Introduction
Photodynamic therapy (PDT), a non-invasive cancer
treatment modality, can effectively eradicate local malignancies.
PDT utilizes a light-absorbing photosensitizer, visible light and
oxygen to generate reactive oxygen species that destroy malignant
cellular targets (1). However,
because tumors recur, PDT needs to be optimized to improve its
therapeutic benefit. Dasatinib, a multi-kinase inhibitor, is an
anticancer agent that has been successfully used for treatment of
chronic myeloid leukemia (2). As a
single agent evaluated in clinical trials for treatment of solid
tumors, including advanced head and neck squamous cell carcinoma
(HNSCC), however, dasatinib has not been shown to be successful
(3). In combination with other
chemotherapeutic agents or radiation dasatinib is a more effective
anticancer treatment in vitro, in vivo and in
clinical trials (4–7).
Bioactive sphingolipids have been implicated in
drug-and radiation-resistance, therefore targeting sphingolipid
metabolism can contribute to increased effectiveness of the current
treatment strategies (8). As shown
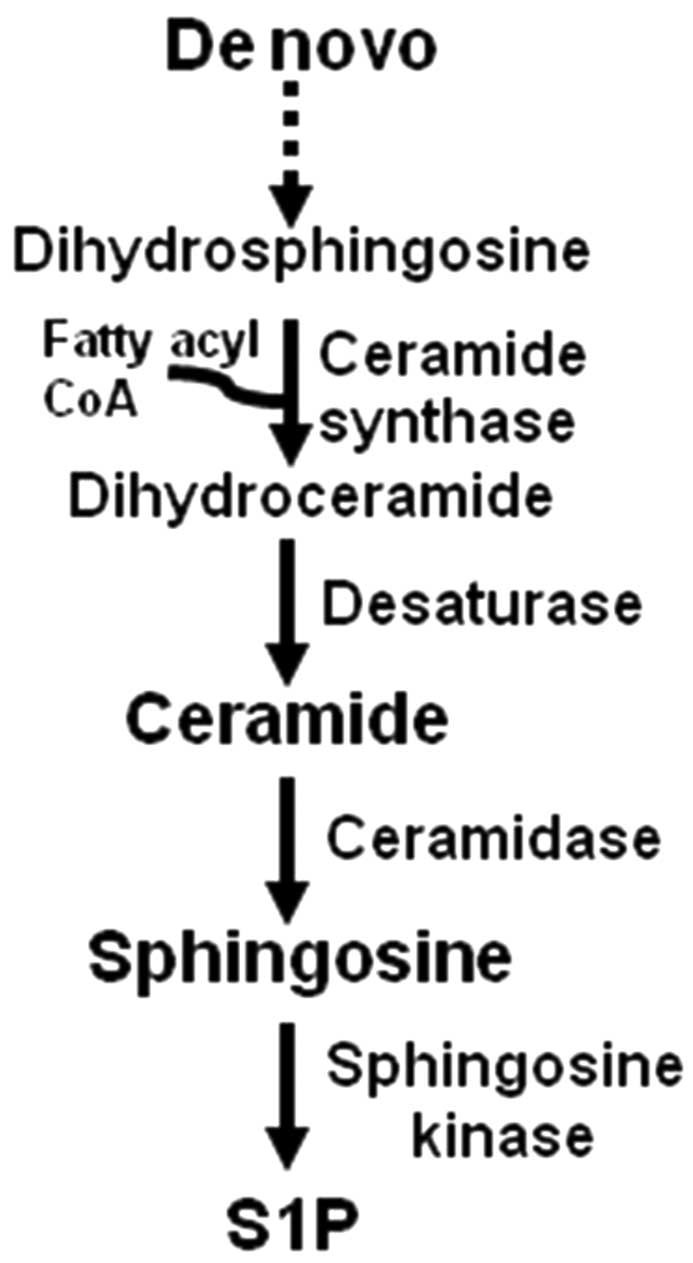
in Fig. 1, the sphingolipid
ceramide is generated in the de novo biosynthesis pathway,
which includes a ceramide synthase-dependent addition of a fatty
acyl group to dihydrosphingosine to form dihydroceramide. Ceramide
is formed from dihydroceramide by a desaturase-dependent insertion
of a double bond in the sphingosine backbone. Six mammalian
ceramide synthases have been identified with distinct specificity
for fatty acyl CoAs and functions (9). For example, C18- and C16-ceramide,
containing an 18- and 16-carbon fatty acid, are generated by
ceramide synthase 1 and 6, respectively, and induce HNSCC
suppression and proliferation, respectively (10). Ceramide is deacylated by
ceramidase, giving rise to sphingosine, and sphingosine is acted
upon by sphingosine kinase to give rise to sphingosine-1-phosphate
(S1P), an antiapoptotic sphingolipid.
We demonstrated that the knockdown of ceramide
synthase 1 or 6 is associated with reduction in ceramides and
dihydroceramides resulting in apoptotic resistance to PDT with Pc 4
(11,12). Dasatinib induces apoptosis via
upregulation of ceramide synthases, including increased expression
of ceramide synthase 1 gene (13). The combination of dasatinib and PDT
with Pc 4 was tested for potential anticancer efficacy in SCCVII
mouse squamous cell carcinoma cells, a preclinical model of HNSCC
(14), using apoptotic markers,
colony formation and ceramide metabolism as experimental
end-points.
Materials and methods
Materials
The phthalocyanine photosensitizer Pc 4,
HOSiPcOSi(CH3)2(CH2)3N(CH3)2,
was supplied by Dr Malcolm E. Kenney (Department of Chemistry, Case
Western Reserve University, Cleveland, OH, USA).
N-[9,10-3H]D-e-C16-ceramide was synthesized at the
Lipidomics Shared Resource (Medical University of South Carolina,
Charleston, SC, USA). RPMI medium and serum were from Life
Technologies (Carlsbad, CA, USA) and Hyclone (Logan, UT, USA),
respectively. The inhibitors zVAD-fmk and dasatinib (BMS-354825)
were from MBL International (Woburn, MA, USA) and Selleck Chemicals
(Houston, TX, USA), respectively.
