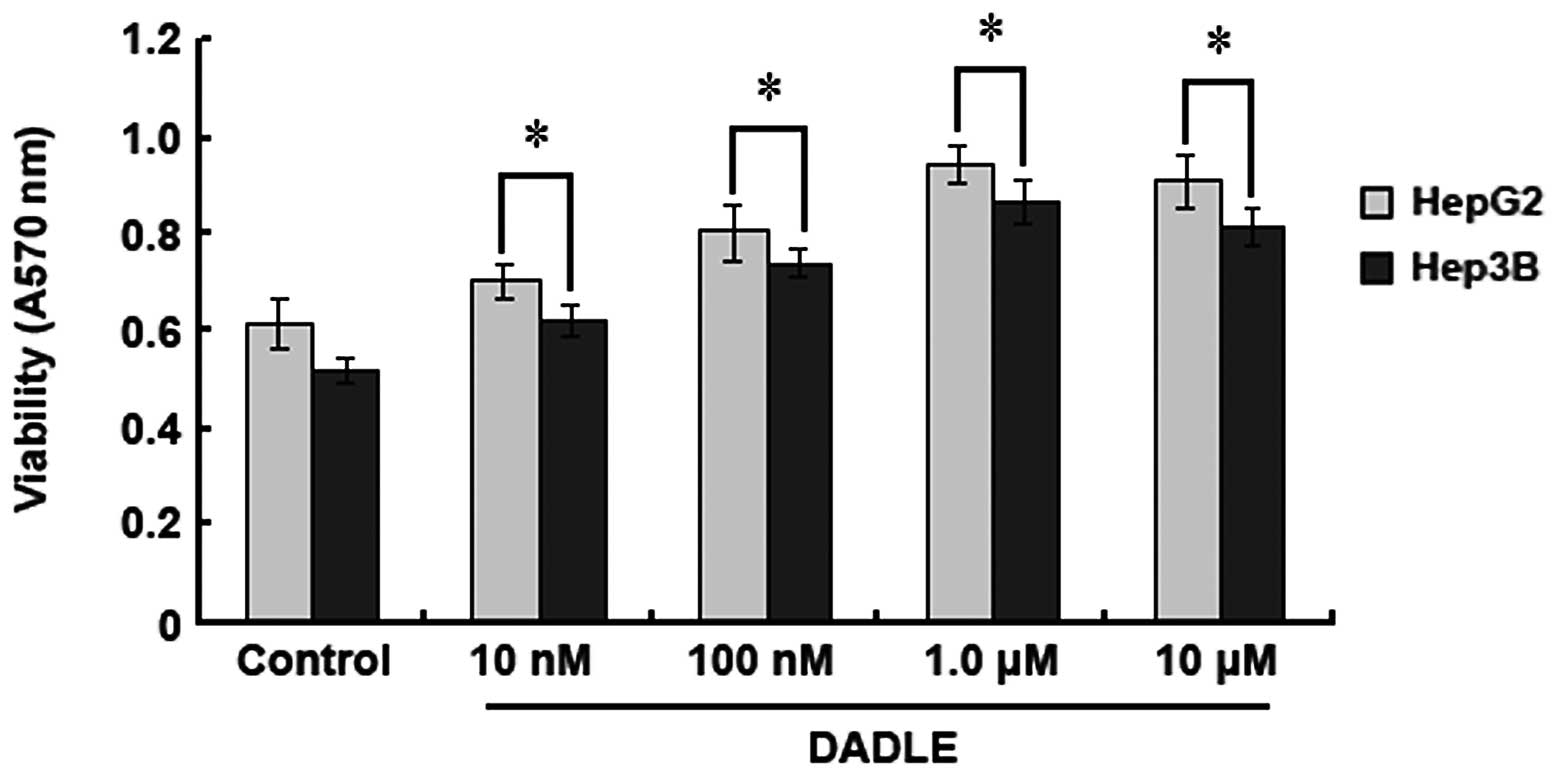
HepG2 and Hep3B HCC cell lines were employed to
study the effect of DOR on HCC cell proliferation. An MTT assay was
used to quantify the cell proliferation rate after DADLE treatment.
When the DADLE concentration was increased from 10 nM to 10
μM, the OD570 values of the DADLE-treated HepG2
and Hep3B cells also increased, but there was no increase in the
control groups (without DADLE treatment). However, when the
concentration of DADLE was above 1.0 μM, the
OD570 values of HepG2 and Hep3B cells did not increase
further, indicating that the activation of DOR can promote HCC cell
proliferation and that 1.0 μM of DADLE induces maximal cell
proliferation (Fig. 2).
