Contents
Telomere structure and function
Telomerase holoenzyme
TERT regulation
Nuclear transport of TR
Assembly of telomerase complex
Regulation of telomerase by telomere binding
Conclusion/summary
Telomere structure and function
Telomeres are regions of repetitive nucleotide
sequences at each end of chromosomes, this region is a repetition
of a guanine-rich sequences. In humans, telomeres are comprised of
a repetitive TTAGGG sequence with a 3′ G-rich single-stranded
overhang (1,2). The ends of telomeres form a
lariat-like structure called t-loop (3), which is postulated to be formed by
strand invasion of the 3′ single strand overhang into the preceding
double stranded telomeric DNA that is then stabilized by telomeric
proteins (4). A six-protein
complex known as ‘shelterin’ has remarkable specificity for
telomeres and some members of the complex specifically binds to
telomeric DNA. These proteins are: telomere repeat binding factor 1
(TRF1), telomere repeat binding factor 2 (TRF2),
repressor/activator protein 1 (RAP1), protection of telomeres 1
(POT1), TRF1 interacting nuclear factor 2 (TIN2), and TPP1 [also
known POT1 and TIN2 organizing protein (5–8)].
The functions of shelterin protein components are to maintain
telomere length, promote t-loop formation, recruit telomerase to
telomeric ends, and protect the ends of chromosomes from being
recognized as DNA damage (5,8,9). The
cell replication apparatus is not able to provide for complete
replication of chromosome ends; also, telomeres are subject to the
action of nucleases and other deleterious factors. As a result,
telomeres shorten during each cell division. In most organisms the
main mechanism of telomere length maintenance is completion of DNA
telomere repeats by telomerase (10). In other cases there is a
non-telomerase mechanism, known as alternative lengthening of
telomeres (ALT) which involves the use of a DNA template (11). Most cancer cells have a chromosome
end renewal mechanism involving the telomerase complex, which
utilizes its integral RNA molecule as a template for reverse
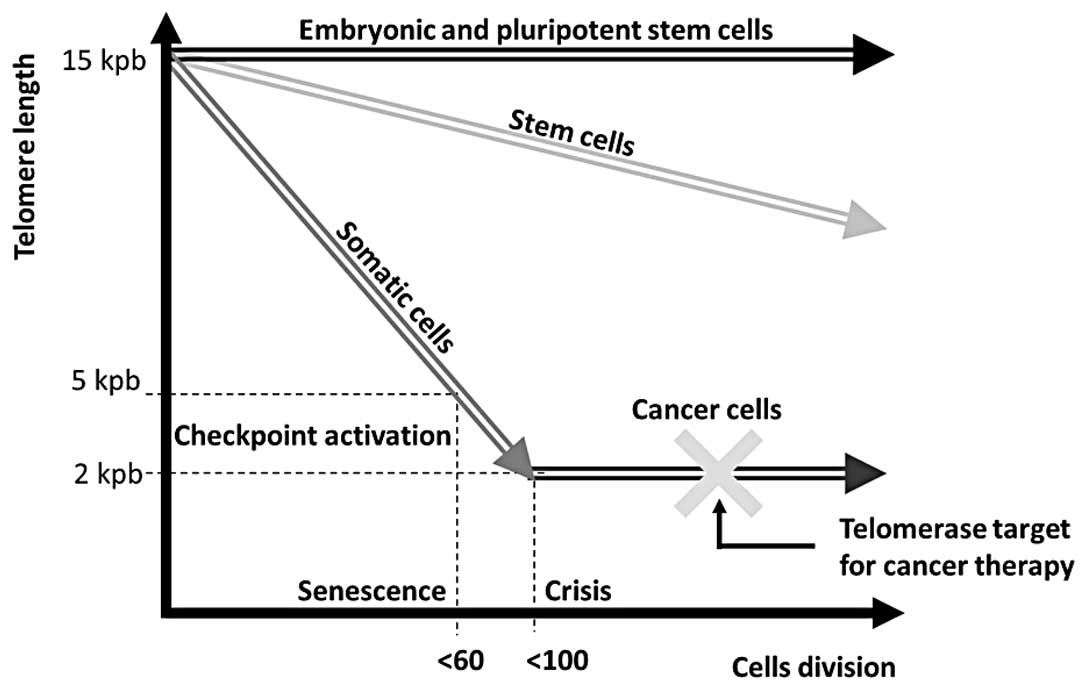
transcription of new telomeric DNA. In normal, healthy cells
telomerase activity is mostly limited to embryonic cells, adult
germline cells, and stem cells but is virtually absent in somatic
cells (12) while it is rather
common in the vast majority of cancer cells (13,14),
providing important targets for detection and treatment (Fig. 1).
