Introduction
Gastric cancer (GC) is one of the most common
malignancies worldwide, with an estimated 934,000 cases reported
globally in 2002, and is the second most common cause of death from
cancer. The prognosis of GC is poor with an estimated relative
5-year survival rate of <20% (1). GC is a genetic disease developing
from a multi-step process. Single or multiple mutations in genes
related to growth control, invasion and metastasis form the
molecular genetic basis of malignant transformation and tumor
progression (2). Therefore,
identification of key genes and targets related to tumorigenesis is
crucial for diagnosis and prevention of GC.
Apoptosis plays a pivotal role in sustaining proper
tissue development and homeostasis. Evading apoptosis by cancer
cells is a part of their adaption to microenvironment and
therapies. Pro-survival molecules including Livin can protect tumor
cells from apoptosis and mediate metastatic processes, thus
enhancing aggressive phenotype (3). Livin, an identified member of the
inhibitor-of-apoptosis protein (IAP) family of anti-apoptosis
proteins, is expressed in a variety of tumors including melanoma,
neuroblastoma, mesothelioma and osteosarcoma (4–6). A
high level of Livin protein expression plays a role in the
progression of melanoma and correlates with survival and a poor
prognosis (7). Overexpression of
Livin stimulates cell proliferation and inhibits chemical induced
apoptosis in bladder cancer, suggesting it as a promising marker to
identify the relapse risk in bladder cancer (8). Livin expression also increases
resistance to apoptotic stimuli, and contributes significantly to
the proliferation and invasive capacity of hepatocellular carcinoma
(HCC) cells (9), while targeted
inhibition of Livin by peptides represents a viable approach for
the apoptotic sensitization and growth inhibition of tumor cells
(10). Thus, targeting Livin may
offer a therapeutic benefit in apoptosis-inducing treatment.
Livin plays an important role in drug resistance and
radiation sensitivity of some cancers. It is highly expressed in
colon cancer cells resistant to several antitumor drugs and
knockdown of the expression reverses drug resistance phenotype of
tumor cells (11). Livin increases
resistance to doxorubicin and etoposide in MYCN oncogene amplified
neuroblastoma (12).
Overexpression of Livin inhibits the activation of caspase-3 and
leads to resistance to cisplatin, while Livin knockdown enhances
its sensitive in colorectal cancer (CRC) cells (13). The IAP Livin is also an important
molecule in anti-radiotherapy, and Livin-specific gene silencing is
likely to be an effective means to enhance radiation sensitivity of
lung cancer (14). These studies
highlight the potential of Livin for cancer therapy.
However, some studies have shown that Livin plays a
dual role in tumorigenicity. Livin α promotes tumor initiation,
while the growth of tumors originating from cells expressing Livin
β is inhibited (15). Livin
expression does not correlate with pathological or clinical
parameters and are not predictive of patient outcome (16,17).
Thus, we need to explore further the clinical significance and
function of Livin in GC. We hypothesized that Livin expression
correlated with pathological or clinical parameters of patients
with GC, and knockdown of Livin suppressed cell growth and
invasion, and induced cell apoptosis in GC cells via inhibition of
the MAPK pathway.
Materials and methods
Materials
The human SGC-7901 GC cell line used in the
experiments was from Institute of Biochemistry and Cell Biology
(Shanghai, China). Lv-shLivin, negative control vector and
virion-packaging elements were from Genechem (Shanghai, China). The
primers of Livin, VEGF and CAS-3 were synthesized by ABI Prism
(USA). All antibodies were from Santa Cruz Biotechnology (Santa
Cruz, CA, USA).
Drugs and reagents
Dulbecco’s modified Eagle’s medium (DMEM) and fetal
bovine serum (FBS) were from Thermo Fisher Scientific Inc.
(Waltham, MA, USA); TRIzol reagent and Lipofectamine 2000 were from
Invitrogen (Carlsbad, CA, USA); M-MLV Reverse Transcriptase was
from Promega (Madison, WI, USA); SYBR Green Master Mixture was from
Takara (Otsu, Japan). ECL-PLUS/kit was from GE Healthcare
(Piscataway, NJ, USA). In-situ cell death kit was from
Boehringer-Mannheim (Germany).
Clinical samples and data
Human GC tissues and corresponding ANCT were
obtained from biopsy prior to chemotherapy in a total of 78
consecutive cases of GC admitted in our hospital from January 2008
to December 2011. The study was approved by Medical Ethics
Committee of Shanghai Jiaotong University and written informed
consent was obtained from the patients or their parents before
sample collection. Two pathologists respectively reviewed all of
the cases.
Immunohistochemical (IHC) staining
Anti-Livin and CAS-3 antibodies were used for IHC
detection of the expression of Livin and CAS-3 proteins in tissue
microarrays. Tissue micro-array sections were processed for IHC
analysis of Livin and CAS-3 protein as follows: IHC examinations
were carried out on 3-mm thick sections. For anti-Livin and CAS-3
IHC, unmasking was performed with 10 mM sodium citrate buffer, pH
6.0, at 90°C for 30 min. For anti-Livin and CAS-3 IHC, antigen
unmasking was not necessary. Sections were incubated in 0.03%
hydrogen peroxide for 10 min at room temperature, to remove
endogenous peroxidase activity and then in blocking serum (0.04%
bovine serum albumin, A2153, Sigma-Aldrich, Shanghai, China and
0.5% normal goat serum X0907, Dako Corp., Carpinteria, CA, USA, in
PBS) for 30 min at room temperature. Anti-Livin and CAS-3
antibodies were used at a dilution of 1:200. The antibodies were
incubated overnight at 4°C. Sections were then washed three times
for 5 min in PBS. Non-specific staining was blocked with 0.5%
casein and 5% normal serum for 30 min at room temperature. Finally,
staining was developed using diaminobenzidine substrate and
sections were counterstained with hematoxylin. Normal serum or PBS
was used to replace anti-Livin and CAS-3 antibodies in negative
controls. Livin and CAS-3 expression was semi-quantitatively
estimated as the total IHC staining score. The proportion score
reflected the fraction of positive staining cells (score 0, <5%;
score 1, 5–10%; score 2, 10–50%; score 3, 50–75%; score 4,
>75%), and the intensity score represented the staining
intensity (score 0, no staining; score 1, weak positive; score 2,
moderate positive; score 3, strong positive). Finally, a total
expression score was given ranging from 0 to 12. Based on the
analysis in advance, Livin and CAS-3 were regarded as negative
expression in GC if the score was <2, and positive expression if
the score was ≥2.
Cell culture and transfection
GC SGC-7901 cells were cultured in DMEM medium
supplemented with 10% heat-inactivated FBS, 100 U/ml of penicillin
and 100 μg/ml of streptomycin. They were all placed in a
humidified atmosphere containing 5% CO2 at 37°C. Cells
were subcultured at a 1:5 dilution in medium containing 300
μg/ml G418 (an aminoglycoside antibody, commonly used stable
transfection reagent in molecular genetic testing). On the day of
transduction, GC cells were replated at 5×104 cells/well
in 24-well plates containing serum-free growth medium with
polybrene (5 mg/ml). When confluence reached 50%, cells were
transfected with recombinant experimental virus or control virus at
the optimal MOI (multiplicity of infection) of 50, and cultured at
37°C and 5% CO2 for 4 h. Then supernatant was discarded
and serum containing growth medium was added. At 4 days of
post-transduction, transduction efficiency was measured by the
frequency of green fluorescent protein (GFP)-positive cells.
Positive stable transfectants were selected and expanded for
further study. The clone in which the Lv-shLivin was transfected
was named as shLivin group, the negative control vector transfected
was named as NC group, and SGC-7901 cells were named as CON
group.
