Introduction
Radiotherapy is used in >50% of patients during
the course of cancer treatment both as a curative modality and for
palliation (1). However,
radioresistance is a major obstacle to the success of radiation
therapy and contributes significantly to tumor relapse and
treatment failure. Therefore, there is a critical need for the
development of novel radiosensitizers that can be used clinically
to overcome tumor radioresistance and thus improve the efficacy of
radiotherapy. Resveratrol (RV) is a small molecule natural
component of grapes and red wine that has been shown to be a
potential anticancer and chemopreventive agent (2–5).
Interestingly, recent studies have indicated that treatment with RV
can sensitize tumor cells to chemotherapeutic agents and IR induced
cell death (6–9). However, the mechanisms by which RV
increases the radiation sensitivity of cancer cells remain to be
determined.
Cellular senescence is characterized as an
irreversible cell cycle arrest that can be triggered by many types
of intrinsic and extrinsic stresses, including IR (10–12).
Senescence limits the life span and proliferative capacity of
cells, therefore the induction of senescence is regarded as an
important mechanism of cancer prevention (13,14).
Notably, it has been suggested that therapy-induced senescence is
an important mechanism through which many anticancer agents
including IR suppresses tumor cell growth (15). Moreover, our recent studies have
revealed that IR-induced tumor cell killing is largely attributed
to the induction of senescence but not apoptosis in lung cancer
cells, suggesting that the induction of premature senescence may
play a pivotal role in mediating the anticancer effects of
radiation therapy (16).
Furthermore, we and others have demonstrated that RV is a potent
small molecule inducer of cellular senescence (17,18).
However, it is largely unknown if and how RV treatment may affect
IR-induced premature senescence in tumor cells. Therefore, the goal
of this study was to determine if RV treatment could sensitize lung
cancer cells to radiotherapy by increasing IR-induced premature
senescence. Here, we show that RV treatment enhances IR-induced
cell killing in NSCLC cells through an apoptosis-independent
mechanism. Our subsequent investigations further demonstrate that
the radiosensitizing effect of RV is associated with increased
DNA-DSBs and SA-β-gal staining in irradiated NSCLC cells,
suggesting that RV treatment may sensitize lung cancer cells to
radiotherapy by enhancing IR-induced senescence.
Materials and methods
Reagents
Resveratrol (trans-3,4′,5-trihydroxystilbene) and
all other chemicals were purchased from Sigma (St. Louis, MO, USA).
Dulbecco’s modified Eagle’s medium (DMEM) and other culture media
were obtained from Invitrogen (Carlsbad, CA, USA). Rabbit
anti-human phospho-p53, phospho-Akt, phospho-chk2 and phospho-mTOR
monoclonal antibodies along with total Akt, chk2 and mTOR
antibodies were purchased from Cell Signaling (Danvers, MA, USA).
Mouse anti-human p53 and p21 monoclonal antibodies were obtained
from Santa Cruz Biotechnology. Monoclonal β-actin antibody was
purchased from Sigma. Senescence-associated β-galactosidase
(SA-β-gal) staining kit was purchased from Cell Signaling.
Cell lines and culture
Human non-small cell lung cancer (NSCLC) cell lines
A549 and H460 were purchased from American Type Culture Collection.
A549 cells were cultured in DMEM medium containing 10% FBS, 2 mM
L-glutamine and 100 μg/ml of penicillin-streptomycin (Invitrogen).
H460 cells were grown in RPMI-1640 medium containing 10% FBS, 2 mM
L-glutamine and 100 μg/ml of penicillin-streptomycin
(Invitrogen).
Clonogenic survival assay
Clonogenic assays were performed to determine the
effects of RV treatment on IR-induced cell death in NSCLC cells.
Briefly, cells were seeded in 60-mm dishes at appropriate densities
in triplicate and exposed to different doses (0–8 Gy) of
irradiation at 4 h after RV pre-treatment using a 137Cs
irradiator (J.L. Shepherd and Associates, Glendale, CA, USA) at a
rate of 2.1 Gy/min. Twenty-four hours after irradiation, culture
media were replaced with fresh complete media to remove drug and
followed by incubation at 37°C for 8–12 days to allow the formation
of colonies. Colonies were fixed and stained with 0.5% crystal
violet (Sigma) in methanol for 30 min. The number of colonies (≥50
cells) was scored using a microscope. The surviving fraction was
calculated as the ratio of the plating efficiency of the treated
cells to that of control cells. The dose enhancement ratio (DER)
was calculated as the dose (Gy) of radiation that yielded a
surviving fraction of 0.3 for control divided by that for the RV
treated cells.
Senescence-associated β-galactosidase
(SA-β-gal) staining
In situ staining of SA-β-gal was performed to
determine the senescent cells in irradiated NSCLC cells using a
senescence β-galactosidase staining kit (Cell Signaling) as we
previously reported (18,19).
Comet assay
Neutral comet assay was employed to determine
DNA-DSBs in irradiated NSCLC cells by using a Comet
Assay® kit (Trevigen, Gaithersburg, MD, USA) according
to the manufacturer’s instructions. Briefly, cells were mixed with
Comet Assay™ low-melting agarose at a ratio of 1:10 (v/v) and
spread evenly on slides. The cells were treated with CometAssay
lysis solution at 4°C for 1 h, submerged in cold neutral
electrophoresis buffer and subjected to electrophoresis at 21 V for
30 min. The cells were stained with SYBR® Green I and
viewed using a Zeiss Axio Observer Z1 microscope. The images were
captured and processed using the AxioVision (4.7.1.0) software
(Carl Zeiss). The percentage of DNA tail moment were evaluated with
the TriTek Comet ScoreTM software (Version 1.5.2.6;
TriTek Corp., VA, USA).
Western blot analysis
Protein samples were extracted using cell lysis
buffer (Cell Signaling) supplemented with a cocktail of proteinase
inhibitors (Sigma). The protein concentrations were quantified
using the Bio-Rad Dc protein assay kit (Bio-Rad Laboratories,
Hercules, CA, USA). Western blot analysis was performed as
previously described (18).
