Introduction
Squamous cell carcinoma (SCC) is the most frequent
malignant tumor of the head and neck region. Head and neck squamous
cell carcinoma (HNSCC) is the sixth leading cancer by incidence
worldwide, with approximately 500,000 new cases per year (1).
The most life-threatening aspects of cancer are
invasion and metastasis. Cervical lymph node metastasis is
frequently detected in HNSCC, including oral squamous cell
carcinoma (OSCC). The presence or absence of cervical lymph node
metastasis has a great impact on the prognosis of the patient,
therefore, it is important to control cervical lymph node
metastasis. However, little is known about the molecular mechanisms
underlying lymph node metastasis in HNSCC.
The metastatic spread of tumor cells is a sequential
multistep process beginning with the detachment of individual tumor
cells from the primary tumor. The progression to metastasis
requires that the individual tumor cells adhere to and invade
through the basement membrane, migrate through the extracellular
matrix, and then intravasate into blood or lymphatic vessels,
throught which they can disseminate to distant sites. Tumor cells
must then extravasate out of the vessel and invade the target organ
before forming a metastatic tumor, which most commonly occurs in
the cervical lymph nodes in HNSCC (2,3).
Many signal transduction systems are associated with
the invasion and metastasis of cancer. Among them, the Wnt
signaling pathway, which is conserved in various species from worms
to mammals, and involved in various differentiation events during
embryonic development. Aberrant Wnt activation can lead to tumor
formation and malignant transformation. Wnt ligands bind to their
cognate receptors on the cell surface and transduce signals through
at least 3 distinct pathways: the canonical β-catenin pathway, the
non-canonical planar cell polarity (PCP) pathway, and the
Ca2+ pathway (4–6).
Wnt proteins are cysteine-rich secreted
glycoproteins that play critical roles in both carcinogenesis and
embryonic development. At least 19 Wnt members have been shown to
be present in humans and mice (7).
Wnt family members can be divided into 2 distinct
types based on their ability to induce transformation of the mouse
mammary epithelial cell line C57MG (8). The highly transforming members
include Wnt1, Wnt3, Wnt3A, and Wnt7A, while the intermediately
transforming or non-transforming members include Wnt2, Wnt4, Wnt5a,
Wnt5b, Wnt6, Wnt7b, and Wnt11. It is thought that the Wnts that
show high transforming activity in C57MG cells activate the
canonical Wnt signals, and that those that show intermediately
transforming or non-transforming activity in C57MG cells activate
the non-canonical Wnt signals (8).
In addition to cancer, abnormal Wnt signaling has
been reported to cause various kinds of disease, including neuronal
disease, bone and cartilage disease, diabetes, and renal disease
(9).
In cancer, mutations in genes involved in canonical
Wnt signaling have been found in several cancer types, often
resulting in the intracellular accumulation of β-catenin (9–11).
The non-canonical Wnt signals were traditionally thought not to be
involved in tumorigenesis because of their failure to induce
transformation of C57MG cells. However, recent evidence has shown
that some Wnts involved in non-canonical Wnt signaling are
upregulated in human cancers; it is thought that abnormal
activation of non-canonical Wnt signaling pathways might play a
role in malignant transformation (8).
In this study, we used human OSCC cell lines with
different metastatic potential to investigate the involvement of
the canonical and non-canonical Wnt signals in the metastatic
potential of cancer cells.
Materials and methods
Cell lines and culture
The SAS cell line was derived from a poorly
differentiated SCC that was originally isolated from the surgical
specimens of a Japanese woman with a primary tongue lesion
(12). Human SCC cell lines,
SAS-Venus and SAS-LM8, were kindly provided by Professor T. Yoneda,
Department of Biochemistry, Osaka University Graduate School of
Dentistry, Japan. Morita et al (13) stably overexpressed Venus protein
into SAS cells (SAS-Venus) and established highly metastatic SAS
cells (named SAS-LM3) after three rounds of in vivo
selection. SAS-LM8 used here was also derived from SAS-Venus but
after 8 rounds of in vivo selection. SAS-LM8 cells have
metastatic ability and motility almost equivalent to SAS-LM3 cells
in vivo.
The SAS-Venus and, SAS-LM8 cell lines were grown in
Dulbecco’s modified Eagle’s medium (DMEM) with 10% fetal bovine
serum (FBS) at 37°C in a humidified atmosphere with 5%
CO2.
Proliferation assay
Cells (2×104) were plated on 6-well
plates and allowed to grow and expand. The cells were then
trypsinized and counted after 8 days and evaluated using a growth
curve.
Cell migration and invasion assay
To measure the cell migration activity, transwell
chamber assays were performed using BD BioCoat cell culture inserts
(Becton-Dickinson, MA, USA). Cells were resuspended in serum-free
DMEM and then added to the upper chamber at a density of
5×104 cells/insert. DMEM containing 10% FBS was added to
the lower chamber. After incubation at 37°C for 48 h, the number of
cells that invaded into the lower chamber was counted. To measure
the cell invasion activity, transwell chamber assays were performed
using BD BioCoat Matrigel invasion chambers (Becton-Dickinson) in a
similar manner as that described for the cell migration assay.
After incubation at 37°C, the cells that penetrated the membrane
onto the lower side were fixed with formalin. The invasiveness of
the cells was determined by counting the area occupied by the cells
on the lower side of the filter through the pores under a
fluorescence microscope at magnification ×100. Seven fields were
randomly selected for each assay.
Immunocytochemical staining
Cells grown on cover slips were fixed with PBS
containing 4% paraformaldehyde for 15 min and then rendered
permeable with PBS containing 0.1% Triton X-100 for 3 min at 4°C.
For staining of β-catenin, after blocking with 2% bovine serum
albumin in PBS for 30 min, the cells were incubated with
anti-β-catenin mouse monoclonal antibody (Transduction
Laboratories, KY, USA; diluted at 1:500) in the blocking solution
at 4°C overnight. After washing, the cells were incubated with a
rhodamin-conjugated goat anti-mouse secondary antibody (Leinco
Technologies, MO, USA; diluted at 1:150) in PBS for 30 min at room
tempurature.
For staining of actin filaments, Alexa Fluor
546-conjugated phalloidin (Invitrogen, CA, USA) was used. After
blocking, the cells were incubated with the Alexa Fluor 546
phalloidin (diluted at 1:40) in the blocking solution at room
temperature for 20 min. Fluorescent images were obtained using a
confocal laser scanning microscope (Carl Zeiss, Germany).
GTPase activity assays
The activity of the Cdc42, Rac1, and RhoA GTPases
was determined using a RhoA/Rac1/Cdc42, Activation Assay Combo
Biochem kit (Cytoskeleton, CO, USA) according to the manufacturer’s
protocol. Briefly, cell lysates were incubated with a GST fusion
protein corresponding to either the p21-binding domain (amino acid
residues 67–150) of PAK1 or the Rho-binding domain (amino acid
residues 7–89) of Rhotekin, and the precipitates were bound to
glutathione-coupled sepharose beads. The bound Cdc42, Rac1, and
RhoA were separated by SDS-PAGE and detected by immunoblotting with
antibodies against Cdc42, Rac1 or RhoA (Cytoskeleton). The bands
were scanned and their intensities were quantified using the public
domain ImageJ program.