Quantitative real-time PCR
To quantitatively determine the mRNA expression
levels of Livin, VEGF and CAS-3 in GC SGC-7901 cells, real-time PCR
was used. Total RNA of each clone was extracted with TRIzol
according to the manufacturer’s protocol. Reverse-transcription was
carried out using M-MLV and cDNA amplification was carried out
using SYBR Green Master Mix kit according to the manufacturer’s
protocol. Target genes were amplified using a specific
oligonucleotide primer and human glyceraldehyde-3-phosphate
dehydrogenase (GAPDH) gene was used as an endogenous control. The
PCR primer sequences were as follows: Livin, 5′-CGCACGGCACA
AAGACGA-3′ and 5′-GTCAGTTCCTGCTCCGGTCAA-3′; p38 MAPK,
5′-AACCTGTCCCCGGTGGGCTCG-3′ and 5′-CGATGTCCCGTCTTTGTATGA-3′; VEGF,
5′-GGTGAG AGGTCTAGTTCCCGA-3′ and 5′-CCATGAACTTTCTG CTCTTC-3′;
MMP-2, 5′-GGCCCTGTCACTCCTGAGAT-3′ and 5′-GGCATCCAGGTTATCGGGGA-3′;
CAS-3, 5′-CAG ACAGTGGAACTGACGAT-3′ and 5′-TTTCAGCATGGC GCAAAGTG-3′;
β-actin, 5′-AGCCATGTACGTAGCCA TCC-3′ and
5′-CTCTCAGCTGTGGTGGTGAA-3′. Data were analyzed using the
comparative Ct method (2−ΔΔCt). Three separate
experiments were performed for each clone.
Western blot assay
GC SGC-7901 cells were harvested and extracted using
lysis buffer (Tris-HCl, SDS, mercaptoethanol, glycerol). Cell
extracts were boiled for 5 min in loading buffer and then equal
amount of cell extracts were separated on 15% SDS-PAGE gels.
Separated protein bands were transferred into polyvinylidene
fluoride (PVDF) membranes and the membranes were blocked in 5% skim
milk powder. The primary antibodies against Livin, MAPK, p-MAPK,
VEGF, MMP-2 and CAS-3 were diluted according to the instructions of
antibodies and incubated overnight at 4°C. Then, horseradish
peroxidase-linked secondary antibodies were added at a dilution
ratio of 1:1,000, and incubated at room temperature for 2 h. The
membranes were washed with PBS three times and the immunoreactive
bands were visualized using ECL-PLUS/kit according to the kit
instructions. The relative protein level in different cell lines
was normalized to β-actin concentration. Three separate experiments
were performed for each clone.
Fluorescence microscopy
Twenty-four hours after transfection, cells were
plated on glass cover slips and 48 h post transfection the cover
slips were washed extensively in phosphate-buffered saline (PBS)
and fixed with 4% paraformaldehyde in PBS. After additional
washing, the cells were permeabilized with 1% Triton X-100 in PBS
for 10 min. The cover slips were then washed and blocked with 1%
BSA for 30 min. Cells were incubated in the appropriate primary
antibodies (Livin, VEGF and CAS-3) overnight at 4°C. Samples were
then washed and incubated with species-specific secondary
rhodamine-labeled antibodies (TRITC) in PBS (1:100 dilution) for 60
min. Nuclei were stained with DAPI at RT for 10 min and cover slips
mounted with Antifade solution prior to imaging on a confocal
microscope.
MTT assay
Cell growth in vitro was evaluated using a
3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT;
Sigma) assay. Briefly, 1×104 cells were plated in
96-well plates and grown overnight. Twenty microliters of MTT (5
g/l) was added into each well for 4 h. After the medium containing
MTT was aspirated, the formazan crystals were dissolved in 200
μl dimethyl sulfoxide. The absorbance was recorded using a
Teacan 96-well spectrophotometer at wavelength of 570 nm, using a
wavelength of 630 nm as the reference. Data are presented as the
mean ± SD, derived from triplicate samples of at least three
independent experiments.
Transwell invasion assay
Transwell filters were coated with Matrigel (3.9
μg/μl, 60–80 μl) on the upper surface of a
polycarbonic membrane (diameter 6.5 mm, pore size 8 μm).
After incubating at 37°C for 30 min, the Matrigel solidified and
served as the extracellular matrix for analysis of tumor cell
invasion. Harvested cells (1×105) in 100 μl of
serum-free DMEM were added into the upper compartment of the
chamber. A total of 200 μl conditioned medium derived from
NIH3T3 cells was used as a source of chemoattractant and was placed
in the bottom compartment of the chamber. After 24-h incubation at
37°C with 5% CO2, the medium was removed from the upper
chamber. The non-invaded cells on the upper side of the chamber
were scraped off with a cotton swab. The cells that had migrated
from the Matrigel into the pores of the inserted filter were fixed
with 100% methanol, stained with hematoxylin, and mounted and dried
at 80°C for 30 min. The number of cells invading through the
Matrigel was counted in three randomly selected visual fields from
the central and peripheral portion of the filter using an inverted
microscope (×200 magnification). Each assay was repeated three
times.
Detection of cell apoptosis
Apoptosis was detected by the TdT-mediated dUTP
nick-end labeling (TUNEL) method. Briefly, cells were dewaxed,
incubated with blocking solution (0.3% H2O2
in double distilled water) for 30 min and permeabilized with 0.1%
Triton X-100 in PBS for 2 min on ice. Apoptosis was detected using
an in-situ cell death kit. Positive cells were visualized by
fluorescence microscopy. As a control, the reaction mixture was
incubated without enzyme to detect non-specific staining. The
apoptotic index was calculated from the ratio of the number of
positively stained tumor cells to the total number of tumor
cells.
Subcutaneous tumor model and gene
therapy
Six-week-old female immune-deficient nude mice
(BALB/c-nu) were bred at the Laboratory Animal Facility
(Haematology Institute of Chinese Academy of Sciences, Shanghai,
China) and were housed individually in microisolator ventilated
cages with free access to water and food. All experimental
procedures were performed according to the regulations and internal
biosafety and bioethics guidelines of Shanghai Jiaotong University
and the Shanghai Municipal Science and Technology Commission. Two
mice were injected subcutaneously with 1×108 GC cells in
50 μl of PBS pre-mixed with an equal volume of Matrigel
matrix (Becton-Dickinson). Mice were monitored daily and developed
a subcutaneous tumor. When the tumor size reached ∼5 mm in length,
they were surgically removed, cut into 1–2 mm3 pieces
and re-seeded individually into other mice. When tumor size reached
∼5 mm in length, the mice were randomly assigned as PBS group and
Lv-shLivin-treated group. In Lv-shLivin group, 15 μl of
lentivirus was injected into subcutaneous tumors using a multi-site
injection format. Injections were repeated every other day after
initial treatment. The tumor volume every three days was measured
with a caliper, using the formula volume = (length ×
width)2 / 2.
Statistical analysis
SPSS 20.0 was used for the statistical analysis.
Kruskal-Wallis H test and χ2 test were used to analyze
the expression rate in all groups. One-way analysis of variance
(ANOVA) was used to analyze the differences between groups. The LSD
method of multiple comparisons was used when the probability for
ANOVA was statistically significant. Statistical significance was
set at P<0.05.