Briefly, 50 μg of protein samples were resolved on 10% Mini-Protean
TGX gels (Bio-Rad) and transferred onto 0.2 μm PVDF membrane
(Millipore). Blots were blocked with 5% non-fat milk for 1-2 h at
room temperature and then probed with primary antibodies and
incubated at 4°C overnight. After extensive washing with TBS-T,
blots were incubated with appropriate HRP-conjugated secondary
antibody for 1 h at room temperature. Protein bands were detected
using an ECL Plus Western Blotting Detection System (GE Healthcare
Life Science).
Flow cytometric analysis of ROS
Intracellular levels of ROS were measured by flow
cytometric analysis as we previously reported (20). Briefly, cells were loaded with 5 μM
of 2′,7′-dichlorodihydrofluorescein diacetate (DCF-DA) and
incubated at 37°C for 30 min. The levels of ROS in NSCLC cells were
analyzed by measuring the mean fluorescence intensity (MFI) of DCF
using a FACSCalibur flow cytometer (Becton-Dickinson, San Jose, CA,
USA).
Statistical analysis
All experiments were repeated independently at least
three times. Paired comparisons were carried out using Student’s
t-test. Multiple group comparisons were performed using analysis of
variance (ANOVA). Differences were considered statistically
significant at p<0.05. All analyses were carried out with the
GraphPad Prism program (GraphPad Software, Inc. San Diego, CA,
USA).
Results
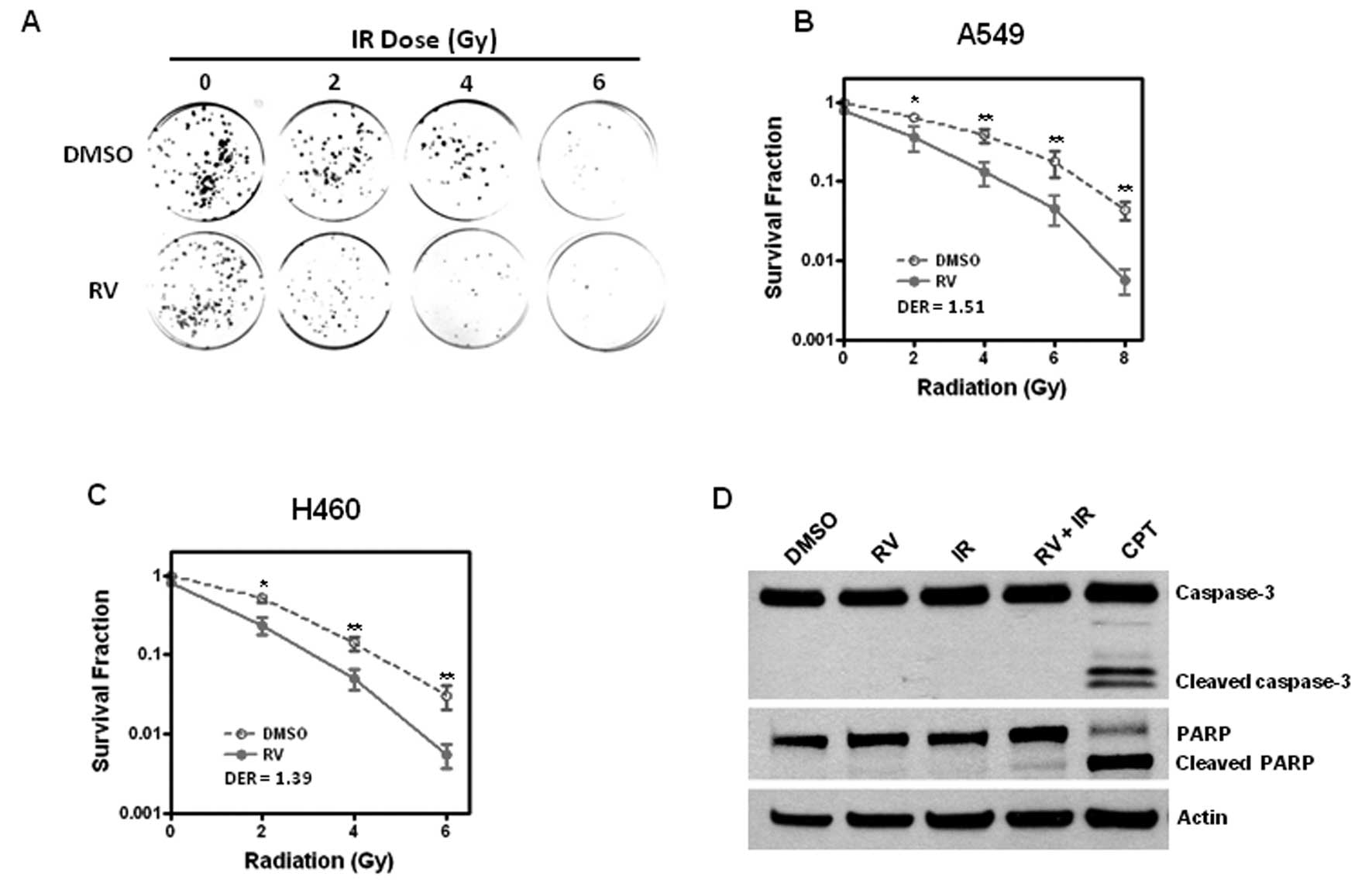
RV enhances IR-induced cell killing in
lung cancer cells via an apoptosis-independent mechanism
Previous studies showed that RV treatment increased
the sensitivity of tumor cells to chemotherapy and IR induced cell
death (6–9). Here, we sought to investigate whether
RV treatment could sensitize NSCLC cells to IR-induced cell
killing. To this end, A549 and H460 cells were pre-incubated with
RV (20 μM) or DMSO as a vehicle control for 4 h prior to exposure
to different doses of IR treatment. Then clonogenic assays were
performed to determine if RV treatment has any impact on IR-induced
tumor cell killing. The results show that preincubation with RV
significantly enhances the cell killing effects of IR with a DER of
1.51 for A549 cells and 1.39 for H460 cells (Fig. 1A–C), suggesting that RV is a
potential radiosensitizer that can increase the sensitivity of lung
cancer cells to IR-induced cell killing.