Telomerase holoenzyme
The telomerase is a specific reverse transcriptase
responsible for the maintenance of telomere length in most mammals;
this enzyme was initially identified in ciliates (15). The human telomerase is comprised of
two main subunits, the RNA template and the catalytic enzyme
(16). The telomerase RNA template
(hTR or hTERC) contains a complementary sequence to the human
telomere that serves as the base for replication of short
repetitive telomere sequences d(TTAGGG) (17). The extension of telomeres is
completed through the catalytic component, its reverse
transcriptase (hTERT) (18). Along
with these two main components are additional telomere/telomerase
associated proteins. These accessory proteins regulate telomerase
biogenesis, their subcellular localization, and function in
vivo. Formation of the functional holoenzyme complex requires
associated proteins including the box H/ACA small nucleolar RNA
proteins: dyskerin, nucleolar protein 10 (NOP10), non-histone
protein 2 (NHP2), and glycine-arginine rich 1 (GAR1) (19). Other proteins involved in the
telomerase complex are pontin/reptin and telomerase Cajal body
protein 1 (TCAB1) (20). Pontin
and reptin are two ATPases, which interact with TERT in the S phase
of the cell cycle, showing a TERT dependent regulation of cell
cycle. Pontin and reptin interact with TERT, suggesting that pontin
and reptin also serve to assemble or remodel a telomerase complex
containing TERT. This process may occur in a stepwise fashion in
which pontin and reptin facilitate assembly of TERT with a
TR-dyskerin RNP, or remodel this maturing telomerase complex
(21). Finally the factor TCAB1
regulates the subcellular location of telomerase (22).
Telomerase levels are regulated at every step of
protein and RNA processing, as well as at the level of complex
assembly and subcellular localization (23). Telomerase activity at the telomere
is also regulated at the level of telomerase recruitment to the
telomere. While the exact mechanism of telomerase recruitment is
still not fully known, it is likely part of a negative feedback
loop created by shelterin proteins bound at the telomere that serve
as negative regulators of telomerase extension of telomeres
(reviewed in ref. 24). The
prevailing model of shelterin mediated telomere length regulation
is that longer telomeres recruit more shelterin complex, which in
turn limits future telomerase elongation. This negative feedback
loop is thought to be responsible for the stable telomere length
found in cancer cells, and is likely responsible for at least
partially maintaining telomere length homeostasis in germ cells and
other stem-like cells in which telomerase is active (25). Thus, it would be possible that
other mechanisms regulating telomerase recruitment or processivity
exist depending on whether cells are at equilibrium conditions
versus non-equilibrium conditions.
TERT regulation
Intensive studies of telomerase functioning in human
cells gave new perspectives on the mechanism of senescence, stem
cells and cancer therapy. The studies show that numerous enzymes
are required for telomerase functioning that facilitate new
approaches for inhibiting telomerase in cancer treatment. Probably
there are still numerous unrevealed proteins that contribute to
regulation of such a dynamic complex that still are to be
discovered. As already reported, TERT splice variants may be
expressed in normal, pre-crisis and alternative lengthening of
telomeres cells (ALT) that lack detectable telomerase activity
(26–28). Thus, transcriptional control of
TERT is supposed to play a crucial role in the complex regulation
of telomerase activity. Post-translational regulation of telomerase
activity can occur via reversible phosphorylation of TERT catalytic
subunit at specific serine/threonine or tyrosine residues. Due to
multiple kinase and phosphatase activators and inhibitors the
telomerase phosphorylation status may affect its structure,
localization and enzyme activity (29). Numerous non-specific
phosphorylation sites within TERT protein are postulated but only a
few of them appear to be the key residues, and their
phosphorylation influences telomerase activity (activation and
inhibition) (30). Specific
phosphorylation site at TERT is present at the proline rich region
(29). It was revealed that the
contribution of c-Abl tyrosine kinase to TERT phosphorylation at
specific tyrosine residues led to decreased telomerase activity. It
was shown that overexpression of c-Abl inhibited cell growth by
causing cell cycle arrest (30).
Because of the role of c-Abl in stress response to DNA damage,
exposure of cells to ionizing radiation led to a significant
increase in TERT phosphorylation by c-Abl. It was also demonstrated
that c-Abl phosphorylated TERT is leading to inhibition of
telomerase activity and decrease in telomere length (31) suggesting a direct association
between c-Abl and TERT. In conclusion, TERT expression is regulated
at both, transcriptional and post-transcriptional levels, with the
alternative splicing of TERT also involved in the control of
telomerase activity. However, contradictive reports concern the
correlation of telomere length with telomerase activity or TERT
expression in different cells which might confirm the
tissue-specificity of those regulatory mechanisms.
Nuclear transport of TR
As shown, translocation of TR and TERT is regulated
and multiple nuclear structures participate in transport and
biogenesis of telomerase (32).
