Contents
Introduction
Epidemiological trends, etiology and risk factors of
NBNC-HCC
Alcohol-related HCC
NAFLD and NASH
DM
Obesity
Iron
Other causes
Mechanism of carcinogenesis in NBNC-HCC
Clinicopathological features and prognosis in
patients with NBNC-HCC
Conclusion
Introduction
Hepatocellular carcinoma (HCC) is a common
malignancy in Asia and South Africa. HCC usually develops in
patients with hepatitis B virus (HBV) infection, hepatitis C virus
(HCV) infection and alcoholic liver disease (1–3). HCC
is diagnosed in more than half a million people worldwide each
year, and therefore it is a major global health problem. HCC is the
fifth most common cancer in the world and the third most common
cause of cancer-related death, behind only lung cancer and gastric
cancer (1–5). Japan has one of the highest rates of
incidence of HCC among developed countries (4–6).
Although most HCC is related to viral infection,
there is a substantial population of HCC patients (5–20%) who are
negative for both markers of HBV and HCV infection [non-B, non-C
(NBNC) hepatitis] in Japan and the incidence of NBNC-HCC has
recently tended to increase (7–12).
Furthermore, investigations in the US assessing risk factors for
chronic liver disease and HCC have failed to identify HBV, HCV or
excessive alcohol intake in a large population (13,14).
The most common cause of liver disease in developed
countries is non-alcoholic fatty liver disease (NAFLD), which
includes non-alcoholic steatohepatitis (NASH) and its related
complications (7,15). The incidence of NASH is reported to
be 1–3% among the adult Japanese population, and ∼6% in Western
countries (7,15). Increased body mass index (BMI) and
diabetes mellitus (DM) are associated with developing NAFLD and
NASH, which is a severe form of NAFLD (17). Increasing clinical evidence
supports the fact that NAFLD and NASH can progress to liver
cirrhosis and HCC (7,13–16).
The exponentially growing incidence of HCC may be partially
attributable to increased numbers of patients with NASH-related
cirrhosis, although recent evidence demonstrates that NAFLD or NASH
may directly promote liver carcinogenesis independent of the
presence of liver cirrhosis (15).
Obesity and the metabolic syndrome are growing
epidemics related to an increased risk for several types of cancer
including HCC (16). In the liver,
inflammatory and angiogenic changes caused by insulin resistance
and fatty liver disease are associated with an increased incidence
of HCC (17,18). In contrast, regardless of
underlying liver disease, liver cirrhosis remains the most
important risk factor for the development of HCC, although as
mentioned earlier, HCC arising without liver cirrhosis raises the
possibility of direct carcinogenesis.
A detailed understanding of the epidemiology,
etiology, molecular mechanism, clinical features and prognosis
associated with NBNC-HCC could improve our screening and therapy of
this disease. In this review, we primarily focus on clinical
aspects of NBNC-HCC and refer to our current knowledge of this
cancer.
Epidemiological trends, etiology, and risk
factors of NBNC-HCC
The major causes of cirrhosis in HCC are HBV, HCV
and alcohol. The risk of HCC increases sharply in response to
chronic liver damage at the fibrosis stage (2). HCV infection is the most prevalent
risk factor for HCC in Japan (2,4,5,19).
In the US, the leading cause of underlying liver disease among HCC
patients is HCV (51%), and the second most common is cryptogenic
cirrhosis (CC) (29%) (14).
Although most HCC still occurs in patients with
chronic hepatitis C in Japan, the incidence of HCV-related HCC has
been decreasing in recent years because of the improvement of
therapy for chronic hepatitis C and a decrease in the number of
patients newly diagnosed with chronic hepatitis C (6,20–22).
In addition, there has been a recent increasing trend in NBNC-HCC
in Japan (7). Nagaoki et al
reported in 1,374 consecutive HCC patients in their institution
that 17 and 67% of HCC was related to HBV and HCV, respectively,
and 15% was related to NBNC-HCC (10). Tokushige et al conducted a
nationwide survey of 14,530 Japanese HCC patients. They reported
that alcohol-related HCC accounted for 7.2% of all HCC, followed by
unknown causes (5.1%) and NAFLD-related HCC (2.0%). The
characteristics of these three groups were clearly different
(median age, 72 years for NAFLD-related HCC, 68 years for
alcohol-related HCC, and 73 years for unknown HCC; female sex, 38,
4 and 37%, respectively) and obesity and lifestyle-related diseases
were significantly more frequent in NAFLD- than alcohol-related HCC
and unknown HCC (7). Ertle et
al reported in 162 HCC patients that HCV-related HCC accounted
for 23.3%, HBV-related HCC for 19.9%, alcohol-related HCC for
12.7%, and NAFLD-related HCC for 24.0% (23).
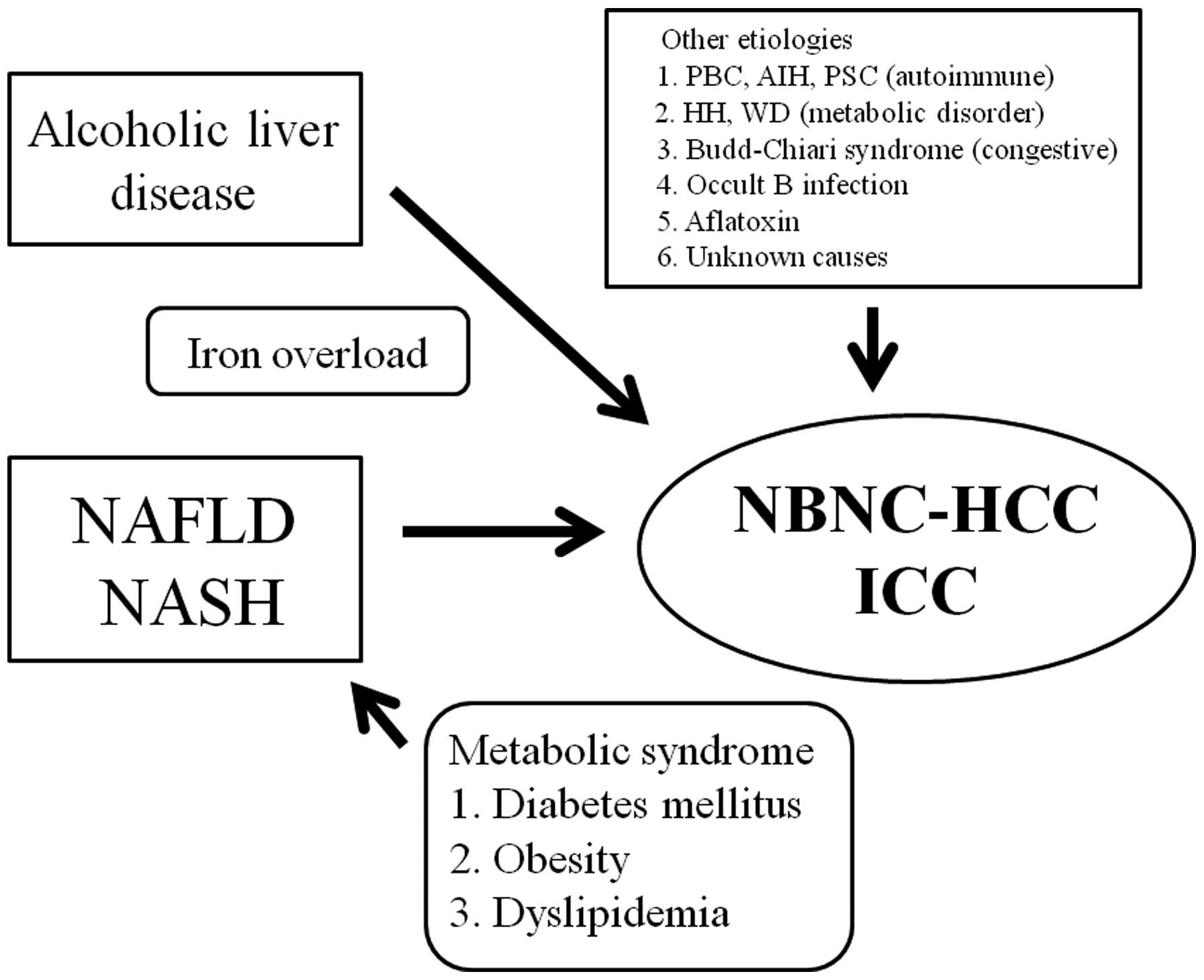
The background liver diseases of NBNC-HCC vary
considerably and they include NAFLD, NASH, alcoholic liver disease,
autoimmune liver disease such as autoimmune hepatitis (AIH),
primary biliary cirrhosis (PBC), primary sclerosing cholangitis
(PSC), congestive liver disease such as Budd-Chiari syndrome (BCS),
congenital metabolic liver disease such as hereditary
hemochromatosis and Wilson disease, occult HBV infection (OBI) and
aflatoxins, as well as liver disease of unknown etiology. Different
etiologies of HCC may cause different clinical characteristics and
clinical outcomes. Fig. 1 shows a
schematic representation of the different etiologies of
NBNC-HCC.
