Introduction
Induced pluripotent stem cells (IPSCs) are derived
from nonpluripotent cells, such as adult somatic cells; they
include fibroblasts, adipocytes and keratinocytes (1–3).
IPSCs are similar to embryonic stem cells (ESCs) in their capacity
to renew themselves, differentiate into various cell types and
migrate actively (4). Studies have
reported that various intra-cellular and extracellular factors
activate multiple signaling networks during the increase in cell
proliferation to generate active self-renewal activity. For
example, Welham et al (5)
reported that phosphoinositide 3-kinase (PI3K) and glycogen
synthase kinase 3 are important in ESC proliferation and
pluripotency. Lee et al (6)
revealed that the self-renewal of ESCs is dependent on PI3K/Akt,
Smad and Wnt signaling pathways. The PI3K/Akt signaling pathway has
also been reported to play a pivotal role in the induction of
pluripotent cells from primordial germ cells and ESCs (7,8).
Selenium is a trace nutrient element that is
involved in many biochemical pathways. It protects cells against
oxidative damage by optimizing the activity of glutathione
peroxidase and thioredoxin reductase, as well as several other
seleno-proteins (9,10). It has been reported to have
anticarcinogenic effects by preventing oxidative damage, regulating
immune responses and inducing apoptosis (11–13).
Studies have also found that selenium has preventive effects in
cardiovascular diseases, viral infections, fertility and aging
(10,14). More recently, selenium was shown to
induce stem cell behavior in human adipose-tissue stromal cells
(15).
Although IPSCs can be an attractive target to
develop regenerative medicines for aplastic diseases, the molecular
mechanisms regulating their pluripotency, proliferation and
differentiation are not fully understood. Thus, in the present
study, we investigated whether selenium, as an extracellular
factor, can improve the potency of stem cells by stimulating the
proliferation and migration of 3T3-L1 preadipocytes. We also
investigated the mechanisms underlying its activity. In the
process, we demonstrated that selenium plays a key role in the
self-renewal activity of stem cells via cell cycle progression and
the activation of the PI3K/Akt signaling pathway.
Materials and methods
Reagents and antibodies
Dulbecco’s modified Eagle’s medium (DMEM), bovine
calf serum (BCS), Dulbecco’s phosphate buffered saline (DPBS), and
1% penicillin-streptomycin were purchased from Gibco-BRL
(Gaithersburg, MD, USA). Selenium, trypan blue, paraformaldehyde
and toluidine blue O were purchased from Sigma-Aldrich Chemical Co.
(St. Louis, MO, USA). LY294002 and PD98059 were obtained from
Calbiochem (San Diego, CA, USA). All primers were purchased from
Bioneer Co. (Daejeon, Korea), and all primary and secondary
antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz,
CA, USA) except p-Akt antibody and p-ERK antibody (Cell Signaling
Technology, Beverly, MA, USA). All other chemicals not specifically
mentioned here were obtained from Sigma-Aldrich.
Cell culture and selenium treatment
Mouse 3T3-L1 preadipocytes (American Type Culture
Collection, Rockville, MD, USA) were cultured at 37°C in humidified
95% air and 5% CO2 in DMEM with 10% BCS and 1%
penicillin-streptomycin. Selenium was dissolved in sterilized
distilled water and stored at −20°C. For selenium treatment, 3T3-L1
cells were seeded and stabilized for 24 h. The cells were then
treated with selenium at various concentrations in serum-free DMEM
for different time-points. Then the optimum concentration of
selenium was selected on the basis of cell viability studies using
the trypan blue exclusion assay.
Trypan blue exclusion assay
3T3-L1 cells were seeded in 6-well plates at a
density of 7×104 cells per well and incubated for 24 h.
The medium was then replaced with serum-free DMEM containing
different concentrations of selenium. Following incubation for
another 24 h, cells were collected and viable cells were counted
with a hematocytometer by excluding the stained cells with 0.4%
trypan blue dye. Triplicate wells were used in all cell viability
assays and each experiment was repeated at least three times.
Colony-forming cell assay
3T3-L1 cells were seeded in 10-cm cell culture
dishes at a density of 3×103 and stabilized for 24 h.
Following stimulation with selenium (15 ng/ml) for another 24 h in
serum-free DMEM, media was replaced with fresh media containing 10%
BCS. After 15 days, the culture media was discarded and the
attached cells were fixed with 4% paraformaldehyde. Then the cells
were stained with 0.1% toluidine blue O in 1% paraformaldehyde for
3 days. After washing with distilled water several times, we
determined the proliferation efficiency by counting the visual
colonies.
Cell migration assay
To evaluate the migration activity of
selenium-treated 3T3-L1 cell, we conducted a simple cell-scraped
wound model assay. Cells were seeded in 35-mm cell culture dishes
at a density of 1.5×105 and stabilized for 24 h. Then
cells were scraped in a straight line across the dish using 200
μl tips and washed with fresh media for three times. After
incubation with selenium (15 ng/ml) for 24 h in serum-free DMEM,
the migrated cells onto the wounded region were photographed under
the microscope at ×40 magnification.
Protein extraction and western blot
analysis
Selenium-treated 3T3-L1 cells were collected and
lysed with lysis buffer [25 mM Tris-Cl (pH 7.5), 250 mM NaCl, 5 mM
EDTA, 1% NP-40, 1 mM phenymethylsulfonyl fluoride (PMSF), 5 mM
dithiothreitol (DTT)]. Then the protein concentrations were
quantified using a Bio-Rad protein assay (Bio-Rad Lab., Hercules,
CA, USA) according to the manufacturer’s instructions. For western
blot analysis, an equal amount of protein was loaded on
SDS-polyacrylamide gel and transferred onto a nitrocellulose
membrane (Schleicher & Schuell, Keene, NH, USA) by
electroblotting. The blots were then probed with the specific
primary antibodies and incubated overnight at 4°C. Following 1 h of
incubation with the secondary antibodies, the blots were visualized
by enhanced chemiluminescence (ECL, Amersham) according to the
manufacturer’s procedure.