Throughout most of the cell cycle TR is present in Cajal bodies
(CBs) that act as its transmitters to telomeres (33). These subnuclear structures are
general sites of RNP assembly and RNA modification (34). In contrary to TR, TERT is located
in distinct nucleoplasmic foci and therefore, the two main subunits
of telomerase are separated during almost the whole cell cycle. In
early S phase TERT is translocated to nucleoli. At the same time
CBs containing TR accumulate at the periphery of nucleoli. TR
accumulates at the pole of CBs that precedes localization to
telomeres in mid-S phase when CBs deliver telomerase to individual
telomeres. Furthermore, it was revealed that the same kinases and
phosphatases that act during S-phase may modify telomerase subunits
(32). However, the mechanisms
involved in targeting and accumulation of TR are not fully
understood (35). To date, within
telomerase RNA molecule the CAB box and H/ACA motif has been
identified to influence the TR translocation to CBs and nucleoli
(36,37). In the same way, one of TERT domains
is known to mediate nucleolar translocation (38,39).
Assembly of telomerase complex
Human telomerase assembly occurs by an
energy-dependent complex mechanism that involves first the
stabilization of TR and then its association with TERT (40,41).
Only TERT and TR are necessary to gain telomerase activity in
vitro. However, in vivo telomerase complex is composed
of additional multiple proteins, that facilitate the enzyme to act
(42). Similarly to the transport
of TERT to the nucleus, assembly of the telomerase complex may be
regulated during the cell cycle. Telomerase assembly could take
place during S phase and it is disassembled probably during M phase
(35). Prevention of premature
binding of the essential telomerase subunits (TERT and TR) is
possible due to different sites of their compartmentalization and
keeping them away from their substrates (telomeres) (32). Thus, two telomerase assembling
sites are possible during S phase at the telomere ends (43) or in CBs (32). It has been suggested that survival
of motor neuron (SMN) complex, an RNP assembly factor present in
CBs, takes part in telomerase biogenesis. It was demonstrated that
TR is associated with GAR1, a protein which interacts with SMN
complex (44). However, further
studies are needed to establish exactly where the telomerase
assembly occurs. Recent studies showed that the localization of TR
in CBs and near telomeres depends onTERT. This suggests that TR
assembles a complex with TERT and then both proteins are
transported to telomeres. Alternatively, TERT is supposed to
indirectly influence the trafficking of TR or a transient
interaction of the two components that contribute to TR
localization (45).
The 3′ end of the vertebrate TR contains two
stem-loop structures separated by a box H and box ACA moiety, aptly
named the H/ACA domain (36). Each
of the two structures in the TR H/ACA domain binds a copy of the
protein complex formed by dyskerin, NOP10, NHP2 and GAR1 proteins.
This protein complex is important for RNA maturation, 3′ processing
and RNP biogenesis (46). CB
location of TR is dependent on the TCAB1 protein binding to the CAB
(Cajal body box) (47). Dyskerin
is the mammalian ortholog of the archaeal H/ACA RNA pseudouridine
synthase which contains the catalytic TruB domain and the
pseudouridine synthase and archaeosine transglycosylase (PUA)
domain involved in RNA modification (48). NOP10 is a small basic protein with
a conserved zinc ribbon domain in the N-terminal region. This
protein does not directly bind to the RNA, and instead binds to
dyskerin (49,50). NHP2 is another small basic protein,
which binds to the RNA (49–51).
GAR1 is defined by, and named for, the glycine and arginine rich
(GAR) domains which flank the highly conserved central domain
(50). As with NOP10, GAR1 also
does not directly bind to the RNA and instead binds to the
dyskerin. GAR1 is not required for H/ACA snoRNP stability in
vivo or snoRNP assembly in vitro (51,52).
While dyskerin bound H/ACA snoRNAs localize to both nucleoli and
CBs, TCAB1 bound scaRNAs exclusively localize to CBs. TCAB1 is
responsible for TR localization to CBs since the depletion of TCAB1
alters the localization of the TR to nucleoli (47).
Numerous unique mutations have been identified
within the DKC1 gene, encoding for dyskerin (53,54).
Mutations in a limited number of families have also been reported
in NOLA2, encoding for NHP2 (55);
and NOLA3, encoding for NOP10 (56). These mutations retain wild-type
telomerase activity in vitro while reducing the amount of
active telomerase within the cell. No reports of mutations within
NOLA1, encoding for GAR1, have been linked to telomere-mediated
disorders. Mutations within the DKC1 gene are associated with
X-linked recessive dyskeratosis congenita (DC) (57), while mutations in the NOLA2 and
NOLA3 genes correlate to autosomal recessive DC (58,59).
Dyskerin and other associated proteins are crucial for ribosomal,
as well as telomerase biogenesis (58). However, mutations within these
genes appear to have no significant negative effect upon ribosome
maturation in human cells (59).
Since the discovery of TCAB1 and its gene, WRD79, there has been a
report of mutations linked to two cases of autosomal recessive DC.
These mutations produce defects in TR trafficking, reducing the
amount of active enzyme (22).