RNA extraction and reverse transcriptase
PCR
A 0.5-μg aliquot of total RNA was
reverse-transcribed into single-stranded cDNA and subsequent PCR
was performed using a Takara RNA PCR kit (AMV) Version 3.0 (Takara,
Shiga, Japan) by monitoring GAPDH levels as a quantitative control.
The primer sequences used for PCR amplification were as follows:
5′-CAACTACATGGTTTACATGTTC-3′ and 5′-GCC AGTGGACTCCACGAC-3′ for
GAPDH; 5′-CCAGCGTGGA CAATGGCTAC-3′ and 5′-TGAGCTCGAGTCATTGCA
TAC-3′ for β-catenin; 5′-AGACTCCAGCGCCTTCTCT CCG-3′ and
5′-CTGTGAGGAGGTTTGCTGTGGCC-3′ for c-myc;
5′-TCTAAGATGAAGGAGACCATC-3′ and 5′-GCG GTAGTAGGACAGGAAGTTGTT-3′ for
cyclin D1; 5′-AAC TCCCGCGTCATAGAAATAATG-3′ and 5′-ACCCAAAGA
ATGGCCAAGTTCATG-3′ for MMP7; 5′-CTGGAGCTT GAAAATCTGCCG-3′
and 5′-GGTTTTTCGGTTCGTGAG TGC-3′ for uPAR;
5′-AATGGGAAGTCCAGGCAGTGT ATC-3′ and 5′-ACAGCGTTCTCCAGTAACAGCTG-3′
for laminin-5γ2 chain; 5′-GGGTTTTTCGGTTCGTGAGT GC-3′ and
5′-CCATTGGGCATCCAGAAGAGAGC-3′ for MT1-MMP;
5′-CAAGTACTCGGGCAAAGAGG-3′ and 5′-CTTCCTCTGCTTATCTG-3′ for
S100A4; 5′-CAGTTC AAGACCGTGCAGAC-3′ and 5′-TGGAACCTACCC
ATCCCATA-3′ for Wnt5a; 5′-CGGGAGCGAGAGAAGA ACT-3′ and
5′-TACACCTGACGAAG CAGCAC-3′ for Wnt5b;
5′-TGACCTCAAGACCCGATACC-3′ and 5′-CAAGTG AAGGCAAAGCACAA-3′ for
Wnt11.
Silencing by siRNA
The Wnt-5b siRNA (Qiagen, Valencia, CA, USA)
used in this study is a 21-bp duplex oligoribonucleotide
corresponding to the human Wnt-5b mRNA sequence and has the
sense sequence 5′-CUCCUGGUGGUCAUUAGC UUU-3′.
Logarithmically growing cells were seeded at a
density of 1×106 cells per 10-cm dish and transfected
with 5 nM Wnt-5b siRNA using HiPerFect HTS reagent (Qiagen)
according to the manufacturer’s instructions. Seventy-two hours
after transfection, the cells were used for an in vitro
migration assay and immunocytochemical staining of actin filaments
as described above. AllStars Negative Control siRNA (Qiagen) was
used as a negative control. The efficiency of the siRNA was checked
by RT-PCR.
Cell stimulation with recombinant
Wnt5b
For stimulation with Wnt5b, cells were cultured with
recombinant human Wnt5b (500 ng/ml) (R&D Systems, Minneapolis,
MN, USA) for 48 h and then used for shape change observations, the
in vitro migration assay, immunocytochemical staining of
actin filaments and the GTPase activity assays as described
above.
Statistical analysis
The Student’s t-test was used to compare data
between 2 groups. p-values of <0.05 were considered to be
statistically significant.
Results
Morphology, migration, and invasion of
SAS-Venus and SAS-LM8 cells
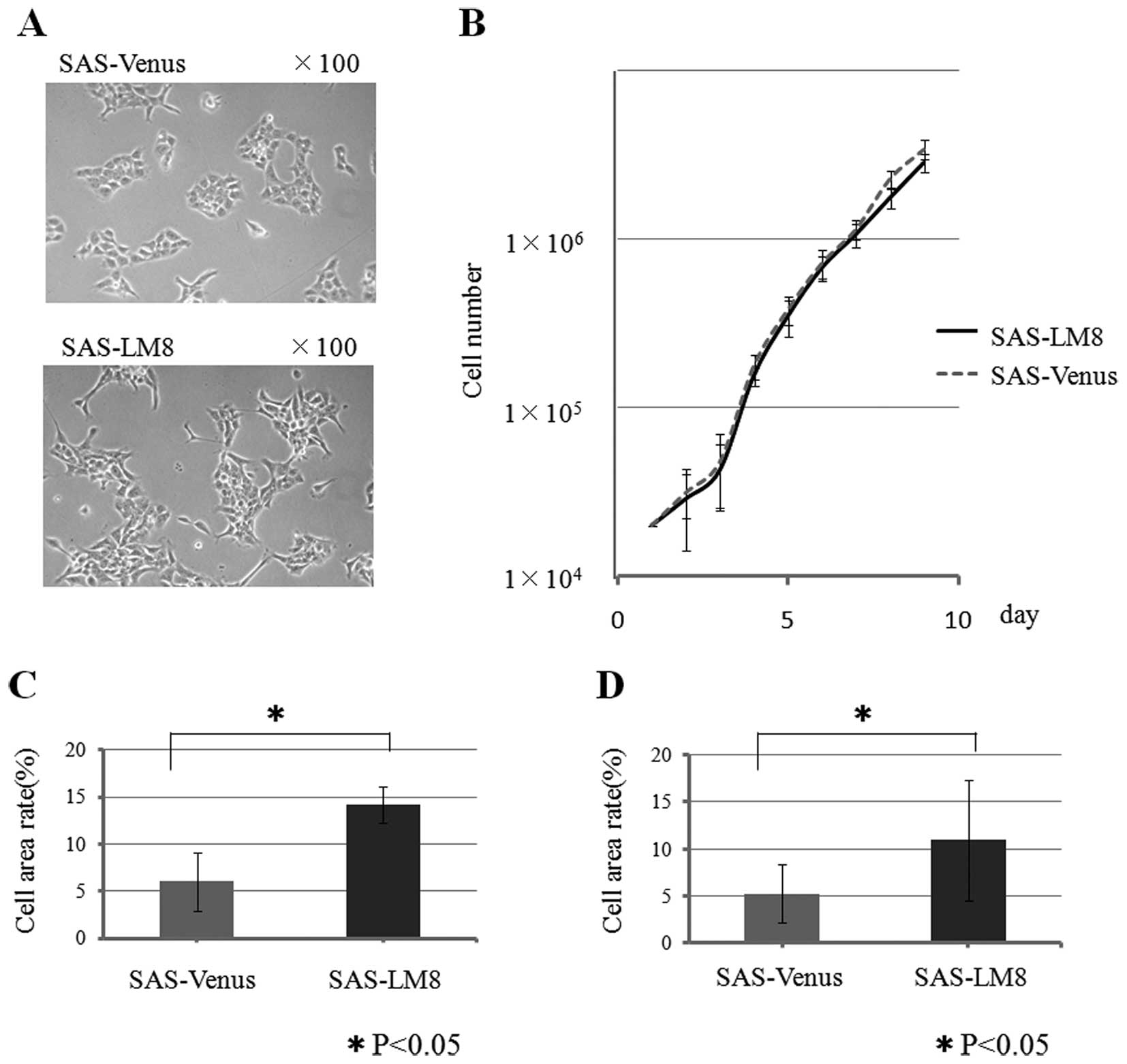
SAS-Venus cells showed a polygonal shape with few
pseudopod-like protrusive structures around the colonies, while
SAS-LM8 cells showed a slightly spindle-shaped morphology with many
pseudopod-like protrusive structures. However, both cell type
maintained cell-cell contact in the colonies (Fig. 1A), and no difference was observed
between their proliferative capacities (Fig. 1B).
