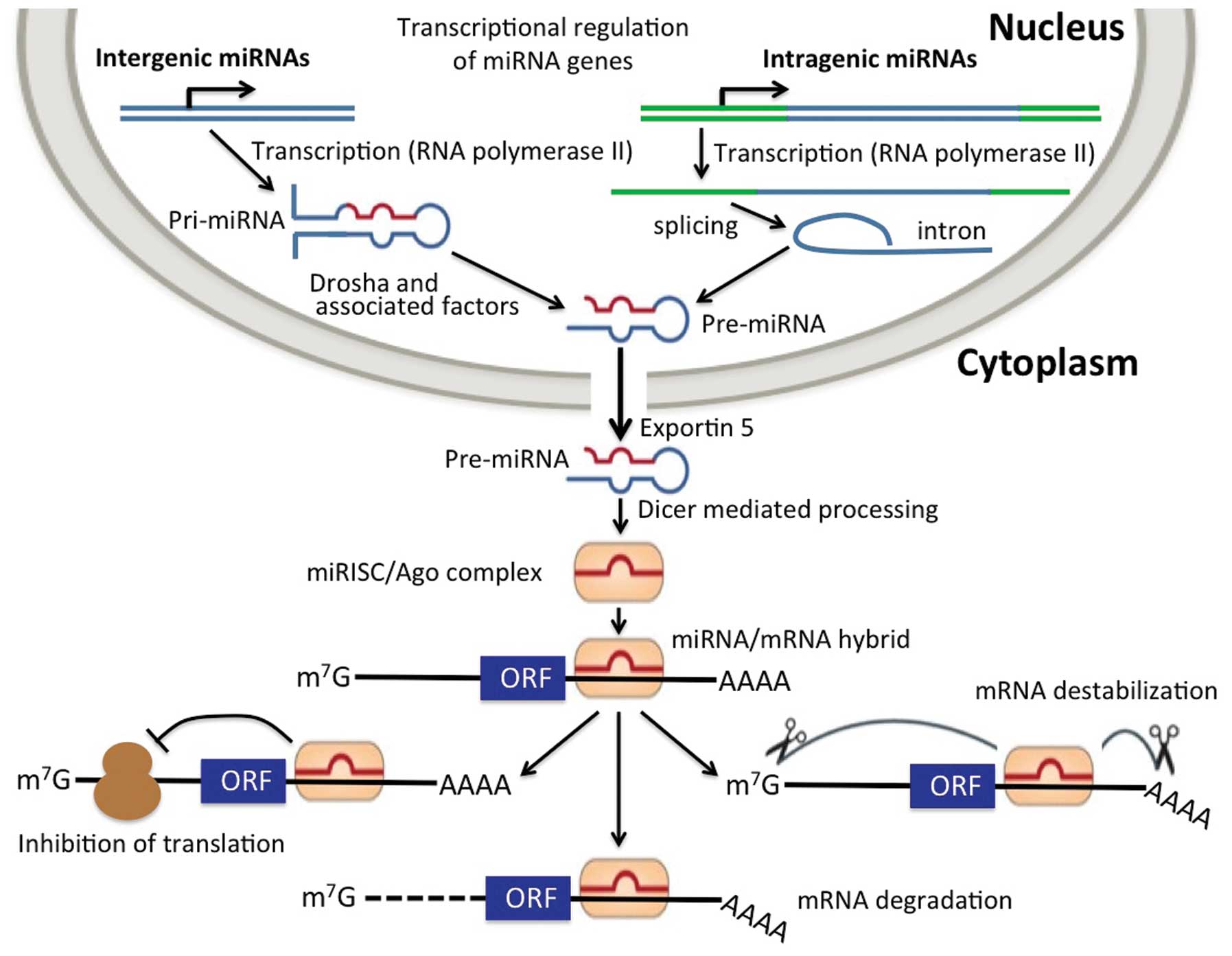
Connecting miRNA-221 with the expression of cellular
genes altered in breast cancer cells: the Slug/miR-221 network
This specific field of miRNA research has confirmed
that the complex networks constituted by miRNAs and mRNA targets
coding for structural and regulatory proteins lead to the control
of highly regulated biological functions, such as differentiation,
cell cycle and apoptosis (14–16).
The low expression of a given miRNA is expected to be linked with a
potential expression of target mRNAs. Conversely, the high
expression of miRNAs is expected to negatively affect the
biological functions of target mRNAs (1–5).
Alterations in miRNA expression have been
demonstrated to be associated with a variety of human pathologies,
and the guided alterations of specific miRNAs have been suggested
as novel approaches for the development of innovative therapeutic
protocols. miRNA therapeutics is a novel field in which miRNA
activity is the major target of intervention (17–21).
The inhibition of miRNA activity can be readily achieved by the use
of small miRNA inhibitors, oligomers, including RNA, DNA and DNA
analogues (miRNA antisense therapy) (19,22–28).
On the contrary, an increase in miRNA function (miRNA replacement
therapy) can be achieved by the use of modified miRNA mimetics,
such as plasmid or lentiviral vectors carrying miRNA sequences
(20,21,29–37).
miRNAs play a pivotal role in all the stages of
cancer. The literature on this specific issue is impressive
(22–37). As a first example, miR-372 and
miR-373 were identified as oncogenes, after a screening of hundreds
of miRNAs in testicular germ cell tumors (38). The mechanisms of action of these
miRNAs involve the negative regulation of the expression of the
LAST2 tumor suppressor gene, blocking the pathway of one of the key
tumor suppressors, p53 (39).
Accordingly, using breast cancer MCF-7 cells as a model system,
Huang et al demonstrated that miR-373 promotes tumor
invasion and metastasis (40). A
similar tumor-promoting activity has been exhibited by miR-221 and
miR-222, which can stimulate the proliferation of human prostate
carcinoma cell lines following the inhibition of the expression of
the tumor suppressor p27Kip1(41).
An opposite effect on tumor development has been
displayed by other miRNAs; for instance miR-31 expression levels
inversely correlate with the metastatic ability of breast tumor
cell lines and the inhibition of miR-31 promotes metastasis.
Another study revealed that miR-31 blocks several steps of
metastasis, including local invasion, extravasation or initial
survival at a distant site, and metastatic colonization (42). Taken together, these data
demonstrate that miRNAs play a double role in cancer, behaving both
as oncogenes or tumor suppressor genes.
In general, a miRNA able to promote cancer targets
mRNAs encoding tumor suppressor proteins, while miRNAs exhibiting
tumor suppressor properties usually target mRNAs encoding
oncoproteins. miRNAs which have been demonstrated to play a crucial
role in the initiation and progression of human cancer are defined
as oncogenic miRNAs (oncomiRs) (22–28).
Moreover, miRNAs have been firmly demonstrated to be involved in
cancer metastasis (metastamiRs) (43–46).
Thus, therapeutic strategies involving miRNA silencing have been
suggested, based on the roles of these small non-coding RNAs as
oncogenes (22–28).