Statistical analysis
All data are presented as the mean ± SD from at
least three independent experiments. Statistical significance of
the differences between groups was calculated using the Student’s
two tailed t-test.
Results
Selenium stimulates the cell growth and
the proliferation of 3T3-L1 preadipocytes
As stem cells have self-renewal activity
demonstrated by enhanced cell proliferation, we first investigated
the effects of selenium on the growth of the 3T3-L1 cells. To
evaluate the degree of proliferation, a trypan blue exclusion assay
was performed following treatment with various concentrations of
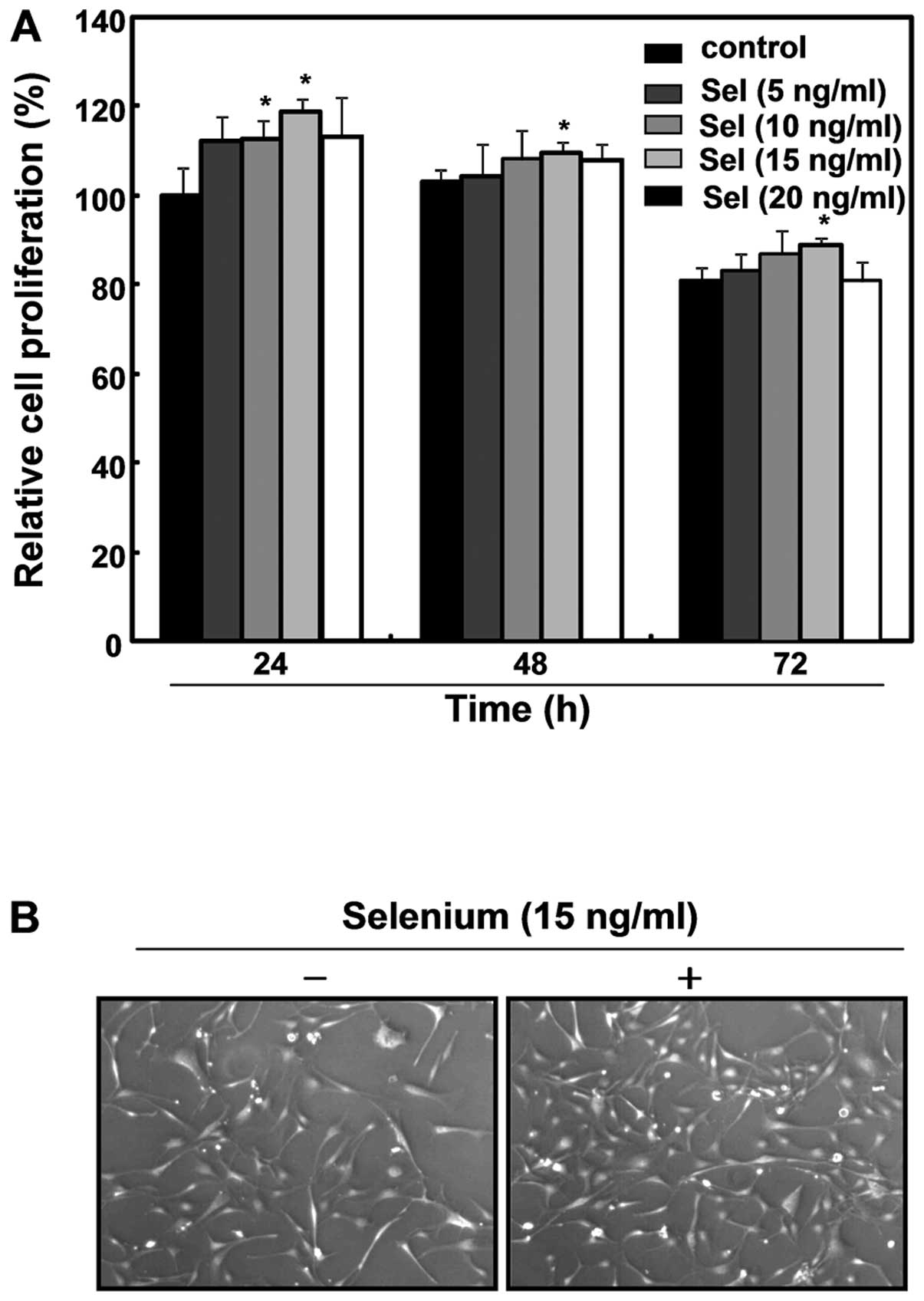
selenium for 72 h. As shown in Fig.
1A, the selenium treatment enhanced the cell growth, with 15
ng/ml of selenium the most effective in stimulating cell
proliferation among various concentrations of selenium tested. The
proliferative action of selenium (15 ng/ml) lasted 72 h, although
the effect was more significant at 24 h. Microscopic observations
of selenium-treated cells revealed active proliferation compared
with untreated control cells (Fig.