The motility of the SAS-LM8 and SAS-Venus cells was
assessed by a transwell chamber migration assay. In the migration
assay, the area occupied by the cells on the lower side of the
filter after migration through the pores was measured. The cell
area rate of the SAS-LM8 cells (14.2%) was significantly higher
than that of the parental cell line SAS-Venus (6.0%: p<0.05)
(Fig. 1C). Similarly, in the
invasion assay, the cell area rate of the SAS-LM8 cell line (11.0%)
was significantly higher than that of the parental cell line
SAS-Venus (5.3%: p<0.05) (Fig.
1D).
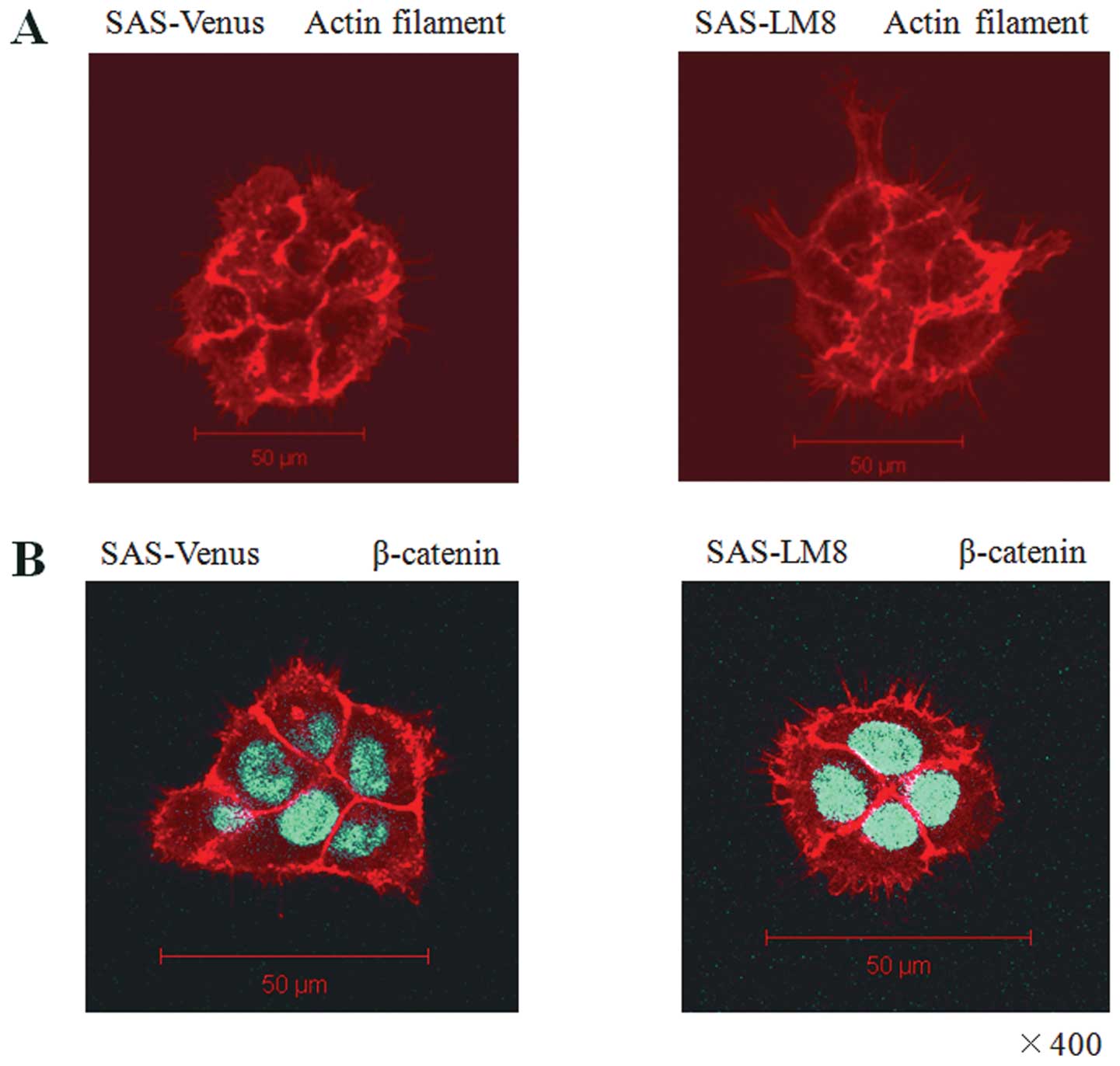
Immunofluorescence analysis of actin
filaments and ß-catenin
Having observed that the cell lines exhibit
significant differences in cell motility, we next assessed the
rearrangement of the actin cytoskeleton and filopodia-like
protrusive structures in SAS-Venus and SAS-LM8 cells via
immunofluorescent staining of actin filaments using Alexa Fluor
546-conjugated phalloidin. Actin rearrangements and the formation
of filopodia-like protrusions were more evident in SAS-LM8 than in
SAS-Venus (Fig. 2A).
Immunofluorescence analysis of β-catenin revealed that it was
located at the cell membranes and in the cytoplasm in SAS-LM8 and
SAS-Venus cells; no difference in β-catenin was observed (Fig. 2B).
Activation of Rho family members
Rho family members including the Cdc42, Rac1 and
RhoA GTPases have been reported to contribute to the rearrangement
of actin filaments. Therefore, the levels of active Cdc42, Rac1,
and RhoA GTPases in SAS-LM8 and SAS-Venus cells were measured by
GTPase activity assays. The level of active Cdc42 and active RhoA
was higher in SAS-LM8 than in SAS-Venus (Fig. 2C).