Results
The expression of Livin and CAS-3
proteins in human GC
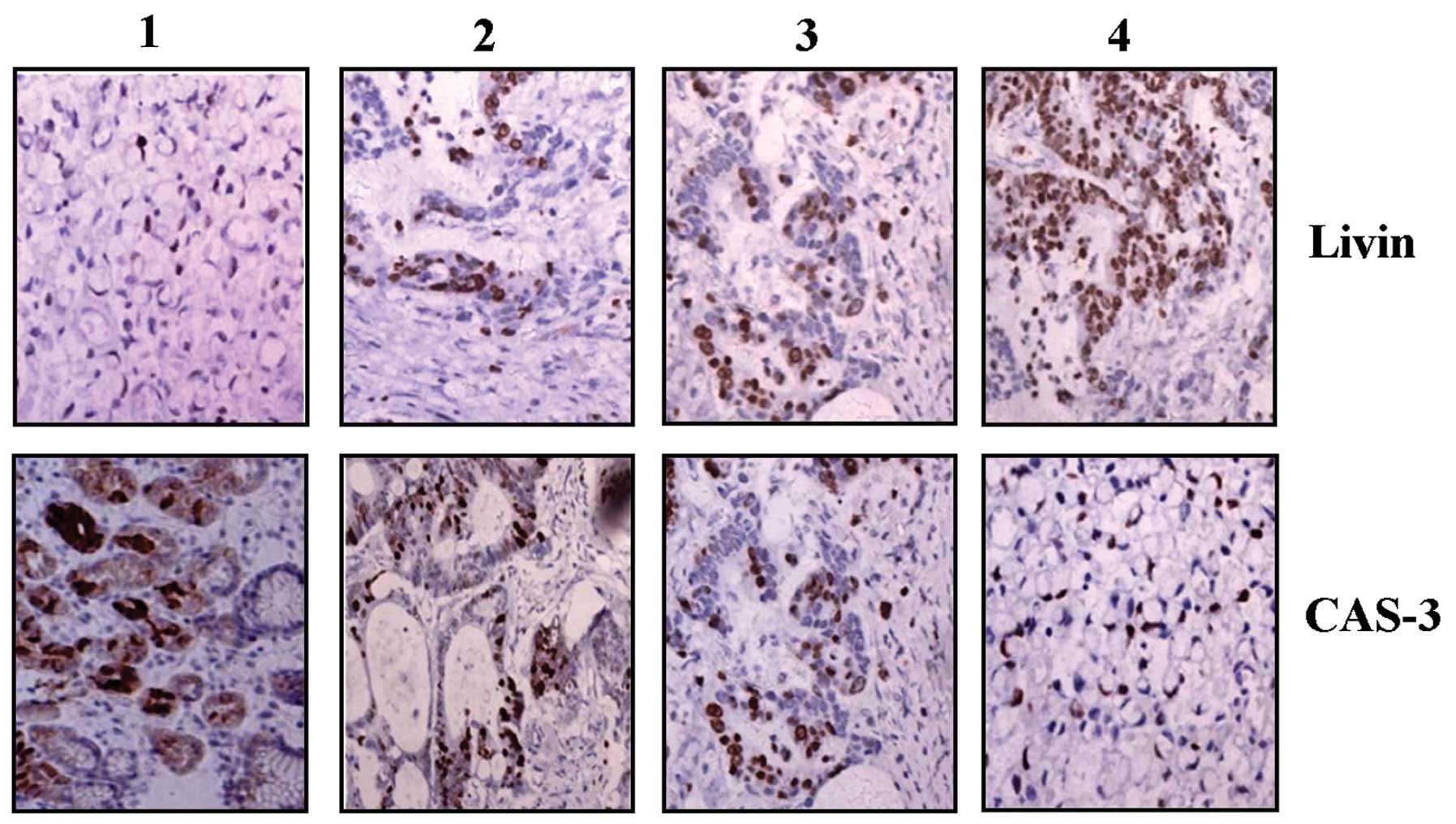
The expression of Livin and CAS-3 proteins was
examined by IHC staining. Positive staining of Livin was found in
the cytoplasm and nucleus, whereas that of CAS-3 protein was mainly
observed in the cytoplasm in GC tissues (Fig. 1). As shown in Table I, the positive expression of Livin
was detected in 64.1% (50/78) of the GC tissues, and 30.8% (24/78)
in a small fraction of ANCT (P<0.001). In contrast, CAS-3 was
found in 66.7% (52/78) of ANCT tissues and 33.3% (26/78) of GC
tissues (P=0.001). Spearman correlation analysis revealed the
negative correlation between Livin and CAS-3 expression in GC.
 | Table I.Expression of Livin and CAS-3
proteins in GC. |
Table I.
Expression of Livin and CAS-3
proteins in GC.
| Target | Group | Total | N
| Positive rate
(%) | χ2 | P-value |
|---|
| − | + | ++ | +++ |
|---|
| Livin | GC | 78 | 28 | 20 | 12 | 18 | 64.1 | 13.562 | <0.001 |
| ANCT | 78 | 54 | 8 | 5 | 11 | 30.8 | | |
| CAS-3 | GC | 78 | 52 | 14 | 8 | 4 | 33.3 | 11.478 | 0.001 |
| ANCT | 78 | 26 | 38 | 11 | 3 | 66.7 | | |
The correlation of Livin and CAS-3
protein expression with clinicopathological characteristics
The association between Livin and CAS-3 expression
and various clinical and histopathological features was analyzed.
As shown in Tables II and III, no significant link was found between
Livin and CAS-3 expression with the factors including age and
gender of the patients, or the size and TNM staging of the tumors
(each P>0.05). However, Livin expression was positively
correlated with tumor differentiation and lymph node metastases
(P=0.009; P=0.007), but CAS-3 was negatively associated with them
(P=0.036; P=0.002).
 | Table II.The correlation of Livin protein with
clinicopathological features of patients with GC. |
Table II.
The correlation of Livin protein with
clinicopathological features of patients with GC.
| Variables | Cases (n) | Livin expression
| P-value |
|---|
| − | + |
|---|
| Total | 78 | 28 | 50 | |
|---|
| No. of
patients | | | | |
| Age (years) | | | | |
| ≤55 | 25 | 8 | 17 | |
| >55 | 53 | 20 | 33 | 0.624 |
| Sex | | | | |
| Male | 54 | 19 | 35 | |
| Female | 24 | 9 | 15 | 0.845 |
| Tumor size
(cm) | | | | |
| ≤3.5 | 36 | 16 | 20 | |
| >3.5 | 42 | 12 | 30 | 0.148 |
| Tumor
differentiation | | | | |
| Well +
moderately | 32 | 17 | 15 | |
| Poorly | 46 | 11 | 35 | 0.009 |
| TNM staging | | | | |
| I+II | 40 | 13 | 27 | |
| III+IV | 38 | 15 | 23 | 0.524 |
| Metastatic lymph
node | | | | |
| Negative | 29 | 16 | 13 | |
| Positive | 49 | 12 | 37 | 0.007 |
 | Table III.The correlation of CAS-3 protein with
clinicopathological features of patients with GC. |
Table III.