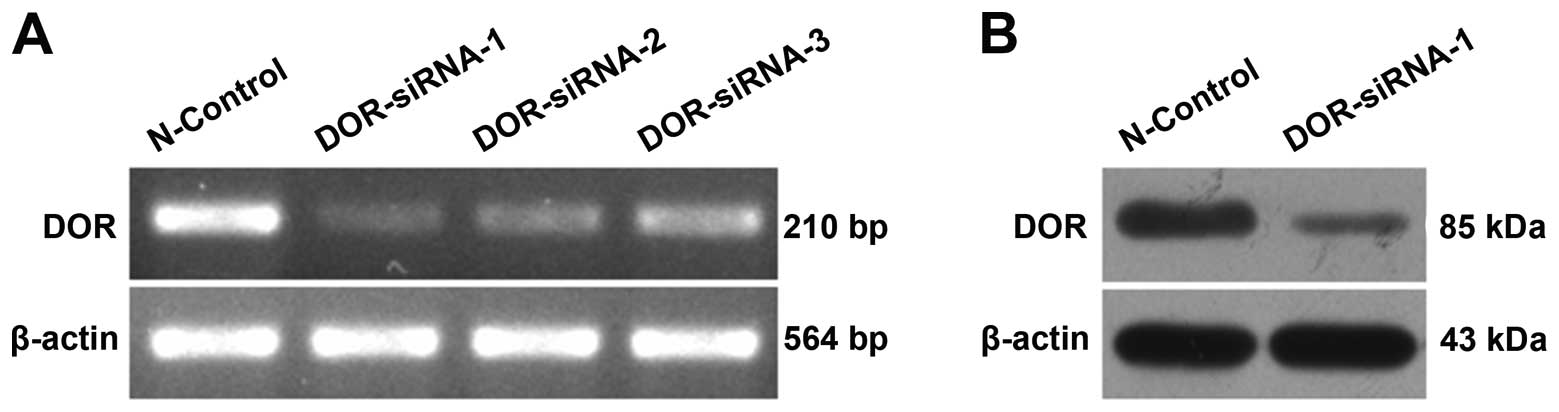
Silencing DOR expression with RNAi
Three siRNAs (DOR-siRNA-1, DOR-siRNA-2 and
DOR-siRNA-3) and the control siRNA (N-control) were used to
transfect HCC cells. Post-transfection, the total RNA was extracted
for use in the RT-PCR analysis of DOR expression in HCC
cells. Upon siRNA transfection, DOR expression decreased
relative to cells transfected with the control siRNA. DOR-siRNA-1
gave the most obvious silencing effect (Fig. 3A). Western blot analysis further
illustrated that DOR protein expression also decreased after
DOR-siRNA-1 transfection (Fig.
3B).
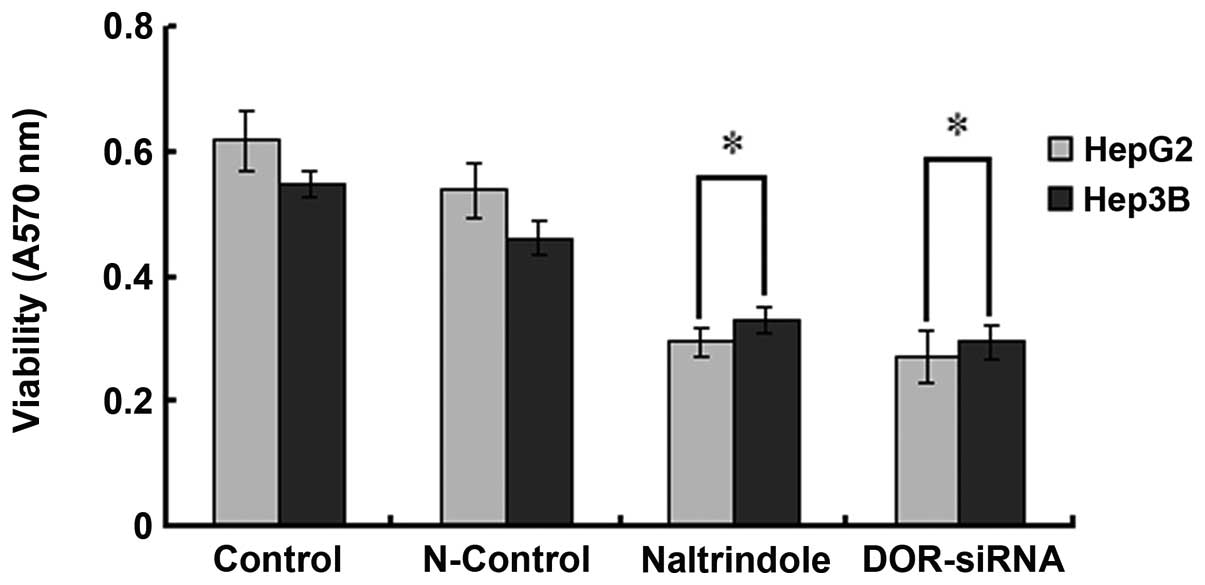
Downregulation of DOR inhibits HCC cell
growth
OD570 values of DOR-siRNA-transfected
HepG2 and Hep3B cells were significantly reduced relative to those
from the HepG2 and Hep3B cells transfected with the control siRNA
(p<0.05). Similar results were observed in naltrindole (specific
DOR inhibitor) treated cells (p<0.05) (Fig. 4), suggesting that the
downregulation of DOR can significantly suppress HCC cell
proliferation.
