Introduction
Metastasis is a multistep process based on a series
of events called the metastatic cascade. Neoplastic cells break
away from the primary tumor, invade the neighboring tissue and
intravasate into existing or newly formed lymph or blood vessels,
circulate within the bloodstream and adhere to vascular walls
somewhere else in the body. At these distant sites, the adherent
cells extravasate through the endothelium of specific target
organs, invade the surrounding tissue and proliferate to form a
secondary or metastatic tumor. This chain of events requires the
cells to have migration ability.
Tumor cell migration is a complex process that
includes the degradation of the extracellular matrix (ECM) by the
activity of tumor cell-secreted matrix metalloproteinases (MMPs) as
well as a coordinated formation and release of focal adhesion
contacts to the extracellular matrix mediated by receptor molecules
such as integrin dimers (1). Focal
adhesion contacts are small, stable, yet transient interactions
between the cell and the ECM (2,3). In
addition to a number of cytosolic proteins attached to the actin
filament network such as the proteins of the ezrin/moesin/radixin
family (4), the ubiquitously
expressed Na+/H+ exchanger isoform 1, NHE1,
is also part of these focal adhesion contacts where it may interact
with integrins either directly or indirectly via the translocated
protons (5–8).
The activity of NHE1 is important for various tumor
cell behavior (9,10). The insufficient tumor
vascularization characteristic of solid tumors and cancer-related
anaemia lead to an inadequate O2 supply so that the
metabolism of the tumor cells is mostly glycolytic. But also in the
presence of oxygen tumor cells can perform glycolysis [Warburg
effect (11)]. Hence, tumor cells
are often confronted with an increased acid load (12). The protons initially accumulating
in the cytosol are extruded into the extracellular space by NHE1
which then causes an extracellular acidification of the surrounding
tissue.
Activation of NHE1 is required for cell polarity and
migration in fibroblasts (13),
epithelial MDCK cells (14) and
human melanoma cells (15). It
enhances the invasiveness of human breast carcinoma cells (16,17)
and affects migration, morphology and adhesion of human melanoma
cells (15). These observations
imply that NHE1 is involved in metastasis and tumor malignancy.
One phenomenon in metastasis is the so-called
organotropism (18), i.e. the
preference of cancer cells to metastasize to characteristic target
organs. Thus, in addition to regional metastasis along the draining
lymph nodes close to the primary tumor, distant melanoma metastases
are primarily found in the lungs including the area between the
lungs, in liver, skin and brain (19). The more than a century-old ‘seed
and soil’ theory postulates that while the cancer cells (‘the
seed’) can disseminate into various organs through circulation,
they can thrive only in permissive tissues (‘the soil’) that match
the intrinsic properties of the tumor cell (20). However, the reasons for this
organo- or tissue tropism appear to be diverse and for the most
part the underlying mechanisms are hardly understood. The direct,
physical tumor-stroma interaction strongly affects the development
of metastases in different tissues and organs. Composition,
structure and stiffness of the extracellular matrix can modulate
cell motility by exerting haptic stimuli (21). An ECM that is optimally resistant
to cell-generated tractional forces is capable of promoting maximal
migration speeds. At the same time invasion requires an ECM that is
digestible for the matrix metalloproteinase set that the cells are
equipped with. Both, adhesion to the ECM as well as its digestion
require an optimum pH at the cell surface (22). In addition to the overall proton
concentration present in the stroma, NHE1 activity represents a
tunable proton source (15,23,24).
Taking advantage of an in vivo approach we
show that inhibiting NHE1 activity does, indeed, reduce invasion of
B16V melanoma cells into liver tissue by 50% while directing
metastases to the lungs. To explain this striking observation and
to learn more about the compatibility of a tumor cell with its
surrounding ECM the present study compares the NHE1-dependent
adhesion, migration, invasion and ECM digestion of the murine B16V
melanoma cell line on two different matrices whose composition
resemble either that of the basement membrane or that of the ECM of
the dermis.
Materials and methods
Cells and cell culture
Cells of the mouse melanoma cell line B16V (25) were grown in bicarbonate buffered
RPMI-1640 (Sigma, Taufkirchen, Germany) supplemented with 10% (v/v)
FBS at 37°C in a humidified atmosphere of 5% CO2/95%
air.
Experimental solutions
In vitro cell migration, adhesion and
invasion were observed in serum free RPMI-1640 medium. Desired pH
values were adjusted by adding appropriate amounts of
NaHCO3.
pH measurements were performed using HEPES-buffered
Ringer solutions containing (mmol l−1): 122.5 NaCl, 5.4
KCl, 0.8 MgCl2, 1.2 CaCl2, 1.0
NaH2PO4*2H2O, 5.0 glucose, 10
HEPES. A pH of 7.0 was adjusted by adding 1 M NaOH.
Where applicable the NHE1 inhibitor cariporide
(HOE642, final concentration: 10 μmol l−1) was
added to either the serum free media or to the HEPES-buffered
Ringer solution.
Detection of NHE1 by western blot
analysis
We followed a protocol by Fafournoux et al
(26). Confluent cells were washed
twice with an ice-cold, hypotonic solution containing (mmol
l−1): 3 KCl, 5 EDTA, 10 Tris-HCl (pH 7.4). Cells were
lysed at 4°C for 10 min in lysis buffer consisting of the hypotonic
solution containing 1.0 mmol l−1 Pefabloc SC Plus (Roche
Molecular Biochemicals, Mannheim, Germany) and 0.2% (v/v) of a
protease inhibitor cocktail (Sigma, P8340). Lysates were scraped
off 10-cm culture dishes (BD Falcon, Franklin Lakes, NJ, USA),
homogenized and spun down at 20,800 × g. The pellets were
resuspended in the hypotonic solution and mixed with reducing
sample buffer (4:1, v/v) containing 500 mmol l−1 Tris,
100 mmol l−1 dithiothreitol, 8.5% SDS, 27.5% sucrose and
0.03% bromophenol blue indicator. SDS-PAGE was performed using
acrylamide gels (7.5%) and a Minigel System (Bio-Rad Laboratories,
Hercules, CA, USA). Equal amounts of protein were loaded.
Electroblotting was performed at 0.8 mA cm−2 for 50 min.
The nitrocellulose membranes (Schleicher & Schuell, Dassel,
Germany) carrying the blotted proteins were bathed in 5% (w/v) milk
in 0.1% (v/v) Tween in PBS for 1 h at room temperature and then
washed with 0.1% Tween in PBS. Overnight incubation with the
primary antibody to the NHE1 (BD Biosciences Pharmingen, 1:500) at
4°C was followed by a 1-h incubation with a horseradish
peroxidase-conjugated goat anti-mouse IgG (1:10,000) at room
temperature. Blots were developed using an ECL immunoblotting
detection reagent kit (Amersham, Arlington Heights, IL, USA).
Measuring intracellular pH
(pHi)
pHi was measured using video imaging
techniques and the fluorescent pH indicator BCECF (Molecular
Probes, Eugene, OR, USA) as previously described (15). Cells were treated as for migration
experiments, resuspended in HEPES-buffered Ringer solution (pH
7.0), plated onto collagen coated coverslips and allowed to adapt
for 3 h. Cells were then incubated with BCECF-AM (final
concentration: 2 μmol l−1) for 5 min. The
coverslips were placed on the stage of an inverted microscope
(Axiovert 200; Carl Zeiss Inc., Göttingen, Germany) and
continuously superfused with prewarmed (37°C) HEPES-buffered Ringer
solutions (for composition see Experimental solutions in Materials
and methods). The excitation wavelengths alternated between 440 and
488 nm, respectively, while the emitted fluorescence intensities
were monitored at 520 nm using a Photometrics camera (CoolSnapfx,
Visitron Systems, Puchheim, Germany). The different wavelengths
were generated by a high speed polychromator system (Visichrome,
Visitron Systems). Polychromator and data acquisition were
controlled by Metafluor software (Visitron Systems). Fluorescence
intensities were measured in 35-sec intervals and corrected for
background fluorescence. At the end of each experiment, the pH
measurements were calibrated by superfusing the B16 cells
successively with modified Ringer solutions of pH 7.5, 7.0 and 6.5
containing (mmol l−1): 125 KCl, 1 MgCl2, 1
CaCl2, 20 HEPES and 10 μmol l−1
nigericin (Sigma-Aldrich).
Invasion into rat liver parenchyma
Male Sprague-Dawley rats (200 to 250 g, Charles
River) were cared for in accordance with standards of the German
Council on Animal Care, under an approved protocol of the local
Animal Welfare Committee. Rats were anesthetized using inhalation
of isoflurane (Curamed, Karlsruhe, Germany) and N2O and
prepared as previously described (27,28).
Vital signs and oxygenation status were kept stable throughout the
experiments. For intravital observation of adhesive interactions
between circulating tumor cells and the hepatic microcirculation,
single-cell suspensions of 1×106 CalceinAM
fluorescent-labeled B16V cells with or without 600 μmol
l−1 cariporide were injected intra-arterially over 60
sec. Mobilized left liver lobes of the laparotomized rats were
placed on a specific holder in order to investigate the lower lobe
surface while avoiding disturbances of hepatic blood flow.
Throughout the experiments the liver was continuously irrigated
with isotonic saline solution. A ×20 objective was located above a
cover slip covering the organ surfaces. A semi-quantitative
analysis of tumor cell adhesions and extravasation was performed
over a 30 min observation period. Using a standardized procedure,
all fields were analyzed in each of the 5-min intervals. Cells were
divided into i) merely adherent and ii) extravasating cells and
their average numbers in 30 microscopic fields were counted.