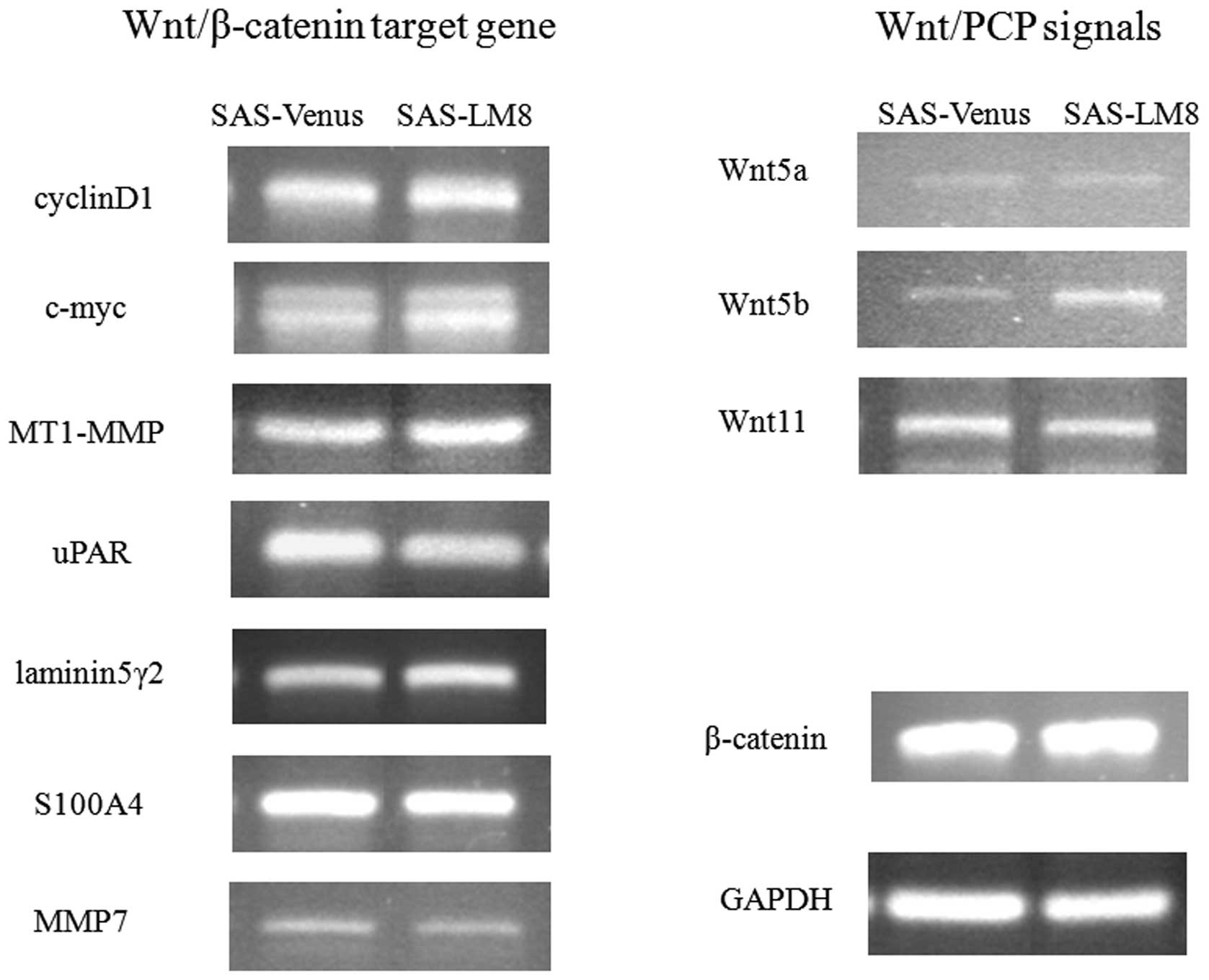
Expression of Wnt signaling related
gene
To investigate whether canonical Wnt/β-catenin
signaling might contribute to the cell migration differences
between the cell lines, the expression of several target genes of
the Wnt/β-catenin pathway was examined using semi-quantitative
RT-PCR. The selected Wnt-related genes included β-catenin,
cyclin D1 (14,15), c-myc (16), MT1-MMP (17), MMP-7(18), laminin-5γ2(19), uPAR(20,21)
and S100A4(22). However,
no significant changes were observed in the expression of the
tested target genes of the Wnt/β-catenin pathway (Fig. 3).
We also assessed the expression of Wnt5a, Wnt5b, and
Wnt11, which are representative non-canonical Wnts that transduce
Wnt/PCP signals (23). The mRNA
level of Wnt5b was higher in SAS-LM8 than in SAS-Venus,
whereas no significant changes were observed in the expression of
Wnt5a and Wnt11 (Fig.
3).
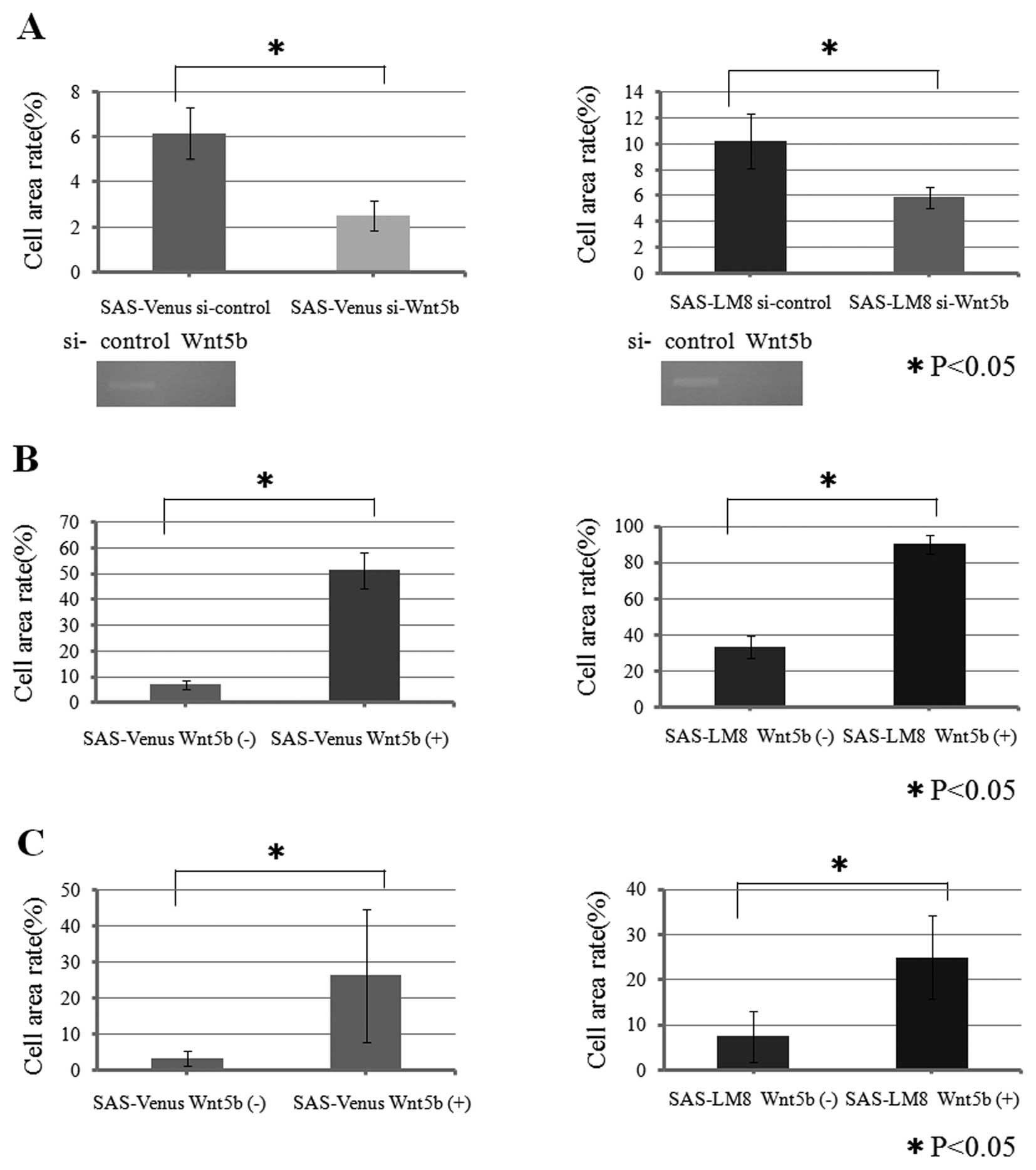
Changes in cell motility result in
siRNA-mediated knockdown of Wnt5b
Having found that Wnt5b was differentially
expressed in the OSCC cell lines used in this study, we next
examined the role of Wnt5b in their migration ability via
siRNA-mediated knockdown of Wnt5b. We first confirmed via
RT-PCR that treatment with the Wnt5b siRNA reduced the expression
of Wnt5b mRNA in the cell lines by comparison with treatment with a
control siRNA (si-control) (Fig.