Alcohol-related HCC
The alcohol consumption criterion for defining
alcoholic liver disease as proposed by the Japanese Study Group on
Alcoholic Liver Disease is an ethanol intake of >70 g/day for
>5 years. As compared with Western countries, the prevalence of
alcohol-related HCC is lower in Japan (19). This is partly because of the high
incidence of hepatitis virus-related HCC (19).
The mechanism by which alcohol consumption increases
the risk of HCC is primarily due to the development of liver
cirrhosis. It has been shown that excessive alcohol consumption of
>80 g/day ethanol for >5 years increases the risk of HCC by
nearly 5-fold (24). According to
a meta-analysis from Italy, hazard ratios (HRs) for HCC development
of 1.19 [95% confidence interval (CI)=1.12–1.27], 1.40 (95%
CI=1.25–1.56), and 1.81 (95% CI=1.50–2.19) were associated with
alcohol consumption of 25, 50 and 100 g/day, respectively. This
indicates that the risk of HCC development is proportional to the
amount of alcohol consumed, although the risk in those who consume
low or moderate levels remains unclear (25).
NAFLD and NASH
NAFLD is characterized by liver steatosis without a
history of significant alcohol use or liver disease of unknown
etiology (26). NAFLD is the most
common cause of chronic liver disease worldwide and it is a hepatic
manifestation of the metabolic syndrome, which is a constellation
of problems that includes hypertension, obesity, insulin resistance
and dyslipidemia (26). The
prevalence of NAFLD increases with age, however, it has been
described in persons of all ages (27). The prevalence of NAFLD and its
related complications is expected to increase in the future
(28).
The prevalence of NAFLD is reported to range from 10
to 30% in adults and its prevalence is increasing in Japan as well
as in Western countries, because of the epidemic rise in DM and
obesity (29). NASH is part of the
spectrum of NAFLD and it is a severe form of NAFLD. Approximately
10% of patients with NAFLD progress to NASH and 20% of NASH cases
can slowly progress to liver cirrhosis and even HCC (13,30).
Powell et al reported the first case of NASH-related HCC
(31). Since then, several case
series of NASH-related HCC have been reported and it has attracted
the attention of oncologists (32,33).
The majority of CC cases are thought to be end-stage NASH because
several clinical features such as obesity and DM in patients with
CC are associated with NASH. However, histological findings often
are not informative when liver cirrhosis is already established
because it is hypothesized that CC often represents ‘burned out’
NASH (34,35). Thus, the impact of NASH on the
incidence of liver cirrhosis and HCC may be underestimated. Marrero
et al reported that 20% of patients in the cryptogenic liver
disease group had evidence of NASH on liver biopsies prior to HCC
occurrence (14). In addition,
half of the patients with CC had prior NASH or suspected NAFLD and
they concluded that NAFLD was the underlying liver disease in 13%
of patients with HCC.
The natural history and prognosis of NASH remains
elusive, because there are few data from prospective cohort studies
(36). Ashca et al reported
that yearly cumulative incidence of HCC was 2.6% in patients with
NASH-related cirrhosis (n=195), compared with 4.0% in patients with
HCV-related cirrhosis (n=315) (37). Likewise, Yatsuji et al
conducted a comparative study between 68 patients with NASH-related
cirrhosis and 69 with HCV-related cirrhosis, to clarify the
incidence of HCC and clinical outcomes (38). They reported that the 5-year rate
of HCC development was 11.3% for NASH-related cirrhosis and 30.5%
for HCV-related cirrhosis, and the 5-year survival rates were 75.2%
for NASH-related cirrhosis and 73.8% for HCV-related cirrhosis. The
hepatocarcinogenesis rate in patients with NASH-related cirrhosis
is considered to be lower than that in patients with HCV-related
cirrhosis.
Metabolic syndrome is reported to be associated with
development of HCC and intrahepatic cholangiocarcinoma (ICC). A
population-based study from the US comprising 3,649 HCC cases, 743
ICC cases and 195,953 comparative persons demonstrated that
metabolic syndrome was significantly more common among persons who
developed HCC (37.1%) and ICC (29.7%) than in the comparison group
(17.1%). After adjusted multiple logistic regression analyses,
metabolic syndrome remained significantly associated with increased
risk of HCC (HR=2.13; 95% CI=1.96–2.31) and ICC (HR=1.56; 95%
CI=1.32–1.83) (39).
DM
El-Serag et al conducted a large longitudinal
study comprising 173,643 patients with DM and 650,620 without DM
(98% male) to elucidate an association between DM and chronic liver
disease and/or HCC (41). They
demonstrated that DM was associated with an HR of 1.98 (95%
CI=1.88–2.09) for chronic non-alcoholic liver disease and an HR of
2.16 (95% CI=1.86–2.52) for HCC development (40). Furthermore, Wang et al
recently conducted a meta-analysis including a total of 25 cohort
studies to examine the relationship between DM and HCC (41). They reported that DM was associated
with an increased incidence of HCC (HR=2.01, 95% CI=1.61–2.51),
compared with individuals without DM and it was also positively
associated with HCC mortality (HR=1.56, 95% CI=1.30–1.87). Thus, DM
was demonstrated to be an independent risk factor for progression
of chronic liver disease and HCC development.
Up to 70% of patients with type II DM have some
degree of fatty liver disease (42). About 10% of patients with liver
cirrhosis have overt DM and a larger percentage of patients have
impaired glucose tolerance (43).
DM may be the result of liver cirrhosis, because in patients with
liver cirrhosis, insulin is not cleared properly (44).
El-Serag et al conducted a matched
case-control study comprising 1,303 cases with DM and 5,212
controls to investigate the effect of statins on HCC development.
The adjusted HR for statin reduction of HCC development was 0.74
(95% CI=0.64–0.87) and they concluded that statin use is associated
with a significant reduction in the risk of HCC in patients with DM
(45).
Obesity
Up to 90% of obese individuals have some degree of
chronic fatty liver disease and hepatic steatosis correlated
significantly with increasing BMI (42,46).
Obesity and related metabolic abnormalities, including chronic
inflammatory conditions, increase the risk of HCC development.
Dysregulation of tumor necrosis factor-α and interleukin-6
expressed in adipose tissue, which are essential cancer promoters
in inflammation-related carcinogenesis, is associated with the
development of steatosis and liver inflammation. These cytokines
are also pivotal in the development of obesity-related HCC
(47).
Obesity is reported to be linked to HCC development
and HCC patients with obesity may have worsened clinical outcomes
(16,48). Based on the prevalence of HCC, it
was estimated that 28% of male HCC cases and 27% of female cases
were due to overweight or obesity (49). Calle et al indicated that
obesity is associated with significantly increased HCC death rates
with an HR of 4.52 in patients with BMI >35 kg/m2 (16). Another large population-based study
from Denmark demonstrated in >40,000 obese patients that the HR
of developing liver cancer was increased to 1.9 compared with the
general population (50).
