Introduction
According to the American Cancer Society, ∼11,280
new soft tissue sarcomas would be diagnosed in 2012 (6,110 cases in
males and 5,170 cases in females) and 3,900 Americans (2,050 males
and 1,850 females) were expected to die of soft tissue sarcomas
(1). Fibrosarcoma and liposarcoma
are among the most common types of sarcoma in adults (1). Forty percent of primary bone cancers
are chondrosarcomas, malignancies of cartilaginous origin,
primarily affecting the cartilage cells of femur, arm, pelvis, knee
and spine (2) and 4% of bone
cancers are fibrosarcomas, aggressive and highly metastatic cancers
of the connective tissue that primarily develops in metaphases of
long tubular bones (2,3). Synovial sarcoma, accounting for
<5–10% of all soft tissue sarcomas, develops in the synovial
membrane of the joints, mainly in the lower limbs, but can also
occur in the trunk and head/neck (4). Poor prognosis is attributed to both
the aggressive metastatic spread characteristic of these cancers
and the lack of efficacy in current treatment modalities to prevent
or counteract tumor progression. The overall relative 5-year
survival rate of people with soft tissue sarcomas is ∼50% according
to statistics from the National Cancer Institute (1).
Numerous clinical and experimental studies have
demonstrated that elevated levels of MMPs are associated with tumor
growth, cancer progression, metastasis and shortened survival in
patients (4,5). Among various MMP types, MMP-2 and
MMP-9 play pivotal roles in tumor cell invasion and metastasis by
degradation of type IV collagen, a major component of the ECM
(6–8). MMP-2 (72 kDa) and MMP-9 (92 kDa) are
secreted in their latent zymogenic form and cleaved by other MMPs
or proteases to yield the activated forms of 68, 58 and 54 kDa for
MMP-2 and 94 kDa for MMP-9.
MMP activity is regulated by and dependent upon
environmental influences from surrounding stroma cells, ECM
proteins, systemic hormones and other factors (6,9,10). A
variety of cytokines and growth factors, such as transforming
growth factor (TGF-β), hepatocyte growth factor (HGF), epidermal
growth factor (EGF) and tumor necrosis factor (TNF-α) also control
MMP activity (11,12). One of the most potent inducers of
cancer cell proliferation is the chemical agent phorbol
12-myristate 13-aceteate (PMA). In addition, activity of MMPs is
regulated at multiple levels, including transcription, modulation
of messenger RNA half-life (translation), secretion, localization,
activation and inhibition (13).
There is little information available on the effects of various
biological and chemical inducers and inhibitors in sarcomas. Among
the few studies available, Rutkowski et al (14) investigated the correlations between
serum levels of selected pro-inflammatory, hematopoietic and
angiogenic cytokines and soluble cytokine receptors with the
clinicopathological features and prognosis in soft tissue sarcoma
patients. They found significant correlations of serum cytokine
levels with tumor size and grade suggesting cytokines may be
directly or indirectly involved in the progression of soft tissue
sarcomas.
In this study, we investigated the effects of
selected cytokines, inducers and inhibitors affecting cancer cell
metabolism on the regulation of MMP-2 and MMP-9 activities in
chondrosarcoma, fibrosarcoma, liposarcoma and synovial sarcoma cell
lines.
Materials and methods
Materials
Human adult sarcoma cell lines chondrosarcoma
(SW-1353), fibrosarcoma (HT-1080), liposarcoma (SW-872) and
synovial sarcoma (SW-982) along with their culture media were
obtained from ATCC. Antibiotics, penicillin and fetal bovine serum
(FBS), were obtained from Gibco (BRL, Long Island, NY, USA).
Twenty-four well tissue culture plates were obtained from Costar
(Cambridge, MA, USA). Gelatinase zymography was performed in 10%
Novex pre-cast SDS polyacrylamide gel (Invitrogen Inc.) with 0.1%
gelatin in non-reducing conditions. Interleukin 1β (IL-1β), tumor
necrosis factor-α (TNF-α), PMA, lipopolysaccharide (LPS),
doxycycline, epigallocatechin gallate (EGCG), cyclohexamide,
actinomycin-D, retinoic acid and dexamethasone, were purchased from
Sigma (St. Louis, MO, USA). The nutrient mixture (NM), prepared by
VitaTech (Hayward, CA, USA) was composed of the following
ingredients in the relative amounts indicated: Vitamin C (as
ascorbic acid and as Mg, Ca and palmitate ascorbate) 700 mg;
L-lysine 1000 mg; L-proline 750 mg; L-arginine 500 mg; N-acetyl
cysteine 200 mg; standardized green tea extract (80% polyphenol)
1000 mg; selenium 30 µg; copper 2 mg; manganese 1 mg. All
other reagents used were of high quality and were obtained from
Sigma, unless otherwise indicated.
Cell cultures
The sarcoma cell lines were grown in their
respective media: fibrosarcoma in MEM, chondrosarcoma in DEM,
liposarcoma in MEM and synovial sarcoma in DME, supplemented with
10% FBS, penicillin (100 U/ml) and streptomycin (100 µg/ml)
in 24-well tissue culture plates. The cells were plated at a
density of 1x105 cells/ml and grown to confluency in a
humidified atmosphere at 5% CO2 at 37°C.
Serum-supplemented media were removed and the cell monolayer was
washed once with PBS and with the recommended serum-free media. The
cells were then incubated in 0.5 ml of serum-free medium with
various cytokines, mitogens, inducers and inhibitors in
triplicates, as indicated: PMA (10, 25, 50, 100 ng/ml); TNF-α (0.1,
1, 10, 25 ng/ml); IL-1β (0.1, 1, 10, 25 ng/ml); LPS (10, 25, 50,
100 µg/ml); EGCG (10, 25, 50, 100 µM) without and
with PMA 100 ng/ml; doxycycline (10, 25, 50, 100 µM) without
and with PMA 100 ng/ml; NM (10, 50, 100, 500, 1000 µg/ml)
with PMA 100 ng/ml, TNF-α 10 ng/ml, or IL-1β 10 ng/ml; retinoic
acid (50 µM); dexamethasone (50 µM); actinomycin-D (2
and 4 µg/ml); and cyclohexamide (2 and 4 µg/ml). The
plates were then returned to the incubator. The conditioned medium
from each treatment was collected separately, pooled and
centrifuged at 4°C for 10 min at 3000 rpm to remove cells and cell
debris. The clear supernatant was collected and used for gelatinase
zymography, as described below.
Gelatinase zymography
Gelatinase zymography was utilized because of its
high sensitivity to gelatinolytic enzymatic activity and ability to
detect both pro and active forms of MMP-2 and MMP-9. Upon
renaturation of the enzyme, the gelatinases digest the gelatin in
the gel and reveal clear bands against an intensely stained
background. Gelatinase zymography was performed in 10% Novex
pre-cast SDS polyacrylamide gel in the presence of 0.1% gelatin
under non-reducing conditions. Culture media (20 µl) were
mixed with sample buffer and loaded for SDS-PAGE with tris glycine
SDS buffer, as suggested by the manufacturer (Novex). Samples were
not boiled before electrophoresis. Following electrophoresis the
gels were washed twice in 2.5% Triton X-100 for 30 min at room
temperature to remove SDS. The gels were then incubated at 37°C
overnight in substrate buffer containing 50 mM Tris-HCl and 10 mM
CaCl2 at pH 8.0 and stained with 0.5% Coomassie Blue
R250 in 50% methanol and 10% glacial acetic acid for 30 min and
destained.
Protein standards were run concurrently and
approximate molecular weights were determined by plotting the
relative mobilities of known proteins. Gelatinase zymograms were
scanned using CanoScan 9950F Canon scanner at 300 dpi. The
intensity of the bands was evaluated using the pixel-based
densitometer program Un-Scan-It, version 5.1, 32-bit, by Silk
Scientific Corp. (Orem, UT, USA), at a resolution of 1 Scanner Unit
(1/100 of an inch for an image that was scanned at 100 dpi).
Results
Inducers and cytokines
Table I shows the
quantitative densitometry results from the effects of PMA, TNF-α,
IL-1β and LPS on MMP-2 and MMP-9 expression in chondrosarcoma,
fibrosarcoma, liposarcoma and synovial sarcoma cell lines
 | Table I.Effect of inducers on adult sarcoma
MMPs. |
Table I.
