Introduction
Osteosarcoma is the most common, frequent and
refractory malignant bone tumor (1). Although various treatment options are
available, it is still a tumor with a high mortality rate ascribed
to the development of metastasis to the lungs and frequent
recurrence after surgery (2,3).
Therefore, identification of factors which are crucial for
osteosarcoma progression is necessary for the development of new
therapeutic strategies for the treatment of osteosarcoma.
Lysyl oxidase (LOX) is a key enzyme which is
required for the normal biosynthesis of collagen and elastin
maturation in the extracellular compartment (4,5). It
is synthesized and secreted as a 50-kDa inactive pro-enzyme (LOX)
and then is processed to a functional 32-kDa LOX enzyme and an
18-kDa LOX pro-peptide (LOX-PP). LOX pro-peptide was widely
confirmed as a tumor suppressive factor (6–8) but
a paradoxical role for LOX in tumorigenesis and metastasis were
reported. Several studies have suggested that LOX contributes to
distant metastasis (9–11) and an increased expression of LOX is
positively correlated with disease progression and survival in
colorectal cancer (11), prostate
cancer (12), ovarian cancer
(13) and renal clear cells
(14). However, earlier research
found that the injection of LOX−/− normal rat kidney
fibroblasts into nude mice could promote tumor formation and
metastases to the peritoneum and lungs (15). In addition, the tumor-suppressor
activity of LOX was confirmed in basal and squamous skin cell
carcinoma (16). Taken together,
these above studies demonstrate that LOX has both tumor suppressors
and metastasis promoters and the biological response of cancer
cells to LOX may depend on the particular cell type and other
factors that are not yet defined.
To date, however, the exact role of LOX in
osteosarcoma is not well understood (17). In the present study, we examined
the endogenous expression level of LOX in human osteosarcoma cell
lines and clinical tumor samples. Then, by overexpressing LOX in
U-2OS and HOS cells we analyzed the effects of LOX on osteosarcoma
cell proliferation, cell cycle, apoptosis and invasion, and also
explored the underlying signaling pathway.
Materials and methods
Materials
Human bone tumor samples (n=95) were obtained as
anonymous specimens and the normal bone tissues (n=20) were
obtained from the resected and discarded bone samples from
individuals who underwent total knee arthroplasty with no history
of tumors from Hospital. The study was reviewed and approved by the
Institutional Review Board of the Hospital. SaO2, U-2OS and HOS
human osteosarcoma cell lines used in the experiments were
purchased from American Type Culture Collection (ATCC, Manassas,
VA, USA). Adenovirus vector with LOX expression (adLOX), negative
control vector with GFP expression (adGFP) and virion-packaging
elements were purchased from Genechem Company (Shanghai, China).
All primary antibodies were purchased from Cell Signaling
Technologies (Beverly, MA, USA).
Reagents
β-APN was purchased from Sigma-Aldrich Chemical Co.
(St. Louis, MO, USA); apoptosis detection kit (Hoechst 33258),
3-(4,5-dimethylthiazol-2-yl)-3,5-diphenyl tetrazolium bromide (MTT)
and ECL-PLUS kit were purchased from Beyotime (Haimen, China);
Dulbecco’s modified Eagle’s medium and fetal bovine serum (FBS)
were purchased from Gibco (Gibco, Montevideo, Uruguay); M-MLV
Reverse Transcriptase was purchased from Promega (Madison, WI,
USA); TRIzol Reagent and Lipofectamine 2000 were purchased from
Invitrogen (Carlsbad, CA, USA); SYBR-Green Master Mixture and the
primers were purchased from Takara (Otsu, Japan).
Cell culture, adenovirus transfection and
study protocol
SaO2, U-2OS and HOS human osteosarcoma cell lines
were cultured in DMEM medium supplemented with 10% FBS, and
antibiotics (100 IU/ml of penicillin G and 100 Ag/ml of
streptomycin). They were all incubated at 37°C humidified
atmosphere of 5% CO2 and the medium was replaced every 2
days. U-2OS and HOS cell lines were infected with recombinant
adenovirus vector expressing LOX or GFP (adLOX and adGFP). In
preliminary experiments, cells were co-infected with recombinant
adenovirus vector adLOX or negative control adGFP at MOIs of 10–100
pfu/cell according to the manufacturer’s instructions. Cells were
subcultured at a 1:5 dilution in 400 μg/ml G418-containing
medium and positive stable transfectants were selected. In this
study, a recombinant adenovirus vector containing a LOX gene and
the negative control adenovirus vector were used to transfect
osteosarcoma cells. U-2OS and HOS cells without gene transfection
were used as a control group. Subsequently, followed by 24-h
recovery after transfection, the proliferation, metastasis and
invasion were assessed, respectively.
Real-time PCR
To quantitatively determine the mRNA expression
level of LOX in vivo and in vitro, real-time PCR
using SYBR-Premix Ex Taq (Takara, Shiga, Japan) was used according
to the manufacturer’s protocol. Total RNA of each specimen was
extracted with TRIzol and assessed by an ABI Prism 7500 sequence
detection system (Applied Biosystems, San Francisco, CA, USA). The
genes were amplified using specific oligonucleotide primer and
human β-actin (actin) gene was used as an internal control. The PCR
primer sequences were as follows: LOX, 5′-ACTGCACACACACAGGGATTG-3′
and 5′-GCCTT CTAATACGGTGAAATTG-3′; β-actin, 5′-CTGGGACGACA
TGGAGAAA-3′ and 5′-AAGGAAGGCTGGAAGAGTGC-3′; Data were analyzed
using the comparative Ct method (2−ΔΔCt).
Immunohistochemistry
The localization of LOX protein in bone tumor
tissues and normal tissues were assessed by immunohistochemistry.
Bone tumor tissues and normal tissues excised from patients were
embedded in paraffin and cut into 6 μM sections. After
deparaffinization, the sections were incubated in 3%
H2O2 and 0.1% Triton X-100 and microwaved in
Tris-buffer for 10 min to retrieve the antigen. The sections were
then blocked in 5% goat serum (Invitrogen) for 30 min and incubated
with LOX antibody (diluted 1:200) for 2 h at room temperature.
While a negative control was performed using a section without
primary antibody. Following incubation with secondary antibodies,
bound antibodies were visualized by a Power Vision Two-step
Histostaining Reagent (Dako, Carpinteria, CA, USA) and antibody
binding was visualized by incubation with DAB (Boster, Wuhan,
China) for 3 min at room temperature followed by counterstaining
with hematoxylin (Basa, Zhuhai, China). The LOX immunoreactive and
non-immunoreactive cells with a nucleus were counted in 3 sections
(with interval 3 sections) per patient in each group. The positive
staining of LOX was shown as brown particles. The sections were
observed and photographed with the optical microscope (Olympus,
Tokyo, Japan). A percentage was calculated by the
immunostained/total neuronal ratio × 100%.