1B). Thus, the application of 15 ng/ml of selenium for 24 h was
determined to be the optimal treatment for further study. In
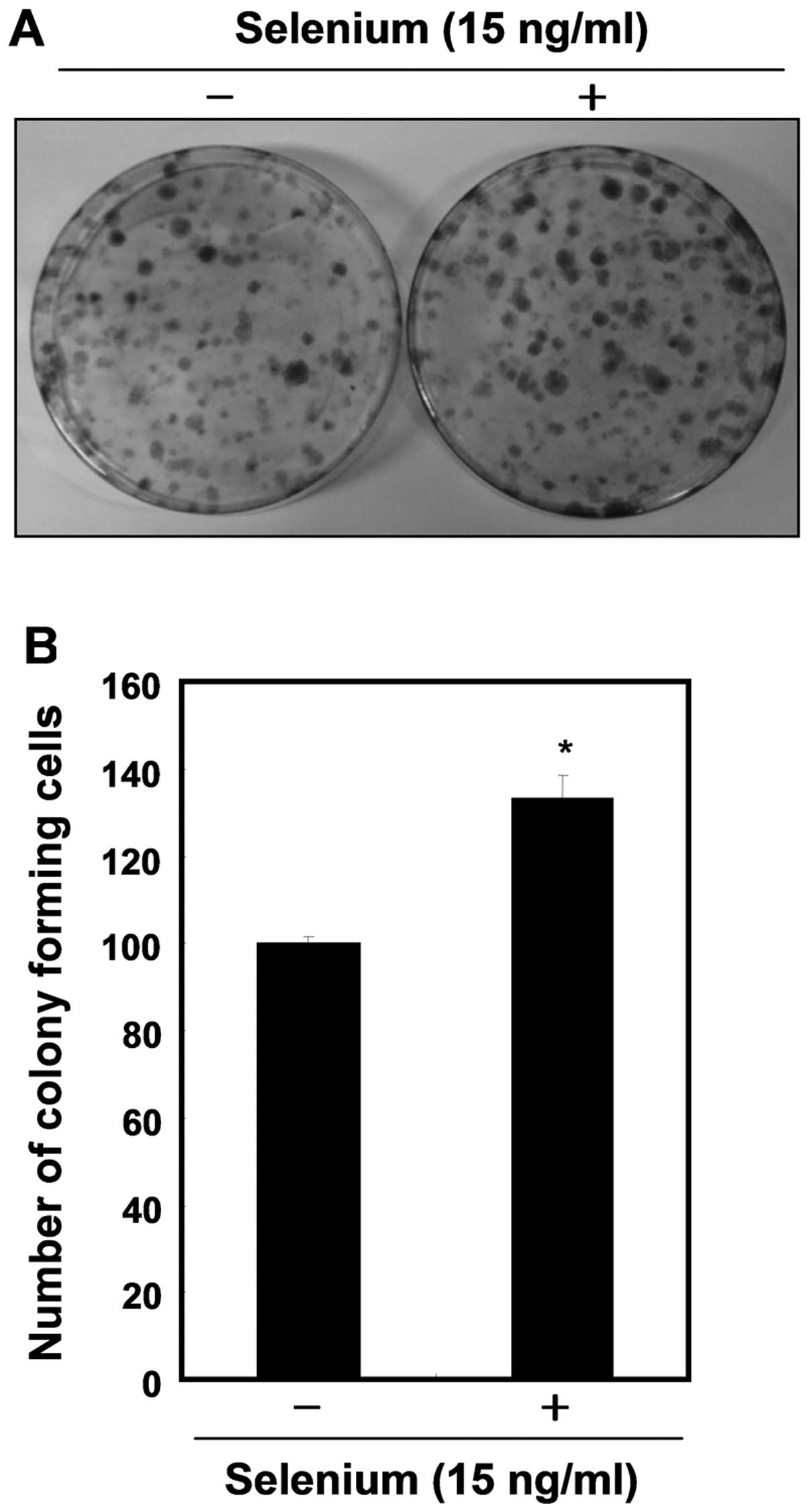
addition, the selenium-treated cells formed more colonies, showing
an ∼1.4-fold increase compared with the control cells (Fig. 2). These results clearly indicate
that selenium induced active cell proliferation of the 3T3-L1
cells.
Selenium stimulated the cell cycle
progression of 3T3-L1 preadipocytes
Cell cycle progression is critically regulated by a
sequential activation of cyclin-dependent kinase (CDKs), the
activities and specificities of which are determined by
phosphorylation of their corresponding catalytic subunits and by
their associations with Cdks inhibitors and cyclins, which are
differentially expressed during the cell cycle. CDK4 and CDK6
modulate the G1/S checkpoint by binding D-type cyclins. In addition
to regulating the G1/S checkpoint by interacting with cyclin E,
CDK2, together with cyclin A, controls the S phase and the G2/M
checkpoint. The CDK1-cyclin B complex is known to regulate G2/M
progression (16,17). In order to investigate the
mechanism by which selenium induced the cell proliferation of the
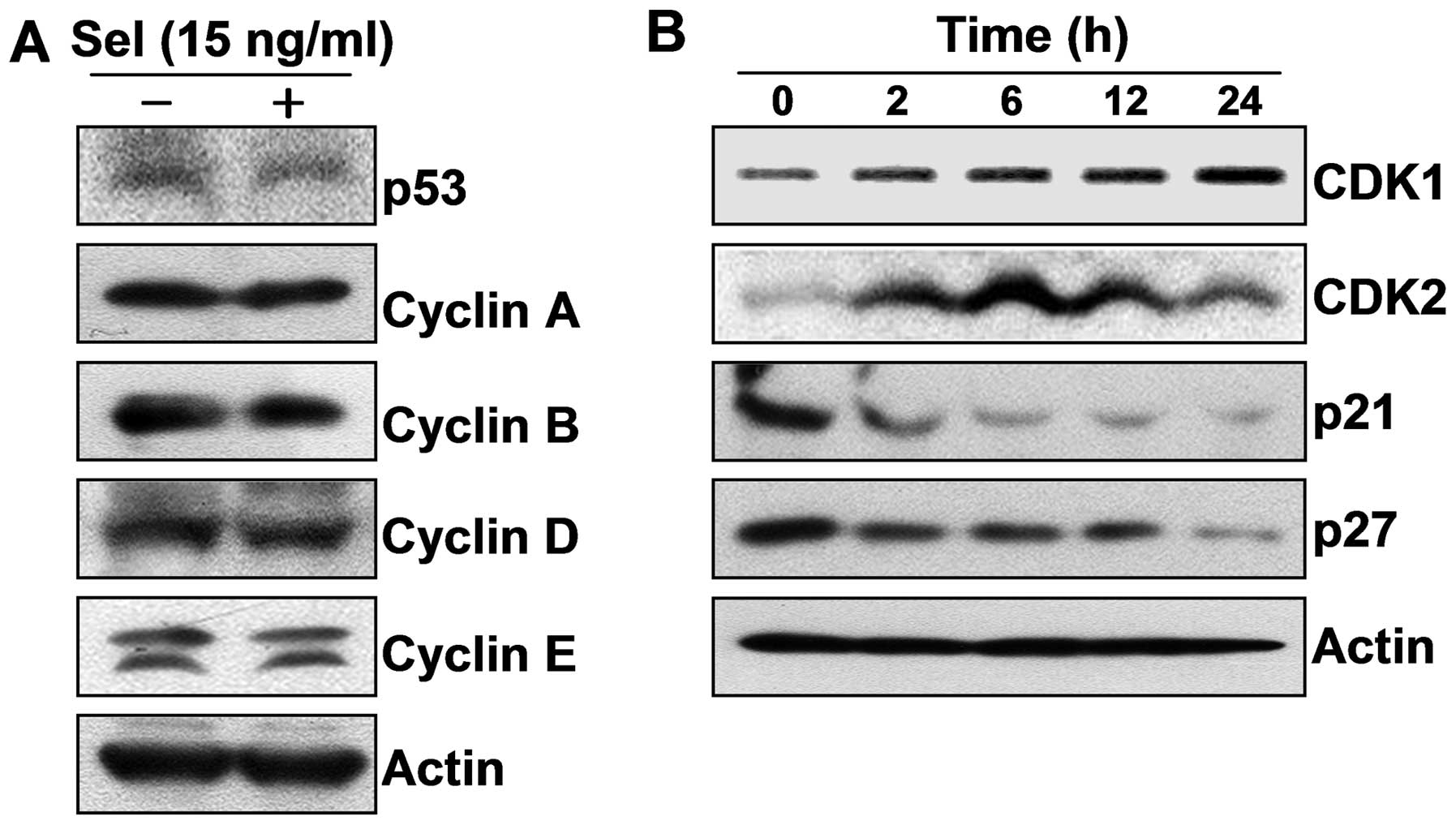
3T3-L1 cells, we focused on the expression of cell cycle-related
proteins including CDKs, cyclins, tumor suppressor p53 and CDK
inhibitors following the selenium treatment. Our results showed