4A). In the migration assay, the cell area rate of SAS-Venus
si-Wnt5b (2.5%) was significantly lower than that of SAS-Venus
si-control (6.1%: p<0.05), and the cell area rate of SAS-LM8
si-Wnt5b (5.9%), was significantly lower than that of SAS-LA8
si-control (10.2%; p<0.05), indicating that knockdown of
Wnt5b significantly inhibited cell migration in both cell
lines (Fig. 4A).
Changes in the ability of migration and
invasion of cells stimulated with recombinant Wnt5b
To confirm the siRNA result indicating that
Wnt5b was involved in migration and invasion, we carried out
assays of cells stimulated with recombinant Wnt5b protein.
In the migration assay, the cell area rates of
SAS-Venus and SAS-LM8 cells stimulated with Wnt5b (51.3 and 90.3%,
respectively) were significantly higher than those of the SAS-Venus
and SAS-LM8 not stimulated with Wnt5b (7.0 and 33.1%, respectively;
p<0.05) (Fig. 4B). These values
correspond to 7.3- and 2.7-fold increase in migration upon Wnt5b
stimulation of SAS-Venus and SAS-LM8 cells, respectively.
Similarly, in the invasion assay, SAS-Venus and
SAS-LM8 cells stimulated with Wnt5b showed significantly higher
cell area rates (26.3 and 25.0%, recpectively) than SAS-Venus and
SAS-LM8 cells not stimulated with Wnt5b (3.3 and 7.4%,
respectively; p<0.05) (Fig.
4C). These results correspond to 8.0-and 3.4-fold increase in
invasion by SAS-Venus and SAS-LM8 cells, respectively, and indicate
that stimulation with Wnt5b promoted cell invasion in both cell
lines.
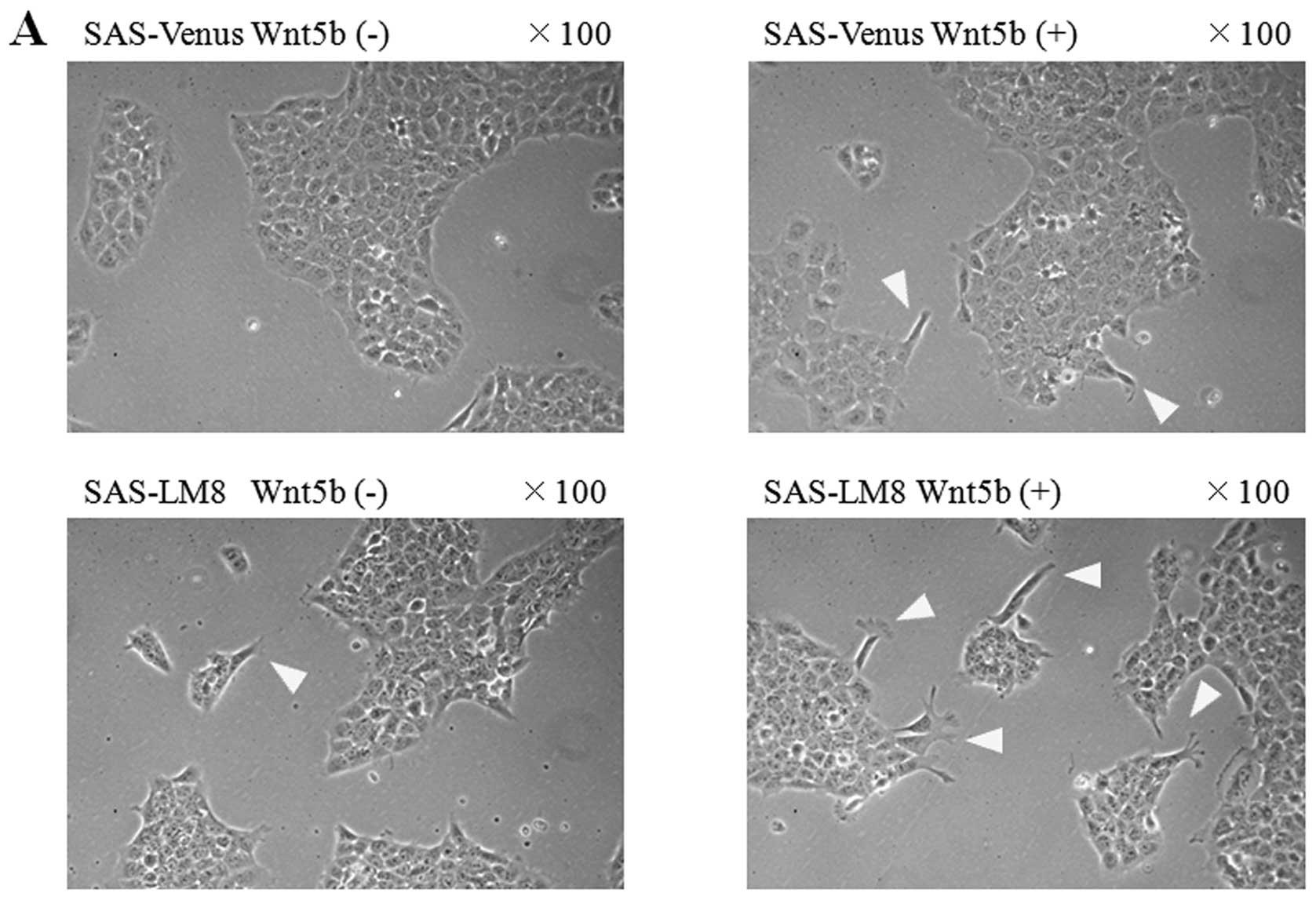
Morphological changes upon stimulation of
cells with recombinant Wnt5b
SAS-Venus and SAS-LM8 cells stimulated with
recombinant Wnt5b, showed an increase in the formation of the
pseudopod-like protrusive structures around the colonies, while
cell-cell interaction was maintained in both cell lines (Fig. 5A).
Changes in actin filaments upon Wnt5b
knockdown or stimulation
Immunofluorescent staining of actin filaments
revealed that treatment with Wnt5b siRNA significantly
inhibited the formation of filopodia-like protrusive structures in
both SAS-Venus and SAS-LM8 cells. Conversely, stimulation with
recombinant Wnt5b resulted in a clear increase in filopodia-like
protrusions in both SAS-Venus and SAS-LM8 cells (Fig. 5B).