Western blot assay
The cells were harvested and extracted at indicated
time using lysis buffer (Tris-HCl, mercaptoethanol, SDS and
glycerol). Cell extracts were boiled for 8 min in loading buffer
and then equal amount of cell extracts were separated on 8–15%
SDS-PAGE gels according to the molecular weight. Separated protein
bands were transferred into polyvinylidene fluoride (PVDF)
membranes and the membranes were blocked in 5% skim milk powder.
The primary antibodies against LOX, Ki-67, PCNA, COX-2, MMP-2,
MMP-9, PI3Kp85α and p-AKT were diluted according to the
instructions of antibodies and incubated overnight at 4°C. Then,
horseradish peroxidase-linked secondary antibodies were added at a
dilution ratio of 1:800, and incubated at room temperature for 3 h.
The membranes were washed with PBS for three times and the
immunoreactive bands were visualized using ECL-PLUS kit according
to the kit instructions. The relative protein level in different
cell lines was normalized to β-actin (actin) concentration.
Cell proliferation assay
Cell proliferation was analyzed with the MTT assay.
Briefly, the cells were incubated in 96-well plates at a density of
1×105 cells per well with DMEM medium supplemented with
10% FBS. Cells were treated with 20 μl MTT dye at 0, 12, 24,
48, 72 and 96 h and then incubated with 150 μl of DMSO for 5
min). The absorbance of optical densities at each time point was
measured at 570 nm with enzyme immunoassay analyzer (Bio-Rad,
Hercules, CA, USA).
Cell cycle analysis
For cell cycle analysis, 106 cells were
harvested, re-suspended, washed with phosphate-buffered saline and
fixed in 70% ethanol. Cells were then treated with RNase (10
μg/ml) and propidium iodide (50 μg/ml) for 30 min.
The cell cycle phase distribution was determined by analytical DNA
flow cytometry. The percentage of cells in each phase of the cell
cycle was analyzed using Modfit software (Verity Software House,
Topsham, ME, USA).
Wound-healing assay
U-2OS and HOS cells were plated in each well of a
6-well culture plate and allowed to grow to 80% confluence.
Treatment with adLOX was then performed. On the next day, a wound
was created using a 10 μl micropipette tip. The migration of
cells towards the wound was monitored at 24 and 48 h.
Transwell invasion assay
Briefly, the cells were removed by trypsinizing and
suspended with medium supplemented with 10% FBS, then
1×105 were added into the Transwell innserts with
8-μm pore size (BD Biosciences) for 24-well plates coated
with 50 μl Matrigel (BD Biosciences), and 200 μl
medium containing 15% FBS were added in the bottom chamber.
Undergoing migration for 24 and 48 h, a cotton swab was used to
remove the non-migrated cells in the upper chamber then the filters
were individually fixed with 4% paraformaldehyde and were
determined with hematoxylin and eosin staining. The cell number was
counted in five random fields of each chamber under the
microscope.
Hoechst 33342 assay
The cells were prepared at a density of 5,000 cells
per well in a 24-well plate. Apoptotic cells were detected by using
the Hoechst 33258 staining. The cells were fixed with 4%
paraformaldehyde for 10 min, washed with PBS for three times and
then stained with 2 μg/ml Hoechst 33258 for 5 min.
Morphologic changes in apoptotic nuclei were evaluated under a
fluorescence microscope (excitation wavelength 350 nm, emission
filter 460 nm) (FluoView, Olympus).
Statistical analysis
All data are presented as the means ± standard error
(SE) for at least three independent experiments. T-test was used
for two group comparison and one-way analysis of variance (ANOVA)
using Fisher’s exact test was employed for multiple comparisons.
The LSD method of multiple comparisons was used when the
probability for ANOVA was statistically significant. P<0.05 was
considered with statistical significance.
Results
Low mRNA and protein expression of LOX in
advanced-stage human osteosarcoma tissues
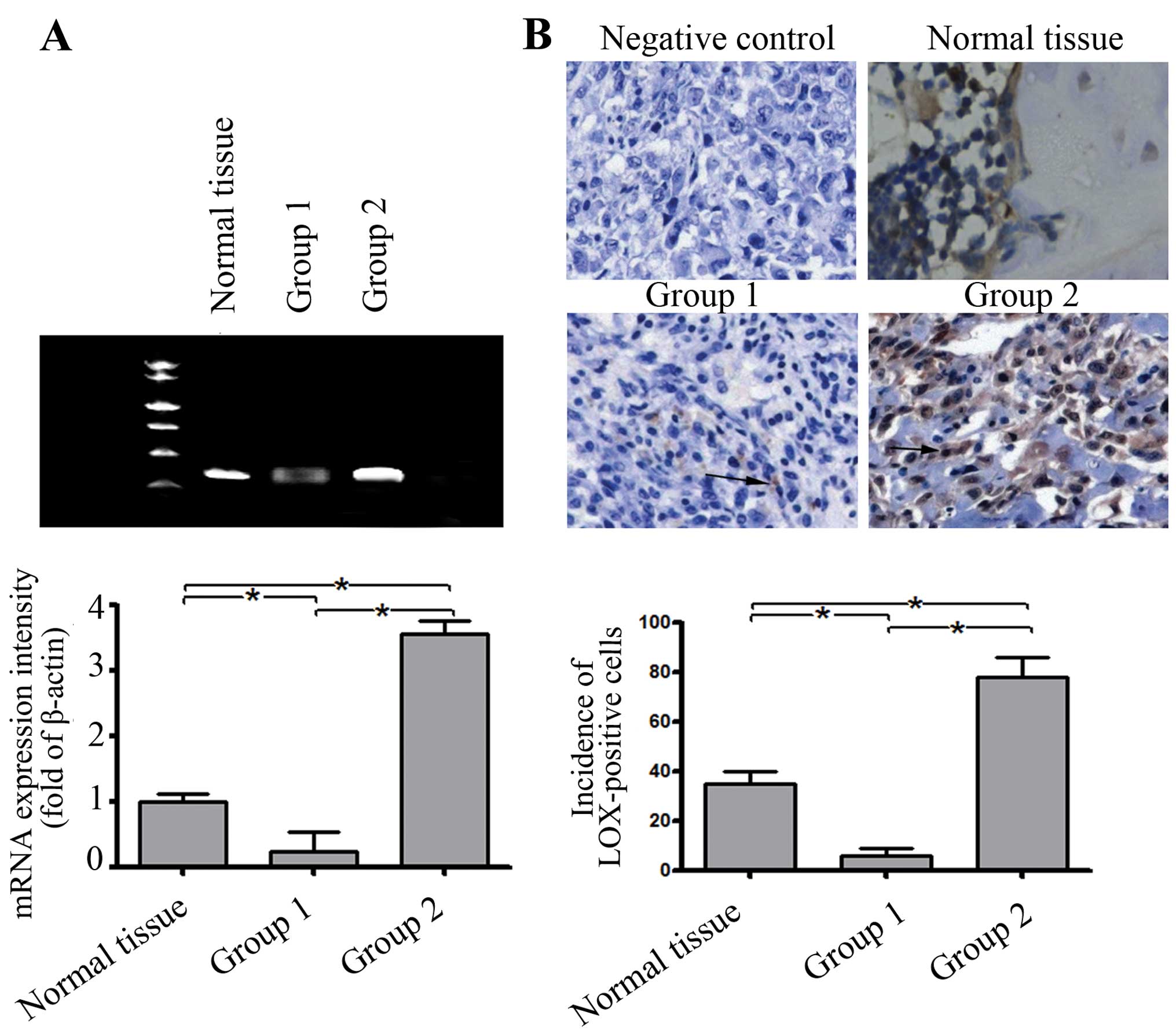
To ascertain whether abnormal LOX expression occurs
in osteosarcoma tissues, the mRNA levels of LOX were examined in
tumor/normal paired tissue samples by RT-PCR analysis (95/20). As a
result, the expression level of LOX in 18% (17/95) was similar to
that of the mean level of normal tissues (>0.5-fold and
<2-fold). However, the expression level of LOX in 57% (55/95) of
the tumor tissue was significantly lower than that of the mean
level of normal tissues (<0.5-fold). A higher level of LOX
expression (>2-fold) was observed in 30% (23/78) of tumor
tissues (Fig. 1A). On the basis of
the results of real-time PCR, the cases were divided into group 1
(55 cases, low level of LOX expression) and group 2 (23 cases, high
level of LOX).