Effect of inducers on adult sarcoma
MMPs.
| Chondrosarcoma
(SW-1353) | Fibrosarcoma
(HT-1080) | Liposarcoma
(SW-872) | Synovial sarcoma
(SW-982) |
|---|
|
|
|
|
|---|
| MMP-2 (%) | MMP-9 (%) | MMP-2 (%) | MMP-9 (%) | MMP-2 (%) | MMP-9 (%) | MMP-2 (%) | MMP-9 (%) |
|---|
| PMA (ng/ml) | | | | | | | | |
| Control | 2.9 | 0.1 | 4.2 | 0.1 | 0.7 | 1.2 | 11.9 | 0.0 |
| 10 | 3.8 | 7.0 | 4.5 | 11.7 | 0.9 | 20.9 | 12.4 | 0.0 |
| 25 | 6.6 | 19.0 | 10.5 | 17.1 | 0.2 | 23.1 | ND | ND |
| 50 | 5.1 | 23.1 | 8.5 | 18.0 | 0.6 | 28.0 | 10.0 | 5.0 |
| 100 | 4.1 | 28.3 | 6.3 | 19.1 | 0.6 | 23.7 | 5.6 | 55.1 |
| TNF-α (ng/ml) | | | | | | | | |
| Control | 14.9 | 14.5 | 4.8 | 0.1 | 3.4 | 8.7 | 25.3 | 0.0 |
| 0.1 | 6.0 | 0.2 | 5.6 | 0.3 | 3.9 | 9.1 | 29.9 | 0.0 |
| 1 | 14.9 | 0.3 | 6.2 | 1.1 | 2.2 | 5.1 | 27.5 | 0.0 |
| 10 | 15.4 | 0.5 | 5.7 | 32.0 | 3.6 | 31.8 | 15.4 | 1.8 |
| 25 | 23.8 | 19.8 | 6.0 | 38.1 | 2.7 | 29.5 | ND | ND |
| IL-1β (ng/ml) | | | | | | | | |
| Control | 9.0 | 0.3 | 18.6 | 0.6 | 5.5 | 14.1 | 24.7 | 0.0 |
| 0.1 | 19.3 | 1.2 | 14.6 | 0.2 | 3.8 | 13.0 | 27.1 | 0.0 |
| 1 | 13.8 | 3.5 | 25.4 | 0.9 | 4.2 | 19.7 | 24.7 | 0.0 |
| 10 | 26.8 | 8.1 | 23.4 | 0.7 | 3.3 | 23.0 | 22.4 | 1.1 |
| 25 | 14.8 | 3.2 | 15.3 | 0.5 | 2.3 | 11.0 | ND | ND |
| LPS
(µg/ml) | | | | | | | | |
| Control | 11.5 | 6.1 | 17.1 | 0.5 | 9.0 | 15.4 | ND | ND |
| 10 | 12.7 | 6.2 | 12.8 | 0.5 | 3.5 | 5.0 | ND | ND |
| 25 | 16.5 | 6.4 | 21.6 | 0.8 | 4.5 | 7.1 | ND | ND |
| 50 | 15.2 | 6.5 | 23.6 | 1.1 | 10.6 | 23.9 | ND | ND |
| 100 | 13.0 | 5.9 | 21.6 | 0.5 | 5.9 | 14.9 | ND | ND |
Effect of PMA, TNF-α, IL-1β and LPS on
MMP-2 and MMP-9 expression in chondrosarcoma SW-1353 cell line
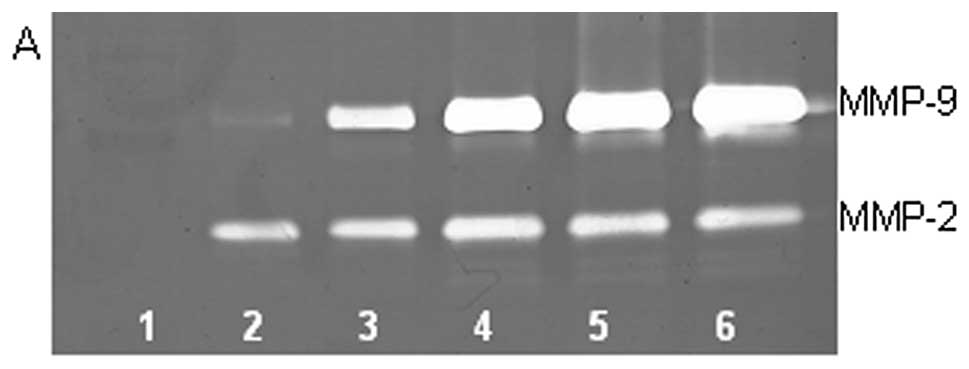
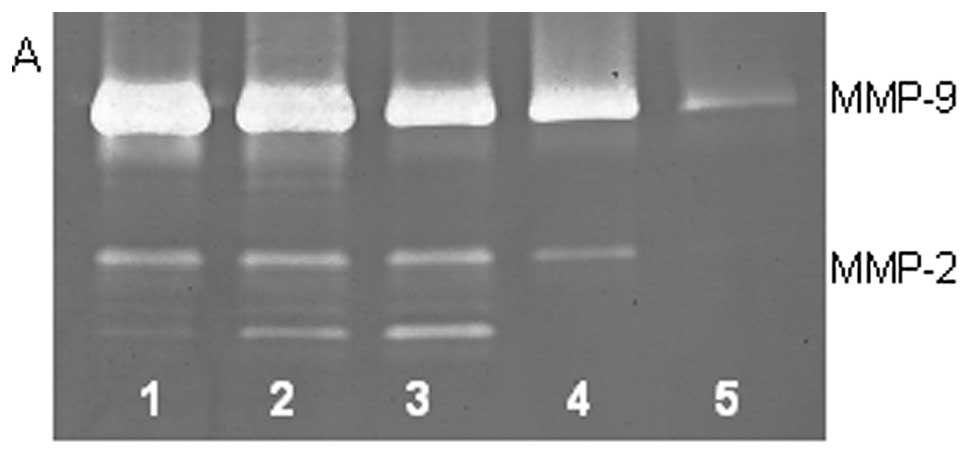
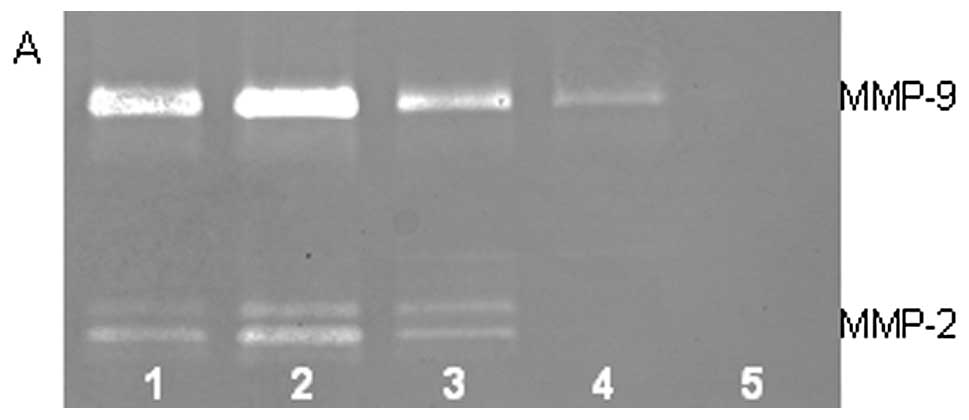
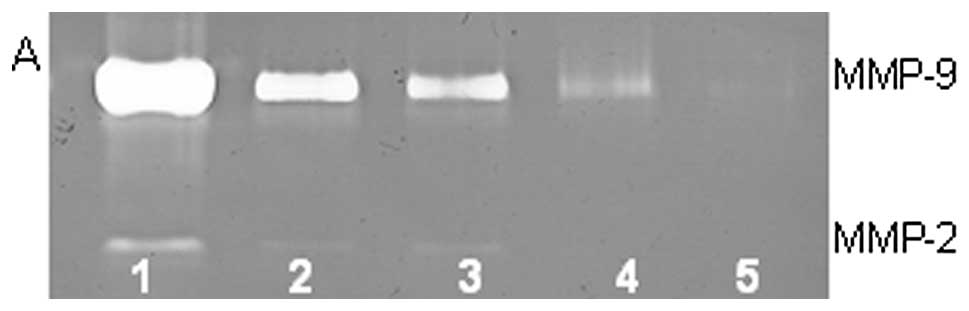
On gelatinase zymography, SW-1353 cells demonstrated
strong expression of MMP-2 and slight expression of MMP-9. PMA
treatment had no significant effect on expression of MMP-2 but
stimulated MMP-9 expression in a dose-dependent manner (linear
trend R2=0.9673), as shown in Fig. 1. TNF-α had a stimulatory effect on
MMP-9 (linear trend R2=0.5135) and insignificant effect
on MMP-2. IL-1β stimulated MMP-9 and MMP-2. LPS showed slight
stimulation of MMP-2 but no significant effect on MMP-9.