While the intricate details of telomerase RNP
assembly have yet to be fully elucidated, much progress has been
made in uncovering many of the steps necessary for individual
component maturation and the assembly of these components into an
active ribonucleoprotein enzyme. TERT protein expression follows
the canonical mRNA transcription, maturation and cytoplasmic
translation. The TERT protein is then recruited to nucleoli and
then CBs for RNP assembly (38,60).
The accumulation of TERT in the nucleoli reduces the levels of
active telomerase, supposedly by sequestering TERT from TR
(61,62). TR begins as a precursor RNA
polymerase II transcript capped by trimethyl-guanosine (TMG)
(63). Binding by RNA Helicase
associated with AU-rich element (RHAU) to the 5′ end resolves the
G-quadruplex structure while binding dyskerin and other proteins to
the 3′ end, trim and internally modify the RNA to produce a mature
TR (64). The initial binding of
dyskerin to the TR, and other H/ACA snoRNA species, relies on the
sequential binding of snoRNA H/ACA family quantitative accumulation
1 (SHQ1), followed by nuclear assembly factor 1 (NAF1) to dyskerin.
NAR1 is exchanged for GAR1 and SHQ1 is lost prior to the
localization of the mature TR to CBs (65). TCAB1 binding is thought to then
direct the mature TR to CBs (47,65).
TERT is then localized near the CBs where telomerase RNP assembly
occurs (60). The assembled
telomerase localize to the telomere for the nucleotide addition to
the process (66). The regulation
of each component of the telomerase holoenzyme has implications for
the accumulation of active telomerase within the cell (67).
ATPases and DNA helicases pontin and reptin reveal
an essential role in telomerase assembly. The amount of TERT bound
to pontin and reptin peaks in S phase (21). When the two pivotal subunits of
telomerase are stabilized and bound with auxiliary proteins the
TERT and TR dimerization occurs. Two regions of TR are necessary
for its binding with TERT: the template region (nucleotides 44–186)
and a putative double hairpin element in the 50 stem of the H/ACA
domain, where TR stabilizing H/ACA proteins bind (nucleotides
243–326) (68).
Regulation of telomerase by telomere
binding
Interaction of telomerase with numerous telomere
binding proteins (TBP) that may influence telomerase enzyme
activity is considered another form of telomerase activity
regulation. It is supposed that binding some of them to telomeres,
therefore making it impossible for telomerase to access the
chromosome ends, is an indirect way of regulation (69). Three-state model of telomere length
regulation were studied (70).
Firstly, POT1 is directly bound at the 3′ end of telomere and
associates with TPP1. This POT1-TPP1 position prevents binding of
telomerase to chromosome ends (5).
Secondly, TPP1-POT1 association enhanced POT1 affinity for
telomeric sDNA and TPP1 associates with the telomerase, providing a
physical link between telomerase and the shelterin complex
(71). According to this model, in
the next state these proteins are removed from their binding sites
by an unidentified mechanism. Posttranslational modification or
disruption of shelterin might be involved in the process. Thistly,
released POT1-TPP1 complex may serve as an activator of telomerase
during telomere extension. When elongated, telomere reaches a
certain threshold, the newly synthesized repeats bind shelterin
complexes and the 3′ end of the overhang is re-bound by POT1-TPP1.
This causes telomerase inhibition and return of the telomere to the
first state of the complex (71).
TRF1 and TRF2 are the main proteins responsible for telomerase
negative feedback control in mammals. They are bound to double
stranded DNA at T-loop, which is a ‘closed’ state of telomere,
which telomerase cannot access and therefore extends the telomere
terminus. TRF1 and TRF2 act as negative regulators of telomere
length because they are involved in T-loop formation (72,73).
TRF1 and TRF2 were shown to act in cis to repress telomere
elongation. TRF1 was reported to repress telomerase action on
telomeres while, on the contrary, TRF2 appears to activate a
telomeric degradation without showing any influence on telomerase
(74). Other proteins with
negative-feedback regulation of telomere length have been
identified in human cells. The proteins acting on TRF1 are
tankyrase 1 and 2 (TANK 1 and 2), TIN2, PINX1, three
TRF1-interacting factors but also hRAP1 which interacts with TRF2
(9,74). PINX1 can inhibit telomerase by
forming a stable complex with catalytic subunit of telomerase and
TRF1 molecule. It binds with TERT by its telomerase inhibitory
domain (TID) placed at C terminal 74 aa (22). The human repressor activator
protein 1 (hRap1) was identified as a protein that specifically
interacts with TRF2 and negatively regulates telomere length in
vivo.