Downregulation of DOR promotes HCC
cellular apoptosis
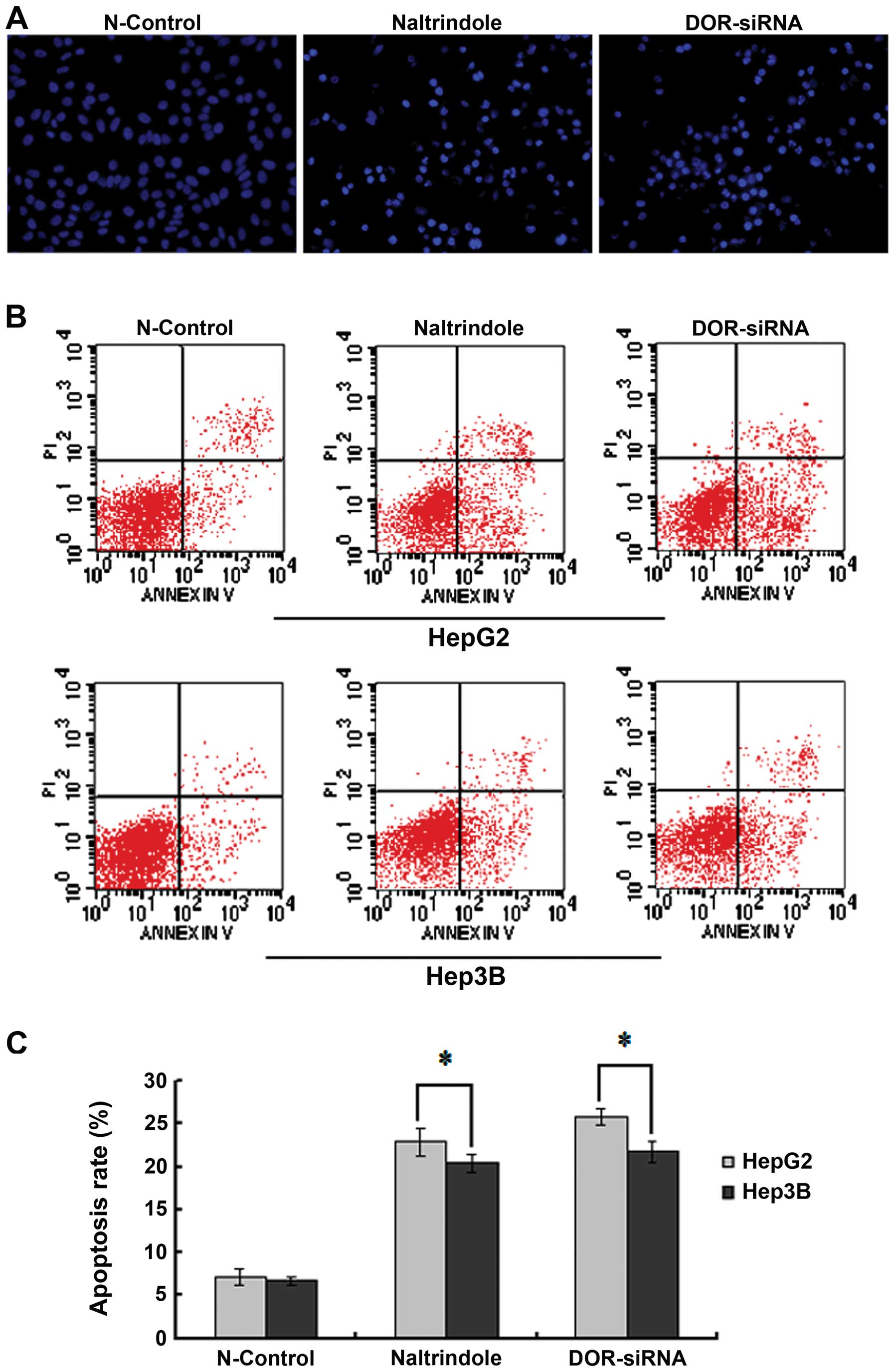
To study the function of DOR in HCC cellular
apoptosis, Hoechst 33342 staining was used to measure the effect of
the downregulation of DOR on apoptotic HCC cell morphology
using fluorescence microscopy. The HCC cell nucleus in the
N-control group was circular or oval in shape with the chromatin
evenly distributed in light blue fluorescence. The silencing of the
DOR gene or the inhibition of DOR using the specific
antagonist naltrindole led to a significant increase in the number
of apoptotic cells. The chromatin in the apoptotic HCC cells was
condensed and unevenly distributed. Chromatin accumulation near the
nuclear membrane, chromatin condensation, increased intensity of
fluorescence staining, nuclear condensation and apoptotic cell
bodies were also detected when DOR was downregulated
(Fig. 5A).
The Annexin V-FITC/PI double-labeling method was
used to study the effect of DOR downregulation on the HCC
cell apoptotic rate. DOR silencing increased the rate of early
apoptosis in HepG2 and Hep3B cells relative to the N-control group
(p<0.05). We also observed that the rate of early apoptosis in
the HepG2 and Hep3B cells increased with naltrindole treatment
(p<0.05) (Fig. 5B and C). These
results suggest that the downregulation of DOR can promote
apoptosis in HCC cells.