Cell culture and treatments
SCCVII cells, initially derived from the spontaneous
abdominal wall tumor of a C3H mouse (15), were grown in RPMI medium containing
10% fetal bovine serum, 100 U/ml penicillin and 100 μg/ml
streptomycin (Life Technologies). Cells were maintained at 37°C in
a 5% CO2 atmosphere and were treated in the growth
medium. For PDT experiments, after overnight incubation with Pc 4
at 37°C, cells were irradiated with red light (2 mW/cm2;
λmax ∼670 nm) using a light-emitting diode array light
source (EFOS, Mississauga, ON, Canada) at the fluence of 200
mJ/cm2 at room temperature and then incubated at 37°C
for indicated periods of time. For PDT + dasatinib, dasatinib was
added to the cells 22 h prior to irradiation, unless indicated
otherwise. After treatments, cells were collected on ice and
processed for various analyses. For mass spectroscopy (MS)
analysis, cells were washed twice with cold phosphate-buffered
saline (Corning Life Sciences, New York, NY, USA), resuspended in
the mixture of ethyl acetate/methanol (1:1, v/v; EMD Chemicals,
Billercia, MA, USA), dried under nitrogen and shipped overnight on
dry ice to the Lipidomics Shared Resource (Medical University of
South Carolina, SC, USA) for further processing.
Electrospray ionization/double mass
spectrometry (MS) analysis
After extraction, sphingolipids were separated by
high performance liquid chromatography, introduced to electrospray
ionization source and then analyzed by double MS using TSQ Quantum
Access Max triple stage quadrupole mass spectrometer (Thermo-Fisher
Scientific, Pittsburg, PA, USA) as described previously (16).
RNA extraction and quantitative real-time
polymerase chain reaction (RT-PCR)
Total RNA isolation was performed with
RNeasy® Mini kit (Qiagen, Valencia, CA, USA) according
to the manufacturer’s instructions. cDNA was synthesized from 1
μg of the total RNA using iScript™ cDNA Synthesis kit
(Bio-Rad, Hercules, CA, USA). The concentration and quality of
total RNA preparations were evaluated spectrophotometrically.
RT-PCR was performed on a Bio-Rad CFX96 detection system using
Bio-Rad SsoFast Probes Supermix™ and TaqMan® Gene
Expression Assays (Life Technologies) with the primers for ceramide
synthases 1, 2, 4, 5, 6, the housekeeping gene products RPL37A and
hypoxanthine-guanine phosphoribosyltransferase (HGPRT), and the
fluorophore probe FAM-490 (6-carboxyflurescein; all obtained from
Life Technologies). Initial steps of RT-PCR were 30 sec at 85°C,
followed by 40 cycles consisting of a 5 sec at 95°C, followed by 10
sec at 60°C. Determination of the relative normalized expression of
corresponding ceramide synthase mRNAs against the expression of
housekeeping gene-encoded proteins RPL37A and HGPRT was performed
by ΔΔCT provided by CFX96 manager software 3.0 from
Bio-Rad.
Acid ceramidase activity assay
Acid ceramidase activity was performed as described
previously (17). Cells were lysed
under acidic condition (pH 4.5). Equal amounts of
N-[9,10-3H] D-e-C16-ceramide were mixed with 0.2% Triton
X-100 and 0.4% cholate and dried down under nitrogen. The lipid
film was dissolved by mixing and sonication in deionized water.
After additions of acidic assay buffer (0.2 M acetic acid, 0.2 M
sodium acetate and 0.5% Triton X-100, pH 4.5) and cell lysate, the
reaction was carried at 37°C for 1 h and stopped by adding Dole’s
alkaline solution. [3H]palmitic acid, a hydrolytic
product of acid ceramidase, was extracted and processed to
calculate the enzyme activity (17). Quantitation of radioactivity was
performed using LS 6500 multipurpose scintillation counter (Beckman
Coulter, Brea, CA, USA).
DEVDase (caspase-3) activity assay
As described previously (18), DEVDase activity was determined in
the cytosol by an assay based on the enzyme’s cleavage of a
fluorogenic derivative of the tetrapeptide substrate
N-acetyl-Asp-Glu-Val-Asp (DEVD; Enzo Life Sciences, Farmingdale,
NY, USA). The peptide sequence is based on the cleavage site
Asp216 of the caspase-3 substrate poly(ADP-ribose)
polymerase (PARP). The fluorescence of the cleaved DEVD substrate
was measured using a spectrofluorometer (F-2500 Hitachi, New York,
NY, USA; 380 nm excitation, 460 nm emission).
Mitochondrial depolarization
measurement
The lipophilic cationic dye JC-1
(5,5′,6,6′-tetrachloro-1,1′3,3′-tetraethylbenzimidazolylcarbocyanine
iodide; BD Biosciences, San Diego, CA, USA) was used to determine
mitochondrial membrane potential by flow cytometry, as we described
previously (11,19,20).
After treatments, cells were harvested and processed for flow
cytometry according to the manufacturer’s instructions (BD
Biosciences). BD LSR II flow cytometer was used for analysis (BD
Biosciences).
Apoptosis detection
As previously described (11,20,21),
to detect apoptosis, the exposure of phosphatidylserine in the
outer leaflet of the cell membrane and cell membrane integrity loss
were measured using Annexin V and DNA-binding propidium iodide
fluorescent dyes (BD Biosciences), respectively. Early apoptotic
(Annexin V+/propidium iodide−) were
distinguished from late apoptotic or necrotic cells (Annexin
V+/propidium iodide+). The kit was obtained
from BD Biosciences and the flow cytometric protocol was followed,
as described by the manufacturer.
Clonogenic assay
Long-term cell viability was assessed using
clonogenic assay according to the modified protocol that we
described previously (20).
Plating density was 250 cells/plate. Plating efficiency was 34%
(n=16).
Protein determination
Protein content was determined by a modified
Bradford assay (Bio-Rad) or, for acid ceramidase assay, by a
bicinchoninic acid protein assay kit (Thermo-Fisher
Scientific).
Statistical analysis
Data are shown as the mean ± SEM. Statistical
analyses were performed by Student’s t-test. Significance was
defined as a two-tailed p<0.05.