Fluorescence images were recorded employing a video-enhancer-zoom
lens system, a low-light charge-coupled device video camera
allowing real-time imaging via a separate monitor and a timer
containing S-VHS video system for further analysis.
Reconstituting extracellular
matrices
Two different matrices were prepared, i) a basement
membrane-like matrix and ii) a dermis-like matrix. In detail, the
basement membrane-like matrix consisted of HEPES-buffered, serum
free RPMI-1640 containing (mg ml−1): 0.32 collagen type
IV (BD Biosciences, Heidelberg, Germany), 0.0025 laminin
(Sigma-Aldrich) and 0.025 fibronectin (Roche). The dermis-like
matrix, also mixed in serum-free RPMI-1640, contained (mg
ml−1): 2.53 collagen type I (Biochrom AG, Berlin,
Germany), 0.083 collagen type IV and 0.01875 laminin. The pH of
both collagen mixtures was adjusted to 7.4 by adding 1 M NaOH.
For migration experiments the bottoms of 25-ml
culture flasks (12.5 cm2, BD Falcon) were covered with
200 μl of the collagen mixture each, and the matrices were
allowed to polymerize overnight at 37°C in a humidified
atmosphere.
For adhesion assays each well of a 24-multiwell
plate (BD Falcon) was coated with 80 μl of the collagen
mixture, and for invasion assays filter membranes with a pore size
of 8.0 μm (ThinCertTM, Greiner Bio-One) were
coated with 60 μl of the collagen mixture. The matrices were
allowed to polymerize overnight at 37°C in a humidified
atmosphere.
Scanning the matrix surfaces
Atomic force microscopy was employed to scan the
surfaces of the two matrices. To this end the matrices polymerized
on glass coverslips overnight at 37°C in a humidified atmosphere.
They were then air-dried. The coverslips coated with the air-dried
matrices were mounted on little metal plates using double-faced
adhesive tape. In order to scan the surfaces of the matrices we
used a Multimode AFM (NanoScope V controller, Veeco, Santa Barbara,
CA, USA) equipped with a stereo microscope. Gold coated, V-shaped
silicon nitride cantilevers (MSCT, Veeco) with a spring constant of
0.03 N m−1 and pyramidal tips with an estimated tip
radius of 10 nm were used. The images were recorded with 512 scan
lines per area, at constant force (height mode) in contact mode in
air with a scan rate of 3–5 Hz. Images were processed using the
Nanoscope 7.0 software (Veeco). High-resolution imaging of the
matrices demanded fine-tuning of the scanning process. This
comprised mainly the minimizing of the scanning force between tip
and sample to values below 3 nN.
Cell adhesion and invasion
For adhesion, B16V cells grown to confluency were
resuspended in serum free culture medium with or without 10
μmol l−1 cariporide and then seeded on
matrix-coated plates (10×104 cells per well) inserted in
a 24-multiwell plate (BD Falcon). After 3 h the media including the
non-adhesive cells were washed off with cold PBS buffer, the
remaining cells were fixed with 3.5% paraformaldehyde in PBS and
counted.
For invasion, 90,000 cells were seeded on
matrix-coated filter-membranes with pore sizes of 8.0 μm
(ThinCert, Greiner Bio-One) at the three pH values, pH 6.8, 7.0 and
7.5. After a 48-h incubation cells were fixed and stained with
crystal violet. The matrix and the remaining cells on the upper
side of the filter were removed carefully and excess crystal violet
was washed away with distilled water. The invasive cells that
remained on the lower side of the filter were counted. To exclude
that possible differences in the cell numbers are based on
pH-dependent proliferation rates a proliferation assay was
performed. Cells (90,000) were seeded in small cell culture dishes
(area 1.9 cm2). After a 48-h incubation including
changing the media after 24 h, the cells were trypsinized and the
cell density was determined. From the numbers obtained for the cell
density the factor by which the number of cells had increased due
to proliferation was calculated. The number of cells found on the
lower side of the matrix-coated filter membrane was divided by this
‘proliferation factor’ in order to receive the correct number of
invasive cells.
Analyzing cell migration
The matrix-coated culture flasks were put into
heated chambers on stages of inverted microscopes (Axiovert25, Carl
Zeiss Inc.). Cell migration was recorded in 10-min intervals for 5
h at 37°C using video cameras (Models XC-ST70CE and XC-77CE,
Hamamatsu/Sony, Japan) and PC-vision frame grabber boards
(Hamamatsu, Herrsching, Germany). Acquisition of images was
controlled by HiPic and WASABI software (Hamamatsu). The cell
contours were labeled applying AMIRA software (TGS, San Diego, CA,
USA) and served as the basis for further analysis. Parameters such
as structural index (SI), migratory velocity (μm
min−1) and translocation (μm) were analyzed using
self-made JAVA programs and the NIH ImageJ software (http://rsb.info.nih.gov/ij/). Migration was determined
as the movement of the cell center per time unit, the velocity was
estimated from the 10-min time intervals applying a three point
difference quotient and the cell area was measured as the number of
pixels. The structural index (SI) represents the cell shape and was
calculated as follows: SI = (4 π A)/p2, where A is the
area covered by the cell and p is the perimeter of A. Values close
to 1 correspond to a spherical cell shape whereas values close to 0
correspond to a spindle or a dendritic cell shape.
Detecting extracellular matrix digestion
using in situ zymography
The quenched fluorophore Bodipy (Invitrogen,
Darmstadt, Germany) was used for analysis of ECM proteolysis in
vitro. In the intact molecule, the fluorescence is quenched by
about 95% so that fluorescence occurs only when the protein is
proteolyzed.
The in situ zymography was performed as
described previously by Busco et al (29). The two matrices were prepared as
described above. Prior to the polymerization step,
BSA-Bodipy-fluorescein conjugate (Invitrogen) was added cautiously
to the matrix components (final concentration 30 μg
ml−1) and mixed by gentle shaking. A total of 200
μl of the mix was distributed uniformly on a coverslip (Ø 30
mm) and allowed to polymerize overnight at 37°C in a humidified
atmosphere. Per coverslip approximately 30,000 cells were seeded
and allowed to adhere and spread on the matrix at 37°C in a
humidified atmosphere of 5% CO2/95% air. Three or six
hours after seeding cells were fixed with paraformaldehyde (3.5% in
PBS) and focal digestion was visualized by fluorescence microscopy.
To this end, the coverslips were placed on the stage of an inverted
microscope (Axiovert 200, Carl Zeiss Inc.). The excitation
wavelength of 488 nm was set by a high speed polychromator
(Visichrome, Visitron Systems, Puchheim, Germany). Emission
intensity was monitored at 510 nm using a Photometrics camera
(CoolSnapfx). Polychromator and data acquisition were controlled by
Metafluor software (Visitron Systems). The overall fluorescence
intensity underneath the entire cell was corrected for background
fluorescence by subtracting background intensities.
Metastasis in mice
C57BL/6 mice (Harlan, Horst, The Netherlands) were
cared for in accordance with standards of the German Council on
Animal Care, under an approved protocol of the local Animal Welfare
Committee. A total of 80 μl of a B16V cell suspension
containing 12.8×106 cells ml−1 in PBS were
intradermally transplanted into the right flanks of 10-week-old
female C57BL/6 mice. Mice fed standard or a cariporide containing
(0.6% v/v) diet (Altromin, Lage, Germany), starting immediately
after transplantation. After 3 weeks, the primary tumor was removed
and metastasis was monitored over a period of 7 weeks employing a
small-animal PET followed by autopsies of the eventually sacrificed
animals. 2-Deoxy-2-[18F]fluoro-D-glucose
(18F-FDG)-PET was used to evaluate the glucose
utilization of tumors. Uptake of radioactivity was visualized using
the high resolution quadHIDAC small animal PET scanner [Oxford
Positron Systems; spatial resolution 0.7 mm FWHM (30)]. Mice were anesthetized with
isoflurane inhalation (2% in oxygen) and temperature was maintained
by using a heating pad during the experimental procedure.
Radiotracer (10 MBq) was injected intravenously and image
acquisition at 60–75 min p.i. was performed.
Statistics
All experiments were repeated three to thirteen
times. Data are presented as the mean values ± SD or SEM as
indicated. The data were tested for significance employing
Student’s unpaired or paired t-test or analysis of variance (ANOVA)
where applicable. The level of significance was set at p<0.05
(*p<0.05; **p<0.01;