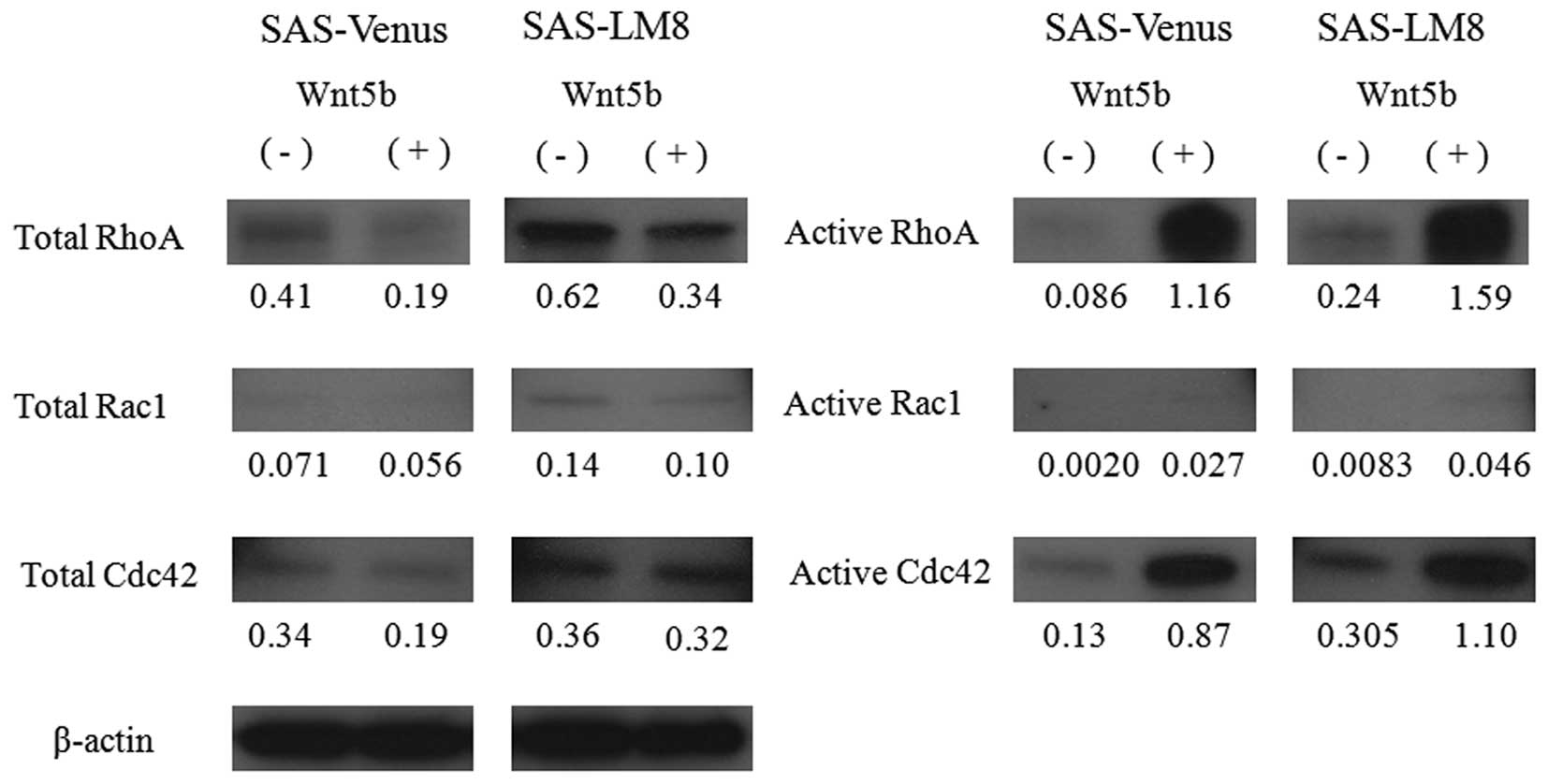
Activation of Rho family members upon
stimulation with recombinant Wnt5b
Having found that stimulation with recombinant Wnt5b
promoted the rearrangement of the actin cytoskeleton and the
formation of filopodia-like protrusive structures, we next carried
out GTPase activity assays on cells stimulated with recombinant
Wnt5b. While the total protein levels of Cdc42, Rac1, and RhoA were
similar in Wnt5b-stimulated and unstimulated cells, the levels of
the active forms of these proteins were clearly higher in the
Wnt5b-stimulated cells than in the unstimulated cells (Fig. 6).
Discussion
It is important to control the invasion and
metastasis of cancer cells. However, little is known about the
molecular mechanisms underlying invasion and lymph node metastasis
in HNSCC. We aimed to clarify the characteristic biological
features related to high metastatic potential and identify new
target molecules for the suppression of lymph node metastasis of
OSCC. To this end, we used cell lines with different metastatic
potential to investigate the involvement of canonical and
non-canonical Wnt signaling in OSCC metastasis.
First, we compared the cell shape, proliferation,
and motility of the cell lines. SAS-LM8 cells exhibited a slight
spindle shape, while SAS-Venus cells showed a polygonal shape.
However, in both cell types, colonies formed with intimate
intercellular interaction, and the cell-cell interactions were
maintained (Fig. 1A). Moreover,
there was no difference in proliferative capacity of the cell lines
(Fig. 1B). Distinct migration and
invasion differences were observed between the cell lines; SAS-LM8
showed significantly higher migration and invasion ability than
SAS-Venus (Fig. 1C and D).
Migration of the cancer cell is essential to the
metastatic process, particularly for the detachment from the
primary tumor, invasion into surrounding tissue and intravasation
steps. Increased motility of cancer cells is one of the malignant
transformation steps of cancer, and is a direct cause of cancer
invasion and metastasis.
Therefore, the differences in migration ability of
the SAS-Venus and SAS-LM8 cells are likely to be largely involved
in the difference in metastatic potential of these cell lines. It
is known that when cells migrate, reconstitution of the actin
cytoskeleton occurs. In our study, immunofluorescent staining of
actin filaments revealed that the rearrangement of the actin
cytoskeleton and formation of filopodia-like protrusive structures
were more pronounced in SAS-LM8 cells than in SAS-Venus cells
(Fig. 2A). Rho family GTPases,
including Cdc42, Rac1, and RhoA, have been reported to contribute
to the rearrangement of actin filaments (24–26).
Rho proteins cycle between an inactive GDP-bound form and an active
GTP-bound form. In their active form, they can interact with a
variety of effector proteins (24–26).
The levels of active Cdc42, and active RhoA in SAS-LM8 were higher
than in SAS-Venus (Fig. 2C),
whereas Rac1 was present at a very low level in both of the cell
lines. These results suggest that the activation of Cdc42 and RhoA
was associated with the high migration ability of the SAS-LM8
cells.
We previously reported that the aberrant cytoplasmic
accumulation of β-catenin can induce Tcf/Lef-mediated
transcriptional activity, and the Rho family member-mediated
rearrangement of the actin cytoskeleton in OSSC (27).
Canonical Wnt signals are transduced through the
Wnt/GSK3β signaling pathway, which determines cell fate through the
activation of the β-catenin/Tcf and Snail/EMT signaling cascades.
Non-canonical Wnt signals are transduced through the Wnt/PCP
signaling pathway, which regulates tissue polarity and cell
movement and includes RhoA, Rac1, Cdc42 as downstream effectors
(23). Therefore, we investigated
whether canonical and/or non-canonical Wnt signaling contributed to
the difference between the migration ability of SAS-Venus and
SAS-LM8 cells.