Results
PDT + dasatinib enhances overall cell
killing. Effect of zVAD-fmk on PDT ± dasatinib-induced cell
killing
To test whether killing of SCCVII cells is increased
by the combination of Pc 4-PDT with dasatinib, colony formation
assay was used as the experimental end-point. The treatments were
first used as single agents to determine whether they induce
dose-dependent cell killing. Incubation of SCCVII cells with 100,
200 and 500 nM dasatinib led to 2, 28 and 53% cell killing,
respectively (Fig. 2A). Similarly,
PDT with 100, 250 or 500 nM Pc 4, at the light fluence of 200
mJ/cm2, induced 16, 26 and 87% cell killing,
respectively (Fig. 2A). Treatment
of cells with the combination of PDT and dasatinib, each used at LD
<30, led to 72% cell killing, which was significantly greater
than that of each treatment alone (Fig. 2B). Because both agents are
apoptotic inducers (11,12,22–26),
the requirement of caspases in cell killing by each agent and the
combination was assessed using the pan caspase inhibitor zVAD-fmk.
As shown in Fig. 2B, zVAD-fmk
substantially inhibited cell killing after dasatinib, but not after
either PDT alone or the combination. Overall, the data demonstrate
that each agent induces dose-dependent cell killing, the
combination enhances cell killing, and that, unlike PDT alone or
the combination, dasatinib induces zVAD-fmk-dependent cell
killing.
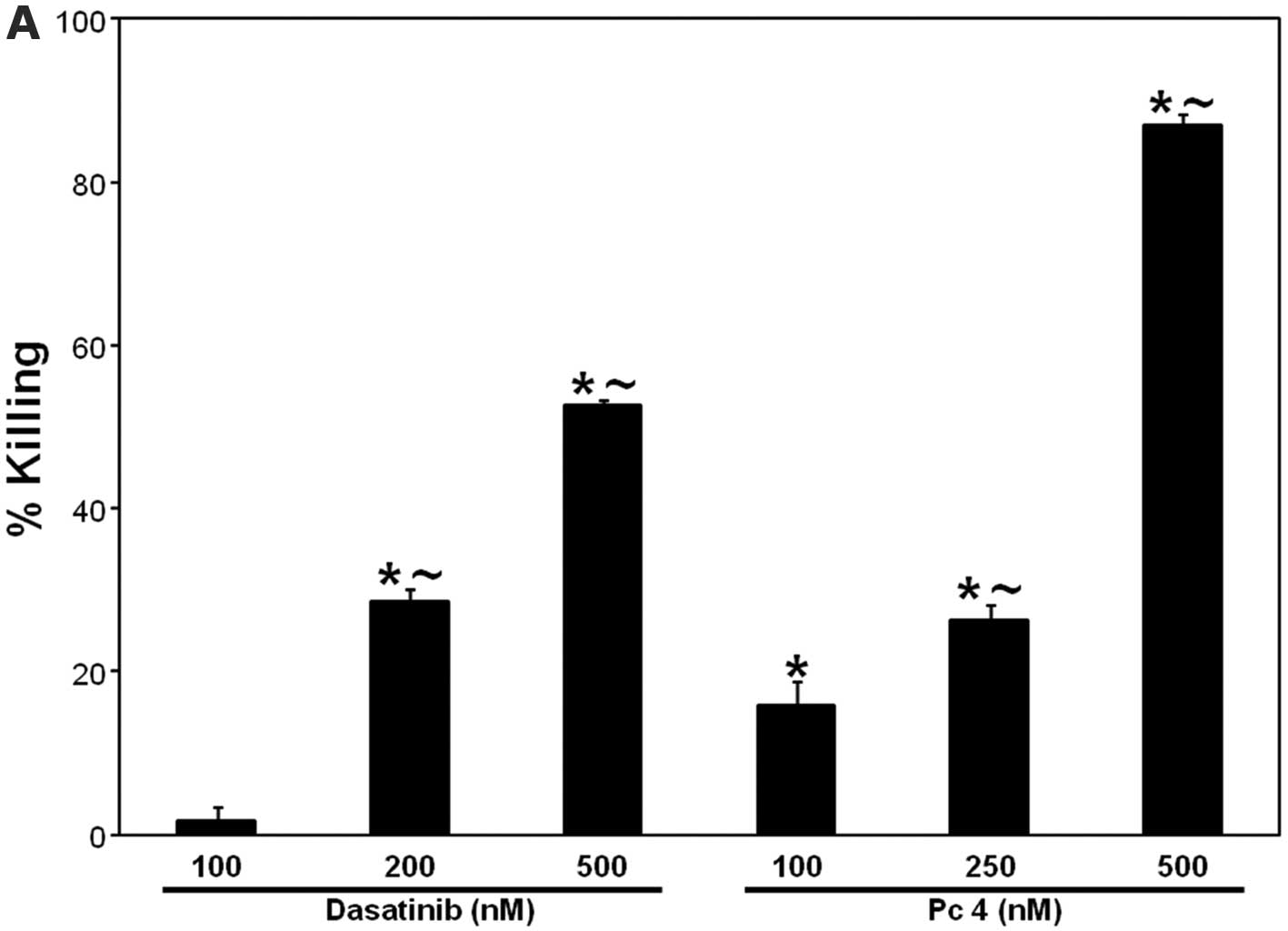 | Figure 2.(A) PDT and dasatinib, respectively,
induce dose-dependent cell killing. (B) Effect of zVAD-fmk on PDT ±
dasatinib-induced cell killing. SCCVII cells were plated after
appropriate dilutions into P60-mm dishes and allowed to attach
overnight in the growth medium. For PDT, cells were plated in the
growth medium containing Pc 4 at the indicated concentrations (A)
or at 250 nM (B), incubated overnight at 37°C, and irradiated with
red light (200 mJ/cm2). (B) zVAD-fmk (25 μM), was
added 1 h prior to treatments; For PDT + dasatinib, dasatinib (200
nM) was added immediately prior to irradiation. (A and B) After
8–10 days of growth at 37°C, colonies (≥50 cells) were stained with
crystal violet (0.1%) and counted. The data are expressed as the
percentage of killing and are shown as the mean ± SEM, n=3–12. The
significance (p<0.05) is shown as follows:
∼difference between corresponding doses of dasatinib or
PDT; *treated are different from untreated control;
+(PDT + dasatinib) is different from PDT or dasatinib
alone; −zVAD-fmk is different from dasatinib alone. Das,
dasatinib. |
Dasatinib-induced caspase-3 activation is
inhibited by zVAD-fmk. The combination potentiates PDT- or
dasatinib-induced activation of caspase-3 in the absence of
appearance of other apoptotic markers
Because zVAD-fmk inhibits effector caspases,
including caspase-3 (27), we
verified caspase-3 as a zVAD-fmk target in dasatinib-induced cell
death using DEVDase assay. Caspase-3 activation began at 2 h and
peaked at 24 h after dasatinib (not shown). As depicted in Fig. 3A, dasatinib induced activation of
DEVDase was abolished by zVAD-fmk. The data suggest that
zVAD-fmk-sensitive cell killing after dasatinib involves
caspase-3.