***p<0.001).
Results
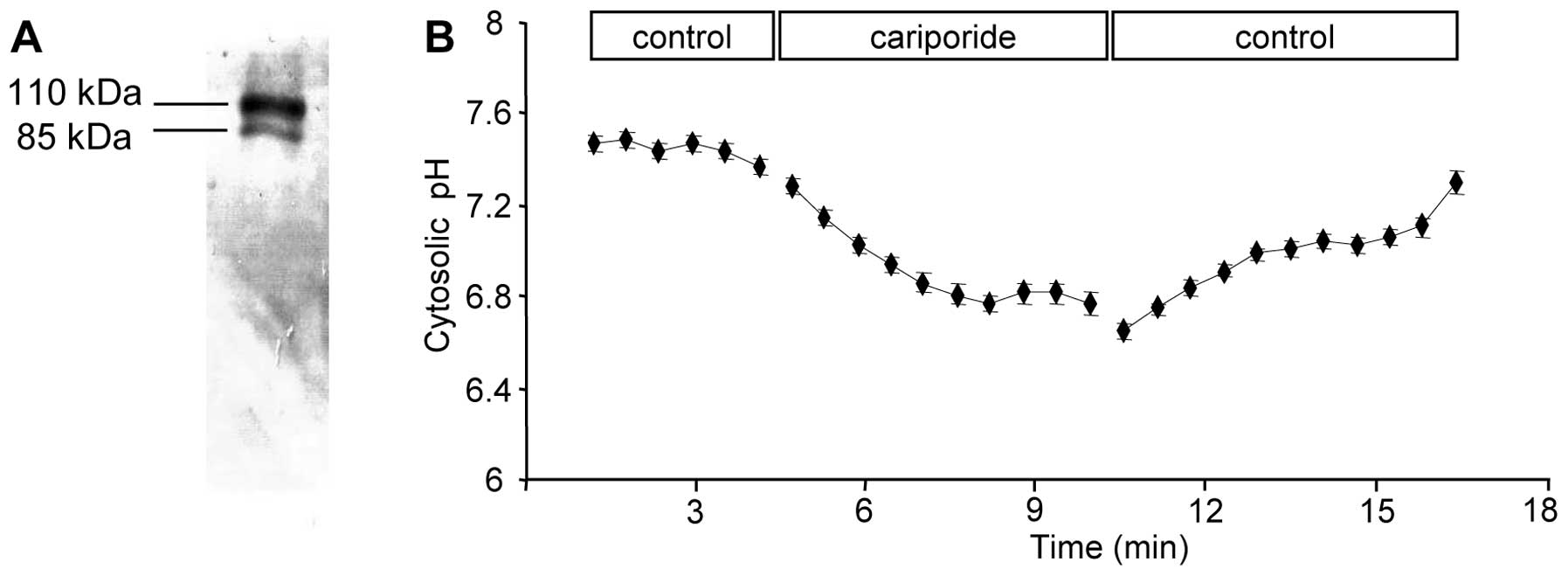
NHE1 expression and activity in B16V
cells
The present study intended to transfer the knowledge
on the function of NHE1 in single human melanoma (MV3) cell
migration to a murine melanoma cell line and to translate it into
an in vivo system. We chose B16V cells as they fulfil two
requirements: they express NHE1 as shown by western blot analysis
(Fig. 1A) and inhibiting their
NHE1 activity with the specific inhibitor cariporide induces the
expected reversible intracellular acidification (Fig. 1B).
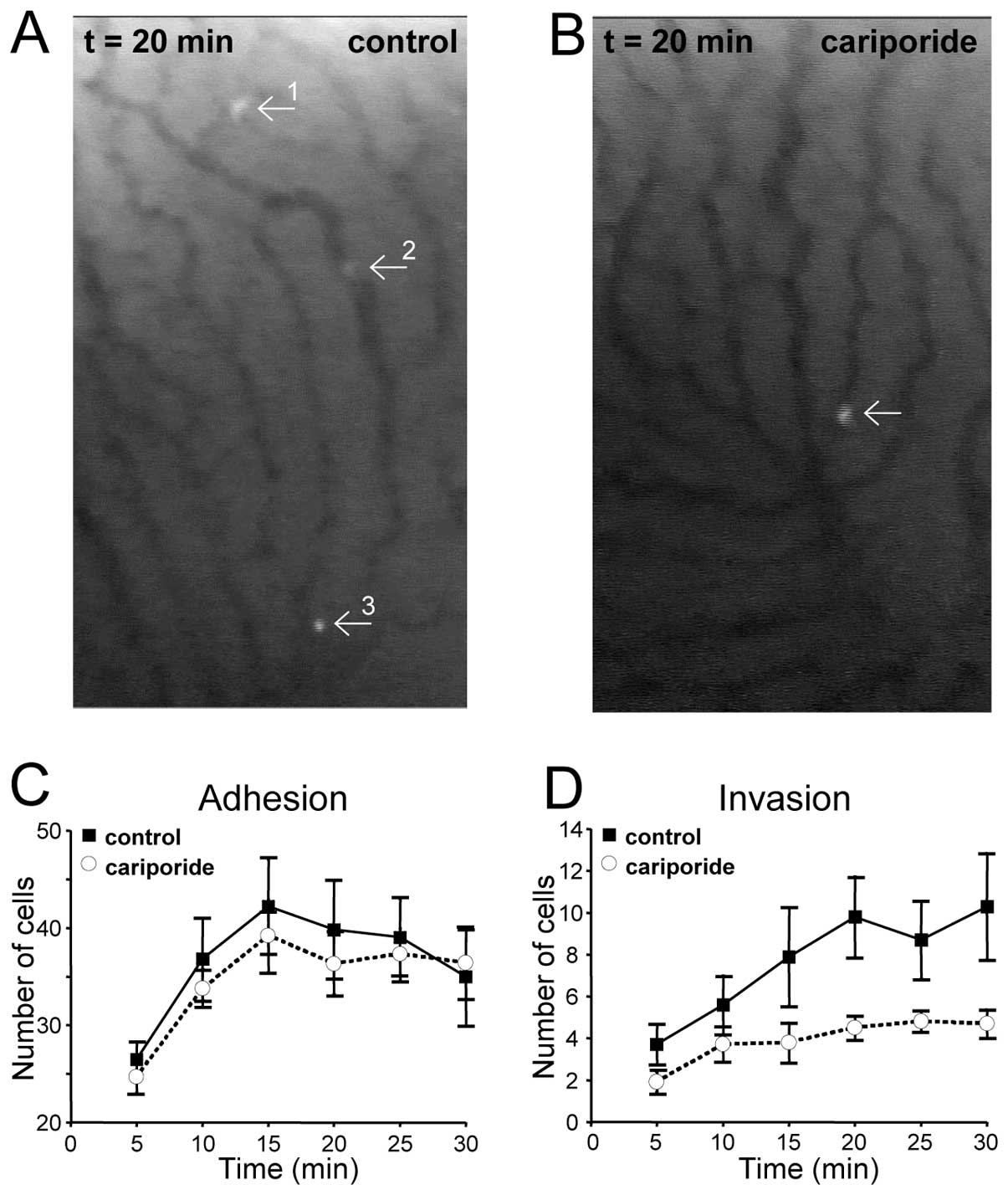
B16V in vivo invasion requires NHE1
activity
As the main aim of the present study was to prove
that inhibition of NHE1 affects B16V cell adhesion and invasion not
only in vitro but also in vivo, fluorescently labeled
B16V cells and cariporide or its solvent alone were injected into
the carotid arteries of rats. As previously demonstrated this route
of injection ensured highest reproducibility and avoided a relevant
artificial first pass effect within the lungs. The number of cells
adhering to the vessel walls of liver sinusoids and that of cells
extravasating into the surrounding liver parenchyma were counted.
During the first 5 min after injection of the cell suspension about
25 cells (total number, obtained from 30 visual fields per rat) had
already adhered to the vessel wall (Fig. 2A and C). After 15 min cell adhesion
had reached its maximum at about 40 adherent cells. There was no
significant difference in the cell adhesiveness in the presence or
absence of the NHE1-inhibitor cariporide (39±3 vs. 42±5,
respectively). In contrast, invasion starting within the initial 5
min after injection was clearly dependent on NHE1 activity
(Fig. 2B and D). In the presence
of cariporide the number of cells found to be migrating inside the
liver parenchyma was reduced by 50%. This held true for the entire
time span beginning at t=5 min until the end of the observation
period at t=30 min.
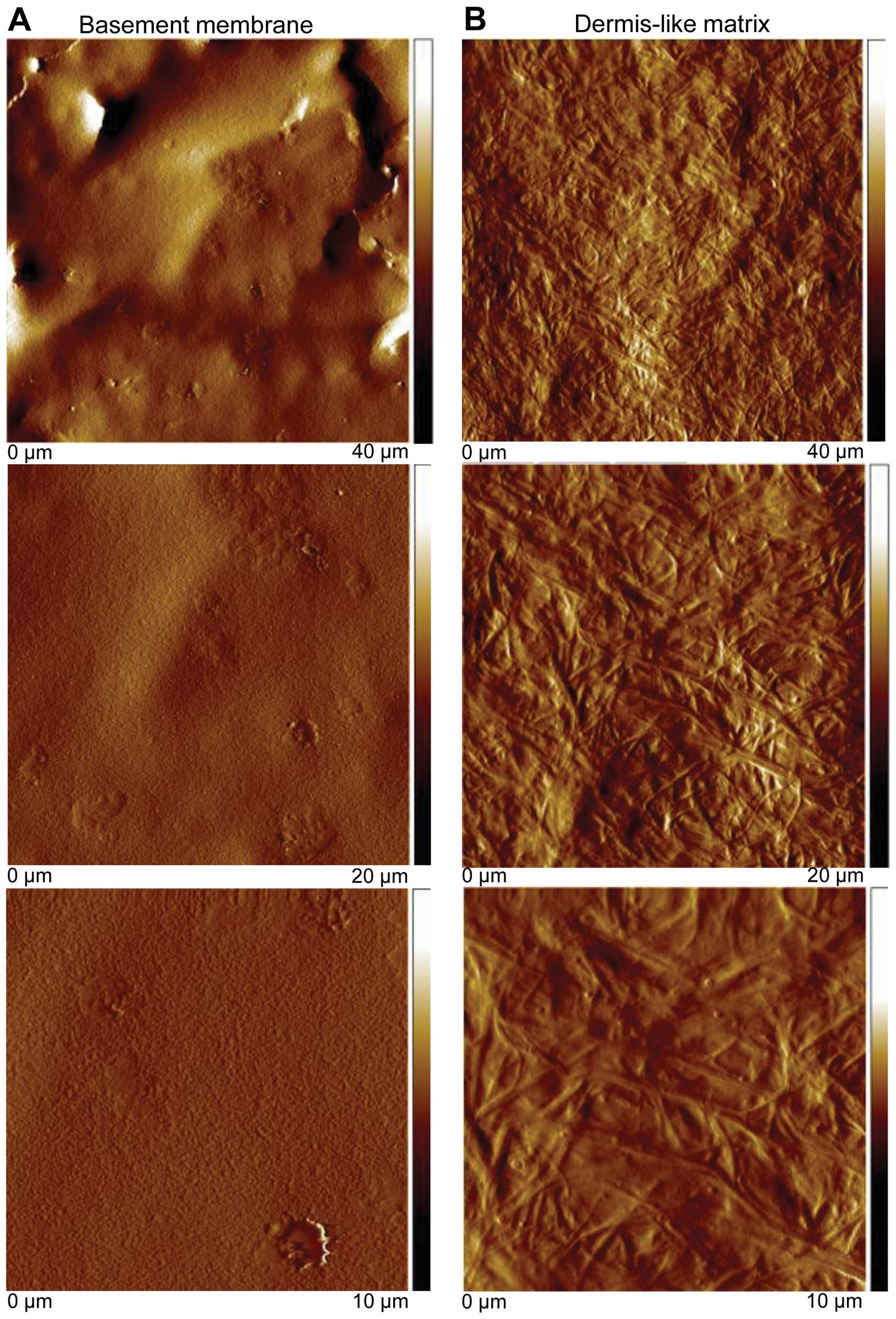
Surface structure of reconstituted
matrices
Since human melanoma cell adhesion to a
reconstituted collagen type I matrix depends on NHE1 activity
(31) we theorized that the lack
of the cariporide effect on B16V adhesion to the sinusoidal wall
could be caused by the composition of the local, perisinusoidal
matrix, known to be rich in fibronectin (32). To identify how the ECM composition
affects the behavior of B16V cells, two different ECMs, a basement
membrane-like and a dermis-like matrix, were prepared. Their relief
was scanned utilizing the AFM technique. The basement membrane-like
matrix formed a dense gel with hardly visible, very small pores
compared to the dimension of a cell (Fig. 3A) whereas the dermis-like matrix
formed a fibrillar meshwork with large pores (Fig. 3B). This observation is perfectly
consistent with electron micrographs published by Poincloux et
al (33) who compared the
matrix architecture of Matrigel commonly used to imitate the
basement membrane with that of acid-solubilized collagen I often
used as a model for the interstitial matrix.
