Introduction
Osteosarcoma is a high-grade bone neoplasm that
originates from mesenchymal tissue. It is the third most common
malignancy in children and adolescents, the most frequent highly
malignant bone-tumor and reaches a peak manifestation during the
second and third decade of life. Although survival rates increased
up to 70% in patients with localized disease, non-responsiveness to
chemotherapy is repeatedly encountered and paired with poor outcome
(1). Currently, the treatment of
osteosarcoma involves surgery with pre- and postoperative
chemotherapy including multiple chemotherapeutic agents. If
metastases are present, results are poor with long-term survival of
less than 20% (2).
The neurokinin-1 (NK-1) receptor (NK1R) has recently
been discovered to play an integral role in the maintenance of a
favorable tumor microenvironment (3,4). The
NK1R is a tachykinin receptor; its natural ligand is substance P
(SP). Tachykinins belong to the family of neuropeptides that are
responsible for a myriad of physiological responses. They bind to
specific seven transmembrane, G protein-coupled receptors (GPCRs)
on target cells. Three mammalian tachykinin receptor subtypes have
been characterized, TACR1 (NK1R), TACR2 and TACR3, which show
preferential but not absolute selectivity for SP, neurokinin A
(NK-A) and neurokinin B (NK-B), respectively (5,6).
Tachykinin receptors are expressed by many different cell types and
respond to tachykinins in a cell type-specific manner. These
receptors are linked to a variety of physiological and biological
processes such as regulation of neurotransmission, pain,
inflammation, cell growth and differentiation as well as
oncogenesis, among others (3,4).
SP and the subsequent activation of NK1R
(SP/NK-receptor complex) strongly influence the tumor
microenvironment (3,4). Activation of NK1R by SP leads to
phosphoinositide hydrolysis, calcium mobilization and
mitogen-activated protein kinase (MAPK) activation (7–10).
Moreover, the activation of the NK1R was found to be involved in a
myriad of processes related to oncogenesis such as mitogenesis,
angiogenesis, cell migration and metastasis. In this sense, it has
been demonstrated that SP acts through the NK1R as a mitogen in
several human cancer cell lines, including astrocytoma, melanoma,
neuroblastoma, glioma, retinoblastoma as well as pancreatic,
larynx, colon and gastric carcinoma, leukemia and breast cancer
(11–18). In these cell lines, the
pharmaceutical blockage of this receptor was found to robustly
inhibit tumor growth, making this receptor an attractive target for
future anticancer strategies (4).
Aprepitant is a selective high-affinity antagonist
of the human NK-1 receptor. Currently, this drug is used in
clinical practice as an antiemetic (19–22).
Additionally, in an experimental setting, it was found to have
great potential as an antitumor agent for a broad spectrum of
cancers (20).
Other NK1R antagonist, such as L-733,060 (a
piperidine derivative) and L-732,138 show affinity for the human
NK1R in vitro (14). Both
induce antitumor activity in human cancer cell lines (4,12–15,23,24).
It has been demonstrated in in vitro studies
that co-administration of L-733,060 with vinblastine or
microtubuledestabilizing agents resulted in synergistic inhibition
of cancer cell growth in cells that express NK1R. Interestingly,
this effect was not observed in non-cancerous cells (3,25).
Moreover, measuring the growth inhibition of
MDA-MB-231 tumor cell xenografts the antitumor activity of NK1R and
NK-2 receptor (NK2R) antagonists has been demonstrated in nude mice
(26,27).
However, nothing is known regarding the expression
of the NK1R in osteosarcomas and whether this receptor can serve as
a target for future anticancer strategies against this tumor,
either alone or together with cytostatic drugs. Likewise, no data
exist regarding a potential therapeutic effect of NK1R antagonists
against osteosarcoma in vivo. Therefore, in this study we
investigated the role of the NK1R as well as its therapeutic effect
in a human osteosarcoma cell line (MG-63) in vitro and in a
mouse xenograft in vivo.
Materials and methods
Cell culture
MG-63 osteosarcoma and other cell lines (HEK293,
GAMG, MRC-5) were purchased from European Collection of Cell
Cultures (ECACC). We used standard DMEM medium with 10% FCS, 1%
glutamine and 1% pentamycin/streptomycin (medium and all
supplements from Life Technologies) for all cell lines except
HEK293. HEK293 cell line was cultured in DMEM containing additional
glutamine. All cell lines were cultivated in either 25 or 75
cm2 culture flasks and incubated at 37°C in a humidified
atmosphere of 95% air/5% CO2 according to the
manufacturer’s instruction and exclusively grew in adherent
monolayers.
Primary human fibroblasts were isolated from skin
biopsies from health adult donors. Informed consent was obtained
prior to all biopsies and reviewed by the ethics committee of our
hospital. Cells were cultured in a similar way as described for
cell lines and grew in adherent monolayers exclusively. Medium was
DMEM containing 10% FCS, 1% glutamine and 1%
pentamycin/streptomycin (medium and all supplements from Life
Technologies). Experiments were carried out prior to 10 passages in
all cases.
Drug treatments
The NK1R antagonist aprepitant [MW 534.43 (5-[[(2R,
3S)-2-[(1R)-1-[3,5-bis(trifluoromethyl)phenyl]
ethoxy]-3-(4-fluorophenyl)-4-morpholinyl]methyl]-1,2-dihydro-3H-1,2,4-triazol-3-one))
was obtained from Merck Research Laboratories (Madrid, Spain), and
was dissolved in distilled water containing 0.1% acetonitrile
before sample treatment. In order to determine the IC50,
different concentrations (5 to 80 μM) of aprepitant were
evaluated. Fosaprepitant dimeglumin is a lyophilized prodrug of
aprepitant and is chemically described as
1-Deoxy-1(methylamino)-D-glucitol[3-[[(2R,3S)-2-[(1R)-1-[3,5bis(trifluoromethyl)phenyl]ethoxy]-3-(4-fluorophenyl)
4-morpholinyl]methyl]-2,5-dihydro-5-oxo-1H-1,2,4-triazol-1-yl]phosphonate
(2:1) salt. It was purchased from Merck Sharp & Dohme.
Fosaprepitant was dissolved and diluted according to the
manufacturer’s instruction.
The NK1R antagonist N-acetyl-L-tryptophan 3, 5-bis
(trifluoromethyl) benzyl ester, molecular weight 472.39 (L-732,138;
Sigma-Aldrich, Madrid, Spain) and
(2S,3S)-3-{[3,5-bis(trifluoromethyl)benzyl]oxy}-2-phenylpiperidine,
molecular weight 403.36 (L-733,060: Sigma-Aldrich), was dissolved
in distilled water containing 2.5% dimethyl sulfoxide (DMSO) before
sample treatment.
SP and acetate salt (Sigma-Aldrich) were dissolved
in distilled water and different concentrations (5, 10, 50, 100 and
500 nM) were used. In experiments investigating synergistic or
competing effects, the MG-63 cell line was incubated with SP for 1
h before the addition of other stimuli.
The common cytostatics adriamycin, mitomycin,
ifosfamide and cisplatin were supplied by the general farmacy of
the Virgen del Rocío Children’s Hospital and were used at the doses
indicated in the text and figure legends.
Animals
Female athymic nu/nu nude mice, 5–7-week-old were
purchased from Harlan Iberia (Barcelona, Spain), maintained in
mircoisolator cages and supplied with sterile materials and food.
Permission for animal experiments in the described setting was
obtained from the ethics committee of Virgen del Rocío University
Hospital (Seville, Spain).
