Introduction
Pancreatic cancer is a disease with poor prognosis
and still overall 5-year survival rate of <20% (1) without any substantial treatment
improvement during the past 30 years. This is in contrast to many
other tumor diseases where earlier diagnosis and significant
adjuvant treatment usually improved survival (2–4).
Pancreatic carcinomas are virtually resistant to radiotherapy as
well as chemotherapy, and surgery remains the only option for cure
(1,5,6).
Therefore, alternative treatment strategies are highly needed and
immunomodulatory methods among others are in focus. However, to
optimize immunotherapy of pancreatic carcinoma a further
understanding of mechanisms behind tumor-host interactions are
required (7–9).
MICA is a glycosylated, polymorphic and membrane
anchored non-classical MHC class I molecule. It is a stress induced
protein normally expressed in intestinal epithelial cells only, but
found to be broadly expressed in a variety of malignant tumors as
melanoma, breast, colon and hepatocellular cancers (10–12).
It functions as a ligand for the NKG2D receptor, which is an
important immunoreceptor on NK cells, CD 8 and γδ T-cells. The
molecule can be cleaved by matrix metalloproteinases and ADAM
proteinase, and may then be released into the blood stream or
tissue culture medium as a soluble molecule (sMICA) (13–15).
Accordingly, it has been suggested that high levels of sMIC in
serum from patients with certain gastrointestinal malignancies can
cause systemic downregulation of NKG2D surface expression on CD8 αβ
T cells and NK cells, which may impair lysis of tumor cells
(11,16). Therefore, our present aim was to
analyze MICA/B expression in pancreatic tumor tissue and serum
associated to tumor stage.
Materials and methods
Patient material, tumor specimens and
blood samples
The use of specimens from human subjects was
approved by the Institutional Review Board of Lithuanian University
of Medical Sciences (Kaunas, Lithuania) and the regional ethics
board in Gothenburg (Sweden). Pancreatic cancer tissue samples used
in IHC and western blot analyses were derived from the surgical
specimens removed from 22 patients undergoing pancreatoduodenal
resections for pancreatic cancer at Department of Surgery at the
Hospital of Lithuanian University of Medical Sciences (Kaunas,
Lithuania). Six specimens of normal pancreas tissues were collected
from liver and/or kidney organ donors. All tissue samples were
snap-frozen in liquid nitrogen and stored at −80°C until analysis.
Paraffin-embedded tissue blocks from the same patients were
obtained from the Department of Pathology and used to assess tumor
differentiation and stage of the pancreatic adenocarcinomas
according to TNM classification by certified independent
pathologists. Blood samples were collected from 13 individuals with
pancreatic ductal adenocarcinoma undergoing surgery at Sahlgrenska
University Hospital (Gothenburg, Sweden). Pancreatic cancer tissue
as well as non-tumor pancreatic tissue from the same patients were
also collected. Tissues were put in RNAlater solution and stored at
−20°C until analysis. Tissue samples from 4 of the 13 patients were
used in the Western blot analysis. Serum from 10 healthy blood
donors was collected as controls (although such individuals are
younger than the average cancer-patients they are systematically
screened for abnormalities). Serum samples were centrifuged,
aliquoted, and stored at −80°C until analysis of sMICA using a
sandwich ELISA as described below. Patient characteristics are
given in Table I.
 | Table I.Patient characteristics (mean ±
SE). |
Table I.
Patient characteristics (mean ±
SE).
| General
characteristics | Lithuania: Tumor
patients | Sweden: Tumor
patients | Sweden: Blood
donors |
|---|
| Gender | | | |
| Female | 14 | 9 | 2 |
| Male | 8 | 4 | 8 |
| Age (years) | 66±2 | 67±2 | 40±4 |
| S-CRP (mg/l) | 24±6 | 16±8 | <5 |
Immunohistochemical (IHC) analysis
Frozen pancreatic tissue sections (5–7-μm)
were air-dried and fixed in a mixture of ice-cold acetone and 10%
formaldehyde for 5 min at −20°C. Tissue sections were stained with
an anti-MICA/B (H-300) rabbit polyclonal antibodies in 1:200
dilution (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) as
previously described (17). Rabbit
IgG in an equivalent concentration was used as a negative control
employing a similar immunohistochemical staining procedure. Two
investigators graded MICA/B expression in a blinded fashion.
Negative MICA/B expression was graded as no MICA/B signal (−).
MICA/B-positive tumors were defined as having weak (+), moderate
(++) or strong (+++) MICA/B signal.