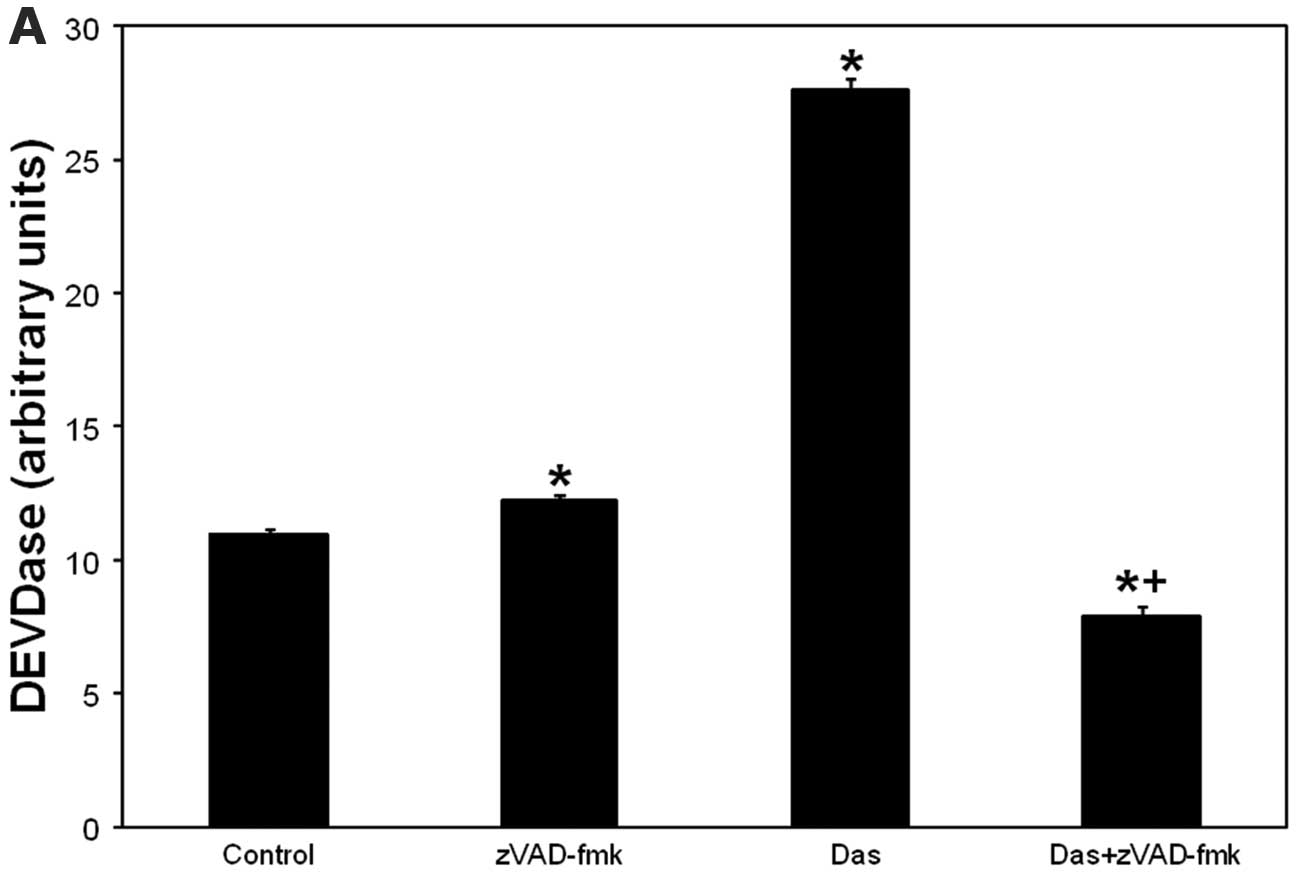 | Figure 3.(A) Dasatinib-induced caspase-3
activation is abolished by zVAD-fmk. (B) PDT- or dasatinib-induced
caspase-3 activation is potentiated after PDT + dasatinib. (C)
PDT-induced mitochondrial depolarization is abolished after the
combination. (D) Annexin V+ and propidium iodide+ cells
remain at control levels after treatments. SCCVII cells were
treated with dasatinib (200 nM) for 24 h (A–C) or 72 h (D). For
PDT, after overnight incubation with Pc 4 (100 or 250 nM), cells
were irradiated with red light (200 mJ/cm2) and then
incubated for 2 h (B and C) or 48 h (D). For dasatinib + zVAD-fmk,
the inhibitor was added 1 h prior to dasatinib (A). For PDT +
dasatinib, dasatinib was added to the cells 22 h (B and C) or 24 h
prior to irradiation (D). After incubations, cells were collected,
lysed and DEVDase assay was carried out to assess caspase-3
activity (A and B). Alternatively, collected cells were processed
for flow cytometry using JC-1 or Annexin V/propidium iodide
staining for mitochondrial depolarization (C) and apoptosis
detection (D), respectively. (C and D) Cells were treated overnight
with camptothecin (5 μM). (A and B) The data are shown as
the mean ± SEM, n=3–23. The significance (p<0.05) is shown as
follows: *treated is different from untreated control;
+(PDT + dasatinib) or (dasatinib + zVAD-fmk) is
different from individual treatments. Con, untreated control; Das,
dasatinib. (C) Percentage of cells with depolarized mitochondria is
shown in lower right dot plot. (D) Percentage of Annexin
V+/propidium iodide− and Annexin
V+/propidium iodide+ cells is shown in lower
right and upper right dot plot, respectively. |
To further assess induction of apoptosis after
treatments, caspase-3 activation, mitochondrial depolarization and
the appearance of Annexin V+ and propidium iodide+ cells
were determined. PDT induced a dose-dependent activation of
caspase-3 and the effect was potentiated after PDT + dasatinib
(Fig. 3B). PDT alone induced
mitochondrial depolarization and the effect was inhibited after the
combination (Fig. 3C). Annexin
V+ and/or propidium iodide+ cells remained at control
levels after treatments (Fig. 3D).