Likewise, the Korea National Health Insurance Corporation Study
reported that there was an HR of 1.53 for development of HCC in men
with BMI >30 kg/m2 as compared with normal controls,
even after controlling for HBV infection, which is the most common
cause of HCC in Korea (51).
Iron
Liver iron overload is suspected when the levels of
serum iron and ferritin are high. In patients with hepatitis virus
infection, iron overload, which is distinct from hereditary
hemochromatosis, is associated with poor prognosis (52). Furthermore, Sorrentino et al
measured hepatic iron retrospectively in liver biopsies of 153
patients with NASH-related cirrhosis (51 with HCC and 102 controls
without HCC) (53). They reported
that iron deposits were more frequent in HCC patients than in
controls and the median corrected total iron score was
significantly higher in HCC patients. Excessive alcohol consumption
and iron overload may act in synergy to promote liver fibrosis and
carcinogenesis (54). Ioannou
et al demonstrated that elevated serum transferrin-iron
saturation is associated with an increased incidence of liver
cirrhosis or HCC; particularly in patients with heavy alcohol
consumption (54). Liver iron
overload may be associated with the progression of liver disease
and the development of HCC in patients with underlying liver
disease of various etiologies. Iron overload is not a benign
condition regardless of etiology, and when recognized, surveillance
for HCC and adequate therapy for reducing iron overload should be
undertaken.
Other causes
PBC
There are several reports of NBNC-HCC with other
causes than alcohol or NAFLD/NASH. According to the Japanese
national data of patients with PBC, the HCC incidence was 2.4%
(71/2946) and the HCC incidence according to sex was 5.1% (19/370)
in men and 2.0% (52/2576) in women (55). Multivariate analysis of risk
factors associated with PBC-related HCC development according to
sex revealed histological fibrosis stage at the time of PBC
diagnosis as an independent risk factor in women, but not in men
(55). The authors concluded that
male PBC patients should be particularly carefully screened for HCC
from the early stages of PBC.
AIH
Although the clinical outcome in patients with AIH
is generally good, there have been several patients with AIH who
developed HCC (56). The National
Hospital Organization Liver Network Study Group in Japan reported
in 193 AIH patients that seven (3.6%) developed HCC during
follow-up, and the presence of liver cirrhosis at presentation was
an independent risk factor for HCC in patients with AIH.
PSC
PSC is a chronic inflammatory disease involving the
biliary tract. PSC can lead to liver cirrhosis due to persistent
inflammation in the liver, therefore, it is not surprising that
PSC-related cirrhosis can develop into HCC. The risk of HCC
development in PSC patients with liver cirrhosis is estimated to be
up to 2% per year (57). However,
the incidence of HCC for patients with PSC has not been fully
studied (58).
Hereditary hemochromatosis and Wilson
disease
Hereditary hemochromatosis is one of the most common
autosomal recessive genetic disorders. It is caused by mutations in
the HFE gene and/or other mutations in the iron metabolism system
and is characterized by excess iron absorption and storage in the
liver (59,60). Several population-based and
case-control studies have demonstrated that hereditary
hemochromatosis markedly elevates the risk of HCC (61–64).
A large population-based study from Sweden demonstrated that
patients with hereditary hemochromatosis had a 20-fold increased
risk of HCC (HR=21, 95% CI=16–22) but an almost unaltered risk of
all other cancers (HR=1.2, 95% CI=1.0–1.4) (64).
Wilson disease is an autosomal recessive disorder of
copper metabolism (65). A
nationwide survey to examine the etiology of liver cirrhosis in
Japan found Wilson disease in two (0.01%) of 16,117 patients with
liver cirrhosis and HCC (66).
Liver cirrhosis is a well-recognized complication of Wilson
disease, but HCC is extremely rare (66).
Budd-Chiari syndrome
BCS is a rare hepatic disease caused by occlusion of
the hepatic venous outflow. Several reports indicate that hepatic
congestion caused by obstruction of hepatic venous outflow can lead
to liver cirrhosis and even HCC (67,68).
A meta-analysis from China including 16 studies in patients with
BCS revealed that the prevalence of HCC in BCS was 2.0–46.2% in 12
Asian studies, 40.0–51.6% in two African studies, 11.3% in one
European study and 11.1% in one American study (69). These results suggest that the
prevalence of HCC in patients with BCS varies depending on
geographical location. However, because a relatively high incidence
of HCC in patients with BCS was observed in each study, routine
radiological surveillance for HCC is warranted in patients with
BCS.
OBI
In a small proportion of individuals, detectable HBV
DNA in the serum and/or liver is observed in the absence of
circulating hepatitis B surface antigen (HBsAg) (70–72).
OBI is defined by the presence of HBV DNA in the liver tissue of
individuals who test negative for HBsAg, regardless of the
detection of HBV DNA in the serum. The clinical implications of OBI
involve causing cryptogenic liver disease and contributing to the
progression of liver disease or even HCC (71,73).
OBI may maintain direct mechanisms of HBV-related carcinogenesis
via the ability to integrate into the host genome, and production
of transforming proteins including mainly X and preS-S proteins
(73–75). In addition, OBI may exert
pro-oncogenic properties through indirect mechanisms (72,74,75).
These are associated with its propensity to induce persistent
necroinflammation in the liver and to promote the progression of
chronic hepatitis to liver cirrhosis. This indicates the step
preceding HCC occurrence in the majority of cases.
Aflatoxins
Aflatoxins are naturally occurring mycotoxins
produced by Aspergillus species. They commonly contaminate foods
such as grain, peanuts and corn, and aflatoxin exposure is reported
to elevate the risk of HCC (76).
Chen et al conducted a community-based cohort study combined
with molecular dosimetry of aflatoxin exposure to elucidate the
relationship between the risk of HCC development and aflatoxins
(77). Elevated aflatoxin exposure
measured by detectable aflatoxin B1-albumin adducts was an
independent risk factor for HCC development after adjusting for
important confounders (HR=5.5, 95% CI=1.2–24.5). However, in Japan,
aflatoxin-associated HCC is extremely rare (7).
Mechanism of carcinogenesis in NBNC-HCC
Although the detailed mechanism of liver
carcinogenesis in patients with NBNC chronic liver disease remains
elusive, insulin resistance and oxidative stress may be involved,
especially in patients with NASH. NASH is characterized by insulin
resistance with hyperinsulinemia, and the insulin resistance is
reported to be associated with liver carcinogenesis (26,78).
Insulin-like growth factor (IGF)-1 significantly activates
mitogen-activated protein kinase, and increases over-expression of
the c-Fos and c-Jun proto-oncogenes in cultured hepatoma cells, and
IGF-1 is potentially involved in the development of HCC (78–82).
c-Jun N-terminal kinase (JNK)1 has also recently attracted
attention because it is linked with obesity, insulin resistance,
NASH and HCC. Obesity is linked to abnormal elevation of JNK
activity (83). In addition, Puri
et al reported that JNK activation increases hepatic
inflammation and apoptosis (84).
JNK1 may thus be the most essential kinase that is upregulated in
HCC.
Adipose tissue is thought to be an endocrine organ
because of its ability to secrete adipokines such as adiponection
and leptin (85). Adiponectin and
leptin are related to insulin resistance and obesity (85,86).
Adiponectin has emerged as the most abundant circulating
adipocytokine and is an anti-inflammatory polypeptide in adipose
tissue (85). It is decreased in
the presence of insulin resistance and inhibits angiogenesis
through modulation of apoptosis in animal models (87). Severe liver steatosis and fibrosis
are found in adiponectin knockout mice as compared with wild-type
mice (86). In addition, liver
adenoma and hyperplastic nodules develop within the liver in
adiponectin knockout mice, whereas no tumor formation is found in
wild-type mice (86). These
observations suggest that adiponectin is inversely associated with
liver disease progression. Leptin is the product of the obese gene
and is mainly produced by adipose tissue, and promotes angiogenesis
and mediates the progression of NASH to HCC in animal models
(88,89). Leptin-mediated neovascularization,
which coordinates with vascular endothelial growth factor, may
accelerate liver fibrosis and cause liver carcinogenesis in
patients with NASH, although its role in NAFLD or NASH is still
unclear (88,89).