Western blot analysis
Freshly frozen or RNAlater treated pancreatic tumor
tissue was homogenized with a rotor-stator homogenizer to five
times the tissue weight of RIPA buffer (50 mM Tris-HCl at pH 8.0,
0.1% sodium dodecyl sulfate, 1% Nonidet P-40, 150 mM NaCl and 0.5%
deoxycholic acid) with complete protease inhibitors added (Roche
Diagnostics Gmbh, Germany). Homogenates were then centrifuged at
10,000 g for 10 min. Protein concentrations were determined with
Bradford Coomassie assay (Bio-Rad Laboratories Inc., Hercules, CA,
USA). Next 30 μg total protein of each sample was separated
by electrophoresis in 4–12% Bis-Tris sodium dodecyl
sulfate-polyacrylamide gels (Invitrogen, Carlsbad, CA, USA) at 200
V for 50 min and transferred to 0.2 μM PVDF membranes
(Bio-Rad Laboratories). After blocking in 10% non-fat dry milk,
membranes were incubated overnight at 4°C with a rabbit polyclonal
anti-MICA/B (sc-20931, Santa Cruz Biotechnology) 1:600 dilution in
3% dry milk. Membranes were incubated for 1 h at room temperature
with peroxidase labeled anti-rabbit IgG as secondary antibody (GE
Healthcare). Immunocomplexes were visualized using the Amersham
Hyperfilm ECL Western blotting analysis system (GE Healthcare). The
film was scanned in a GS-710 calibrated densitometer (Bio-Rad
Laboratories) and quantified using Quantity One software. The
optical density was measured and expressed in arbitrary units. Two
lanes on each gel were loaded with Magic Mark XP protein standard
(Invitrogen Inc.). The average density of a standard band was used
to normalize signal intensity between the blots.
Protein digestion and identification by
LC-MS/MS
Proteins were electrophoretically separated as
described for western blots and thereafter Coomassie stained. The
protein gel band was excised, washed and dried prior to digestion
with trypsin (50 mM NH4HCO3, 10 ng/μl
trypsin) at 37°C overnight. Peptides were extracted, dried and
reconstituted in 0.2% HCOOH. The sample was analyzed with nanoflow
LC-MS/MS using a column packed in-house with 3 μm
Reprosil-Pur C18-AQ particles connected to an
LTQ-Orbitrap XL. An acetonitrile gradient in 0.2% HCOOH was used
for separation of the peptides.
The LTQ-Orbitrap was operated in a data-dependent
mode, switching between one MS1 FTMS scan precursor ions scan
followed by CID (collision induced dissociation) fragmentation
(MS/MS) of the six most intense, protonated ions in each FTMS scan.
All the tandem mass spectra were searched by MASCOT (Matrix
Science, London) against the Uniref100 protein database. The search
parameters were set to: human, MS accuracy 5 ppm, MS/MS accuracy
0.5 Da, one missed cleavage by trypsin allowed and variable
modification of propionamide modification of cysteine and oxidized
methionine.
ELISA analysis
MICA concentrations in serum from pancreatic cancer
patients and from healthy blood donors (13 and 10 individuals,
respectively) were determined by using DuoSet ELISA (R&D
systems Abington, UK) according to the manufacturer’s
instructions.
Quantification of serum proteins
Quantification of CRP and serum protein
electrophoresis were performed as routine analyses at the
Laboratory for Clinical Chemistry at Sahlgrenska University
Hospital.
Statistical analysis
Results are reported as the mean ± SEM. Chi-square
tests were performed to analyze the difference in the
clinicopathological parameters of MICA/B positive and
MICA/B-negative pancreatic ductal adenocarcinomas for significant
association. Factorial ANOVA followed by Fisher’s PLSD post
hoc test was used to analyze differences among groups in MIC
A/B western blotting protein expression. Mann-Whitney U test was
used to compare serum MICA levels between pancreatic cancer
patients and controls. A p<0.05 was considered statistically
significant in two-tailed tests. All statistics were conducted by
using SPSS 11.5.
Results
Tissue expression of MICA/B in pancreatic
tumors
Pancreatic cancer specimens from 22 patients with
primary adenocarcinomas (8 male and 14 female) with a mean age of
66±8 years (range 50–79 years) and six samples of normal pancreas
from liver and/or kidney donors (4 male and 2 female) were analyzed
for MICA/B expression by immunohistochemical staining (IHC)
according to the above described protocols. The mean survival time
of the cancer patients after surgery was 479±275 days (range
124–1,210 days).
