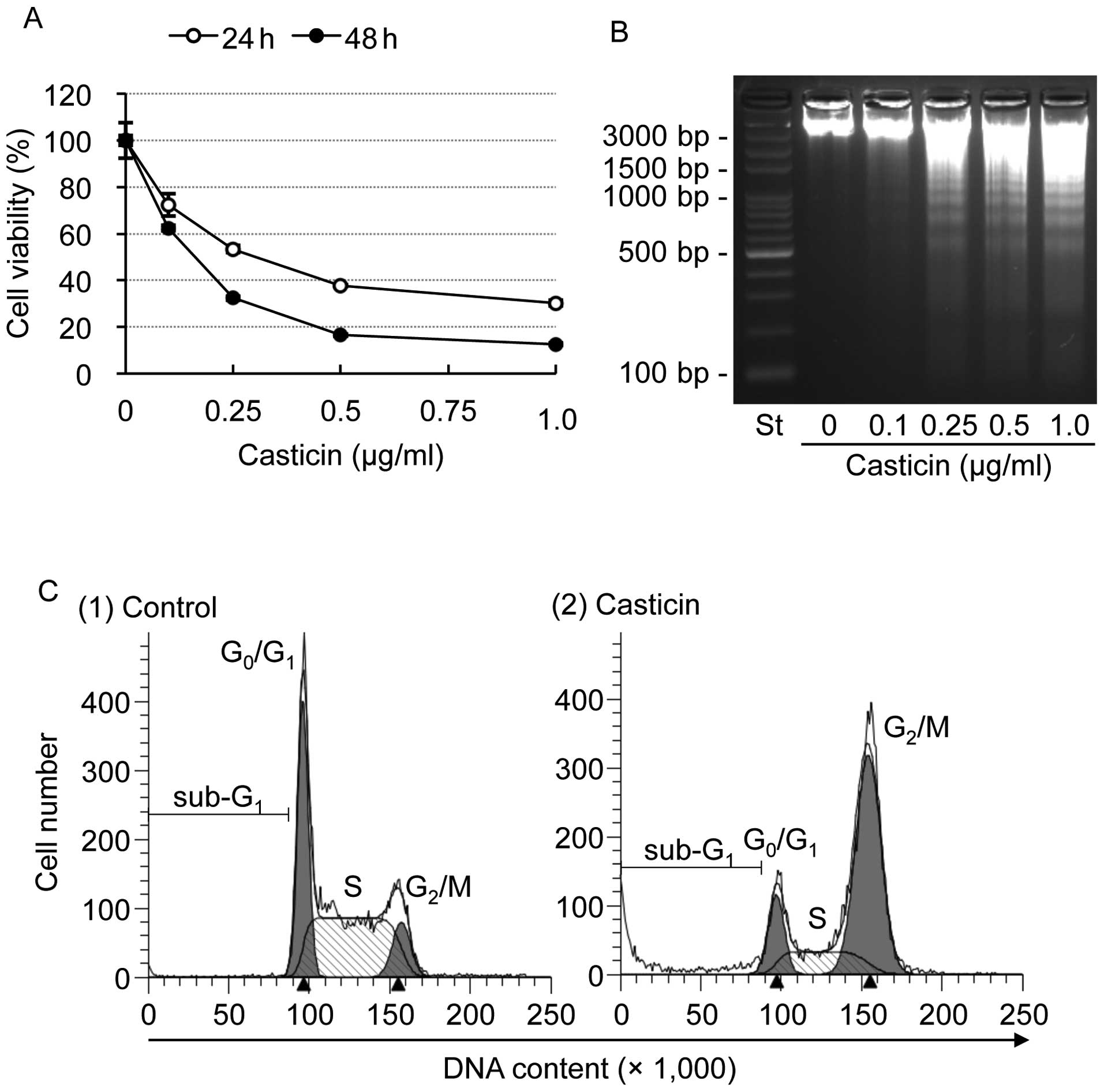
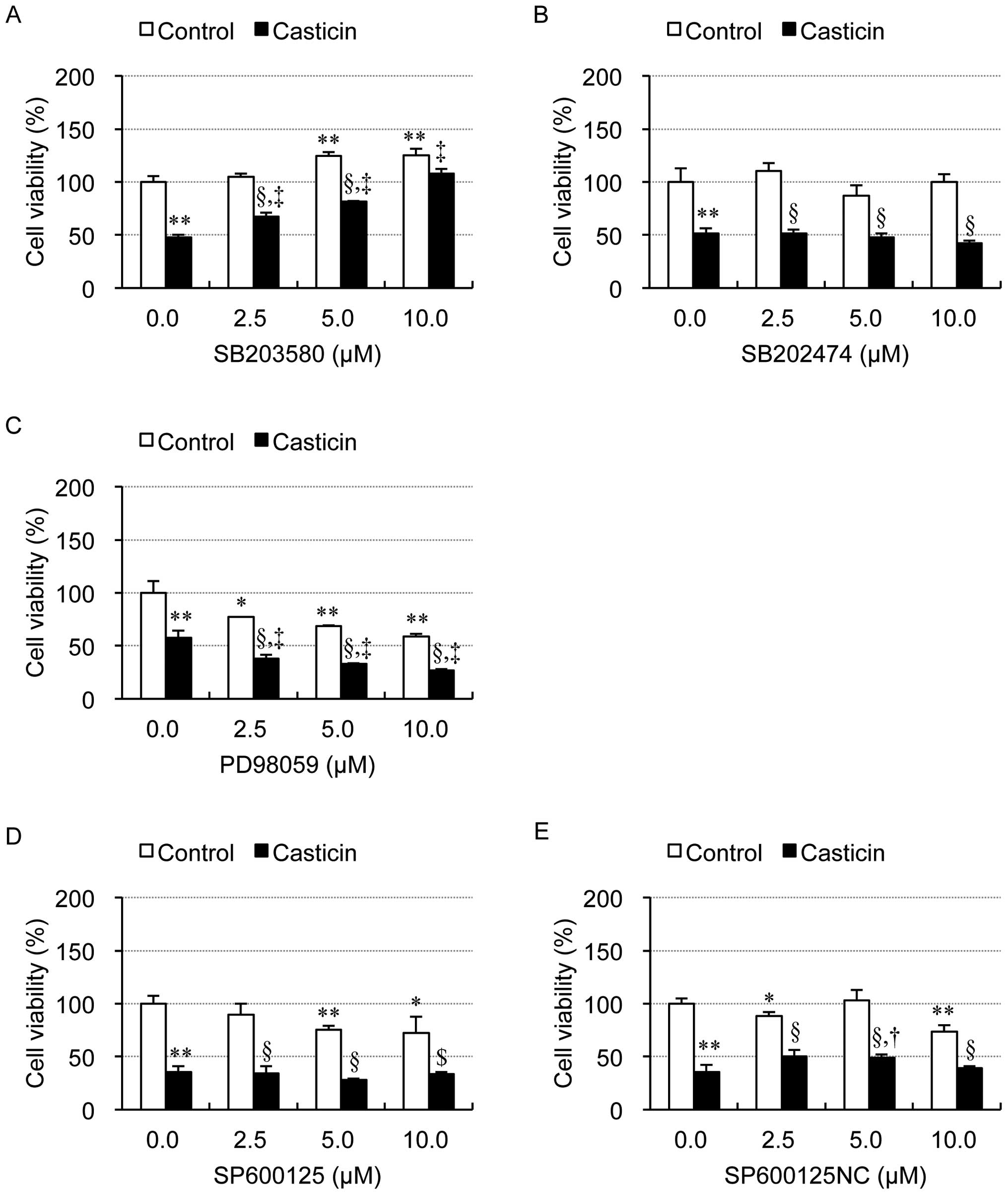
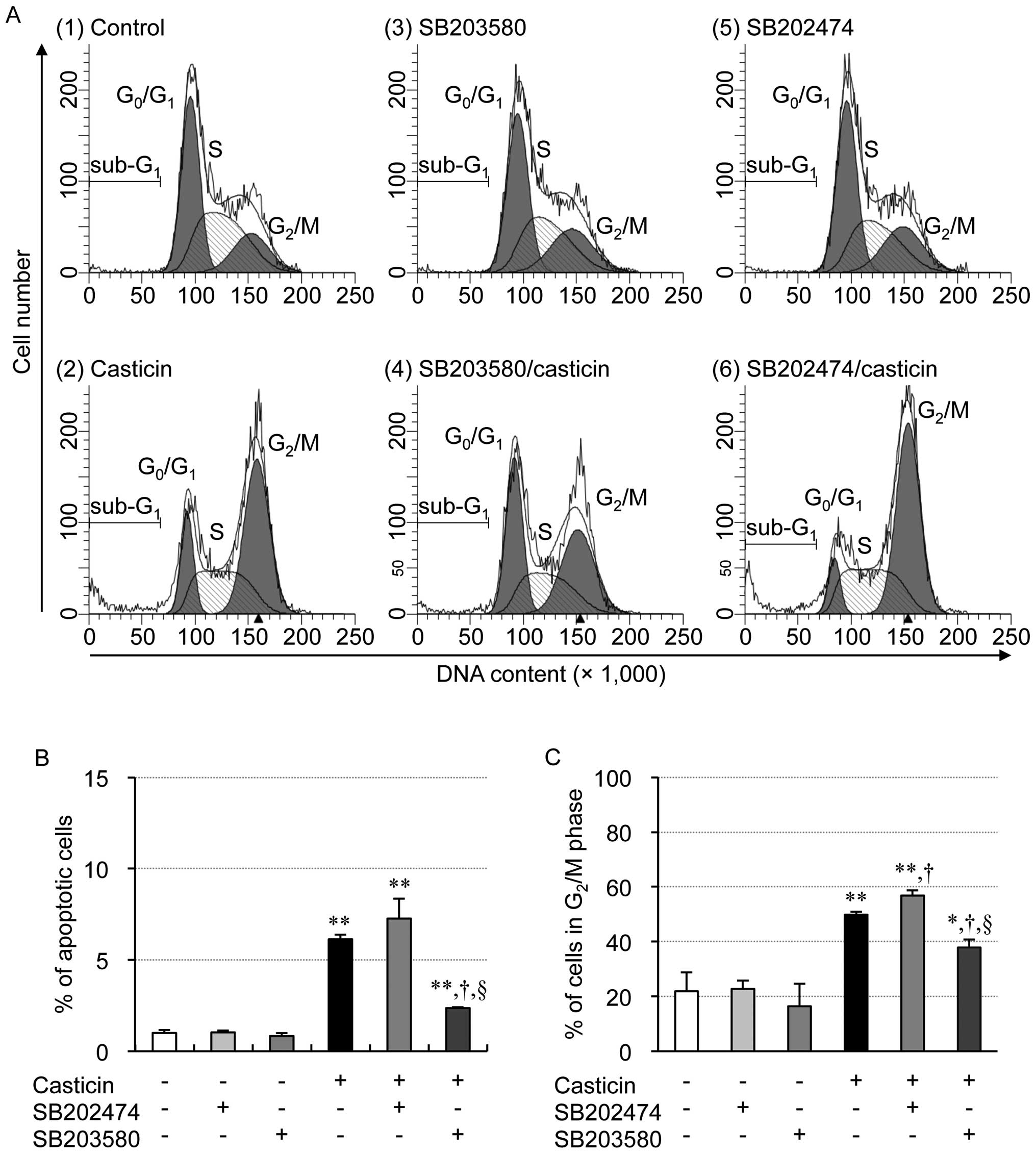
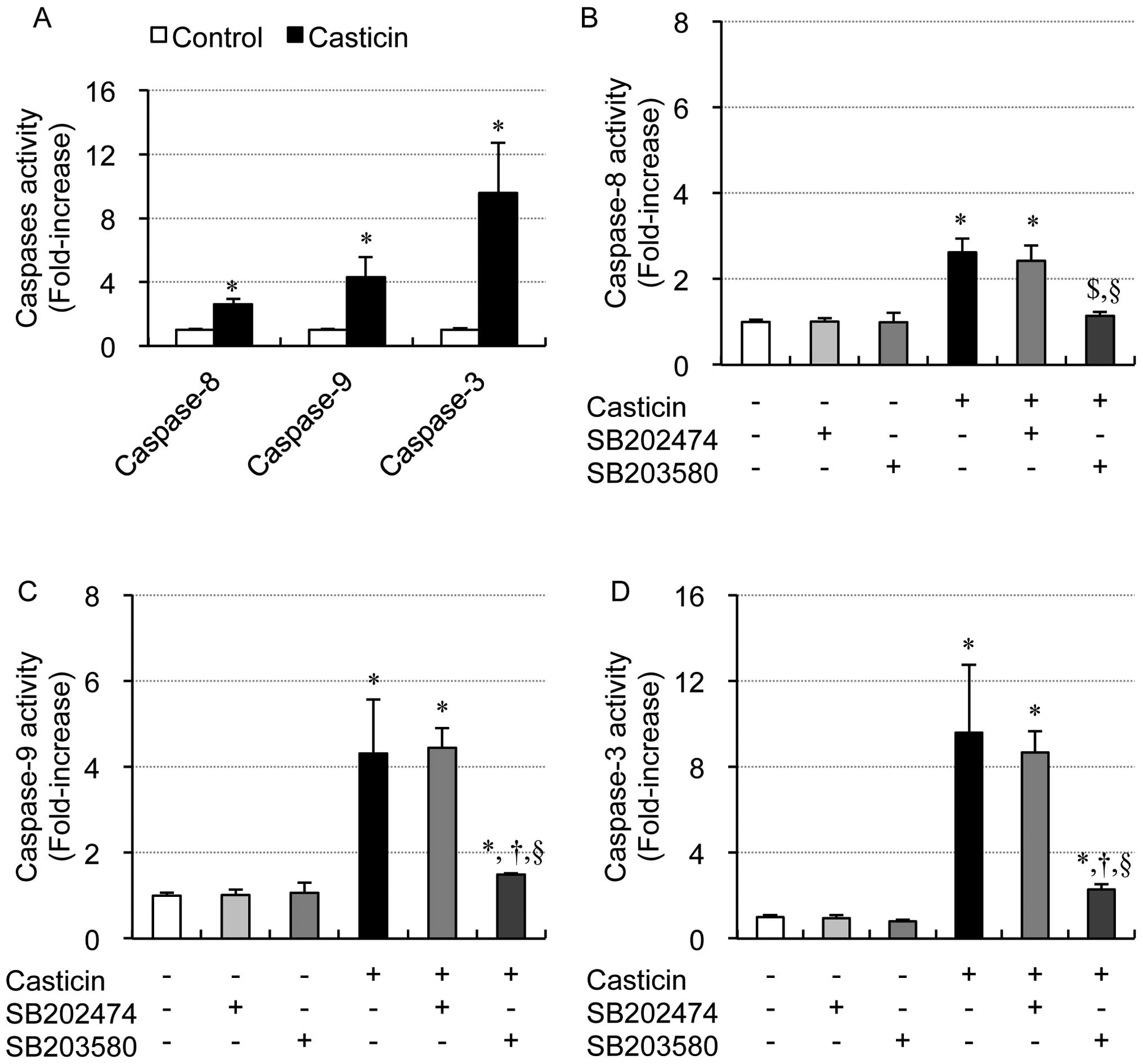
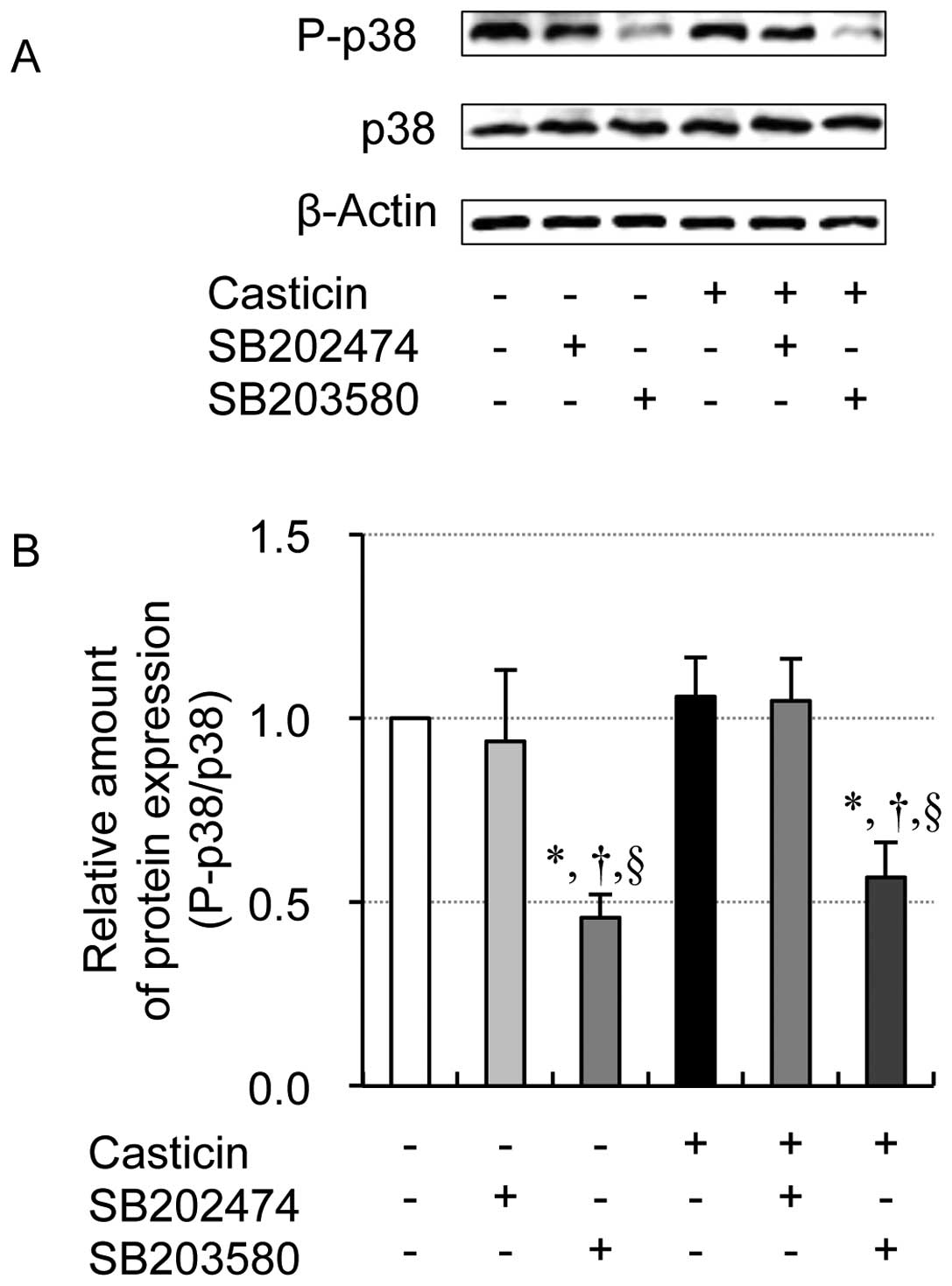