Downregulation of DOR causes liver cancer
cell cycle arrest
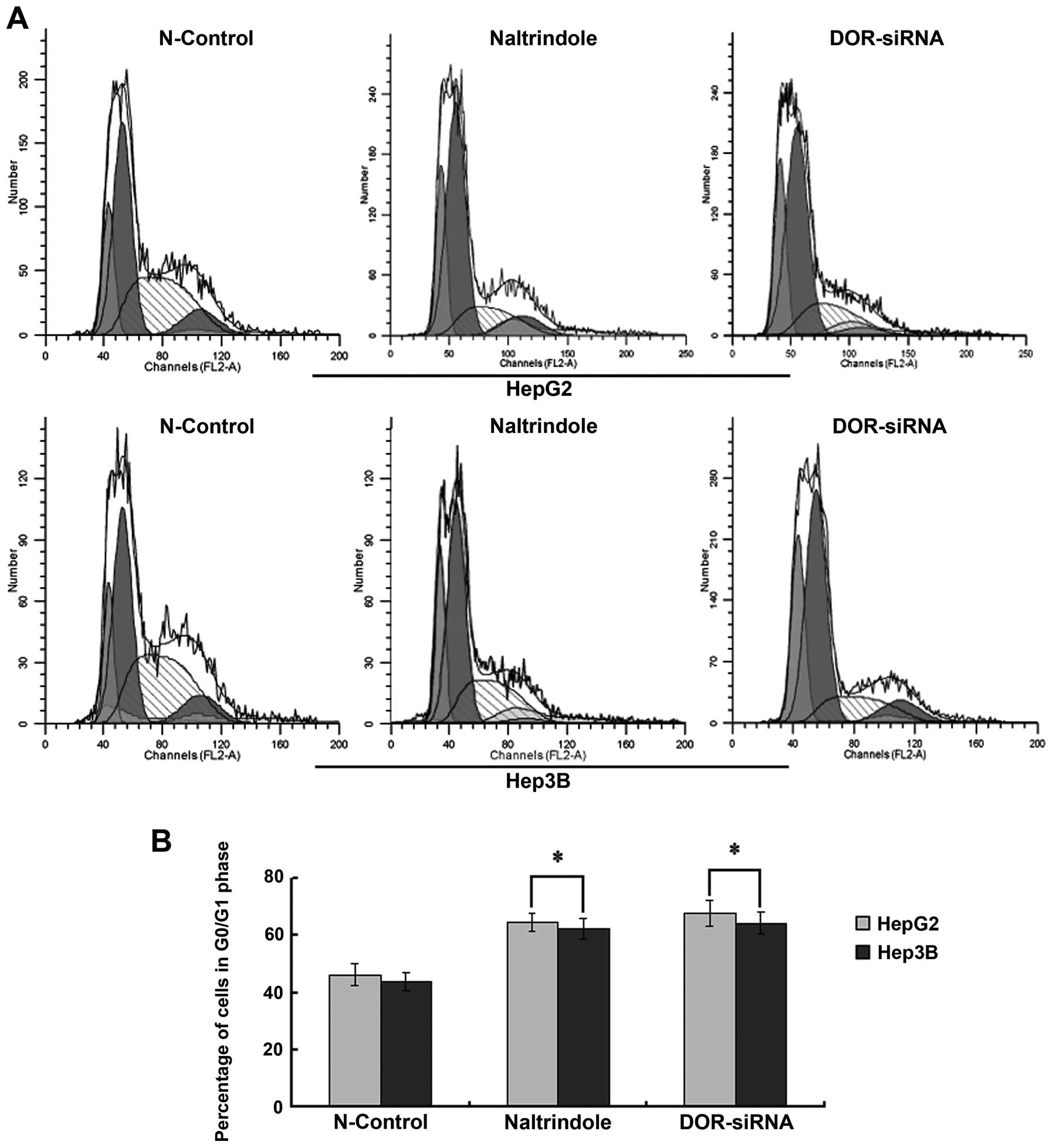
Flow cytometry analysis was used to determine
whether DOR affected the cell cycle in HCC cells. When the
DOR gene was silenced using siRNAs or the cells were treated
with naltrindole, the cell cycle of HepG2 and Hep3B cells was
arrested at G0/G1 (p<0.05), suggesting that the downregulation
of DOR increased the percentage of HCC cells in G0/G1. This
down-regulation also inhibited HCC cell growth (Fig. 6).
Downregulation of DOR inhibits HCC cell
invasion and migration
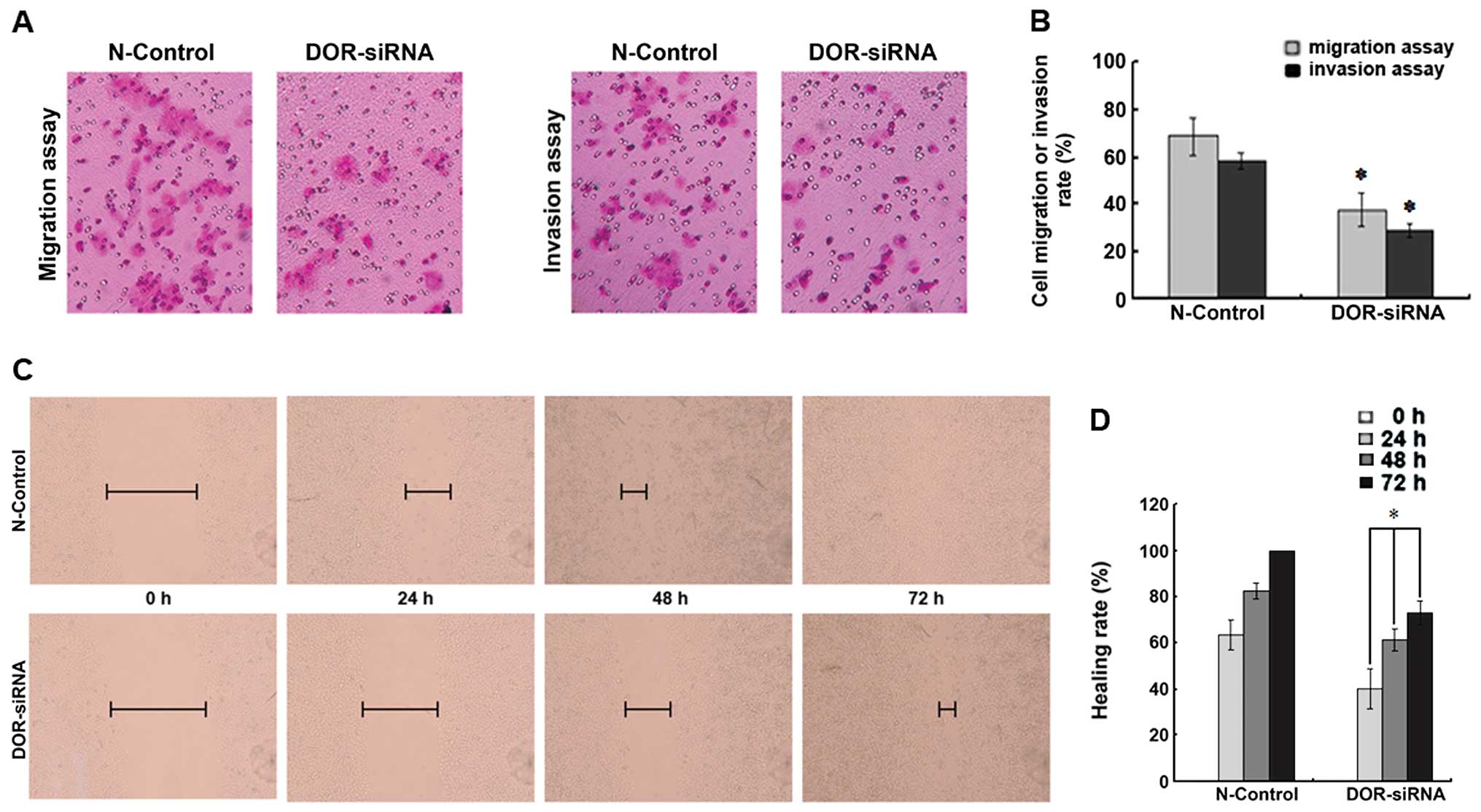
To determine if the downregulation of DOR can
affect HCC cell invasion and migration, we compared the number of
cells passing through an artificial human basal membrane before and
after siRNA transfection. Five fields of cells were randomly chosen
for this analysis. The downregulation of DOR decreased the
invasion ability of HCC cells and significantly reduced the number
of cells passing through the artificial human basal membrane
(p<0.05). Moreover, the downregulation of DOR also
reduced the migration of HCC cells (p<0.05) (Fig. 7A and B).
The cell monolayer was scratched and the distance
between scratch margins was measured at 0, 24, 48 and 72 h. The
downregulation of DOR led to a significant decrease in the
migration of HCC cells at the four different time-points
(p<0.05), which suggested that the downregulation of DOR
led to a significant reduction in liver cancer cell migration
(Fig. 7C and D).
Effect of DOR downregulation on cancer
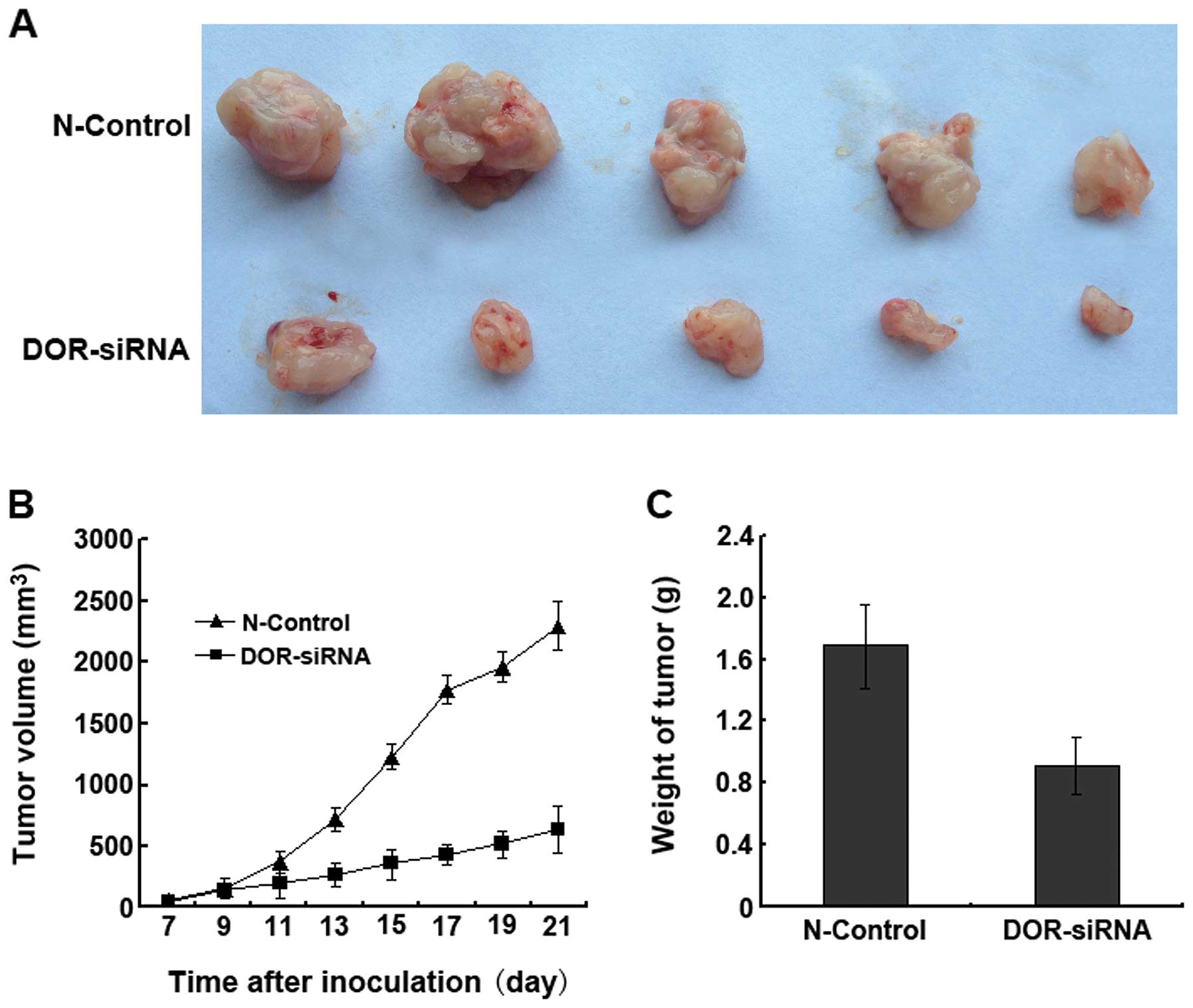
progression in nude mice
After four weeks of inoculation, the tumor formation
rates reached 100% in all nude mice (n=5) in the N-control group
and 80% in the nude mice inoculated with the DOR-siRNA-transfected
cancer cells. Tumor progression in the nude mice of the N-control
group was more rapid than in the nude mice inoculated with
DOR-siRNA-transfected cancer cells (Fig. 8A). We also found that the tumor