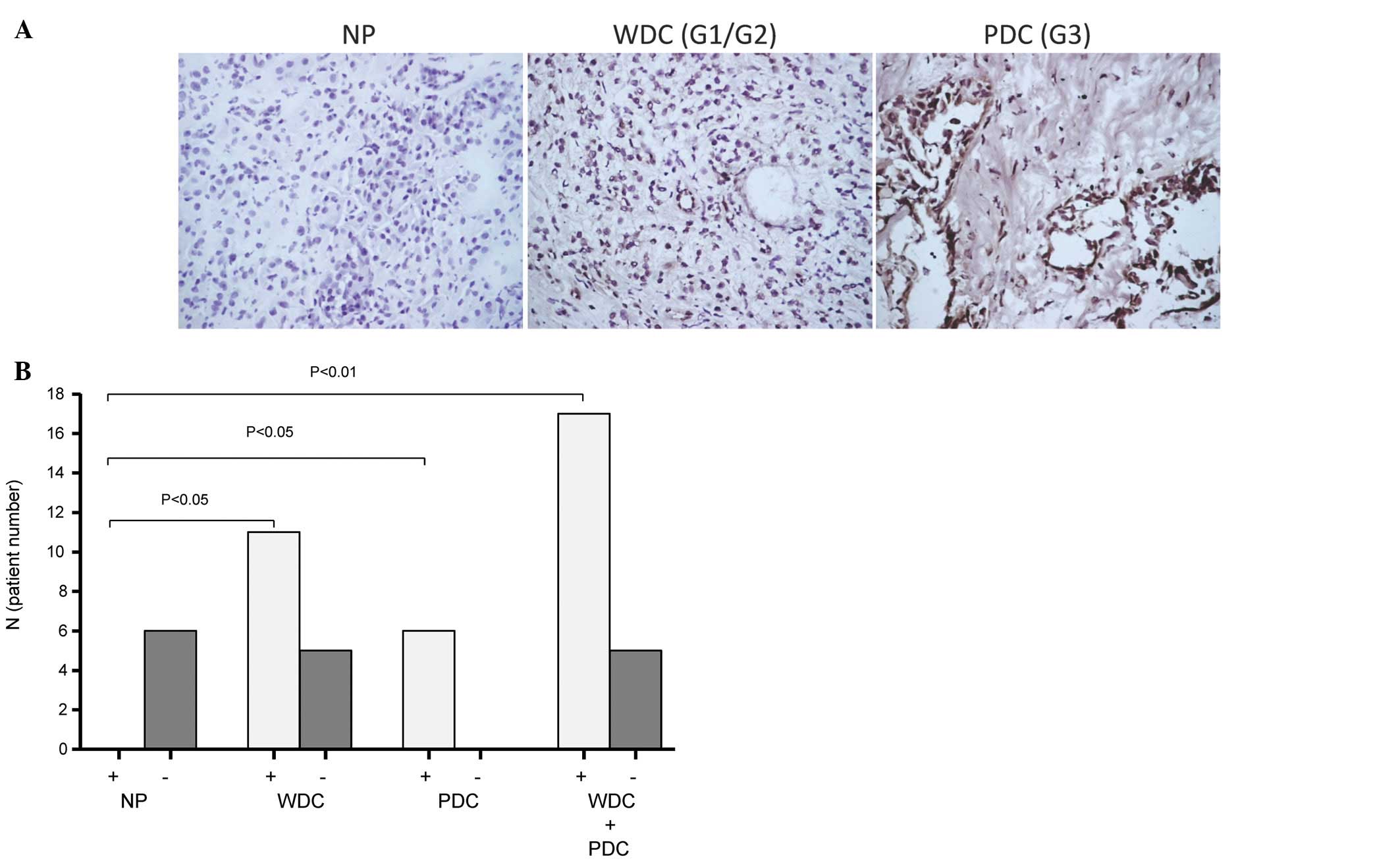
IHC staining with rabbit polyclonal anti-MICA/B
revealed a strong specific dark brown signal of MICA/B expression
present in 17 (77%) of 22 pancreatic adenocarcinomas. Specific
staining was only observed in tumor cells but not in normal
pancreatic tissue cells (Fig. 1A).
The negative control by rabbit IgG did not show any signal.
Negative pancreatic adenocarcinomas were defined according to a
lack of specific staining in tumor cells. More than half (69%) of
the well differentiated tumor specimens (G1–G2) and all (100%) of
the poorly differentiated tumor specimens (G3) showed positive
staining for MICA/B (Fig. 1).