In addition, the level and localization of LOX
protein were further assessed by immunohistochemisty in groups 1
and 2. Consistently, the incidence of LOX-positive cells in the
tumor tissues and the mean level of LOX protein expression in group
1 were significantly lower than those in the normal tissues
(Fig. 1B). In contrast, the
incidence of LOX-positive cells and the mean level of LOX protein
expression in group 2 were higher than those in the normal tissues
(Fig. 1).
Subsequently, to further evaluate whether the
expression levels of LOX mRNA and protein were related to clinical
therapeutic outcomes, the clinical status of each specimen was
determined according to tumor-node-metastasis classification (TNM)
(Table I). Our results indicated
that the tumor size in group 1 was significantly larger than that
in group 2 and there were more cases in the poor nodal status in
group 2. In addition, the patients in group 2 had a significantly
higher occurrence of pulmonary metastasis. These results revealed a
decrease in LOX mRNA expression in most advanced-stage tumor
tissues.
 | Table I.The analysis of prognostic factors
according to LOX mRNA expression. |
Table I.
The analysis of prognostic factors
according to LOX mRNA expression.
| Group 1 (no. of
cases) | Group 2 (no. of
cases) | P-value |
|---|
| Age (years) | 18.9±5 | 22.8±6.7 | 0.19 |
| Gender | | | |
| Male | 38 | 19 | 0.27 |
| Female | 17 | 4 | |
| Size of tumor | | | |
| T1 | 17 | 12 | 0.23 |
| T2 | 25 | 6 | |
| T3 | 16 | 5 | |
| T4 | 1 | 0 | |
| Nodal status | | | |
| N0 | 43 | 23 | 0.04 |
| N1 | 10 | 0 | |
| N2 | 2 | 0 | |
| N3 | 0 | 0 | |
| Metastasis | | | |
| M0 | 39 | 23 | 0.16 |
| M1 | 16 | 0 | |
| 5-year
survival | | | |
| Alive | 21 | 16 | 0.01 |
| Dead | 34 | 7 | |
The expression of LOX in osteosarcoma
cell lines and construction of LOX overexpression vector
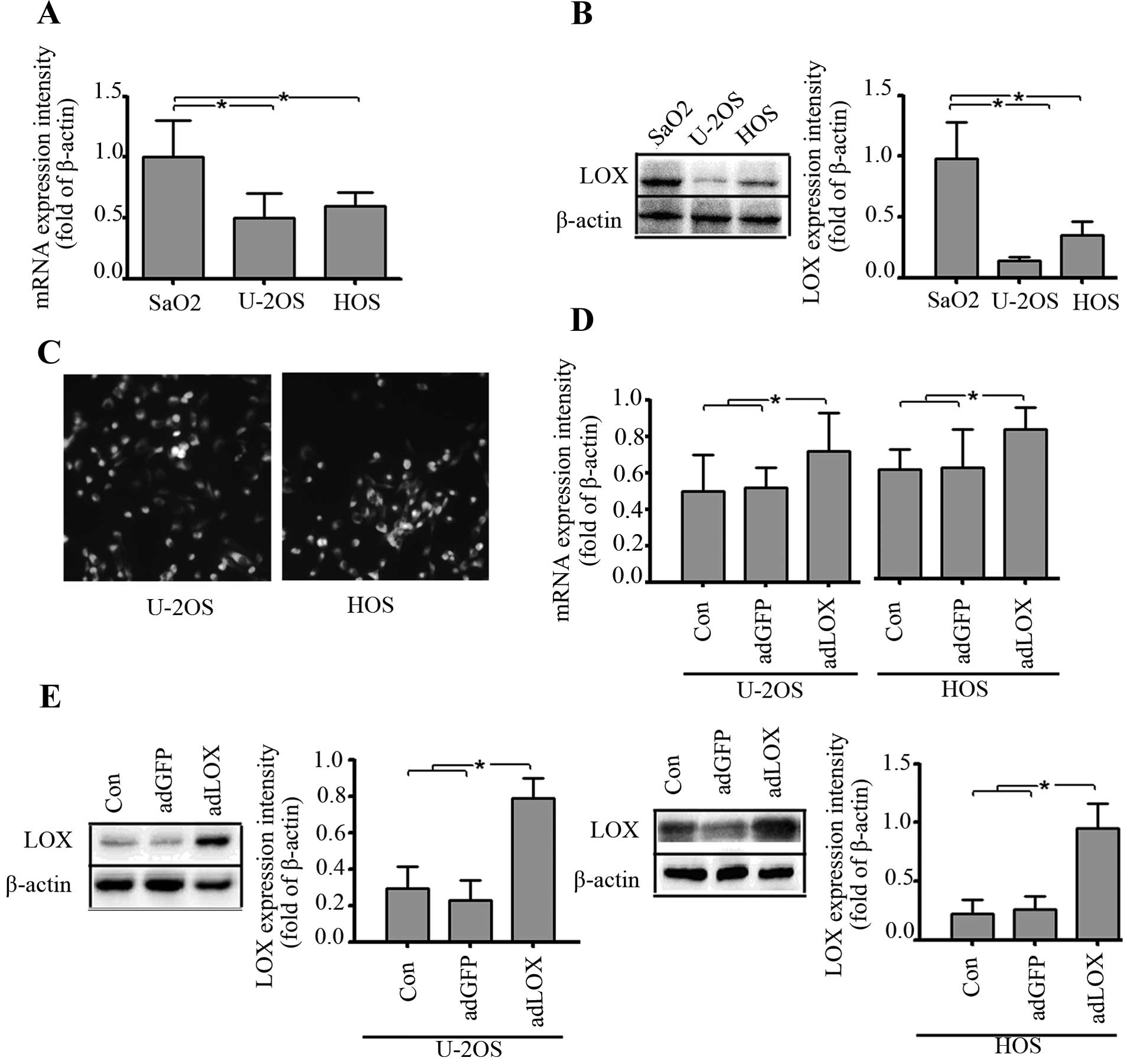
The endogenous expression of LOX in human
osteosarcoma cell lines, SaO2, U-2OS and HOS, was first evaluated
using RT-PCR and western blot analysis. As shown in Fig. 2A and B, there were different levels
of mRNA and protein expression of LOX in SaO2, U-2OS and HOS cell
lines, but the expression levels of LOX were significantly higher
in SaO2 cell line than those in U-2OS and HOS cell lines. Thus,
U-2OS and HOS were used as osteosarcoma cell lines for infection by
recombinant adenovirus containing LOX gene (Fig. 2A and B).