Effect of PMA, TNF-α, IL-1β and
LPS on MMP-2 and MMP-9 expression in fibrosarcoma HT-1080 cell
line
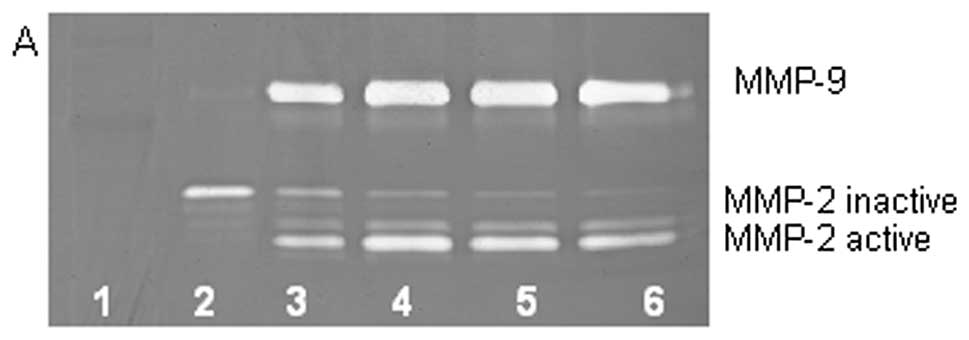
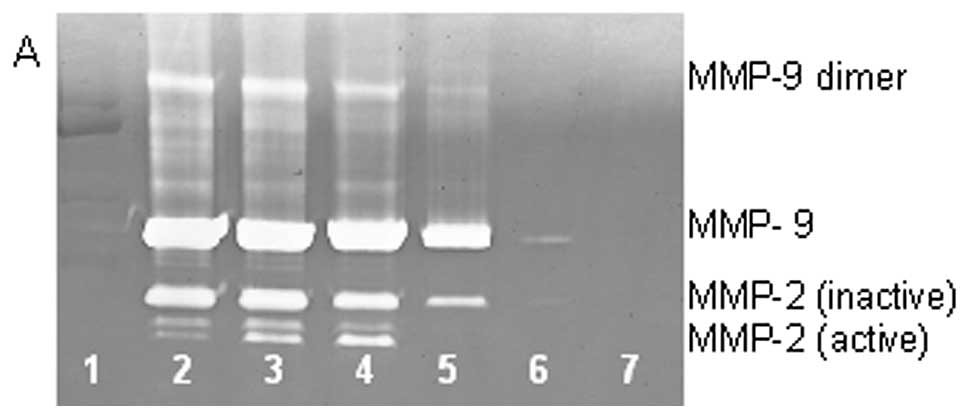
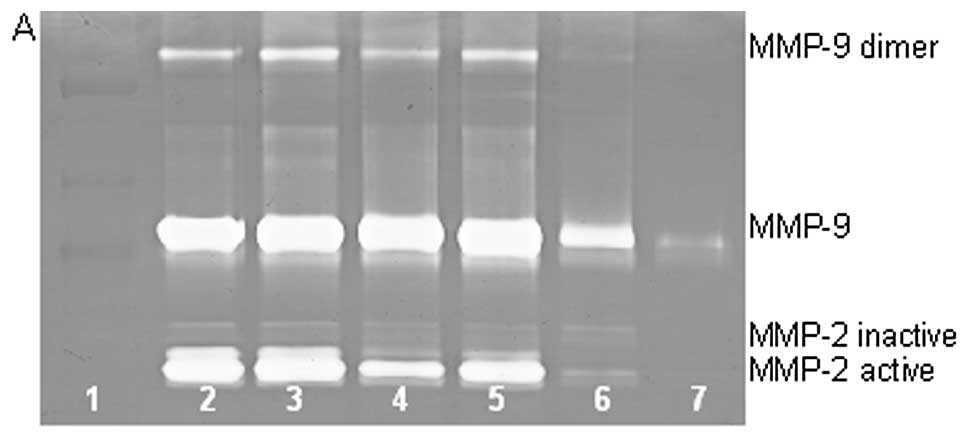
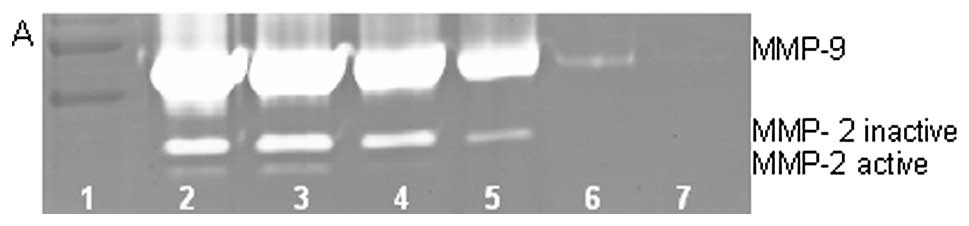
On gelatinase zymography, HT-1080 cells demonstrated
strong expression of MMP-2 inactive and faint MMP-2 active, both
greater than MMP-9. PMA treatment strongly stimulated MMP-9
expression in a dose-dependent manner (linear trend
R2=0.7952) and slightly enhanced MMP-2 active and
inhibited MMP-2 inactive, as shown in Fig. 2. TNF-α had a strong stimulatory
dose-dependent effect on MMP-9 and a slight enhancement of MMP-2.
IL-1β slightly stimulated MMP-9 and had no discernible effect on
MMP-2. LPS showed slight stimulation of MMP-2 but no significant
effect on MMP-9.
Effect of PMA, TNF-α, IL-1β and
LPS on MMP-2 and MMP-9 expression in liposarcoma SW-872 cell
line
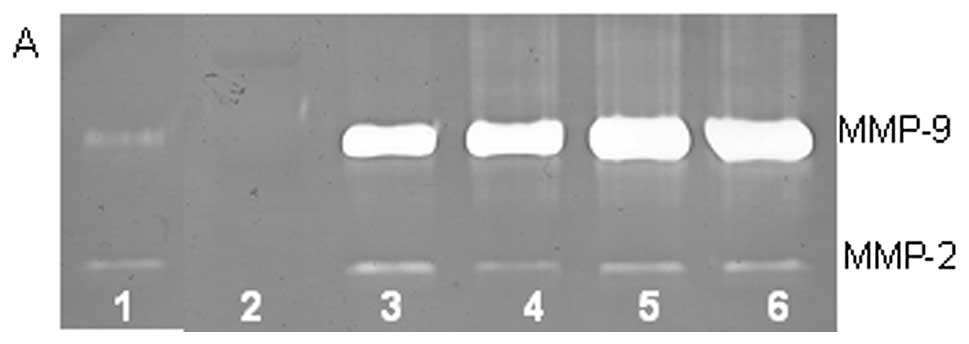
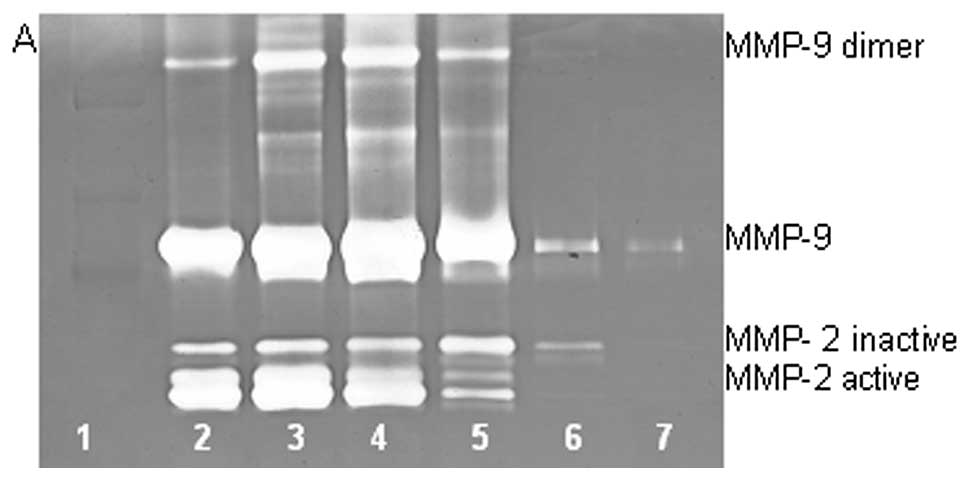
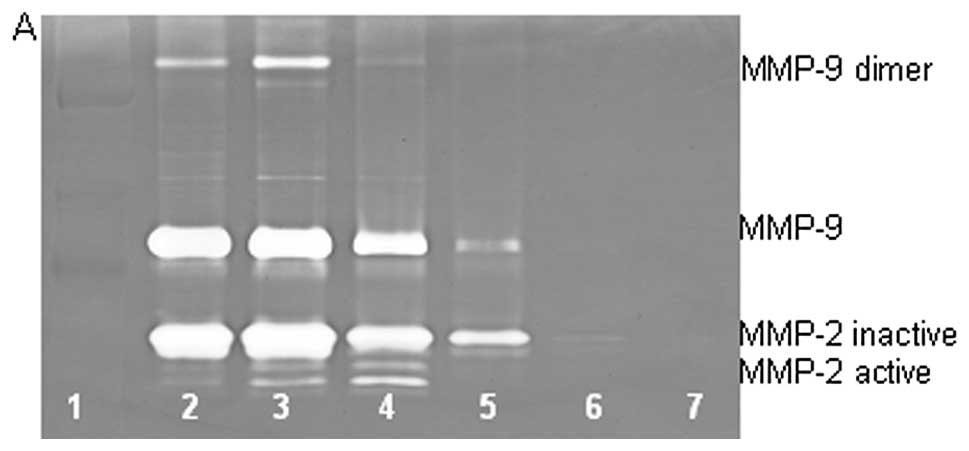
On gelatinase zymography, SW-872 cells demonstrated
MMP-2 and MMP-9 expression. PMA treatment strongly stimulated MMP-9
expression in a dose-dependent manner (linear trend
R2=0.617) but had no significant effect on MMP-2, as
shown in Fig. 3. TNF-α had a
strong stimulatory dose-dependent effect on MMP-9 (linear trend
R2=0.6358) and a slight reduction in MMP-2. IL-1β
slightly stimulated MMP-9 expression at 1 and 10 ng/ml, then
inhibited it at 25 ng/ml and inhibited MMP-2 in dose-dependent
manner (linear trend R2=0.865). LPS showed slight
dose-dependent inhibition in MMP-2, except at 50 ng/ml, but no
significant effect on MMP-9.