The correlation of CAS-3 protein with
clinicopathological features of patients with GC.
| Variables | Cases (n) | CAS-3 expression
| P-value |
|---|
| − | + |
|---|
| Total | 78 | 52 | 26 | |
|---|
| No. of
patients | | | | |
| Age (years) | | | | |
| ≤55 | 25 | 17 | 8 | |
| >55 | 53 | 35 | 18 | 0.865 |
| Sex | | | | |
| Male | 54 | 34 | 20 | |
| Female | 24 | 18 | 6 | 0.301 |
| Tumor size
(cm) | | | | |
| ≤3.5 | 36 | 26 | 10 | |
| >3.5 | 42 | 26 | 16 | 0.338 |
| Tumor
differentiation | | | | |
| Well +
moderately | 32 | 17 | 15 | |
| Poorly | 46 | 35 | 11 | 0.036 |
| TNM staging | | | | |
| I+II | 40 | 28 | 12 | |
| III+IV | 38 | 24 | 14 | 0.524 |
| Metastatic lymph
node | | | | |
| Negative | 29 | 13 | 16 | |
| Positive | 49 | 39 | 10 | 0.002 |
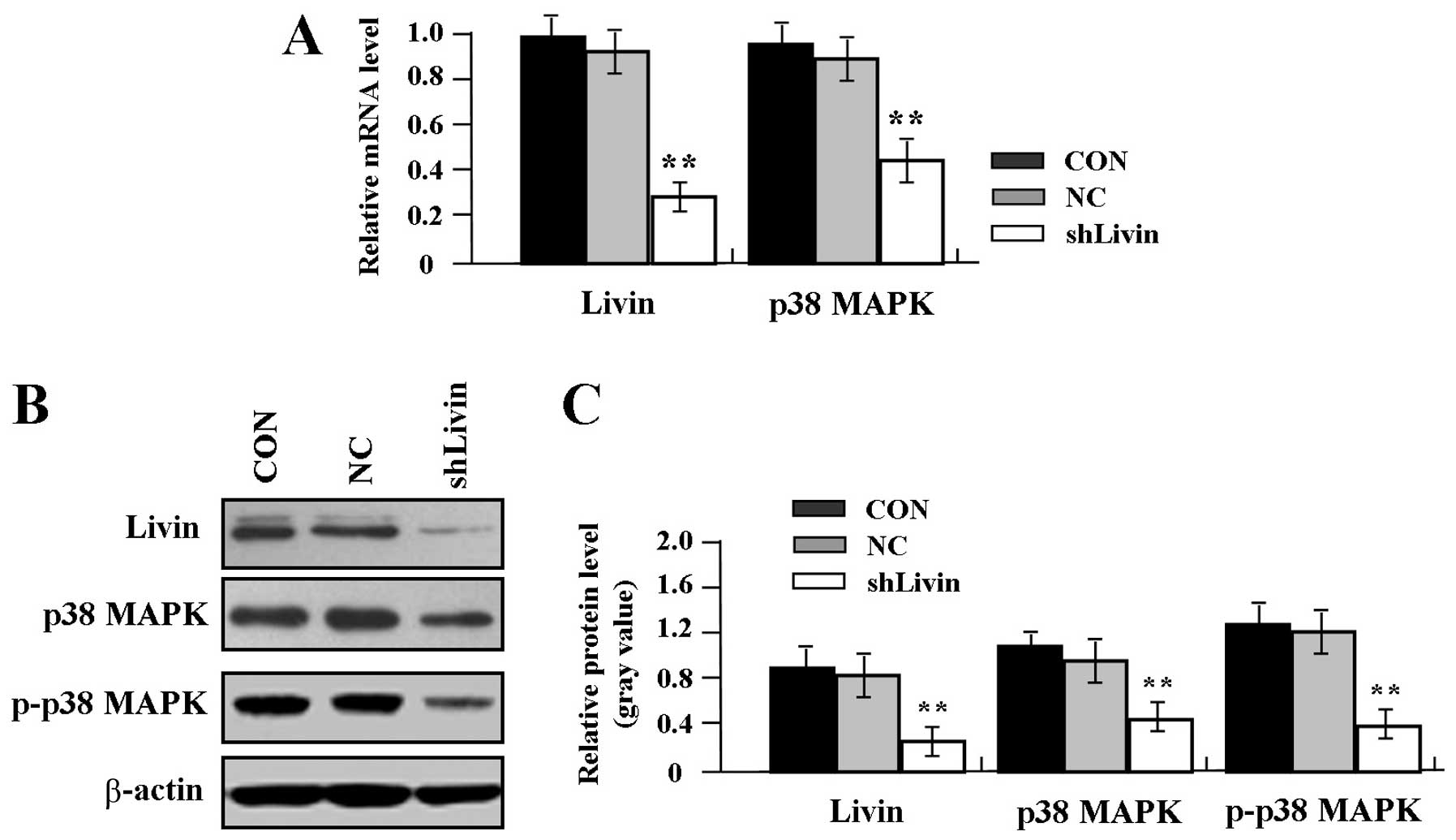
Effect of Livin knockdown on p38 MAPK and
p-p38 MAPK expression
To confirm the effect of Livin knockdown on the
expression of p38 MAPK and p-p38 MAPK in GC SGC-7901 cells, the
mRNA expression levels of Livin and p38 MAPK were measured by
real-time PCR. An obvious inhibition of Livin and p38 MAPK mRNA
expression was observed in shLivin group compared with the NC and
CON groups (Fig. 2A, P<0.01).
Western blot assay indicated that Livin, p38 MAPK and p-p38 MAPK
were found downregulated in shLivin group compared with the NC and
CON groups) in GC cells (Fig. 2B and
C, P<0.01).
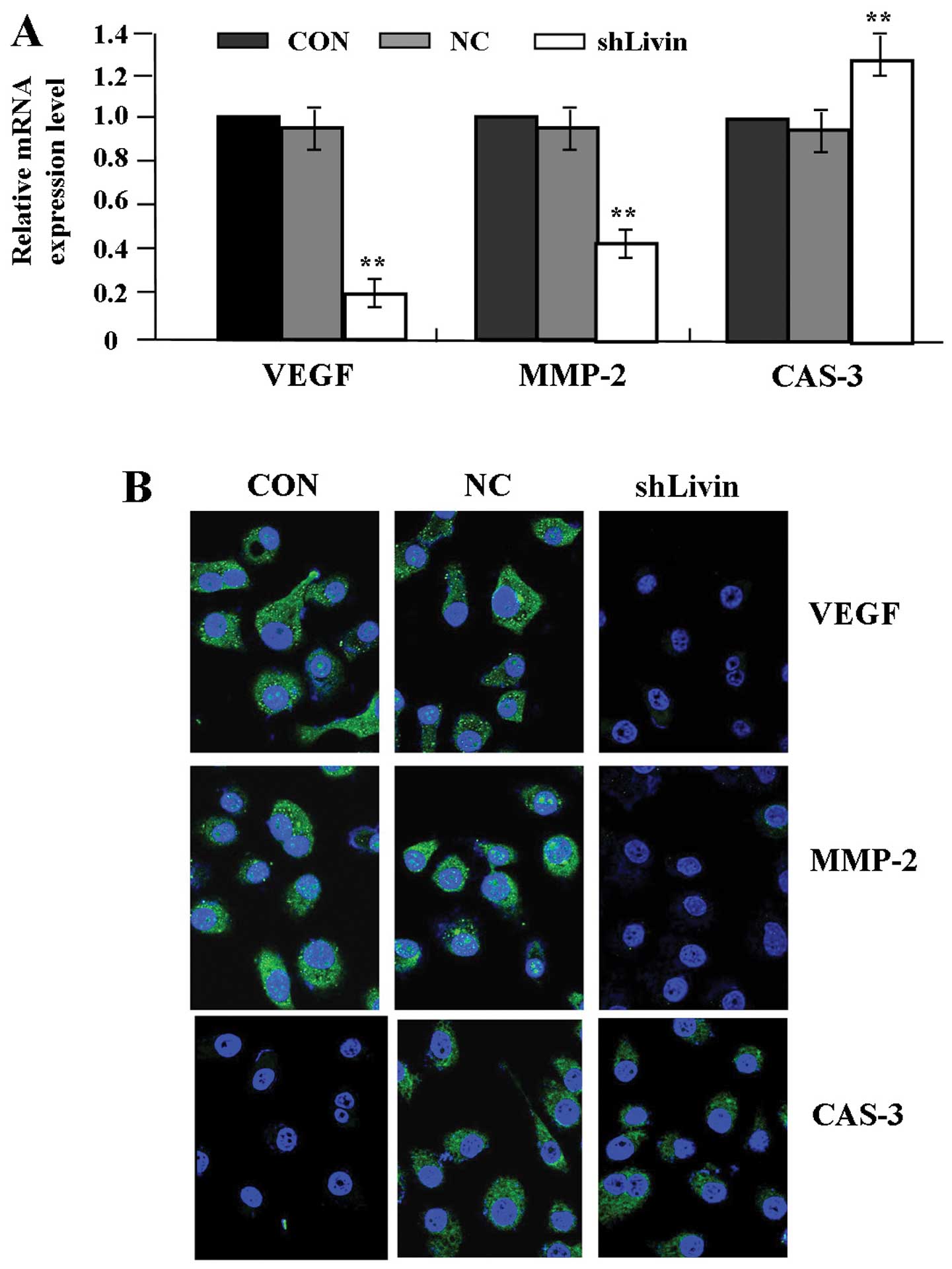
Effect of Livin knockdown on VEGF, MMP-2
and CAS-3 expression
To examine the effect of Livin knockdown on the
expression of VEGF, MMP-2 and CAS-3 in GC cells, GC SGC-7901 cells
were transfected with Lv-shLivin. The mRNA expression levels of
VEGF, MMP-2 and CAS-3 were evaluated by quantitative real-time PCR
(Fig. 3A) and their protein
expression was identified by fluorescence microscopy (Fig. 3B). The expression levels of VEGF
and MMP-2 were decreased, while that of CAS-3 was increased in
shLivin group compared with the NC and CON groups (each
P<0.01).