Clinicopathological analyses indicated that
expression of MICA/B was not related to patient gender, age or
post-operative survival, tumor size, tumor differentiation or
perineural invasion. However, statistical analysis confirmed that
MICA/B-negative tumors (23%) were associated with significantly
lower mortality during postoperative follow-up (p=0.021), and lower
incidence of regional lymph node metastases (p=0.003; Table II).
 | Table II.MICA/B expression in pancreatic cancer
and clinico-pathological significance (mean ± SE). |
Table II.
MICA/B expression in pancreatic cancer
and clinico-pathological significance (mean ± SE).
| Clinical
characteristics |
MICA/B+ |
MICA/B− | Total | p-value |
|---|
| Gender | | | | |
| Female | 11 | 3 | 14 | 0.620 |
| Male | 6 | 2 | 8 | |
| Age (years) | 64±2 | 71±3 | 66±2 | 0.120 |
| Tumor size | | | | |
| T3 | 16 | 4 | 20 | 0.441 |
| T4 | 1 | 1 | 2 | |
| Regional lymph
nodes | | | | |
| N0 | 1 | 4 | 5 | 0.003 |
| N1 | 16 | 1 | 17 | |
| Distant
metastases | | | | |
| M0 | 17 | 5 | 22 | NS |
| M1 | 0 | 0 | 0 | |
| Tumor
differentiation | | | | |
| G1–G2 | 10 | 5 | 15 | 0.114 |
| G3 | 7 | 0 | 7 | |
| Perineural
infiltration | | | | |
| Yes | 9 | 4 | 13 | 0.293 |
| No | 8 | 1 | 9 | |
| Death during
follow-up | | | | |
| Yes | 14 | 1 | 15 | 0.021 |
| No | 3 | 4 | 7 | |
| Survival
(days) | 461±65 | 542±146 | 479±59 | 0.704 |
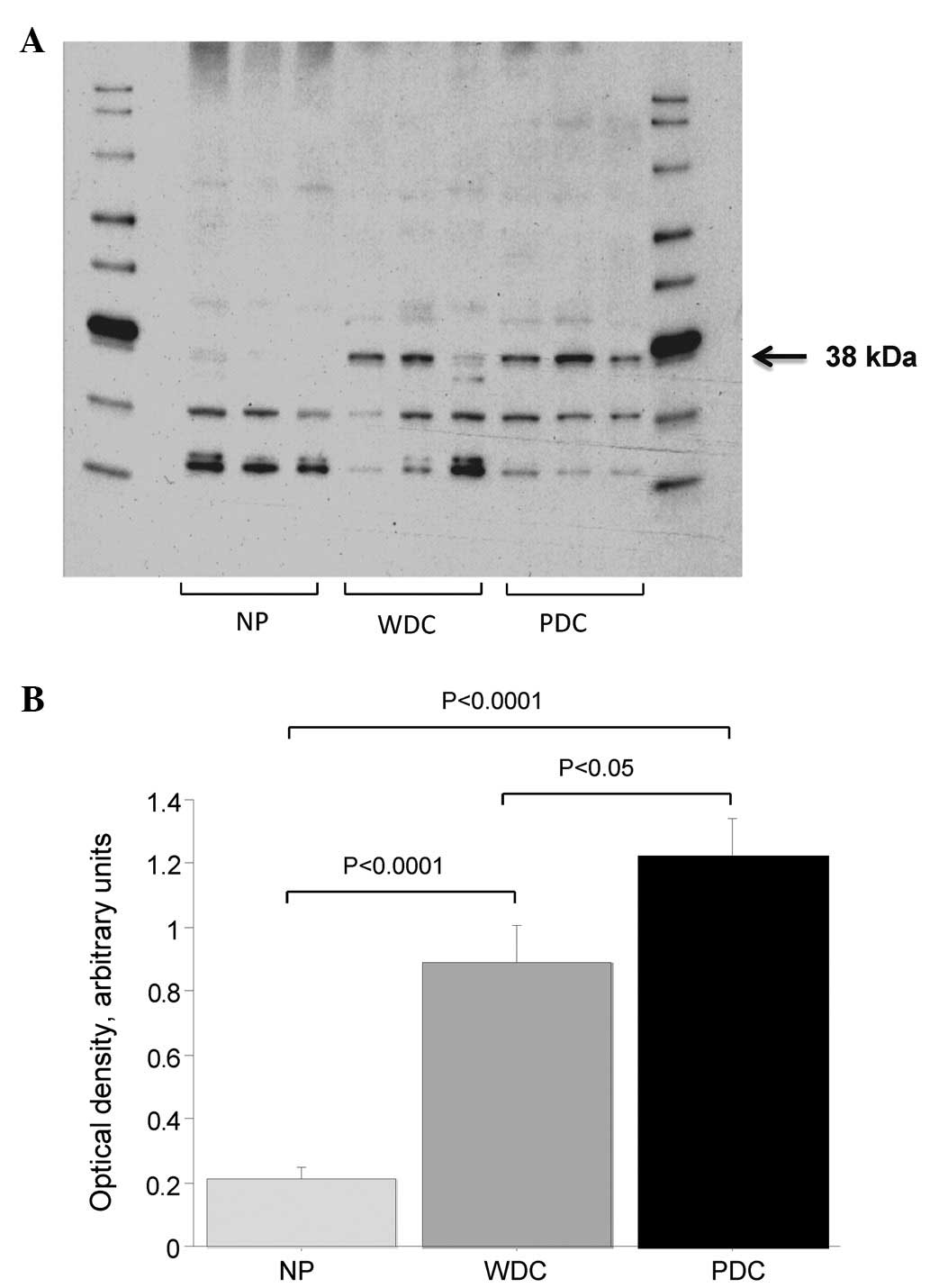
Western blot analysis was performed on RIPA
extracted homogenized pancreatic tissue in order to quantify tissue
levels of MICA/B in normal (donor), non-tumor and tumor pancreatic
tissue. The MICA/B antibody showed three bands with strong
immunoreactivity. Optical density was analyzed for the band at ∼38
kDA. A representative blot is shown in Fig. 2A. Visual inspection indicated that
expression of MICA/B in the 38-kDa band was lower in normal
pancreas tissue compared to tumor tissue (Fig. 2A). It was evident that protein
levels of MICA/B were significantly higher in both poorly and well
differentiated pancreatic tumor tissue when compared to non-tumor
pancreatic tissue (p<0.001 for both; Fig. 2B). Poorly differentiated tumors
(G3) had higher MICA/B expression than well differentiated tumors
(G1–G2; p<0.05). No difference was found in MICA/B expression
from normal pancreas from organ donors compared to ‘next-to-cancer’
non-tumor pancreatic tissue (pancreatic tissue obtained from
resected specimens of cancer patients but containing no tumor).