Effect of PMA, TNF-α, IL-1β and
LPS on MMP-2 and MMP-9 expression in synovial sarcoma SW-982 cell
line
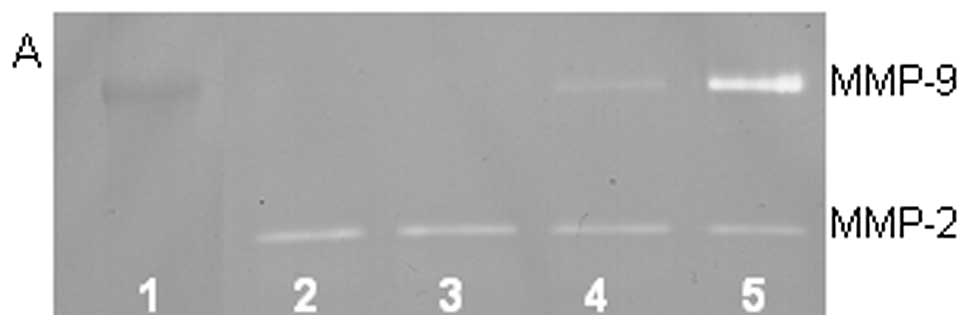
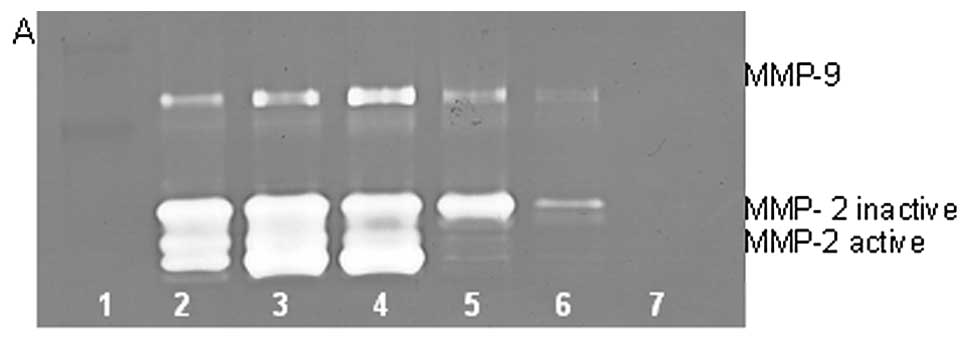
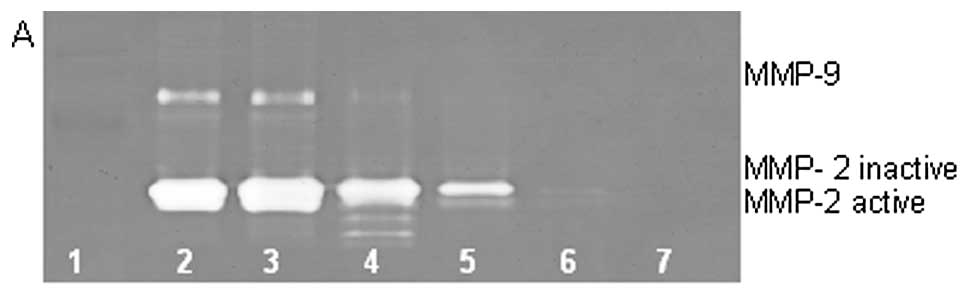
On gelatinase zymography, SW-982 cells demonstrated
moderate MMP-2 and no MMP-9 expression. PMA treatment strongly
stimulated MMP-9 expression in a dose-dependent manner (linear
trend R2=0.672) and slightly inhibited MMP-2 expression
(linear trend R2=0.797), as shown in Fig. 4. TNF-α had a moderate inhibitory
dose-dependent effect on MMP-2 (linear trend R2=0.425)
and a slight stimulatory effect on MMP-9 at the highest dose
tested, 10 ng/ml (linear trend R2=0.425). IL-1β had no
significant effect on MMP-2 or MMP-9 expression. The effects of LPS
were not determined.
Chemical inhibitors
Table II shows the
quantitative densitometry results from the effects of chemical
inhibitors doxycycline, dexamethasone, actinomycin-D and
cyclohex-amide on MMP-2 and MMP-9 expression in chondrosarcoma,
fibrosarcoma, liposarcoma and synovial sarcoma cell lines
 | Table II.Effect of chemical inhibitors on
adult sarcoma MMPs. |
Table II.
Effect of chemical inhibitors on
adult sarcoma MMPs.
| Chondrosarcoma
(SW-1353) | Fibrosarcoma
(HT-1080) | Liposarcoma
(SW-872) | Synovial sarcoma
(SW-982) |
|---|
|
|
|
|
|---|
| MMP-2 (%) | MMP-9 (%) | MMP-2 (%) | MMP-9 (%) | MMP-2 (%) | MMP-9 (%) | MMP-2 (%) | MMP-9 (%) |
|---|
| Doxycycline
(µM) |
| Control | 35.6 | 4.5 | 26.3 | 5.5 | 8.3 | 12.7 | ND | ND |
| 10 | 35.4 | 4.2 | 35.6 | 3.4 | 10.0 | 20.7 | ND | ND |
| 25 | 11.1 | 1.8 | 22.7 | 0.0 | 8.8 | 28.4 | ND | ND |
| 50 | 4.9 | 1.9 | 6.5 | 0.0 | 1.8 | 7.3 | ND | ND |
| 100 | 0.4 | 0.2 | 0.0 | 0.0 | 0.1 | 1.8 | ND | ND |
| Doxycycline
(µM) with PMA (100 ng/ml) |
| Control | 0.9 | 26.8 | 1.4 | 17.7 | 33.5 | 2.0 | ND | ND |
| 10 | 1.2 | 26.1 | 8.9 | 24.9 | 16.8 | 0.3 | ND | ND |
| 25 | 1.1 | 22.3 | 5.9 | 17.4 | 1.8 | 0.0 | ND | ND |
| 50 | 2.0 | 18.5 | 6.0 | 17.3 | 92.8 | 6.6 | ND | ND |
| 100 | 0.1 | 1.1 | 0.1 | 0.4 | 0.0 | 0.0 | ND | ND |
| Dexamethasone
(µM) |
| Control | 43.4 | 7.3 | 61.8 | 0.0 | 24.6 | 66.4 | ND | ND |
| 100 | 45.7 | 3.6 | 38.2 | 0.0 | 3.9 | 5.0 | ND | ND |
| Actinomycin-D
(µg/ml) |
| Control | 42.5 | 13.6 | 39.1 | 0.0 | ND | ND | ND | ND |
| 2 | 24.3 | 3.2 | 32.4 | 0.0 | ND | ND | ND | ND |
| 4 | 22.9 | 2.7 | 28.5 | 0.0 | ND | ND | ND | ND |
| Cyclohexamide
(µg/ml) |
| Control | 80.5 | 13.6 | ND | ND | ND | ND | ND | ND |
| 2 | 3.9 | 0.0 | ND | ND | ND | ND | ND | ND |
| 4 | 2.0 | 0.0 | ND | ND | ND | ND | ND | ND |
Effect of chemical inhibitors:
doxycycline, dexamethasone, actinomycin-D and cyclohexamide on
MMP-2 and MMP-9 expression in chondrosarcoma SW-1353 cell line
On gelatinase zymography, SW-1353 cells demonstrated
strong expression of MMP-2 and slight expression of MMP-9, with
enhanced MMP-9 expression with PMA (100 ng/ml) treatment.
Doxycycline with and without PMA (100 ng/ml) treatment showed
dose-dependent inhibition of MMP-2 and MMP-9 (linear trends
R2=0.894 and 0.910, respectively). MMP-2 was inhibited
by 99% and MMP-9 by 96% at 100 µM doxycycline compared to
control; PMA-treated SW-1353 showed dose-dependent inhibition of
MMP-9 (R2=0.785) with doxycycline treatment compared to
control and no significant change in MMP-2. Actinomycin-D showed
dose-dependent inhibition of both MMP-2 and -9 (linear trends
R2=0.804 and 0.785, respectively). Cyclohexamide showed
dose-dependent inhibition of both MMP-2 and -9 (linear trends
R2=0.768 and 0.750, respectively). Dexamethasone 50
µM demonstrated no effect on MMP-2 but inhibition of
MMP-9.