In NAFLD patients, mitochondrial dysfunction also
leads to free radical production and oxidative stress, which may
provide the ‘second hit’ that allows progression from steatosis to
steatohepatitis, liver cirrhosis and even HCC (90). NASH-related insulin resistance
causes inhibition of liver mitochondrial fatty acid oxidation, and
increased intra-cellular fatty acids can lead to oxidative DNA
damage via stimulating microsomal peroxidases (91). Oxidative stress may also promote
carcinogenesis (92). Insulin
resistance, hepatic steatosis, oxidative stress and imbalances in
adipokines/cytokines interplay, which are the most essential
factors involved in NAFLD pathogenesis and progression, could also
have a pivotal role in liver carcinogenesis, through DNA damage and
promoting cellular growth (90,93,94).
In HCC patients with obesity, these correlations indicate a
possible association between the metabolic syndrome and poor
clinical outcomes.
Reactive oxygen species (ROS) can also activate
fibrosis (90). Ishii et al
demonstrated in animal models that eicosapentaenoic acid (EPA)
improved steatohepatitis with decreasing serum ROS, which is
associated with inhibited development of HCC (95). Treatment with EPA may minimize the
risk of HCC development in patients with NASH. However, there are
few promising drugs with the potential to reduce the risk of HCC
development in patients with NASH.
Overall, obesity and insulin resistance are known to
be related significantly to hepatic steatosis (96). Increased levels of hepatic
steatosis are linked to more severe necroinflammatory activity and
liver fibrosis, and several studies reported that the increase in
steatosis may be a predictor for liver fibrosis progression
(46,97,98).
Subsequently, liver disease occurs more frequently in patients with
more severe metabolic disorders, possibly leading to a higher rate
of development of HCC.
Clinicopathological features and prognosis
in patients with NBNC-HCC
Several studies have investigated the
clinicopathological features of NBNC-HCC. Takuma et al
reviewed 11 patients with NASH-associated HCC (6 male, 5 female;
mean age, 73.8 years) who received curative treatment (99). They reported that most (91%)
patients were diagnosed with obesity, DM, hypertension or
dyslipidemia, and 7 patients (64%) also had a non-cirrhotic liver.
Duan et al reported 169 patients with NAFLD-associated HCC
(68 with non-cirrhotic liver and 101 with cirrhosis); 72.8% were
male with a median age at abnormal liver function tests and
diagnosis of NAFLD and HCC of 60, 64 and 67 years, respectively
(100). Most patients had obesity
(75%) and DM (59.8%), 32.3% had dyslipidemia, and 53% had
hypertension. Nearly all patients were complicated with at least
one metabolic disorder. In terms of tumor characteristics, the
majority (76%) of the HCC patients had a solitary tumor nodule
0.8-20 cm in diameter (mean 3.4 cm) and most (61.1%) patients had
moderately differentiated HCC. Reddy et al compared 52
patients with NASH-related HCC and 162 with HCV and/or
alcohol-related HCC (101).
NASH-related HCC patients were older, more often female, had higher
BMI at HCC diagnosis, and more frequently had DM, dyslipidemia and
the metabolic syndrome. Liver function at presentation was worse in
patients with HCV/alcohol-related HCC.
Whether patients with NBNC-HCC have comparable
prognosis to patients with HCC with other causes remains
controversial. In a single-center retrospective study of patients
with a maximum tumor size <5 cm, who received curative surgery,
Kaibori et al reported that patients with NBNC-HCC tended to
have a higher overall survival rate than those with HCV-related HCC
(8). Patients with NBNC-HCC had a
significantly higher disease-free survival rate than those with
HCV-related HCC, although the difference in overall and
disease-free survival between the two groups was not
significant.
In a large retrospective comparative study, Li et
al investigated 675 patients with NBNC-HCC and 3529 with
hepatitis B surface antigen-positive/HCV-antibody-negative HCC who
underwent curative resection (102). There were no significant
differences between the two groups regarding overall survival,
cumulative incidence of HCC-specific death and recurrence.
Furthermore, in their multivariate analysis they found that female
sex, serum γ-glutamyl transpeptidase level, tumor size, tumor
capsule and tumor differentiation were independent risk factors
associated with HCC-specific survival in patients with NBNC-HCC.
They also claimed that women with NBNC-HCC should be closely
monitored even after curative surgery.
Malik et al reported in a single-center
prospective study that survival after liver transplantation in
patients with HCC and NASH-related liver cirrhosis was 88%, with a
mean follow-up of 2.5 years (103). There was no significant
difference in 5-year survival between patients transplanted for
NASH-related liver cirrhosis with and without HCC. There was no
significant difference in 5-year survival after liver
transplantation between HCC patients with and without NASH-related
cirrhosis. They therefore concluded that patients with NASH and HCC
have a favorable clinical outcome after liver transplantation.
Giannini et al demonstrated that HCC patients
with CC had a significantly greater prevalence of advanced HCC
stage, lower amenability to any treatment, and shorter survival
compared with HCV-related HCC patients (104). This was because HCC in patients
with CC is often diagnosed at an advanced stage owing to the lack
of imaging surveillance systems.
Tokushige et al conducted prospective studies
to clarify the outcomes and recurrence of HCC in NASH, compared
with patients with HCV-related HCC (105). The 5-year survival rate was 55.2%
and cumulative recurrence of HCC at 5 years was 69.8% in treated
NASH-HCC, and both groups showed similar survival and recurrence
rates.
Overall, owing to the lack of adequate surveillance
of HCC in patients with NBNC liver disease, NBNC-HCC tends to be
diagnosed at an advanced stage. However, in NBNC-HCC patients who
undergo curative therapy, clinical outcomes after HCC therapy in
NBNC-HCC patients are comparable or even better than those in
patients with hepatitis-related HCC. Previous reports of clinical
characteristics and clinical outcomes in patients with NBNC-HCC are
summarized in Table I.
 | Table I.Reported studies of clinical
characteristics and outcomes in non-B and non-C hepatocellular
carcinoma. |
Table I.
Reported studies of clinical
characteristics and outcomes in non-B and non-C hepatocellular
carcinoma.
|
Authors/(Refs.) | No. of patients
(m/f) | Age | Treatment | LC (yes/no) | Tumor size
(cm) | Tumor no.