To further explore the mechanisms by which RV
increases the radiation sensitivity of lung cancer cells, we
investigated whether RV could promote IR-induced apoptosis. Because
activated caspase-3 and cleaved PARP are well-documented
measurements of apoptosis (21,22),
we examined whether RV treatment affects the expression of
activated caspase-3 and cleaved PARP in irradiated NSCLC cells.
Western blot analyses show that RV treatment has no significant
effect on the expression of cleaved PARP and activated caspase-3 in
H460 cells regardless of IR treatment. In contrast, camptothecin
(CPT) treatment results in a pronounced increase in the expression
levels of cleaved PARP and activated caspase-3 (Fig. 1D). These results demonstrate for
the first time that RV enhances IR-induced tumor cell killing via
an apoptosis-independent mechanism.
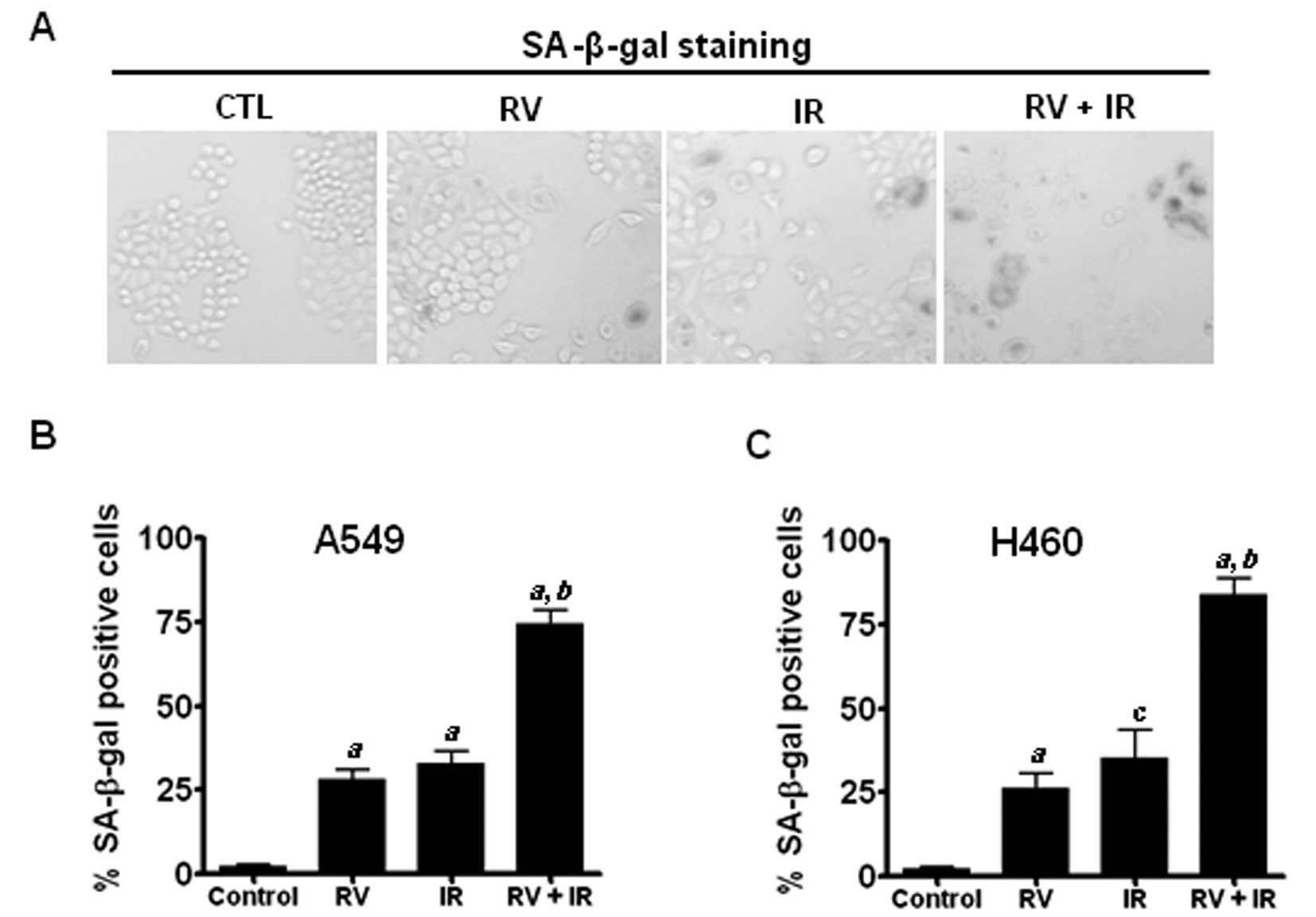
RV promotes IR-induced premature
senescence in lung cancer cells
Our recent studies have shown that IR induces
premature senescence in lung cancer cells in a dose-dependent
manner, suggesting that the induction of senescence plays an
important role in IR-induced tumor suppression (16). However, it remains to be determined
whether RV radiosensitizes lung cancer cells by augmenting
IR-induced premature senescence. To this end, SA-β-gal staining was
employed to detect senescent cells in irradiated A549 and H460
cells with or without RV treatment. The results show that RV and IR
combined treatment induces more SA-β-gal positive senescent cells
than either RV or IR treatment alone in NSCLC cells (Fig. 2). These results suggest that RV may
radiosensitize lung cancer cells by enhancing IR-induced premature
senescence.