Proliferation assays
Cell proliferation was evaluated using the
tetrazolium compound
3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)2-(4-sulfophenyl)-2H-tetrazolium,
inner salt (MTS), according to the manufacturer’s instructions
(CellTiter 96 Aqueous One-Solution Cell Proliferation Assay,
Promega Corp., Madison, WI, USA). Cell numbers were quantified
using a Coulter counter. The plate included blank wells (0
cells/0.1 ml), control wells (104 cells/0.1 ml), control
wells with acetonitrile, control wells treated with aprepitant and
control wells treated with the most effective SP concentration and
aprepitant. The plates were inoculated with aprepitant (5–80
μM for tumor cell lines) and were incubated for the first
doubling time specific for each tumor cell line. Plates were also
inoculated with the most mitogenic exogenous SP nM concentration
with or without the 50% μM inhibition concentration
(IC50) of aprepitant for their first doubling times. For
the proliferation assay, 20 μl of the MTS reagent was added
to each well 90 min before reading the samples on a multiscanner
microplate reader (Tecan Spectra Classic, Barcelona, Spain) at 492
nm. Each experimental condition was assayed in duplicate and all
experiments were performed at least three times.
For experiments analyzing synergistic effects in
MG-63 and HEK293 cell lines, cytostatics and NK1R antagonists were
co-cultured at the doses described in the text. For pretreatment
experiments of HEK293, cells were pre-incubated for 24 h with NK1R
antagonist L-733,060. After this, cisplatin and ifosfamide were
added for an additional 24 h and cytotoxicity assay was conducted
as described.
DAPI staining
In order to determine whether apoptosis was induced,
DAPI staining was performed. Briefly, after treatment with
aprepitant for their first doubling times, the cells were fixed in
4% paraformaldehyde. Following a second wash in PBS, cells were
incubated in DAPI solution (Sigma-Aldrich) at a dilution of 1/1,000
(1 μg/ml) for 30 min in the dark. Cells were then analyzed
through a fluorescence microscope (Zeiss, Oberkochen, Germany).
Apoptotic cells were defined by chromatin condensation and nuclear
fragmentation. We counted the number of apoptotic cells, repeating
the counts on three different slides. Finally, on each slide we
counted the number of apoptotic cells located in five different
sequential fields.
Western blot analyses
Total protein was prepared from cell cultures from
MG-63, GAMG, MRC-5 and HEK293 cell lines as previously reported
(13). Protein concentrations were
determined using the protein assay kit from Bio-Rad according to
the manufacturer’s instructions. From each sample, 40 μg of
protein was separated by electrophoresis on 12% SDS-polyacrylamide
gels and electroblotted onto PVDF membranes. Blots were incubated
in blocking solution [5% non-fat milk in PBS, 0.1% Tween-20
(PBS-T)], followed by overnight incubation with an antibody against
the KTMTESSSFYSNMLA conserved domain, corresponding to the
C-terminus of the NK1R (Sigma-Aldrich) and diluted 1:4,000.
Membranes were then washed with PBS-T and incubated with a
horseradish peroxidase-conjugated goat anti-rabbit IgG antibody for
2 h at room temperature (1:10,000 dilution). Antibody detection was
performed with an enhanced chemiluminescence reaction (ECL Western
blotting detection; Amersham Life Science, Amersham, UK).
Pan-cadherin and the secondary antibody alone served as a
control.
Polymerase chain reaction (PCR)
Real-time PCR was carried out for the cell lines
MG-63, GAMG and HEK293 as well as primary human fibroblasts.
RNA extraction and reverse
transcription
From cultured cells, total RNA isolation was
obtained after using the NucleoSpin RNA II Kit (Macherey-Nagel),
allowing approximately the purification of 5×106
cultured cells. Final RNA was dissolved in RNase-free water. The
purity and quality of the RNA were determined with
Nanodrop®. Reverse transcription with elimination of
genomic DNA was performed according to the manufacturer’s
instructions (QuantiTect Reverse transcription Handbook, Qiagen).
We carried out all the reactions on ice in order to minimize the
risk of RNA degradation. The cDNA obtained was kept at −80°C.
Amplification
From the cDNA preparation, 2 μl was used in
PCR with specifics primers (according to the modified method of
Bigioni et al (27) based
on the common sequence of the TAC1 human isoforms (NM 001058, NM
015727) TAC1-F (CTG CTG GTG ATT GGC TAT GC) and
TAC1-R (AGG AGG AAG AAG ATG TGG AAG G) which yield a 186 bp
fragment (28). The amplification
of the specimen was performed in a final reaction volume of 20
μl, and was incubated at 95°C for 7 min, subjected to 40
cycles of 95°C for 30 sec, 60°C for 30 sec and 72°C for 30 sec,
followed by a final extension cycle at 72°C for 7 min. The
amplification products were visualized by electrophoresis on 2%
agarose gel stained with ethidium bromide.
Real-time quantitative RT-PCR
The PCR was performed with the LightCycler FastStart
DNA SYBR-Green I kit (Roche) according to the protocol provided in
the parameter-specific kits and as described previously (12). The transcript numbers were
normalized according to the expression of β-actin per microliter of
cDNA.
Osteosarcoma xenograft
A human osteosarcoma tumor xenograft was generated
from subcutaneous (s.c.) in vivo injection of human MG-63
osteosarcoma cell line (20×106 cells/flank/0.2 ml) in
the right flank of adult athymic female nude mice. Five days after
tumor cell inoculation MG-63 tumor-bearing mice were randomly
divided into treatment and control group. For treatments, all s.c.
injections of fosaprepitant were placed at about 5 mm from the
tumor. Fosaprepitant was administered at doses that have been
previously shown to produce a complete blockade of tachykinin
NK-1-mediated IC100 response maximum inhibition. Tumor
growth was followed by caliber measurement of length and width
twice weekly. Tumor volume (TV) was calculated by using the
formula: volume (mm3) = width2 × length/2.
Fosaprepitant was administered s.c. at doses of 80 mg/kg. Treatment
intervals were once weekly. At the end of each experiment, tumors
were excised and fixed in formalin (37%) for histological
analyses.
Histology
Sections of 3 μm of the tumor were stained
with hematoxylin and eosin. Then, tumor slides were blinded and
interpreted separately by a trained pathologist.
Statistical analyses
Results are expressed as the mean ± standard error
of the mean (SEM). All statistical comparisons were made with a
standard t-test and Mann-Whitney U test, with significance at
p<0.05 using biostatistics software from GraphPad
Prism® (La Jolla, CA, USA). The criterion for
significance was *p<0.05 and **p<0.01
for all comparisons.