We validated that SCCVII cells display depolarized mitochondria and
undergo apoptosis using camptothecin as a positive control
(Fig. 3C and D). The results show
that the combined treatment augments PDT or dasatinib-induced
caspase-3 activation in the absence of appearance of other
apoptotic markers.
Dasatinib-induced upregulation of mRNA
ceramide synthase 1 is enhanced after the combination
Dasatinib upregulates expression levels of
ceramide synthase genes 1, 2, 5 and 6 (13). We showed that knockdown of ceramide
synthase 1 or 6 leads to apoptotic resistance to PDT (11,12).
To test whether ceramide synthases are affected by treatments, mRNA
levels of ceramide synthases 1, 2 4, 5 and 6 were measured using
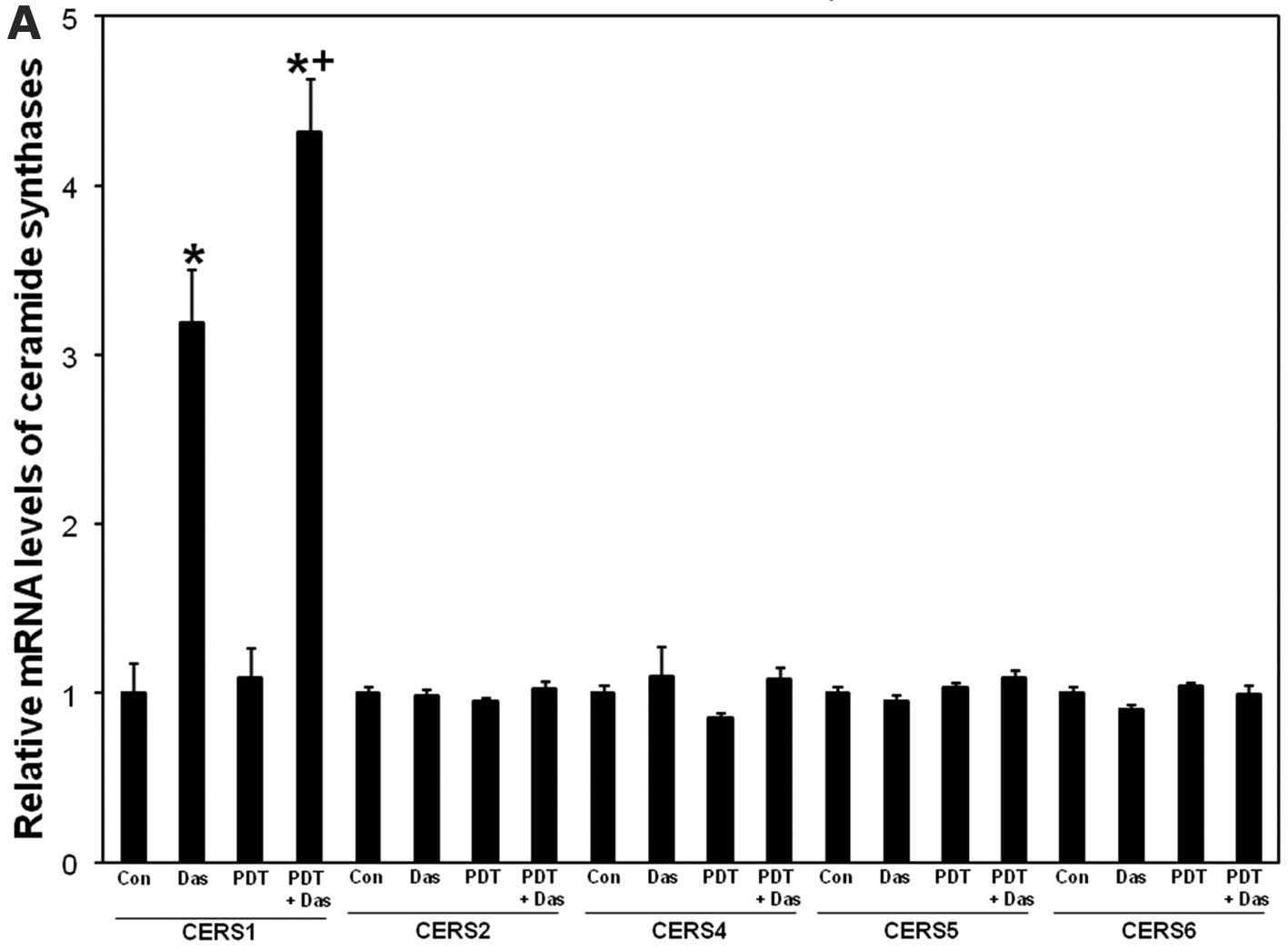
RT-PCR. As depicted in Fig. 4A,
ceramide synthase 1 mRNA levels were upregulated after dasatinib
and the effect was further increased after PDT + dasatinib. PDT
alone did not significantly increase ceramide synthase 1 levels.
None of the treatments had any effect on mRNA levels of ceramide
synthase 2, 4, 5 and 6 (Fig. 4A).
The data show that dasatinib-induced upregulation of ceramide
synthase 1 mRNA levels is enhanced after the combination.
Effects of treatments on the sphingolipid
profile
To assess whether enhanced upregulation of ceramide
synthase 1 mRNA after PDT + dasatinib is associated with increased
ceramide production, ceramide levels were measured using MS.
Dasatinib alone induced a modest increase in C20:1- and
C22-ceramide but had no effect on total ceramide levels (Table I and Fig. 4B). PDT alone increased the levels
of all 12 individual ceramides that were measured, as well as total
ceramides. PDT-induced increases in ceramide levels were not, for
the most part, further changed after the combination, with the
exception of attenuated levels of C26- and C26:1-ceramide after PDT
+ dasatinib compared to individual treatments (Table I and Fig. 4B).
 | Table I.Effect of PDT± dasatinib on
sphingolipids in SCCVII cells. |
Table I.