In order to enhance the exogenous expression of LOX
in osteosarcoma U-2OS and HOS cells, recombinant adenovirus
containing LOX gene (adLOX) was constructed and used for
upregulation of LOX in the U-2OS and HOS cell lines. The infection
efficiency of adLOX (at a multiplicity of infection = 100) in U-2OS
and HOS cell lines were both greater than 80% by fluorescence
microscopy (Fig. 2C). Real-time
PCR and western blot assays were performed to measure the exogenous
expression of LOX after 48 h following adenovirus infection and an
obvious increase of LOX mRNA and protein expression was observed in
adLOX group as shown in Fig. 2D and
E.
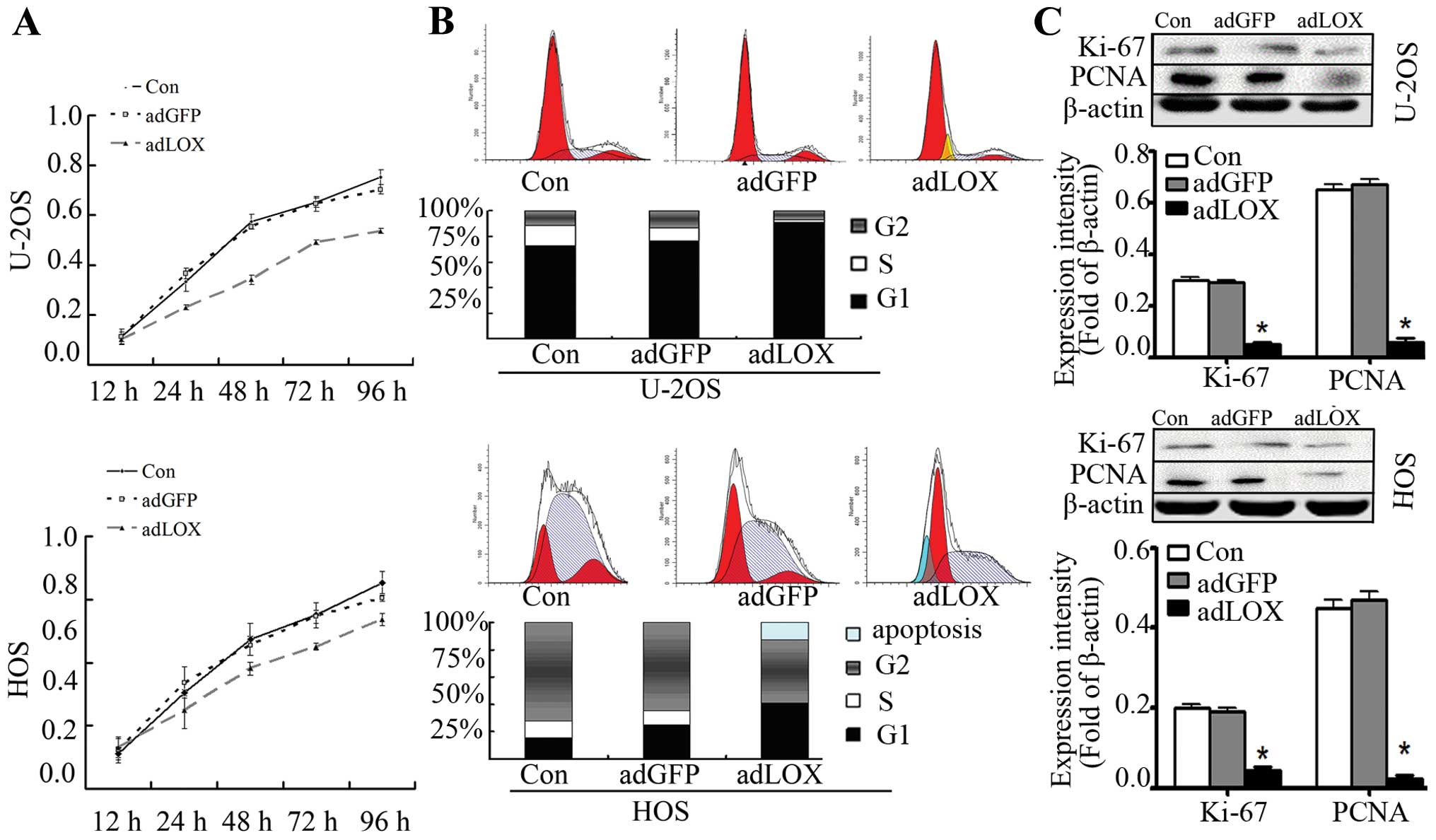
Proliferation of osteosarcoma cells was
inhibited by upregulation of LOX expression
Deregulation of cell proliferation and cell cycle is
one of the hallmarks of cancer (18). In order to detect the effect of LOX
on cell proliferation, we investigated the proliferative activities
of adLOX-infected U-2OS and HOS cells by MTT assay. As a result,
the cell proliferative ability of adLOX-infected U-2OS and HOS was
significantly suppressed as compared with the adGFP group and
control group. It was indicated that LOX overexpression would
reduce the proliferative activities of U-2OS and HOS cell lines.
However, no difference was found between adGFP group and control
group in U-2OS and HOS cell lines (Fig. 3A). To further confirm whether LOX
overexpression inhibited cell cycle progression, flow cytometry was
employed. As shown in Fig. 3B, the
apoptotic incidence of U-2OS and HOS cells in the adLOX group was
remarkably higher than that in the adGFP group and control group.
Cell cycle kinetics showed that the G0/G1 phase fraction was
increased, while S phase fraction was decreased. Cell cycle was
arrested in G0/G1 phase more in the adLOX group than in the adGFP
group and control group (Fig.
3B).
In order to investigate the mechanisms of
proliferative inhibition of LOX, we evaluated effects of LOX on two
independent proliferative markers: Ki-67 and proliferating cell
nuclear antigen (PCNA). Ki-67 is identified as a critical mediator
in tumor progression in Ewing’s sarcoma (19) and is widely used to evaluate the
outcome of anticancer treatment (1,20,21).
Similarly, PCNA has been employed to evaluate cell proliferation
(22). The expression of Ki-67 and
PCNA was examined by western blot assay and the results indicated
that the expression level of Ki-67 and PCNA was significantly
inhibited by LOX overexpression in U-2OS and HOS cell lines and no
difference was found between adGFP group and control group
(Fig. 3C). These data suggested
that overexpression of LOX might inhibit osteosarcoma cell
proliferation via downregulation of Ki-67 and PCNA.