(s/m) | Prevalence of
comorbid disease (%) | Survival after
therapy |
|---|
|
|---|
| DM | BMI ≥ 25
kg/m2 | DL |
|---|
| Kusakabe et
al (106) | 45 (28/17) | 65.8 (mean) | NA | NA | 4.4 cm (mean) | 30/15 | 17/45 (38%) | 15/45 (33%) | NA | NA |
| Hatanaka et
al (107) | 240 (186/54) | 67.4 (mean) | NA | NA | 5.1 cm (mean) | NA | 43/80 (53.8%) | NA | NA | NA |
| Abe et al
(9) | 64 (51/13) | 69 (median) | NA | 53/64 (82.8%) | NA | 32/64 | 29/64 (45.3%) | 12/64 (18.8%) | NA | NA |
| Tokushige et
al (105) | 34 (21/13) | 70 (median) | NA | F3 or F4 (88%) | NA | NA | 25/34 (74%) | 21/34 (62%) | 10/34 (29%) | 5-year OS, 69.8%
5-year RFS, 30.2% |
| Reddy et al
(101) | 52 (27/25) | 65 (median) | TACE, surgery,
RFA | 38/14 | 3.0 cm
(median) | NA | 28/52 (53.8%) | Median, 31.3
kg/m2 Range, 27.6–33.9 kg/m2 | 17/52 (32.7%) | 3-year OS,
60.9% |
| Duan et al
(100) | 169 (123/46) | 67 (median) | TACE, surgery, LT,
RFA, PEI | 101/68 | 3.4 cm (mean) | 128/41 | 101/169
(59.8%) | 127/169 (75%) | 32.3% | NA |
| Kaibori et
al (8) | 60 (52/8) | 66.6 (mean) | Surgery | 15/45 | 5.57 cm (mean) | 50/10 | 25/60 (41.7%) | NA | NA | 3-year OS, 75%
3-year RFS, 45% |
| Li et al
(102) | 675 (521/154) | 181 (≤ 50 years)
494 (>50 years) | Surgery | 367/308 | <5 cm, 263 (39%)
>5 cm, 412 (61%) | 599/76 | NA | NA | NA | 3-year OS, 57%
5-year OS, 48.8% |
| Nishikawa et
al (108) | 260 (199/61) | 70 (median) | TACE, surgery, RFA,
PEI | NA | 3.0 cm
(median) | 169/91 | 128/260
(49.2%) | 116/260
(44.6%) | NA | 3-year OS, 77%
3-year RFS, 36% |
| Cauchy et al
(109) | 62 (58/4) | 70 (median) | Surgery | F0-2, 24/62 F3,4,
38/62 | Range, 2.5–3.5
cm | NA | 52/62 (84%) | Median, 30.4
kg/m2 Range, 20.2–42.0 kg/m2 | 40/62 (65%) | 3-year OS, 75%
3-year RFS, 70% |
Conclusion
Various factors unrelated to hepatitis virus are
implicated in the development of HCC. Cumulative evidence suggests
that NAFLD and NASH, which are hepatic manifestations of the
metabolic syndrome that includes hypertension, obesity, insulin
resistance and hyperlipidemia, can progress to cirrhosis and HCC.
Obesity or DM itself can be an independent risk factor for the
development of HCC. Insulin resistance, oxidative stress and
adipokines are closely associated with liver carcinogenesis. Most
patients with NBNC-HCC may have at least one metabolic disorder.
Owing to the lack of adequate surveillance of NBNC-HCC, HCC tends
to be diagnosed at an advanced stage. However, in NBNC-HCC patients
who undergo curative therapy, clinical outcomes after HCC therapy
in NBNC-HCC patients may be comparable or even better than in
patients with hepatitis-related HCC. Furthermore, the ability to
decide which patients with liver disease with non-viral causes will
develop HCC will have screening implications in the future.
Acknowledgements
The authors would like to thank all
the staff in their hospital for their valuable support.
References
|
1.
|
Livraghi T, Mäkisalo H and Line PD:
Treatment options in hepatocellular carcinoma today. Scand J Surg.
100:22–29. 2011.PubMed/NCBI
|
|
2.
|
El-Serag HB: Epidemiology of viral
hepatitis and hepatocellular carcinoma. Gastroenterology.
142:1264–1273. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
De Lope CR, Tremosini S, Forner A, Reig M
and Bruix J: Management of HCC. J Hepatol. 56(Suppl 1): S75–S87.
2012.
|
|
4.
|
Nishikawa H, Osaki Y, Iguchi E, Takeda H,
Matsuda F, Nakajima J, Sakamoto A, Hatamaru K, Saito S, Nasu A,
Kita R and Kimura T: Radiofrequency ablation for hepatocellular
carcinoma: the relationship between a new grading system for the
ablative margin and clinical outcomes. J Gastroenterol. Oct
12–2012.(Epub ahead of print).
|
|
5.
|
Nishikawa H, Arimoto A, Wakasa T, Kita R,
Kimura T and Osaki Y: Surgical resection for hepatocellular
carcinoma: clinical outcomes and safety in elderly patients. Eur J
Gastroenterol Hepatol. 25:912–919. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Umemura T, Ichijo T, Yoshizawa K, Tanaka E
and Kiyosawa K: Epidemiology of hepatocellular carcinoma in Japan.
J Gastroenterol. 44(Suppl 19): 102–107. 2009. View Article : Google Scholar
|
|
7.
|
Tokushige K, Hashimoto E, Horie Y, Taniai
M and Higuchi S: Hepatocellular carcinoma in Japanese patients with
nonalcoholic fatty liver disease, alcoholic liver disease, and
chronic liver disease of unknown etiology: report of the nationwide
survey. J Gastroenterol. 46:1230–1237. 2011. View Article : Google Scholar
|
|
8.
|
Kaibori M, Ishizaki M, Matsui K and Kwon
AH: Clinicopathologic characteristics of patients with non-B non-C
hepatitis virus hepatocellular carcinoma after hepatectomy. Am J
Surg. 204:300–307. 2012. View Article : Google Scholar
|
|
9.
|
Abe H, Yoshizawa K, Kitahara T, Aizawa R,
Matsuoka M and Aizawa Y: Etiology of non-B non-C hepatocellular
carcinoma in the eastern district of Tokyo. J Gastroenterol.
43:967–974. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Nagaoki Y, Hyogo H, Aikata H, Tanaka M,
Naeshiro N, Nakahara T, Honda Y, Miyaki D, Kawaoka T, Takaki S,
Hiramatsu A, Waki K, Imamura M, Kawakami Y, Takahashi S and Chayama
K: Recent trend of clinical features in patients with
hepatocellular carcinoma. Hepatol Res. 42:368–375. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Suzuki Y, Ohtake T, Nishiguchi S,
Hashimoto E, Aoyagi Y, Onji M and Kohgo Y: The Japan Non-B, Non-C
Liver Cirrhosis Study Group: Survey of non-B, non-C liver cirrhosis
in Japan. Hepatol Res. Dec 26–2012.(Epub ahead of print).
|
|
12.
|
Kim SK, Marusawa H, Eso Y, Nishikawa H,
Ueda Y, Kita R, Kimura T, Chiba T, Osaki Y and Kudo M: Clinical
characteristics of non-B non-C hepatocellular carcinoma: a
single-center retrospective study. Digestion. 84(Suppl 1): 43–49.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Bugianesi E, Leone N, Vanni E, Marchesini
G, Brunello F, Carucci P, Musso A, De Paolis P, Capussotti L and
Salizzoni M: Expanding the natural history of nonalcoholic
steatohepatitis: from cryptogenic cirrhosis to hepatocellular
carcinoma. Gastroenterology. 123:134–140. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Marrero JA, Fontana RJ, Su GL, Conjeevaram
HS, Emick DM and Lok AS: NAFLD may be a common underlying liver
disease in patients with hepatocellular carcinoma in the United
States. Hepatology. 36:1349–1354. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Torres DM and Harrison SA: Nonalcoholic
steatohepatitis and noncirrhotic hepatocellular carcinoma: fertile
soil. Semin Liver Dis. 32:30–38. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Calle EE, Rodriguez C, Walker-Thurmond K
and Thun MJ: Overweight, obesity, and mortality from cancer in a
prospectively studied cohort of US adults. N Engl J Med.
348:1625–1638. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Kaji K, Yoshiji H, Ikenaka Y, Noguchi R,
Aihara Y, Shirai Y, Douhara A and Fukui H: Possible involvement of
angiogenesis in chronic liver diseases: interaction among
renin-angiotensin-aldosterone system, insulin resistance and
oxidative stress. Curr Med Chem. 19:1889–1898. 2012. View Article : Google Scholar
|
|
18.
|
Yasui K, Hashimoto E, Tokushige K, Koike
K, Shima T, Kanbara Y, Saibara T, Uto H, Takami S, Kawanaka M and
Komorizono Y: Clinical and pathological progression of
non-alcoholic steatohepatitis to hepatocellular carcinoma. Hepatol
Res. 42:767–773. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Chung H, Ueda T and Kudo M: Changing
trends in hepatitis C infection over the past 50 years in Japan.