volumes in the DOR-siRNA group were significantly smaller than in
the N-control group (p<0.05) at all time-points (Fig. 8B). The tumor weights of the
DOR-siRNA group were significantly smaller than the weights of the
N-control group (Fig. 8C). These
results suggested that the downregulation of DOR in vivo can
significantly decrease cancer progression.
Discussion
Since the discovery of the opioid receptor and
opioid receptor drugs, researchers have studied their roles in
neurological diseases and pain control. DOR, a key member of the
opioid receptor superfamily and a G protein-coupled receptor, is
mainly expressed in the central nervous system (CNS) and is
involved in the functional regulation of the CNS and pain control
(23–25). Studies have shown that DOR is also
expressed in the circulatory system (26), digestive system (27,28),
reproductive system (29,30) and immune system (31), suggesting that apart from the CNS,
DOR may play different roles in these peripheral tissues.
In addition to the being expressed in the stomach,
small intestine, large intestine and pancreas, DOR was
reported to be expressed in the liver (27,28).
Our previous studies (21)
determined that DADLE (a specific DOR agonist) had a dose-dependent
protective effect on human liver cells by suppressing liver cell
apoptosis. However, naltrindole (a highly selective DOR antagonist)
can suppress the function of DOR, which suggests that DOR is
expressed and regulates function in normal human livers, consistent
with previous studies.
It was reported that DOR is widely expressed in
multiple human malignant cancers (12–15,16,17).
DOR is also involved in malignant transformation and cancer
progression. However, there is not enough evidence on the
functional effects of DOR in liver cancers. The aim of this study
was to investigate DOR expression in human liver cancers and its
effects on human liver cancer progression. First, we found that DOR
is expressed in human HCC tissues and cells. The expression level