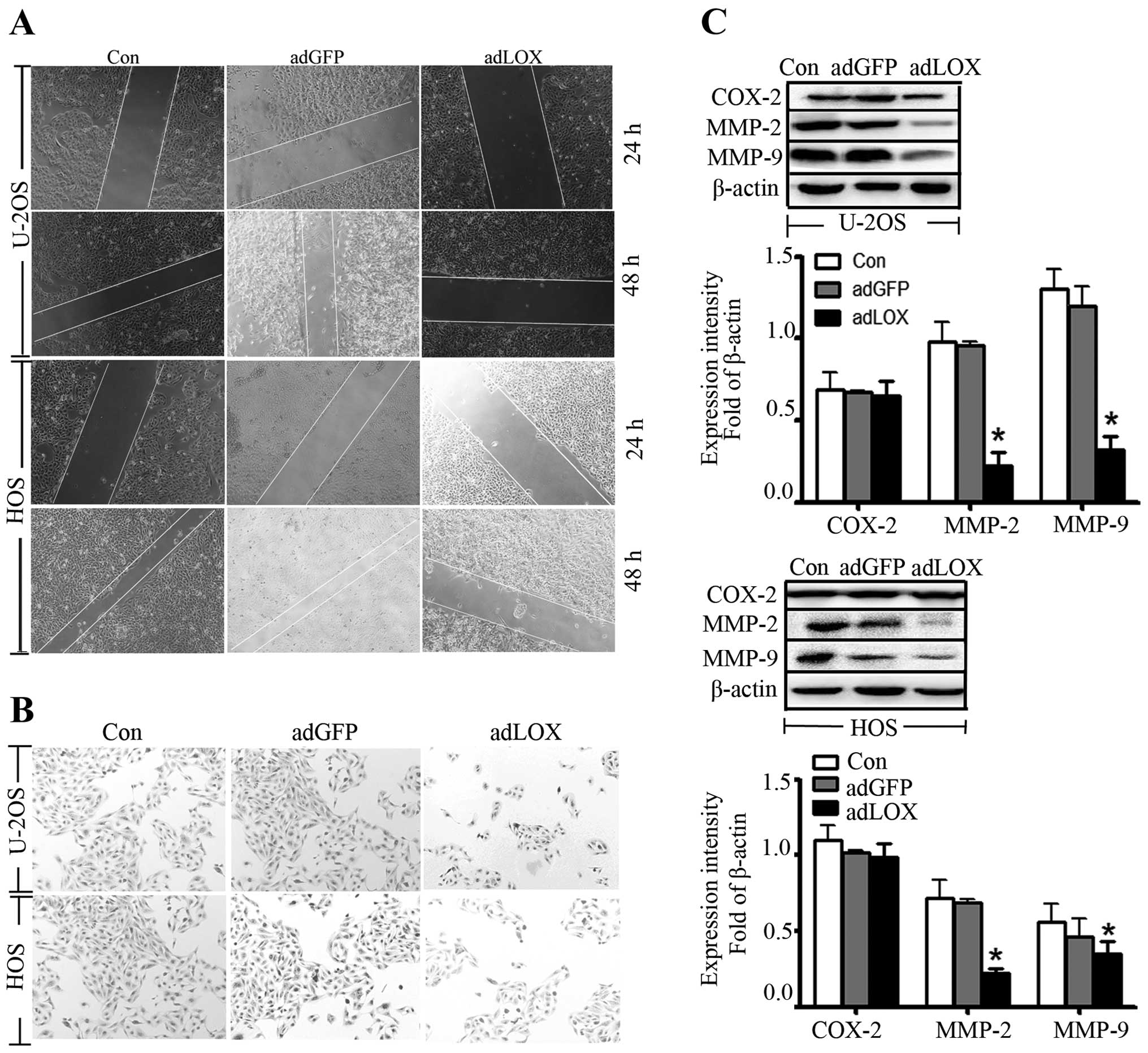
Cell migration was suppressed by LOX
To determine the effect of LOX on U-2OS and HOS cell
migration, wound-healing assay was performed and the results showed
that the migrative capacities of osteosarcoma cells with adLOX
infection were markedly suppressed. However, no significant changes
were detected between adGFP group and control group (Fig. 4A). Moreover, Transwell invasion
assay was performed to examine the ability of adLOX-infected 2U-2OS
and HOS cells to traverse the Matrigel-coated Transwell chamber. As
shown in Fig. 4B, compared with
the adGFP group and control group, invasion through Matrigel in
adLOX group was reduced by 48%.
It has been reported that cyclooxygenase-2 (COX-2)
(23) and MMPs were correlated
with tumor invasive progression or metastasis in several types of
cancers (22,24,25).
To investigate whether the activities of cyclooxygenase-2 and MMPs
were affected by LOX, western blot analysis was used to examine the
expressions of COX-2, MMP-9 and MMP-2. As shown in Fig. 4C, the expression of MMP-9 and MMP-2
proteins was significantly suppressed in adLOX group as compared
with the adGFP group and control group. However, no significant
difference in expression of COX-2 was found among the three groups.
These data indicated that overexpression of LOX might inhibit the
migration of osteosarcoma cells via downregulation of MMP-9 and
MMP-2 expression.