Another very interesting feature of miRNAs has been
found by studying cancer-associated miRNAs in different
experimental model systems; cancer-specific miRNAs are present in
extracellular body fluids and may play a crucial role in the
cross-talk between cancer cells and surrounding normal cells
(47–52). Of note, evidence of the presence of
miRNAs in serum, plasma and saliva supports their potential as an
additional set of biomarkers for cancer. Extracellular miRNAs are
protected by exosome-like structures, small intraluminal vesicles
shed from a variety of cells (including cancer cells), with a
biogenesis connected with the endosomal sorting complex required
for transport machinery in multivesicular bodies. These
extracellular structures, originally considered as a ‘garbage bag’
devoted to discarding degraded proteins, are now considered to play
an important role as an intercellular communication tool. It is
still unclear as to whether these exosome-associated miRNAs occur
as a result of tumor cell death and lyses, or are actively excreted
from tumor cells into the microenvironment. However, this novel
secretory machinery of miRNAs may be involved in tumor-associated
features, such as the enhancement of angiogenesis, the increase of
cytokine secretion and migration to pre-metastatic niche. Table I illustrates a summarized list of
oncomiRs and metastamiRs.
In addition to oncogenic activities, miRNAs exhibit,
as has already been pointed out, oncosuppressor properties by
targeting mRNAs encoding oncoproteins (29–37).
Piovan et al recently explored the interaction between
certain miRNAs and transcriptional factors involved in determining
cell fate, including the well known ‘genome guardian’, p53
(53). They demonstrated that
miR-205, an oncosuppressive miRNA lost in breast cancer, is
directly transactivated by the oncosuppressor p53. Moreover,
evaluating miR-205 expression in a panel of cell lines belonging to
the highly aggressive triple-negative [estrogen receptor (ER),
progesterone receptor (PR) and Her2/neu] breast cancer subtype,
which still lacks an effective targeted therapy and is
characterized by an extremely undifferentiated mesenchymal
phenotype, the authors demonstrated that this miRNA is critically
downregulated compared with a normal cell line. The re-expression
of miR-205 strongly reduced cell proliferation, cell cycle
progression and clonogenic potential in vitro, and inhibited
tumor growth in vivo. The tumor suppressor activity of
miR-205 is partially exerted by targeting of E2F1, one of the
master regulators of cell cycle progression, and LAMC1, a component
of the extracellular matrix involved in cell adhesion,
proliferation and migration. In another study, Lee et al
(54), demonstrated that an
estrogen-downregulated miRNA, miR-34b, acts as an oncosuppressor
that targets cyclin D1 and Jagged-1 (JAG1) in an
ERα-positive/wild-type p53 breast cancer cell line (MCF-7), as well
as in ovarian and endometrial cells, but not in ERα-negative or
mutant p53 breast cancer cell lines (T47D, MBA-MB-361 and
MDA-MB-435). The negative association between ERα and miR-34b
expression levels has also been found in ERα-positive breast cancer
patients. In addition, the overexpression of miR-34b has been shown
to inhibit ERα-positive breast tumor growth in an orthotopic
mammary fat pad xenograft mouse model. Table II illustrates a summarized list of
oncosuppressor miRNAs (29–37,55–58).
As already presented in the previous chapters,
miRNAs play a crucial role in breast tumors (59–73).
Several studies have been undertaken with the objective of
determining the correlation between the expression profile of
oncomiRs and tumor suppressor miRNAs, and, in particular, the
tumorigenic potential of triple-negative primary breast cancers. In
the study by Radojicic et al (68) 49 primary triple-negative breast
cancer cases, along with 34 matched tumor-associated normal samples
were investigated for the expression of 9 miRNAs using qRT-PCR.
Correlations between the expression of miR-10b, miR-21, miR-122a,
miR-145, miR-205, miR-210, miR-221, miR-222 and miR-296 and the
pathological features of the tumors were examined, as well as the
effects of miRNA expression on patient overall and cancer-specific
survival. miR-21, miR-210 and miR-221 were significantly
overexpressed, whereas miR-10b, miR-145, miR-205 and miR-122a were
significantly underexpressed in the triple-negative primary breast
cancers. Significant correlations among all the studied miRNAs were
scored both in the breast cancer and control tissues. The
expression of miR-222 and miR-296 did not exhibit any significant
difference between the breast cancer and normal tissue.
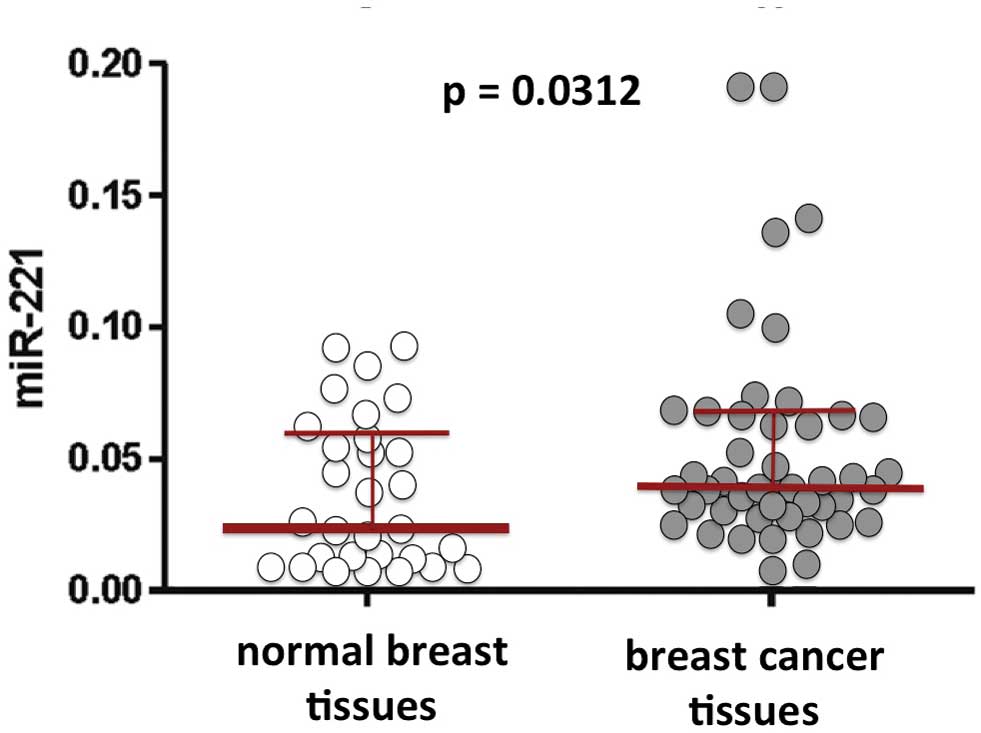
Furthermore, the expression of miR-221 is clearly
involved in chemoresistence. Studies have revealed an elevated
expression of miR-221 in adriamycin-resistant MCF-7/ADR cells. In
conclusion, several studies have indicated that miR-221 is one of
the major miRNAs involved in breast cancer (75). Representative results concerning
miR-221 in breast cancer tissues are illustrated in Fig. 2.
In consideration of the importance of miR-221 in the
tumor phenotype of breast cancer, several studies have been
performed with the objective of analyzing miR-221 in biological
fluids as a marker of breast cancer. Zhao et al (75) demonstrated that plasma miR-221 can
be considered as a predictive biomarker for chemoresistance in
breast cancer patients who have previously received neoadjuvant
chemotherapy. The expression levels of circulating miR-221 were
assessed in the plasma of 93 breast cancer patients who had
previously received neoadjuvant chemotherapy (NAC), as well as in
32 healthy individuals. The correlation between miR-221 and
clinicopathological features and chemosensitivity was also
analyzed. The expression level of miR-221 was significantly
associated with the hormone receptor (HR) status. Patients with
higher plasma miR-221 levels tended to be HR-negative. Patients
with varying miR-221 levels had significant differences in the
overall response rate but not in the pathological complete response
rate. These results indicate that plasma miR-221 may be a
predictive biomarker for sensitivity to NAC in breast cancer
patients.