of DOR in human HCC tissues and cells was significantly higher than
in normal liver. Our immunofluorescent data for the intracellular
localization of DOR determined that DOR is mostly localized to the
liver cell membrane. These results suggest that DOR may be involved
in HCC formation and tumor progression. It may also be involved in
other physiological functions in the human body.
Among the members of the opioid receptor
superfamily, DOR is most closely involved in cell survival and
proliferation (32,33). Studies have shown that DOR can
protect liver damage in cholestatic liver disease by promoting
liver generation and liver cell proliferation (18,34).
The protective effect in the liver was due to the activation of DOR
on the liver cell membrane. We speculated that DOR may have some
effects on liver cancer progression. Further studies are needed to
investigate this effect of DOR. Surprisingly, the activation of DOR
significantly increased HCC cell proliferation and promoted cancer
cell growth in our study. We suggest that DOR may also play an
important role in HCC cell proliferation. This finding may provide
an effective method to suppress HCC progression in clinical
practice.
Kuniyasu et al suggested that
methionine-enkephalin can suppress colorectal cancer (CRC) cell
growth and invasion based on their finding from a model of the
liver metastasis of CRC (35),
which is contradictory to our results. We found that the
downregulation of DOR suppressed HCC cell proliferation and
that the tumor cells underwent apoptosis. We also found that the
HCC cell cycle was arrested at G0/G1. In addition, our results were
contradictory to results from Hatzoglou et al (36). This group showed that opioid
receptor agonists can suppress cancer cell proliferation. This
controversy could be caused by the differences between liver
cancers and other cancers as well as the multiple subtypes of DOR.
Whether the downregulation of DOR in vivo can suppress
proliferation in other cancer cell types still needs to be
tested.