The basement membrane-like matrix
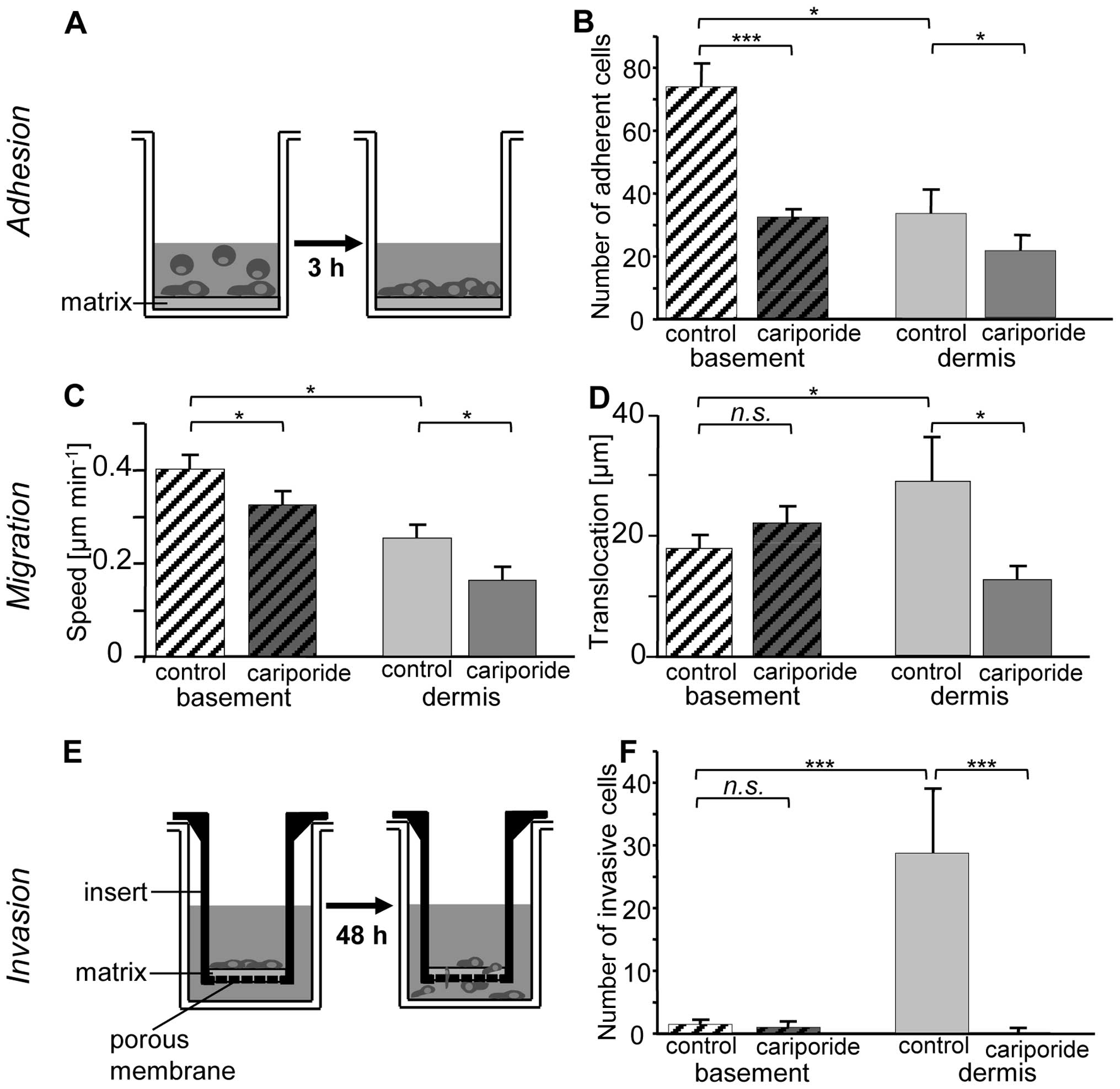
facilitates adhesion and migration
Fig. 4 shows that
B16V cells were significantly more adhesive (p<0.05) on the
basement membrane-like as compared to the dermis-like matrix
(Fig. 4A and B). On both matrices,
particularly on the basement membrane-like matrix, inhibition of
NHE1 activity by cariporide led to a decrease in adhesiveness
[95.8±8.7 vs. 29.1±2.3 cells per visual field on the basement
membrane-like matrix (p<0.001), 34.0±7.6 vs. 21.5±5.2 cells per
visual field on the dermis-like matrix (p<0.05)]. We chose two
parameters to describe cell motility: i) the migratory speed
(Fig. 4C); and ii) the
translocation that represents the distance covered during the
course of a 5-h experiment (Fig.
4D). B16V cells travelled almost twice as fast when seeded on
the basement membrane-like matrix (0.4±0.03 vs. 0.25±0.03 μm
min−1, p<0.05). On both matrices the migratory speed
was clearly reduced by the specific NHE1 inhibitor cariporide
(basement: −20%; 0.32±0.03 μm min−1, p=0.04;
dermis: −38%; 0.16±0.02 μm min−1, p=0.04). In
contrast, the translocation remained nearly unaffected on the
basement membrane but was significantly lowered on the dermis-like
matrix (from 27.3±7.0 to 11.4±2.2 μm, p<0.05).
The dermis-like matrix facilitates
invasion
Next, we investigated whether the matrix-dependent
differences in the migratory behavior of B16V cells are reflected
also in their invasive behavior (Fig.