This issue has great impact on the design of novel
therapeutic approaches. On the one hand, it is very important to
determine whether the expression of the miR-221/miR-222 cluster is
under the transcriptional regulation of cellular proteins (for
instance tumor-associated transcription factors). On the other
hand, it is imperative to determine which mRNAs are specifically
targeted by miR-221 (for instance tumor-suppressor mRNAs)
determining the tumorigenic potential of this miRNA. Finally, it
should be verified whether mRNAs regulated by miR-221 encode
proteins able to regulate upstream miR-221 modifiers, therefore
activating a ‘vicious intracellular cycle’. As regards these
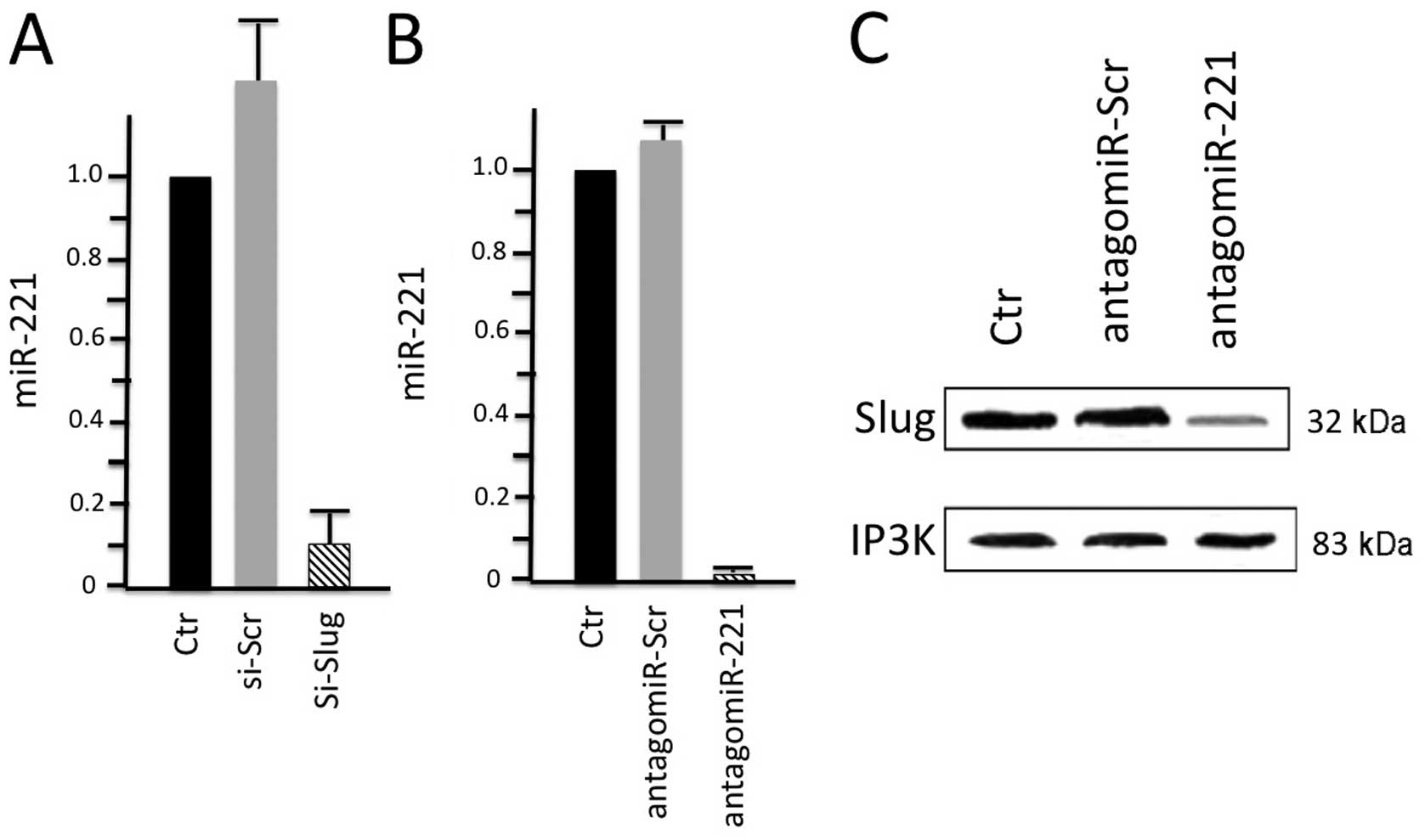
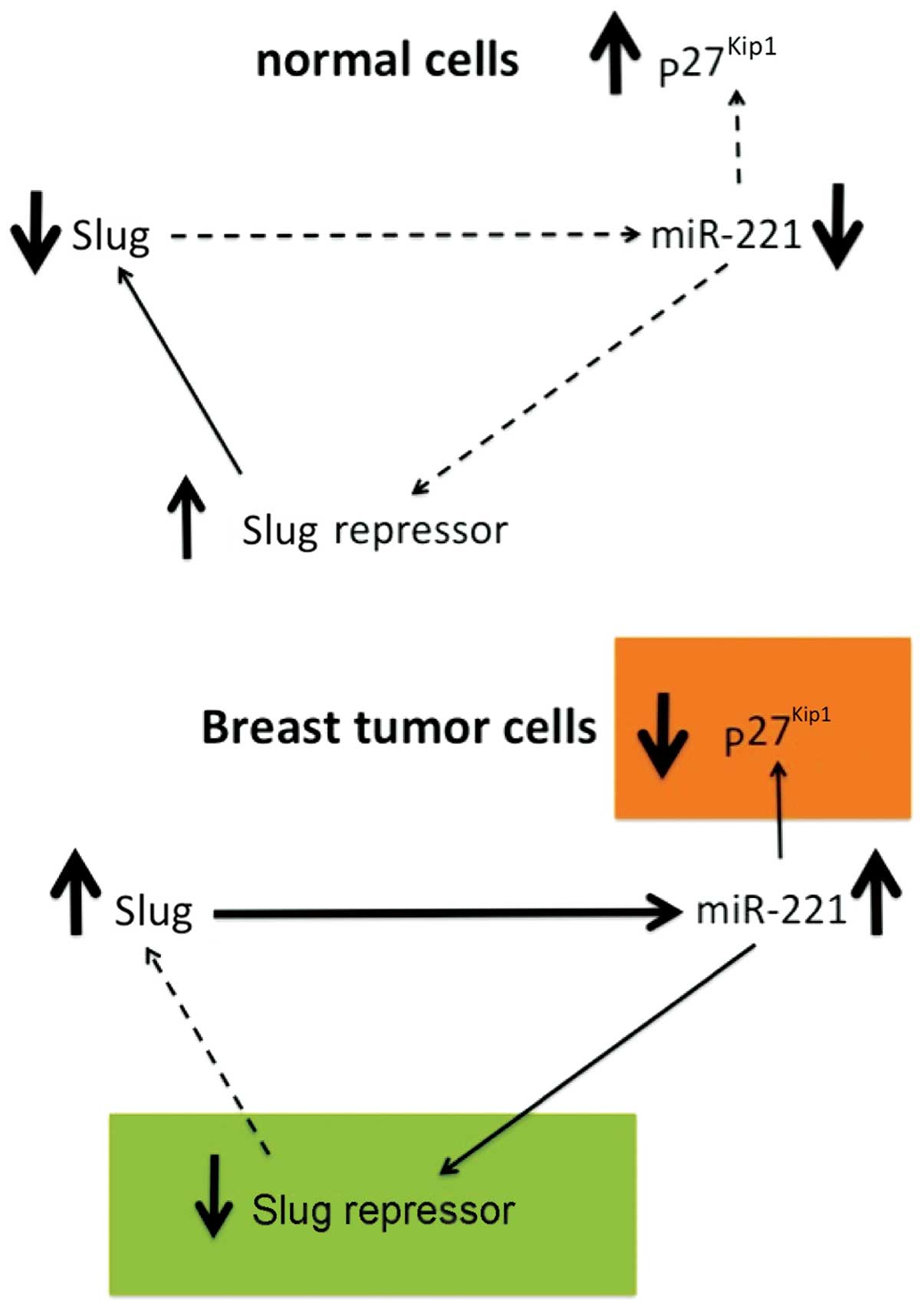
issues, a number of studies have been published. Lambertini et
al (74) recently demonstrated
that the Slug transcription factor binds to the miR-221/miR-222
promoter and is responsible for the high expression of the
miR-221/miR-222 cluster in breast cancer cells. In order to
investigate the possible correlation between the Slug transcription
factor and miR-221, they performed Slug gene silencing in
MDA-MB-231 breast cancer cells and evaluated the expression of
genes involved in supporting the breast cancer phenotype by qRT-PCR
and western blot analysis. Chromatin immunoprecipitation and wound
healing assays were employed to determine a functional link between
these two molecules. The results of their study (Fig. 3) revealed that Slug silencing
significantly decreased the level of miR-221 and vimentin,
reactivated ERα and increased E-cadherin and TRPS1 expression
(74). It was demonstrated that
miR-221 is a Slug target gene, and the authors identified a
specific region of the miR-221 promoter that is transcriptionally
active and binds the transcription factor Slug in vivo. In
addition, they observed a more potent inhibiton of cell migration
in the Slug-silenced cells, which retained residual miR-221
(approximately 38%), compared with antagomiR-221-treated cells with
a complete knockdown of miR-221. As a whole, their study reported
for the first time evidence of a correlation between the Slug
transcription factor and miR-221 in breast cancer cells, suggesting
that miR-221 expression is, at least in part, dependent on Slug,
which is more effective than miR-221 in sustaining cell migration
and invasion.
In miRNA therapeutics, by targeting oncomiRs and
metastamiRs, several strategies have been performed to inhibit the
functions of oncomiRs and metastamiRs. One of the most common
approaches involves the use of antisense miRNAs (antagomiRs)
capable of knocking down miRNAs. Velu et al (76) demonstrated the efficacy of the
knockdown of miR-21, which is involved in myelopoiesis, using
antagomiRs in primary murine bone marrow stem/progenitor cells.
This approach has a clear potential impact in anticancer therapy,
as demonstrated in a very recent study by Poltronieri et al
