Introduction
Recent studies on regenerative medicine have
indicated that mesenchymal tissues contain multipotent stem or
progenitor cells that can give rise to neural and skin tissues,
adipocytes, myocytes, cardiomyocytes, blood vessels, chondrocytes
and hepatocytes (1). Given that
bone marrow (BM) and adipose tissues are major sources of
mesenchymal stem or progenitor cells (2), the observation of BM transplantation
in mice and humans can provide adequate biological and biophysical
information concerning cells of origin. Reportedly, bone
marrow-derived cells (BMDCs) accumulate in the gastric epithelium
as a result of Helicobacter pylori infection and can
contribute to tumor development, indicating that infection can lead
to the development of hyperplasia, metaplasia and dysplasia
associated with BMDC recruitment and accumulation in the gastric
epithelial mucosa, which occurs against a background of chronic
inflammation (3). However, the
contribution of BMDSs to head and neck cancers, including
esophageal cancer, remains unknown.
GVHD is a major complication of allogeneic
hematopoietic stem cell transplantation (HSCT), with significant
morbidity and mortality (4).
Therefore, adequate control of GVHD is critical to the continued
success of transplantation. GVHD shares its molecular basis with
chronic inflammation. This molecular basis includes the induction
of intrinsic damage to tissue stem or progenitor cells and
deleterious effects on immune surveillance, all extensive and
dynamic alterations that accumulate in the course of carcinogenesis
(5). The use of immunosuppressant
therapy exerts a favorable effect by ameliorating chronic GVHD, but
it is associated with a higher relapse rate of hematopoietic and
secondary malignancies, thus posing a major threat in the long term
(6).
Cells for HSCT are obtained from BM, peripheral
blood, or umbilical cord blood, which is proposed to contain
mesenchymal stem or progenitor cells as well as other cell types
(7,8). Secondary malignancies following HSCT
are common late complications (9).
With regard to the cells of origin, secondary tumors are considered
to be derived from recipient-derived cells because there are very
few epithelial cells in normal BM and peripheral blood (10). Nevertheless, the contribution of
human BM cells, including HSCs, to epithelium, dysplasia and cancer
is poorly understood (11). To
study the involvement of BM cells in solid tumors developing after
HSCT and distinguish the origins of epithelial cancer cells in
humans, we performed highly sensitive FISH using gender
chromosome-specific probes and histopathological analyses in five
cases of head and neck tumors that developed subsequent to
gender-mismatched BM transplantation. Our study allowed the
identification of donor-derived epithelium, dysplasia, and cancer
of the esophagus against a background of chronic inflammation due
to GVHD, demonstrating that BMDCs can contribute to the development
of precancerous lesions and various cancers.
Patients and methods
Patients
Patient characteristics are summarized in Table I. All patients had received
gender-mismatched HSCTs and developed GVHD. Two clinical samples
were obtained from patients with esophageal squamous cell carcinoma
(SCC) treated at our hospital. Three clinical samples from two
patients with oral SCC and one patient with tongue Diseases (Osaka,
Japan). All clinical samples used in this study were acquired after
obtaining written informed consent from each patient.
 | Table I.Characteristics of cases with
secondary SCCs after HSCT. |
Table I.
Characteristics of cases with
secondary SCCs after HSCT.
| Case no. | Blood diseases | Secondary SCCs |
|---|
|
|
|---|
| Gender of
donor/recipient | Diagnosis | Type of HSCT | GVHD | Location | Age at
diagnosis | Time after
transplantation (months) |
|---|
| 1 | F/M | Non-Hodgkin’s
lymphoma | PBSCT | Chronic | Esophagus | 42 | 115 |
| 2 | F/M | Non-Hodgkin’s
lymphoma | PBSCT | Chronic | Esophagus | 77 | 120 |
| 3 | F/M | MDS | BMT | Chronic | Oral cavity | 38 | 76 |
| 4-1 | M/F | CML | BMT | Chronic | Tongue | 45 | 150 |
| 4-2 | Oral cavity | 46 | 163 |
FISH analysis
FISH analysis was performed by Chromosome Science
Labo, Inc. (Sapporo, Japan) using formalin-fixed, paraffin-embedded
tissue sections as described previously (12). Briefly, 5-mm-thick sections were
deparaffinized, dehydrated, microwaved (600 W) in 2X saline sodium
citrate (SSC) for 10 min, cooled in PBS, digested in pepsin
solution containing 0.1 N HCl at 37°C (0.1% pepsin for 10 min for
samples 4-1 and 4-2; 0.02% pepsin for 5 min for the other samples),
and dehydrated. Human XY FISH probes (Chromosome Science Labo,
Inc.) were applied to the pretreated sections, covered with cover
slips, and simultaneously denatured at 90°C for 13 min.