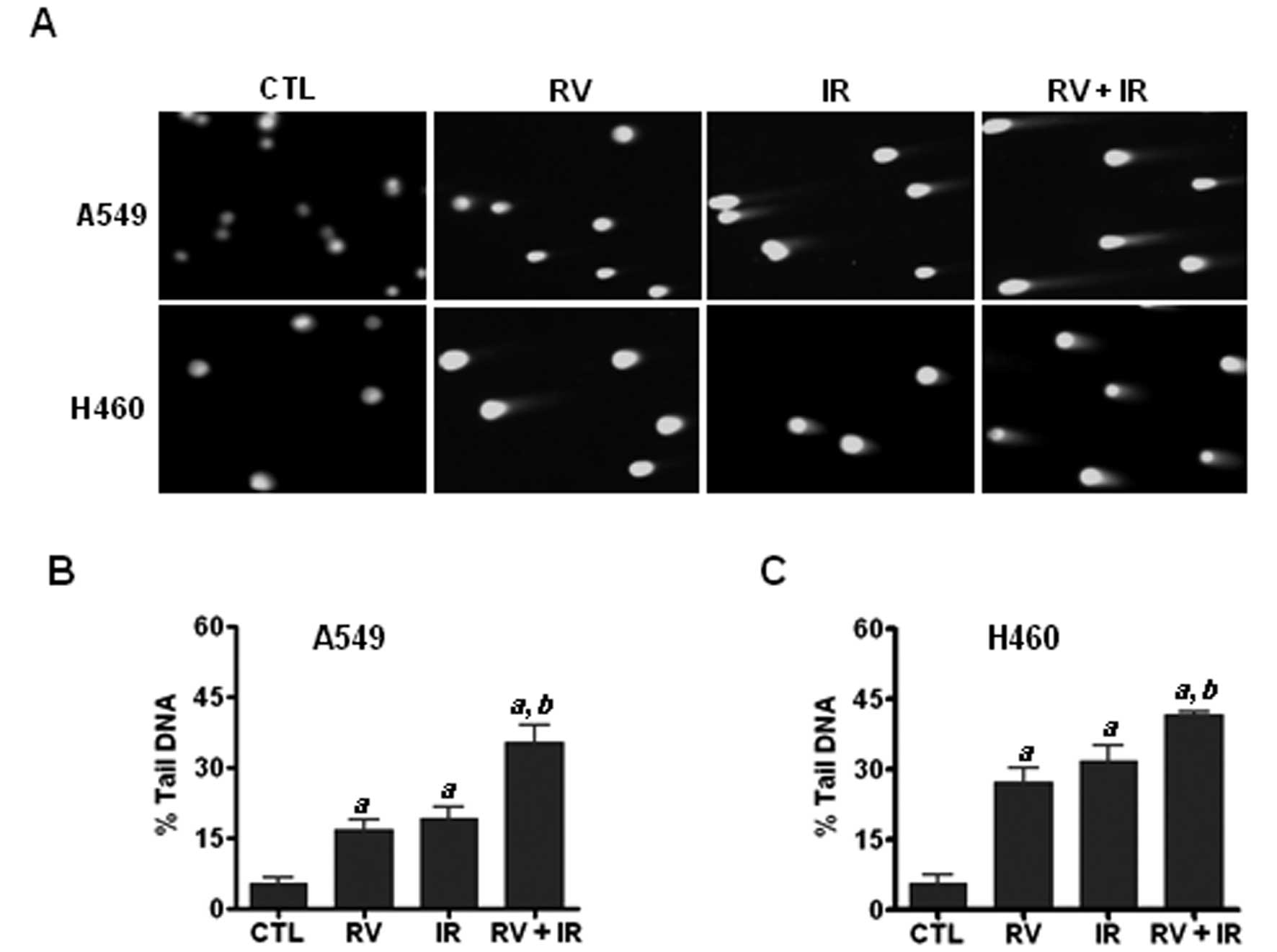
RV treatment increases IR-induced
DNA-DSBs in NSCLC cells
Next we asked the question as to how RV enhances
IR-induced premature senescence in lung cancer cells. Given that
DNA damage is a major cause underlying chemotherapy and ionizing
radiation induced premature senescence (18,20,23),
we hypothesized that RV treatment may enhance IR-induced senescence
via increasing DNA damage in irradiated NSCLC cells. To test this
hypothesis, we performed neutral comet assays to measure DNA-DSBs
in irradiated NSCLC cells with or without RV treatment. The results
demonstrate that RV and IR combined treatment results in more
DNA-DSBs than either IR or RV treatment alone (Fig. 3). These results strongly support
the hypothesis that RV treatment may promote the induction of
senescence in irradiated lung cancer cells by increasing IR-induced
DNA damage.
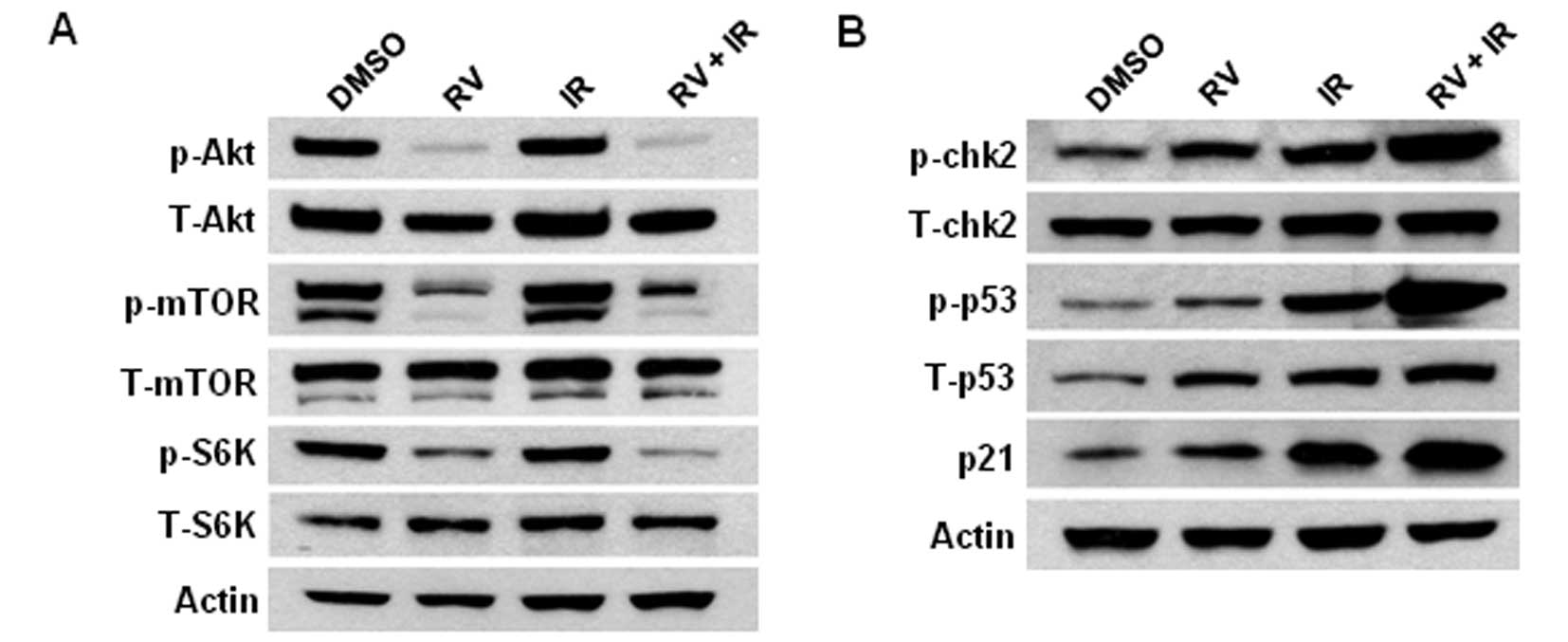
RV inhibits the phosphorylation of Akt
and mTOR in lung cancer cells
It has been shown that the inhibition of Akt
activity sensitizes tumor cells to anticancer therapy (24). However, it has yet to be determined
if RV treatment affects the activities of Akt and mTOR in NSCLC