Although progress has been made in the study of HCC
formation and progression, the 5-year survival rate of liver cancer
is still very low, which may be due to the high invasion and
metastasis rates of HCC (37). In
the present study, we established that DOR expression in human HCC
cells (MHCC97-H) with a high metastatic rate was higher than in
human HCC cells (MHCC97-L) with a low metastatic rate, which
suggests that DOR might be closely involved in cancer cell
malignancy and invasion. However, in this previous study, there was
no evidence that demonstrated that DOR plays a functional role in
human HCC invasion and metastasis. In the present study, we found
that silencing the DOR gene decreased liver cancer cell
invasion and migration. Nude mice inoculated with cells stably
expressing low levels of DOR displayed low tumor formation
rates and reduced cancer progression. These results suggest that
DOR may play some role in HCC cell invasion and migration. This can
provide a new theoretical basis for the prevention of HCC invasion
and migration.
In conclusion, high expression levels of DOR were
observed in HCC tissues and cells and DOR was mainly localized to
the tumor cell membrane. Our results demonstrated that
downregulation of DOR can suppress liver cancer progression.
Therefore, DOR may become a new marker and target for HCC treatment
and provide a potential treatment method to reduce or inhibit tumor
malignancy.
Acknowledgements
This study was supported in part by
The National Natural Science Foundation of China (nos. 81160066 and
30870719), Science & Technology Planning Project of Guang Xi
Province (1140003-79 and 1298003-2-1), National High Technology
Research and Development Program (863 Program) funding
(2006AA02A309), Opening fund Special Project from Experimental
Center of Guangxi Medical Sciences Key Laboratory (KFJJ2010-49),
Science & Technology Planning Project of Guilin City
(20110119-1-8), Scientific Research Foundation for Returned
Scholars, Ministry of Education of China (jyb2010-01).
References
|
1.
|
El-Serag HB and Rudolph KL: Hepatocellular
carcinoma: epidemiology and molecular carcinogenesis.
Gastroenterology. 132:2557–2576. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Rampone B, Schiavone B, Martino A, Viviano
C and Confuorto G: Current management strategy of hepatocellular
carcinoma. World J Gastroenterol. 15:3210–3216. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Rahbari NN, Mehrabi A, Mollberg NM, Müller
SA, Koch M, Büchler MW and Weitz J: Hepatocellular carcinoma:
current management and perspectives for the future. Ann Surg.
253:453–469. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Xu G, Qi FZ, Zhang JH, Cheng GF, Cai Y and
Miao Y: Meta-analysis of surgical resection and radiofrequency
ablation for early hepatocellular carcinoma. World J Surg Oncol.
10:1632012. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
DuBray BJ Jr, Chapman WC and Anderson CD:
Hepatocellular carcinoma: a review of the surgical approaches to
management. Mo Med. 108:195–198. 2011.PubMed/NCBI
|
|
6.
|
Salhab M and Canelo R: An overview of
evidence-based management of hepatocellular carcinoma: a
meta-analysis. J Cancer Res Ther. 7:463–475. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Law PY, Wong YH and Loh HH: Molecular
mechanisms and regulation of opioid receptor signaling. Annu Rev
Pharmacol Toxicol. 40:389–430. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Kieffer BL, Befort K, Gaveriaux-Ruff C and
Hirth CG: The delta-opioid receptor: isolation of a cDNA by
expression cloning and pharmacological characterization. Proc Natl
Acad Sci USA. 89:12048–12052. 1992. View Article : Google Scholar
|
|
9.
|
Evans CJ, Keith DE Jr, Morrison H,
Magendzo K and Edwards RH: Cloning of a delta opioid receptor by
functional expression. Science. 258:1952–1955. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Bzdega T, Chin H, Kim H, Jung HH, Kozak CA
and Klee WA: Regional expression and chromosomal localization of
the delta opiate receptor gene. Proc Natl Acad Sci USA.
90:9305–9309. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Simonin F, Befort K, Gavériaux-Ruff C,
Matthes H, Nappey V, Lannes B, Micheletti G and Kieffer B: The
human delta-opioid receptor: genomic organization, cDNA cloning,
functional expression, and distribution in human brain. Mol
Pharmacol. 46:1015–1021. 1994.PubMed/NCBI
|
|
12.
|
Madar I, Bencherif B, Lever J, Heitmiller
RF, Yang SC, Brock M, Brahmer J, Ravert H, Dannals R and Frost JJ:
Imaging delta- and mu-opioid receptors by PET in lung carcinoma
patients. J Nucl Med. 48:207–213. 2007.PubMed/NCBI
|
|
13.
|
Zagon IS, McLaughlin PJ, Goodman SR and
Rhodes RE: Opioid receptors and endogenous opioids in diverse human
and animal cancers. J Natl Cancer Inst. 79:1059–1065.
1987.PubMed/NCBI
|
|
14.
|
Bostwick DG, Null WE, Holmes D, Weber E,
Barchas JD and Bensch KG: Expression of opioid peptides in tumors.
N Engl J Med. 317:1439–1443. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Lundberg JM, Hamberger B, Schultzberg M,
Hökfelt T, Granberg PO, Efendić S, Terenius L, Goldstein M and Luft
R: Enkephalin- and somatostatin-like immunoreactivities in human
adrenal medulla and pheochromocytoma. Proc Natl Acad Sci USA.
76:4079–4083. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Heiss A, Ammer H and Eisinger DA:
delta-opioid receptor-stimulated Akt signaling in neuroblastoma x
glioma (NG108-15) hybrid cells involves receptor tyrosine
kinase-mediated PI3K activation. Exp Cell Res. 315:2115–2125. 2009.