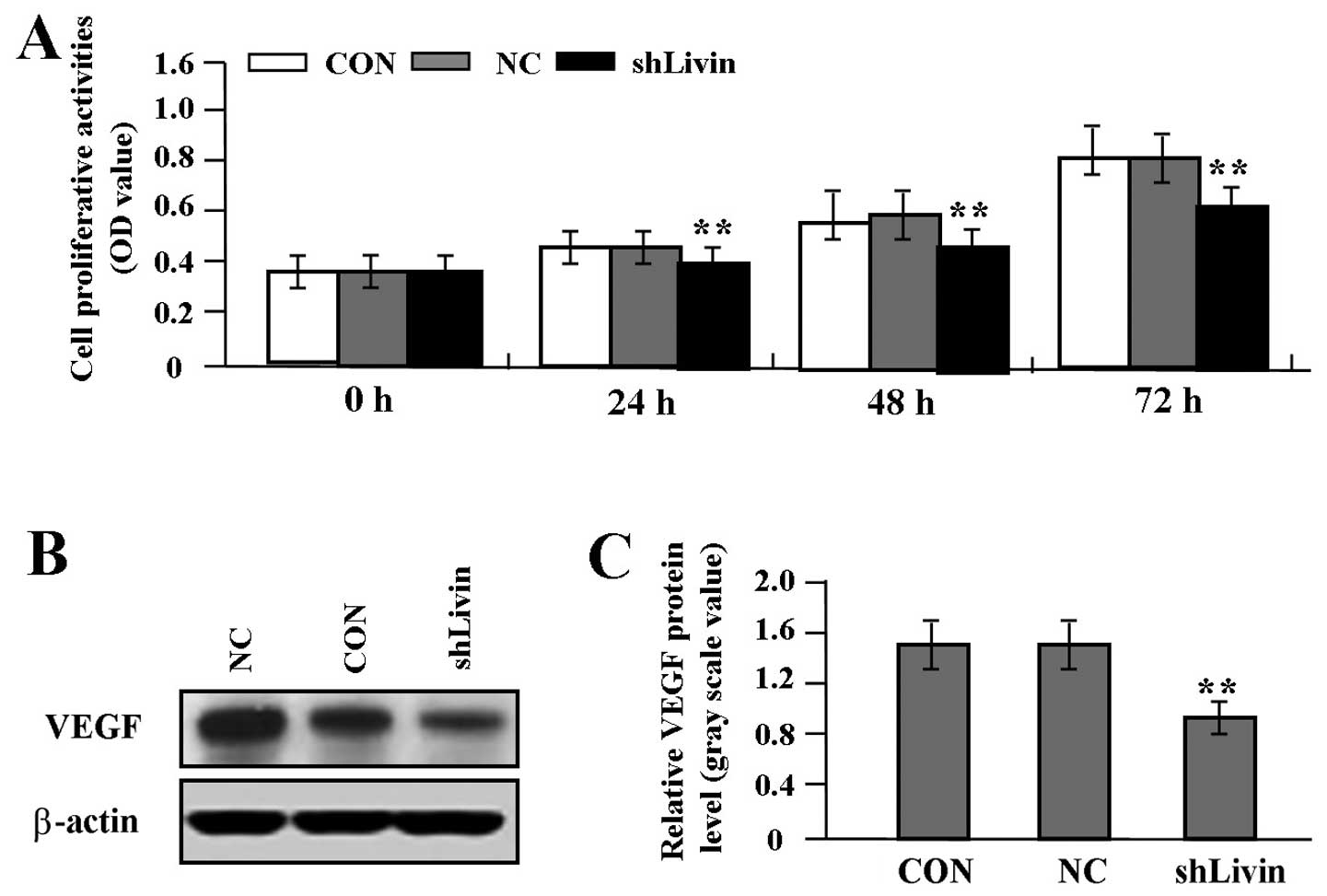
Effect of Livin knockdown on cell
proliferation and invasion
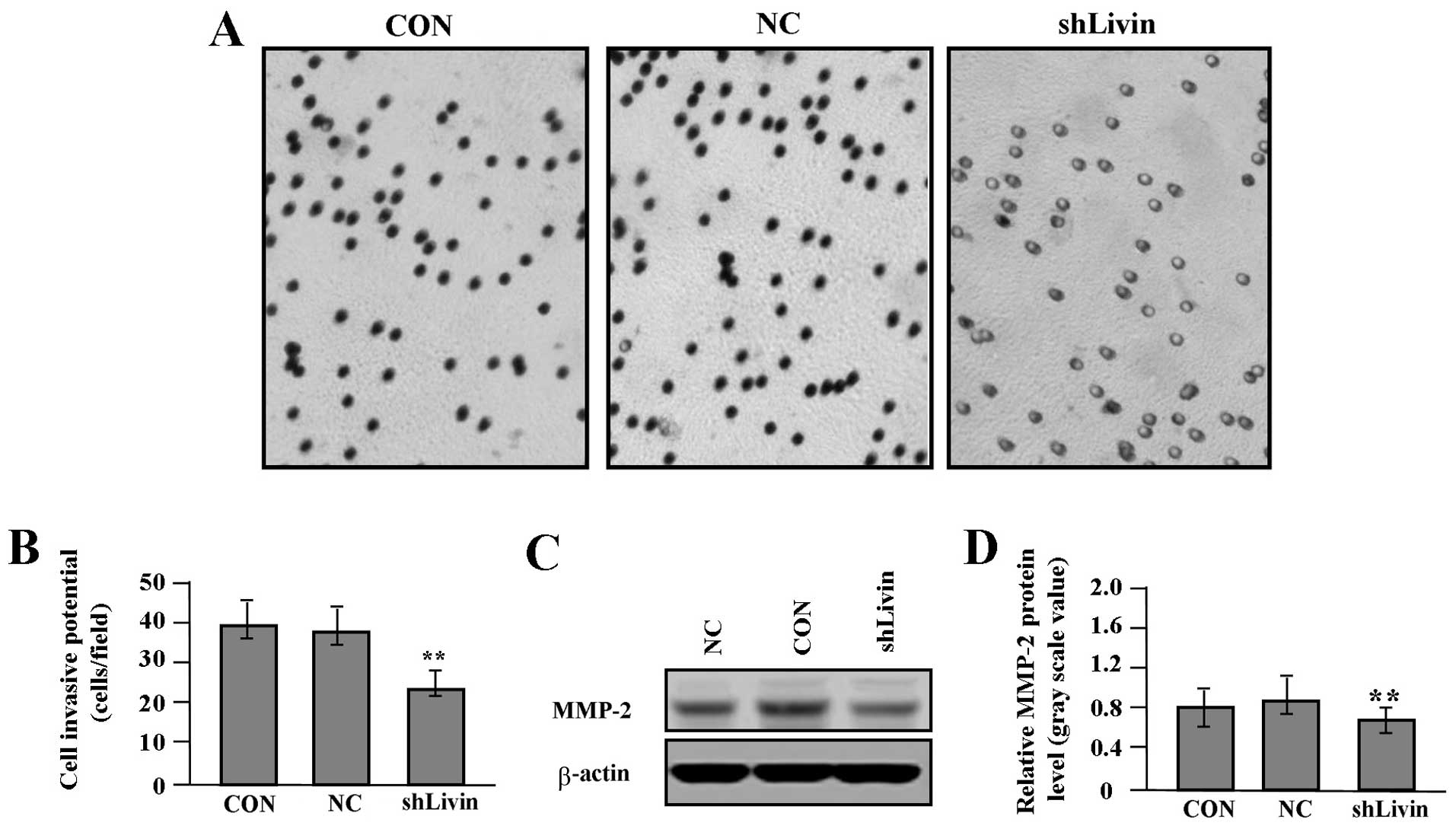
To gain knowledge on the effect of Livin knockdown
on tumor growth and invasion in GC SGC-7901 cells, we assessed cell
proliferative activities by MTT assay and cell invasive potential
by Transwell assay. It was found that Livin knockdown markedly
suppressed cell proliferative activities in a time-dependent manner
(Fig. 4A) and cell invasive
potential (Fig. 5A and B) in GC
cells compared with NC and CON groups (each P<0.01). In
addition, we detected the protein expression of VEGF (Fig. 4B and C) and MMP-2 (Fig. 5C and D) by western blot assay to
determine whether Livin knockdown affected their expression through