cells. To address this issue, western blot analyses were performed
to determine the expression levels of phosphorylated Akt (p-Akt,
Ser473) and phosphorylated mTOR (p-mTOR, Ser2448) in lung cancer
cells. The results show that RV treatment significant inhibits the
expression levels of p-Akt and p-mTOR in irradiated H460 lung
cancer cells (Fig. 4A). Moreover,
our studies also show that RV treatment results in a significant
decline in the phosphorylation of p70 S6 kinase (p-S6K, T389), a
downstream target of mTOR. Furthermore, we show that RV and IR
combined treatment leads to marked increases of phosphorylated p53
(p-p53, Ser15) and phosphorylated chk2 (p-chk2, T78) levels in
irradiated NSCLC cells than in cells treated with IR or RV alone
(Fig. 4B). Because phosphorylation
of p53 and chk2 are important biomarkers of DNA damage, these
results further confirm that RV treatment enhances IR-induced DNA
damage in NSCLC cells.
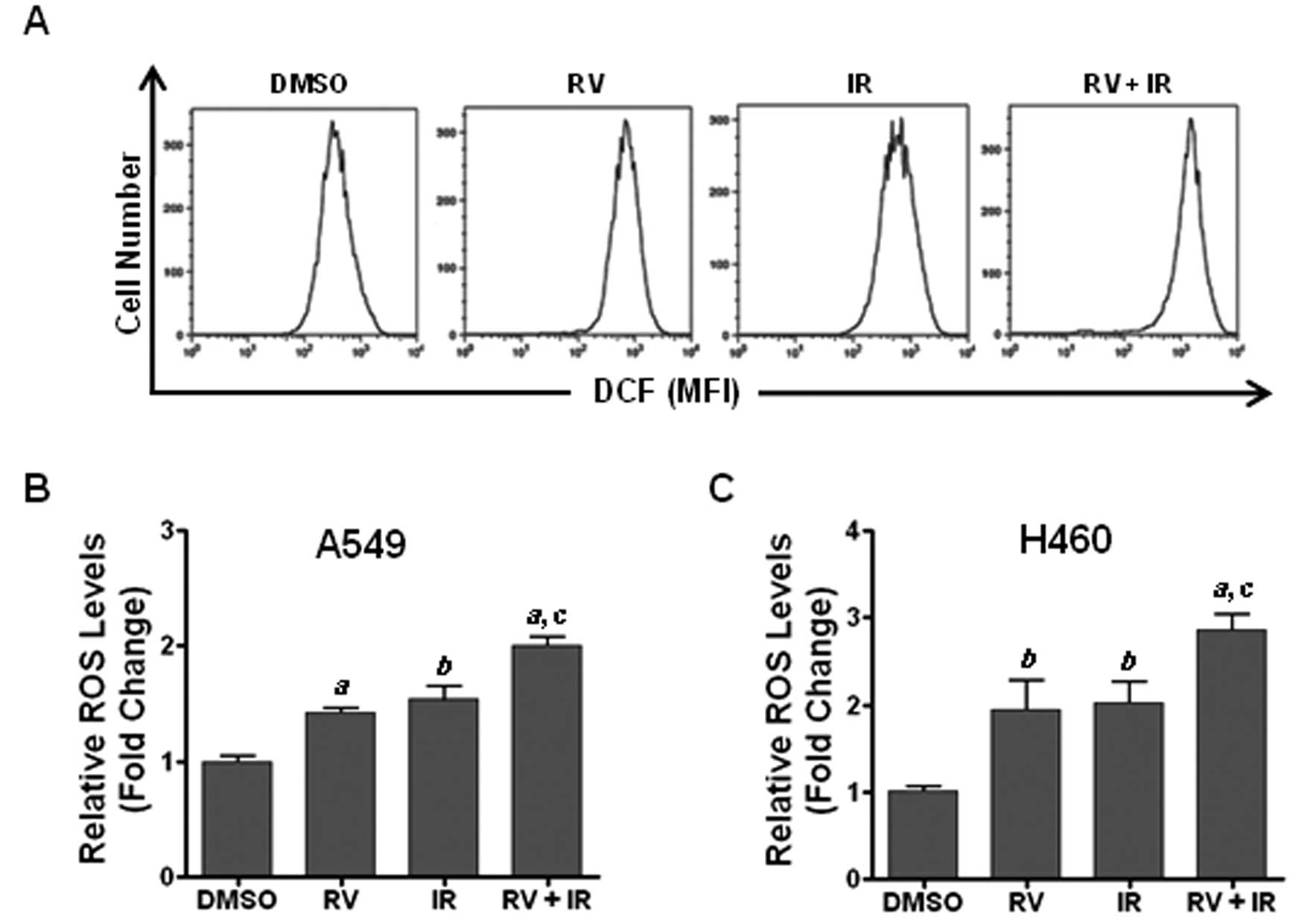
RV treatment increases ROS production in
irradiated lung cancer cells
Previous studies have shown that ROS play a critical
role in modulating genotoxic stress-induced DNA damage and that DNA
damage is able to induce premature senescence in tumor cells
(16,20,23).