View Article : Google Scholar
|
|
17.
|
Debruyne D, Leroy A, DE Wever O, Vakaet L,
Mareel M and Bracke M: Direct effects of delta opioid receptor
agonists on invasion-associated activities of HCT-8/E11 colon
cancer cells. Anticancer Res. 30:9–17. 2010.PubMed/NCBI
|
|
18.
|
Nicoll J, Axiotis CA and Bergasa NV: The
delta opioid receptor 1 is expressed by proliferating bile ductules
in rats with cholestasis: implications for the study of liver
regeneration and malignant transformation of biliary epithelium.
Med Hypotheses. 65:1099–1105. 2005. View Article : Google Scholar
|
|
19.
|
Bergasa NV and Boyella VD: Liver derived
endogenous opioids may interfere with the therapeutic effect of
interferon in chronic hepatitis C. Med Hypotheses. 70:556–559.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
De Minicis S, Candelaresi C, Marzioni M,
Saccomano S, Roskams T, Casini A, Risaliti A, Salzano R, Cautero N,
di Francesco F, Benedetti A and Svegliati-Baroni G: Role of
endogenous opioids in modulating HSC activity in vitro and liver
fibrosis in vivo. Gut. 57:352–364. 2008.PubMed/NCBI
|
|
21.
|
Tang B, Zhang Y, Liang R, Yuan P, Du J,
Wang H and Wang L: Activation of the δ-opioid receptor inhibits
serum deprivation-induced apoptosis of human liver cells via the
activation of PKC and the mitochondrial pathway. Int J Mol Med.
28:1077–1085. 2011.
|
|
22.
|
Zhang B, Zhang X, Tang B, Zheng P and
Zhang Y: Investigation of elemene-induced reversal of tamoxifen
resistance in MCF-7 cells through oestrogen receptor α (ERα)
re-expression. Breast Cancer Res Treat. 136:399–406.
2012.PubMed/NCBI
|
|
23.
|
Alvira-Botero MX and Garzón M: Cellular
and subcellular distributions of delta opioid receptor activation
sites in the ventral oral pontine tegmentum of the cat. Brain Res.
1123:101–111. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Wang HB, Guan JS, Bao L and Zhang X:
Distinct subcellular distribution of delta-opioid receptor fused
with various tags in PC12 cells. Neurochem Res. 33:2028–2034. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Gaveriaux-Ruff C, Nozaki C, Nadal X, Hever
XC, Weibel R, Matifas A, Reiss D, Filliol D, Nassar MA, Wood JN,
Maldonado R and Kieffer BL: Genetic ablation of delta opioid
receptors in nociceptive sensory neurons increases chronic pain and
abolishes opioid analgesia. Pain. 152:1238–1248. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Howells RD, Kilpatrick DL, Bailey LC, Noe
M and Udenfriend S: Proenkephalin mRNA in rat heart. Proc Natl Acad
Sci USA. 83:1960–1963. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Neidle A, Manigault I and Wajda IJ:
Distribution of opiate-like substances in rat tissues. Neurochem
Res. 4:399–410. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Wittert G, Hope P and Pyle D: Tissue
distribution of opioid receptor gene expression in the rat. Biochem
Biophys Res Commun. 218:877–881. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Kilpatrick DL, Howells RD, Noe M, Bailey
LC and Udenfriend S: Expression of preproenkephalin-like mRNA and
its peptide products in mammalian testis and ovary. Proc Natl Acad
Sci USA. 82:7467–7469. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Civelli O, Douglass J, Goldstein A and
Herbert E: Sequence and expression of the rat prodynorphin gene.
Proc Natl Acad Sci USA. 82:4291–4295. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Radulović J and Janković BD: Opposing
activities of brain opioid receptors in the regulation of humoral
and cell-mediated immune responses in the rat. Brain Res.
661:189–195. 1994.PubMed/NCBI
|
|
32.
|
Su TP: Delta opioid
peptide[D-Ala(2),D-Leu(5)]enkephalin promotes cell survival. J
Biomed Sci. 7:195–199. 2000.
|
|
33.
|
Kim H, Lee SW, Park JS, Min JH and Kim HK:
Genomic analysis of [d-Ala(2), d-Leu(5)] enkephalin preconditioning
in cortical neuron and glial cell injury after oxygen deprivation.
Brain Res. 1447:91–105. 2012.
|
|
34.
|
Marzioni M, Alpini G, Saccomanno S, de
Minicis S, Glaser S, Francis H, Trozzi L, Venter J, Orlando F, Fava
G, Candelaresi C, Macarri G and Benedetti A: Endogenous opioids
modulate the growth of the biliary tree in the course of
cholestasis. Gastroenterology. 130:1831–1847. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Kuniyasu H, Luo Y, Fujii K, Sasahira T,
Moriwaka Y, Tatsumoto N, Sasaki T, Yamashita Y and Ohmori H: CD10
enhances metastasis of colorectal cancer by abrogating the
anti-tumoural effect of methionine-enkephalin in the liver. Gut.
59:348–356. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Hatzoglou A, Kampa M and Castanas E:
Opioid-somatostatin interactions in regulating cancer cell growth.
Front Biosci. 10:244–256. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Zhou XD: Recurrence and metastasis of
hepatocellular carcinoma: progress and prospects. Hepatobiliary
Pancreat Dis Int. 1:35–41. 2002.PubMed/NCBI
|