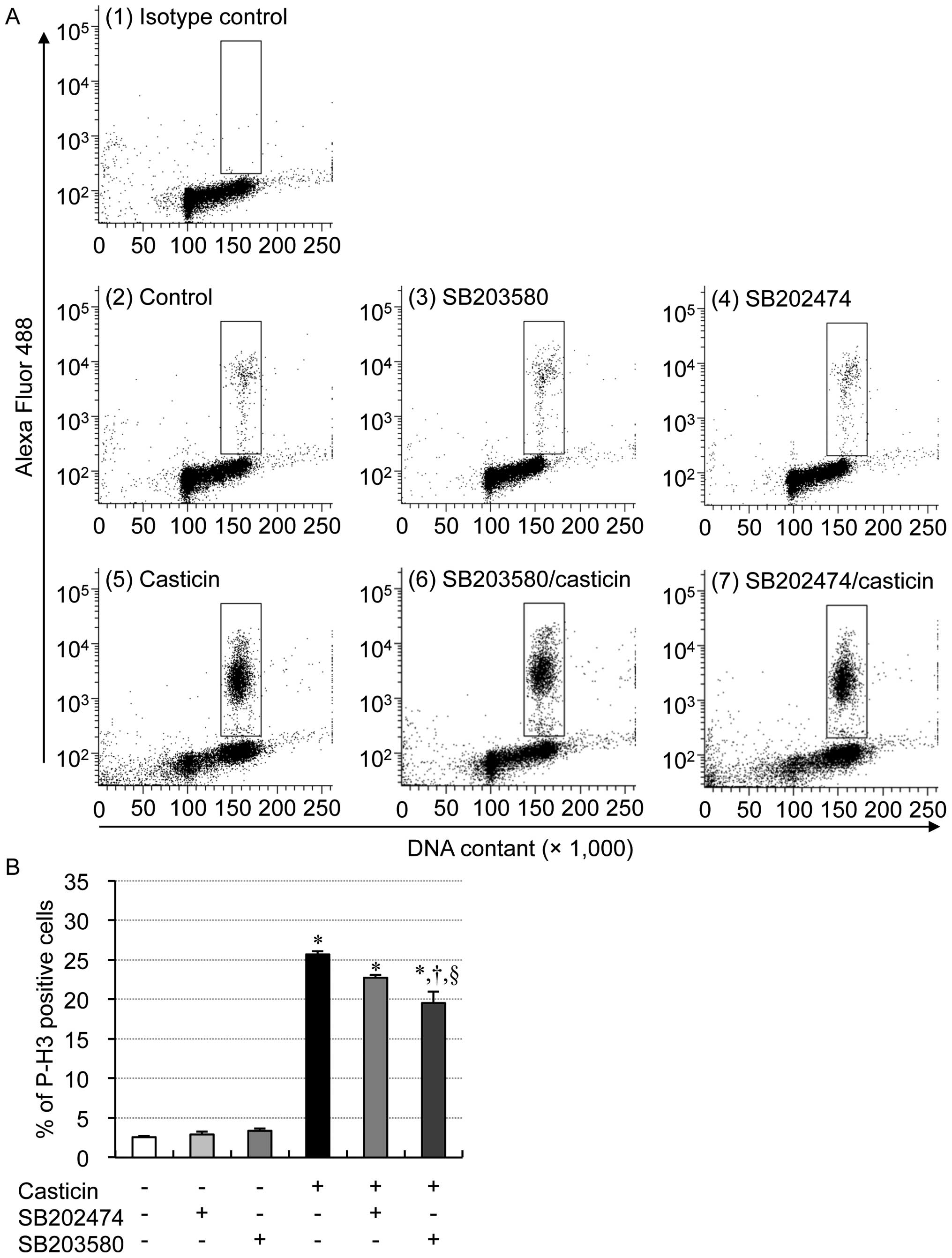
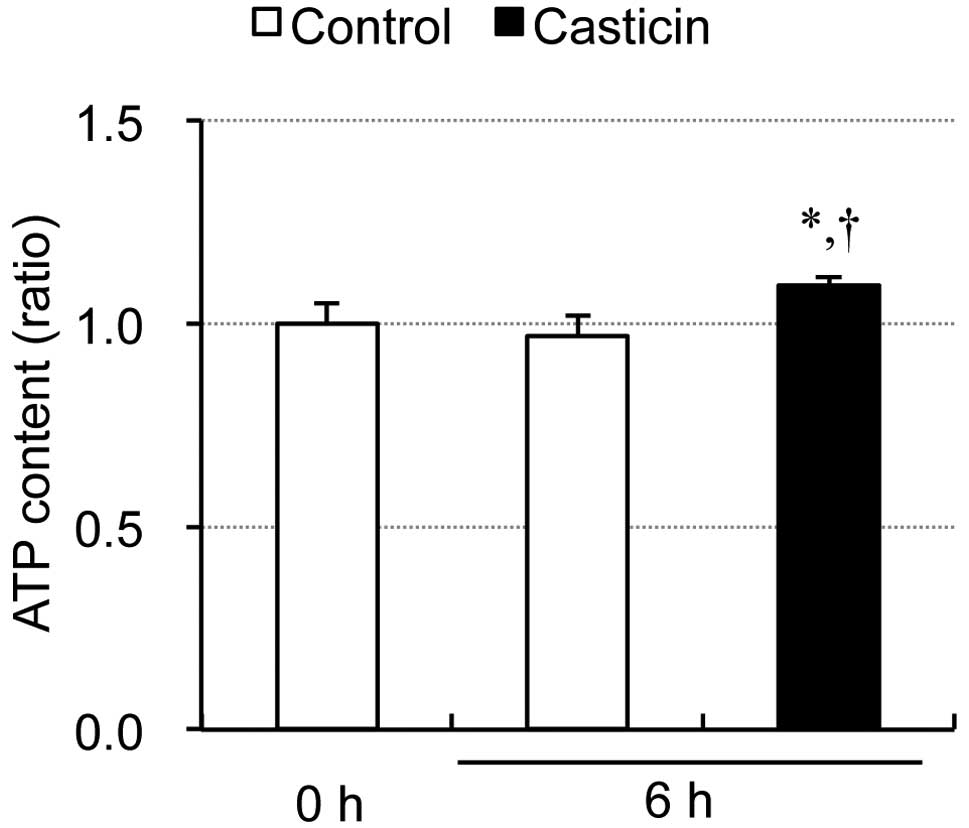
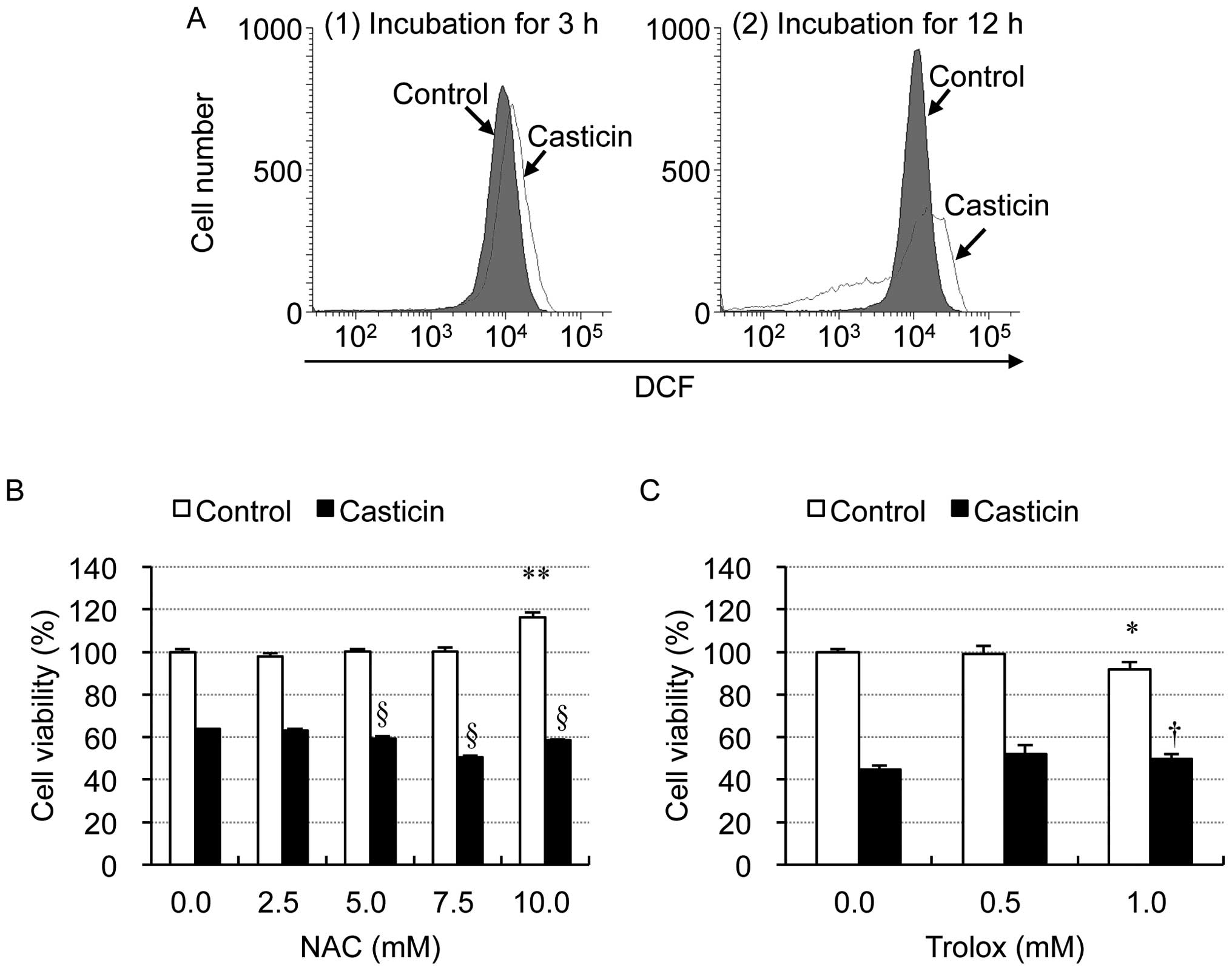
|
1.
|
Indran IR, Tufo G, Pervaiz S and Brenner
C: Recent advances in apoptosis, mitochondria and drug resistance
in cancer cells. Biochim Biophys Acta. 1807:735–745. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Yuan B, Yoshino Y, Kaise T and Toyoda H:
Application of arsenic trioxide therapy for patients with leukemia.
Biological Chemistry of Arsenic, Antimony and Bismuth. Sun H: John
Wiley & Sons Ltd; Chichester: pp. 263–292. 2011
|
|
3.
|
Wang ZY and Chen Z: Acute promyelocytic
leukemia: from highly fatal to highly curable. Blood.
111:2505–2515. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Ryter SW, Kim HP, Hoetzel A, Park JW,
Nakahira K, Wang X and Choi AM: Mechanisms of cell death in
oxidative stress. Antioxid Redox Signal. 9:49–89. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Kantari C and Walczak H: Caspase-8 and
bid: caught in the act between death receptors and mitochondria.