Effect of chemical inhibitors:
doxycycline, dexamethasone, actinomycin-D and cyclohexamide on
MMP-2 and MMP-9 expression in fibrosarcoma HT-1080 cell line
On gelatinase zymography, normal HT-1080 cells
demonstrated strong expression of MMP-2 with PMA-induced expression
of MMP-9. Doxycycline treatment of HT-1080 cells showed
dose-dependent inhibition of MMP-2 and MMP-9 (linear trends
R2=0.780 and 0.798, respectively). PMA-treated HT-1080
showed dose-dependent inhibition of MMP-9 (R2=0.543)
with doxycycline treatment compared to control and no significant
change in MMP-2. Actinomycin-D showed dose-dependent inhibition of
MMP-2 (linear trend R2=0.978). Dexamethasone 50
µM demonstrated no effect on MMP-9 but inhibited MMP-2 by
38%. The effect of cyclohexamide was not determined.
Effect of chemical inhibitors:
doxycycline, dexamethasone, actinomycin-D and cyclohexamide on
MMP-2 and MMP-9 expression in liposarcoma SW-872 cell line
On gelatinase zymography, normal SW-872 cells
demonstrated MMP-2 and MMP-9 expression. Doxycycline treatment of
SW-872 cells showed enhanced secretion of MMP-9 at 10 and 25
µM and inhibition of MMP-9 at 50 and 100 µM. MMP-2
expression was not appreciably affected at lower concentrations of
doxycycline, but was significantly inhibited at 50 and 100
µM (linear trend R2=0.739). PMA-treated SW-872
cells showed dose-dependent inhibition of MMP-9
(R2=0.922) with doxycycline treatment compared to
control and no significant change in MMP-2. Dexamethasone 50
µM inhibited SW-873 secretion of MMP-2 by 84% and MMP-9 by
99%. The effects of cyclohexamide and actinomycin-D were not
determined.
Effect of chemical inhibitors
doxycycline, dexamethasone, actinomycin-D and cyclohexamide on
MMP-2 and MMP-9 expression in synovial sarcoma SW-982 cell
line
On gelatinase zymography, normal SW-982 cells
demonstrated strong expression of MMP-2 and undetectable level of
MMP-9. The effects of doxycycline, actinomycin-D, dexamethasone,
cyclohexamide and retinoic acid on SW-982 cell expression of MMP-2
and -9 were not determined.
Natural inhibitors
Table III shows the
quantitative densitometry results from the effects of natural
inhibitors EGCG, the NM and retinoic acid on MMP-2 and MMP-9
expression in chondrosarcoma, fibrosarcoma, liposarcoma and
synovial sarcoma cell lines.
 | Table III.Effect of natural inhibitors on adult
sarcoma MMPs. |
Table III.
Effect of natural inhibitors on adult
sarcoma MMPs.
| Chondrosarcoma
(SW-1353) | Fibrosarcoma
(HT-1080) | Liposarcoma
(SW-872) | Synovial sarcoma
(SW-982) |
|---|
|
|
|
|
|---|
| MMP-2 (%) | MMP-9 (%) | MMP-2 (%) | MMP-9 (%) | MMP-2 (%) | MMP-9 (%) | MMP-2 (%) | MMP-9 (%) |
|---|
| EGCG
(µM) |
| Control | 23.9 | 9.5 | 30.6 | 0.0 | 19.7 | 25.6 | 56.5 | 0.0 |
| 10 | 30.7 | 8.7 | 45.6 | 0.0 | 16.1 | 27.9 | 43.5 | 0.0 |
| 25 | 17.6 | 4.6 | 23.9 | 0.0 | 7.0 | 3.7 | 0.0 | 0.0 |
| 50 | 3.8 | 0.3 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| 100 | 1.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| EGCG (µM)
with PMA (100 ng/ml) |
| Control | 1.7 | 29.8 | 2.4 | 22.0 | 1.7 | 64.4 | 8.8 | 24.8 |
| 10 | 2.4 | 23.5 | 6.4 | 54.2 | 0.1 | 20.8 | 6.7 | 56.2 |
| 25 | 3.6 | 20.0 | 1.4 | 11.8 | 0.2 | 10.9 | 0.0 | 0.0 |
| 50 | 0.6 | 17.0 | 0.0 | 1.9 | 0.0 | 1.8 | 0.0 | 0.0 |
| 100 | 0.0 | 1.4 | 0.0 | 0.0 | 0.0 | 0.2 | 0.0 | 0.0 |
| Nutrient mixture
(µg/ml) |
| Control | 23.8 | 7.2 | 19.4 | 5.4 | 15.6 | 16.3 | 39.4 | 0.7 |
| 10 | 27.8 | 2.1 | 22.5 | 6.3 | 17.7 | 17.3 | 38.1 | 0.0 |
| 50 | 23.4 | 1.5 | 17.5 | 8.4 | 13.7 | 10.4 | 19.2 | 0.0 |
| 100 | 12.0 | 0.7 | 10.1 | 7.9 | 7.0 | 1.9 | 2.6 | 0.0 |
| 500 | 1.4 | 0.0 | 1.1 | 1.2 | 0.1 | 0.0 | 0.0 | 0.0 |
| 1000 | 0.1 | 0.0 | 0.1 | 0.2 | 0.0 | 0.0 | 0.0 | 0.0 |
| Nutrient mixture
(µg/ml) with PMA (100 ng/ml) |
| Control | 5.8 | 22.5 | 12.6 | 13.2 | 4.7 | 24.4 | 33.9 | 8.6 |
| 10 | 7.4 | 23.0 | 12.9 | 13.8 | 6.1 | 25.2 | 35.6 | 3.9 |
| 50 | 2.9 | 18.6 | 6.2 | 12.9 | 3.5 | 20.2 | 16.4 | 0.2 |
| 100 | 1.4 | 17.5 | 8.0 | 13.9 | 0.6 | 14.4 | 1.4 | 0.0 |
| 500 | 0.1 | 0.8 | 0.2 | 6.0 | 0.0 | 0.8 | 0.0 | 0.0 |
| 1000 | 0.0 | 0.1 | 0.0 | 0.3 | 0.0 | 0.0 | 0.0 | 0.0 |
| Nutrient mixture
(µg/ml) with TNF-α (10 ng/ml) |
| Control | 9.9 | 12.4 | 16.5 | 13.4 | ND | ND | ND | ND |
| 10 | 13.8 | 14.5 | 21.0 | 15.8 | ND | ND | ND | ND |
| 50 | 7.9 | 17.3 | 13.8 | 9.0 | ND | ND | ND | ND |
| 100 | 3.4 | 11.4 | 5.18 | 1.74 | ND | ND | ND | ND |
| 500 | 0.2 | 0.7 | 0.69 | 0.0 | ND | ND | ND | ND |
| 1000 | 0.0 | 0.15 | 0.02 | 0.0 | ND | ND | ND | ND |
| Nutrient mixture
(µg/ml) with IL-1 β (10 ng/ml) |
| Control | 15.3 | 0.85 | 30.3 | 7.15 | ND | ND | ND | ND |
| 10 | 24.3 | 2.1 | 28.7 | 7.0 | ND | ND | ND | ND |
| 50 | 26.9 | 4.2 | 16.0 | 3.8 | ND | ND | ND | ND |
| 100 | 24.9 | 0.7 | 6.9 | 0.0 | ND | ND | ND | ND |
| 500 | 0.57 | 0.13 | 0.15 | 0.0 | ND | ND | ND | ND |
| 1000 | 0.0 | 0.0 | 0.0 | 0.0 | ND | ND | ND | ND |
| Retinoic acid
(µM) |
| Control | 81.6 | 13.9 | ND | ND | ND | ND | ND | ND |
| 50 | 4.5 | 0.0 | ND | ND | ND | ND | ND | ND |
Effect of EGCG, nutrient mixture and
retinoic acid on MMP-2 and MMP-9 expression in chondrosarcoma
SW-1353 cell line treated with inducers
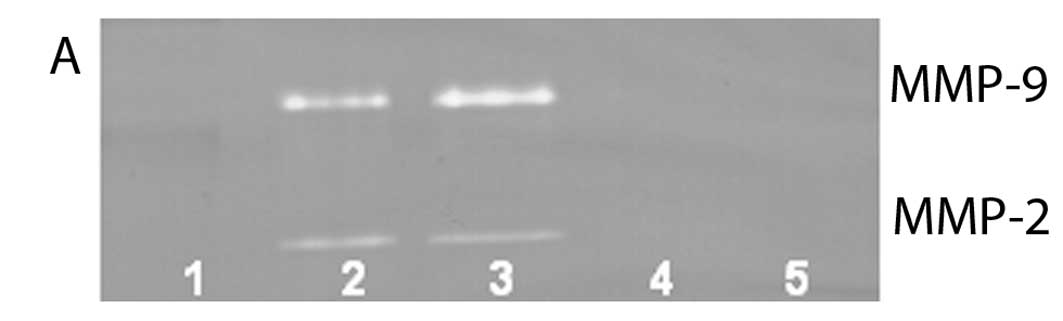
On gelatinase zymography, SW-1353 cells demonstrated
strong expression of MMP-2 and slight expression of MMP-9. EGCG
inhibited MMP-9 and MMP-2 in a dose-dependent manner, with total
inhibition of MMP-2 and 95% inhibition of MMP-9 at 100 µM
(linear trends R2=0.809 and 0.933, respectively). NM
inhibited MMP secretion in a dose-dependent manner with virtual
total inhibition of MMP-9 at 500 µg/ml (linear trend
R2=0.713) and MMP-2 at 1000 µg/ml (linear trend
R2=0.860). PMA (100 ng/ml) treatment profoundly enhanced
MMP-9 expression. EGCG inhibited MMP-9 and MMP-2 in a
dose-dependent manner, with total inhibition of MMP-9 and 96%
inhibition of MMP-2 at 100 µM (linear trends
R2=0.892 and 0.336, respectively), as shown in Fig. 5. NM demonstrated dose-dependent
inhibition of MMP-2 and MMP-9 expression levels of PMA-treated
SW-1353 total block of MMP-2 and virtually total inhibition of
MMP-9 secretion at 1000 µg/ml (linear trends
R2=0.831 and 0.833, respectively), as shown in Fig. 6. SW-1353 cells treated with TNF-α
(10 ng/ml) showed strong expression levels of MMP-9 and MMP-2.