Hybridization was performed at 37°C overnight. Sections were then
washed with 50% formamide/2X SSC at 37°C for 20 min and 1X SSC for
15 min at room temperature. The slides were treated with antibodies
at 37°C for 30 min, washed three times with 0.1% Nonidet P-40/2X
SSC, counterstained with 4′,6-diamidino-2-phenylindole (DAPI), and
mounted. The FISH images were captured using the CW4000 FISH
application program (Leica Microsystems Imaging Solution, Ltd.,
Cambridge, UK) using a cooled charge-coupled device camera mounted
on a Leica DMRA2 microscope (Leica Microsystems, Wetzlar, Germany).
The enumeration probes for the X chromosomes were labeled with
cyanine 3 (Cy3), SpectrumGold™ (Abbott Laboratories,
Abbott Park, IL, USA), or Cy5, whereas the enumeration probes for
the Y chromosomes were labeled with SpectrumGreen™ or
SpectrumRed™ (Abbott Laboratories). The number of cells
showing FISH signals was counted by the observation of at least
five fields under the microscope (×100). All data were evaluated by
at least three pathologists.
Histopathological analyses
Pathological diagnoses of DAPI- or hematoxylin and
eosin-stained samples were performed by at least three pathologists
to identify normal and malignant cells.
Results
BM significantly contributes to normal
epithelium and SCC of the esophagus
Esophageal cancer developed in a male recipient with
non-Hodgkin’s lymphoma 115 months after gender-mismatched HSCT
using cells from a female donor (Table
I, case no. 1). To assess normal epithelium in the esophagus,
FISH was performed using gender chromosome-specific probes (Cy5 and
SpectrumGreen for the X chromosome; SpectrumRed for the Y
chromosome; Fig. 1). A comparison
of FISH and histopathological analyses indicated that all
infiltrating lymphocytes in an examination of 50 fields displayed
green (X) signals but not red (Y) signals, suggesting that the
lymphocytes were replaced with donor hematopoietic cells after HSCT
(representative data in Fig. 1A–C;
Tables II and III). The data indicated that both
epithelial cells and basal cells were also positive for green (X)
signals but not red (Y) signals, whereas mesenchymal cells in the
stroma were positive for both green (X) and red (Y) signals,
suggesting that the recipient cells were integrated in the
mesenchymal tissues (Fig. 1A–C).
The infiltration of lymphocytes with only green (X) signals in the
epithelial and mesenchymal tissues was compatible with chronic
GHVD. The present data propose that epithelial regions within the
esophagus were replaced predominantly with donor-derived cells
after HSCT. Next, we examined tumor tissues obtained from case 1.
Comparative FISH and histopathological analyses indicated that all
SCC cells within the tumor displayed green (X) signals but not red
(Y) signals, suggesting that esophageal cancer cells in case 1 were
derived from donor cells in the regions examined. Infiltrating
recipient-derived cells were not detected while lymphocytes with
green (X) signals were present; this was compatible with the
replacement of recipient hematopoietic cells by donor cells
(Fig. 1D–G; Tables II and III). An examination of the mesenchymal
tissues indicated that stromal cells, which include fibroblasts as
well as lymphocytes and coexist in flanking regions of epithelial
cancer cells, displayed red (Y) signals showing the contribution of
the recipient cells. On the other hand, approximately 30% of the
stromal cells displayed green (X) signals but not red signals,
suggesting the involvement of donor-derived cells. We found that
tumor-associated fibroblasts displayed both green (X) and red (Y)
signals, indicating that they were recipient cells (Fig. 1H and I).