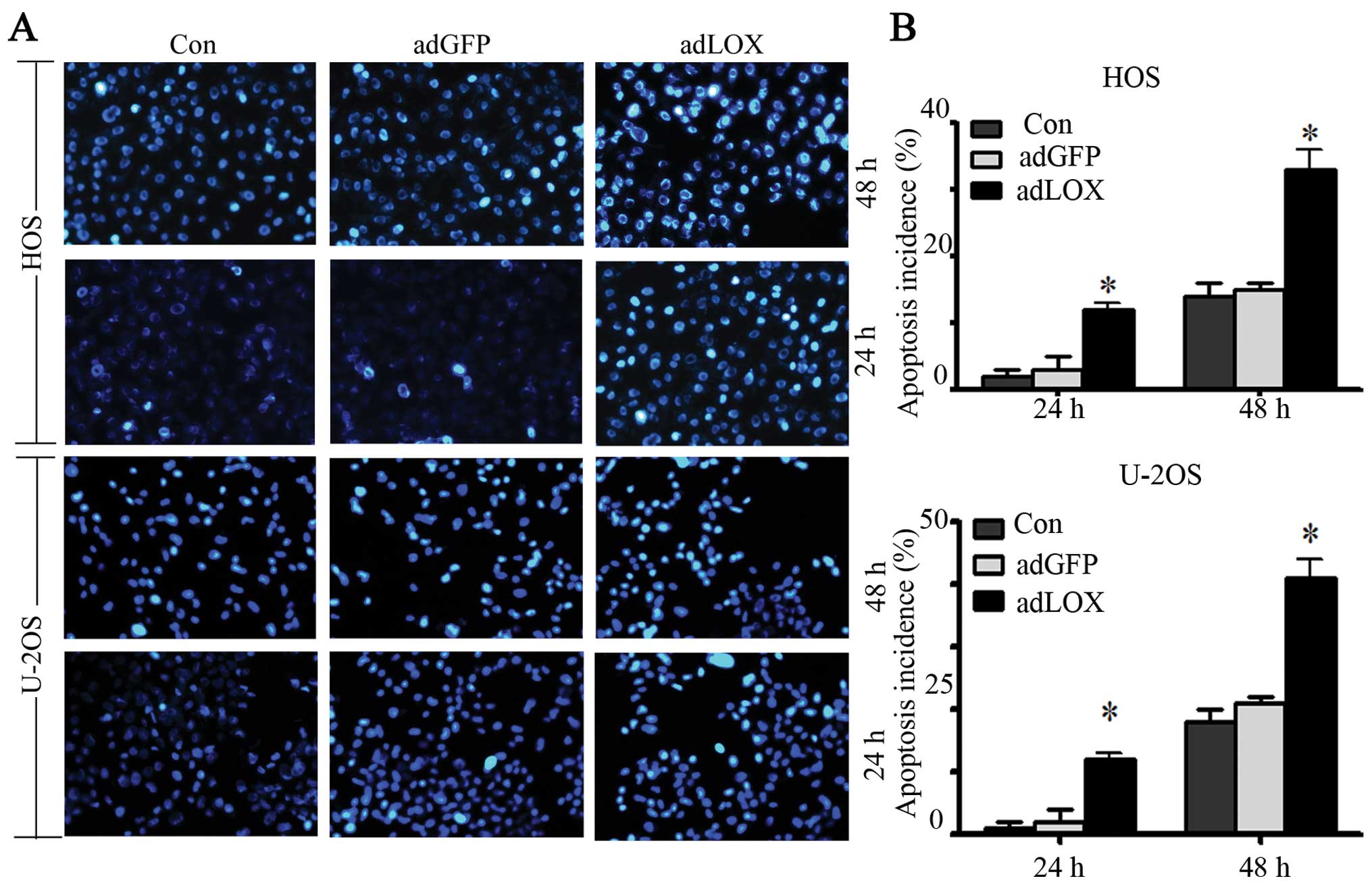
The anti-apoptotic ability was suppressed
by LOX
It has been shown that the mechanism of action of
tumorigenesis factors is associated with their ability to suppress
apoptosis. Thus, in this study, apoptosis was detected by Hoechst
33342 staining. After culture for 24 and 48 h, the cells were
stained with Hoechst 33342 and their cell nucleus was observed
under the microscope for apoptosis. The representative micrographs
(Fig. 5A) and quantitative scoring
of all data (Fig. 5B) showed that
the number of U-2OS and HOS apoptotic cells and necrotic cells
(strong blue staining) in adLOX group significantly increased
compared with that in adGFP group and control group in a
time-dependent manner. No difference was found between adGFP group
and control group (P>0.05). These data suggested that
overexpression of LOX could decrease the ability of osteosarcoma
cells against apoptosis.
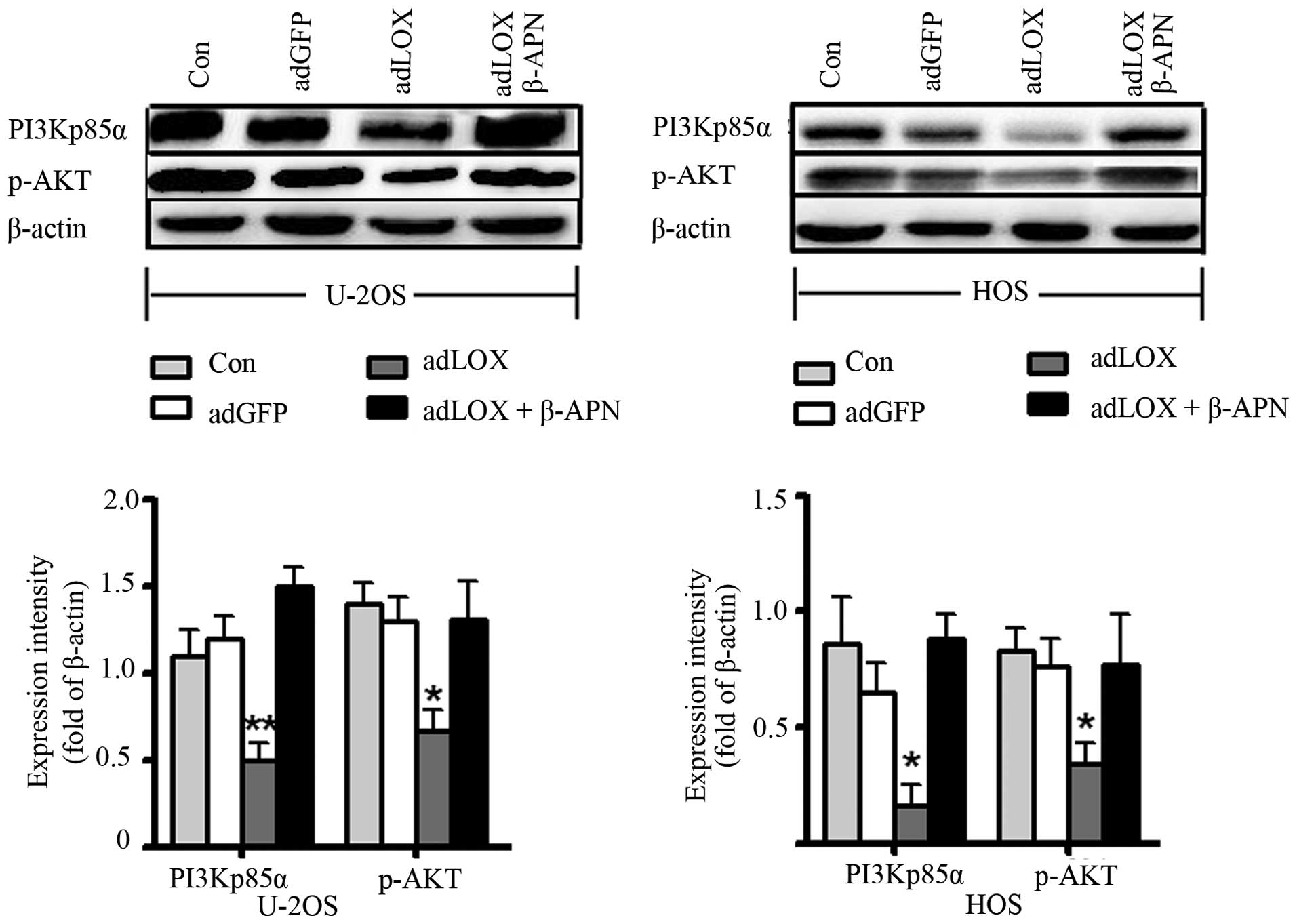
The effect of LOX was mediated via
PI3K/AKT signaling pathway
The PI3K/Akt signaling pathway plays a critical role
in regulating cellular proliferation, growth, survival and motility
for normal cells and its dysfunction is deeply connected with
tumorigenesis (20,26). To directly test the effects of
PI3K/AKT signaling, the expression of PI3Kp85α and p-AKT was
assessed by western blot assay. As shown in Fig. 6, an obvious decrease of PI3Kp85α
and p-AKT was observed in adLOX group compared with that in the
adGFP group and control group (*P<0.05). But, no
difference was found between adGFP group and control group
(P>0.05). To further confirm this result, 300 μM
β-aminopropionitrile (β-APN), a LOX inhibitor, was used to treat
the adLOX-infected U-2OS and HOS cells. Western blot results showed
that the expressions of PI3Kp85α and p-AKT were significantly
suppressed by β-APN. In addition, the inhibitory effect of LOX on
proliferation and migration of human osteosarcoma cells also could
be reversed by β-APN (data not shown). These data suggested that
overexpression of LOX might inhibit the proliferation and migration
of human osteosarcoma cells through PI3K/AKT signaling pathway.