Intervirology. 53:39–43. 2010.PubMed/NCBI
|
|
20.
|
Schaefer EA and Chung RT: Anti-hepatitis C
virus drugs in development. Gastroenterology. 142:1340–1350. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Jacobson IM, Pawlotsky JM, Afdhal NH,
Dusheiko GM, Forns X, Jensen DM, Poordad F and Schulz J: A
practical guide for the use of boceprevir and telaprevir for the
treatment of hepatitis C. J Viral Hepat. 19(Suppl 2): 1–26. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Rosen HR: Clinical practice. Chronic
hepatitis C infection N Engl J Med. 364:2429–2438. 2011.PubMed/NCBI
|
|
23.
|
Ertle J, Dechêne A, Sowa JP, Penndorf V,
Herzer K, Kaiser G, Schlaak JF, Gerken G, Syn WK and Canbay A:
Non-alcoholic fatty liver disease progresses to hepatocellular
carcinoma in the absence of apparent cirrhosis. Int J Cancer.
128:2436–2443. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Donato F, Tagger A, Gelatti U, Parrinello
G, Boffetta P, Albertini A, Decarli A, Trevisi P, Ribero ML,
Martelli C, Porru S and Nardi G: Alcohol and hepatocellular
carcinoma: the effect of lifetime intake and hepatitis virus
infections in men and women. Am J Epidemiol. 155:323–331. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Corrao G, Bagnardi V, Zambon A and La
Vecchia C: A meta-analysis of alcohol consumption and the risk of
15 diseases. Prev Med. 38:613–619. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Sookoian S and Pirola CJ: The genetic
epidemiology of nonalcoholic fatty liver disease: toward a
personalized medicine. Clin Liver Dis. 16:467–485. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Shen L, Fan JG, Shao Y, Zeng MD, Wang JR,
Luo GH, Li JQ and Chen SY: Prevalence of nonalcoholic fatty liver
among administrative officers in Shanghai: an epidemiological
survey. World J Gastroenterol. 9:1106–1110. 2003.PubMed/NCBI
|
|
28.
|
Ong JP and Younossi ZM: Epidemiology and
natural history of NAFLD and NASH. Clin Liver Dis. 11:1–16. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Kojima S, Watanabe N, Numata M, Ogawa T
and Matsuzaki S: Increase in the prevalence of fatty liver in Japan
over the past 12 years: analysis of clinical background. J
Gastroenterol. 38:954–961. 2003.PubMed/NCBI
|
|
30.
|
Harrison SA, Torgerson S and Hayashi PH:
The natural history of nonalcoholic fatty liver disease: a clinical
histopathological study. Am J Gastroenterol. 98:2042–2047. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Powell EE, Cooksley WG, Hanson R, Searle
J, Halliday JW and Powell LW: The natural history of nonalcoholic
steatohepatitis: a follow-up study of forty-two patients for up to
21 years. Hepatology. 11:74–80. 1990.PubMed/NCBI
|
|
32.
|
Hashimoto E, Yatsuji S, Tobari M, Taniai
M, Torii N, Tokushige K and Shiratori K: Hepatocellular carcinoma
in patients with nonalcoholic steatohepatitis. J Gastroenterol.
44(Suppl 19): 89–95. 2009. View Article : Google Scholar
|
|
33.
|
Hashizume H, Sato K, Takagi H, Hirokawa T,
Kojima A, Sohara N, Kakizaki S, Mochida Y, Shimura T and Sunose Y:
Primary liver cancers with nonalcoholic steatohepatitis. Eur J
Gastroenterol Hepatol. 19:827–834. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Yoshioka Y, Hashimoto E, Yatsuji S, Kaneda
H, Taniai M, Tokushige K and Shiratori K: Nonalcoholic
steatohepatitis: cirrhosis, hepatocellular carcinoma, and burnt-out
NASH. J Gastroenterol. 39:1215–1218. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Ayata G, Gordon FD, Lewis WD, Pomfret E,
Pomposelli JJ, Jenkins RL and Khettry U: Cryptogenic cirrhosis:
clinicopathologic findings at and after liver transplantation. Hum
Pathol. 33:1098–1104. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Starley BQ, Calcagno CJ and Harrison SA:
Nonalcoholic fatty liver disease and hepatocellular carcinoma: a
weighty connection. Hepatology. 51:1820–1832. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Ascha MS, Hanouneh IA, Lopez R, Tamimi TA,
Feldstein AF and Zein NN: The incidence and risk factors of
hepatocellular carcinoma in patients with nonalcoholic
steatohepatitis. Hepatology. 51:1972–1978. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Yatsuji S, Hashimoto E, Tobari M, Taniai
M, Tokushige K and Shiratori K: Clinical features and outcomes of
cirrhosis due to non-alcoholic steatohepatitis compared with
cirrhosis caused by chronic hepatitis C. J Gastroenterol Hepatol.
24:248–254. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39.
|
Welzel TM, Graubard BI, Zeuzem S, El-Serag
HB, Davila JA and McGlynn KA: Metabolic syndrome increases the risk
of primary liver cancer in the United States: a study in the
SEER-Medicare database. Hepatology. 54:463–471. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40.
|
El-Serag HB, Tran T and Everhart JE:
Diabetes increases the risk of chronic liver disease and
hepatocellular carcinoma. Gastroenterology. 126:460–468. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
41.
|
Wang C, Wang X, Gong G, Ben Q, Qiu W, Chen
Y, Li G and Wang L: Increased risk of hepatocellular carcinoma in
patients with diabetes mellitus: a systematic review and
meta-analysis of cohort studies. Int J Cancer. 130:1639–1648. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
42.
|
Neuschwander-Tetri BA and Caldwell SH:
Nonalcoholic steatohepatitis: summary of an AASLD Single Topic
Conference. Hepatology. 37:1202–1219. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
43.
|
Garcia-Compean D, Jaquez-Quintana JO,
Gonzalez-Gonzalez JA and Maldonado-Garza H: Liver cirrhosis and
diabetes: risk factors, pathophysiology, clinical implications and
management. World J Gastroenterol. 15:280–288. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44.
|
Allison ME, Wreghitt T, Palmer CR and
Alexander GJ: Evidence for a link between hepatitis C virus
infection and diabetes mellitus in a cirrhotic population. J
Hepatol. 21:1135–1139. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
45.
|
El-Serag HB, Johnson ML, Hachem C and
Morgana RO: Statins are associated with a reduced risk of
hepatocellular carcinoma in a large cohort of patients with
diabetes. Gastroenterology. 136:1601–1608. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46.
|
Ohata K, Hamasaki K, Toriyama K, Matsumoto
K, Saeki A, Yanagi K, Abiru S, Nakagawa Y, Shigeno M, Miyazoe S,
Ichikawa T, Ishikawa H, Nakao K and Eguchi K: Hepatic steatosis is
a risk factor for hepatocellular carcinoma in patients with chronic
hepatitis C virus infection. Cancer. 97:3036–3043. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
47.
|
Shimizu M, Tanaka T and Moriwaki H:
Obesity and hepatocellular carcinoma: targeting obesity-related
inflammation for chemoprevention of liver carcinogenesis. Semin
Immunopathol. 35:191–202. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48.
|
Bianchini F, Kaaks R and Vainio H:
Overweight, obesity, and cancer risk. Lancet Oncol. 3:565–574.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
49.
|
Larsson SC and Wolk A: Overweight, obesity
and risk of liver cancer: a meta-analysis of cohort studies. Br J
Cancer. 97:1005–1008. 2007.PubMed/NCBI
|
|
50.
|
Moller H, Mellemgaard A, Lindvig K and
Olsen JH: Obesity and cancer risk: a Danish record-linkage study.
Eur J Cancer. 30A:344–350. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
51.
|
Oh SW, Yoon YS and Shin SA: Effects of
excess weight on cancer incidences depending on cancer sites and
histologic findings among men: Korea National Health Insurance
Corporation Study. J Clin Oncol. 23:4742–4754. 2005. View Article : Google Scholar
|
|
52.
|
Drakesmith H and Prentice A: Viral
infection and iron metabolism. Nat Rev Microbiol. 6:541–552. 2008.