However, it remains to be determined whether the generation of ROS
is involved in mediating the radiosensitization effect of RV. To
address this question, we examined the levels of ROS in lung cancer
cells using DCF-DA staining along with flow cytometric analyses as
previously described (20). The
results show that RV treatment markedly increases ROS production in
irradiated lung cancer cells compared with those cells treated with
RV or IR alone (Fig. 5). These
results suggest that increasing of ROS production may play a
pivotal role in RV-induced radiosensitization.
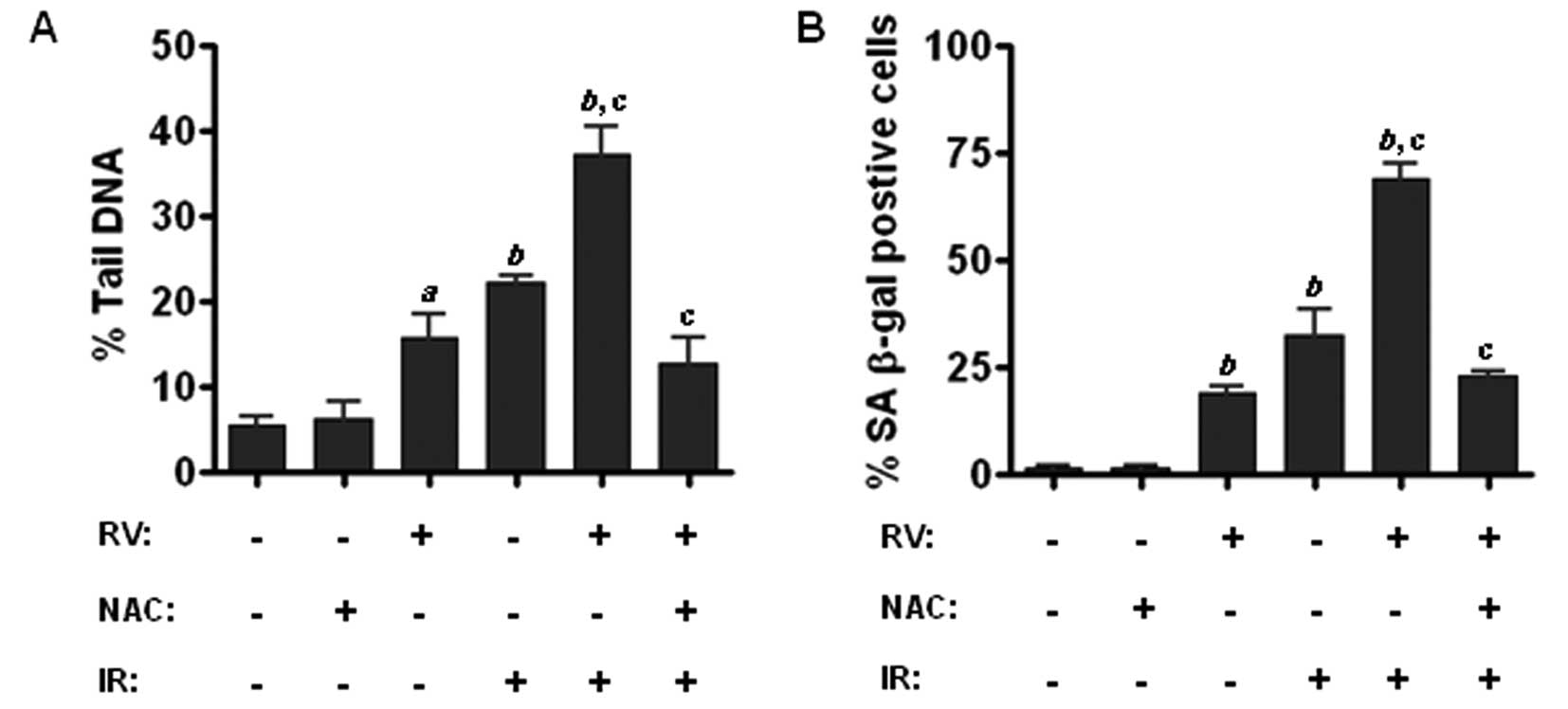
Inhibition of ROS by NAC attenuates the
radiosensitizing effect of RV in lung cancer cells
To determine the role of ROS in RV-mediated
radiosensitization, we sought to examine whether inhibition of ROS
production by antioxidant NAC has any impact on RV-mediated
enhancement of IR-induced DNA damage and premature senescence in
lung cancer cells. Because comet assay is a well characterized
approach to measure DNA-DSBs, we performed comet assays and found
that RV and IR combined treatment results in more DNA damage in
lung cancer cells than either IR or RV treatment alone (Fig. 6A). More importantly, our data
further demonstrate that preincubation with NAC attenuates the
enhancement of RV on IR-induced DNA-DSBs (Fig. 6A). Furthermore, SA-β-gal staining
assays reveal that inhibition of ROS production by NAC markedly
diminishes the enhancement effect of RV on IR-induced senescence in
lung cancer cells (Fig. 6B).
Together, these findings suggest that RV treatment may enhance
IR-induced premature senescence via increasing ROS-mediated DNA
damage in lung cancer cells.
Discussion
Because of the high cost, debilitating side effects,
and therapeutic limitations of conventional chemotherapy and
radiotherapy, it is estimated that ∼40% of Americans use
complementary and alternative medicine (CAM), including herbal
medicine and natural products (NPs), for cancer prevention and
treatment (4). The use of NPs as
antitumor agents for the management of human cancers is an
attractive idea because they are readily available and exhibit
little or no toxicity. RV is such an NP that has been shown to
exhibit both anticancer and chemopreventive potentials (4,25). A
number of previous studies indicated that RV treatment may
sensitize tumor cells to chemotherapeutic agents and radiation
induced cell death (6–9). However, the mechanisms whereby RV
sensitizes tumor cells to radiotherapy are poorly understood. In
this report, we provide evidence demonstrating that RV treatment
markedly increases DNA-DSBs and SA-β-gal staining in irradiated
NSCLC cells, suggesting that RV may sensitize lung cancer cells to
radiotherapy via enhancing IR-induced premature senescence.