Discussion
LOX is a key enzyme that control extracellular
matrix, collagen and elastin maturation, but, the role of LOX is
not limited to these functions and it also plays a critical role in
the development and invasion of osteosarcoma. A previous microarray
analysis suggested that the gene expression of LOX was associated
with osteosarcoma (27).
Furthermore, a study in vitro identified that LOX expression
could be increased by suramin (an anticancer drug) in a
dose-dependent manner, indicating that suramin might inhibit
tumorigenesis by increase of LOX (28). Consistently, our results also
showed that patients with low LOX expression were more likely to
develop poor nodal status (P=0.04) and survival was significantly
reduced (P=0.01). These findings indicated that LOX might serve as
a tumor suppressive gene and a prognostic marker for survival in
patients with osteosarcoma.
In addition, studies have shown that LOX is
upregulated in invasive breast cancer cells, and induced
proliferation in vitro (29). However, our finding demonstrated
that the overexpression of LOX significantly reduced the
proliferative activity of U-2OS and HOS cell lines in a
time-dependent manner. The result was consistent with previous
reports that LOX inhibited proliferation in smooth muscle cells
(30,31). At the same time, we found that the
expression of Ki-67 and PCNA were downregulated in U-2OS and HOS
cell lines when LOX was overexpressed. These data suggested that
LOX might inhibit proliferation through downregulation of Ki-67 and
PCNA expression.
Failure of cells to undergo apoptosis is a feature
of cancers and it has been reported that LOX has apoptosis-inducing
effects in lung cancer and breast cancer cells (6,32,33).
Similarly, our results also identified that LOX overexpression
resulted in increased apoptosis in U-2OS and HOS cell lines. On the
other hand, cell cycle is a highly-ordered and tightly-regulated
process involving multiple checkpoints that assesses extracellular
growth signals, cell size, and DNA integrity and its deregulation
would lead to tumorigenesis. Our results demonstrated that the cell
cycle of osteosarcoma cells in the adLOX group had more cells
arrested in G0/G1 phase and had higher apoptotic incidence compared
with the adGFP group and control. Transwell invasion and
wound-healing assay indicated that LOX overexpression inhibited the
migration and invasiveness of osteosarcoma cells. The expression of
MMP-9 and MMP-2 proteins was significantly suppressed in U-2OS and
HOS cell lines with LOX overexpression. This suggested that LOX
inhibited the migration of osteosarcoma cells via downregulation of
MMP-9 and MMP-2 expression.
PI3K/AKT is a major pathway for malignant
progression in various tumors (20,26).
It mediated survival signals to rescue Ewing tumor cells from
fibroblast growth factor 2-induced cell death (34). It was reported that several
cyclooxygenase-2 inhibitors could induce tumor cell apoptosis via
inhibition of PI3K/AKT signaling pathway (35,36).
Furthermore, it had been reported that the LOX gene might inhibit
migration and proliferation via PI3K/AKT signaling pathway in tumor
cells (37–39). Our study also indicated that
overexpression of LOX could lead to a marked decrease of PI3Kp85α
and p-AKT, accompanied by a reduced proliferative activity and
migration capacity in U-2OS and HOS cell lines. The β-APN could
reverse the effect of LOX on proliferation and migration of human
osteosarcoma cells. This suggested that overexpression of LOX
inhibited osteosarcoma cell growth and migration via blockade of
the PI3K/AKT signaling pathway. To our knowledge, this is the first
report on the role of LOX in the growth and migration of
osteosarcoma cells. But, the small sample size of 95 cases and the
use of only two osteosarcoma cell lines provide limited evidence.
Further research with larger sample size and more cell lines is
required to confirm our findings.
In conclusion, our investigations revealed that the
expression level of LOX mRNA and protein is downregulated in human
osteosarcoma tissues and the enhanced expression of LOX in
osteosarcoma cells exerts inhibitory effects on growth and
migration of osteosarcoma cells via blockade of the PI3K/AKT
signaling pathway.
References
|
1.
|
Thompson L, Wang S, Tawfik O, et al:
Effect of 25-hydroxyvitamin D3 and 1 α,25 dihydroxyvitamin D3 on
differentiation and apoptosis of human osteosarcoma cell lines. J
Orthop Res. 30:831–844. 2012.
|
|
2.
|
Chugh R: Experimental therapies and
clinical trials in bone sarcoma. J Natl Compr Canc Netw. 8:715–725.
2010.PubMed/NCBI
|
|
3.
|
Dai X, Ma W, He X, et al: Review of
therapeutic strategies for osteosarcoma, chondrosarcoma, and
Ewing’s sarcoma. Med Sci Monit. 17:RA177–190. 2011.
|
|
4.
|
Kagan HM and Trackman PC: Properties and
function of lysyl oxidase. Am J Respir Cell Mol Biol. 5:206–210.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Kim YM, Kim EC and Kim Y: The human lysyl
oxidase-like 2 protein functions as an amine oxidase toward
collagen and elastin. Mol Biol Rep. 38:145–149. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Bais MV, Nugent MA, Stephens DN, et al:
Recombinant lysyl oxidase propeptide protein inhibits growth and
promotes apoptosis of pre-existing murine breast cancer xenografts.
PLoS One. 7:e311882012. View Article : Google Scholar
|
|
7.
|
Palamakumbura AH, Vora SR, Nugent MA, et
al: Lysyl oxidase propeptide inhibits prostate cancer cell growth
by mechanisms that target FGF-2-cell binding and signaling.
Oncogene. 28:3390–3400. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Hurtado PA, Vora S, Sume SS, et al: Lysyl
oxidase propeptide inhibits smooth muscle cell signaling and
proliferation. Biochem Biophys Res Commun. 366:156–161. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Erler JT, Bennewith KL, Nicolau M, et al:
Lysyl oxidase is essential for hypoxia-induced metastasis. Nature.
440:1222–1226. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Kirschmann DA, Seftor EA, Fong SF, et al:
A molecular role for lysyl oxidase in breast cancer invasion.