View Article : Google Scholar
|
|
53.
|
Sorrentino P, D'Angelo S, Ferbo U, Micheli
P, Bracigliano A and Vecchione R: Liver iron excess in patients
with hepatocellular carcinoma developed on non-alcoholic
steatohepatitis. J Hepatol. 50:351–357. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
54.
|
Ioannou GN, Weiss NS and Kowdley KV:
Relationship between transferrin-iron saturation, alcohol
consumption, and the incidence of cirrhosis and liver cancer. Clin
Gastroenterol Hepatol. 5:624–629. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
55.
|
Harada K, Hirohara J, Ueno Y, Nakano T,
Kakuda Y, Tsubouchi H, Ichida T and Nakanuma Y: Incidence of and
risk factors for hepatocellular carcinoma in primary biliary
cirrhosis: national data from Japan. Hepatology. 57:1942–1949.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
56.
|
Migita K, Watanabe Y, Jiuchi Y, Nakamura
Y, Saito A, Yagura M, Ohta H, Shimada M, Mita E, Hijioka T,
Yamashita H, Takezaki E, Muro T, Sakai H, Nakamuta M, Abiru S,
Komori A, Ito M, Yatsuhashi H, Nakamura M and Ishibashi H: Japanese
NHO-Liver-network study group: Hepatocellular carcinoma and
survival in patients with autoimmune hepatitis (Japanese National
Hospital Organization-autoimmune hepatitis prospective study).
Liver Int. 32:837–844. 2012. View Article : Google Scholar
|
|
57.
|
Harnois DM, Gores JG, Ludwig J, Steers JL,
LaRusso NE and Wiesner RH: Are patients with cirrhotic stage
primary sclerosing cholangitis at risk for the development of
hepatocellular cancer? J Hepatol. 27:512–516. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
58.
|
Razumilava N, Gores GJ and Lindor KD:
Cancer surveillance in patients with primary sclerosing
cholangitis. Hepatology. 54:1842–1852. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
59.
|
Edwards CQ, Griffen LM, Goldgar D,
Drummond C, Skolnick MH and Kushner JP: Prevalence of
hemochromatosis among 11,065 presumably healthy blood donors. N
Engl J Med. 318:1355–1362. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
60.
|
Powell LW, Subramaniam VN and Yapp TR:
Haemochromatosis in the new millennium. J Hepatol. 32:48–62. 2000.
View Article : Google Scholar
|
|
61.
|
Hsing AW, McLaughlin JK, Olsen JH,
Mellemkjar L, Wacholder S and Fraumeni JF Jr: Cancer risk following
primary hemochromatosis: a population-based cohort study in
Denmark. Int J Cancer. 60:160–162. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
62.
|
Fracanzani AL, Conte D, Fraquelli M,
Taioli E, Mattioli M, Losco A and Fargion S: Increased cancer risk
in a cohort of 230 patients with hereditary hemochromatosis in
comparison to matched control patients with non-iron-related
chronic liver disease. Hepatology. 33:647–651. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
63.
|
Yang Q, McDonnell SM, Khoury MJ, Cono J
and Parrish RG: Hemochromatosis-associated mortality in the United
States from 1979 to 1992: an analysis of multiple-cause mortality
data. Ann Intern Med. 129:946–953. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
64.
|
Elmberg M, Hultcrantz R, Ekbom A, Brandt
L, Olsson S, Olsson R, Lindgren S, Lööf L, Stål P, Wallerstedt S,
Almer S, Sandberg-Gertzén H and Askling J: Cancer risk in patients
with hereditary hemochromatosis and in their first-degree
relatives. Gastroenterology. 125:1733–1741. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
65.
|
Wang Y, Xie CL, Fu DL, Lu L, Lin Y, Dong
QQ, Wang XT and Zheng GQ: Clinical efficacy and safety of Chinese
herbal medicine for Wilson's disease: a systematic review of 9
randomized controlled trials. Complement Ther Med. 20:143–154.
2012.
|
|
66.
|
Michitaka K, Nishiguchi S, Aoyagi Y, Hiasa
Y, Tokumoto Y and Onji M: Japan Etiology of Liver Cirrhosis Study
Group: Etiology of liver cirrhosis in Japan: a nationwide survey. J
Gastroenterol. 45:86–94. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
67.
|
Tanaka M and Wanless IR: Pathology of the
liver in Budd-Chiari syndrome: portal vein thrombosis and the
histogenesis of veno-centric cirrhosis, veno-portal cirrhosis, and
large regenerative nodules. Hepatology. 27:488–496. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
68.
|
Okuda K: Inferior vena cava thrombosis at
its hepatic portion (obliterative hepatocavopathy). Semin Liver
Dis. 22:15–26. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
69.
|
Ren W, Qi X, Yang Z, Han G and Fan D:
Prevalence and risk factors of hepatocellular carcinoma in
Budd-Chiari syndrome: a systematic review. Eur J Gastroenterol
Hepatol. 25:830–841. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
70.
|
Raimondo G, Pollicino T, Cacciola I and
Squadrito G: Occult hepatitis B virus infection. J Hepatol.
46:160–170. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
71.
|
Raimondo G, Allain JP, Brunetto MR,
Buendia MA, Chen DS, Colombo M, Craxì A, Donato F, Ferrari C, Gaeta
GB, Gerlich WH, Levrero M, Locarnini S, Michalak T, Mondelli MU,
Pawlotsky JM, Pollicino T, Prati D, Puoti M, Samuel D, Shouval D,
Smedile A, Squadrito G, Trépo C, Villa E, Will H, Zanetti AR and
Zoulim F: Statements from the Taormina expert meeting on occult
hepatitis B virus infection. J Hepatol. 49:652–657. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
72.
|
Nishikawa H, Arimoto A, Wakasa T, Kita R,
Kimura T and Osaki Y: Lack of correlation between the antibody to
hepatitis B core antigen and survival after surgical resection for
hepatitis C virus-related hepatocellular carcinoma. Oncol Rep.
30:91–98. 2013.
|
|
73.
|
Raimondo G, Caccamo G, Filomia R and
Pollicino T: Occult HBV infection. Semin Immunopathol. 35:39–52.
2013. View Article : Google Scholar
|
|
74.
|
Squadrito G, Pollicino T, Cacciola I,
Caccamo G, Villari D, La Masa T, Restuccia T, Cucinotta E, Scisca
C, Magazzu D and Raimondo G: Occult hepatitis B virus infection is
associated with the development of hepatocellular carcinoma in
chronic hepatitis C patients. Cancer. 106:1326–1330. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
75.
|
Pollicino T, Squadrito G, Cerenzia G,
Cacciola I, Raffa G, Craxi A, Farinati F, Missale G, Smedile A,
Tiribelli C, Villa E and Raimondo G: Hepatitis B virus maintains
its pro-oncogenic properties in the case of occult HBV infection.
Gastroenterology. 126:102–110. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
76.
|
Wu HC and Santella R: The role of
aflatoxins in hepatocellular carcinoma. Hepat Mon.
12:e72382012.PubMed/NCBI
|
|
77.
|
Chen CJ, Wang LY, Lu SN, Wu MH, You SL,
Zhang YJ, Wang LW and Santella RM: Elevated aflatoxin exposure and
increased risk of hepatocellular carcinoma. Hepatology. 24:38–42.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
78.
|
Kawaguchi T, Izumi N, Charlton MR and Sata
M: Branched-chain amino acids as pharmacological nutrients in
chronic liver disease. Hepatology. 54:1063–1070. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
79.
|
Price JA, Kovach SJ, Johnson T, Koniaris
LG, Cahill PA, Sitzmann JV and McKillop IH: Insulin-like growth
factor I is a comitogen for hepatocyte growth factor in a rat model
of hepatocellular carcinoma. Hepatology. 36:1089–1097. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
80.
|
Buzzelli G, Dattolo P, Pinzani M, Brocchi
A, Romano S and Gentilini P: Circulating growth hormone and
insulin-like growth factor-I in nonalcoholic liver cirrhosis with
or without superimposed hepatocarcinoma: evidence of an altered
circadian rhythm. Am J Gastroenterol. 88:1744–1748. 1993.
|
|
81.
|
Kasprzak A and Adamek A: The insulin-like
growth factor (IGF) signaling axis and hepatitis C virus-associated
carcinogenesis (Review). Int J Oncol. 41:1919–1931. 2012.PubMed/NCBI
|
|
82.
|
Shimizu M, Kubota M, Tanaka T and Moriwaki
H: Nutraceutical approach for preventing obesity-related colorectal
and liver carcinogenesis. Int J Mol Sci. 13:579–595. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
83.
|
Hirosumi J, Tuncman G, Chang L, Görgün CZ,
Uysal KT, Maeda K, Karin M and Hotamisligil GS: A central role for
JNK in obesity and insulin resistance. Nature. 420:333–336. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
84.
|
Puri P, Mirshahi F, Cheung O, Natarajan R,
Maher JW, Kellum JM and Sanyal AJ: Activation and dysregulation of
the unfolded protein response in nonalcoholic fatty liver disease.