translational repression. It was shown that the amount of VEGF and
MMP-2 proteins was significantly decreased in shLivin group
compared with NC and CON groups (P<0.01).
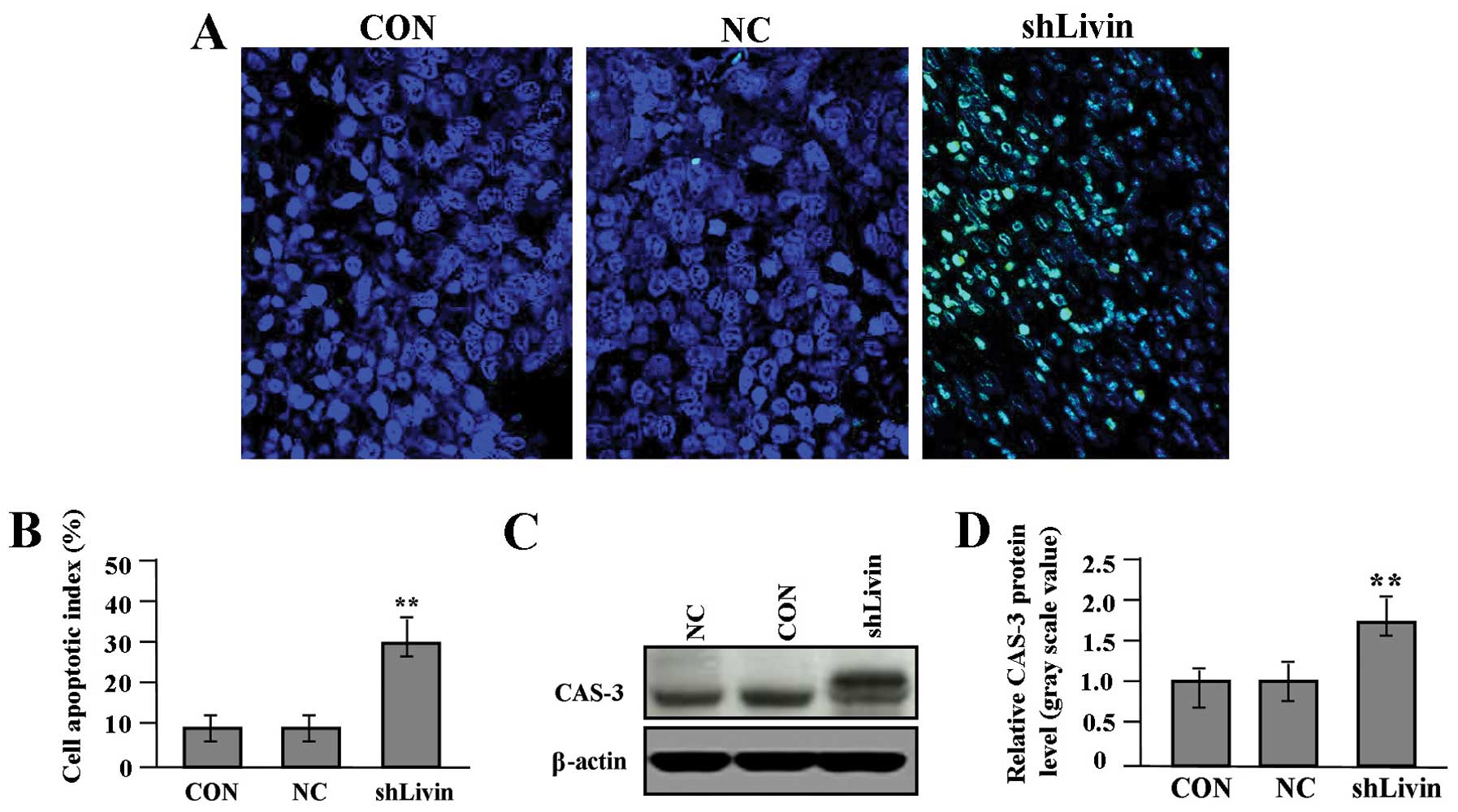
Effect of Livin knockdown on cell
apoptosis
To evaluate whether Livin knockdown influenced cell
in situ apoptosis, TUNEL method was performed. Cell
apoptotic index in shLivin group was remarkably increased compared
with the NC and CON groups (Fig. 6A
and B, P<0.01). We detected the protein expression of CAS-3
(Fig. 6C and D) by western blot
assay to determine the effect of Livin knockdown on CAS-3
expression through translational repression. It was shown that the
amount of CAS-3 protein was significantly increased in shLivin
group compared with NC and CON groups (P<0.01).