Results
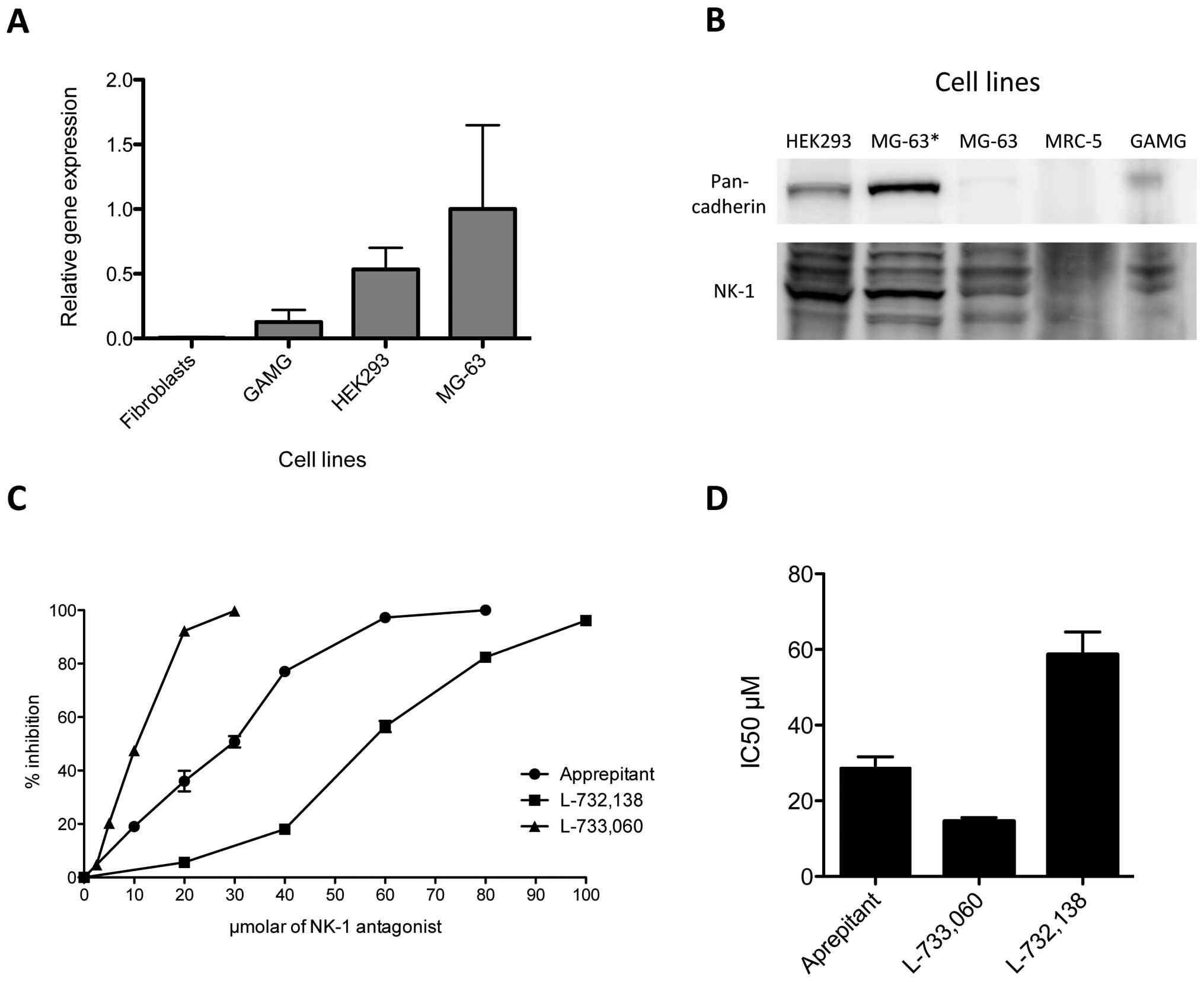
NK-1 receptor expression in human MG-63
osteosarcoma cell line
In order to identify the expression of NK1R in the
human MG-63 osteosarcoma cell line, we performed both RT-PCR and
western blot analyses. RT-PCR was carried out as described and
expression was detected for mRNA encoding the NK1R. β-actin
functioned as a housekeeping gene. We compared expression levels
for human fibroblasts, GAMG cell line, HEK293 and MG-63. Relative
gene expression levels for NK1R were highest in MG-63, followed by
HEK293 with levels of roughly 50% of what was observed in MG-63.
NK1R copies in GAMG were 0.2 compared to MG-63, and human
fibroblasts expressed only marginal amounts of NK1R (Fig. 1A).
For western blot analysis, total cell protein
extracts were loaded onto polyacrylamide gels, resolved and
transferred to membranes as described in Materials and methods.
After incubation with the specific antibody, we observed strong
expression of the NK1R at 46 kDa in MG-63 and HEK293 cell line
(Fig. 1B). Slight expression was
found for the GAMG cell line, and essentially no expression could
be detected for the fibroblast-like cell line MRC-5. Therefore, the
observations made for the expression of mRNA correlated with
protein levels analyzed by western blotting. Pan-cadherin antibody
served as a control.
Growth inhibition of NK-1 receptor
antagonists in the MG-63 cell line
Growth inhibition of the MG-63 cell line by
aprepitant, L-733,060 and L-732,138 was analyzed with MTS
proliferation assay. MG-63 was incubated with increasing
concentrations of all three NK1R antagonists for 48 h. We observed
a concentration-dependent growth inhibition for all three
antagonists (Fig. 1C). L-733,060
showed the strongest effect and reached 47.45% growth inhibition at
10 μM and 99.78% growth inhibition at 30 μM.
L-732,138 showed the weakest antitumor effect, resulting in 56.43%
growth inhibition at 60 μM and 96.17% at 100 μM.
Aprepitant showed robust growth inhibition, with 50.78% inhibition
at 30 μM and 97.25% at 60 μM. Thus, the
concentrations required for a 50% reduction in optical density
(IC50) observed in the controls treated with aprepitant,
L-733,060 and L-732,138 were 28.65, 14.55 and 58.61 μM for
48 h, respectively (Fig. 1D).
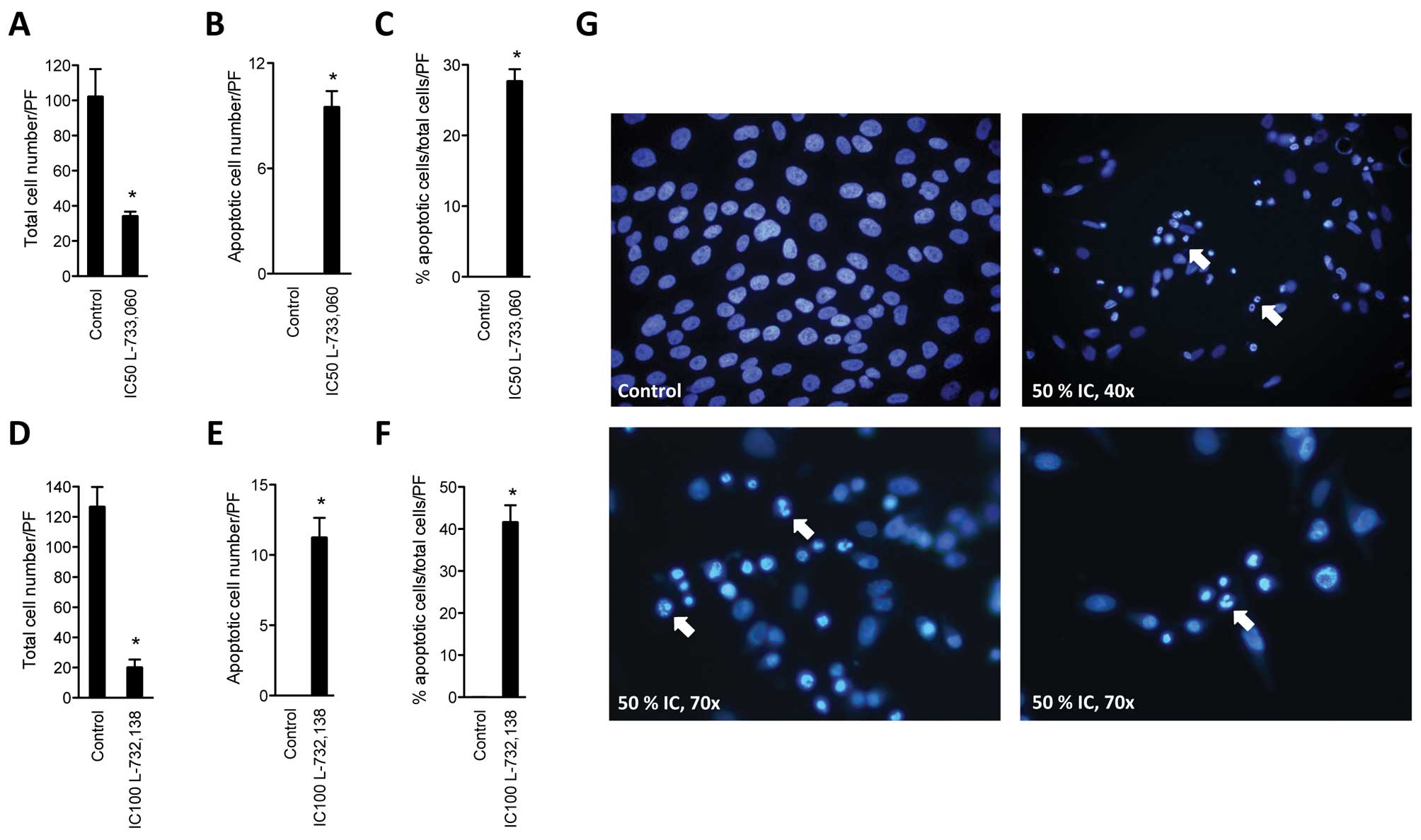
NK-1 receptor antagonists induce
apoptosis of MG-63 cell line
After having confirmed substantial growth inhibition
of MG-63 cell line with NK1R antagonists, the cell line was
cultured with NK1R antagonists and stained with DAPI in order to
determine the rate of induction of apoptosis. After administration
of either NK1R antagonist L-733,060 or L-732,138, a significant
number of apoptotic cells was found in the MG-63 cell line
(Fig. 2). After treatment with
antagonist L-733,060 at IC50 dose, we observed 34.3±2.5
(SEM) living cells per high power field (PF) compared to
102.25±15.6 in the control. Of these, none were apoptotic in the
control (0%). Treatment with L-733,060 lead to 27.7±1.69% apoptotic
cells (Fig. 2A–C). After
administration of L-732,138, we found a total cell number of
20.25±5.1 and 126±13.0 for the control. Again, no apoptotic cells
were found in the control group, but 41.66±4.0% apoptotic cells
were found after treatment with the antagonist (Fig. 2D–F).