Gastroenterology. 134:568–576. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
85.
|
Duan XF, Tang P, Li Q and Yu ZT: Obesity,
adipokines and hepatocellular carcinoma. Int J Cancer. Feb
12–2013.(Epub ahead of print).
|
|
86.
|
Asano T, Watanabe K, Kubota N, Gunji T,
Omata M, Kadowaki T and Ohnishi S: Adiponectin knockout mice on
high fat diet develop fibrosing steatohepatitis. J Gastroenterol
Hepatol. 24:1669–1676. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
87.
|
Brakenhielm E, Veitonmäki N, Cao R, Kihara
S, Matsuzawa Y, Zhivotovsky B, Funahashi T and Cao Y:
Adiponectin-induced antiangiogenesis and antitumor activity involve
caspase-mediated endothelial cell apoptosis. Proc Natl Acad Sci
USA. 101:2476–2481. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
88.
|
Kitade M, Yoshiji H, Kojima H, Ikenaka Y,
Noguchi R, Kaji K, Yoshii J, Yanase K, Namisaki T and Asada K:
Leptin-mediated neovascularization is a prerequisite for
progression of nonalcoholic steatohepatitis in rats. Hepatology.
44:983–991. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
89.
|
Ikejima K, Takei Y, Honda H, Hirose M,
Yoshikawa M, Zhang YJ, Lang T, Fukuda T, Yamashina S, Kitamura T
and Sato N: Leptin receptor-mediated signaling regulates hepatic
fibrogenesis and remodeling of extracellular matrix in the rat.
Gastroenterology. 122:1399–1410. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
90.
|
Angulo P: Nonalcoholic fatty liver
disease. N Engl J Med. 346:1221–1231. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
91.
|
Yang S, Zhu H, Li Y, Lin H, Gabrielson K,
Trush MA and Diehl AM: Mitochondrial adaptations to obesity-related
oxidant stress. Arch Biochem Biophys. 378:259–268. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
92.
|
Nowsheen S, Aziz K, Kryston TB, Ferguson
NF and Georgakilas A: The interplay between inflammation and
oxidative stress in carcinogenesis. Curr Mol Med. 12:672–680. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
93.
|
Marnett LJ: Oxyradicals and DNA damage.
Carcinogenesis. 21:361–370. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
94.
|
Petta S and Craxì A: Hepatocellular
carcinoma and non-alcoholic fatty liver disease: from a clinical to
a molecular association. Curr Pharm Des. 16:741–752. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
95.
|
Ishii H, Horie Y, Ohshima S, Anezaki Y,
Kinoshita N, Dohmen T, Kataoka E, Sato W, Goto T and Sasaki J:
Eicosapentaenoic acid ameliorates steatohepatitis and
hepatocellular carcinoma in hepatocyte-specific Pten-deficient
mice. J Hepatol. 50:562–571. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
96.
|
Ratziu V, Giral P, Charlotte F, Bruckert
E, Thibault V, Theodorou I, Khalil L, Turpin G, Opolon P and
Poynard T: Liver fibrosis in overweight patients. Gastroenterology.
118:1117–1123. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
97.
|
Adinolfi LE, Gambardella M, Andreana A,
Tripodi MF, Utili R and Ruggiero G: Steatosis accelerates the
progression of liver damage of chronic hepatitis C patients and
correlates with specific HCV genotype and visceral obesity.
Hepatology. 33:1358–1364. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
98.
|
Westin J, Nordlinder H, Lagging M,
Norkrans G and Wejstal R: Steatosis accelerates fibrosis
development over time in hepatitis C virus genotype 3 infected
patients. J Hepatol. 37:837–842. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
99.
|
Takuma Y and Nouso K: Nonalcoholic
steatohepatitis-associated hepatocellular carcinoma: our case
series and literature review. World J Gastroenterol. 16:1436–1441.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
100.
|
Duan XY, Qiao L and Fan JG: Clinical
features of nonalcoholic fatty liver disease-associated
hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 11:18–27.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
101.
|
Reddy SK, Steel JL, Chen HW, DeMateo DJ,
Cardinal J, Behari J, Humar A, Marsh JW, Geller DA and Tsung A:
Outcomes of curative treatment for hepatocellular cancer in
nonalcoholic steatohepatitis versus hepatitis C and alcoholic liver
disease. Hepatology. 55:1809–1819. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
102.
|
Li T, Qin LX, Gong X, Zhou J, Sun HC, Qiu
SJ, Ye QH, Wang L and Fan J: Hepatitis B virus surface
antigen-negative and hepatitis C virus antibody-negative
hepatocellular carcinoma: clinical characteristics, outcome, and
risk factors for early and late intrahepatic recurrence after
resection. Cancer. 119:126–135. 2013. View Article : Google Scholar
|
|
103.
|
Malik SM, Gupte PA, de Vera ME and Ahmad
J: Liver transplantation in patients with nonalcoholic
steatohepatitis-related hepatocellular carcinoma. Clin
Gastroenterol Hepatol. 7:800–806. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
104.
|
Giannini EG, Marabotto E, Savarino V,
Trevisani F, di Nolfo MA, Del Poggio P, Benvegnù L, Farinati F,
Zoli M and Borzio F: Hepatocellular carcinoma in patients with
cryptogenic cirrhosis. Clin Gastroenterol Hepatol. 7:580–585. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
105.
|
Tokushige K, Hashimoto E, Yatsuji S,
Tobari M, Taniai M, Torii N and Shiratori K: Prospective study of
hepatocellular carcinoma in nonalcoholic steatohepatitis in
comparison with hepatocellular carcinoma caused by chronic
hepatitis C. J Gastroenterol. 45:960–967. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
106.
|
Kusakabe A, Tanaka Y, Orito E, Sugauchi F,
Kurbanov F, Sakamoto T, Shinkai N, Hirashima N, Hasegawa I, Ohno T,
Ueda R and Mizokami M: A weak association between occult HBV
infection and non-B non-C hepatocellular carcinoma in Japan. J
Gastroenterol. 42:298–305. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
107.
|
Hatanaka K, Kudo M, Fukunaga T, Ueshima K,
Chung H, Minami Y, Sakaguchi Y, Hagiwara S, Orino A and Osaki Y:
Clinical characteristics of nonBnonC-HCC: comparison with HBV and
HCV related HCC. Intervirology. 50:24–31. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
108.
|
Nishikawa H, Osaki Y, Takeda H, Sakamoto
A, Saito S, Nishijima N, Nasu A, Arimoto A, Kita R and Kimura T:
Effect of body mass index on survival after curative therapy for
non-B non-C hepatocellular carcinoma. J Gastrointestin Liver Dis.
22:173–181. 2013.PubMed/NCBI
|
|
109.
|
Cauchy F, Zalinski S, Dokmak S, Fuks D,
Farges O, Castera L, Paradis V and Belghiti J: Surgical treatment
of hepatocellular carcinoma associated with the metabolic syndrome.
Br J Surg. 100:113–121. 2013. View Article : Google Scholar : PubMed/NCBI
|