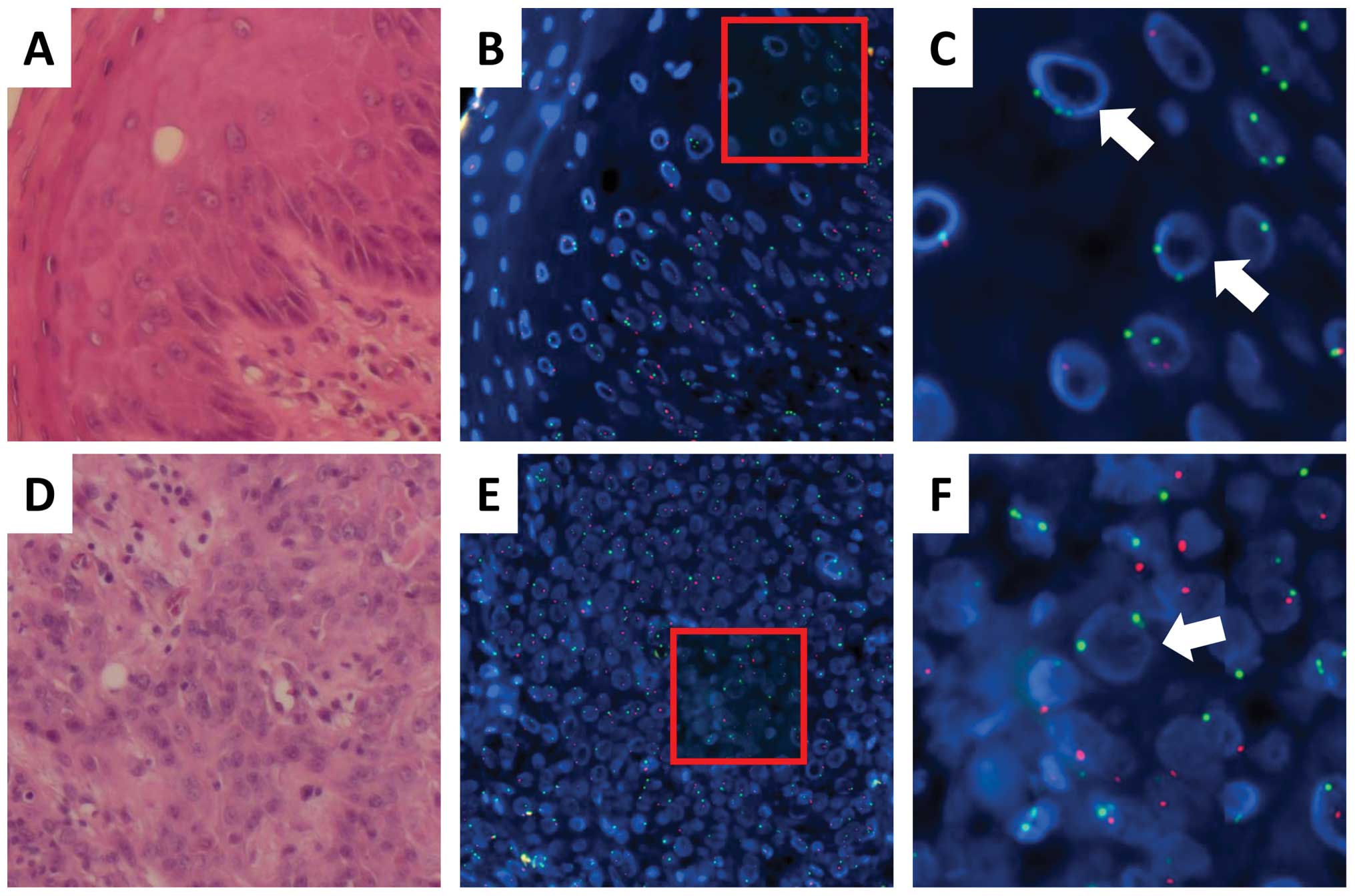
 | Figure 1.Donor-derived esophageal epithelial
cells, SCCs, and recipient-derived fibroblasts from a male
recipient (case 1) of peripheral blood stem cells from a female
donor. (A) Tissue sections of the normal mucosa are stained with
hematoxylin and eosin. (B) Neighboring tissue sections of (A) are
examined by FISH to determine the centromeres of the X (Cy5, green)
and Y (SpectrumRed, red) chromosomes. (C) A magnified image of the
red-framed square in (B). (D) Tumor tissue sections are stained
with hematoxylin and eosin. (E) Neighboring tissue sections of (D)
are examined by FISH to determine the centromeres of the X (Cy5,
green) and Y (SpectrumRed, red) chromosomes. (F) A magnified image
of the cancerous region, i.e., the red-framed square in (E). (G) A
magnified image of the stromal region, i.e., the green-framed
square in (E). (H) Fibroblasts from the stromal region in a dish.