The 38-kDa band of a Coomassie stained gel was
subjected to masspectrometric analysis to verify the specificity of
the MICA/B antibody since immune based analyses may be hampered by
unspecific binding of antibodies. Protein fragments in the gel-band
could be matched to the MICA/B protein sequence which supports that
the immunoreactive 38-kDa band represents MCA/B (data not
shown).
Serum proteins and sMICA in pancreatic
cancer patients and healthy blood donors
Electrophoretic separation of serum proteins was
performed to check the extent pancreatic cancer patients were
systemically affected by their disease. Serum proteins showed
decreased levels of IgM (0.54±0.06 vs 1.04±0.16 g/l), IgG (6.6±0.6
vs 10.8±0.5 g/l) and albumin (25±1 vs. 45±1 g/l); increased levels
of haptoglobin (1.1±0.1 vs 0.7±0.1 g/l) without a difference in
orosomucoid (0.7±0.1 vs 0.8±0.1 g/l) or α1-antitrypsin levels
(1.2±0.1 vs 1.3±0.1 g/l) in pancreatic cancer patients compared to
blood donors. CRP levels were increased in 70% of the patient
samples (mean 16±8 mg/l; normal values <5 mg/l). Concentrations
of sMICA in serum from the cancer patients were normal, despite the
well-recognized alterations in serum acute phase proteins. The mean
serum concentration of sMICA in blood donors was 50±14 and 40±13
pg/ml in cancer patients. Thus, there was no inference between
serum MICA and tumor stage.
Discussion
It has been reported that expression of MICA/B is
increased in several types of malignancies (10), although studies examining MICA/B
expression in pancreatic cancer are few, particularly in patients
from European countries. In the present study we focused on MICA/B
expression in pancreatic tumor tissue and found that in 17 of 22
(77%) of the tumor specimens MICA/B were detected by
immunohistochemistry, while it was entirely absent in normal
pancreatic duct epithelial cells. This agrees with the study of Xu
et al, performed on North Americans who found MICA/B
expressed in 17 of 25 (68%) tumor specimens (18). In addition to this, a large Chinese
study performed by Duan et al on 103 pancreatic ductal
adenocarcinomas reported that MICA/B expression was observed in 92
of 103 patients (89%) (19).
Tissue distributions of MICA in normal tissue is
restricted to intestinal epithelial cells (20), but the protein may be upregulated
in various types of tumor cells of epithelial origin as well as in
tumor cell lines, possibly due to physical stress (10,21).