that selenium upregulated the expression of cyclin-dependent kinase
1 (CDK1) and CDK2, without affecting the levels of p53 and cyclins;
however selenium markedly downregulated the levels of p21 and p27,
which are well-known CDK inhibitors and negatively control a broad
range of CDKs (Fig. 3). Taken
together, these data suggest that selenium induced active cell
proliferation by stimulating cell cycle progression.
Selenium enhances the cell proliferation
of 3T3-L1 preadipocytes through the PI3K/Akt pathway
The PI3K/Akt signaling cascade plays a critical role
in various physiological processes, including cell cycle
progression, transcription, translation, differentiation,
apoptosis, and metabolism (18).
In particular, the PI3K/Akt pathway is known to control the
function of numerous substrates, which regulate cell cycle
progression and cellular growth (19). Thus, we hypothesized that selenium
stimulates cell growth by modulating the PI3K/Akt signaling
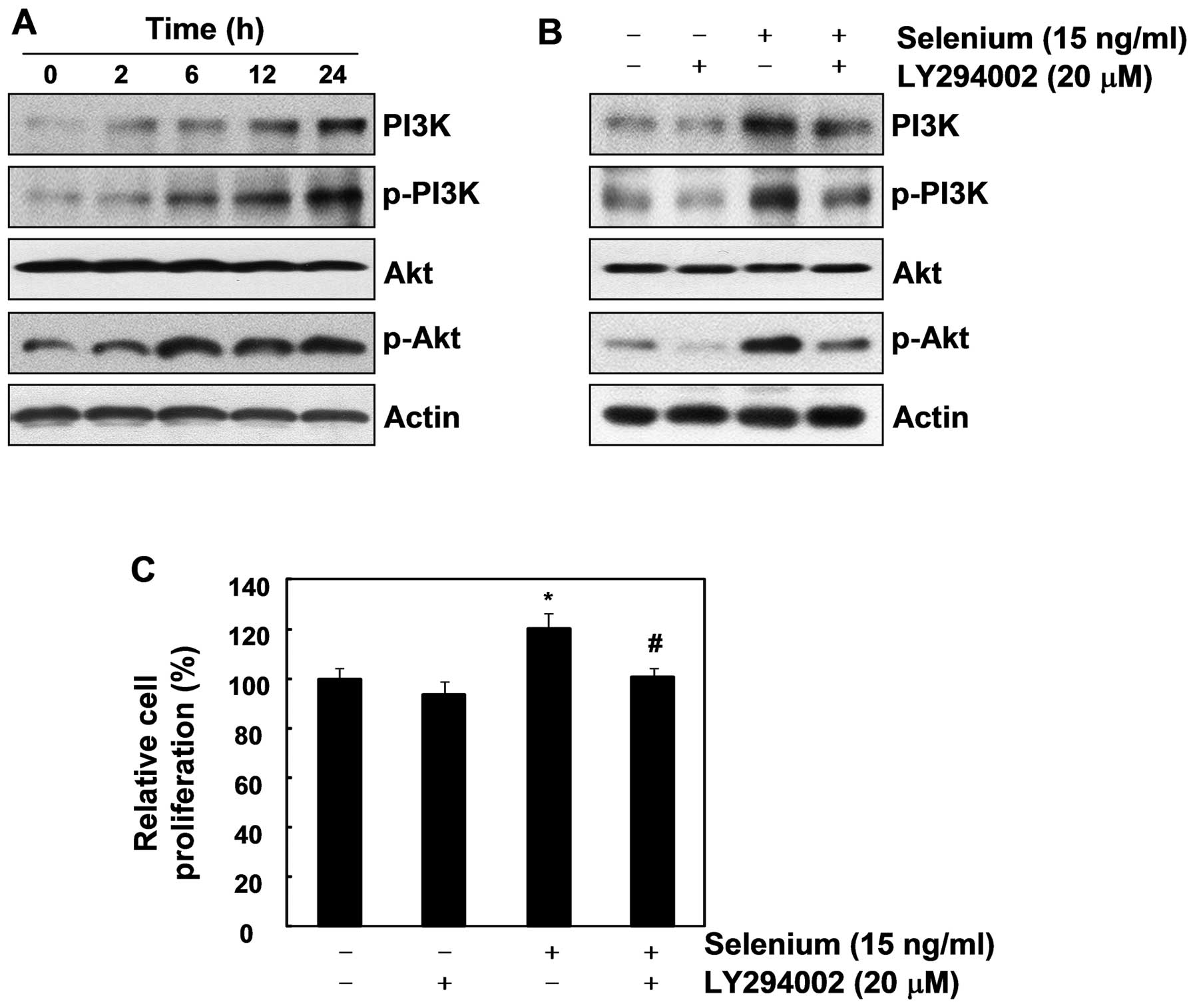
pathway. As shown in Fig. 4A, the
selenium treatment upregulated PI3K, p-PI3K, and p-Akt, indicating
that selenium activates the PI3K/Akt pathway. To confirm that the
PI3K/Akt pathway is important in the cell proliferation induced by
selenium, we used LY294002, an inhibitor of PI3K. As we expected,
LY294002 not only suppressed the activation of the PI3K/Akt
pathway, it also completely blocked the selenium-stimulated cell
proliferation (Fig. 4B and C).