Effect of PDT± dasatinib on
sphingolipids in SCCVII cells.
| Sphingolipid | Untreated | Dasatinib | PDT | PDT +
dasatinib |
|---|
| C14-ceramide | 13.1±1.4 | 15.2±1.0 | 23.6±1.6a | 21.8±1.4a |
| C16-ceramide | 94.8±11.4 | 103.9±8.1 |
196.5±6.1a |
179.2±20.4a |
| C18-ceramide | 6.8±0.9 | 8.2±0.5 | 21.5±1.6a | 21.5±1.8a |
| C18:1-ceramide | 4.3±0.5 | 4.4±0.9 | 10.5±0.1a | 9.5±0.6a |
| C20-ceramide | 5.8±0.8 | 7.3±0.7 | 15.8±0.6a | 17.6±0.9a |
| C20:1-ceramide | 0.9±0.1 | 1.1±0.1a | 2.3±0.2a | 2.5±0.2a |
| C22-ceramide | 32.5±3.8 | 44.7±3.5a | 80.4±4.2a | 84.7±4.7a |
| C22:1-ceramide | 10.0±1.1 | 11.2±1.0 | 18.4±0.7a | 17.5±0.7a |
| C24-ceramide | 147.6±15.1 | 172.3±14.4 |
270.6±10.2a |
239.4±12.2a |
| C24:1-ceramide | 171.7±18.8 | 205.3±15.5 |
313.0±14.3a |
307.7±16.6a |
| C26-ceramide | 2.9±0.3 | 3.4±0.3 | 6.7±0.3a |
5.1±0.4a,b |
| C26:1-ceramide | 4.8±0.5 | 5.5±0.7 | 11.7±0.4a |
10.1±0.5a,b |
|
C16-dihydroceramide | 10.3±1.5 | 9.6±0.6 | 22.4±0.5a | 19.7±1.1a |
|
Dihydrosphingosine | 14.2±2.2 | 16.9±1.4 | 17.2±1.3 | 17.4±0.6 |
|
Dihydrosphingosine-1-phosphate | 0.4±0.1 | 0.3±0.1 | 0.6±0.1 | 0.5±0.1 |
| Sphingosine | 118.8±19.5 | 150.3±8.7 | 15.1±1.0a | 19.6±1.8a |
|
Sphingosine-1-phosphate | 1.3±0.1 | 1.8±0.2 | 0.5±0.1a | 0.7±0.1a |
The effects of treatments on other sphingolipids
were also measured by MS and are shown in Table I. Unlike dasatinib, and
irrespective of the presence of dasatinib, PDT increased the levels
of C16-dihydroceramide, an intermediate from the de novo
sphingolipid biosynthesis pathway. Moreover, unlike dasatinib, and
irrespective of the presence of dasatinib, PDT induced an 87%
decrease in sphingosine levels. PDT also induced a 62% decrease in
S1P levels, and the effect was not changed after the combination.
Overall, the data show that in SCCVII cells PDT induced substantial
changes in the sphingolipid profile that were not modulated by the
addition of dasatinib.
Acid ceramidase activity is inhibited
after PDT
The substantial decrease in sphingosine levels after
PDT could result from inhibition of ceramidase, a
sphingosine-producing enzyme (Fig.
1). The inhibition of acid ceramidase has been implicated in
radiosensitization of prostate cancer cells (28). We measured the activity of acid
ceramidase in cell lysates from untreated and PDT-treated cells. As
depicted in Fig. 4C, acid
ceramidase activity was reduced by 52% post-PDT. Thus, PDT-induced
decrease in sphingosine correlated with inhibition of acid
ceramidase.
Discussion
Dasatinib-induced caspase-3 activation and cell
killing was zVAD-fmk-dependent. PDT induced caspase-3 activation
and the effect was potentiated after the combination. As shown
previously (29), PDT-induced
caspase-3 activation was abolished by zVAD-fmk. However, PDT- or
PDT + dasatinib-induced cell killing was zVAD-fmk-insensitive.
zVAD-fmk-insensitivity of PDT is consistent with the findings that
caspase-3 is not required for the lethal effect of PDT (30). Incidentally, zVAD-fmk has been
reported to upregulate caspase-9 activity in cell death after
etoposide (27). We found no
activation of caspase-9 after PDT in SCCVII cells (31). Therefore, it is unlikely that
zVAD-fmk would have such an effect, especially since the inhibitor
was used in our studies at a non-toxic, and comparably, lower
concentration than in the etoposide study, i.e., 25 vs. 50
μM, respectively. Overall, the data suggest that, unlike PDT
or the combination, dasatinib requires caspase-3 activation for
cell killing. Apparently, PDT rather than dasatinib determined
zVAD-fmk sensitivity of cell killing after the combination.
Dasatinib-induced upregulation of ceramide synthase
1 mRNA correlated with increased production of C20:1- and
C22-ceramide concomitant with activation of caspase-3. This is
consistent with the finding that ceramide synthase 1 gene is
upregulated during apoptosis after dasatinib (13). However, there was no correlation
between combination-induced enhanced ceramide synthase 1 mRNA
upregulation and ceramide production, suggesting that the enzyme
might modulate other cellular functions. Ceramide synthase 1 has
been associated with sensitization to cisplatin via activation of
p38 mitogen-activated protein kinase (MAPK) and concomitant
translocation of the enzyme from the endoplasmic reticulum to the
Golgi apparatus (32). Activation
of p38 MAPK is critical for the antileukemic effects of dasatinib
(33). Activation of p38 MAPK
pathway has been associated with triggering apoptosis after Pc
4-PDT (34). The potential link
between ceramide synthase 1 upregulation, p38 MAPK pathway and
sensitization to PDT should be addressed in our future studies.
In HNSCC in vitro and in vivo models
ceramide synthase 1-dependent C18-ceramide production has a
proapoptotic role (35) and
inhibits xenograft growth (36),
respectively. We showed in HNSCC cells that knockdown of CERS1
induced apoptotic resistance to PDT and reduced the levels of total
ceramide and several individual ceramides, including C18-ceramide
(12). Our findings that the
levels of the whole spectrum of ceramides are increased after PDT
in the absence of upregulation of ceramide synthase mRNAs imply the
involvement of other enzymes. Accordingly, in the present study we
have demonstrated that PDT-induced inhibition of acid ceramidase
correlates with decrease in sphingosine and increase in ceramide
levels, concomitant with activation of caspase-3. Consistent with
these findings, upregulation of acid ceramidase confers
radioresistance in prostate cancer cells (28). As suggested in the same study,
inhibition of acid ceramidase could be a potential target for
treatment of cancers with overexpressed acid ceramidase.