Cancer Res. 62:4478–4483. 2002.PubMed/NCBI
|
|
11.
|
Baker AM, Bird D, Lang G, et al: Lysyl
oxidase enzymatic function increases stiffness to drive colorectal
cancer progression through FAK. Oncogene. 73:583–594.
2013.PubMed/NCBI
|
|
12.
|
Lapointe J, Li C, Higgins JP, et al: Gene
expression profiling identifies clinically relevant subtypes of
prostate cancer. Proc Natl Acad Sci USA. 101:811–816. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Wu J, Cai C, Tong D, et al: Lysyl oxidase
G473A polymorphism is associated with increased risk of ovarian
cancer. Genet Test Mol Biomarkers. 16:915–919. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Stassar MJ, Devitt G, Brosius M, et al:
Identification of human renal cell carcinoma associated genes by
suppression subtractive hybridization. Br J Cancer. 85:1372–1382.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Giampuzzi M, Botti G, Cilli M, et al:
Down-regulation of lysyl oxidase-induced tumorigenic transformation
in NRK-49F cells characterized by constitutive activation of ras
proto-oncogene. J Biol Chem. 276:29226–29232. 2001. View Article : Google Scholar
|
|
16.
|
Bouez C, Reynaud C, Noblesse E, et al: The
lysyl oxidase LOX is absent in basal and squamous cell carcinomas
and its knockdown induces an invading phenotype in a skin
equivalent model. Clin Cancer Res. 12:1463–1469. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Barker HE, Cox TR and Erler JT: The
rationale for targeting the LOX family in cancer. Nat Rev Cancer.
12:540–552. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Novello C, Pazzaglia L, Cingolani C, et
al: miRNA expression profile in human osteosarcoma: role of miR-1
and miR-133b in proliferation and cell cycle control. Int J Oncol.
42:667–675. 2013.PubMed/NCBI
|
|
19.
|
Sollazzo MR, Benassi MS, Magagnoli G, et
al: Increased c-myc oncogene expression in Ewing’s sarcoma:
correlation with Ki67 proliferation index. Tumori. 85:167–173.
1999.
|
|
20.
|
Li B, Yang Y, Jiang S, et al:
Adenovirus-mediated overexpression of BMP-9 inhibits human
osteosarcoma cell growth and migration through downregulation of
the PI3K/AKT pathway. Int J Oncol. 41:1809–1819. 2012.PubMed/NCBI
|
|
21.
|
Liu ZL, Wang G, Peng AF, et al: Fatty acid
synthase expression in osteosarcoma and its correlation with
pulmonary metastasis. Oncol Lett. 4:878–882. 2012.PubMed/NCBI
|
|
22.
|
Kamei S, Sakayama K, Tamashiro S, et al:
Ketoprofen in topical formulation decreases the matrix
metalloproteinase-2 expression and pulmonary metastatic incidence
in nude mice with osteosarcoma. J Orthop Res. 27:909–915. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Duan DP, Dang XQ, Wang KZ, et al: The
cyclooxygenase-2 inhibitor NS-398 inhibits proliferation and
induces apoptosis in human osteosarcoma cells via downregulation of
the survivin pathway. Oncol Rep. 28:1693–1700. 2012.PubMed/NCBI
|
|
24.
|
Korpi JT, Hagstrom J, Lehtonen N, et al:
Expression of matrix metalloproteinases-2, -8, -13, -26, and tissue
inhibitors of metalloproteinase-1 in human osteosarcoma. Surg
Oncol. 20:e18–e22. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Zhang Y, Song L, Cai L, et al: Effects of
baicalein on apoptosis, cell cycle arrest, migration and invasion
of osteosarcoma cells. Food Chem Toxicol. 53C:325–333.
2012.PubMed/NCBI
|
|
26.
|
Rasmussen N and Rathmell WK: Looking
beyond inhibition of VEGF/mTOR: emerging targets for renal cell
carcinoma drug development. Curr Clin Pharmacol. 6:199–206. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Kubista B, Klinglmueller F, Bilban M, et
al: Microarray analysis identifies distinct gene expression
profiles associated with histological subtype in human
osteosarcoma. Int Orthop. 35:401–411. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Buchinger B, Spitzer S, Karlic H, et al:
Lysyl oxidase (LOX) mRNA expression and genes of the differentiated
osteoblastic phenotype are upregulated in human osteosarcoma cells
by suramin. Cancer Lett. 265:45–54. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Payne SL, Fogelgren B, Hess AR, et al:
Lysyl oxidase regulates breast cancer cell migration and adhesion
through a hydrogen peroxide-mediated mechanism. Cancer Res.
65:11429–11436. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Gacheru SN, Thomas KM, Murray SA, et al:
Transcriptional and post-transcriptional control of lysyl oxidase
expression in vascular smooth muscle cells: effects of TGF-beta 1
and serum deprivation. J Cell Biochem. 65:395–407. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Peinado H, Moreno-Bueno G, Hardisson D, et
al: Lysyl oxidase-like 2 as a new poor prognosis marker of squamous
cell carcinomas. Cancer Res. 68:4541–4550. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Yu Z, Sato S, Trackman PC, et al: Blimp1
activation by AP-1 in human lung cancer cells promotes a migratory
phenotype and is inhibited by the lysyl oxidase propeptide. PLoS
One. 7:e332872012. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Palamakumbura AH, Jeay S, Guo Y, et al:
The propeptide domain of lysyl oxidase induces phenotypic reversion
of ras-transformed cells. J Biol Chem. 279:40593–40600. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Hotfilder M, Sondermann P, Senss A, et al:
PI3K/AKT is involved in mediating survival signals that rescue
Ewing tumour cells from fibroblast growth factor 2-induced cell
death. Br J Cancer. 92:705–710. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Liu B, Shi ZL, Feng J, et al: Celecoxib, a
cyclooxygenase-2 inhibitor, induces apoptosis in human osteosarcoma
cell line MG-63 via down-regulation of PI3K/Akt. Cell Biol Int.
32:494–501. 2008. View Article : Google Scholar
|
|
36.
|
Jin S, Pang RP, Shen JN, et al: Grifolin
induces apoptosis via inhibition of PI3K/AKT signalling pathway in
human osteosarcoma cells. Apoptosis. 12:1317–1326. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Jeay S, Pianetti S, Kagan HM, et al: Lysyl
oxidase inhibits ras-mediated transformation by preventing
activation of NF-kappa B. Mol Cell Biol. 23:2251–2263. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Pez F, Dayan F, Durivault J, et al: The
HIF-1-inducible lysyl oxidase activates HIF-1 via the Akt pathway
in a positive regulation loop and synergizes with HIF-1 in
promoting tumor cell growth. Cancer Res. 71:1647–1657. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
39.
|
Voloshenyuk TG, Landesman ES, Khoutorova
E, et al: Induction of cardiac fibroblast lysyl oxidase by
TGF-beta1 requires PI3K/Akt, Smad3, and MAPK signaling. Cytokine.
55:90–97. 2011. View Article : Google Scholar : PubMed/NCBI
|