TNF-α-treated cells showed slight stimulation of MMP-2 and -9 at
low concentrations of NM and strong inhibition at concentrations of
NM 100 µg/ml and higher, with linear trends
R2=0.796 and 0.641, respectively, as shown in Fig. 7. SW-1353 cells treated with IL-1β
(10 ng/ml) showed strong expression of MMP-9 and slight expression
of MMP-2. NM treatment of IL-1 β -treated cells showed enhanced
expression of both MMPs at low concentrations of NM and strong
inhibition of both MMP-2 and MMP-9 at concentrations of NM 100
µg/ml and higher, as shown in Fig. 8. Retinoic acid showed strong
inhibition of MMP-2 and MMP-9 at the dose tested (50
µM).
Effect of EGCG, the nutrient mixture and
retinoic acid on MMP-2 and MMP-9 expression in fibrosarcoma HT-1080
cell line treated with inducers
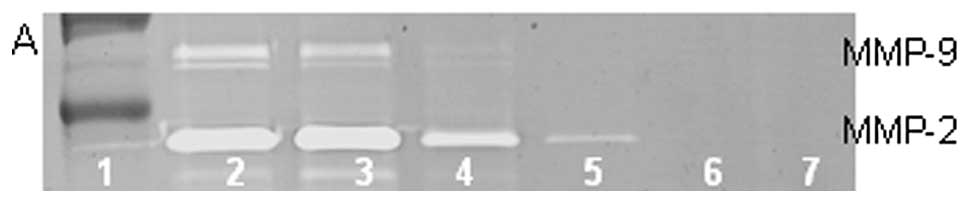
On gelatinase zymography, normal HT-1080 cells
demonstrated strong expression of MMP-2 with PMA-induced expression
of MMP-9. EGCG inhibited MMP-2 in a dose-dependent manner, with
linear trend R2=0.720; PMA-treated HT-1080 cells showed
MMP-2 and induced MMP-9 expression, which were inhibited in a
dose-dependent manner, with linear trends R2=0.452 and
0.475, respectively, as shown in Fig.
9. NM inhibited secretion of MMP-2 and MMP-9 by uninduced
HT-1080 cells in a dose-dependent manner, with linear trends
R2=0.510 and 0.546, respectively. NM showed
dose-dependent inhibition of MMP-2 and -9 expression in PMA-treated
cells with linear trends R2=0.866 and 0.678,
respectively, as shown in Fig.
10. HT-1080 cells treated with TNF-α (10 ng/ml) showed strong
expression levels of MMP-9 and MMP-2. TNF-α-treated cells showed
slight stimulation of MMP-2 and -9 at low concentrations of NM and
strong inhibition at concentrations of NM 100 µg/ml and
higher, with linear trends R2=0.880 and 0.855,
respectively, as shown in Fig.
11. HT-1080 cells treated with IL-1β (10 ng/ml) showed a strong
expression of MMP-9 greater than MMP-2. NM treatment of
IL-1β-treated cells showed dose-dependent inhibition of both MMP-2
and MMP-9, with linear trends R2=0.934 and 0.861,
respectively, as shown in Fig.
12. The effect of retinoic acid was not determined.
Effect of EGCG, the nutrient mixture and
retinoic acid on MMP-2 and MMP-9 expression in liposarcoma SW-872
cell line treated with inducers
On gelatinase zymography, SW-872 cells demonstrated
MMP-2 and strongly stimulated MMP-9 with PMA (100 ng/ml) treatment.
EGCG inhibited both MMP-2 and -9 in a dose-dependent manner, with
linear trends R2=0.914 and 0.787, respectively; PMA (100
ng/ml)-treated SW-872 cells showed MMP-2 and strongly enhanced
MMP-9 expression, which were inhibited by EGCG in a dose-dependent
manner, with linear trends R2=0.560 and 0.782,
respectively, as shown in Fig.
13. NM treatment of SW-872 cells treated with PMA showed
dose-dependent inhibition of MMP-2 and MMP-9, with linear trends
R2=0.820 and 0.898, respectively, as shown in Fig. 14. The effects of retinoic acid
were not determined.
Effect of natural inhibitors: EGCG, the
nutrient mixture and retinoic acid on MMP-2 and MMP-9 expression in
synovial sarcoma SW-892 cell line treated with inducers
On gelatinase zymography, SW-892 cells showed strong
MMP-2 expression and induction of MMP-9 with PMA (100 ng/ml)
treatment. EGCG inhibited MMP-2 in a dose-dependent manner, with
linear trend R2=0.878; PMA (100 ng/ml)-treated SW-982
cells showed MMP-2 and induced MMP-9 expression, which were
inhibited by EGCG in a dose-dependent manner, with linear trends
R2=0.881 and 0.459, respectively, as shown in Fig. 15. SW-982 cells treated with PMA
showed dose-dependent inhibition of MMP-2 and MMP-9 expression with
NM treatment, with linear trends, R2=0.855 and 0.694,
respectively, as shown in Fig.
16. Effects of retinoic acid were not determined.
Discussion
Experimental and clinical studies have shown a
correlation between increased MMPs and tumor progression and
metastasis (6,7). Thus, knowledge of MMP regulation is
of importance for developing therapeutic strategies. MMP expression
is regulated at both pre- and post-transcriptional levels.
Extracellular factors, including cytokines, growth factors and
inducers and inhibitors, have been implicated in the regulation of
MMP expression in different types of tumor cells (15,16).
Though few cytokine and growth factor studies have been conducted
on sarcomas, some research has documented elevated serum levels of
VEGF, IL-2 and bFGF in sera of patients with soft tissue sarcomas
(17,18); VEGF serum levels correlated
significantly with tumor size and histological grade (17). Serum cytokine levels significantly
correlated with tumor size and grade suggesting involvement of
cytokines in the progression of soft tissue sarcomas (14). Rutkowski et al found
elevated cytokines and soluble cytokine receptors involved in bone
destruction and bone formation in 46% of adult bone sarcoma
patients, suggesting they have an essential role in the progression
of malignant bone tumors (19).
In this study, we compared MMP secretion patterns by
cytokines, PMA and LPS in four adult sarcoma cell lines that
express MMP-2 and MMP-9 to different extents. In addition, we
investigated the effect of inhibitors doxycycline and EGCG and
others, such as dexamethasone, retinoic acid and agents that affect
transcription and translation levels, such as actinomycin-D and
cyclohexamide. Furthermore, we tested a nutrition mixture that had
inhibitory effects on MMP-2 and MMP-9 expression. We found that
fibrosarcoma HT-1080, chondrosarcoma SW-1353, liposarcoma SW-872
and synovial sarcoma SW-982 normally expressed both MMP-2 and
MMP-9. Treatment of all these cell lines with PMA strongly
upregulated expression of MMP-9 in a dose-dependent manner.