MICA functions as a ligand for the NKG2D receptor and may promote
signals for lysis, by both NK cells and CD8+ T cells.
In vitro studies have provided strong evidence that MICA is
important for the susceptibility of target T cells to NK cells, CD8
cytotoxic T cells, and γδ T cells. Tumor cells that stably express
NKG2D ligands at high level may be rejected by CD8 T cells and/or
NK cells and mice immunized with NKG2/D ligand-transfected tumor
cells were reported to develop adaptive immunity against
re-challenge with the parental tumor cell lines (22,23).
Accordingly, blocked interactions between MICA and NKG2D with
antibodies inhibit NK and T cell-mediated cytolysis (24). It seems, however, rather
contradictory that pancreatic tumors, associated with poor
prognosis, should induce expression of MIC, which is assumed to
make tumor cells more susceptible to immune attack and
apoptosis.
Studies on MICA/B expression in tumors demonstrate
somewhat conflicting results. Increased expression of tumor MICA/B
has been shown to correlate with both increased survival and
improved prognosis in colorectal carcinomas (25), while others have found opposite
results in pancreatic cancers (19). In the present study there was no
significant difference in post-operative survival time between
patients with MICA/B positive and negative tumors, but such
calculations must be based on large number of patients, but
significantly higher 3-year mortality in patients with
MICA/B-positive tumors was found. Such discrepancies might be
explained by the fact that MICA/B tumor expression was associated
with regional lymph node involvement, since tumor spread to
regional lymph nodes was significantly less frequent among patients
with MIC-negative tumors.
It has been suggested that MICA/B expression is
associated with poor prognosis, because MICA/B can be released from
the tumor cells by proteolytic shedding, perhaps with final
appearance of its soluble form in blood. This would help tumors to
escape immune surveillance, since the release of sMICA/B and other
NKG2D ligands has the ability to inhibit interaction of NK cells
with the activating receptor, thereby, protecting the ligand
expressing tumor cells from cytolysis (19,26).
However, our present study showed normal levels of sMICA in serum
from pancreatic cancer patients, confirmed by comparisons to
healthy blood donors. Others have reported significantly elevated
levels of MICA in serum from patients with pancreatic
adenocarcinomas, without any correlation to tumor stage and
survival (18). However, Duan
et al reported that mean survival time of pancreatic cancer
patients, with low serum levels of MICA, was significantly longer
than the mean survival time of patients with high levels (19). Some studies have reported that
elevated sMICA correlated significantly to tumor stage in
pancreatic cancer patients and that sMICA levels dropped in serum
from patients following tumor resection (26,27).
However, sMIC levels in serum have been argued not to be specific
enough as an individual prognostic tumor marker (26), although, a recent study claimed
that both sMICA and sMICB are potential biomarkers for pancreatic
ductal adenocarcinoma, and that serum levels of sMICA correlated
with distant metastases and sMICB with unresectability (28). So far, there is no obvious reason
for described inconsistence in observations among reported studies
on sMICA. However, the definite relationships between MICA and
pancreatic tumor progression must await further technical
considerations, since we have observed variation between
specificity and serum components among patient samples analyzed by
the commercially available analysis kits. This may introduce
particular hazards for samples with increased acute phase proteins,
as often found to various extent in cancer patients. Another factor
may be the fact that the highly glycosylated MIC molecule is among
the most polymorphic mammalian MHC genes known (29). MICA is highly polymorphic in
transcellular and extracellular domains, resulting in several
different MICA variants more or less common among different
populations (30,31). Thus, different antibodies used in
the ELISA might detect different forms of diverse MICA/B proteins
since reported levels of MICA showed large variations even in
healthy individuals (18,32). Recent studies in cultured cells
imply that MICA/B shedding is far more complex than initially
thought (33,34). Chitadze et al showed that
shedding of MICA varied substantially among different epithelial
cancer cell lines. Furthermore, they failed to detect sMICA from
cells expressing the allelic variant MICA*008; the most
common allelic variant in Caucasian populations (35).
Molecular mechanisms for increased expression of
MICA/B in tumor cells are not known, but studies indicate that MAP
kinase activation through BRAF gene mutation as well as STAT 3
transcriptional regulation may be involved (17,36).
Observations have also supported suggestions that uric acid
accumulation in DNA-damaged pancreatic cancer cells play an
important role for induction of MICA/B expression (18).