Biochim Biophys Acta. 1813:558–563. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Viswanath V, Wu Y, Boonplueang R, Chen S,
Stevenson FF, Yantiri F, Yang L, Beal MF and Andersen JK: Caspase-9
activation results in downstream caspase-8 activation and bid
cleavage in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced
Parkinson’s disease. J Neurosci. 21:9519–9528. 2001.PubMed/NCBI
|
|
7.
|
Reddy L, Odhav B and Bhoola KD: Natural
products for cancer prevention: a global perspective. Pharmacol
Ther. 99:1–13. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Cargnello M and Roux PP: Activation and
function of the MAPKs and their substrates, the MAPK-activated
protein kinases. Microbiol Mol Biol Rev. 75:50–83. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Zhuang S, Demirs JT and Kochevar IE: p38
mitogen-activated protein kinase mediates bid cleavage,
mitochondrial dysfunction, and caspase-3 activation during
apoptosis induced by singlet oxygen but not by hydrogen peroxide. J
Biol Chem. 275:25939–25948. 2000. View Article : Google Scholar
|
|
10.
|
Yuan B, Ohyama K, Takeichi M and Toyoda H:
Direct contribution of inducible nitric oxide synthase expression
to apoptosis induction in primary smooth chorion trophoblast cells
of human fetal membrane tissues. Int J Biochem Cell Biol.
41:1062–1069. 2009. View Article : Google Scholar
|
|
11.
|
Lu JJ, Meng LH, Cai YJ, Chen Q, Tong LJ,
Lin LP and Ding J: Dihydroartemisinin induces apoptosis in HL-60
leukemia cells dependent of iron and p38 mitogen-activated protein
kinase activation but independent of reactive oxygen species.
Cancer Biol Ther. 7:1017–1023. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Milella M, Kornblau SM, Estrov Z, Carter
BZ, Lapillonne H, Harris D, Konopleva M, Zhao S, Estey E and
Andreeff M: Therapeutic targeting of the MEK/MAPK signal
transduction module in acute myeloid leukemia. J Clin Invest.
108:851–859. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Yao J, Qian CJ, Ye B, Zhang X and Liang Y:
ERK inhibition enhances TSA-induced gastric cancer cell apoptosis
via NF-κB-dependent and Notch-independent mechanism. Life Sci.
91:186–193. 2012.PubMed/NCBI
|
|
14.
|
Yuan B, Imai M, Kikuchi H, Fukushima S,
Hazama S, Akaike T, Yoshino Y, Ohyama K, Hu X, Pei X and Toyoda H:
Cytocidal effects of polyphenolic compounds, alone or in
combination with, anticancer drugs against cancer cells: Potential
future application of the combinatory therapy. Apoptosis and
Medicine. Ntuli TM: InTech; Rijeka: pp. 155–174. 2012
|
|
15.
|
Imai M, Kikuchi H, Yuan B, Aihara Y,
Mizokuchi A, Ohyama K, Hirobe C and Toyoda H: Enhanced growth
inhibitory effect of 5-fluorouracil in combination with Vitex
agnus-castus fruits extract against a human colon
adenocarcinoma cell line, COLO 201. J Chin Clin Med. 6:14–19.
2011.
|
|
16.
|
Cassileth B, Yeung KS and Gubili J: Herbs
and other botanicals in cancer patient care. Curr Treat Options
Oncol. 9:109–116. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Haïdara K, Zamir L, Shi QW and Batist G:
The flavonoid Casticin has multiple mechanisms of tumor
cytotoxicity action. Cancer Lett. 242:180–190. 2006.PubMed/NCBI
|
|
18.
|
Kobayakawa J, Sato-Nishimori F, Moriyasu M
and Matsukawa Y: G2-M arrest and antimitotic activity mediated by
casticin, a flavonoid isolated from Viticis Fructus
(Vitex rotundifolia Linne fil.). Cancer Lett. 208:59–64.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Chen SN, Friesen JB, Webster D, Nikolic D,
van Breemen RB, Wang ZJ, Fong HH, Farnsworth NR and Pauli GF:
Phytoconstituents from Vitex agnus-castus fruits.
Fitoterapia. 82:528–533. 2011.
|
|
20.
|
Ohyama K, Akaike T, Hirobe C and Yamakawa
T: Cytotoxicity and apoptotic inducibility of Vitex
agnus-castus fruit extract in cultured human normal and cancer
cells and effect on growth. Biol Pharm Bull. 26:10–18.
2003.PubMed/NCBI
|
|
21.
|
Ohyama K, Akaike T, Imai M, Toyoda H,
Hirobe C and Bessho T: Human gastric signet ring carcinoma
(KATO-III) cell apoptosis induced by Vitex
agnus-castus fruit extract through intracellular
oxidative stress. Int J Biochem Cell Biol. 37:1496–1510. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Imai M, Kikuchi H, Denda T, Ohyama K,
Hirobe C and Toyoda H: Cytotoxic effects of flavonoids against a
human colon cancer derived cell line, COLO 201: a potential natural
anti-cancer substance. Cancer Lett. 276:74–80. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Shen JK, Du HP, Yang M, Wang YG and Jin J:
Casticin induces leukemic cell death through apoptosis and mitotic
catastrophe. Ann Hematol. 88:743–752. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Yuan B, Ohyama K, Bessho T, Uchide N and
Toyoda H: Imbalance between ROS production and elimination results
in apoptosis induction in primary smooth chorion trophoblast cells
prepared from human fetal membrane tissues. Life Sci. 82:623–630.