It has been well documented that the induction of
senescence is an important alternative mechanism underlying chemo-
and radiotherapy-induced tumor suppression (11,12,15,16,18).
In contrast to most of the previous studies in which high
concentrations of RV were used to induce tumor cell apoptosis
(26,27), in this study we utilized a
relatively lower dose of RV to sensitize cancer cells to IR-induced
senescence. The advantage of using low concentration of RV is that
it could be clinically achievable (28,29).
Notably, therapy-induced senescence can be achieved at much lower
doses of chemotherapy than those required to induce apoptosis
(15,18). Compared to the traditional
apoptosis inducing strategies, this low dose approach may
significantly reduce the side effects of cancer therapy and thus
improve the quality of life for cancer patients.
Radioresistance is a major obstacle to successful
treatment in lung cancer radiotherapy (30–32).
The use of radiosensitizer holds the promise of overcoming
radioresistance and thus improving treatment outcomes. A variety of
kinase inhibitors such as Akt, mTOR, and chk1 inhibitors have been
extensively investigated as potential radiosensitizers (33–37).
However, the clinical benefits of these inhibitors to improve
treatment outcomes are limited. In addition, there are growing
concerns as to the potential toxicity and side effects of these
kinase inhibitors because of their broad biological activities.
Therefore, there is a critical need for the development of novel
and more effective radiosensitizers to meet this challenge. In
contrast to the kinase inhibitors, natural compounds such as RV
have been presumed to be safer than synthetic compounds due to
their presence in diet, wide availability and tolerability
(25,38). In fact, it has been demonstrated in
a phase I study that it is clinically safe to orally take ≤5 g of
RV per day (39). Together, these
studies support a further development of RV as a safer, affordable
and effective radiosensitizer.
We and others have shown that ROS plays a critical
role in mediating chemotherapy- and IR-induced DNA damage and cell
killing (18,20,40,41).
Therefore, we hypothesized that RV treatment may sensitize tumor
cells to IR-induced premature senescence via increasing
ROS-mediated DNA damage. In agreement with this hypothesis, we
found that RV-induced enhancement on IR-induced DNA damage was
associated with a significant increase in ROS generation in
irradiated NSCLC cells (Fig. 5).
Moreover, our studies also show that inhibition of ROS by NAC
attenuates the sensitizing effects of RV on IR-induced DNA damage
and premature senescence in lung cancer cells, suggesting that ROS
may play an important role in mediating the radiosensitizing effect
of RV (Fig. 6). Consistent with
these observations, it was reported that treatment of bladder
cancer cells with buthionine sulphoximine (BSO), a glutathione
synthesis inhibitor, significantly increases ROS production and
enhances cisplatin-induced cytotoxicity (42). These observations support the
concept that modulating oxidative stress in tumor cells could be an
effective therapeutic strategy for cancer treatment. Taken
together, these results demonstrate for the first time that
RV-induced radiosensitization is associated with marked increases
in ROS production, DNA-DSBs, and senescence induction in irradiated
NSCLC cells, suggesting that increasing the IR-induced premature
senescence could be exploited as a novel strategy to sensitize lung
cancer cells to radiotherapy.
Acknowledgements
The authors thank Mr. Richard Peppler
for flow cytometric analyses. This study was supported in part by
NIH grants CA138313, HL106451 and UL1TR000062.
References
|
1.
|
Hall EJ and Wu CS: Radiation-induced
second cancers: the impact of 3D-CRT and IMRT. Int J Radiat Oncol
Biol Phys. 56:83–88. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Gupta SC, Kannappan R, Reuter S, et al:
Chemosensitization of tumors by resveratrol. Ann NY Acad Sci.
1215:150–160. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Sale S, Tunstall RG, Ruparelia KC, et al:
Comparison of the effects of the chemopreventive agent resveratrol
and its synthetic analog trans 3,4,5,4′-tetramethoxystilbene
(DMU-212) on adenoma development in the Apc(Min+) mouse and
cyclooxygenase-2 in human-derived colon cancer cells. Int J Cancer.
115:194–201. 2005.PubMed/NCBI
|
|
4.
|
Gullett NP, Ruhul Amin AR, Bayraktar S, et
al: Cancer prevention with natural compounds. Semin Oncol.
37:258–281. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Bhat KP, Lantvit D, Christov K, et al:
Estrogenic and antiestrogenic properties of resveratrol in mammary
tumor models. Cancer Res. 61:7456–7463. 2001.PubMed/NCBI
|
|
6.
|
Scarlatti F, Sala G, Ricci C, et al:
Resveratrol sensitization of DU145 prostate cancer cells to
ionizing radiation is associated to ceramide increase. Cancer Lett.
253:124–130. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Baatout S, Derradji H, Jacquet P, et al:
Enhanced radiation-induced apoptosis of cancer cell lines after
treatment with resveratrol. Int J Mol Med. 13:895–902.
2004.PubMed/NCBI
|
|
8.
|
Zoberi I, Bradbury CM, Curry HA, et al:
Radiosensitizing and anti-proliferative effects of resveratrol in
two human cervical tumor cell lines. Cancer Lett. 175:165–173.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Rashid A, Liu C, Sanli T, et al:
Resveratrol enhances prostate cancer cell response to ionizing
radiation. Modulation of the AMPK, Akt and mTOR pathways. Radiat
Oncol. 6:1442011. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Wang Y, Scheiber MN, Neumann C, et al:
MicroRNA regulation of ionizing radiation-induced premature
senescence. Int J Radiat Oncol Biol Phys. 81:839–848. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Jones KR, Elmore LW, Jackson-Cook C, et
al: p53-dependent accelerated senescence induced by ionizing
radiation in breast tumor cells. Int J Radiat Biol. 81:445–458.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Schmitt CA: Cellular senescence and cancer
treatment. Biochim Biophys Acta. 1775:5–20. 2007.PubMed/NCBI
|
|
13.
|
Braig M, Lee S, Loddenkemper C, et al:
Oncogene-induced senescence as an initial barrier in lymphoma
development. Nature. 436:660–665. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Guo X, Keyes WM, Papazoglu C, et al: TAp63
induces senescence and suppresses tumorigenesis in vivo. Nat Cell
Biol. 11:1451–1457. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Ewald JA, Desotelle JA, Wilding G and
Jarrard DF: Therapy-induced senescence in cancer. J Natl Cancer
Inst. 102:1536–1546. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Luo H, Yount C, Lang H, et al: Activation
of p53 with Nutlin-3a radiosensitizes lung cancer cells via
enhancing radiation-induced premature senescence. Lung Cancer.