However, the effect on MMP-2 was variable; PMA-treated fibrosarcoma
and chondrosarcoma cells showed slightly stimulated MMP-2
expression, while PMA showed no effect on liposarcoma expression of
MMP-2 and inhibition of MMP-2 was seen in PMA treatment of synovial
sarcoma. TNF-α had an inhibitory effect at 0.1-10 ng/ml and a
stimulatory effect at 25 ng/ml on MMP-9 and an inhibitory effect at
0.1 ng/ml and stimulatory effect at 10–25 ng/ml on MMP-2 in
chondrosarcoma SW1353 cells. In fibrosarcoma cells TNF-α strongly
stimulated MMP-9 and slightly stimulated MMP-2. In liposarcoma
cells, TNF-α strongly stimulated MMP-9 and showed slight up and
down activity on MMP-2. Synovial sarcoma showed inhibition of MMP-2
with TNF-α and slight stimulation of MMP-9 at 10 ng/ml. IL-1β
stimulated MMP-9 and MMP-2 in chondrosarcoma cells, enhanced levels
of both MMPs at 1 ng/ml but decreased levels at 25 ng/ml in
fibrosarcoma, inhibited MMP-2 and enhanced MMP-9 at 1 and 10 ng/ml
and downregulated at 25 ng/ml in liposarcoma cells. LPS showed
slight stimulation of MMP-2 in chondrosarcoma and fibrosarcoma and
slight inhibition of MMP-2 in lipo sarcoma, but no significant
effect on MMP-9 in these cell lines.
Doxycycline and EGCG inhibited MMP-2 and MMP-9
expression in a dose-dependent fashion in all cell lines tested.
Sensitivity to doxycycline was nearly equivalent in MMP-2
expression, but fibrosarcoma expression of MMP-9 was significantly
more sensitive to doxycycline than were the other cell lines. MMP-2
expression was downregulated by 99% by doxycycline 100 µM in
chondrosarcoma by 100% in fibrosarcoma and by 99% in liposarcoma.
MMP-9 expression was downregulated by doxycycline 100 µM in
chondrosarcoma and liposarcoma by 96 and 86%, respectively, while
fibrosarcoma MMP-9 expression was completely blocked at 25
µM. Sensitivity of cell lines to EGCG varied in MMP-2
expression, with total block at 25 µM in synovial sarcoma,
50 µM in fibrosarcoma and liposarcoma and 96% block in
chondrosarcoma at 100 µM. PMA-induced MMP-9 expression was
blocked by EGCG at 25 µM in synovial sarcoma and 100
µM in fibrosarcoma and virtually blocked at 100 µM in
chondrosarcoma and liposarcoma. Actinomycin-D, cyclohexamide,
retinoic acid and dexamethasone inhibited MMP-2 and -9 in
chondrosarcoma and fibrosarcoma cells.
The nutrition mixture inhibited MMP-2 and MMP-9
expression in a dose-dependent fashion in all PMA-treated cell
lines. Synovial sarcoma was most sensitive to NM with block of
MMP-2 at 500 µg/ml and MMP-9 at 100 µg/ml.
Liposarcoma showed block of MMP-2 at 500 µg/ml and MMP-9 at
1000 µg/ml, while fibrosarcoma and chondrosarcoma showed
block of MMP-2 at 1000 µg/ml and virtual block of MMP-9 at
1000 µg/ml. MMP-2 and-9 expression of chondrosarcoma and
fibrosarcoma cells treated with TNF-α 10 ng/ml were downregulated
by NM treatment in a dose-dependent manner with MMP-2 block at 1000
µg/ml NM and MMP-9 total block at 500 µg/ml NM in
fibrosarcoma and 99% block at 1000 µg/ml in chondrosarcoma.
MMP-2 and-9 expression of chondrosarcoma and fibrosarcoma cells
treated with IL-1β 10 ng/ml were downregulated by NM treatment in a
dose-dependent manner with MMP-2 block at 1000 µg/ml NM and
MMP-9 total block at 100 µg/ml NM in fibrosarcoma and at
1000 µg/ml in chondrosarcoma.
The nutrition mixture studied, which contains
lysine, proline, ascorbic acid and green tea extract among other
micro-nutrients, has been shown to have anti-tumor and
anti-invasive potential in vivo and in vitro
(20). Of interest, a previous
study demonstrated significant correlation between NM inhibition of
Matrigel invasion and NM modulation of the MMP-2 and -9 activities
of the sarcoma cell lines studied (21). Significant negative correlations
were found between NM modulation of Matrigel invasion inhibition
and MMP-2 secretion with fibrosarcoma HT-1080 (r= −0.911),
chondrosarcoma SW-1353 (r= −0.942), liposarcoma SW-872 (r= −0.957)
and synovial sarcoma SW-982 (r= −0.878). Previous in vivo
studies of the dietary effects of NM 0.5% on xenograft tumor growth
of fibrosarcoma and synovial sarcoma cells in nude mice support
these results in that they demonstrated significant inhibition of
xenograft tumor growth: 59%, p=0.0005 in fibrosarcoma HT-1080
xenografts (22) and 44%, p=0.01
in synovial sarcoma Hs 701.T xenografts (23).
NM was designed by focusing on physiological targets
in cancer progression and metastasis as documented in clinical and
experimental studies. The nutrient mixture was formulated by
selecting nutrients that act on critical physiological targets in
cancer progression and metastasis, as documented in both clinical
and experimental studies. Combining these micro-nutrients expands
metabolic targets, maximizing biological impact with lower doses of
components. A previous study of the comparative effects of NM,
green tea extract and EGCG on inhibition of MMP-2 and MMP-9
secretion of different cancer cell lines with varying MMP secretion
patterns, revealed the superior potency of NM over GTE and EGCG at
equivalent doses (24). These
results can be understood from the more comprehensive treatment
offered by the combination of nutrients in NM over individual
components of NM since MMP-2 and MMP-9 are mediated by differential
pathways.
Optimal ECM structure depends upon adequate supplies
of ascorbic acid and the amino acids lysine and proline to ensure
proper synthesis and hydroxylation of collagen fibers. In addition,
lysine contributes to ECM stability as a natural inhibitor of
plasmin-induced proteolysis (25,26).
Manganese and copper are also essential for collagen formation.
There is considerable documentation of the potency of green tea
extract in modulating cancer cell growth, metastasis, angiogenesis
and other aspects of cancer progression (27–31).
N-acetyl cysteine and selenium have demonstrated inhibition of
tumor cell MMP-9 and invasive activities, as well as migration of
endothelial cells through ECM (32–34).
Ascorbic acid demonstrates cytotoxic and antimetastatic actions on
malignant cell lines (35–39) and cancer patients have been found
to have low levels of ascorbic acid (40,41).
Low levels of arginine, a precursor of nitric oxide (NO), can limit
the production of NO, which has been shown to predominantly act as
an inducer of apoptosis (42).
In conclusion, our results show that cytokines and
inhibitors regulate MMP-2 and MMP-9 expression in adult sarcoma
cell lines, suggesting the clinical value of targeting these
proteases for management of sarcomas and their pathogenesis.
Acknowledgements
This study was funded by Dr. Rath
Health Foundation (Santa Clara, CA), a non-profit organization.
References
|
1.
|
American Cancer Society: Adult soft tissue
cancer. What are the key statistics about soft tissue sarcomas?
http://www.cancer.org/cancer/sarcoma-adultsofttissuecancer/detailedguide/sarcoma-adult-soft-tissue-cancer-key-statistics.
Last revised October 2, 2012. Accessed January 21, 2013.
|
|
2.
|
American Cancer Society: Bone cancer. What
are the key statistics about bone cancer? http://www.cancer.org/cancer/bonecancer/detailedguide/bone-cancer-key-statistics.
Last revised November 29, 2012. Accessed January 21, 2013.
|
|
3.
|
Papagelopoulos PJ, Galanis E, Frassica FJ,
Sim FH, Larson DR and Wold LE: Primary fibrosarcoma of bone.
Outcome after primary surgical treatment. Clin Orthop Relat Res.
373:88–103. 2000.PubMed/NCBI
|
|
4.
|
Benassi MS, Gamberi G, Magagnoli G,
Molendini L, Ragazzini P, Merli M, Chiesa F, Balladelli A, Manfrini
M, Bertoni F, Mercri M and Picci P: Metalloproteinase expression
and prognosis in soft tissue sarcomas. Ann Oncol. 12:75–80. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Nelson AR, Fingleton B, Rothenberg ML and
Matrisian LM: Matrix metalloproteinases: biologic activity and
clinical implications. J Clin Oncol. 18:1135–1149. 2000.PubMed/NCBI
|
|
6.
|
Liotta LA, Tryggvason K, Garbisa A, Hart
I, Foltz CM and Shafie S: Metastatic potential correlates with
enzymatic degradation of basement membrane collagen. Nature.
284:67–68. 1980. View
Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Stetler-Stevenson WG: The role of matrix
metalloproteinases in tumor invasion, metastasis, and angiogenesis.
Surg Oncol Clin N Am. 10:383–392. 2001.PubMed/NCBI
|
|
8.
|
Stetler-Stevenson WG: Type IV collagenases
in tumor invasion and metastasis. Cancer Metastasis Rev. 9:289–303.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Sato T, Sakai T, Noguchi Y, Takta M,
Hirakawa S and Ito A: Tumor-stromal cell contact promotes invasion
of human uterine cervical carcinoma cells by augmenting the
expression and activation of stromal matrix metalloproteinases.