Collectively, our results demonstrate that the PI3K/Akt signaling
pathway plays a crucial role in active cell proliferation of 3T3-L1
cells.
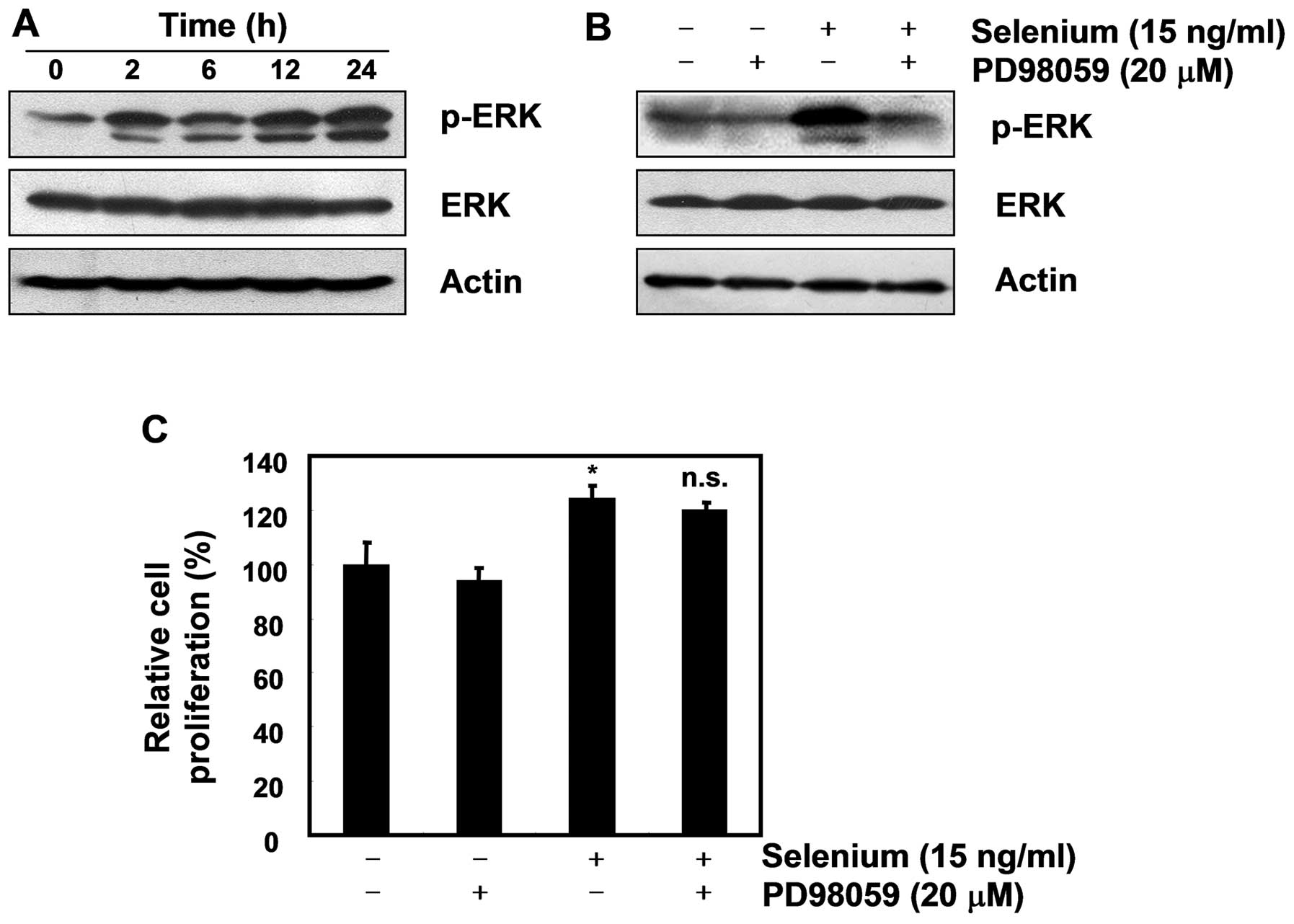
Selenium-induced ERK phosphorylation did
not affect the cell proliferation of 3T3-L1 preadipocytes
Extracellular signal-related kinase (ERK) is a key
player in mitogenic signaling following growth factor stimulation,
and studies have reported that it functions as a cell survival
mechanism against apoptosis (20,21).
As many researchers have reported that ERK plays a critical role in
cellular proliferation through the Ras/Raf/MEK/ERK pathway
(22,23), we next focused on the activation of
ERK. As displayed in Fig. 5A, our
data showed that selenium induced ERK phosphorylation in the 3T3-L1
cells. Although the ERK inhibitor PD98059 did not block the active
cell proliferation induced by the selenium treatment, it completely
inhibited the expression of pERK (Fig.
5B and C). Taken together, these data suggest that ERK has no
effect on the selenium-stimulated cell proliferation of 3T3-L1
cells.