(77), who hypothesized that, as
oncomiRs promote the growth of cancer cells and support survival
during chemotherapy, thus miRNA-silencing therapies may be a
valuable approach in conjunction with anticancer drugs and
chemotherapy treatments. Specifically, they focused on miR-155,
which they found overexpressed in different types of cancer. Of
particular interest was the finding that GABA-A receptor
downregulation was found to correlate with the glioma grade, with
decreasing levels being associated with a higher grade of
malignancy. The demonstration that the knockdown of miR-155
involves the re-expression of GABRA 1 protein in vivo has a
great implication on the effectiveness of RNA-silencing approaches
against miR-155, with the aim to control proliferation and
signalling pathways regulated by the GABA-A receptor.
Another study also focused on potential anticancer
therapy based on miRNA knockdown. Ma et al aimed to control
mammary tumor metastasis (78).
They demonstrated that the systemic treatment of tumor-bearing mice
with miR-10b antagomiRs suppresses breast cancer metastasis, both
in vitro and in vivo. The silencing of miR-10b with
antagomiRs significantly decreased miR-10b levels and increased the
levels of a functionally important miR-10b target, Hoxd10. Of note,
the administration of miR-10b antagomiRs to mice bearing highly
metastatic cells did not reduce primary mammary tumor growth but
markedly suppressed the formation of lung metastases in a
sequence-specific manner. The miR-10b antagomiR, which is well
tolerated by healthy animals, appears to be a promising candidate
for the development of novel anti-metastatic agents.
In the case of the development of PNA-based miRNA
therapeutics for altering gene expression in breast cancer cells,
PNA targeting miR-221 has shown to specifically interact with
miR-221 expressed in aggressive breast cancer cell lines (94). In order to maximize uptake in
target cells, a polyarginine-peptide (R8) was conjugated,
generating an anti-miR-221 PNA (R8-PNA-a221) displaying very high
affinity for RNA and efficient uptake within target cells without
the need of transfection reagents. Unmodified PNA with the same
sequence displayed RNA binding, but cellular uptake was very poor.
Consistently, only R8-PNA-a221 markedly inhibited miR-221 in
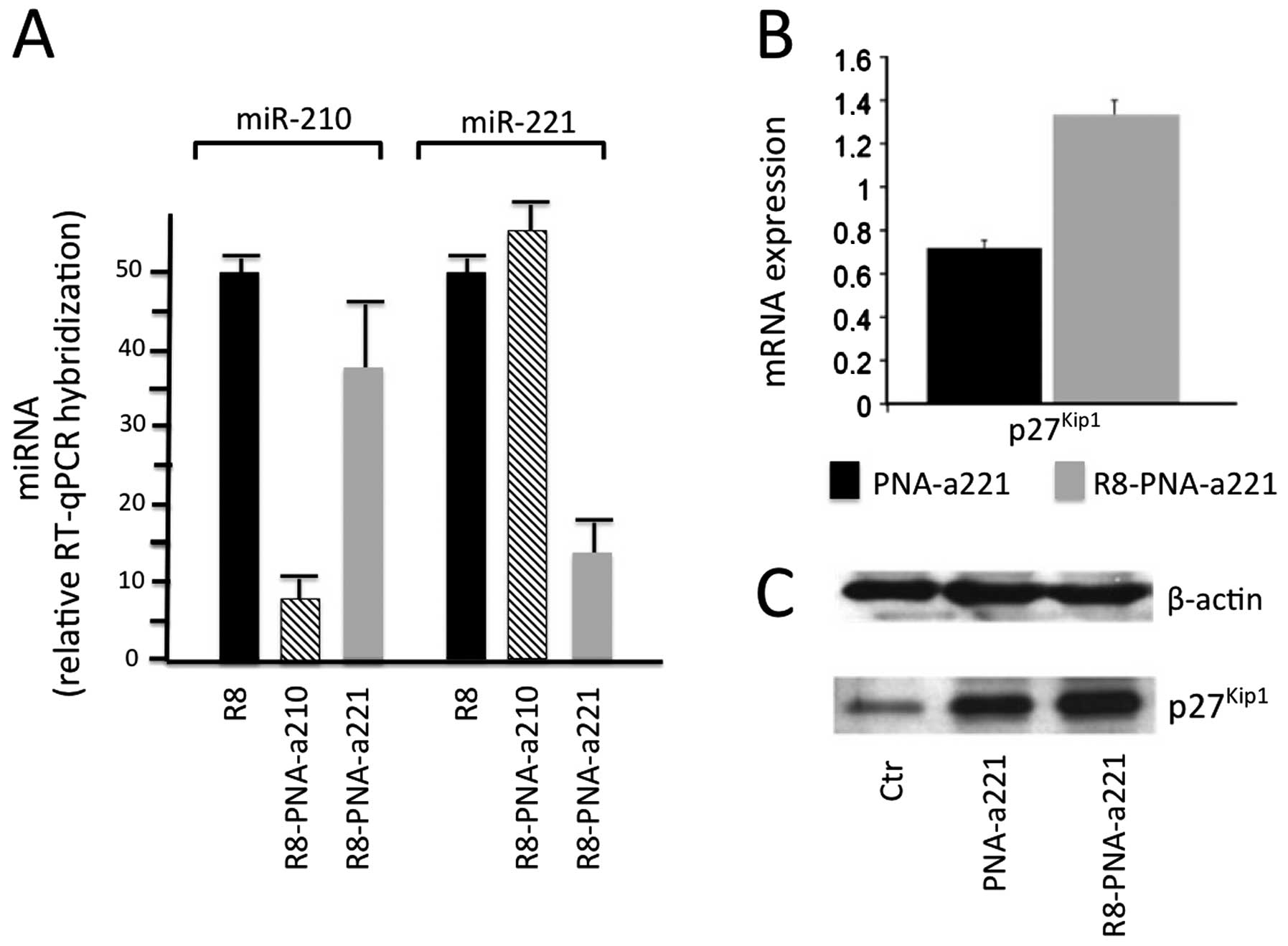
MDA-MB-231 breast cancer cells. This is illustrated in Fig. 5A, describing the effects of two
PNA-based antagomiRs, R8-PNA-a210 and R8-PNAa221, targeting miR-210
and miR-221, respectively. As it is clearly evident, R8-PNA-a210
inhibits miR-210 but not miR-221 and vice-versa, R8-PNAa221
inhibits miR-221 but not miR-210. Therefore, targeting miR-221 with
R8-PNAa221 resulted in i) a specific decrease in the hybridization
levels of miR-221 measured by qRT-PCR; and ii) the upregulation of
p27Kip1, mRNA and protein, measured by qRT-PCR and
western blot analysis (Fig. 5B and
C).