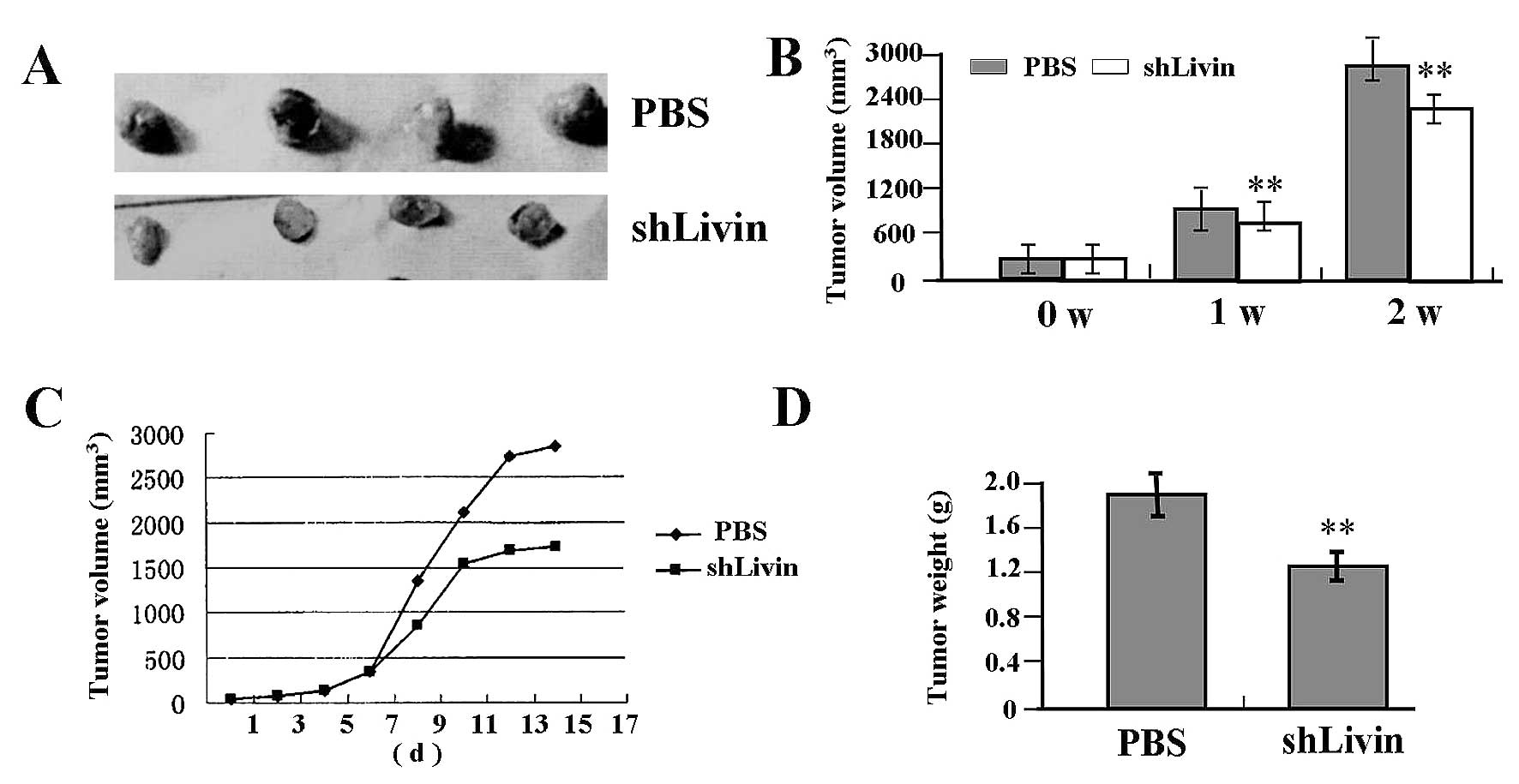
Antitumor effect of Lv-shLivin on
SGC-7901 xenograft model
Our in vitro experiments demonstrated that
knockdown of Livin could efficiently inhibit proliferation and
invasion in GC SGC-7901 cells. Therefore, we further investigated
antitumor effect of Lv-shLivin in vivo using the SGC-7901
xenograft model and letivirus-mediated gene therapy. The mean
volume of tumors in all experimental mice before treatment was
38.20±9.40 mm3. Each mouse was challenged by in
situ injection of PBS (control; n=4), or Lv-shLivin (n=4).
During the first two weeks recovery, the tumors in Lv-shLivin group
grew slowly compared with NC group (Fig. 7A and C). There was a significant
difference in tumor volumes and weight between Lv-shLivin group and
PBS group over the observation period (P<0.01) (Fig. 7B and D).
Discussion
Livin, a member of the IAP family, plays crucial
roles in apoptosis, cell proliferation and cell cycle control.
Abnormal Livin expression is detected during the process of cancer
formation and progression (18).
Livin is highly expressed in CRC tissues, and may influence the
prognosis of CRC as a biomarker or potential therapeutic target
(19). Livin is also expressed in
75% of bladder cancer, and its detection in bronchial aspirates
shows 63% sensitivity and 92% specificity, suggesting that Livin
may be valuable diagnostic marker for the early diagnosis of lung
cancer (20) and predict early
recurrence in invasive bladder cancer (21). Thus, Livin research may provide an
opportunity for the development of potential therapy for
Livin-relevant cancers.
Interestingly, Livin is a member of CAS inhibitors
that selectively binds the endogenous CAS-3 (22). It is negatively associated with
CAS-3 expression and contributes to the tumor progression (23). To further clarify the clinical
significance of Livin and CAS-3 in GC, in the present study, our
findings showed that, Livin expression was increased, while CAS-3
was decreased in human GC tissues compared to the ANCT. Moreover,
Livin expression was positively correlated with tumor
differentiation and lymph node metastases, but CAS-3 was negatively
associated with them, which has been confirmed by Wang et al
(24) and Liang et al
(25). In addition, as for the
cellular localization, the positive expression of Livin and CAS-3
was mainly localized in the cytoplasm, suggesting that cytoplasmic
accumulation of Livin may contribute to the development of GC.
In regard to the function of Livin in cancer, some
studies have demonstrated that Livin promotes tumor cell
proliferation by regulating G1-S cell cycle transition (26) and mediates cell invasion via
nuclear NF-κB signaling (27).
Inversely, silencing Livin gene leads to apoptosis induction, cell
cycle arrest and proliferation inhibition in malignant tumor cells
(28–30). However, few reports have shown the
function of Livin in GC. To confirm the effect of Livin knockdown
on GC cells, the present study showed that knockdown of Livin
inhibited cell proliferation and the invasive potential, and
induced cell apoptosis in GC cells in vitro and in
vivo, suggesting that Livin might serve as a novel therapeutic
target for the treatment of GC.
Accumulating data indicate that VEGF participates in
the pathogenesis of many neoplastic diseases, and correlates with
aggressiveness and prognosis as a tumor biomarker for tumor
invasion (31). However, there is
little evidence demonstrating the direct regulation of Livin on
VEGF expression in GC cells. Knockdown of Livin inhibits cell
invasion via decrease of MMP-2/-9 expression in osteosarcoma cells
(32). Regarding the effect of
Livin on CAS-3 expression, antisense oligonucleotide targeting
Livin induces apoptosis of human bladder cancer cell with increased
expression of CAS-3 (33). In the
present study, our findings showed that knockdown of Livin
downregulated the expression of VEGF and MMP-2, but upregulated the
expression of CAS-3 in GC cells. Moreover, our finding indicated
that knockdown of Livin decreased the activity of MAPK signaling in
GC cells, while MAPK upregulates MMP-2 and VEGF expression and
downregulates CAS-3 expression in cancer cells (34–36),
suggesting that Livin may be implicated in the development and
progression of GC cells possibly via regulation of MAPK
signaling-mediated VEGF, MMP-2 and CAS-3 expression.
Overall, our findings indicate that the expression
of Livin is increased in human GC and correlates with tumor
differentiation and lymph node metastases, while knockdown of Livin
inhibits cell growth and invasion through blockade of the MAPK
pathway in GC cells in vitro and in vivo, suggesting
that Livin may be a potential therapeutic target for the treatment
of GC.
Acknowledgements
This study was supported by Shanghai
Science and Technology Committee Scientific and Technological
Innovation Project (no. 12140901102) and Shanghai City Board of
Education Research and Innovation Project (no. 12YZ042).