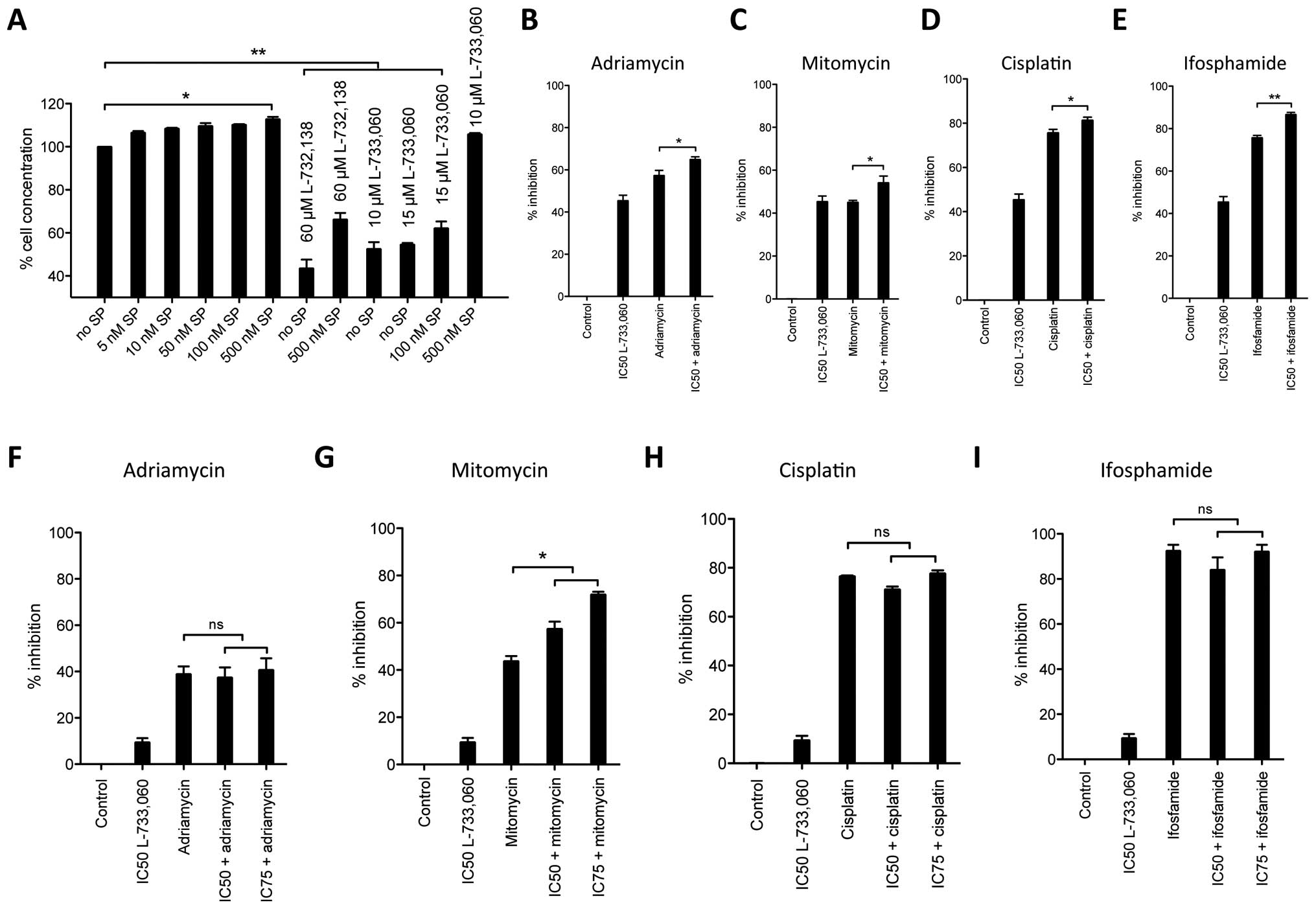
The NK-1 receptor antagonists L-733,060
and L-732,138 block substance P-induced mitogenic stimulation
Knowing that SP, the natural ligand for the NK1R,
acts as a mitogen in other cell lines, we investigated whether this
was also true for the MG-63 cell line. We stimulated cell cultures
with increasing concentrations of SP and observed that nanomolar
concentrations of SP induced cell proliferation as compared to the
untreated controls (Fig. 3A). Even
though small, cell proliferation induced by SP stimulation was
evident at 5 nM (6.56%) and then continuously increased to maximum
level of 12.77% in cellular density at 500 nM. Hence, the
activation of NK1R with its natural ligand SP leads to
proliferation in the human osteosarcoma MG-63 cell line.
We then analyzed whether blockage of NK1R with
antagonists could reverse the SP-induced cell proliferation. In
these experiments, we stimulated MG-63 cell line with 100 and 500
nM SP and blocked the NK1R with different concentrations of NK1R
antagonists L-733,060 and L-732,138. We observed that the mitogenic
action of SP can be reversed in the MG-63 cell line with NK1R
blockage at IC50 concentrations even at high stimulatory
doses of SP [stimulation with 500 nM SP and blockage with
IC50 (60 μM) L-732,138] but not if NK1R
antagonist dose is negligible (stimulation with 500 nM SP and
blockage with 10 μM L-733,060). Therefore, since L-733,060-
and L-733,138-induced growth inhibition was partially reversed by
the administration of nanomolar doses of exogenous SP, these
results indicate that both L-733,060 and L-732,138 block the SP
mitogenic stimulation.