Gynecol Oncol. 92:47–56. 2004. View Article : Google Scholar
|
|
10.
|
Pyke C, Kristensen P, Ralfkiaer E,
Gröndahl-Hansen J, Eriksen J, Blasi F and Danø K: Urokinase-type
plasminogen activator is expressed in stromal cells and its
receptor in cancer cells at invasive foci in human colon
adenocarcinomas. Am J Pathol. 138:1059–1067. 1991.PubMed/NCBI
|
|
11.
|
Harvey P, Clark IM, Jourand MC, Warn RM
and Edwards DR: Hepatocyte growth factor/scatter factor enhances
the invasion of mesothelioma cell lines and the expression of
matrix metalloproteinases. Br J Cancer. 83:1147–1153. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Liu Z, Ivanoff A and Klominek J:
Expression and activity of matrix metalloproteases in human
malignant mesothelioma cell lines. Int J Cancer. 91:638–643. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Vincenti MP, White LA, Schroen DJ, Benbow
U and Brinckerhoff CE: Regulating expression of the gene for matrix
metalloproteinase-1 (collagenase): mechanisms that control enzyme
activity, transcription and mRNA stability. Crit Rev Eukaryot Gene
Expr. 6:391–411. 1996. View Article : Google Scholar
|
|
14.
|
Rutkowski P, Kaminska J, Kowalska M, Ruka
W and Steffen J: Cytokine and cytokine receptor serum levels in
soft tissue sarcoma patients: correlations with
clinico-pathological features and prognosis. Int J Cancer.
100:463–471. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Ray JM and Stetler-Stevenson WG: The role
of matrix metalloproteinase and their inhibitors in tumour
invasion, metastasis and angiogenesis. Eur Respir J. 7:2062–2072.
1994.PubMed/NCBI
|
|
16.
|
Apodaca G, Rutka JT, Bouhana K, Berens ME,
Giblin JR, Rosenblum ML, McKerrow JH and Banda MJ: Expression of
metalloproteinases and metalloproteinase inhibitors by fetal
astrocytes and glioma cells. Cancer Res. 50:2322–2329.
1990.PubMed/NCBI
|
|
17.
|
Linder C, Linder S, Munck-Wickland E and
Strander H: Independent expression of serum vascular endothelial
growth factor (VEGF) and basic fibroblast growth factor (bFGF) in
patients with carcinoma and sarcoma. Anticancer Res. 18:2063–2068.
1998.PubMed/NCBI
|
|
18.
|
Graeven U, Andre N, Achilles E, Zornig C
and Schmeigel W: Serum levels of vascular endothelial growth factor
and basic fibroblast growth factor in patients with soft-tissue
sarcoma. J Cancer Res Clin Oncol. 125:577–581. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Rutkowski P, Kaminska J, Kowalska M, Ruka
W and Steffen J: Cytokine and cytokine receptor serum levels in
adult bone sarcoma patients: correlations with local tumor extent
and prognosis. J Surg Oncol. 84:151–159. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Niedzwiecki A, Roomi MW, Kalinovsky T and
Rath M: Micronutrient synergy - a new tool in effective control of
metastasis and other key mechanisms of cancer. Cancer Metastasis
Rev. 29:529–543. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Roomi MW, Monterrey JC, Kalinovsky T,
Niedzwiecki A and Rath M: Inhibition of invasion and MMPs by a
nutrient mixture in human cancer cell lines: a correlation study.
Exp Oncol. 32:243–248. 2010.PubMed/NCBI
|
|
22.
|
Roomi MW, Ivanov V, Kalinovsky T, Rath M
and Niedzwiecki A: In vivo and in vitro antitumor effect of
ascorbic acid, lysine, proline, arginine and green tea extract on
human fibrosarcoma cells HT-1080. Med Oncol. 23:105–112. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Roomi MW, Ivanov V, Kalinovsky T,
Niedzwiecki A and Rath M: In vitro and in vivo anti-tumor effect of
a nutrient mixture containing ascorbic acid, lysine, proline and
green tea extract on human synovial sarcoma cancer cells. JANA .
9:22006.
|
|
24.
|
Roomi MW, Monterrey JC, Kalinovsky T, Rath
M and Niedzwiecki A: Comparative effects of EGCG, green tea and a
nutrient mixture on the patterns of MMP-2 and MMP-9 expression in
cancer cell lines. Oncol Rep. 24:747–757. 2010.PubMed/NCBI
|
|
25.
|
Rath M and Pauling L: Plasmin-induced
proteolysis and the role of apoprotein(a), lysine and synthetic
analogs. Orthomolecular Med. 7:17–23. 1992.
|
|
26.
|
Sun Z, Chen YH, Wang P, Zhang J, Gurewich
V, Zhang P and Liu JN: The blockage of high-affinity lysine binding
sites of plasminogen by EACA significantly inhibits
prourokinase-induced plasminogen activation. Biochem Biophys Acta.
1596:182–192. 2002.PubMed/NCBI
|
|
27.
|
Valcic S, Timmermann BN, Alberts DS,
Wachter GA, Krutzsch M, Wymer J and Guillen JM: Inhibitory effect
of six green tea catechins and caffeine on the growth of four
selected human tumor cell lines. Anticancer Drugs. 7:461–468. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Mukhtar H and Ahmed N: Tea polyphenols:
prevention of cancer and optimizing health. Am J Clin Nutr.
71(Suppl 6): 1698–1704. 2000.PubMed/NCBI
|
|
29.
|
Yang GY, Liao J, Kim K, Yurtow EJ and Yang
CS: Inhibition of growth and induction of apoptosis in human cancer
cell lines by tea polyphenols. Carcinogenesis. 19:611–616. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Taniguchi S, Fujiki H, Kobayashi H, Go H,
Miyado K, Sadano H and Shimikawa R: Effect of (−) epigallocatechin
gallate, the main constituent of green tea, on lung metastasis with
mouse B16 melanoma cell lines. Cancer Lett. 65:51–54. 1992.
|
|
31.
|
Hara Y: Green Tea: Health Benefits and
Applications . Marcel Dekker; New York, Basel: 2001, View Article : Google Scholar
|
|
32.
|
Kawakami S, Kageyama Y, Fujii Y, Kihara K
and Oshima H: Inhibitory effects of N-acetyl cysteine on invasion
and MMP 9 production of T24 human bladder cancer cells. Anticancer
Res. 21:213–219. 2001.PubMed/NCBI
|
|
33.
|
Morini M, Cai T, Aluigi MG, Noonan DM,
Masiello L, De Floro S, D'Agostinin F, Albini A and Fassima G: The
role of the thiol N-acetyl cysteine in the prevention of tumor
invasion and angiogenesis. Int J Biol Markers. 14:268–271.
1999.PubMed/NCBI
|
|
34.
|
Yoon SO, Kim MM and Chung AS: Inhibitory
effects of selenite on invasion of HT 1080 tumor cells. J Biol
Chem. 276:20085–20092. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Naidu KA, Karl RC and Coppola D:
Antiproliferative and proapoptotic effect of ascorbyl stearate in
human pancreatic cancer cells: association with decreased
expression of insulin-like growth factor 1 receptor. Dig Dis Sci.
48:230–237. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Anthony HM and Schorah CJ: Severe
hypovitaminosis C in lung-cancer patients: The utilization of
vitamin C in surgical repair and lymphocyte-related host
resistance. Br J Cancer. 46:354–367. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Maramag C, Menon M, Balaji KC, Reddy PG
and Laxmanan S: Effect of vitamin C on prostate cancer cells in
vitro: effect on cell number, viability and DNA synthesis.
Prostate. 32:188–195. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Koh WS, Lee SJ, Lee H, Park C, Park MH,
Kim WS, Yoon SS, Park K, Hong SI, Chung MH and Park CH:
Differential effects and transport kinetics of ascorbate
derivatives in leukemic cell lines. Anticancer Res. 8:2487–2493.
1998.PubMed/NCBI
|
|
39.
|
Chen Q, Espey MG, Krishna MC, Mitchell JB,
Corpe CP, Buettner GR, Shacter E and Levine M: Pharmacologic
ascorbic acid concentrations selectively kill cancer cells: action
as a pro-drug to deliver hydrogen peroxide to tissues. Proc Natl
Acad Sci USA. 102:13604–13609. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40.
|
Nunez C, Ortiz de Apodaca Y and Ruiz A:
Ascorbic acid in the plasma and blood cells of women with breast
cancer. The effect of consumption of food with an elevated content
of this vitamin. Nutr Hosp. 10:368–372. 1995.(In Spanish).
|
|
41.
|
Kurbacher CM, Wagner U, Kolster B,
Andreotti PE, Krebs D and Bruckner HW: Ascorbic acid (vitamin C)
improves the anti neoplastic activity of doxorubicin, cisplatin and
paclitaxel in human breast carcinoma cells in vitro. Cancer Lett.
103:183–189. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
42.
|
Cooke JP and Dzau VJ: Nitric oxide
synthase: Role in the genesis of vascular disease. Annu Rev Med.
48:489–509. 1997. View Article : Google Scholar : PubMed/NCBI
|