The present study shows for the first time enhanced
additive killing of SCCVII cells after the combination of PDT and
dasatinib and paves the way for testing the combination in
vivo. This combination has the potential to achieve what is
hoped by combination therapy, i.e. maximizing the efficacy of each
single anticancer agent while minimizing their systemic toxicity
through the delivery of lower drug doses (37). This will have to be validated in
vivo. Regardless, our novel findings imply the translational
potential of the combination for cancer treatment.
Acknowledgements
This study was supported by U.S.
Public Health Service Grants: to D.S., R01 CA77475 from the
National Cancer Institute (NCI), National Institutes of Health
(NIH); to J.M.K., P20-RR17677 (NIH), grants from the Rally
Foundation for Childhood Cancer Research, Hyundai Hope on Wheels,
Monica Kreber Golf Tournament, and Chase after a Cure Foundation;
to T.I.G., the Veterans Administration Merit Awards from RR&D
and BLRD programs; to the flow cytometry-related work at the
Microscopy, Imaging, and Cytometry Resources Core (Karmanos Cancer
Institute, Wayne State University), NCI Grant P30 CA22453; to the
MS-related work at the Lipidomics Shared Resource (Medical
University of South Carolina), NCI Grants IPO1CA097132 and P30 CA
138313, NIH/NCRR SC COBRE Grant P20 RR017677, C06 RR018823 from the
Extramural Research Facilities Program of the National Center for
Research Resources.
References
|
1.
|
Agostinis P, Berg K, Cengel KA, Foster TH,
Girotti AW, Gollnick SO, Hahn SM, Hamblin MR, Juzeniene A, Kessel
D, Korbelik M, Moan J, Mroz P, Nowis D, Piette J, Wilson BC and
Golab J: Photodynamic therapy of cancer: an update. CA Cancer J
Clin. 61:250–281. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Wei G, Rafiyath S and Liu D: First-line
treatment for chronic myeloid leukemia: dasatinib, nilotinib, or
imatinib. J Hematol Oncol. 3:472010. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Brooks HD, Glisson BS, Bekele BN, Johnson
FM, Ginsberg LE, El-Naggar A, Culotta KS, Takebe N, Wright J, Tran
HT and Papadimitrakopoulou VA: Phase 2 study of dasatinib in the
treatment of head and neck squamous cell carcinoma. Cancer.
117:2112–2119. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Kopetz S, Lesslie DP, Dallas NA, Park SI,
Johnson M, Parikh NU, Kim MP, Abbruzzese JL, Ellis LM, Chandra J
and Gallick GE: Synergistic activity of the SRC family kinase
inhibitor dasatinib and oxaliplatin in colon carcinoma cells is
mediated by oxidative stress. Cancer Res. 69:3842–3849. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Pichot CS, Hartig SM, Xia L, Arvanitis C,
Monisvais D, Lee FY, Frost JA and Corey SJ: Dasatinib synergizes
with doxorubicin to block growth, migration, and invasion of breast
cancer cells. Br J Cancer. 101:38–47. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Haura EB, Tanvetyanon T, Chiappori A,
Williams C, Simon G, Antonia S, Gray J, Litschauer S, Tetteh L,
Neuger A, Song L, Rawal B, Schell MJ and Bepler G: Phase I/II study
of the Src inhibitor dasatinib in combination with erlotinib in
advanced non-small-cell lung cancer. J Clin Oncol. 28:1387–1394.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Raju U, Riesterer O, Wang ZQ, Molkentine
DP, Molkentine JM, Johnson FM, Glisson B, Milas L and Ang KK:
Dasatinib, a multi-kinase inhibitor increased radiation sensitivity
by interfering with nuclear localization of epidermal growth factor
receptor and by blocking DNA repair pathways. Radiother Oncol.
105:241–249. 2012. View Article : Google Scholar
|
|
8.
|
Adan-Gokbulut A, Kartal-Yandim M, Iskender
G and Baran Y: Novel agents targeting bioactive sphingolipids for
the treatment of cancer. Curr Med Chem. 20:108–122. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Hannun YA and Obeid LM: Many ceramides. J
Biol Chem. 286:27855–27862. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Saddoughi SA and Ogretmen B: Diverse
functions of ceramide in cancer cell death and proliferation. Adv
Cancer Res. 117:37–58. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Separovic D, Breen P, Joseph N, Bielawski
J, Pierce JS, Van Buren E and Gudz TI: Ceramide synthase 6
knockdown suppresses apoptosis after photodynamic therapy in human
head and neck squamous carcinoma cells. Anticancer Res. 32:753–760.
2012.PubMed/NCBI
|
|
12.
|
Separovic D, Breen P, Joseph N, Bielawski
J, Pierce JS, Van Buren E and Gudz TI: siRNA-mediated
down-regulation of ceramide synthase 1 leads to apoptotic
resistance in human head and neck squamous carcinoma cells after
photodynamic therapy. Anticancer Res. 32:2479–2485. 2012.
|
|
13.
|
Gencer EB, Ural AU, Avcu F and Baran Y: A
novel mechanism of dasatinib-induced apoptosis in chronic myeloid
leukemia; ceramide synthase and ceramide clearance genes. Ann
Hematol. 90:1265–1275. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Khurana D, Martin EA, Kasperbauer JL,
O’Malley BW Jr, Salomao DR, Chen L and Strome SE: Characterization
of a spontaneously arising murine squamous cell carcinoma (SCC VII)
as a prerequisite for head and neck cancer immunotherapy. Head
Neck. 23:899–906. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Suit HD, Sedlacek RS, Silver G and
Dosoretz D: Pentobarbital anesthesia and the response of tumor and
normal tissue in the C3Hf/sed mouse to radiation. Radiat Res.
104:47–65. 1985. View
Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Separovic D, Semaan L, Tarca AL, Awad
Maitah MY, Hanada K, Bielawski J, Villani M and Luberto C:
Suppression of sphingomyelin synthase 1 by small interference RNA
is associated with enhanced ceramide production and apoptosis after
photodamage. Exp Cell Res. 314:1860–1868. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Bai A, Szulc ZM, Bielawski J, Mayroo N,
Liu X, Norris J, Hannun YA and Bielawska A: Synthesis and
bioevaluation of omega-N-amino analogs of B13. Bioorg Med Chem.