4E and F). The cells preferred to invade the dermis-like
matrix. Invasion into the dermis-like matrix was drastically
reduced upon NHE1 inhibition by cariporide. Normally, invasion is
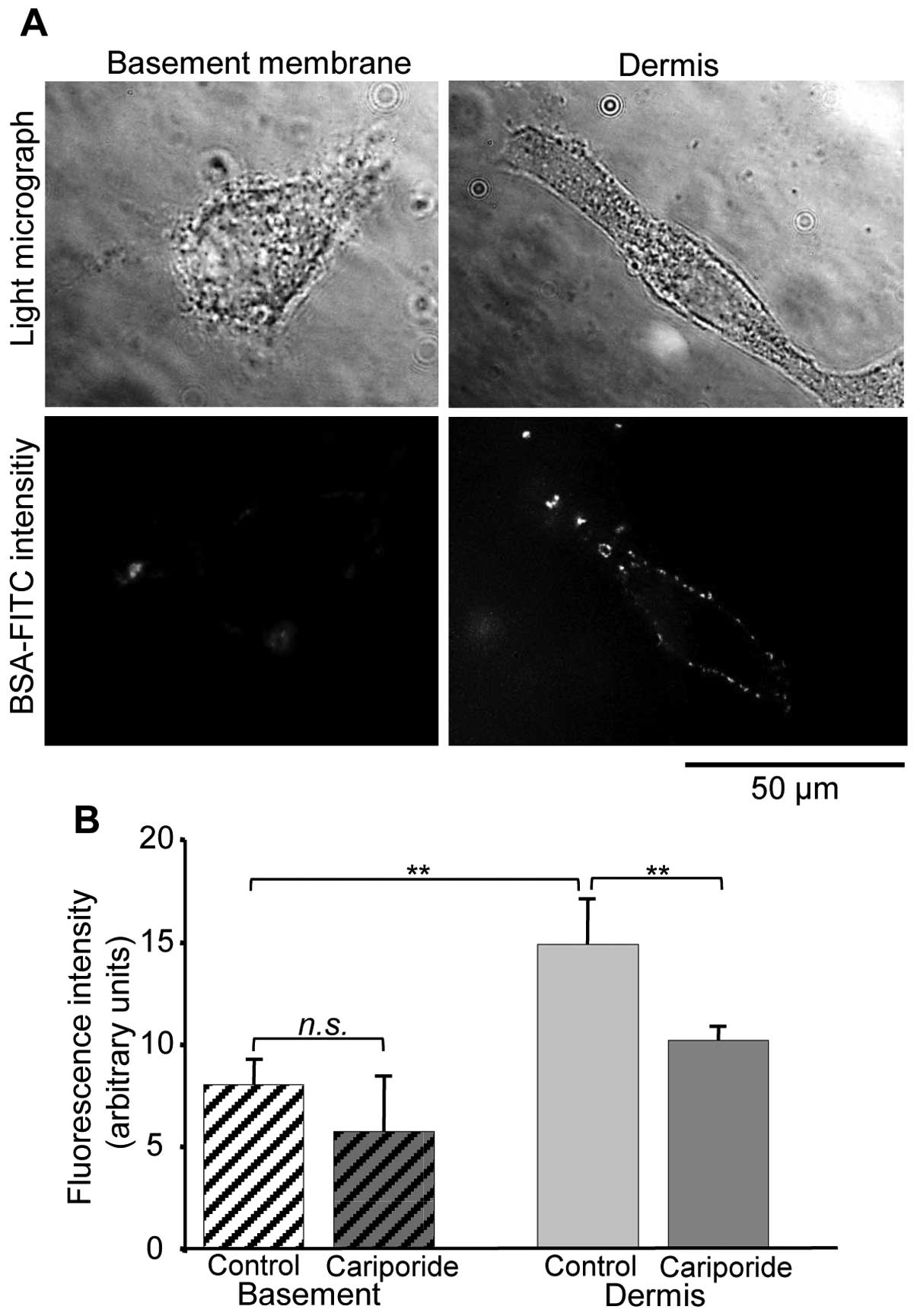
accompanied by digestion of the extracellular matrix (34). According to this and to our
observation that B16V cells prefer to invade the dermis-like
matrix, the cells digested the dermis-like matrix much more
efficiently than the basement membrane-like matrix (Fig. 5A and B). Also the digestion process
is significantly facilitated by the activity of NHE1.
B16V cell adhesion, morphology, migration
and invasion depend on extracellular pHe
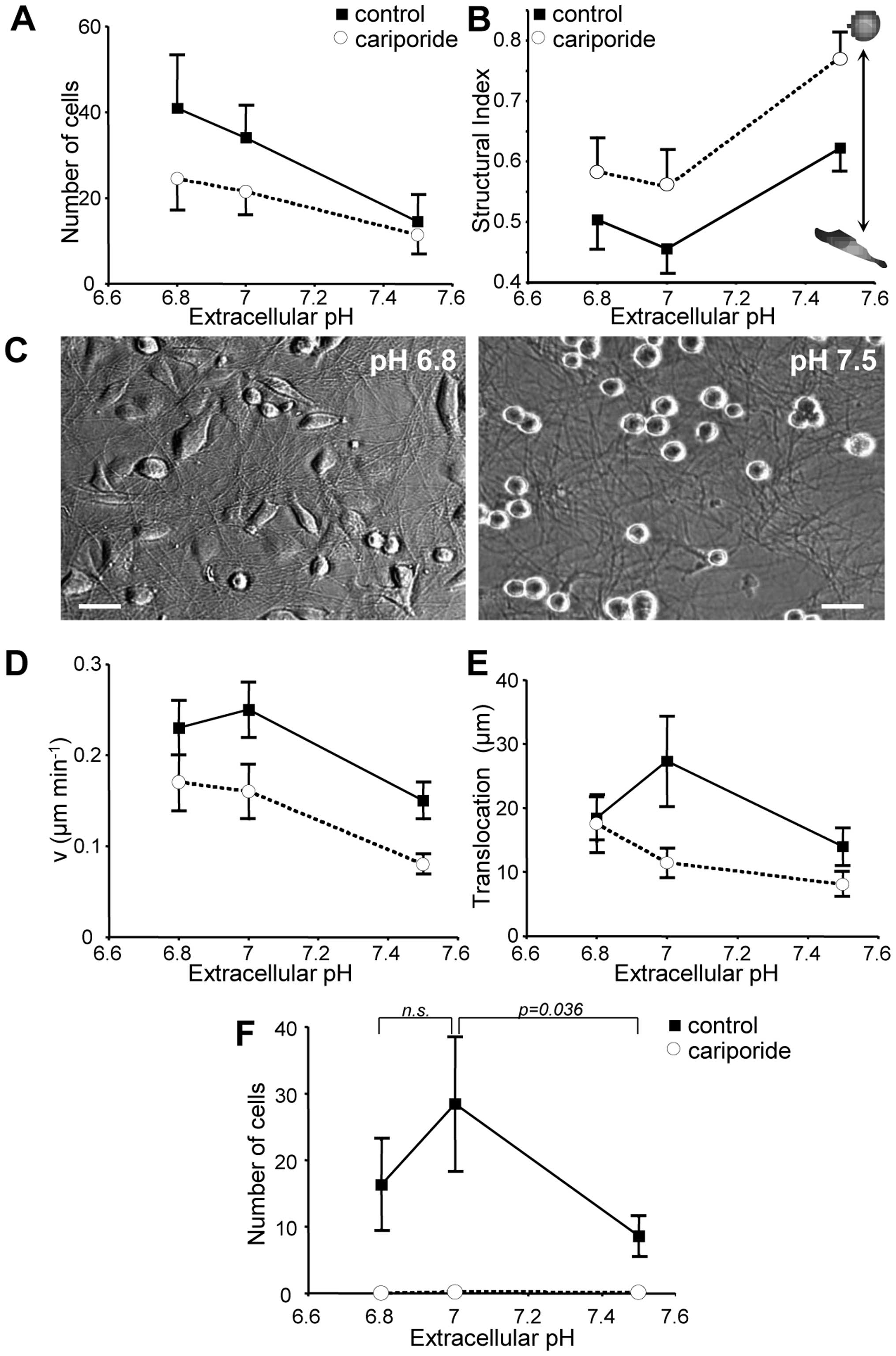
At extracellular pH (pHe) values of 6.8
and 7.0, NHE1 inhibition by cariporide led to a significant
(p<10−3) decrease in in vitro cell adhesion
(Fig. 6A). This cariporide effect
was most distinct at acidic pHe values (pH 6.8) and not
present at pHe 7.5. Adhesion also depended on
pHeper se irrespective of NHE1 activity. It
decreased as pHe increased no matter whether NHE1 was
fully active (pHe 6.8: 40.9±12.2; pHe 7.0:
34.0±7.6; pHe 7.5: 14.5±6.4 cells per well) or inhibited
(pHe 6.8: 24.5±7.3; pHe 7.0: 21.5±5.2;
pHe 7.5: 11.4±4.5 cells per well). At the same time the
morphology altered from spindle-shaped to spherical (Fig. 6B and C). Accordingly, the
structural index (Fig. 6B)
increased by approximately 0.15 units in control and in
cariporide-treated cells when pHe was shifted from pH
7.0 to 7.5. However, in general, cariporide-treated cells were
significantly more spherical than the cells under control
conditions.
Migration of B16V cells depends also on
pHe (Fig. 6D and
E)
Under standard conditions at pHe 7.0
maximum migratory speed was 0.25±0.03 μm min−1
(Fig. 6D) and the translocation
amounted to 27.3±7.0 μm (Fig.
6E). From there, speed and translocation decreased when
pHe was increased to pH 7.5 (speed: 0.15±0.02 μm
min−1, translocation: 10.9±2.0 μm) or decreased
to pH 6.8 (speed: 0.23±0.03 μm min−1,
translocation: 18.4±3.6 μm). NHE1 inhibition led to a
significant reduction in migratory speed at all of the different
pHe values. A lower pHe seems to be able to
compensate for the lack of NHE1 activity: in cariporide treated
cells the migratory activity, i.e. speed and distance, increased
continuously as pHe decreased from pH 7.5 (speed:
0.08±0.01 min−1, translocation: 9.7±1.6 μm) over
pH 7.0 (speed: 0.16±0.02 μm min−1, translocation:
11.4±2.2 μm) to pH 6.8 (speed: 0.17±0.03 μm
min−1, translocation: 14.0±3.2 μm).
Invasion was completely absent at all tested
pHe values when NHE1 was inhibited by cariporide
(Fig. 6F). In contrast, under
control conditions invasion reached its maximum at pHe
7.0 (28.5±10.0 cells per filter). It dropped significantly when
pHe was increased to pH 7.5 (8.6±2.8 cells per filter)
and was also diminished when pHe was decreased to pH 6.8
(16.3±6.7 cells per filter).