This study was supported by a grant
from MIUR (Italian Ministry of University and Research). R.G.
received a grant from AIRC, Fondazione Cariparo (Cassa di Risparmio
di Padova e Rovigo), CIB (Consorzio Interuniversitario di
Biotecnologie).
|
1.
|
Filipowicz W, Jaskiewicz L, Kolb FA and
Pillai RS: Post-transcriptional gene silencing by siRNAs and
miRNAs. Curr Opin Struc Biol. 15:331–341. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
He L and Hannon GJ: MicroRNAs: small RNAs
with a big role in gene regulation. Nat Rev Genet. 5:522–531. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Kozomara A and Griffiths-Jones S: miRBase:
integrating microRNA annotation and deep-sequencing data. Nucleic
Acids Res. 39:D152–D157. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Krol J, Loedige I and Filipowicz W: The
widespread regulation of microRNA biogenesis, function and decay.
Nat Rev Genet. 11:597–610. 2010.PubMed/NCBI
|
|
5.
|
Sontheimer EJ and Carthew RW: Silence from
within: endogenous siRNAs and miRNAs. Cell. 122:9–12. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Taccioli C, Fabbri E, Visone R, Volinia S,
Calin GA, Fong LY, et al: UCbase and miRfunc: a database of
ultracon-served sequences and microRNA function. Nucleic Acids Res.
37:D41–D48. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Griffiths-Jones S: miRBase: the microRNA
sequence database. Methods Mol Biol. 342:129–138. 2006.PubMed/NCBI
|
|
8.
|
Witwer KW: Data submission and quality in
microarray-based microRNA profiling. Clin Chem. 59:392–400. 2013.
View Article : Google Scholar
|
|
9.
|
Sablok G, Milev I, Minkov G, Minkov I,
Varotto C, Yahubyan G and Baev V: isomiRex: Web-based
identification of microRNAs, isomiR variations and differential
expression using next-generation sequencing datasets. FEBS Lett.
Jul 4–2013.(Epub ahead of print).
|
|
10.
|
Russo F, Di Bella S, Nigita G, Macca V,
Laganà A, Giugno R, Pulvirenti A and Ferro A: miRandola:
extracellular circulating microRNAs database. PLoS One.
7:e477862012. View Article : Google Scholar
|
|
11.
|
Krützfeldt J, Kuwajima S, Braich R, Rajeev
KG, Pena J, Tuschl T, Manoharan M and Stoffel M: Specificity,
duplex degradation and subcellular localization of antagomirs.
Nucleic Acids Res. 35:2885–2892. 2007.PubMed/NCBI
|
|
12.
|
Dalmay T: Mechanism of miRNA-mediated
repression of mRNA translation. Essays Biochem. 54:29–38. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Jiang Q, Wang Y, Hao Y, Juan L, Teng M,
Zhang X, Li M, Wang G and Liu Y: miR2Disease: a manually curated
database for microRNA deregulation in human disease. Nucleic Acids
Res. 37:D98–D104. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Subramanian S and Steer CJ: MicroRNAs as
gatekeepers of apoptosis. J Cell Physiology. 223:89–98. 2010.
|
|
15.
|
Wang YM and Blelloch R: Cell cycle
regulation by MicroRNAs in embryonic stem cells. Cancer Res.
69:4093–4096. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Alvarez-Garcia I and Miska EA: MicroRNA
functions in animal development and human disease. Development.
132:4653–4662. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Tsai LM and Yu D: MicroRNAs in common
diseases and potential therapeutic applications. Clin Exp Pharmacol
Physiol. 7:102–107. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Hemida MG, Ye X, Thair S and Yang D:
Exploiting the therapeutic potential of microRNAs in viral
diseases: expectations and limitations. Mol Diagn Ther. 14:271–282.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Kota SK and Balasubramanian S: Cancer
therapy via modulation of micro RNA levels: a promising future.
Drug Discov Today. 15:733–740. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Bader AG, Brown D and Winkler M: The
promise of microRNA replacement therapy. Cancer Res. 70:7027–7030.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Sibley CR, Seow Y and Wood MJ: Novel
RNA-based strategies for therapeutic gene silencing. Mol Ther.
18:466–476. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Ge YF, Sun J, Jin CJ, Cao BQ, Jiang ZF and
Shao JF: AntagomiR-27a targets FOXO3a in glioblastoma and
suppresses U87 cell growth in vitro and in vivo. Asian Pac J Cancer
Prev. 14:963–968. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Rather MI, Nagashri MN, Swamy SS, Gopinath
KS and Kumar A: Oncogenic microRNA-down-regulates tumor suppressor
CDC73 and promotes oral squamous cell carcinoma cell proliferation:
implications for cancer therapeutics. J Biol Chem. 288:608–618.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Shu M, Zheng X, Wu S, Lu H, Leng T, Zhu W,
Zhou Y, Ou Y, Lin X, Lin Y, Xu D, Zhou Y and Yan G: Targeting
oncogenic miR-335 inhibits growth and invasion of malignant
astrocytoma cells. Mol Cancer. 10:592011. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Haug BH, Henriksen JR, Buechner J, Geerts
D, Tømte E, Kogner P, Martinsson T, Flægstad T, Sveinbjørnsson B
and Einvik C: MYCN-regulated miRNA-92 inhibits secretion of the
tumor suppressor DICKKOPF-3 (DKK3) in neuroblastoma.