81:167–173. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Rusin M, Zajkowicz A and Butkiewicz D:
Resveratrol induces senescence-like growth inhibition of U-2 OS
cells associated with the instability of telomeric DNA and
upregulation of BRCA1. Mech Ageing Dev. 130:528–537. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Luo H, Yang A, Schulte BA, et al:
Resveratrol induces premature senescence in lung cancer cells via
ROS-mediated DNA damage. PLoS One. 8:e600652013. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Wang Y, Meng A and Zhou D: Inhibition of
phosphatidylinostol 3-kinase uncouples
H2O2-induced senescent phenotype and cell
cycle arrest in normal human diploid fibroblasts. Exp Cell Res.
298:188–196. 2004.PubMed/NCBI
|
|
20.
|
Wang Y, Liu L, Pazhanisamy SP, et al:
Total body irradiation selectively induces persistent oxidative
stress in murine hematopoietic stem cells. Free Radic Biol Med.
48:348–356. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Nicholson DW, Ali A, Thornberry NA, et al:
Identification and inhibition of the ICE/CED-3 protease necessary
for mammalian apoptosis. Nature. 376:37–43. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Lazebnik YA, Kaufmann SH, Desnoyers S, et
al: Cleavage of poly (ADP-ribose) polymerase by a proteinase with
properties like ICE. Nature. 371:346–347. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
te Poele RH, Okorokov AL, Jardine L, et
al: DNA damage is able to induce senescence in tumor cells in vitro
and in vivo. Cancer Res. 62:1876–1883. 2002.PubMed/NCBI
|
|
24.
|
Kao GD, Jiang Z, Fernandes AM, et al:
Inhibition of phosphatidylinositol-3-OH kinase/Akt signaling
impairs DNA repair in glioblastoma cells following ionizing
radiation. J Biol Chem. 282:21206–21212. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Baur JA and Sinclair DA: Therapeutic
potential of resveratrol: the in vivo evidence. Nat Rev Drug
Discov. 5:493–506. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Jiang H, Zhang L, Kuo J, et al:
Resveratrol-induced apoptotic death in human U251 glioma cells. Mol
Cancer Ther. 4:554–561. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Cui J, Sun R, Yu Y, et al:
Antiproliferative effect of resveratrol in pancreatic cancer cells.
Phytother Res. 24:1637–1644. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Scott E, Steward WP, Gescher AJ and Brown
K: Resveratrol in human cancer chemoprevention - choosing the
‘right’ dose. Mol Nutr Food Res. 56:7–13. 2012.
|
|
29.
|
Patel KR, Scott E, Brown VA, et al:
Clinical trials of resveratrol. Ann NY Acad Sci. 1215:161–169.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Sibley GS, Jamieson TA, Marks LB, et al:
Radiotherapy alone for medically inoperable stage I non-small-cell
lung cancer: the Duke experience. Int J Radiat Oncol Biol Phys.
40:149–154. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Sura S, Yorke E, Jackson A and Rosenzweig
KE: High-dose radiotherapy for the treatment of inoperable
non-small cell lung cancer. Cancer J. 13:238–242. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Rosenzweig KE, Fox JL, Yorke E, et al:
Results of a phase I dose-escalation study using three-dimensional
conformal radiotherapy in the treatment of inoperable nonsmall cell
lung carcinoma. Cancer. 103:2118–2127. 2005. View Article : Google Scholar
|
|
33.
|
Senra JM, Telfer BA, Cherry KE, et al:
Inhibition of PARP-1 by olaparib (AZD2281) increases the
radiosensitivity of a lung tumor xenograft. Mol Cancer Ther.
10:1949–1958. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Yang H, Yoon SJ, Jin J, et al: Inhibition
of checkpoint kinase 1 sensitizes lung cancer brain metastases to
radiotherapy. Biochem Biophys Res Commun. 406:53–58. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Nagata Y, Takahashi A, Ohnishi K, et al:
Effect of rapamycin, an mTOR inhibitor, on radiation sensitivity of
lung cancer cells having different p53 gene status. Int J Oncol.
37:1001–1010. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Calabrese CR, Almassy R, Barton S, et al:
Anticancer chemosensitization and radiosensitization by the novel
poly (ADP-ribose) polymerase-1 inhibitor AG14361. J Natl Cancer
Inst. 96:56–67. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Kim KW, Moretti L, Mitchell LR, et al:
Combined Bcl-2/mammalian target of rapamycin inhibition leads to
enhanced radiosensitization via induction of apoptosis and
autophagy in non-small cell lung tumor xenograft model. Clin Cancer
Res. 15:6096–6105. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Cottart CH, Nivet-Antoine V,
Laguillier-Morizot C and Beaudeux JL: Resveratrol bioavailability
and toxicity in humans. Mol Nutr Food Res. 54:7–16. 2010.
View Article : Google Scholar
|
|
39.
|
Boocock DJ, Faust GE, Patel KR, et al:
Phase I dose escalation pharmacokinetic study in healthy volunteers
of resveratrol, a potential cancer chemopreventive agent. Cancer
Epidemiol Biomarkers Prev. 16:1246–1252. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40.
|
Trachootham D, Zhou Y, Zhang H, et al:
Selective killing of oncogenically transformed cells through a
ROS-mediated mechanism by beta-phenylethyl isothiocyanate. Cancer
Cell. 10:241–252. 2006. View Article : Google Scholar
|
|
41.
|
Trachootham D, Alexandre J and Huang P:
Targeting cancer cells by ROS-mediated mechanisms: a radical
therapeutic approach? Nat Rev Drug Discov. 8:579–591. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
42.
|
Miyajima A, Nakashima J, Yoshioka K, et
al: Role of reactive oxygen species in
cis-dichlorodiammineplatinum-induced cytotoxicity on bladder cancer
cells. Br J Cancer. 76:206–210. 1997. View Article : Google Scholar : PubMed/NCBI
|