NHE1-inhibition affects melanoma
metastasis
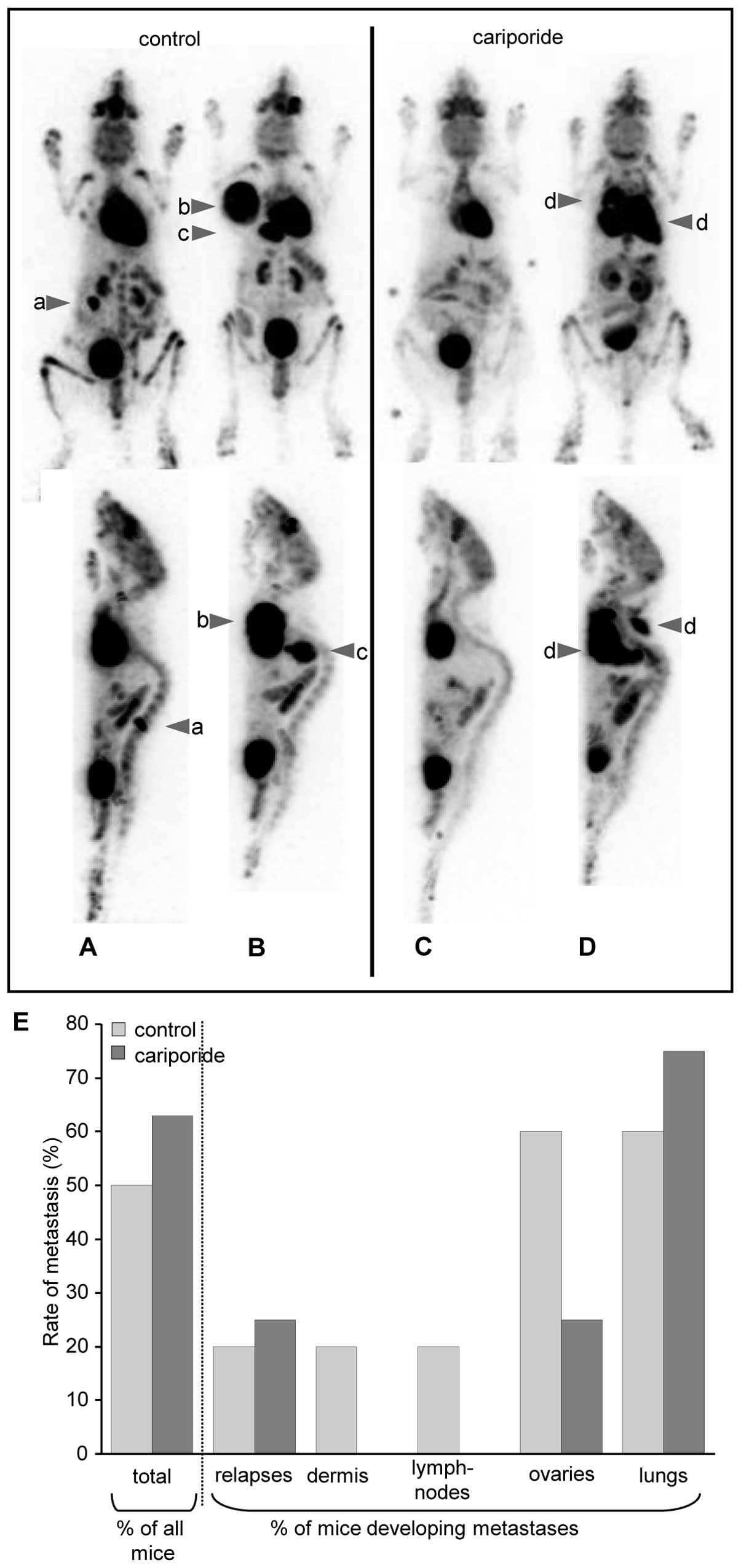
In addition to the intravital imaging of B16V cell
invasion into rat liver parenchyma (Fig. 2), we launched another in
vivo approach investigating NHE1-dependent metastasis in female
C57BL/6 mice. Three weeks after intradermal injection of the B16V
cell suspension into the right flank, the primary tumor was removed
and metastasis was monitored over a period of 7 weeks employing a
small-animal PET and followed by autopsies of the finally
sacrificed animals (Fig. 7). The
percentage of animals developing metastases or local relapses
hardly differed between the two groups. In 4 out of the 8 (50%)
control animals and in 5 out of the 8 (62.5%) cariporide-treated
animals metastases and/or local relapses occurred after removal of
the primary tumor. However, it is particularly noticeable that
counting all visible metastases per animal revealed that in
cariporide-treated animals metastases occurred mainly in the lungs
(in 3 out of the 4 control vs. 5 out of the 5 cariporide-treated
animals), but neither in the dermis nor in lymph nodes. Metastases
in the ovaries were rather rare compared to untreated animals.
Thus, in cariporide-treated cells metastases seem to get directed
to the lungs while control animals show metastases spread all over
the body.
Discussion
Key results of the present study are that NHE1
inhibition in B16V cells i) significantly reduces invasion of rat
liver parenchyma; ii) decreases adhesion, migration and invasion
depending on the composition of the employed reconstituted
matrices; and iii) directs the metastatic spread to the lungs.
NHE1 inhibition efficiently reduces
invasion of liver parenchyma
B16V cell adhesion to the walls of rat liver
sinusoids was observed to remain unaffected by cariporide whereas
invasion of the liver parenchyma was decreased by ∼50% (Fig. 2). Collagen types I, III, IV and V
are the most frequently found in the ECM of the rat liver (35). While collagen types I, III and V
are confined mainly to the portal tract and the central vein wall,
type IV collagen, laminin and fibronectin are components of a
low-density, basement membrane-like material lining the sinusoidal
walls (36,37). The low density of this basement
membrane-like structure could help the tumor cells to adhere to the
sinusoidal wall and to find access to the parenchyma. The ECM of
the liver parenchyma also contains heparan sulfate-proteoglycans
(38,39). The heparan sulfate-poteoglycans are
digested by the B16 cells’ MMPs, thereby allowing efficient B16
invasion of the rat liver parenchyma (40). While NHE1 inhibition by cariporide
hardly affects adhesion to the sinusoidal wall, it significantly
reduces invasion suggesting that under these conditions the protons
extruded by NHE1 function as a digestive catalyzing MMP action
rather than as a glue that strengthens focal adhesions (22). This interpretation finds additional
support by our in vitro studies showing that the
physiological pH close to ∼7.4 in the sinusoidal blood possibly
renders cariporide ineffective in disturbing adhesion (Fig. 6A) whereas invasion is significantly
reduced even at pH 7.4 (Fig.
6F).
The ECM composition arranges motility and
invasion
In order to analyze B16V migration and invasion more
precisely, two types of reconstituted matrices resembling either
the ECM of the dermis or the basement membrane were generated
(Fig. 3). B16V cell behavior was
totally different on these two matrices. Cells were more adhesive
and migrated more quickly on the basement membrane while strongly
invading the dermis-like matrix. These findings support the
observations by Luikart et al (41) who found that a B16 clone derived
from B16F1 remained more spherical when seeded on collagen type I
whereas it tended to spread on collagen type IV. In the present
study, laminin and fibronectin were key constituents of the
basement membrane-like matrix. Both laminin and fibronectin can be
bound by heparin or heparan sulfate present at the cell surface as
a component of the glycocalix (42–44).
Heparan sulfate proteoglycans also represent specific components of
native basement membranes and the degradation of heparan sulfate
chains is required for B16 cell invasion (40). Our reconstituted basement
membrane-like matrix used here did not contain any heparan sulfate
proteoglycans which could account for the lack of invasion
(Fig. 4F).
B16V cell adhesion was twice as strong on the
basement membrane- as on the dermis-like matrix (Fig. 4B). This strong adhesion to the
basement membrane can be correlated with a rather low translocation
(Fig. 4D) as the cells keep moving
vividly, although on the spot (Fig.
4C). α4β1, α6β1 and
ανβ3 integrins preferentially bind to
collagen IV, laminin and fibronectin, respectively (45,46),
and in murine melanoma cells, ανβ3
integrin-binding to fibronectin mediates adhesion and motility
(47,48). Thus, the components of the
reconstituted basement membrane force the cells to use the
appropriate integrin dimers in order to interact specifically with
the respective constituent. The present reconstituted basement
membrane induced adhesion and spreading but did not facilitate
translocation and invasion (Fig.
4) suggesting that either the ανβ3
integrin-binding to fibronectin was too strong or that in addition
other cell-matrix interactions are required for directional
migration or invasion.
The ECM composition determines the
efficiency of NHE1 inhibition
Both migration and invasion of human melanoma (MV3)
cells require well-balanced adhesion strength and depend on NHE1
activity and pHe(31,49).
B16V cell adhesion and migration on the dermis-like matrix also
depend on pHe and NHE1 activity (Fig. 6A–E). The higher the extracellular
proton concentration is the stronger is the adhesion strength, and
adhesion is clearly reduced when NHE1 is inhibited by cariporide.
The cariporide-induced decrease in motility can be compensated for
by an increase in the extracellular H+ concentration,
i.e. a decrease in pHe, especially visible at
pHe 6.8 (Fig. 6D and
E).
In MV3 cells NHE1 modulates the cell surface pH
(8,15), even locally right next to integrins
at focal adhesions (24). These
pH-environments are stabilized by the glycocalix (23). Particularly at focal adhesions at
the cell front, the pericellular H+ concentration is
high. An acidic extracellular pH activates
ανβ3 integrins by opening the head pieces of
the dimers (50). Thus, the
protons extruded by NHE1, predominantly at focal adhesions, could
stabilize the ανβ3 integrin-mediated B16V
cell adhesion on the basement membrane-like matrix. Indeed, NHE1
inhibition by cariporide reduces adhesion by more than 50%
(Fig. 4B).