Carcinogenesis. 32:1005–1012. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Tang H, Liu X, Wang Z, She X, Zeng X, Deng
M, Liao Q, Guo X, Wang R, Li X, Zeng F, Wu M and Li G: Interaction
of hsa-miR-381 and glioma suppressor LRRC4 is involved in glioma
growth. Brain Res. 1390:21–32. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Ma L, Reinhardt F, Pan E, Soutschek J,
Bhat B, Marcusson EG, Teruya-Feldstein J, Bell GW and Weinberg RA:
Therapeutic silencing of miR-10b inhibits metastasis in a mouse
mammary tumor model. Nat Biotechnol. 28:341–347. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Mercatelli N, Coppola V, Bonci D, Miele F,
Costantini A, Guadagnoli M, Bonanno E, Muto G, Frajese GV, De Maria
R, Spagnoli LG, Farace MG and Ciafrè SA: The inhibition of the
highly expressed miR-221 and miR-222 impairs the growth of prostate
carcinoma xenografts in mice. PLoS One. 3:e40292008. View Article : Google Scholar
|
|
29.
|
Scheibner KA, Teaboldt B, Hauer MC, Chen
X, Cherukuri S, Guo Y, Kelley SM, Liu Z, Baer MR, Heimfeld S and
Civin CI: MiR-27a functions as a tumor suppressor in acute leukemia
by regulating 14-3-3θ. PLoS One. 7:e508952012.PubMed/NCBI
|
|
30.
|
Endo H, Muramatsu T, Furuta M, Uzawa N,
Pimkhaokham A, Amagasa T, Inazawa J and Kozaki K: Potential of
tumor-suppressive miR-596 targeting LGALS3BP as a therapeutic agent
in oral cancer. Carcinogenesis. 34:560–569. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Liang Z, Ahn J, Guo D, Votaw JR and Shim
H: MicroRNA-302 replacement therapy sensitizes breast cancer cells
to ionizing radiation. Pharm Res. 30:1008–1016. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Thomas M, Lange-Grünweller K, Weirauch U,
Gutsch D, Aigner A, Grünweller A and Hartmann RK: The
proto-oncogene Pim-1 is a target of miR-33a. Oncogene. 31:918–928.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Ibrahim AF, Weirauch U, Thomas M,
Grünweller A, Hartmann RK and Aigner A: MicroRNA replacement
therapy for miR-145 and miR-33a is efficacious in a model of colon
carcinoma. Cancer Res. 71:5214–5224. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Wiggins JF, Ruffino L, Kelnar K, Omotola
M, Patrawala L, Brown D and Bader AG: Development of a lung cancer
therapeutic based on the tumor suppressor microRNA-34. Cancer Res.
70:5923–5930. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Trang P, Wiggins JF, Daige CL, Cho C,
Omotola M, Brown D, Weidhaas JB, Bader AG and Slack FJ: Systemic
delivery of tumor suppressor microRNA mimics using a neutral lipid
emulsion inhibits lung tumors in mice. Mol Ther. 19:1116–1122.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Wu Y, Crawford M, Mao Y, Lee RJ, Davis IC,
Elton TS, Lee LJ and Nana-Sinkam SP: Therapeutic delivery of
microRNA-29b by cationic lipoplexes for lung cancer. Mol Ther
Nucleic Acids. 2:e842013. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Huang X, Schwind S, Yu B, Santhanam R,
Wang H, Hoellerbauer P, Mims A, Klisovic R, Walker AR, Chan KK,
Blum W, Perrotti D, Byrd JC, Bloomfield CD, Caligiuri MA, Lee RJ,
Garzon R, Muthusamy N, Lee LJ and Marcucci G: Targeted delivery of
microRNA-29b by transferrin-conjugated anionic lipopolyplex
nanoparticles: a novel therapeutic strategy in acute myeloid
leukemia. Clin Cancer Res. 19:2355–2367. 2013.
|
|
38.
|
Voorhoeve PM, le Sage C, Schrier M, Gillis
AJ, Stoop H, Nagel R, Liu YP, van Duijse J, Drost J, Griekspoor A,
Zlotorynski E, Yabuta N, De Vita G, Nojima H, Looijenga LH and
Agami R: A genetic screen implicates miRNA-372 and miRNA-373 as
oncogenes in testicular germ cell tumors. Cell. 124:1169–1181.
2006. View Article : Google Scholar
|
|
39.
|
Voorhoeve PM, le Sage C, Schrier M, Gillis
AJ, Stoop H, Nagel R, Liu YP, van Duijse J, Drost J, Griekspoor A,
Zlotorynski E, Yabuta N, De Vita G, Nojima H, Looijenga LH and
Agami R: A genetic screen implicates miRNA-372 and miRNA-373 as
oncogenes in testicular germ cell tumors. Adv Exp Med Biol.
604:17–46. 2007. View Article : Google Scholar
|
|
40.
|
Huang Q, Gumireddy K, Schrier M, le Sage
C, Nagel R, Nair S, Egan DA, Li A, Huang G, Klein-Szanto AJ,
Gimotty PA, Katsaros D, Coukos G, Zhang L, Puré E and Agami R: The
microRNAs miR-373 and miR-520c promote tumour invasion and
metastasis. Nat Cell Biol. 10:202–210. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41.
|
Galardi S, Mercatelli N, Giorda E,
Massalini S, Frajese GV, Ciafrè SA and Farace MG: miR-221 and
miR-222 expression affects the proliferation potential of human
prostate carcinoma cell lines by targeting p27Kip1. J
Biol Chem. 282:23716–23724. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42.
|
Valastyan S, Reinhardt F, Benaich N,
Calogrias D, Szász AM, Wang ZC, Brock JE, Richardson AL and
Weinberg RA: A pleiotropically acting microRNA, miR-31, inhibits
breast cancer metastasis. Cell. 137:1032–1046. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43.
|
Hurst DR, Edmonds MD and Welch DR:
Metastamir: the field of metastasis-regulatory microRNA is
spreading. Cancer Res. 69:7495–7498. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44.
|
Wotschofsky Z, Liep J, Meyer HA, Jung M,
Wagner I, Disch AC, Schaser KD, Melcher I, Kilic E, Busch J,
Weikert S, Miller K, Erbersdobler A, Mollenkopf HJ and Jung K:
Identification of metastamirs as metastasis-associated microRNAs in
clear cell renal cell carcinomas. Int J Biol Sci. 8:1363–1374.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
45.
|
Taylor MA, Sossey-Alaoui K, Thompson CL,
Danielpour D and Schiemann WP: TGF-β upregulates miR-181a
expression to promote breast cancer metastasis. J Clin Invest.
123:150–163. 2013.
|
|
46.
|
Welch DR and Hurst DR: Unraveling the
‘TGF-β paradox’ one metastamir at a time. Breast Cancer Res.
15:3052013.
|
|
47.
|
Moldovan L, Batte K, Wang Y, Wisler J and
Piper M: Analyzing the circulating microRNAs in
exosomes/extracellular vesicles from serum or plasma by qRT-PCR.
Methods Mol Biol. 1024:129–145. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48.
|
Chen X, Liang H, Zhang J, Zen K and Zhang
CY: Horizontal transfer of microRNAs: molecular mechanisms and
clinical applications. Protein Cell. 3:28–37. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49.
|
Kosaka N and Ochiya T: Unraveling the
mystery of cancer by secretory microRNA: horizontal microRNA
transfer between living cells. Front Genet. 2:972011.PubMed/NCBI
|
|
50.
|
Chen X, Liang H, Zhang J, Zen K and Zhang
CY: Secreted microRNAs: a new form of intercellular communication.