(I) Fibroblasts from (H) are examined by FISH to determine the
centromeres of the X (Cy3, red) and Y (SpectrumGreen, green)
chromosomes. Panels A through F, ×63 magnification; inset in panel
D, ×160 magnification. |
 | Table II.Cell of origin. |
Table II.
Cell of origin.
| Case no. | Donor cells | Recipient
cells |
|---|
|
|
|---|
| E | L | D | C | E | L | D | C |
|---|
| 1 | + | + | ND | + | − | − | ND | − |
| 2 | ± | + | + | ± | + | − | − | + |
| 3 | ± | + | ND | ± | + | − | ND | + |
| 4-1 | − | + | ND | − | + | − | ND | + |
| 4-2 | − | + | ND | − | + | − | ND | + |
 | Table III.Results of this study. |
Table III.
Results of this study.
| Case no. | Normal epithelial
cells | Cancer cells | Dysplasia |
|---|
|
|
|
|---|
| Y | X | XY | XX | Y | X | XY | XX | Y | X | XY | XX |
|---|
| 1 | 0.0 | 94.3 | 0.0 | 5.7 | 0.0 | 58.2 | 0.0 | 41.8 | ND | ND | ND | ND |
| 2 | 36.1 | 18.1 | 41.0 | 1.2 | 27.9 | 34.9 | 65.1 | 2.3 | 0.0 | 86.7 | 0.0 | 13.3 |
| 3 | 43.8 | 30.8 | 24.6 | 0.3 | 35.9 | 37.5 | 18.8 | 1.6 | ND | ND | ND | ND |
| 4-1 | 0.0 | 57.7 | 0.0 | 28.2 | 0.0 | 44.6 | 0.0 | 46.4 | ND | ND | ND | ND |
| 4-2 | 0.0 | 18.9 | 0.0 | 66.2 | 0.0 | 50.9 | 0.0 | 25.5 | ND | ND | ND | ND |
Significant contribution of BM to
dysplasia of esophagus
In case 2, the recipient was a male and the donor
was a female. Esophageal cancer, which was characterized by
dysplastic lesions, developed in the recipient 120 months after
HSCT for malignant lymphoma, although the original disease was in
complete remission (Table I). As
indicated in Fig. 2 (SpectrumGold
for the X chromosome; SpectrumRed for the Y chromosome), FISH and
histopathological analyses indicated that normal epithelial regions
(lesion I in Fig. 2A) were
composed predominantly of squamous cells as indicated by the yellow
(X) and red (Y) signals, whereas a fraction displayed yellow (X)
signals alone, suggesting that the esophageal epithelium was
reconstituted at least partially with donor-derived cells in the
regions examined. As indicated by the representative data in
Fig. 2B–D (Tables II and III), the nuclei were similar to those of
the surrounding epithelial cells in size, indicating that they were
also epithelial cells. Infiltrated lymphocytes displayed only
yellow (X) signals, indicating that the hematopoietic cells in the
recipient were replaced by donor cells and the involvement of GVHD.