In conclusion, the present study confirms induced
expression of MICA/B protein in pancreatic tumor cells, but without
subsequent net appearance of MICA in serum despite the presence of
well-recognized systemic inflammation in our pancreatic cancer
patients as demonstrated by serum acute phase reactants. The
expression in pancreatic cancer cells increased with loss of tumor
differentiation and further disease progression. Our study implies
that induction of MICAB tumor expression was associated with
frequent tumor spread to the regional lymph nodes leading to
increased 3-year mortality following surgical resection.
Acknowledgements
This study was supported in parts by
grants from the Swedish Cancer Society, Assar Gabrielsson
Foundation (AB Volvo) and the Swedish government (LUA-ALF). We
would like to thank The Proteomics Core Facility at Sahlgrenska
Academy, Gothenburg University for performing the mass spectrometry
analysis. The purchase of LTQ-OrbitrapXL was made possible through
a grant from the Knut and Alice Wallenberg Foundation to Professor
Gunnar C. Hansson (KAW2007.0118). We acknowledge Professor Kent
Lundholm for support of this study.
References
|
1.
|
Wagner M, Redaelli C, Lietz M, Seiler CA,
Friess H and Buchler MW: Curative resection is the single most
important factor determining outcome in patients with pancreatic
adenocarcinoma. Br J Surg. 91:586–594. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Bramhall SR, Allum WH, Jones AG, Allwood
A, Cummins C and Neoptolemos JP: Treatment and survival in 13,560
patients with pancreatic cancer, and incidence of the disease, in
the West Midlands: an epidemiological study. Br J Surg. 82:111–115.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Jemal A, Siegel R, Ward E, et al: Cancer
statistics, 2008. CA Cancer J Clin. 58:71–96. 2008. View Article : Google Scholar
|
|
4.
|
McCracken M, Olsen M, Chen MS Jr, et al:
Cancer incidence, mortality, and associated risk factors among
Asian Americans of Chinese, Filipino, Vietnamese, Korean, and
Japanese ethnicities. CA Cancer J Clin. 57:190–205. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Buchler MW, Wagner M, Schmied BM, Uhl W,
Friess H and Z’Graggen K: Changes in morbidity after pancreatic
resection: toward the end of completion pancreatectomy. Arch Surg.
138:1310–1315. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Diener MK, Heukaufer C, Schwarzer G, et
al: Pancreaticoduodenectomy (classic Whipple) versus
pylorus-preserving pancreaticoduodenectomy (pp Whipple) for
surgical treatment of periampullary and pancreatic carcinoma.
Cochrane Database Syst Re. 16:CD0060532008.
|
|
7.
|
Jaffee EM, Hruban RH, Biedrzycki B, et al:
Novel allogeneic granulocyte-macrophage colony-stimulating
factor-secreting tumor vaccine for pancreatic cancer: a phase I
trial of safety and immune activation. J Clin Oncol. 19:145–156.
2001.
|
|
8.
|
Picozzi VJ, Kozarek RA and Traverso LW:
Interferon-based adjuvant chemoradiation therapy after
pancreaticoduodenectomy for pancreatic adenocarcinoma. Am J Surg.
185:476–480. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Dranoff G: Coordinated tumor immunity. J
Clin Invest. 111:1116–1118. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Groh V, Rhinehart R, Secrist H, Bauer S,
Grabstein KH and Spies T: Broad tumor-associated expression and
recognition by tumor-derived gamma delta T cells of MICA and MICB.
Proc Natl Acad Sci USA. 96:6879–6884. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Groh V, Wu J, Yee C and Spies T:
Tumour-derived soluble MIC ligands impair expression of NKG2D and
T-cell activation. Nature. 419:734–738. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Pende D, Rivera P, Marcenaro S, et al:
Major histocompatibility complex class I-related chain A and
UL16-binding protein expression on tumor cell lines of different
histotypes: analysis of tumor susceptibility to NKG2D-dependent
natural killer cell cytotoxicity. Cancer Res. 62:6178–6186.
2002.
|
|
13.
|
Liu G, Atteridge CL, Wang X, Lundgren AD
and Wu JD: The membrane type matrix metalloproteinase MMP14
mediates constitutive shedding of MHC class I chain-related
molecule A independent of A disintegrin and metalloproteinases. J
Immunol. 184:3346–3350. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Salih HR, Rammensee HG and Steinle A:
Cutting edge: down-regulation of MICA on human tumors by
proteolytic shedding. J Immunol. 169:4098–4102. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Waldhauer I, Goehlsdorf D, Gieseke F, et
al: Tumor-associated MICA is shed by ADAM proteases. Cancer Res.
68:6368–6376. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Doubrovina ES, Doubrovin MM, Vider E, et
al: Evasion from NK cell immunity by MHC class I chain-related
molecules expressing colon adenocarcinoma. J Immunol.