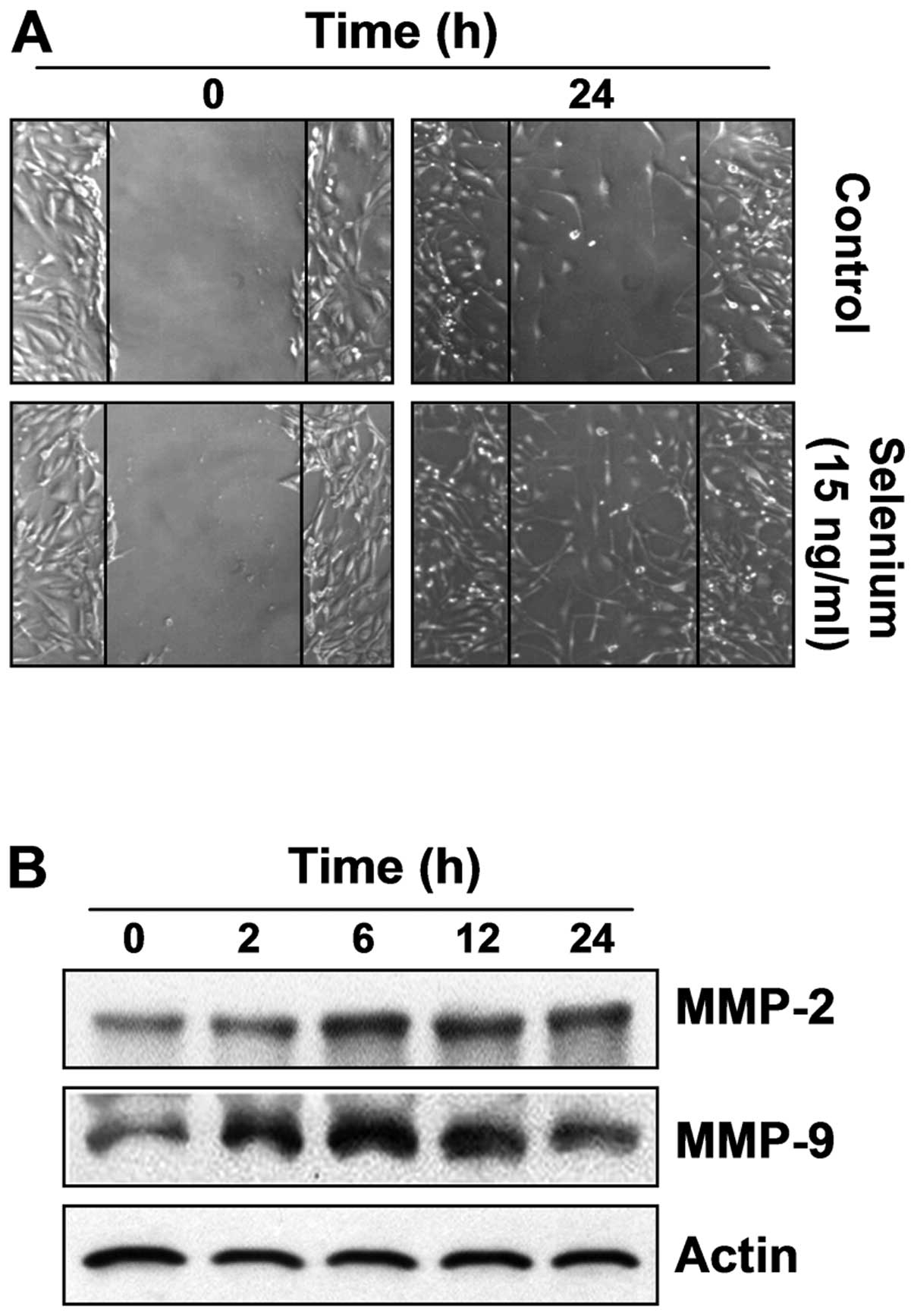
Selenium induces active migration in
3T3-L1 preadipocytes
To assess the migration efficiency of selenium,
which is an important characteristic of stem cells, an in
vitro scratch-inducing cell migration assay was conducted. As
shown in Fig. 6A, the
selenium-treated cells migrated more actively than the control
cells, indicating that selenium significantly increased the
migration efficiency of the 3T3-L1 cells. Next, we investigated the
expression of migration-related proteins, matrix metalloproteinases
(MMPs), which have many physiological functions in cell migration
and invasion (24,25). Our data showed that selenium
upregulated the expression of MMP-2 and MMP-9 in a time-dependent
manner. These results indicate that selenium-induced active
migration is associated with the overexpression of MMP-2 and MMP-9
(Fig. 6B).
Discussion
It is hoped that IPSCs will overcome several
fundamental problems of stem cell therapy, including ethical issues
and the difficulty of obtaining sufficient amounts of adult stem
cells. However, many of the underlying mechanisms of the
reprogramming processes are unknown, making IPSC technology far
from clinical use. Although selenium has been reported to have
various pharmacological actions, its ability to improve stem cell
behavior has not been fully elucidated. Therefore, we investigated
the acquisition of stem cell potency following selenium treatment
in 3T3-L1 adipocytes. We also investigated the effects of selenium
on the cell proliferation and active migration of the 3T3-L1
adipocytes.
Our data demonstrated that selenium stimulated the
cell proliferation of the 3T3-L1 cells, proven by a trypan blue
exclusion assay, microscopic observations and a colony-forming
assay. We investigated the mechanisms of selenium-induced active
proliferation with respect to cell cycle progression, the PI3K/Akt
pathway and ERK phosphorylation. First, we investigated whether
selenium modulated the cell cycle regulators of 3T3-L1 cells. Our
results showed that selenium upregulated CDK1 and CDK2 and
downregulated their inhibitors p21 and p27. Interestingly, selenium
did not affect the expression of cyclins, critical regulators of
the cell cycle, by forming complexes with CDKs (Fig. 3). Dinarina et al (26) reported that contrary to the common
belief, the activation of CDKs does not necessarily depend on
cyclins. For example, CDK1 and CDK2 can be directly activated by a
protein known as RINGO/Speedy (14,27).
Porter et al (28) revealed
that the protein can interact with CDK2 and p27 to play a role in
the G1/S checkpoint. Thus, our results imply that selenium
stimulated cell cycle progression by modulating CDKs, which were
activated independently of cyclins.