Synergism of NK-1 receptor antagonists
with common cytostatics
After having observed robust antiproliferative and
proapoptotic effects of NK1R antagonists, we studied how this
effect compared to common cytostatic drugs (Fig. 3B–E). In an in vitro cell
culture experiment, MG-63 cell line was cultured with L-733,060
alone at IC50 or together with 10 μM of the
cytostatic drugs adriamycin, mitomycin, ifosfamide or cisplatin. In
these particular experiments, the mean (with SEM) of four separate
experiments was analyzed. Our calculated IC50 dose for
L-733,060 induced 45.4±2.6% SEM cell inhibition and served as a
control for reference throughout this experiment. In the case of
adriamycin, we observed 57.4±2.36% cell inhibition for the
cytostatic drug alone, but 65.0±1.22% together with the antagonist
at IC50 dose (p=0.0292). The cell inhibition induced by
mitomycin was similar to the cell inhibition observed for the NK1R
antagonist alone (45.4±2.6% and 45.1±0.9%, respectively). Again, a
synergistic effect was observed when the cytostatic drug was
combined with the NK1R antagonist (54.2±3.1, p=0.0305). As observed
with adriamycin, cisplatin induced more cell inhibition than the
NK1R antagonist alone (75.7±2.6% and 45.4±2.6%, respectively).
Combining the NK1R antagonist and cisplatin induced 81.36±1.4% cell
inhibition (p=0.0349). Ifosfamide induced 75.7±1.1% cell inhibition
when given alone and 86.66±1.0% when given together with the NK1R
antagonist, again showing an increased effect (p=0.030).
Effect of NK-1 receptor antagonists on
non-malignant HEK293 cells
Given the robust inhibitory effect observed in MG-63
cell line we assessed whether this inhibition could be unfolded in
non-malignant cells as well. We incubated HEK293 cells with NK1R
antagonist L-733,060 and common cytostatic drugs and measured
cytotoxicity as described (Fig.
3F–I). We intentionally did not recalculate the IC50
and IC75 dosage for HEK293, but used the same dosage we
had calculated for MG-63 cells. While observing cell inhibition of
only 9.4±1.8% for the NK1R antagonist in these cells, we observed
robust cell inhibition by the cytostatic drugs in the range of
39–92.1% (adriamycin 38.9±3.35%, mitomycin 43.8±2.17%, cisplatin
76.6±0.3% and ifosfamide 92.1±3.1%, respectively). However, in
contrast to what we observed in the malignant MG-63 cell line, when
combined with NK1R antagonists, in HEK293 cytostatic drugs induced
similar (for adriamycin and mitomycin, Fig. 3F and G) or less (for cisplatin and
ifosfamide, Fig. 3H and I) cell
inhibition compared to the cytostatic applied alone
(IC50 L-733,060 with adriamycin 37.4±4.41%, mitomycin
57.4±3.0%, cisplatin 71.2±1.1% and ifosfamide 84.0±5.6%).
Therefore, importantly, with the exception of mitomycin, no
increased effects between the NK1R antagonists used at either
IC50 or IC75 and the cytostatic drugs were
observed in non-malignant HEK293 cells.
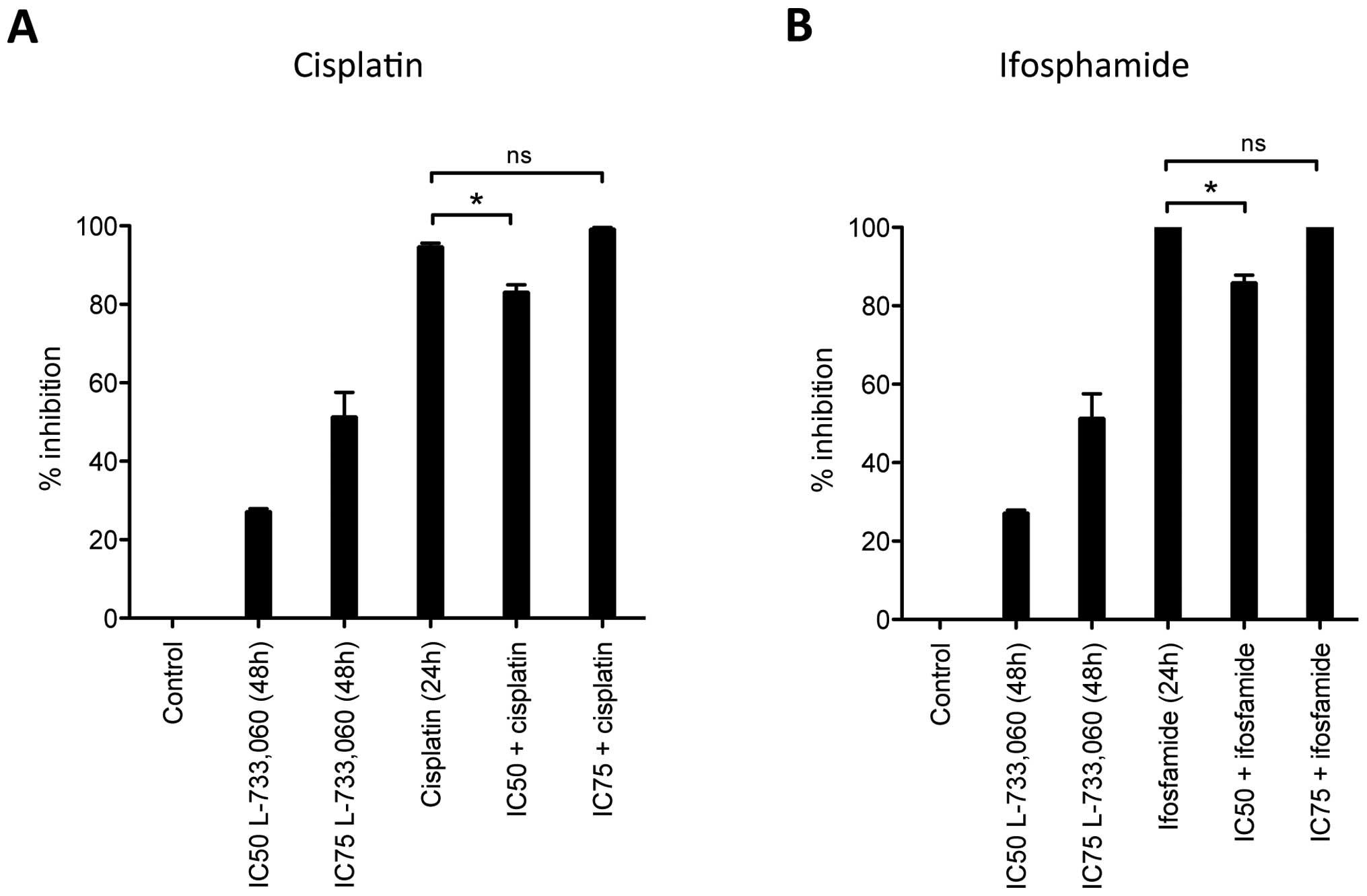
Given the effect observed for L-733,060 in HEK293
cells, in a further experiment, instead of simultaneous incubation
with the NK1R antagonist and the respective cytostatic drug, we
pre-incubated HEK293 cells for 24 h with NK1R antagonist L-733,060
and then added the cytostatic drug for an additional 24 h (Fig 4). Interestingly, we observed a
reduced inhibition after this pre-treatment with NK1R antagonist
resulting in less cytotoxicity from cisplatin and ifosfamide.
Cisplatin alone induced 94.6±0.9% cell inhibition but only
83.1±1.9% cell inhibition, if cells were pre-incubated with
L-733,060 at IC50 dose for 24 h. The reduced inhibition
achieved by the NK1R antagonist for ifosfamide application was even
stronger, showing total cell death (100%) for the cytostatic alone
but only 85.9±2.0% when combined with L-733,060. Of note, this
effect was lost if higher concentrations (IC75) of the
NK1R antagonist were used for pre-treatment.