Trends Cell Biol. 22:125–132. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
51.
|
Ramachandran S and Palanisamy V:
Horizontal transfer of RNAs: exosomes as mediators of intercellular
communication. Wiley Interdiscip Rev RNA. 3:286–293. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
52.
|
Muralidharan-Chari V, Clancy JW, Sedgwick
A and D’Souza-Schorey C: Microvesicles: mediators of extracellular
communication during cancer progression. J Cell Sci1. 23:1603–1611.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
53.
|
Piovan C, Palmieri D, Di Leva G, Braccioli
L, Casalini P, Nuovo G, Tortoreto M, Sasso M, Plantamura I, Triulzi
T, Taccioli C, Tagliabue E, Iorio MV and Croce CM: Oncosuppressive
role of p53-induced miR-205 in triple negative breast cancer. Mol
Oncol. 6:458–472. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
54.
|
Lee YM, Lee JY, Ho CC, Hong QS, Yu SL,
Tzeng CR, Yang PC and Chen HW: miRNA-34b as a tumor suppressor in
estrogen-dependent growth of breast cancer cells. Breast Cancer
Res. 13:R1162011. View Article : Google Scholar : PubMed/NCBI
|
|
55.
|
Iorio MV and Croce CM: Causes and
consequences of microRNA dysregulation. Cancer J. 18:215–222. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
56.
|
Xu X, Chen H, Lin Y, Hu Z, Mao Y, Wu J, Xu
X, Zhu Y, Li S, Zheng X and Xie L: MicroRNA-409-3p inhibits
migration and invasion of bladder cancer cells via targeting c-Met.
Mol Cells. 36:62–68. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
57.
|
He J, Deng Y, Yang G and Xie W:
MicroRNA-203 down-regulation is associated with unfavorable
prognosis in human glioma. J Surg Oncol. 108:121–125. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
58.
|
Iorio MV and Croce CM: MicroRNAs in
cancer: small molecules with a huge impact. J Clin Oncol.
27:5848–5856. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
59.
|
Volinia S, Galasso M, Sana ME, Wise TF,
Palatini J, Huebner K and Croce CM: Breast cancer signatures for
invasiveness and prognosis defined by deep sequencing of microRNA.
Proc Natl Acad Sci USA. 109:3024–3029. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
60.
|
Lu Y, Roy S, Nuovo G, Ramaswamy B, Miller
T, Shapiro C, Jacob ST and Majumder S: Anti-microRNA-222
(anti-miR-222) and -181B suppress growth of tamoxifen-resistant
xenografts in mouse by targeting TIMP3 protein and modulating
mitogenic signal. J Biol Chem. 286:42292–42302. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
61.
|
Shah MY and Calin GA: MicroRNAs miR-221
and miR-222: a new level of regulation in aggressive breast cancer.
Genome Med. 3:562011. View
Article : Google Scholar : PubMed/NCBI
|
|
62.
|
Stinson S, Lackner MR, Adai AT, Yu N, Kim
HJ, O’Brien C, Spoerke J, Jhunjhunwala S, Boyd Z, Januario T,
Newman RJ, Yue P, Bourgon R, Modrusan Z, Stern HM, Warming S, de
Sauvage FJ, Amler L, Yeh RF and Dornan D: miR-221/222 targeting of
trichorhinophalangeal 1 (TRPS1) promotes epithelial-to-mesenchymal
transition in breast cancer. Sci Signal. 4(pt5)2011.PubMed/NCBI
|
|
63.
|
Cochrane DR, Cittelly DM, Howe EN,
Spoelstra NS, McKinsey EL, LaPara K, Elias A, Yee D and Richer JK:
MicroRNAs link estrogen receptor alpha status and Dicer levels in
breast cancer. Horm Cancer. 1:306–319. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
64.
|
Yoshimoto N, Toyama T, Takahashi S,
Sugiura H, Endo Y, Iwasa M, Fujii Y and Yamashita H: Distinct
expressions of microRNAs that directly target estrogen receptor α
in human breast cancer. Breast Cancer Res Treat. 130:331–339.
2011.PubMed/NCBI
|
|
65.
|
Stinson S, Lackner MR, Adai AT, Yu N, Kim
HJ, O’Brien C, Spoerke J, Jhunjhunwala S, Boyd Z, Januario T,
Newman RJ, Yue P, Bourgon R, Modrusan Z, Stern HM, Warming S, de
Sauvage FJ, Amler L, Yeh RF and Dornan D: TRPS1 targeting by
miR-221/222 promotes the epithelial-to-mesenchymal transition in
breast cancer. Sci Signal. 4:ra412011.PubMed/NCBI
|
|
66.
|
Guttilla IK, Phoenix KN, Hong X, Tirnauer
JS, Claffey KP and White BA: Prolonged mammosphere culture of MCF-7
cells induces an EMT and repression of the estrogen receptor by
microRNAs. Breast Cancer Res Treat. 132:75–85. 2012. View Article : Google Scholar
|
|
67.
|
Gordanpour A, Stanimirovic A, Nam RK,
Moreno CS, Sherman C, Sugar L and Seth A: miR-221 is down-regulated
in TMPRSS2: ERG fusion-positive prostate cancer. Anticancer Res.
31:403–410. 2011.PubMed/NCBI
|
|
68.
|
Radojicic J, Zaravinos A, Vrekoussis T,
Kafousi M, Spandidos DA and Stathopoulos EN: MicroRNA expression
analysis in triple-negative (ER, PR and Her2/neu) breast cancer.
Cell Cycle. 10:507–517. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
69.
|
Pelletier C, Speed WC, Paranjape T, Keane
K, Blitzblau R, Hollestelle A, Safavi K, van den Ouweland A,
Zelterman D, Slack FJ, Kidd KK and Weidhaas JB: Rare BRCA1
haplotypes including 3’UTR SNPs associated with breast cancer risk.
Cell Cycle. 10:90–99. 2011.
|
|
70.
|
Rao X, Di Leva G, Li M, Fang F, Devlin C,
Hartman-Frey C, Burow ME, Ivan M, Croce CM and Nephew KP:
MicroRNA-221/222 confers breast cancer fulvestrant resistance by
regulating multiple signaling pathways. Oncogene. 30:1082–1097.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
71.
|
Zhou M, Liu Z, Zhao Y, Ding Y, Liu H, Xi
Y, Xiong W, Li G, Lu J, Fodstad O, Riker AI and Tan M:
MicroRNA-125b confers the resistance of breast cancer cells to
paclitaxel through suppression of pro-apoptotic Bcl-2 antagonist
killer 1 (Bak1) expression. J Biol Chem. 285:21496–21507. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
72.
|
Di Leva G, Gasparini P, Piovan C, Ngankeu
A, Garofalo M, Taccioli C, Iorio MV, Li M, Volinia S, Alder H,
Nakamura T, Nuovo G, Liu Y, Nephew KP and Croce CM: MicroRNA
cluster 221–222 and estrogen receptor alpha interactions in breast
cancer. J Natl Cancer Inst. 102:706–721. 2010.
|
|
73.
|
Pogribny IP, Filkowski JN, Tryndyak VP,
Golubov A, Shpyleva SI and Kovalchuk O: Alterations of microRNAs
and their targets are associated with acquired resistance of MCF-7
breast cancer cells to cisplatin. Int J Cancer. 127:1785–1794.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
74.
|
Lambertini E, Lolli A, Vezzali F,
Penolazzi L, Gambari R and Piva R: Correlation between Slug
transcription factor and miR-221 in MDA-MB-231 breast cancer cells.