Summary and conclusions
Numerous studies on the behavior of telomerase,
created a solid body of evidence about the mechanisms of
senescence, and its activity in tumor cells. These studies showed
the role of accessory proteins in the functionally active
telomerase complex. Since accessory proteins regulate telomerase
there is a chance of creating new approaches for the indirect
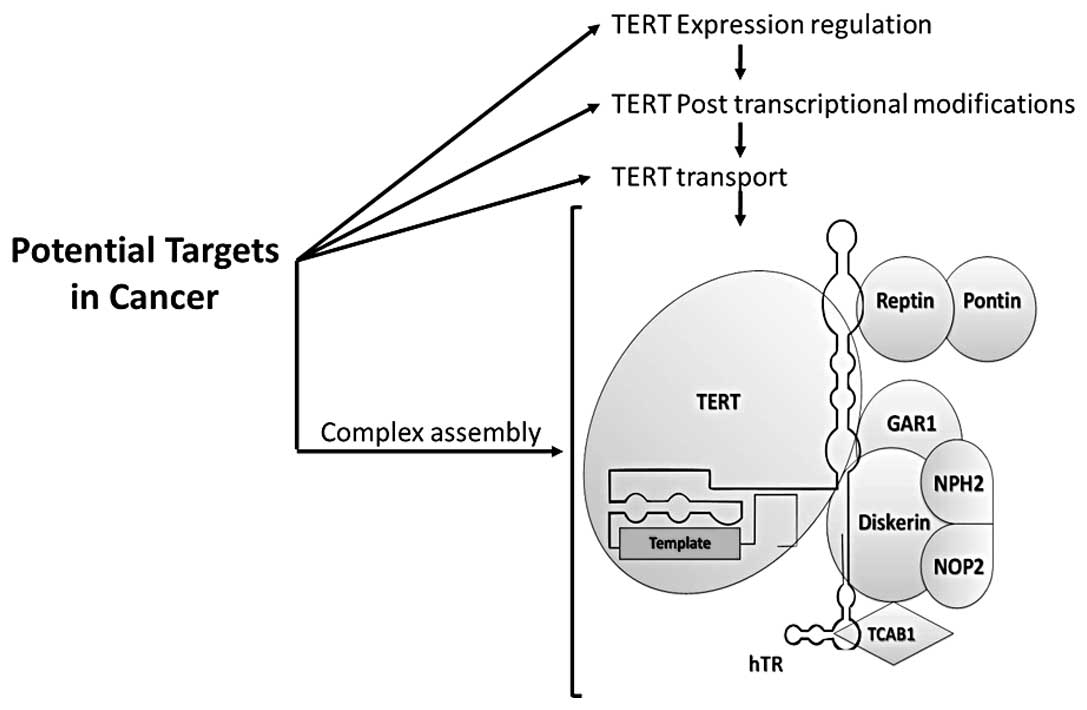
inhibition of telomerase in cancer treatment. In conclusion,
telomerase activity contains multiple factors that allow the
regulation, including the expression of TERT levels,
post-transcriptional modifications, transportation and location and
finally conformation and interactions with accessory proteins
during assembly of the telomerase complex (Fig. 2) providing possible targets to be
approached in the strategy of affecting this enzyme for cancer
treatment. Many of these processes have been studied in detail,
creating the basis to find molecules that regulate telomerase
activity. Imetelstat is a synthetic molecule that binds to the
telomerase RNA component sequence (hTR) in the active site region
of telomerase preventing its action (75). Curcumin has been shown to decrease
the activity of telomerase in several types of cancer (76–78).
This inhibition may be due to the impossibility of the
translocation to the nucleus by dissociating Hsp-90 and p23
chaperones from TERT (79).
Sulforaphane has been shown to cause a decrease in the expression
of TERT and TERT phosphorylation, preventing nuclear translocation
(80). However, there is little
evidence on how we can modulate telomerase activity by targeting
the major components of the complex (diskerin, Gar1, Nop10, and
NHP2), leaving a window of opportunity to the creation and
application of indirect anti-telomeric therapies in cancer.
Acknowledgements
This study was supported by Grants
from Quilmes National University, CONICET and ANPCyT (Argentina).
D.E.G. and H.G.F. are researchers from CONICET. D.E.G. is a member
of the National Cancer Institute of Argentina.
References
|
1.
|
Blackburn EH: Structure and function of
telomeres. Nature. 350:569–573. 1991. View
Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Moyzis RK, Buckingham JM, Cram LS, et al:
A highly conserved repetitive DNA sequence, (TTAGGG)n, present at
the telomeres of human chromosomes. Proc Natl Acad Sci USA.
85:6622–6626. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Griffith JD, Comeau L, Rosenfield S, et
al: Mammalian telomeres end in a large duplex loop. Cell.
97:503–514. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Greider CW: Telomeres do D-loop-T-loop.
Cell. 97:419–422. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
De Lange T: Shelterin: the protein complex
that shapes and safeguards human telomeres. Genes Dev.