Treatment of the human MG-63 mouse
xenograft with fosaprepitant
Having observed a robust antitumor effect in
vitro, we then studied the effect of NK1R antagonist
fosaprepitant in vivo. Nude mice were xenografted with human
MG-63 osteosarcoma cell line as described in Materials and methods
and randomly separated into control and treatment groups once
tumors became visible. Subsequently, treatments with peritumoral
s.c. injections of fosaprepitant were started and were repeated
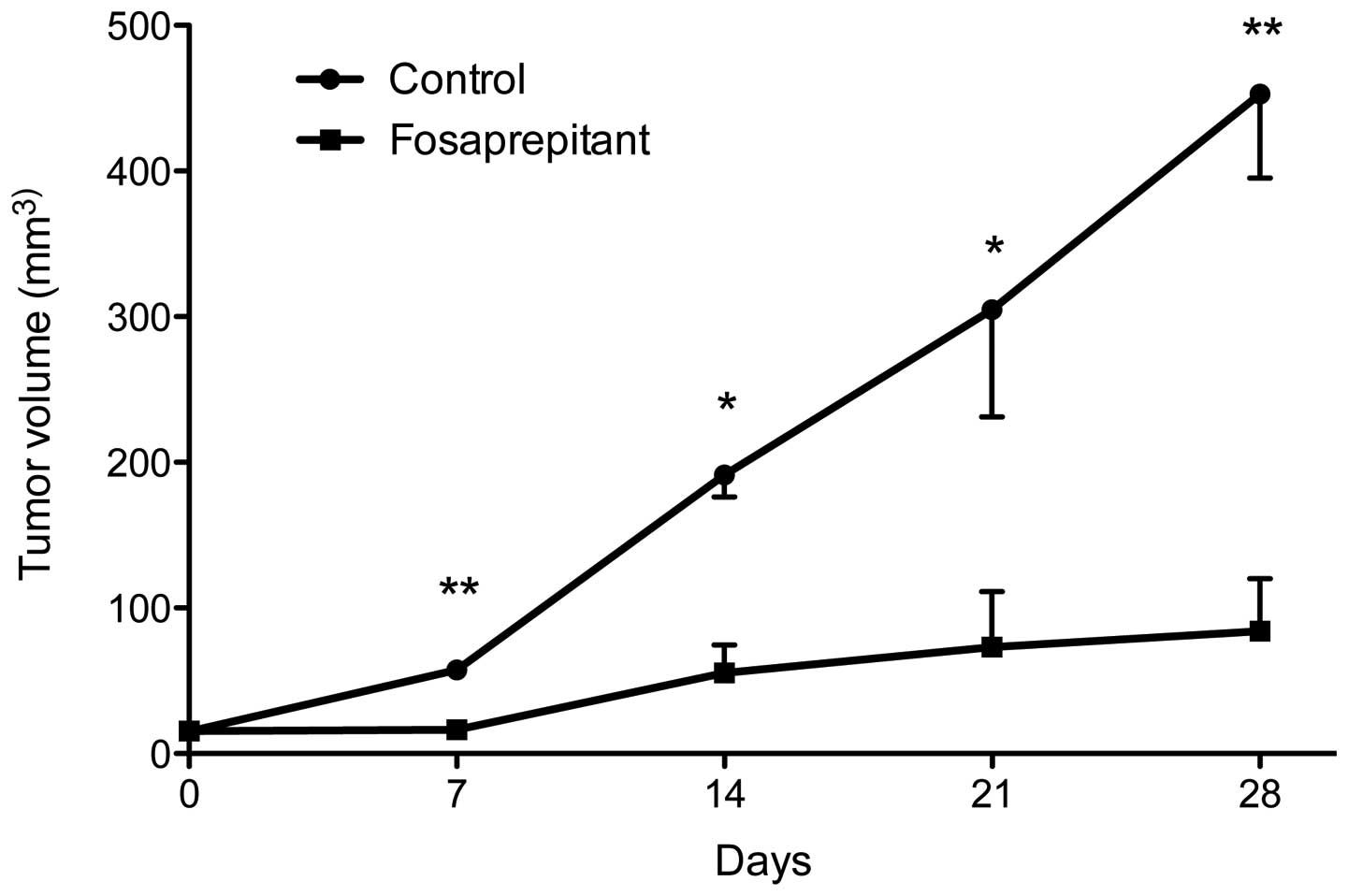
weekly from thereon (days 0, 7, 14, 21 and 28, see Fig. 5). The control group was injected
with phosphate-buffered saline (PBS) at the exact same time points
as the fosaprepitant injections. At each treatment point,
measurement of tumor size was obtained as well as one week after
the last injection (on day 28). The control group consisted of 4
mice, the treatment group consisted of 5 mice. At 7 days, tumor
volume in the control group was 57.6±4.9 and 16.4±4.3
mm3 in the treatment group (p<0.01). After 14 days,
tumors in the control group were 191.4±15.2 and 55.4±19.2
mm3 in the treatment group (p<0.05). At 21 and 28
days, the tumor volume was 304.7±53.6 and 452.9±57.7
mm3, respectively, in the control group and 73.2±38.2
and 84.2±35.8 mm3, respectively, in the treatment group
(p<0.05 at day 21 and p<0.01 at day 28). Taken together, we
saw a strong, statistically highly significant therapeutic effect
in mice xenografted with the osteosarcoma cell line MG-63 after
treatment with NK1R antagonist fosaprepitant.
Histology of treated mice
After completion of the in vivo experiments,
the mice were sacrificed and their histologic features were
analyzed regarding necrosis, apoptosis, mitosis, nuclear grade and
viability (Table I). In the
control group an average necrosis of 18.75±10.3% was found,
compared to 28.0±9.1 in the treatment group. Even though this marks
a clear difference, no statistical significance was found
(p=0.1952). The same is true for the rate of apoptosis, mitosis and
nuclear grade (Table I). Viability
in the control group was higher (62.5±20.6%) compared to the
treatment group (45.0±19.2%), but due to the small sample size,
differences were not statistically significant (p=0.2253).
Histologic analysis of all tumor specimens clearly showed features
of human osteosarcoma.
 | Table I.Histological analysis of in
vivo treated osteosarcomas of human MG-63 xenografted mice. |
Table I.
Histological analysis of in
vivo treated osteosarcomas of human MG-63 xenografted mice.
| Control | Treatment | P-value |
|---|
| Necrosis | 18.75±10.3 | 28.0±9.1 | 0.1952 |
| Apoptosis | 7.5±3.5 | 10.0±5.0 | 0.4186 |
| Mitosis | 13.25±1.5 | 14.4±3.3 | 0.5415 |
| Nuclear grade | 2.25±0.5 | 3.0±0.0 | 0.6845 |
| Viability | 62.5±20.6 | 45.0±19.2 | 0.2253 |
Discussion
Osteosarcoma, especially if metastases are present,
is traditionally known to be a tumor with only modest long-term
survival. Recent therapeutic changes and a multidisciplinary
approach have increased the prognosis and close to two-thirds of
patients can now be cured. Adjuvant chemotherapy has an established
role in osteosarcoma in the control of subclinical metastatic
disease. The most effective chemotherapeutic agents currently in
use include high-dose methotrexate, doxorubicin, cisplatin and
ifosfamide/etoposide (29).
However, if metastatic spread has occurred, especially to other
parts of the skeletal system, the prognosis is poor and survival is
less than 20% (30). Therefore,
additional therapeutic strategies are desperately required as part
of future, multidisciplinary treatment approaches against
osteosarcoma.
The SP/NK-1 receptor system has recently been
identified as a potential antitumor target (3,4).
Blocking this receptor system with NK1R antagonists is currently
under intense investigation for future anticancer strategies. To
the best of our knowledge, until now nothing has been described
regarding the expression of this receptor system in human
osteosarcoma cell lines. Therefore, with the data presented here,
for the first time we describe that NK1R is expressed in human
osteosarcoma cell lines and that blocking of this receptor with
non-peptide derived NK1R antagonists such as the clinical drug
aprepitant and other NK1R antagonists results in robust tumor
inhibition both in vitro and in vivo.