References
|
1.
|
Jemal A, Bray F, Center MM, et al: Global
cancer statistics. CA Cancer J Clin. 61:69–90. 2011. View Article : Google Scholar
|
|
2.
|
Tajima Y, Yamazaki K, Makino R, et al:
Gastric and intestinal phenotypic marker expression in early
differentiated-type tumors of the stomach: clinicopathologic
significance and genetic background. Clin Cancer Res. 12:6469–6479.
2006. View Article : Google Scholar
|
|
3.
|
Hartman ML and Czyz M: Anti-apoptotic
proteins on guard of melanoma cell survival. Cancer Lett.
331:24–34. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Kim DK, Alvarado CS, Abramowsky CR, et al:
Expression of inhibitor-of-apoptosis protein (IAP) livin by
neuroblastoma cells: correlation with prognostic factors and
outcome. Pediatr Dev Pathol. 8:621–629. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Kleinberg L, Lie AK, Flørenes VA, et al:
Expression of inhibitor-of-apoptosis protein family members in
malignant mesothelioma. Hum Pathol. 38:986–994. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Nedelcu T, Kubista B, Koller A, et al:
Livin and Bcl-2 expression in high-grade osteosarcoma. J Cancer Res
Clin Oncol. 134:237–244. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Lazar I, Perlman R, Lotem M, et al: The
clinical effect of the inhibitor of apopotosis protein livin in
melanoma. Oncology. 82:197–204. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Liu HB, Kong CZ, Zeng Y, et al: Livin may
serve as a marker for prognosis of bladder cancer relapse and a
target of bladder cancer treatment. Urol Oncol. 27:277–283. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Liu H, Wang S, Sun H, et al: Inhibition of
tumorigenesis and invasion of hepatocellular carcinoma by
siRNA-mediated silencing of the livin gene. Mol Med Rep. 3:903–907.
2010.PubMed/NCBI
|
|
10.
|
Crnković-Mertens I, Bulkescher J, Mensger
C, et al: Isolation of peptides blocking the function of
anti-apoptotic Livin protein. Cell Mol Life Sci. 67:1895–1905.
2010.PubMed/NCBI
|
|
11.
|
Wang X, Xu J, Ju S, et al: Livin gene
plays a role in drug resistance of colon cancer cells. Clin
Biochem. 43:655–660. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Dasgupta A, Alvarado CS, Xu Z, et al:
Expression and functional role of inhibitor-of-apoptosis protein
livin (BIRC7) in neuroblastoma. Biochem Biophys Res Commun.
400:53–59. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Ding ZY, Liu GH, Olsson B, et al:
Upregulation of the anti-apoptotic factor Livin contributes to
cisplatin resistance in colon cancer cells. Tumour Biol.
234:683–693. 2013. View Article : Google Scholar
|
|
14.
|
Sun JG, Liao RX, Zhang SX, et al: Role of
inhibitor of apoptosis protein Livin in radiation resistance in
nonsmall cell lung cancer. Cancer Biother Radiopharm. 26:585–592.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Abd-Elrahman I, Hershko K, Neuman T, et
al: The inhibitor of apoptosis protein Livin (ML-IAP) plays a dual
role in tumorigenicity. Cancer Res. 69:5475–5480. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Kempkensteffen C, Hinz S, Christoph F, et
al: Expression of the apoptosis inhibitor livin in renal cell
carcinomas: correlations with pathology and outcome. Tumour Biol.
28:132–138. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Dai CH, Li J, Shi SB, et al: Survivin and
Smac gene expressions but not livin are predictors of prognosis in
non-small cell lung cancer patients treated with adjuvant
chemotherapy following surgery. Jpn J Clin Oncol. 40:327–335. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Yan B: Research progress on Livin protein:
an inhibitor of apoptosis. Mol Cell Biochem. 357:39–45. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Xi RC, Biao WS and Gang ZZ: Significant
elevation of survivin and livin expression in human colorectal
cancer: inverse correlation between expression and overall
survival. Onkologie. 34:428–432. 2011. View Article : Google Scholar
|
|
20.
|
Li J, Chen P, Li XQ, et al: Elevated
levels of survivin and livin mRNA in bronchial aspirates as markers
to support the diagnosis of lung cancer. Int J Cancer.
132:1098–1104. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Xi RC, Sheng YR, Chen WH, et al:
Expression of survivin and livin predicts early recurrence in
non-muscle invasive bladder cancer. J Surg Oncol. 107:550–554.
2012.PubMed/NCBI
|
|
22.
|
Chang H and Schimmer AD: Livin/melanoma
inhibitor of apoptosis protein as a potential therapeutic target
for the treatment of malignancy. Mol Cancer Ther. 6:24–30. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Li H, Chen Y, Chen G, et al: Expression of
livin in lung cancer tissue and its relationship with the
expression of caspase-3. Zhongguo Fei Ai Za Zhi. 10:486–490.
2007.PubMed/NCBI
|
|
24.
|
Wang TS, Ding QQ, Guo RH, et al:
Expression of livin in gastric cancer and induction of apoptosis in
SGC-7901 cells by shRNA-mediated silencing of livin gene. Biomed
Pharmacother. 64:333–338. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Liang YZ, Fang TY, Xu HG, et al:
Expression of CD44v6 and Livin in gastric cancer tissue. Chin Med
J. 125:3161–3165. 2012.PubMed/NCBI
|
|
26.
|
Ye L, Song X, Li S, et al: Livin-α
promotes cell proliferation by regulating G1-S cell cycle
transition in prostate cancer. Prostate. 71:42–51. 2011.
|
|
27.
|
Chen F, Yang D, Che X, et al: Livin
mediates tumor cell invasion in the DU-145 cell line via NF-κB.
Oncol Rep. 27:2010–2016. 2012.PubMed/NCBI
|
|
28.
|
Wang H, Tan SS, Wang XY, et al: Silencing
livin gene by siRNA leads to apoptosis induction, cell cycle
arrest, and proliferation inhibition in malignant melanoma LiBr
cells. Acta Pharmacol Sin. 28:1968–1974. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Yang D, Song X, Zhang J, et al:
Suppression of livin gene expression by siRNA leads to growth
inhibition and apoptosis induction in human bladder cancer T24
cells. Biosci Biotechnol Biochem. 74:1039–1044. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Yuan B, Ran B, Wang S, et al: siRNA
directed against Livin inhibits tumor growth and induces apoptosis
in human glioma cells. J Neurooncol. 107:81–87. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Kajdaniuk D, Marek B, Fołtyn W, et al:
Vascular endothelial growth factor (VEGF) in endocrinology and
oncology. Endokrynol Pol. 62:14–22. 2011.PubMed/NCBI
|
|
32.
|
Li X, Fan S, Li L, et al: RNA
interference-mediated knockdown of Livin suppresses cell
proliferation and invasion and enhances the chemosensitivity to
cisplatin in human osteosarcoma cells. Int J Oncol. 43:159–168.
2013.PubMed/NCBI
|
|
33.
|
Liu C, Wu X, Luo C, et al: Antisense
oligonucleotide targeting Livin induces apoptosis of human bladder
cancer cell via a mechanism involving caspase 3. Exp Clin Cancer
Res. 29:632010. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Shin I, Kim S, Song H, et al:
H-Ras-specific activation of Rac-MKK3/6-p38 pathway: its critical
role in invasion and migration of breast epithelial cells. Biol
Chem. 280:14675–14683. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Hideshima T, Akiyama M, Hayashi T, et al:
Targeting p38 MAPK inhibits multiple myeloma cell growth in the
bone marrow milieu. Blood. 101:703–705. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Noguchi K, Yamana H, Kitanaka C, et al:
Differential role of the JNK and p38 MAPK pathway in c-Myc- and
s-Myc-mediated apoptosis. Biochem Biophys Res Commun. 267:221–227.
2000. View Article : Google Scholar : PubMed/NCBI
|