19:2100–2110. 2005.PubMed/NCBI
|
|
6.
|
Neidle S: Human telomeric G-quadruplex:
the current status of telomeric G-quadruplexes as therapeutic
targets in human cancer. FEBS J. 277:1118–1125. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
O’Sullivan RJ and Karlseder J: Telomeres:
protecting chromosomes against genome instability. Nat Rev Mol Cell
Biol. 11:171–181. 2010.PubMed/NCBI
|
|
8.
|
Palm W and de Lange T: How shelterin
protects mammalian telomeres. Annu Rev Genet. 42:301–334. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Smogorzewska A and de Lange T: Regulation
of telomerase by telomeric proteins. Annu Rev Biochem. 73:177–208.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Greider CW and Blackburn EH: The telomere
terminal transferase of Tetrahymena is a ribonucleoprotein enzyme
with two kinds of primer specificity. Cell. 51:887–898. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Bryan TM and Reddel RR: Telomere dynamics
and telomerase activity in in vitro immortalised human cells. Eur J
Cancer. 33:767–773. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Broccoli D, Young JW and de Lange T:
Telomerase activity in normal and malignant hematopoietic cells.
Proc Natl Acad Sci USA. 92:9082–9086. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Ryan KM and Birnie GD: Cell-cycle
progression is not essential for c-Myc to block differentiation.
Oncogene. 14:2835–2843. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Wu KJ, Grandori C, Amacker M, et al:
Direct activation of TERT transcription by c-MYC. Nat Genet.
21:220–224. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Greider CW and Blackburn EH:
Identification of a specific telomere terminal transferase activity
in Tetrahymena extracts. Cell. 43:405–413. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Mason M, Schuller A and Skordalakes E:
Telomerase structure function. Curr Opin Struct Biol. 21:92–100.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Feng J, Funk WD, Wang SS, et al: The RNA
component of human telomerase. Science. 269:1236–1241. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Morin GB: The human telomere terminal
transferase enzyme is a ribonucleoprotein that synthesizes TTAGGG
repeats. Cell. 59:521–529. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Ly H: Genetic and environmental factors
influencing human diseases with telomere dysfunction. Int J Clin
Exp Med. 2:114–130. 2009.PubMed/NCBI
|
|
20.
|
Fu D and Collins K: Purification of human
telomerase complexes identifies factors involved in telomerase
biogenesis and telomere length regulation. Mol Cell. 28:773–785.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Venteicher AS, Meng Z, Mason PJ, Veenstra
TD and Artandi SE: Identification of ATPases pontin and reptin as
telomerase components essential for holoenzyme assembly. Cell.
132:945–957. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Zhong F, Savage SA, Shkreli M, et al:
Disruption of telomerase trafficking by TCAB1 mutation causes
dyskeratosis congenita. Genes Dev. 25:11–16. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Kelleher C, Teixeira MT, Forstemann K and
Lingner J: Telomerase: biochemical considerations for enzyme and
substrate. Trends Biochem Sci. 27:572–579. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
De Boeck G, Forsyth RG, Praet M and
Hogendoorn PC: Telomere-associated proteins: cross-talk between
telomere maintenance and telomere-lengthening mechanisms. J Pathol.
217:327–344. 2009.PubMed/NCBI
|
|
25.
|
Forsyth NR, Wright WE and Shay JW:
Telomerase and differentiation in multicellular organisms: turn it
off, turn it on, and turn it off again. Differentiation.
69:188–197. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Kilian A, Bowtell DD, Abud HE, et al:
Isolation of a candidate human telomerase catalytic subunit gene,
which reveals complex splicing patterns in different cell types.
Hum Mol Genet. 6:2011–2019. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Ulaner GA, Hu JF, Vu TH, Giudice LC and
Hoffman AR: Telomerase activity in human development is regulated
by human telomerase reverse transcriptase (hTERT) transcription and
by alternate splicing of hTERT transcripts. Cancer Res.
58:4168–4172. 1998.
|
|
28.
|
Ulaner GA, Hu JF, Vu TH, Oruganti H,
Giudice LC and Hoffman AR: Regulation of telomerase by alternate
splicing of human telomerase reverse transcriptase (hTERT) in
normal and neoplastic ovary, endometrium and myometrium. Int J
Cancer. 85:330–335. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Cong YS, Wright WE and Shay JW: Human
telomerase and its regulation. Microbiol Mol Biol Rev. 66:407–425.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Sawyers CL, McLaughlin J, Goga A, Havlik M
and Witte O: The nuclear tyrosine kinase c-Abl negatively regulates
cell growth. Cell. 77:121–131. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Kharbanda S, Kumar V, Dhar S, et al:
Regulation of the hTERT telomerase catalytic subunit by the c-Abl
tyrosine kinase. Curr Biol. 10:568–575. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Tomlinson RL, Ziegler TD, Supakorndej T,
Terns RM and Terns MP: Cell cycle-regulated trafficking of human
telomerase to telomeres. Mol Biol Cell. 17:955–965. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Jady BE, Richard P, Bertrand E and Kiss T:
Cell cycle-dependent recruitment of telomerase RNA and Cajal bodies
to human telomeres. Mol Biol Cell. 17:944–954. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Cioce M and Lamond AI: Cajal bodies: a
long history of discovery. Annu Rev Cell Dev Biol. 21:105–131.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Wojtyla A, Gladych M and Rubis B: Human
telomerase activity regulation. Mol Biol Rep. 38:3339–3349. 2011.