The NK1R is a tachykinin receptor with high affinity
for the short neuropeptide SP, which is considered its natural
ligand (31). Interestingly, it
has been observed that presence of NK1R expression was related to
the tumor cell subtype, with higher expression in the luminal human
breast cancer cell lines expressing Her2 (SKBR3, BT474 and
MDA-MD-453) than in the basal-like breast cancer cell lines
(MDA-MB-468, MDA-MB-231, MCF10A and MCF12A). Moreover, the
pro-apoptotic effect of NK1R inhibition was greater in cells with
higher levels of NK1R expression, suggesting ‘oncogenic addiction’
of these cells to NK1R signaling (32).
Substantial efforts have been made to develop
therapeutic inhibitors against NK1R in the last decade. Small
molecules such as antagonists of NK1R represent an important
opportunity to further exploit compounds that are active against
this receptor as novel therapeutic agents (3). One promising compound is aprepitant
(Emend), currently approved by the Food and Drug Administration
(FDA) for the treatment of chemotherapy-induced nausea and vomiting
and is available for oral use (33). It has also been successfully used
as an off-label drug in the treatment of pain, migraine, emesis and
certain psychiatric disorders. Fosaprepitant (Ivemend) is a
water-soluble phosphoryl prodrug of aprepitant and is available for
intravenous use (19,22). In the present study, we
demonstrated that treatment of the human osteosarcoma cell line
with NK1R antagonists L-733,060, L-732,138 and aprepitant produced
robust growth inhibition and cell death by apoptosis in
vitro. On the other hand, exogenous SP induced cellular
proliferation. When combining the two, we were able to show that
cell proliferation induced by exogenous SP was partially reverted
by administration of NK1R antagonists, suggesting a specific effect
through the NK1R.
Our findings are in accordance with the recent
understanding that NK1R antagonists have great antitumor potential
against a large variety of human cancer cell lines such as human
neuroblastoma, glioma, melanoma, retinoblastoma, pancreatic
adenocarcinoma and many others (3). This observation suggests the
possibility of a common mechanism for cancer cell proliferation
mediated by SP and NK1R. If this was truly the case, targeting the
NK1R with competing antagonists could potentially inhibit a large
number of tumor cell types and tumors and could function as
broad-spectrum antineoplastic drugs (3). While these observations are
encouraging, it must be pointed out that at this early stage of
investigation, little is known about possible tumor-escape
mechanisms regarding the SP/NK-1 receptor system. Since this is the
case with essentially all chemotherapeutic agents in a large
variety of tumor types, under favorable circumstances, a
single-target approach will most likely result in tumor-escape.
Whether this will be the case with targeting the SP/NK-1 receptor
system is impossible to predict at the moment, and further
investigation needs to take into account this possibility when
developing NK-1 receptor targeting anticancer drugs.
One of the most interesting findings among our
results is our analysis of NK1R in HEK293 cells and its subsequent
response to NK1R ligands. HEK 293 is a cell line derived from
embryonic kidney tissue. This cell line is considered immortal, but
benign. In our experiments, despite robust expression of the NK1R,
cell inhibition with NK1R antagonists was significantly smaller
compared to the MG-63 osteosarcoma cell line. This finding by
itself endorses that the observed effects in vitro of NK1R
antagonists are not general toxic effects. This becomes even
clearer when interpreting our findings regarding the simultaneous
treatment of cell lines in vitro with NK1R ligands and
common cytostatic drugs. While there was a clear synergism between
cytostatic drugs and NK1R antagonists in the case of a malignant
human osteosarcoma cell line, this effect was not seen in the
benign cell line HEK293. When the NK1R antagonist was given
together with the cytostatic, the sole presence of the NK1R
antagonists seemed to have a protective effect resulting in less
cell inhibition compared to the cytostatic alone at least for some
cytostatic drugs. Indeed and of utmost importance, compared to
cytotoxicity in malignant cells, growth inhibition of the benign
cell line HEK293 was only minimal. Whether this selectivity is
exclusively due to the fact that one cell is malignant and the
other benign, is impossible to determine from our results. Such a
specific selectivity between malignant and benign cells has been
previously hypothesized by others and was explained by
overexpression of the NK1R in several malignant cells compared to
benign cells (20). In our study,
we clearly see a correlation between the relative gene expression
and the response rate of tumor growth to NK1R antagonists.
Therefore, our data provided here support this concept. It will be
interesting and necessary to investigate in closer detail whether
targeting the NK1R truly results in selectivity for malignant
tissue, and what additional mechanisms are responsible.
An in vivo anticancer effect for NK1R
antagonist has previously been reported by Palma et al
(26). In their study, nude mice
xenografted subcutaneously with malignant glioma cell line U373 MG
showed significant reduction of tumor size after treatment with
NK1R antagonists. This therapeutic effect could be achieved both
with intravenous and s.c. treatment and was partially reversible
with administration of SP. In another study by Bigioni et al
a similar in vivo effect was observed for treatment with
NK1R antagonists in the human breast carcinoma cell line MDA-MB-231
xenografted mice. Interesting in their study was the observation
that in vivo targeting of both NK1R and NK-2 resulted in a
therapeutic effect (27). In
accordance with these findings, in our study, we observed a
stunning therapeutic effect for osteosarcoma xenografted nude mice.
However, we have currently no understanding on which method of
application is ideal for treatment of xenografted mice, and whether
conclusions of such experiments in animal models can be drawn
regarding possible treatment approaches in humans. We used
peritumoral s.c. injections as the mode of application, which was
therefore different from intravenous injection used by Palma et
al (26) and Bigioni et
al (27). Nonetheless, all
methods of application resulted in a therapeutic effect.
It is known that those osteosarcomas which show
relatively low degrees of necrosis after administration of
chemotherapy have poorer survival than patients with more
chemotherapy-responsive tumors (29,30).
In our study, we observed histologic changes similar to those found
after chemotherapy in osteosarcoma treatment. Even though no
statistical differences could be calculated between the two groups,
our findings clearly show a higher rate of necrosis and apoptosis
paired with a decreased rate of viable cells in the histology of
the treatment group compared to the control group (Table I). Whether NK1R can have a role as
anticancer agent in those osteosarcomas that show low response
rates to chemotherapy is impossible to say from our data and
requires further, intense investigation.
In summary, we have demonstrated for the first time
that human osteosarcoma cell line MG-63 expresses the NK1R and that
the clinical drug aprepitant and other NK1R antagonists can inhibit
cell growth and induce apoptosis both in vitro and in
vivo in this tumor. This effect was specifically mediated
through the NK1R, and selectivity was observed for malignant cells
compared to benign cells. Also, NK1R antagonist showed a
synergistic effect on osteosarcoma cells when combined with common
cytostatics but not on benign HEK293 cells. Despite these
encouraging results presented here, many questions remain
unanswered. This includes the important question on whether the
SP/NK-1 receptor system is ubiquitously expressed in tumor cells
and represents a universal survival pathway in cancer cells,
whether targeting of NK1R leads to tumor escape mechanisms by the
tumor cell and whether overexpression of NK1R is entirely
responsible for the observed selectivity between malignant and
benign cells, thereby, giving hints on what type of tumors might be
treated with these antagonists. Nevertheless, our result clearly
identify the NK1R as a promising therapeutic target in osteosarcoma
and NK1R antagonists should be further explored for the use in
future anticancer strategies in patients that suffer from this
cancer.
Acknowledgements
We thank Raquel Gómez from the
Instituto de Biomedicina de Sevilla (IBIS) for technical assistance
when performing and analyzing western blot experiments. Michael
Berger was supported by the German Academic Exchange Program
(DAAD).