Histopathological analysis of the surgical specimen from case 2
indicated dysplasia (lesion II in Fig.
2A) as a continuous lesion with the tumor (lesion III). FISH
and histopathological analyses of lesion II indicated that almost
all the dysplastic cells displayed only yellow (X) signals but not
red (Y) signals (Fig. 2E–G;
Tables II and III), suggesting that the donor-derived
cells were recruited to the esophageal epithelium and transformed
or that some damaged donor cells may have been incorporated into
the esophagus. Analysis of the tumor (lesion III; Fig. 2H–M; Tables II and III) showed both yellow (X) and red (Y)
signals in almost all cancer cells, whereas a few cells expressed
only yellow (X) signals (arrows in Fig. 2M; their nuclei were similar to
those of the surrounding epithelial cancer cells in size),
indicating that the tumor was composed predominantly of recipient
cancer cells with a fraction of donor-derived cells. This was
characterized by infiltrating lymphocytes displaying yellow (X)
signals alone (arrows in Fig. 2J;
the nuclei were much smaller than those of epithelial cells,
indicating lymphocytes), thus being compatible with GVHD.
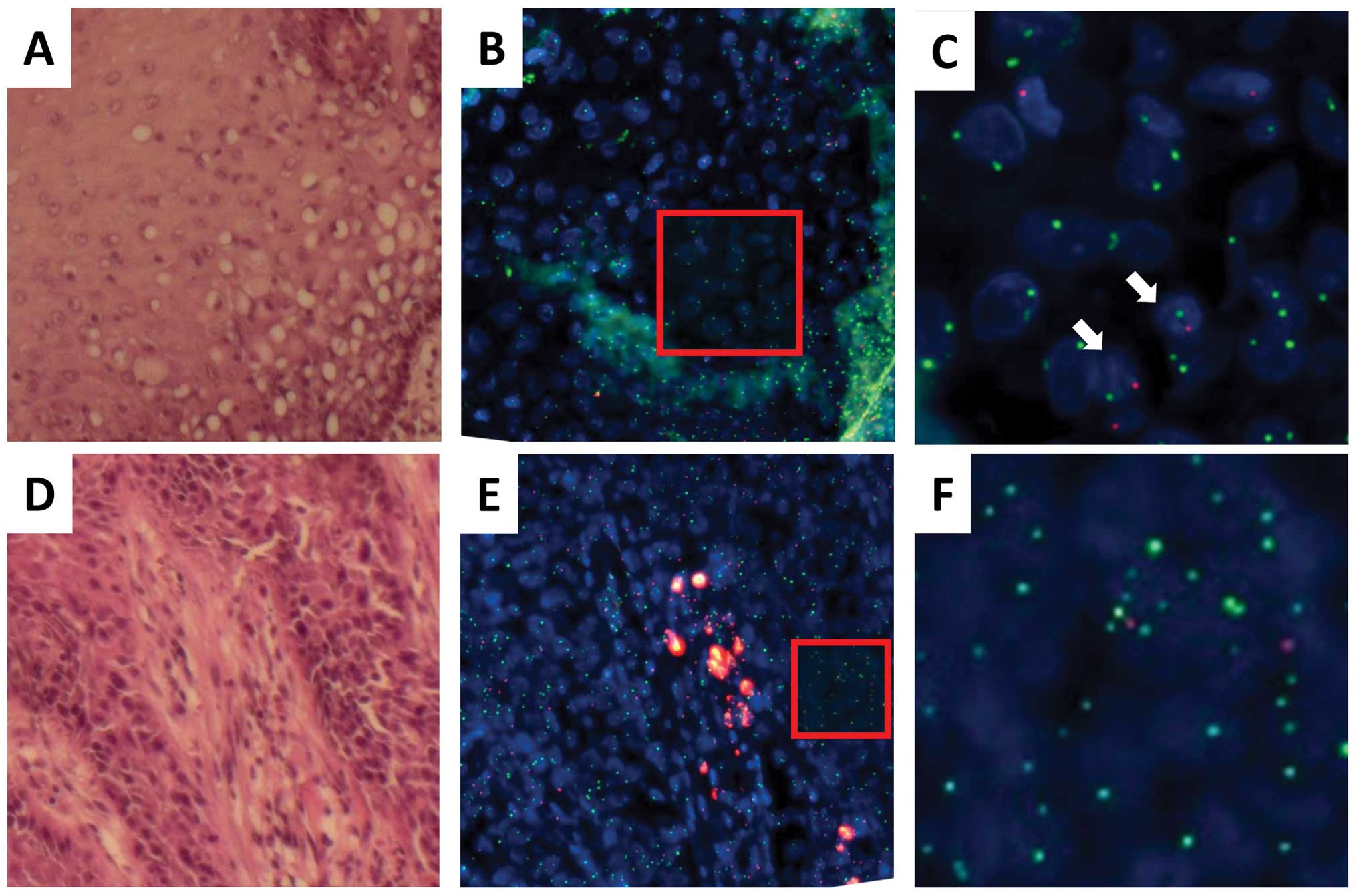
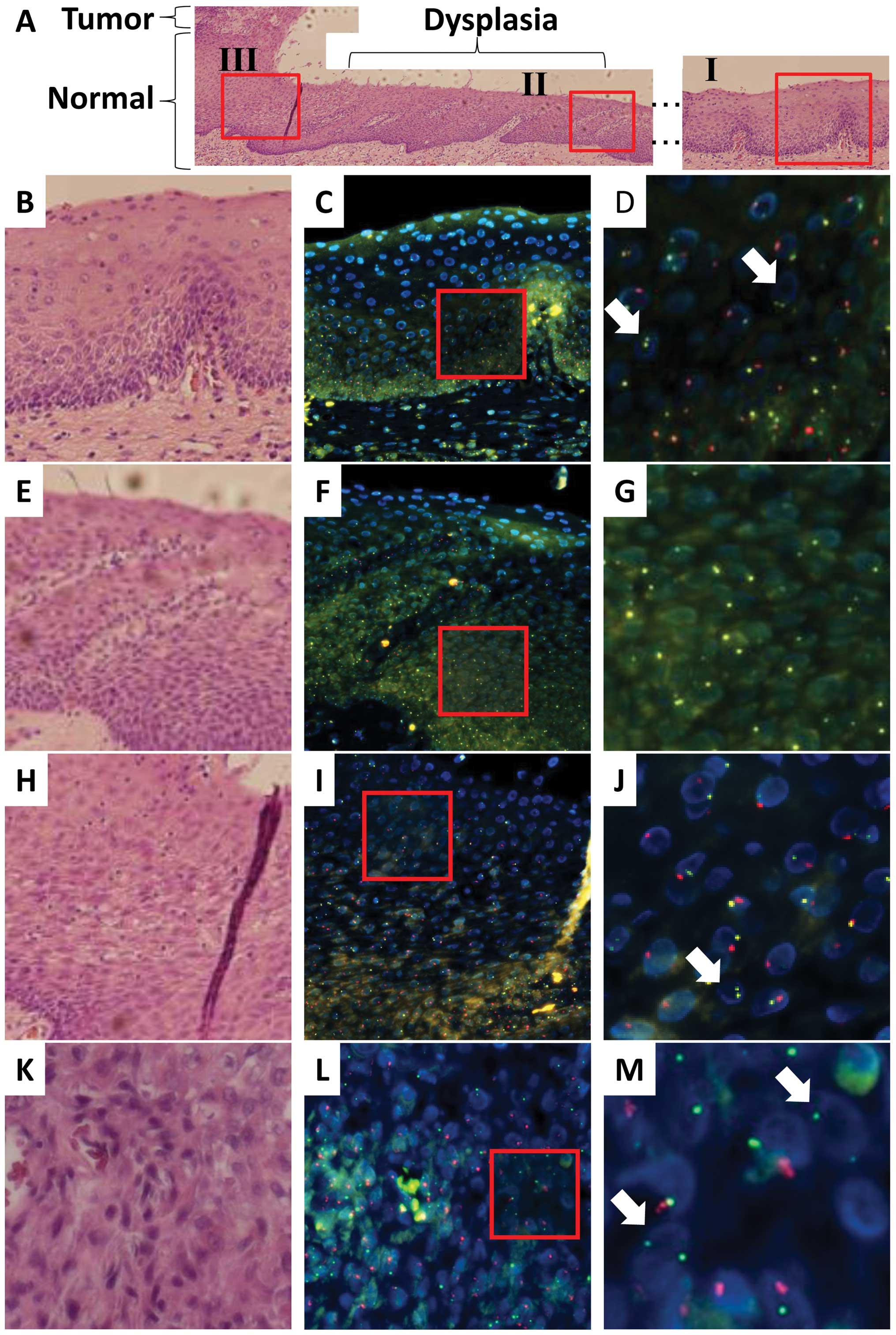 | Figure 2.Recipient- and donor-derived
esophageal epithelial cells and recipient-derived esophageal SCC
from a male recipient (case 2) of peripheral blood stem cells from
a female donor. (A) Tissue sections of the normal mucosa and solid
tumor tissue are stained with hematoxylin and eosin. (B) A
magnified image of normal mucosa (I). (C) Neighboring tissue
sections of (B) are examined by FISH to determine the centromeres
of the X (SpectrumGold, yellow) and Y (SpectrumRed, red)
chromosomes. (D) A magnified image of the red-framed square in (C).
A few donor-derived cells display XX signals, and their nuclei are
similar to those of normal epithelial cells in size (white arrow).
(E) A magnified image of the region with dysplasia (II). (F)
Neighboring tissue sections of (E) are examined by FISH to
determine the centromeres of the X (SpectrumGold, yellow) and Y
(SpectrumRed, red) chromosomes. (G) A magnified image of the
red-framed square in (F). No cell in the epithelium displays Y
signals. (H) A magnified image of normal mucosa (III). (I)