Second, we focused on the PI3K/Akt and ERK signaling
pathways. The PI3K/Akt pathway is activated by growth factors and
involved in various biological functions, including cell
proliferation, survival and cell migration (18). Several reports have suggested that
this signaling pathway plays a crucial role in maintaining the
self-renewal activity of ESCs, as well as the dedifferentiation of
embryonic germ cells (29–31). Liu et al (32) also reported that an inhibitor of
PI3K, LY294002, resulted in the loss of ESC features. Our results
also clearly showed that selenium induced active cell proliferation
of the 3T3-L1 cells by activating the PI3K/Akt pathway. This was
proven by the enhanced expression of p-PI3K and p-Akt, and by the
complete inhibition of cell proliferation following LY294002
treatment. As ERK signaling is known to be a key regulator of cell
proliferation (22,23), and Kim et al (15) reported that selenium changed
adipose-tissue stromal cells into a less mature state via
ERK-mediated pathways, we investigated the effect of ERK on the
proliferation of the 3T3-L1 adipocytes. However, our results
clearly showed that ERK activation did not affect cell
proliferation, although selenium induced ERK phosphorylation.
As additional proof of stem cell potency, selenium
treatment induced active cell migration, which is an important
characteristic of stem cells, in 3T3-L1 cells. The motogenic
potential of stem cells is critical in causing the stem cell
migration to the wounded area. MMPs seem to play an important role
in the active migration of stem cells. Heissig et al
(33) demonstrated that MMP-9
enhanced the motogenic potential of stem cells by translocating
them to a permissive proliferative vascular niche. In addition, Yu
et al (34) reported that
MMP-2 promoted the cell migration of mesenchymal stem cells. Our
results also showed that selenium upregulated the expression of
MMP-2 and MMP-9, both of which are associated with active cell
migration of 3T3-L1 cells. We cautiously suggest that
selenium-activated ERK might be involved in the active migration of
these cells because many researches have reported that ERK is a
critical factor in the regulation of cell migration. For example,
active MEK promoted cell migration in several cell types, and the
mutation or inhibition of ERK suppressed cell migration (35,36).
In conclusion, the present study suggests that
selenium can improve the potency of stem cells by stimulating the
cellular proliferation and active migration of 3T3-L1
preadipocytes. Our results indicate that regulating the PI3K/Akt
pathway might be an attractive method to produce IPSCs. Although
future studies will be required to investigate whether selenium
stimulates the pluripotency of 3T3-L1 cells to differentiate into
various cell types, our results provide knowledge on the novel
function of selenium, as well as the molecular mechanisms
underlying its activity.
Acknowledgements
This study was supported by a grant
from the National Research Foundation of Korea (NRF) grant funded
by the Korea government (2012-0000476), R&D program of MKE/KEIT
(10040391, Development of Functional Food Materials and Device for
Prevention of Aging-associated Muscle Function Decrease), and
Blue-Bio Industry RIC at Dong-Eui University as a RIC (08-06-07)
program of KIAT under Ministry of Knowledge Economy, Republic of
Korea.
References
|
1.
|
Aasen T, Raya A, Barrero MJ, Garreta E,
Consiglio A, Gonzalez F, Vassena R, Bilić J, Pekarik V, Tiscornia
G, Edel M, Boué S and Izpisúa Belmonte JC: Efficient and rapid
generation of induced pluripotent stem cells from human
keratinocytes. Nat Biotechnol. 26:1276–1284. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Im JE, Song SH, Kim JY, Kim KL, Baek SH,
Lee DR and Suh W: Vascular differentiation of multipotent
spermatogonial stem cells derived from neonatal mouse testis. Exp
Mol Med. 44:303–309. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Taura D, Sone M, Homma K, Oyamada N,
Takahashi K, Tamura N, Yamanaka S and Nakao K: Induction and
isolation of vascular cells from human induced pluripotent stem
cells - brief report. Arterioscler Thromb Vasc Biol. 29:1100–1103.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Pittenger MF, Mackay AM, Beck SC, Jaiswal
RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S and
Marshak DR: Multilineage potential of adult human mesenchymal stem
cells. Science. 284:143–147. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Welham MJ, Kingham E, Sanchez-Ripoll Y,
Kumpfmueller B, Storm M and Bone H: Controlling embryonic stem cell
proliferation and pluripotency: the role of PI3K- and
GSK-3-dependent signalling. Biochem Soc Trans. 39:674–678. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Lee MY, Lim HW, Lee SH and Han HJ: Smad,
PI3K/Akt, and Wnt-dependent signaling pathways are involved in
BMP-4-induced ESC self-renewal. Stem Cells. 27:1858–1868. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Chen L and Khillan JS: A novel signaling
by vitamin A/retinol promotes self renewal of mouse embryonic stem
cells by activating PI3K/Akt signaling pathway via insulin-like
growth factor-1 receptor. Stem Cells. 28:57–63. 2010.PubMed/NCBI
|
|
8.
|
Kimura T and Nakano T: Induction of
pluripotency in primordial germ cells. Histol Histopathol.
26:643–650. 2011.PubMed/NCBI
|
|
9.
|
Lee SR, Bar-Noy S, Kwon J, Levine RL,
Stadtman TC and Rhee SG: Mammalian thioredoxin reductase: oxidation
of the C terminal cysteine/selenocysteine active site forms a
thioselenide, and replacement of selenium with sulfur markedly
reduces catalytic activity. Proc Natl Acad Sci USA. 97:2521–2526.