2008. View Article : Google Scholar
|
|
25.
|
Darzynkiewicz Z, Bruno S, Del Bino G,
Gorczyca W, Hotz MA, Lassota P and Traganos F: Features of
apoptotic cells measured by flow cytometry. Cytometry. 13:795–808.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Imai M, Yuan B, Kikuchi H, Saito M, Ohyama
K, Hirobe C, Oshima T, Hosoya T, Morita H and Toyoda H: Growth
inhibition of a human colon carcinoma cell, COLO 201, by a natural
product, Vitex agnus-castus fruits extract, in vivo
and in vitro. Adv Biol Chem. 2:20–28. 2012. View Article : Google Scholar
|
|
27.
|
Ozawa K: Reduction of phosphorylated
histone H3 serine 10 and serine 28 cell cycle marker intensities
after DNA damage. Cytometry A. 73:517–527. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Robinson JP, Bruner LH, Bassoe CF, Hudson
JL, Ward PA and Phan SH: Measurement of intracellular fluorescence
of human monocytes relative to oxidative metabolism. J Leukoc Biol.
43:304–310. 1988.PubMed/NCBI
|
|
29.
|
Uchide N, Ohyama K, Bessho T, Yuan B and
Yamakawa T: Effect of antioxidants on apoptosis induced by
influenza virus infection: inhibition of viral gene replication and
transcription with pyrrolidine dithiocarbamate. Antiviral Res.
56:207–217. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Frantz B, Klatt T, Pang M, Parsons J,
Rolando A, Williams H, Tocci MJ, O’Keefe SJ and O’Neill EA: The
activation state of p38 mitogen-activated protein kinase determines
the efficiency of ATP competition for pyridinylimidazole inhibitor
binding. Biochemistry. 37:13846–13853. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Young PR, McLaughlin MM, Kumar S, Kassis
S, Doyle ML, McNulty D, Gallagher TF, Fisher S, McDonnell PC, Carr
SA, Huddleston MJ, Seibel G, Porter TG, Livi GP, Adams JL and Lee
JC: Pyridinyl imidazole inhibitors of p38 mitogen-activated protein
kinase bind in the ATP site. J Biol Chem. 272:12116–12121. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Kuntzen C, Sonuc N, De Toni EN, Opelz C,
Mucha SR, Gerbes AL and Eichhorst ST: Inhibition of
c-Jun-N-terminal-kinase sensitizes tumor cells to CD95-induced
apoptosis and induces G2/M cell cycle arrest. Cancer Res.
65:6780–6788. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Xia HH, He H, De Wang J, Gu Q, Lin MC, Zou
B, Yu LF, Sun YW, Chan AO, Kung HF and Wong BC: Induction of
apoptosis and cell cycle arrest by a specific c-Jun NH2-terminal
kinase (JNK) inhibitor, SP-600125, in gastrointestinal cancers.
Cancer Lett. 241:268–274. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Zhong S, Goto H, Inagaki M and Dong Z:
Phosphorylation at serine 28 and acetylation at lysine 9 of histone
H3 induced by trichostatin A. Oncogene. 22:5291–5297. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Goto H, Tomono Y, Ajiro K, Kosako H,
Fujita M, Sakurai M, Okawa K, Iwamatsu A, Okigaki T, Takahashi T
and Inagaki M: Identification of a novel phosphorylation site on
histone H3 coupled with mitotic chromosome condensation. J Biol
Chem. 274:25543–25549. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Tikoo K, Lau SS and Monks TJ: Histone H3
phosphorylation is coupled to poly-(ADP-ribosylation) during
reactive oxygen species-induced cell death in renal proximal
tubular epithelial cells. Mol Pharmacol. 60:394–402.
2001.PubMed/NCBI
|
|
37.
|
Waring P, Khan T and Sjaarda A: Apoptosis
induced by gliotoxin is preceded by phosphorylation of histone H3
and enhanced sensitivity of chromatin to nuclease digestion. J Biol
Chem. 272:17929–17936. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Clayton AL and Mahadevan LC: MAP
kinase-mediated phosphoacetylation of histone H3 and inducible gene
regulation. FEBS Lett. 546:51–58. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39.
|
Lee YJ and Shukla SD: Histone H3
phosphorylation at serine 10 and serine 28 is mediated by p38 MAPK
in rat hepatocytes exposed to ethanol and acetaldehyde. Eur J
Pharmacol. 573:29–38. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40.
|
Kumar S, Jiang MS, Adams JL and Lee JC:
Pyridinylimidazole compound SB 203580 inhibits the activity but not
the activation of p38 mitogen-activated protein kinase. Biochem
Biophys Res Commun. 263:825–831. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
41.
|
Li J, Gorospe M, Hutter D, Barnes J, Keyse
SM and Liu Y: Transcriptional induction of MKP-1 in response to
stress is associated with histone H3 phosphorylation-acetylation.
Mol Cell Biol. 21:8213–8224. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
42.
|
Wang D and Lippard SJ: Cisplatin-induced
post-translational modification of histones H3 and H4. J Biol Chem.
279:20622–20625. 2004. View Article : Google Scholar : PubMed/NCBI
|