References
|
1.
|
Wittig JC, Bickels J, Priebat D, et al:
Osteosarcoma: a multidisciplinary approach to diagnosis and
treatment. Am Fam Physician. 65:1123–1132. 2002.PubMed/NCBI
|
|
2.
|
Ritter J and Bielack SS: Osteosarcoma. Ann
Oncol. 21(Suppl 7): vii320–vii325. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Muñoz M, Rosso M and Coveñas R: A new
frontier in the treatment of cancer: NK-1 receptor antagonists.
Curr Med Chem. 17:504–516. 2010.PubMed/NCBI
|
|
4.
|
Muñoz M, Rosso M and Coveñas R: The NK-1
receptor: a new target in cancer therapy. Curr Drug Targets.
12:909–921. 2011.PubMed/NCBI
|
|
5.
|
Vanden Broeck J, Torfs H, Poels J, et al:
Tachykinin-like peptides and their receptors. A review. Ann NY Acad
Sci. 897:374–387. 1999.PubMed/NCBI
|
|
6.
|
Patacchini R and Maggi CA: Peripheral
tachykinin receptors as targets for new drugs. Eur J Pharmacol.
429:13–21. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Rollandy I, Dreux C, Imhoff V and
Rossignol B: Importance of the presence of the N-terminal
tripeptide of substance P for the stimulation of
phosphatidylinositol metabolism in rat parotid gland: a possible
activation of phospholipases C and D. Neuropeptides. 13:175–185.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Pradier L, Heuillet E, Hubert JP, et al:
Substance P-evoked calcium mobilization and ionic current
activation in the human astrocytoma cell line U 373 MG:
pharmacological characterization. J Neurochem. 61:1850–1858. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Luo W, Sharif TR and Sharif M: Substance
P-induced mitogenesis in human astrocytoma cells correlates with
activation of the mitogen-activated protein kinase signaling
pathway. Cancer Res. 56:4983–4991. 1996.
|
|
10.
|
DeFea KA, Zalevsky J, Thoma MS, et al:
Beta-arrestin-dependent endocytosis of proteinase-activated
receptor 2 is required for intracellular targeting of activated
ERK1/2. J Cell Biol. 148:1267–1281. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Palma C: Tachykinins and their receptors
in human malignancies. Curr Drug Targets. 7:1043–1052. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Muñoz M, González-Ortega A and Coveñas R:
The NK-1 receptor is expressed in human leukemia and is involved in
the antitumor action of aprepitant and other NK-1 receptor
antagonists on acute lymphoblastic leukemia cell lines. Invest New
Drugs. 30:529–540. 2012.PubMed/NCBI
|
|
13.
|
Muñoz M, Rosso M, Pérez A, et al: The NK1
receptor is involved in the antitumoural action of L-733,060 and in
the mitogenic action of substance P on neuroblastoma and glioma
cell lines. Neuropeptides. 39:427–432. 2005.PubMed/NCBI
|
|
14.
|
Muñoz M, Rosso M, Pérez A, et al:
Antitumoral action of the neurokinin-1-receptor antagonist
L-733,060 and mitogenic action of substance P on human
retinoblastoma cell lines. Invest Ophthalmol Vis Sci. 46:2567–2570.
2005.PubMed/NCBI
|
|
15.
|
Muñoz M, Rosso M and Coveñas R: The NK-1
receptor is involved in the antitumoural action of L-733,060 and in
the mitogenic action of substance P on human pancreatic cancer cell
lines. Lett Drug Des Discov. 3:323–329. 2006.PubMed/NCBI
|
|
16.
|
Muñoz M, Rosso M, Aguilar F, et al: NK-1
receptor antagonists induce apoptosis and counteract substance
P-related mitogenesis in human laryngeal cancer cell line HEp-2.
Invest New Drugs. 26:111–118. 2008.PubMed/NCBI
|
|
17.
|
Rosso M, Robles-Frias MJ, Coveñas R,
Salinas-Martín MV and Muñoz M: The NK-1 receptor is expressed in
human primary gastric and colon adenocarcinomas and is involved in
the antitumor action of L-733,060 and the mitogenic action of
substance P on human gastrointestinal cancer cell lines. Tumour
Biol. 29:245–254. 2008. View Article : Google Scholar
|
|
18.
|
Muñoz M, Pérez A, Rosso M, et al: The NK-1
receptor is expressed in human melanoma and is involved in the
antitumor action of the NK-1 receptor antagonist aprepitant on
melanoma cell lines. Lab Invest. 90:1259–1269. 2010.
|
|
19.
|
Navari RM: Fosaprepitant (MK-0517): a
neurokinin-1 receptor antagonist for the prevention of
chemotherapy-induced nausea and vomiting. Expert Opin Investig
Drugs. 16:1977–1985. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Muñoz M and Rosso M: The NK-1 receptor
antagonist aprepitant as a broad spectrum antitumor drug. Invest
New Drugs. 28:187–193. 2010.PubMed/NCBI
|
|
21.
|
Quartara L, Altamura M, Evangelista S and
Maggi CA: Tachykinin receptor antagonists in clinical trials.
Expert Opin Investig Drugs. 18:1843–1864. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Quartara L and Altamura M: Tachykinin
receptors antagonists: from research to clinic. Curr Drug Targets.
7:975–992. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Muñoz M, Pérez A, Rosso M, Zamarriego C
and Rosso R: Antitumoral action of the neurokinin-1 receptor
antagonist L-733 060 on human melanoma cell lines. Melanoma Res.
14:183–188. 2004.PubMed/NCBI
|
|
24.
|
Muñoz M, Rosso M, Casinello F and Coveñas
R: Paravertebral anesthesia: how substance P and the NK-1 receptor
could be involved in regional block and breast cancer recurrence.
Breast Cancer Res Treat. 122:601–603. 2010.PubMed/NCBI
|
|
25.
|
Muñoz M and Coveñas R: Neurokinin-1
receptor: a new promising target in the treatment of cancer. Discov
Med. 10:305–313. 2010.PubMed/NCBI
|
|
26.
|
Palma C, Bigioni M, Irrissuto C, et al:
Anti-tumour activity of tachykinin NK1 receptor antagonists on
human glioma U373 MG xenograft. Br J Cancer. 82:480–487.
2000.PubMed/NCBI
|
|
27.
|
Bigioni M, Benzo A, Irrissuto C, Maggi CA
and Goso C: Role of NK-1 and NK-2 tachykinin receptor antagonism on
the growth of human breast carcinoma cell line MDA-MB-231.
Anticancer Drugs. 16:1083–1089. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Muñoz M, Rosso M, Pérez A, et al:
Neurokinin-1 receptors located in human retinoblastoma cell lines:
antitumor action of its antagonist, L-732,138. Invest Ophthalmol
Vis Sci. 48:2775–2781. 2007.PubMed/NCBI
|
|
29.
|
Ayerza MA, Farfalli GL, Aponte-Tinao L and
Muscolo DL: Does increased rate of limb-sparing surgery affect
survival in osteosarcoma? Clin Orthop Relat Res. 468:2854–2859.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Weber K, Damron TA, Frassica FJ and Sim
FH: Malignant bone tumors. Instr Course Lect. 57:673–688. 2008.
|
|
31.
|
Hökfelt T, Pernow B and Wahren J:
Substance P: a pioneer amongst neuropeptides. J Intern Med.
249:27–40. 2001.PubMed/NCBI
|
|
32.
|
Almendro V, García-Recio S and Gascón P:
Tyrosine kinase receptor transactivation associated to G
protein-coupled receptors. Curr Drug Targets. 11:1169–1180. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Tattersall FD, Rycroft W, Cumberbatch M,
et al: The novel NK1 receptor antagonist MK-0869 (L-754,030) and
its water soluble phosphoryl prodrug, L-758,298, inhibit acute and
delayed cisplatin-induced emesis in ferrets. Neuropharmacology.
39:652–663. 2000. View Article : Google Scholar
|