2000. View Article : Google Scholar
|
|
10.
|
Rayman MP: The importance of selenium to
human health. Lancet. 356:233–241. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Combs GF and Gray WP Jr: Chemopreventive
agents: selenium. Pharmacol Ther. 79:179–192. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Ganther HE: Selenium metabolism,
selenoproteins and mechanisms of cancer prevention: complexities
with thioredoxin reductase. Carcinogenesis. 20:1657–1666. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Kim JH, Hue JJ, Kang BS, Park H, Nam SY,
Yun YW, Kim JS and Lee BJ: Effects of selenium on colon
carcinogenesis induced by azoxymethane and dextran sodium sulfate
in mouse model with high-iron diet. Lab Anim Res. 27:9–18. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Ferby I, Blazquez M, Palmer A, Eritja R
and Nebreda AR: A novel p34cdc2-binding and activating protein that
is necessary and sufficient to trigger G2/M progression in
Xenopus oocytes. Genes Dev. 13:2177–2189. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Kim JH, Lee MR, Kim JH, Jee MK and Kang
SK: IFATS collection: selenium induces improvement of stem cell
behaviors in human adipose-tissue stromal cells via SAPK/JNK and
stemness acting signals. Stem Cells. 26:2724–2734. 2008. View Article : Google Scholar
|
|
16.
|
O’Connor PM, Ferris DK, Pagano M, Draetta
G, Pines J, Hunter T, Longo DL and Kohn KW: G2 delay induced by
nitrogen mustard in human cells affects cyclin A/cdk2 and cyclin
B1/cdc2-kinase complexes differently. J Biol Chem. 268:8298–8308.
1993.PubMed/NCBI
|
|
17.
|
Nurse P: A long twentieth century of the
cell cycle and beyond. Cell. 100:71–78. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Yuan TL and Cantley LC: PI3K pathway
alterations in cancer: variations on a theme. Oncogene.
27:5497–5510. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Steelman LS, Pohnert SC, Shelton JG,
Franklin RA, Bertrand FE and McCubrey JA: JAK/STAT, Raf/MEK/ERK,
PI3K/Akt and BCR-ABL in cell cycle progression and leukemogenesis.
Leukemia. 18:189–218. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Chatani Y, Tanimura S, Miyoshi N, Hattori
A, Sato M and Kohno M: Cell type-specific modulation of cell growth
by transforming growth factor beta 1 does not correlate with
mitogen-activated protein kinase activation. J Biol Chem.
270:30686–30692. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Martin P and Pognonec P: ERK and cell
death: cadmium toxicity, sustained ERK activation and cell death.
FEBS J. 277:39–46. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Downward J: Targeting Ras signaling
pathways in cancer therapy. Nat Rev Cancer. 3:11–22. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Steelman LS, Chappell WH, Abrams SL, Kempf
RC, Long J, Laidler P, Mijatovic S, Maksimovic-Ivanic D, Stivala F,
Mazzarino MC, Donia M, Fagone P, Malaponte G, Nicoletti F, Libra M,
Milella M, Tafuri A, Bonati A, Bäsecke J, Cocco L, Evangelisti C,
Martelli AM, Montalto G, Cervello M and McCubrey JA: Roles of the
Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR pathways in controlling growth
and sensitivity to therapy-implications for cancer and aging. Aging
(Albany, NY). 3:192–222. 2011.PubMed/NCBI
|
|
24.
|
Zhong WD, Han ZD, He HC, Bi XC, Dai QS,
Zhu G, Ye YK, Liang YX, Qin WJ, Zhang Z, Zeng GH and Chen ZN:
CD147, MMP-1, MMP-2 and MMP-9 protein expression as significant
prognostic factors in human prostate cancer. Oncology. 75:230–236.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Jezierska A and Motyl T: Matrix
metalloproteinase-2 involvement in breast cancer progression: a
mini-review. Med Sci Monit. 15:RA32–RA40. 2009.PubMed/NCBI
|
|
26.
|
Dinarina A, Perez LH, Davila A, Schwab M,
Hunt T and Nebreda AR: Characterization of a new family of
cyclin-dependent kinase activators. Biochem J. 386:349–355. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Lenormand JL, Dellinger RW, Knudsen KE,
Subramani S and Donoghue DJ: Speedy: a novel cell cycle regulator
of the G2/M transition. EMBO J. 18:1869–1877. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Porter LA, Kong-Beltran M and Donoghue DJ:
Spy1 interacts with p27Kip1 to allow G1/S progression.
Mol Cell Biol. 14:3664–3674. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Paling NR, Wheadon H, Bone HK and Welham
MJ: Regulation of embryonic stem cell self-renewal by
phosphoinositide 3-kinase-dependent signaling. J Biol Chem.
279:48063–48070. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Watanabe S, Umehara H, Murayama K, Okabe
M, Kimura T and Nakano T: Activation of Akt signaling is sufficient
to maintain pluripotency in mouse and primate embryonic stem cells.
Oncogene. 25:2697–2707. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Kimura T, Tomooka M, Yamano N, Murayama K,
Matoba S, Umehara H, Kanai Y and Nakano T: AKT signaling promotes
derivation of embryonic germ cells from primordial germ cells.
Development. 135:869–879. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Liu N, Lu M, Feng XM, Ma FX, Fang ZH, Tian
XM, Ren Q, Zhang L, Liu B, Huang PP, Liu L and Han ZC: Exogenous
Nanog alleviates but is insufficient to reverse embryonic stem
cells differentiation induced by PI3K signaling inhibition. J Cell
Biochem. 106:1041–1047. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Heissig B, Hattori K, Dias S, Friedrich M,
Ferris B, Hackett NR, Crystal RG, Besmer P, Lyden D, Moore MA, Werb
Z and Rafii S: Recruitment of stem and progenitor cells from the
bone marrow niche requires MMP-9 mediated release of kit-ligand.
Cell. 109:625–637. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Yu Q, Chen L, You Y, Zou C, Zhang Y, Liu Q
and Cheng F: Erythropoietin combined with granulocyte
colony-stimulating factor enhances MMP-2 expression in mesenchymal
stem cells and promotes cell migration. Mol Med Rep. 4:31–36.
2011.PubMed/NCBI
|
|
35.
|
Klemke RL, Cai S, Giannini AL, Gallagher
PJ, deLanerolle P and Cheresh DA: Regulation of cell motility by
mitogen-activated protein kinase. J Cell Biol. 137:481–492. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Jo M, Thomas KS, Somlyo AV, Somlyo AP and
Gonias SL: Cooperativity between the Ras-ERK and Rho-Rho kinase
pathways in urokinase-type plasminogen activator-stimulated cell
migration. J Biol Chem. 277:12479–12485. 2002. View Article : Google Scholar : PubMed/NCBI
|