Our examination of canonical Wnt signals, revealed
that there was no difference between the cell lines in the
localization of of β-catenin (Fig.
2B) or the expression of Wnt/β-catenin target genes (Fig. 3).
When we examined Wnts known to be involved in
non-canonical Wnt/PCP signaling, namely, Wnt5a, Wnt5b, and Wnt11,
we found that the mRNA level of Wnt5b was increased in
SAS-LM8 compared to SAS-Venus (Fig.
3). Wnt5b has an 80.5% total-amino-acid identity with Wnt5a;
however, there are many remaining questions about the function of
Wnt5b. Whether the function of Wnt5b is the same as that of Wnt5a.
It has been reported that Wnt5a inhibits cell proliferation,
migration, and invasion in thyroid cancer (28), and colon cancer (29), but promotes cell migration and
invasion in malignant melanoma (30) and gastric cancer (31). These latter findings suggest that
Wnt5a might enhance the migration activity of cancer cells and
promote invasion and metastasis. The Wnt5b gene has been
linked to diseases other than cancer. For example, Wnt5b may
contribute to the susceptibility to type 2 diabetes and may be
involved in the pathogenesis of this disease through the regulation
of adipocyte function (32,33).
However, there are only a few reports on the relationship between
Wnt5b and malignant tumors, and to the best of our
knowledge, there is only one report indicating that Wnt5b
promotes the invasion of HNSCC (34).
In our study, no difference was observed in the
expression of Wnt5a between the cell lines; however,
compared to SAS-Venus, SAS-LM8 cells exhibited higher migration
ability and higher expression of Wnt5b, which shows high sequence
identity with Wnt5a. In order to clarify the role of Wnt5b
in the migration capability of OSCC cell lines, we examined the
effects of Wnt5b knockdown by using siRNA in SAS-Venus and
SAS-LM8 cells. We found that Wnt5b knockdown significantly
inhibited the migration of both SAS-Venus and SAS-LM8 cells
(Fig. 4A). Consistent with these
results, the stimulation of SAS-Venus and SAS-LM8 cells with
recombinant Wnt5b resulted in a significant increase in migration
and invasion (Fig. 4B and C).
Taken together, these results indicate that Wnt5b was involved in
the migration ability of the OSSC cells tested in this study.
The siRNA-mediated knockdown of Wnt5b also
caused a decrease in the formation of filopodia-like protrusive
structures in SAS-Venus and SAS-LM8 (Fig. 5B). Conversely, when stimulated with
recombinant Wnt5b, SAS-Venus and SAS-LM8 cells formed more
filopodia-like protrusions (Fig.
5), and the levels of active Cdc42 and active RhoA increased
(Fig. 6). These results suggest
that the activation of Cdc42 and RhoA is particularly important for
the increase in the cell migration ability induced by Wnt5b.
In the process of cancer invasion, invadopodia are
formed locally and penetrate the basement membrane (35). Once they are through the basement
membrane and interstitial tissue, the tumor cells form
filopodia-like protrusive structures at the invading front, in
addition to local stress fibers. Therefore, it is thought that the
invasion of the cancer is promoted by the activation of Cdc42 and
RhoA, which leads to subsequent rearrangement of the actin
cytoskeleton.
When a cancer cell moves, we can classify its
migration modes into amoeboid, mesenchymal and collective migration
(36,37). Cdc42, RhoA, and Rac1 have been
found to be involved in each of the migration styles (26). The amoeboid and mesenchymal modes
are observed when single cells migrate, while collective migration
is a mode of movement in which cell-cell adhesion of a plurality of
cells is maintained. It is becoming clear that collective migration
is involved in the dissemination of tumor cells, particularly for
tumors such as SCCs. In the classical view of metastasis, it is
thought that tumor cells must undergo EMT to migrate as single
cells. However, imaging of tumor cell behavior in a 3D culture
revealed that epithelial-type tumor cells can spread as groups or
sprouts, and full EMT appears not to be essential for tumors to
spread into the surrounding tissue (38).
In our study, SAS-Venus and SAS-LM8 cells formed
colonies with intimate intercellular interactions. Furthermore,
cell-cell interactions were maintained and no significant changes
were observed in the expression of E-cadherin (data not shown).
Cdc42 has been reported to be involved in the collective migration
of cancer cells via myosin (39).
We observed an increase in the levels of active Cdc42 and active
RhoA in SAS-LM8; thus, in addition to single-cell migration,
SAS-LM8 cells might have a high potential for formation of
invadopodia and collective cell migration, which likely contributes
to the high metastasis potential of SAS-LM8.
In conclusion, we demonstrated that Wnt5b is
involved in cell motility through the activation of Cdc42 and RhoA
via the non-canonical Wnt signaling pathway. Therefore, the
elevated expression of Wnt5b may become an important index for the
evaluation of OSCC invasion and metastasis. Furthermore, Wnt5b
shows promising as a therapeutic target for the prevention of OSCC
metastasis.
Acknowledgements
We would like to thank Dr Yoneda
Toshiyuki (Adjunctive Professor and Emeritus Professor, Department
of Biochemistry, Graduate School of Dentistry, Osaka University,
Japan and Senior Research Professor, Division of
Hematology/Oncology, Indiana University School of Medicine, USA)
and Dr Hata Kenji (Associated Professor, Department of
Biochemistry, Graduate School of Dentistry, Osaka University,
Japan) for providing SAS-LM8 and SAS-Venus cell lines.
References
|
1.
|
Rousseau A and Badoual C: Head and neck:
squamous cell carcinoma: an overview. Atlas Genet Cytogenet Oncol
Haematol. 16:145–155. 2012.
|
|
2.
|
Howell GM and Grandis JR: Molecular
mediators of metastasis in head and neck squamous cell carcinoma.
Head Neck. 27:710–717. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Sahai E: Illuminating the metastatic
process. Nat Rev Cancer. 7:737–749. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Veeman MT, Axelrod JD and Moon RT: A
second canon: functions and mechanisms of β-catenin-independent Wnt
signaling. Dev Cell. 5:367–377. 2003.PubMed/NCBI
|
|
5.
|
Nelson WJ and Nusse R: Convergence of Wnt,
β-catenin, and cadherin pathways. Science. 303:1483–1487. 2004.
|
|
6.
|
Kühl M, Sheldahl LC, Park M, Miller JR and
Moon RT: The Wnt/Ca2+ pathway: a new vertebrate Wnt
signaling pathway takes shape. Trends Genet. 16:279–283. 2000.
|
|
7.
|
Miller JR: The Wnts. Genome Biol. 3(1):
reviews. 3001.1–3001.15. 2001. View Article : Google Scholar
|
|
8.
|
Kikuchi A and Yamamoto H: Tumor formation
due to abnormalities in the β-catenin-independent pathway of Wnt
signaling. Cancer Sci. 99:202–208. 2008.
|
|
9.
|
Kikuchi A: Wnt signaling; its
abnormalities and diseases. Seikagaku. 81:780–792. 2009.PubMed/NCBI
|
|
10.
|
Polakis P: The many ways of Wnt in cancer.