17:1840–1848. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Dolgachev V, Farooqui MS, Kulaeva OI,
Tainsky MA, Nagy B, Hanada K and Separovic D: De novo ceramide
accumulation due to inhibition of its conversion to complex
sphingolipids in apoptotic photosensitized cells. J Biol Chem.
279:23238–23249. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Dolgachev V, Nagy B, Taffe B, Hanada K and
Separovic D: Reactive oxygen species generation is independent of
de novo sphingolipids in apoptotic photosensitized cells. Exp Cell
Res. 288:425–436. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Separovic D, Saad ZH, Edwin EA, Bielawski
J, Pierce JS, Van Buren E and Bielawska A: C16-ceramide analog
combined with Pc 4 photodynamic therapy evokes enhanced total
ceramide accumulation, promotion of DEVDase activation in the
absence of apoptosis, and augmented overall cell killing. J Lipids.
2011:1–9. 2011. View Article : Google Scholar
|
|
21.
|
Separovic D, Mann KJ and Oleinick NL:
Association of ceramide accumulation with photodynamic
treatment-induced cell death. Photochem Photobiol. 68:101–109.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Agarwal ML, Clay ME, Harvey EJ, Evans HH,
Antunez AR and Oleinick NL: Photodynamic therapy induces rapid cell
death by apoptosis in L5178Y mouse lymphoma cells. Cancer Res.
51:5993–5996. 1991.PubMed/NCBI
|
|
23.
|
Wispriyono B, Schmelz E, Pelayo H, Hanada
K and Separovic D: A role for the de novo sphingolipids in
apoptosis of photosensitized cells. Exp Cell Res. 279:153–165.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Nam S, Williams A, Vultur A, List A,
Bhalla K, Smith D, Lee FY and Jove R: Dasatinib (BMS-354825)
inhibits Stat5 signaling associated with apoptosis in chronic
myelogenous leukemia cells. Mol Cancer Ther. 6:1400–1405. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Lin YC, Wu MH, Wei TT, Chuang SH, Chen KF,
Cheng AL and Chen CC: Degradation of epidermal growth factor
receptor mediates dasatinib-induced apoptosis in head and neck
squamous cell carcinoma cells. Neoplasia. 14:463–475.
2012.PubMed/NCBI
|
|
26.
|
Xue T, Luo P, Zhu H, Zhao Y, Wu H, Gai R,
Wu Y, Yang B, Yang X and He Q: Oxidative stress is involved in
Dasatinib-induced apoptosis in rat primary hepatocytes. Toxicol
Appl Pharmacol. 261:280–291. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Rodriguez-Enfedaque A, Delmas E, Guillaume
A, Gaumer S, Mignotte B, Vayssiere JL and Renaud F: zVAD-fmk
upregulates caspase-9 cleavage and activity in etoposide-induced
cell death of mouse embryonic fibroblasts. Biochim Biophys Acta.
1823:1343–1352. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Mahdy AE, Cheng JC, Li J, Elojeimy S,
Meacham WD, Turner LS, Bai A, Gault CR, McPherson AS, Garcia N,
Beckham TH, Saad A, Bielawska A, Bielawski J, Hannun YA, Keane TE,
Taha MI, Hammouda HM, Norris JS and Liu X: Acid ceramidase
upregulation in prostate cancer cells confers resistance to
radiation: AC inhibition, a potential radiosensitizer. Mol Ther.
17:430–438. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Nagy B, Chiu S-M and Separovic D:
Fumonisin B1 does not prevent apoptosis in A431 human epidermoid
carcinoma cells after photosensitization with phthalocyanine 4. J
Photochem Photobiol B. 57:132–141. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Xue LY, Chiu SM and Oleinick NL:
Photodynamic therapy-induced death of MCF-7 human breast cancer
cells: a role for caspase-3 in the late steps of apoptosis but not
for the critical lethal event. Exp Cell Res. 263:145–155. 2001.
View Article : Google Scholar
|
|
31.
|
Separovic D, Joseph N, Breen P, Bielawski
J, Pierce JS, van Buren E, Bhatti G, Saad ZH, Bai A and Bielawska
A: Combining anticancer agents photodynamic therapy and LCL85 leads
to distinct changes in the sphingolipid profile, autophagy,
caspase-3 activation in the absence of cell death, and long-term
sensitization. Biochem Biophys Res Commun. 409:372–377. 2011.
View Article : Google Scholar
|
|
32.
|
Min J, Mesika A, Sivaguru M, Van Veldhoven
PP, Alexander H, Futerman AH and Alexander S: (Dihydro)ceramide
synthase 1 regulated sensitivity to cisplatin is associated with
the activation of p38 mitogen-activated protein kinase and is
abrogated by sphingosine kinase 1. Mol Cancer Res. 5:801–812. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Dumka D, Puri P, Carayol N, Lumby C,
Balachandran H, Schuster K, Verma AK, Terada LS, Platanias LC and
Parmar S: Activation of the p38 Map kinase pathway is essential for
the antileukemic effects of dasatinib. Leuk Lymphoma. 50:2017–2029.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Whitacre CM, Feyes DK, Satoh T, Grossmann
J, Mulvihill JW, Mukhtar H and Oleinick NL: Photodynamic therapy
with the phthalocyanine photosensitizer Pc 4 of SW480 human colon
cancer xenografts in athymic mice. Clin Cancer Res. 6:2021–2027.
2000.PubMed/NCBI
|
|
35.
|
Koybasi S, Senkal CE, Sundararaj K,
Spassieva S, Bielawski J, Osta W, Day TA, Jiang JC, Jazwinski SM,
Hannun YA, Obeid LM and Ogretmen B: Defects in cell growth
regulation by C18:0-ceramide and longevity assurance gene 1 in
human head and neck squamous cell carcinomas. J Biol Chem.
279:44311–44319. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Senkal CE, Ponnusamy S, Bielawski J,
Hannun YA and Ogretmen B: Antiapoptotic roles of
ceramide-synthase-6-generated C16-ceramide via selective regulation
of the ATF6/CHOP arm of ER-stress-response pathways. FASEB J.
24:296–308. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Mayer LD and Janoff AS: Optimizing
combination chemotherapy by controlling drug ratios. Mol Interv.
7:216–223. 2007. View Article : Google Scholar : PubMed/NCBI
|