View Article : Google Scholar
|
|
36.
|
Jady BE, Bertrand E and Kiss T: Human
telomerase RNA and box H/ACA scaRNAs share a common Cajal
body-specific localization signal. J Cell Biol. 164:647–652. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Lukowiak AA, Narayanan A, Li ZH, Terns RM
and Terns MP: The snoRNA domain of vertebrate telomerase RNA
functions to localize the RNA within the nucleus. RNA. 7:1833–1844.
2001.PubMed/NCBI
|
|
38.
|
Etheridge KT, Banik SS, Armbruster BN, et
al: The nucleolar localization domain of the catalytic subunit of
human telomerase. J Biol Chem. 277:24764–24770. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
39.
|
Yang Y, Chen Y, Zhang C, Huang H and
Weissman SM: Nucleolar localization of hTERT protein is associated
with telomerase function. Exp Cell Res. 277:201–209. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
40.
|
Aisner DL, Wright WE and Shay JW:
Telomerase regulation: not just flipping the switch. Curr Opin
Genet Dev. 12:80–85. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
41.
|
Collins K: Physiological assembly and
activity of human telomerase complexes. Mech Ageing Dev. 129:91–98.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
42.
|
McEachern MJ, Krauskopf A and Blackburn
EH: Telomeres and their control. Annu Rev Genet. 34:331–358. 2000.
View Article : Google Scholar
|
|
43.
|
Taggart AK, Teng SC and Zakian VA: Est1p
as a cell cycle-regulated activator of telomere-bound telomerase.
Science. 297:1023–1026. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
44.
|
Bachand F, Boisvert FM, Cote J, Richard S
and Autexier C: The product of the survival of motor neuron (SMN)
gene is a human telomerase-associated protein. Mol Biol Cell.
13:3192–3202. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
45.
|
Tomlinson RL, Abreu EB, Ziegler T, et al:
Telomerase reverse transcriptase is required for the localization
of telomerase RNA to cajal bodies and telomeres in human cancer
cells. Mol Biol Cell. 19:3793–3800. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46.
|
Li H: Unveiling substrate RNA binding to
H/ACA RNPs: one side fits all. Curr Opin Struct Biol. 18:78–85.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
47.
|
Venteicher AS, Abreu EB, Meng Z, et al: A
human telomerase holoenzyme protein required for Cajal body
localization and telomere synthesis. Science. 323:644–648. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
48.
|
Cheng X and Roberts RJ: AdoMet-dependent
methylation, DNA methyltransferases and base flipping. Nucleic
Acids Res. 29:3784–3795. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
49.
|
Hamma T, Reichow SL, Varani G and
Ferre-D’Amare AR: The Cbf5-Nop10 complex is a molecular bracket
that organizes box H/ACA RNPs. Nat Struct Mol Biol. 12:1101–1107.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
50.
|
Pogacic V, Dragon F and Filipowicz W:
Human H/ACA small nucleolar RNPs and telomerase share
evolutionarily conserved proteins NHP2 and NOP10. Mol Cell Biol.
20:9028–9040. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
51.
|
Maiorano D, Brimage LJ, Leroy D and
Kearsey SE: Functional conservation and cell cycle localization of
the Nhp2 core component of H + ACA snoRNPs in fission and budding
yeasts. Exp Cell Res. 252:165–174. 1999.PubMed/NCBI
|
|
52.
|
Girard JP, Caizergues-Ferrer M and Lapeyre
B: The SpGAR1 gene of Schizosaccharomyces pombe encodes the
functional homologue of the snoRNP protein GAR1 of Saccharomyces
cerevisiae. Nucleic Acids Res. 21:2149–2155. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
53.
|
Cossu F, Vulliamy TJ, Marrone A, Badiali
M, Cao A and Dokal I: A novel DKC1 mutation, severe combined
immunodeficiency (T+B-NK- SCID) and bone marrow transplantation in
an infant with Hoyeraal-Hreidarsson syndrome. Br J Haematol.
119:765–768. 2002.PubMed/NCBI
|
|
54.
|
Wong JM, Kyasa MJ, Hutchins L and Collins
K: Telomerase RNA deficiency in peripheral blood mononuclear cells
in X-linked dyskeratosis congenita. Hum Genet. 115:448–455.
2004.PubMed/NCBI
|
|
55.
|
Vulliamy T, Beswick R, Kirwan M, et al:
Mutations in the telomerase component NHP2 cause the premature
ageing syndrome dyskeratosis congenita. Proc Natl Acad Sci USA.