The high levels of basal NHE1 activity in tumor
cells (10,51) and the resulting acidification of
both the cell surface and the extracellular space also elevate the
proteolytic potential leading to an increase of tumor cell
invasiveness (22). A low
pHe of the tumor microenvironment optimizes the activity
of matrix metalloproteinases (MMPs) (52) and cathepsins D (53,54),
B and L (55) as well as the
urokinase-type plasminogen activator (56). In this context, even
ανβ3 integrin expression might facilitate B16
cell invasion of collagen type I-containing matrices. Collagen type
I, denatured by heat or after proteolysis at physiological
temperatures can be ligated by ανβ3 integrins
(57). Thus, initial, unspecific
digestion of dermal collagen type I during the course of tissue
remodeling or infiltration makes cryptic RGD sites accessible
(58) for
ανβ3 integrins. The stimulated
ανβ3 integrins activate MMP2 present on the
cell surface which in turn furthers collagen type I digestion
(59). The proteolytic activity of
MMPs was indeed significantly higher in cells seeded on a
dermis-like matrix (Fig. 5)
strongly suggesting that the molecular composition and/or the
structure of the ECM trigger its own digestion. A considerable role
of NHE1 activity in this setting is supported by the present
findings that on the dermis-like matrix the proteolytic activity is
significantly reduced and the invasion nearly completely blocked
upon NHE1 inhibition. However, during invasion as opposed to
migration, a missing NHE1 activity cannot be compensated for by a
decrease in pHe (Fig.
6F) confirming that the protons locally extruded by NHE1
dominate those present in the bulk solution (15).
NHE1 inhibition is ineffective in the
lungs
In mice, NHE1 inhibition does not inhibit the
overall metastasis rate but seems to direct metastasis
preferentially to the lungs (Fig.
7B), or put differently, cariporide does not inhibit metastasis
to the lungs. Two factors could contribute to this phenomenon: i)
the composition of the lung ECM may support homing; and ii) the
local, alveolar pCO2 and hence the local pHe
might circumvent the blockade of NHE1.
The ECM of the lung is a network of non-uniformly
distributed elastic fibers that loop around alveolar ducts and form
rings at the mouths of alveoli (60). It consists of elastin, a highly
proteinase-resistant protein, interstitial collagen types I and III
that provide tensile strength, and collagen type IV as part of the
basement membrane separating the alveolar endothelium and
epithelium. Also in the lungs proteoglycans, in particular the
heparan-sulfate proteoglycan whose degradation is required for B16
cell invasion (40), are highly
expressed in both the basement membrane and the interstitium
(60,61). Furthermore, numerous studies
indicate that fibronectin, the preferential ligand of
ανβ3 integrins, is present in the basement
membrane of alveolar epithelia (reviewed in ref. 62). Spontaneous metastasis of breast
cancer to lung and bone is enhanced by ανβ3
integrins, partially due to their strong binding to fibronectin and
vitronectin (63–65). In addition, binding of
ανβ3 integrins to ECM proteins prevents
apoptosis in a foreign environment (66).
In a healthy human being pHe values come
to about pH 7.1 in liver, less than pH 6.9 in active muscle, and
only pH 6.7 in the pulmonary extracellular space (67). The in vitro experiments of
the present study show that regarding cell motility (Fig. 6D and E) the proton concentration at
a pHe of 6.8 appears to compensate for NHE1 inhibition.
Consequently, the rather acidic pH in the extravascular space of
the lung could neutralize the effects of NHE1 inhibition by
cariporide which then would lead to the observed formation of lung
metastases in the cariporide treated mice.
Although NHE1 inhibition leads to a decrease in
adhesion, invasion and metastasis, its function as a sole potential
target in cancer therapy remains still questionable. The present
results show that the effectiveness of NHE1 inhibition in reducing
melanoma metastasis depends on both the composition of the ECM,
which varies tremendously between different organs and tissues, and
the local pHe, which is modulated by NHE1 activity,
vascularization, pCO2 and other acid/base regulators.
The locally very different interstitial pHe values
depend not only on the local metabolism and
vascularization/perfusion but also on the buffer capacity
determined by the local, tissue-specific glycosaminoglycan
composition. Due to the interweavement of these numerous parameters
it will be very difficult to analyze and predict the efficacy of
NHE1 inhibition locally and individually. Nevertheless, the use of
NHE1 inhibitors as an adjuvant therapy strategy or as one
constituent of a broader drug cocktail appears to be a promising
approach to further advance anticancer therapies.
Acknowledgements
The authors thank Dr Steve J. Reshkin
(University of Bari) and Dr Uta Schnöckel (UKM) for providing their
expertise on in situ zymography and the PET technique,
respectively. Christine Bätza and Anne Kanzog performed the PET
procedure. Sincere thanks also to Dr Jürgen Pünter at
Sanofi-Aventis for kindly providing cariporide. This study was
supported by the Interdisciplinary Center of Clinical Research
(IZKF core unit PIX), University of Münster, Germany.
References
|
1.
|
Lock JG, Wehrle-Haller B and Stromblad S:
Cell-matrix adhesion complexes: master control machinery of cell
migration. Semin Cancer Biol. 18:65–76. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Broussard JA, Webb DJ and Kaverina I:
Asymmetric focal adhesion disassembly in motile cells. Curr Opin
Cell Biol. 20:85–90. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Arnaout MA, Goodman SL and Xiong JP:
Structure and mechanics of integrin-based cell adhesion. Curr Opin
Cell Biol. 19:495–507. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Niggli V and Rossy J:
Ezrin/radixin/moesin: versatile controllers of signaling molecules
and of the cortical cytoskeleton. Int J Biochem Cell Biol.
40:344–349. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Grinstein S, Woodside M, Waddell TK, et
al: Focal localization of the NHE-1 isoform of the
Na+/H+ antiport: assessment of effects on
intracellular pH. EMBO J. 12:5209–5218. 1993.PubMed/NCBI
|
|
6.
|
Plopper GE, McNamee HP, Dike LE,
Bojanowski K and Ingber DE: Convergence of integrin and growth
factor receptor signaling pathways within the focal adhesion
complex. Mol Biol Cell. 6:1349–1365. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Stock C and Schwab A: Role of the Na/H
exchanger NHE1 in cell migration. Acta Physiol (Oxf). 187:149–157.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Stock C, Mueller M, Kraehling H, et al: pH
nanoenvironment at the surface of single melanoma cells. Cell
Physiol Biochem. 20:679–686. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Harguindey S, Orive G, Luis Pedraz J,
Paradiso A and Reshkin SJ: The role of pH dynamics and the
Na+/H+ antiporter in the etiopathogenesis and
treatment of cancer. Two faces of the same coin - one single
nature. Biochim Biophys Acta. 1756:1–24. 2005.PubMed/NCBI
|
|
10.
|
Stock C, Ludwig FT and Schwab A: Is the
multifunctional Na+/H+ exchanger isoform 1 a
potential therapeutic target in cancer? Curr Med Chem. 19:647–660.
2012.PubMed/NCBI
|
|
11.
|
Warburg O: On the origin of cancer cells.
Science. 123:309–314. 1956. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Helmlinger G, Sckell A, Dellian M, Forbes
NS and Jain RK: Acid production in glycolysis-impaired tumors
provides new insights into tumor metabolism. Clin Cancer Res.
8:1284–1291. 2002.PubMed/NCBI
|
|
13.
|
Meima ME, Mackley JR and Barber DL: Beyond
ion translocation: structural functions of the sodium-hydrogen
exchanger isoform-1. Curr Opin Nephrol Hypertens. 16:365–372. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Klein M, Seeger P, Schuricht B, Alper SL
and Schwab A: Polarization of Na+/H+ and
Cl−/HCO3− exchangers in migrating
renal epithelial cells. J Gen Physiol. 115:599–608. 2000.
|
|
15.
|
Stuwe L, Muller M, Fabian A, et al: pH
dependence of melanoma cell migration: protons extruded by NHE1
dominate protons of the bulk solution. J Physiol. 585:351–360.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Cardone RA, Bellizzi A, Busco G, et al:
The NHERF1 PDZ2 domain regulates PKA-RhoA-p38-mediated NHE1
activation and invasion in breast tumor cells. Mol Biol Cell.
18:1768–1780. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Bourguignon LY, Singleton PA, Diedrich F,
Stern R and Gilad E: CD44 interaction with
Na+-H+ exchanger (NHE1) creates acidic
microenvironments leading to hyaluronidase-2 and cathepsin B
activation and breast tumor cell invasion. J Biol Chem.
279:26991–27007. 2004.PubMed/NCBI
|
|
18.
|
Hu G, Kang Y and Wang XF: From breast to
the brain: unraveling the puzzle of metastasis organotropism. J Mol
Cell Biol. 1:3–5. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Meyers ML and Balch CM: Diagnosis and
treatment of metastatic melanoma. Cutaneous Melanoma. Balch CM,
Houghton AN, Sober AJ and Soong S-J: Quality Medical Publishing,
Inc; St Louis, MO: 1998
|
|
20.
|
Paget S: The distribution of secondary
growths in cancer of the breast. 1889. Cancer Metastasis Rev.
8:98–101. 1989.PubMed/NCBI
|
|
21.
|
Peyton SR and Putnam AJ: Extracellular
matrix rigidity governs smooth muscle cell motility in a biphasic
fashion. J Cell Physiol. 204:198–209. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Stock C, Cardone RA, Busco G, Krahling H,
Schwab A and Reshkin SJ: Protons extruded by NHE1: digestive or
glue? Eur J Cell Biol. 87:591–599. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Krahling H, Mally S, Eble JA, Noel J,
Schwab A and Stock C: The glycocalyx maintains a cell surface pH
nanoenvironment crucial for integrin-mediated migration of human
melanoma cells. Pflugers Arch. 458:1069–1083. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Ludwig FT, Schwab A and Stock C: The
Na+/H+ - exchanger (NHE1) generates pH
nanodomains at focal adhesions. J Cell Physiol. 228:1351–1358.