BMC Cancer. 12:4452012. View Article : Google Scholar : PubMed/NCBI
|
|
75.
|
Zhao R, Wu J, Jia W, Gong C, Yu F, Ren Z,
Chen K, He J and Su F: Plasma miR-221 as a predictive biomarker for
chemoresistance in breast cancer patients who previously received
neoadjuvant chemotherapy. Onkologie. 34:675–680. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
76.
|
Velu CS and Grimes HL: Utilizing antagomiR
(antisense microRNA) to knock down microRNA in murine bone marrow
cells. Methods Mol Biol. 928:185–195. 2012.PubMed/NCBI
|
|
77.
|
Poltronieri P, D’Urso PI, Mezzolla V and
D’Urso OF: Potential of anti-cancer therapy based on anti-miR-155
oligonucleotides in glioma and brain tumours. Chem Biol Drug Des.
81:79–84. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
78.
|
Ma D, Tao X, Gao F, Fan C and Wu D:
miR-224 functions as an onco-miRNA in hepatocellular carcinoma
cells by activating AKT signaling. Oncol Lett. 4:483–488.
2012.PubMed/NCBI
|
|
79.
|
Nielsen PE, Egholm M, Berg RH and Buchardt
O: Sequence-selective recognition of DNA by strand displacement
with a thymine-substituted polyamide. Science. 254:1497–1500. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
80.
|
Demidov VV and Frank-Kamenetskii MD:
Sequence-specific targeting of duplex DNA by peptide nucleic acids
via triplex strand invasion. Methods. 23:108–122. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
81.
|
Gambari R: Peptide-nucleic acids (PNAs): a
tool for the development of gene expression modifiers. Curr Pharm
Des. 7:1839–1862. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
82.
|
Karkare S and Bhatnagar D: Promising
nucleic acid analogs and mimics: characteristic features and
applications of PNA, LNA, and morpholino. Appl Microbiol
Biotechnol. 71:575–586. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
83.
|
Nielsen PE: Antisense peptide nucleic
acids. Curr Opin Mol Ther. 2:282–287. 2002.
|
|
84.
|
Soomets U, Hällbrink M and Langel U:
Antisense properties of peptide nucleic acids. Front Biosci.
4:D782–D786. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
85.
|
Ray A and Nordén B: Peptide nucleic acid
(PNA): its medical and biotechnical applications and promise for
the future. FASEB J. 14:1041–1060. 2000.PubMed/NCBI
|
|
86.
|
Nielsen PE: Targeting double stranded DNA
with peptide nucleic acid (PNA). Curr Med Chem. 8:545–550. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
87.
|
Gambari R: Biological activity and
delivery of peptide nucleic acids (PNA)-DNA chimeras for
transcription factor decoy (TFD) pharmacotherapy. Curr Med Chem.
11:1253–1263. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
88.
|
Corradini R, Sforza S, Tedeschi T,
Totsingan F and Marchelli R: Peptide nucleic acids with a
structurally biased backbone: effects of conformational constraints
and stereochemistry. Curr Top Med Chem. 7:681–694. 2007. View Article : Google Scholar
|
|
89.
|
Sforza S, Tedeschi T, Calabretta A,
Corradini R, Camerin C, Tonelli R, Pession A and Marchelli R: A
peptide nucleic acid embedding a pseudopeptide nuclear localization
sequence in the backbone behaves as a peptide mimic. Eur J Org
Chem. 13:2441–2444. 2010. View Article : Google Scholar
|
|
90.
|
Sforza S, Corradini R, Ghirardi S, Dossena
A and Marchelli R: DNA binding of a D-Lysine-based chiral PNA:
direction control and mismatch recognition. Eur J Org Chem.
16:2905–2913. 2000. View Article : Google Scholar
|
|
91.
|
Sforza S, Tedeschi T, Corradini R and
Marchelli R: Induction of helical handedness and DNA binding
properties of peptide nucleic acids (PNAs) with two stereogenic
centres. Eur J Org Chem. 35:5879–5885. 2007. View Article : Google Scholar
|
|
92.
|
Tedeschi T, Sforza S, Corradini R and
Marchelli R: Synthesis of new chiral PNAs bearing a dipeptide-mimic
monomer with two lysine-derived stereogenic centres. Tetrahedron
Lett. 46:8395–8399. 2005. View Article : Google Scholar
|
|
93.
|
Dragulescu-Andrasi A, Zhou P, He G and Ly
DH: Cell-permeable GPNA with appropriate backbone stereochemistry
and spacing binds sequence-specifically to RNA. Chem Commun.
3:244–246. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
94.
|
Brognara E, Fabbri E, Aimi F, Manicardi A,
Bianchi N, Finotti A, Breveglieri G, Borgatti M, Corradini R,
Marchelli R and Gambari R: Peptide nucleic acids targeting miR-221
modulate p27Kip1 expression in breast cancer MDA-MB-231
cells. Int J Oncol. 41:2119–2127. 2012.PubMed/NCBI
|
|
95.
|
Gambari R, Fabbri E, Borgatti M, Lampronti
I, Finotti A, Brognara E, Bianchi N, Manicardi A, Marchelli R and
Corradini R: Targeting microRNAs involved in human diseases: a
novel approach for modification of gene expression and drug
development. Biochem Pharmacol. 82:1416–1429. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
96.
|
Fabani MM and Gait MJ: miR-122 targeting
with LNA/2′-O-methyloligonucleotide mixmers, peptide nucleic acids
(PNA), and PNA-peptide conjugates. RNA. 14:336–346. 2008.
|
|
97.
|
Fabani MM, Abreu-Goodger C, Williams D,
Lyons PA, Torres AG, Smith KGC, et al: Efficient inhibition of
miR-155 function in vivo by peptide nucleic acids. Nucleic Acids
Res. 38:4466–4475. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
98.
|
Fabbri E, Manicardi A, Tedeschi T, Sforza
S, Bianchi N, Brognara E, Finotti A, Breveglieri G, Borgatti M,
Corradini R, Marchelli R and Gambari R: Modulation of the
biological activity of microRNA-210 with peptide nucleic acids
(PNAs). Chem Med Chem. 6:2192–2202. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
99.
|
Fabbri E, Brognara E, Borgatti M,
Lampronti I, Finotti A, Bianchi N, Sforza S, Tedeschi T, Manicardi
A, Marchelli R, Corradini R and Gambari R: miRNA therapeutics:
delivery and biological activity of peptide nucleic acids targeting
miRNAs. Epigenomics. 3:733–745. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
100.
|
Manicardi A, Fabbri E, Tedeschi T, Sforza
S, Bianchi N, Brognara E, Gambari R, Marchelli R and Corradini R:
Cellular uptakes, biostabilities and anti-miR-210 activities of
chiral arginine-PNAs in leukaemic K562 cells. Chembiochem.
13:1327–1337
|
|
101.
|
Yan LX, Wu QN, Zhang Y, Li YY, Liao DZ,
Hou JH, Fu J, Zeng MS, Yun JP, Wu QL, Zeng YX and Shao JY:
Knockdown of miR-21 in human breast cancer cell lines inhibits
proliferation, in vitro migration and in vivo tumor growth. Breast
Cancer Res. 13:R22011. View Article : Google Scholar : PubMed/NCBI
|