171:6891–6899. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Xu X, Rao G, Gaffud MJ, et al:
Clinicopathological significance of major histocompatibility
complex class I-related chain a and B expression in thyroid cancer.
J Clin Endocrinol Metab. 91:2704–2712. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Xu X, Rao GS, Groh V, et al: Major
histocompatibility complex class I-related chain A/B (MICA/B)
expression in tumor tissue and serum of pancreatic cancer: role of
uric acid accumulation in gemcitabine-induced MICA/B expression.
BMC Cancer. 11:1942011. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Duan X, Deng L, Chen X, et al: Clinical
significance of the immunostimulatory MHC class I chain-related
molecule A and NKG2D receptor on NK cells in pancreatic cancer. Med
Oncol. 28:466–474. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Groh V, Bahram S, Bauer S, Herman A,
Beauchamp M and Spies T: Cell stress-regulated human major
histocompatibility complex class I gene expressed in
gastrointestinal epithelium. Proc Natl Acad Sci USA.
93:12445–12450. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Bauer S, Groh V, Wu J, et al: Activation
of NK cells and T cells by NKG2D, a receptor for stress-inducible
MICA. Science. 285:727–729. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Diefenbach A, Jensen ER, Jamieson AM and
Raulet DH: Rae1 and H60 ligands of the NKG2D receptor stimulate
tumour immunity. Nature. 413:165–171. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Hayakawa Y, Kelly JM, Westwood JA, et al:
Cutting edge: tumor rejection mediated by NKG2D receptor-ligand
interaction is dependent upon perforin. J Immunol. 169:5377–5381.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Raulet DH: Roles of the NKG2D
immunoreceptor and its ligands. Nat Rev Immunol. 3:781–790. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Watson NF, Spendlove I, Madjd Z, et al:
Expression of the stress-related MHC class I chain-related protein
MICA is an indicator of good prognosis in colorectal cancer
patients. Int J Cancer. 118:1445–1452. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Marten A, von Lilienfeld-Toal M, Buchler
MW and Schmidt J: Soluble MIC is elevated in the serum of patients
with pancreatic carcinoma diminishing gammadelta T cell
cytotoxicity. Int J Cancer. 119:2359–2365. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Holdenrieder S, Stieber P, Peterfi A,
Nagel D, Steinle A and Salih HR: Soluble MICA in malignant
diseases. Int J Cancer. 118:684–687. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Chung HW and Lim JB: Clinical significance
of serum levels of immune-associated molecules, uric acid and
soluble MHC class I chain-related molecules A and B, as diagnostic
tumor markers for pancreatic ductal adenocarcinoma. Cancer Sci.
102:1673–1679. 2011. View Article : Google Scholar
|
|
29.
|
Bahram S: MIC genes: from genetics to
biology. Adv Immunol. 76:1–60. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Collins RW, Stephens HA, Clare MA and
Vaughan RW: High resolution molecular phototyping of MICA and MICB
alleles using sequence specific primers. Hum Immunol. 63:783–794.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Stephens HA: MICA and MICB genes: can the
enigma of their polymorphism be resolved? Trends Immunol.
22:378–385. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Arreygue-Garcia NA, Daneri-Navarro A, del
Toro-Arreola A, et al: Augmented serum level of major
histocompatibility complex class I-related chain A (MICA) protein
and reduced NKG2D expression on NK and T cells in patients with
cervical cancer and precursor lesions. BMC Cancer. 8:162008.
View Article : Google Scholar
|
|
33.
|
Chitadze G, Lettau M, Bhat J, et al:
Shedding of endogenous MHC class I-related chain molecules A and B
from different human tumor entities: heterogeneous involvement of
the ‘a disintegrin and metalloproteases’ 10 and 17. Int J Cancer.
133:1557–1566. 2013.PubMed/NCBI
|
|
34.
|
Ashiru O, Boutet P, Fernandez-Messina L,
et al: Natural killer cell cytotoxicity is suppressed by exposure
to the human NKG2D ligand MICA*008 that is shed by tumor
cells in exosomes. Cancer Res. 70:481–489. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Collins RW: Human MHC class I chain
related (MIC) genes: their biological function and relevance to
disease and transplantation. Eur J Immunogenet. 31:105–114. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Bedel R, Thiery-Vuillemin A, Grandclement
C, et al: Novel role for STAT3 in transcriptional regulation of NK
immune cell targeting receptor MICA on cancer cells. Cancer Res.
71:1615–1626. 2011. View Article : Google Scholar : PubMed/NCBI
|