2013.PubMed/NCBI
|
|
25.
|
Supino R, Prosperi E, Formelli F, Mariani
M and Parmiani G: Characterization of a doxorubicin-resistant
murine melanoma line: studies on cross-resistance and its
circumvention. Br J Cancer. 54:33–42. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Fafournoux P, Noel J and Pouyssegur J:
Evidence that Na+/H+ exchanger isoforms NHE1
and NHE3 exist as stable dimers in membranes with a high degree of
specificity for homodimers. J Biol Chem. 269:2589–2596.
1994.PubMed/NCBI
|
|
27.
|
Haier J, Korb T, Hotz B, Spiegel HU and
Senninger N: An intravital model to monitor steps of metastatic
tumor cell adhesion within the hepatic microcirculation. J
Gastrointest Surg. 7:507–515. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Korb T, Schluter K, Enns A, et al:
Integrity of actin fibers and microtubules influences metastatic
tumor cell adhesion. Exp Cell Res. 299:236–247. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Busco G, Cardone RA, Greco MR, et al: NHE1
promotes invadopodial ECM proteolysis through acidification of the
peri-invadopodial space. FASEB J. 24:3903–3915
|
|
30.
|
Schafers KP, Reader AJ, Kriens M, Knoess
C, Schober O and Schafers M: Performance evaluation of the
32-module quadHIDAC small-animal PET scanner. J Nucl Med.
46:996–1004. 2005.PubMed/NCBI
|
|
31.
|
Stock C, Gassner B, Hauck CR, et al:
Migration of human melanoma cells depends on extracellular pH and
Na+/H+ exchange. J Physiol. 567:225–238.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Couvelard A, Scoazec JY, Dauge MC,
Bringuier AF, Potet F and Feldmann G: Structural and functional
differentiation of sinusoidal endothelial cells during liver
organogenesis in humans. Blood. 87:4568–4580. 1996.PubMed/NCBI
|
|
33.
|
Poincloux R, Lizarraga F and Chavrier P:
Matrix invasion by tumour cells: a focus on MT1-MMP trafficking to
invadopodia. J Cell Sci. 122:3015–3024. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Roy R, Yang J and Moses MA: Matrix
metalloproteinases as novel biomarkers and potential therapeutic
targets in human cancer. J Clin Oncol. 27:5287–5297. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Bedossa P and Paradis V: Liver
extracellular matrix in health and disease. J Pathol. 200:504–515.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Martinez-Hernandez A: The hepatic
extracellular matrix. I Electron immunohistochemical studies in
normal rat liver. Lab Invest. 51:57–74. 1984.PubMed/NCBI
|
|
37.
|
Rosenow F, Ossig R, Thormeyer D, et al:
Integrins as antimetastatic targets of RGD-independent snake venom
components in liver metastasis [corrected]. Neoplasia. 10:168–176.
2008.PubMed/NCBI
|
|
38.
|
Schuppan D: Structure of the extracellular
matrix in normal and fibrotic liver: collagens and glycoproteins.
Semin Liver Dis. 10:1–10. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
39.
|
Gressner AM: Hepatic proteoglycans - a
brief survey of their pathobiochemical implications.
Hepatogastroenterology. 30:225–229. 1983.PubMed/NCBI
|
|
40.
|
Kramer RH and Vogel KG: Selective
degradation of basement membrane macromolecules by metastatic
melanoma cells. J Natl Cancer Inst. 72:889–899. 1984.PubMed/NCBI
|
|
41.
|
Luikart SD, Maniglia CA and Sartorelli AC:
Influence of collagen substrata on glycosaminoglycan production by
B16 melanoma cells. Proc Natl Acad Sci USA. 80:3738–3742. 1983.
View Article : Google Scholar : PubMed/NCBI
|
|
42.
|
Culp LA, Rollins BJ, Buniel J and Hitri S:
Two functionally distinct pools of glycosaminoglycan in the
substrate adhesion site of murine cells. J Cell Biol. 79:788–801.
1978. View Article : Google Scholar : PubMed/NCBI
|
|
43.
|
Yamada KM, Kennedy DW, Kimata K and Pratt
RM: Characterization of fibronectin interactions with
glycosaminoglycans and identification of active proteolytic
fragments. J Biol Chem. 255:6055–6063. 1980.PubMed/NCBI
|
|
44.
|
Sakashita S, Engvall E and Ruoslahti E:
Basement membrane glycoprotein laminin binds to heparin. FEBS Lett.
116:243–246. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
45.
|
Ramos DM, Berston ED and Kramer RH:
Analysis of integrin receptors for laminin and type IV collagen on
metastatic B16 melanoma cells. Cancer Res. 50:728–734.
1990.PubMed/NCBI
|
|
46.
|
Tietze L, Borntraeger J, Klosterhalfen B,
et al: Expression and function of beta(1) and beta(3) integrins of
human mesothelial cells in vitro. Exp Mol Pathol. 66:131–139. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
47.
|
Lonsdorf AS, Kramer BF, Fahrleitner M, et
al: Engagement of αIIbβ3 (GPIIb/IIIa) with ανβ3 integrin mediates
interaction of melanoma cells with platelets: a connection to
hematogenous metastasis. J Biol Chem. 287:2168–2178. 2012.
|
|
48.
|
Li X, Regezi J, Ross FP, et al: Integrin
alphavbeta3 mediates K1735 murine melanoma cell motility in vivo
and in vitro. J Cell Sci. 114:2665–2672. 2001.PubMed/NCBI
|
|
49.
|
Friedl P, Maaser K, Klein CE, Niggemann B,
Krohne G and Zanker KS: Migration of highly aggressive MV3 melanoma
cells in 3-dimensional collagen lattices results in local matrix
reorganization and shedding of alpha2 and beta1 integrins and CD44.
Cancer Res. 57:2061–2070. 1997.PubMed/NCBI
|
|
50.
|
Paradise RK, Lauffenburger DA and Van
Vliet KJ: Acidic extracellular pH promotes activation of integrin
alpha(v) beta(3). PLoS One. 6:e157462011. View Article : Google Scholar : PubMed/NCBI
|
|
51.
|
Cardone RA, Casavola V and Reshkin SJ: The
role of disturbed pH dynamics and the Na+/H+
exchanger in metastasis. Nat Rev Cancer. 5:786–795. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
52.
|
Koblinski JE, Ahram M and Sloane BF:
Unraveling the role of proteases in cancer. Clin Chim Acta.
291:113–135. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
53.
|
Tedone T, Correale M, Barbarossa G,
Casavola V, Paradiso A and Reshkin SJ: Release of the aspartyl
protease cathepsin D is associated with and facilitates human
breast cancer cell invasion. FASEB J. 11:785–792. 1997.PubMed/NCBI
|
|
54.
|
Szpaderska AM and Frankfater A: An
intracellular form of cathepsin B contributes to invasiveness in
cancer. Cancer Res. 61:3493–3500. 2001.PubMed/NCBI
|
|
55.
|
Mohamed MM and Sloane BF: Cysteine
cathepsins: multifunctional enzymes in cancer. Nat Rev Cancer.
6:764–775. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
56.
|
Kindzelskii AL, Amhad I, Keller D, et al:
Pericellular proteolysis by leukocytes and tumor cells on
substrates: focal activation and the role of urokinase-type
plasminogen activator. Histochem Cell Biol. 121:299–310. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
57.
|
Pauli BU and Knudson W: Tumor invasion: a
consequence of destructive and compositional matrix alterations.
Hum Pathol. 19:628–639. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
58.
|
Taubenberger AV, Woodruff MA, Bai H,
Muller DJ and Hutmacher DW: The effect of unlocking RGD-motifs in
collagen I on pre-osteoblast adhesion and differentiation.
Biomaterials. 31:2827–2835. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
59.
|
Brooks PC, Stromblad S, Sanders LC, et al:
Localization of matrix metalloproteinase MMP-2 to the surface of
invasive cells by interaction with integrin alpha v beta 3. Cell.
85:683–693. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
60.
|
Shapiro SD: Chairman’s summary.
Proceedings of the American Thoracic Society. 42006.
|
|
61.
|
Thompson SM, Jesudason EC, Turnbull JE and
Fernig DG: Heparan sulfate in lung morphogenesis: The elephant in
the room. Birth Defects Res C Embryo Today. 90:32–44. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
62.
|
Dunsmore SE and Rannels DE: Extracellular
matrix biology in the lung. Am J Physiol. 270:L3–L27.
1996.PubMed/NCBI
|
|
63.
|
Harms JF, Welch DR, Samant RS, et al: A
small molecule antagonist of the alpha(v)beta3 integrin suppresses
MDA-MB-435 skeletal metastasis. Clin Exp Metastasis. 21:119–128.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
64.
|
Sloan EK, Pouliot N, Stanley KL, et al:
Tumor-specific expression of alphavbeta3 integrin promotes
spontaneous metastasis of breast cancer to bone. Breast Cancer Res.
8:R202006. View Article : Google Scholar : PubMed/NCBI
|
|
65.
|
Bentmann A, Kawelke N, Moss D, et al:
Circulating fibronectin affects bone matrix, whereas osteoblast
fibronectin modulates osteoblast function. J Bone Miner Res.
25:706–715. 2010.PubMed/NCBI
|
|
66.
|
Montgomery AM, Reisfeld RA and Cheresh DA:
Integrin alpha v beta 3 rescues melanoma cells from apoptosis in
three-dimensional dermal collagen. Proc Natl Acad Sci USA.
91:8856–8860. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
67.
|
Effros RM and Chinard FP: The in vivo pH
of the extravascular space of the lung. J Clin Invest.
48:1983–1996. 1969. View Article : Google Scholar : PubMed/NCBI
|