105:8073–8078. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
56.
|
Walne AJ, Vulliamy T, Marrone A, et al:
Genetic heterogeneity in autosomal recessive dyskeratosis congenita
with one subtype due to mutations in the telomerase-associated
protein NOP10. Hum Mol Genet. 16:1619–1629. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
57.
|
Heiss NS, Knight SW, Vulliamy TJ, et al:
X-linked dyskeratosis congenita is caused by mutations in a highly
conserved gene with putative nucleolar functions. Nat Genet.
19:32–38. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
58.
|
Meier UT: The many facets of H/ACA
ribonucleoproteins. Chromosoma. 114:1–14. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
59.
|
Parry EM, Alder JK, Lee SS, et al:
Decreased dyskerin levels as a mechanism of telomere shortening in
X-linked dyskeratosis congenita. J Med Genet. 48:327–333. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
60.
|
Tomlinson RL, Li J, Culp BR, Terns RM and
Terns MP: A Cajal body-independent pathway for telomerase
trafficking in mice. Exp Cell Res. 316:2797–2809. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
61.
|
Lin J, Jin R, Zhang B, et al: Nucleolar
localization of TERT is unrelated to telomerase function in human
cells. J Cell Sci. 121:2169–2176. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
62.
|
Wong JM, Kusdra L and Collins K:
Subnuclear shuttling of human telomerase induced by transformation
and DNA damage. Nat Cell Biol. 4:731–736. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
63.
|
Gallardo F and Chartrand P: Telomerase
biogenesis: The long road before getting to the end. RNA Biol.
5:212–215. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
64.
|
Collins K: The biogenesis and regulation
of telomerase holoenzymes. Nat Rev Mol Cell Biol. 7:484–494. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
65.
|
Grozdanov PN, Roy S, Kittur N and Meier
UT: SHQ1 is required prior to NAF1 for assembly of H/ACA small
nucleolar and telomerase RNPs. RNA. 15:1188–1197. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
66.
|
Abreu E, Aritonovska E, Reichenbach P, et
al: TIN2-tethered TPP1 recruits human telomerase to telomeres in
vivo. Mol Cell Biol. 30:2971–2982. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
67.
|
Cifuentes-Rojas C and Shippen DE:
Telomerase regulation. Mutat Res. 730:20–27. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
68.
|
Collins K and Mitchell JR: Telomerase in
the human organism. Oncogene. 21:564–579. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
69.
|
Venteicher AS and Artandi SE: TCAB1:
driving telomerase to Cajal bodies. Cell Cycle. 8:1329–1331. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
70.
|
Wang F, Podell ER, Zaug AJ, et al: The
POT1-TPP1 telomere complex is a telomerase processivity factor.
Nature. 445:506–510. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
71.
|
Xin H, Liu D, Wan M, et al: TPP1 is a
homologue of ciliate TEBP-beta and interacts with POT1 to recruit
telomerase. Nature. 445:559–562. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
72.
|
Shore D and Bianchi A: Telomere length
regulation: coupling DNA end processing to feedback regulation of
telomerase. EMBO J. 28:2309–2322. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
73.
|
Smogorzewska A, van Steensel B, Bianchi A,
et al: Control of human telomere length by TRF1 and TRF2. Mol Cell
Biol. 20:1659–1668. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
74.
|
Ancelin K, Brunori M, Bauwens S, et al:
Targeting assay to study the cis functions of human telomeric
proteins: evidence for inhibition of telomerase by TRF1 and for
activation of telomere degradation by TRF2. Mol Cell Biol.
22:3474–3487. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
75.
|
Roth A, Harley CB and Baerlocher GM:
Imetelstat (GRN163L)--telomerase-based cancer therapy. Recent
Results Cancer Res. 184:221–234. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
76.
|
Chakraborty S, Ghosh U, Bhattacharyya NP,
Bhattacharya RK and Roy M: Inhibition of telomerase activity and
induction of apoptosis by curcumin in K-562 cells. Mutat Res.
596:81–90. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
77.
|
Mukherjee Nee Chakraborty S, Ghosh U,
Bhattacharyya NP, Bhattacharya RK, Dey S and Roy M:
Curcumin-induced apoptosis in human leukemia cell HL-60 is
associated with inhibition of telomerase activity. Mol Cell
Biochem. 297:31–39. 2007.PubMed/NCBI
|
|
78.
|
Ramachandran C, Fonseca HB, Jhabvala P,
Escalon EA and Melnick SJ: Curcumin inhibits telomerase activity
through human telomerase reverse transcritpase in MCF-7 breast
cancer cell line. Cancer Lett. 184:1–6. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
79.
|
Lee JH and Chung IK: Curcumin inhibits
nuclear localization of telomerase by dissociating the Hsp90
co-chaperone p23 from hTERT. Cancer Lett. 290:76–86. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
80.
|
Moon DO, Kang SH, Kim KC, Kim MO, Choi YH
and Kim GY: Sulforaphane decreases viability and telomerase
activity in hepatocellular carcinoma Hep3B cells through the
reactive oxygen species-dependent pathway. Cancer Lett.
295:260–266. 2010. View Article : Google Scholar
|