Curr Opin Genet Dev. 17:45–51. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Kikuch A: Tumor formation by genetic
mutations in the components of the Wnt signaling pathway. Cancer
Sci. 94:225–229. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Takahashi K, Kanazawa H, Akiyama Y, Tazaki
S, Takahara M, Muto T, Tanzawa H and Sato K: Establishment and
characterization of a cell line (SAS) from poorly differentiated
human squamous cell carcinoma of the tongue. J Jpn Stomatol Soc.
38:20–28. 1989.
|
|
13.
|
Morita Y, Hata K, Nakanishi M, Nishisho T,
Yura Y and Yoneda T: Cyclooxygenase-2 promotes tumor
lymphangiogenesis and lymph node metastasis in oral squamous cell
carcinoma. Int J Oncol. 41:885–892. 2012.PubMed/NCBI
|
|
14.
|
Shtutman M, Zhurinsky J, Simcha I,
Albanese C, D’Amico M, Pestell R and Ben-Ze’ev A: The cyclin D1
gene is a target of the β-catenin/LEF-1 pathway. Proc Natl Acad Sci
USA. 96:5522–5527. 1999.
|
|
15.
|
Tetsu O and McCormick F: β-catenin
regulates expression of cyclin D1 in colon carcinoma cells. Nature.
398:422–426. 1999.
|
|
16.
|
He TC, Sparks AB, Rago C, Hermeking H,
Zawel L, Da Costa LT, Morin PJ, Vogelstein B and Kinzler KW:
Identification of c-myc as a target of the APC pathway. Science.
281:1509–1512. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Takahashi M, Tsunoda T, Seiki M, Nakamura
Y and Furukawa Y: Identification of membrane-type matrix
metalloproteinase-1 as a target of the β-catenin/Tcf4 complex in
human colorectal cancers. Oncogene. 21:5861–5867. 2002.
|
|
18.
|
Brabletz T, Jung A, Dag S, Hlubek F and
Kirchner T: β-catenin regulates the expression of the matrix
metalloproteinase-7 in human colorectal cancer. Am J Pathol.
155:1033–1038. 1999.
|
|
19.
|
Hlubek F, Jung A, Kotzor N, Kirchner T and
Brabletz T: Expression of the invasion factor laminin γ2 in
colorectal carcinomas is regulated by β-catenin. Cancer Res.
61:8089–8093. 2001.
|
|
20.
|
Mann B, Gelos M, Siedow A, Hanski ML,
Gratchev A, Ilyas M, Bodmer WF, Moyer MP, Riecken EO, Buhr HJ and
Hanski C: Target genes of beta-catenin-T cell
factor/lymphoid-enhancer-factor signaling in human colorectal
carcinomas. Proc Natl Acad Sci USA. 96:1603–1608. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Hiendlmeyer E, Regus S, Wassermann S,
Hlubek F, Haynl A, Dimmler A, Koch C, Knoll C, van Beest M, Reuning
U, Brabletz T, Kirchner T and Jung A: β-catenin up-regulates the
expression of the urokinase plasminogen activator in human
colorectal tumors. Cancer Res. 64:1209–1214. 2004.
|
|
22.
|
Stein U, Arlt F, Walther W, Smith J,
Waldman T, Harris ED, Mertins SD, Heizmann CW, Allard D, Birchmeier
W, Schlag PM and Shoemaker RH: The metastasis-associated gene
S100A4 is a novel target of beta-catenin/T-cell factor signaling in
colon cancer. Gastroenterology. 131:1486–1500. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Katoh M: WNT/PCP signaling pathway and
human cancer (Review). Oncol Rep. 14:1583–1588. 2005.PubMed/NCBI
|
|
24.
|
Sahai E and Marshall CJ: Rho-GTPases and
cancer. Nat Rev Cancer. 2:133–142. 2002. View Article : Google Scholar
|
|
25.
|
Price LS and Collard JG: Regulation of the
cytoskeleton by Rho-family GTPases: implications for tumour cell
invasion. Cancer Biol. 11:167–173. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Vega FM and Ridley AJ: Rho GTPases in
cancer cell biology. FEBS Lett. 582:2093–2101. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Iwai S, Yonekawa A, Harada C, Hamada M,
Katagiri W, Nakazawa M and Yura Y: Involvement of the Wnt-β-catenin
pathway in invasion and migration of oral squamous carcinoma cells.
Int J Oncol. 37:1095–1103. 2010.
|
|
28.
|
Kremenevskaja N, von Wasielewski R, Rao
AS, Schöfl C, Andersson T and Brabant G: Wnt-5a has tumor
suppressor activity in thyroid carcinoma. Oncogene. 24:2144–2154.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Dejmek J, Dejmek A, Säfholm A, Sjölander A
and Andersson T: Wnt-5a protein expression in primary dukes B colon
cancers identifies a subgroup of patients with good prognosis.
Cancer Res. 65:9142–9146. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Weeraratna AT, Jiang Y, Hostetter G,
Rosenblatt K, Duray P, Bittner M and Trent JM: Wnt5a signaling
directly affects cell motility and invasion of metastatic melanoma.
Cancer Cell. 1:279–288. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Kurayoshi M, Oue N, Yamamoto H, Kishida M,
Inoue A, Asahara T, Yasui W and Kikuchi A: Expression of Wnt-5a is
correlated with aggressiveness of gastric cancer by stimulating
cell migration and invasion. Cancer Res. 66:10439–10448. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Kanazawa A, Tsukada S, Kamiyama M,
Yanagimoto T, Nakajima M and Maeda S: Wnt5b partially inhibits
canonical Wnt/β-catenin signaling pathway and promotes adipogenesis
in 3T3-L1 preadipocytes. Biochem Biophys Res Commun. 330:505–510.
2005.PubMed/NCBI
|
|
33.
|
van Tienen F, Laeremans H, van der Kallen
C and Smeets H: Wnt5b stimulates adipogenesis by activating PPARγ,
and inhibiting the β-catenin dependent Wnt signaling pathway
together with Wnt5a. Biochem Biophys Res Commun. 387:207–211.
2009.PubMed/NCBI
|
|
34.
|
Deraz EM, Kudo Y, Yoshida M, Obayashi M,
Tsunematsu T, Tani H, Siriwardena SBSM, Kiekhaee MR, Qi G, Iizuka
S, Ogawa I, Campisi G, Muzio LL, Abiko Y, Kikuchi A and Takata T:
MMP-10/stromelysin-2 promotes invasion of head and neck cancer.
PLoS One. 6:1–14. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Weaver AM: Invadopodia: specialized cell
structures for cancer invasion. Clin Exp Metastasis. 23:97–105.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Friedl P and Wolf K: Plasticity of cell
migration: a multiscale tuning model. J Cell Biol. 188:11–19. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Sahai E: Mechanisms of cancer cell
invasion. Curr Opin Genet Dev. 15:87–96. 2005. View Article : Google Scholar
|
|
38.
|
Rørth P: Collective cell migration. Annu
Rev Cell Dev Biol. 25:407–429. 2009.
|
|
39.
|
Gaggioli C, Hooper S, Hidalgo-Carcedo C,
Grosse R, Marshall JF, Harrington K and Sahai E: Fibroblast-led
collective invasion of carcinoma cells with differing roles for
RhoGTPases in leading and following cells. Nat Cell Biol.
9:1392–1400. 2007. View Article : Google Scholar : PubMed/NCBI
|