Neighboring tissue sections of (H) are examined by FISH to
determine the centromeres of the X (SpectrumGold, yellow) and Y
(SpectrumRed, red) chromosomes. (J) A magnified image of the
red-framed square in (H). Infiltrating donor-derived cells display
XX signals and smaller nuclei than those of normal epithelial cells
(white arrow). (K) Tumor tissue sections are stained with
hematoxylin and eosin. (L) Neighboring tissue sections of (K) are
examined by FISH to determine the centromeres of the X (Cy5, green)
and Y (SpectrumRed, red) chromosomes. (M) A magnified image of the
cancer region, i.e., the red-framed square in (L). A few
donor-derived cells display XX signals, and their nuclei are
similar to those of SCC cells in size (white arrow). |
A mixed tumor of recipient- and
donor-derived cells
In case 3, the donor was a female and the recipient
was a male who developed oral SCC 76 months after HSCT for
myelodysplastic syndrome (Table
I). FISH and histopathological analyses indicated that all
infiltrating lymphocytes displayed green (X) signals but not red
(Y) signals, suggesting that the lymphocytes were replaced after
transplantation; this was again compatible with GVHD (Cy5 for the X
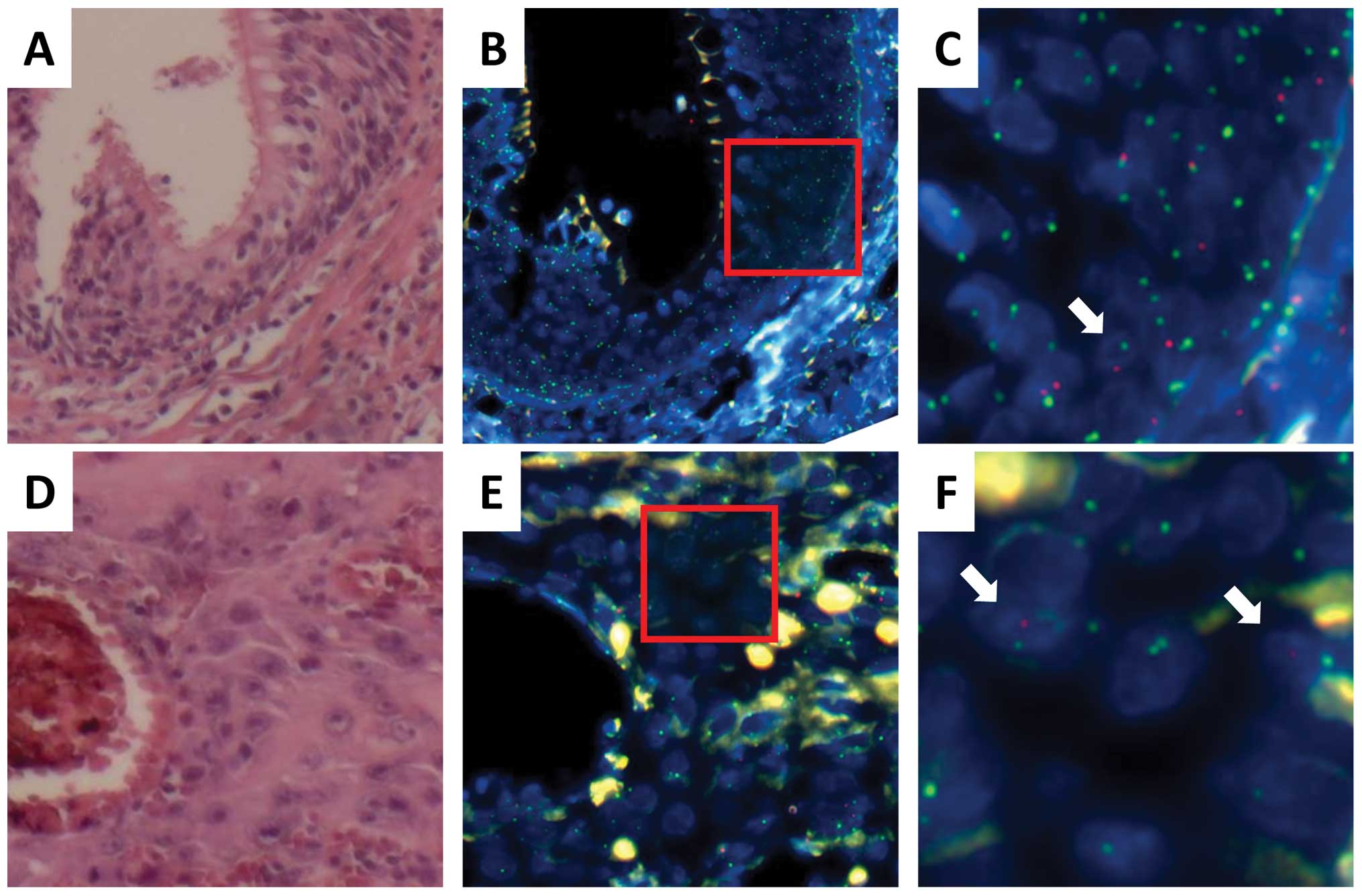
chromosome; SpectrumRed for the Y chromosome; Fig. 3). Analysis of the normal epithelium
indicated that almost all epithelial cells displayed red (Y)
signals (Fig. 3A–C; Tables II and III), whereas approximately 5% cells
displayed green (X) signals alone. The nuclei of the latter cells
were similar to those of the surrounding epithelial cells in size
(Fig. 3C), indicating that normal
epithelium in the surgical specimen of the oral cavity was composed
predominantly of recipient cells. In the tumor, approximately 80%
cancer cells expressed red (Y) signals (Fig. 3D–F; Tables II and III), whereas 20% cells displayed green
(X) signals alone. The nuclei of the latter cells were similar size
to those of the surrounding SCC cells in size (Fig. 3F), suggesting that the tumor had
two different origins: a predominant contribution from the
recipient cells and a partial contribution from the donor cells,
which may have developed after HSCT presumably through the
involvement of GVHD.
Sequentially occurring oral cancer after
HSCT
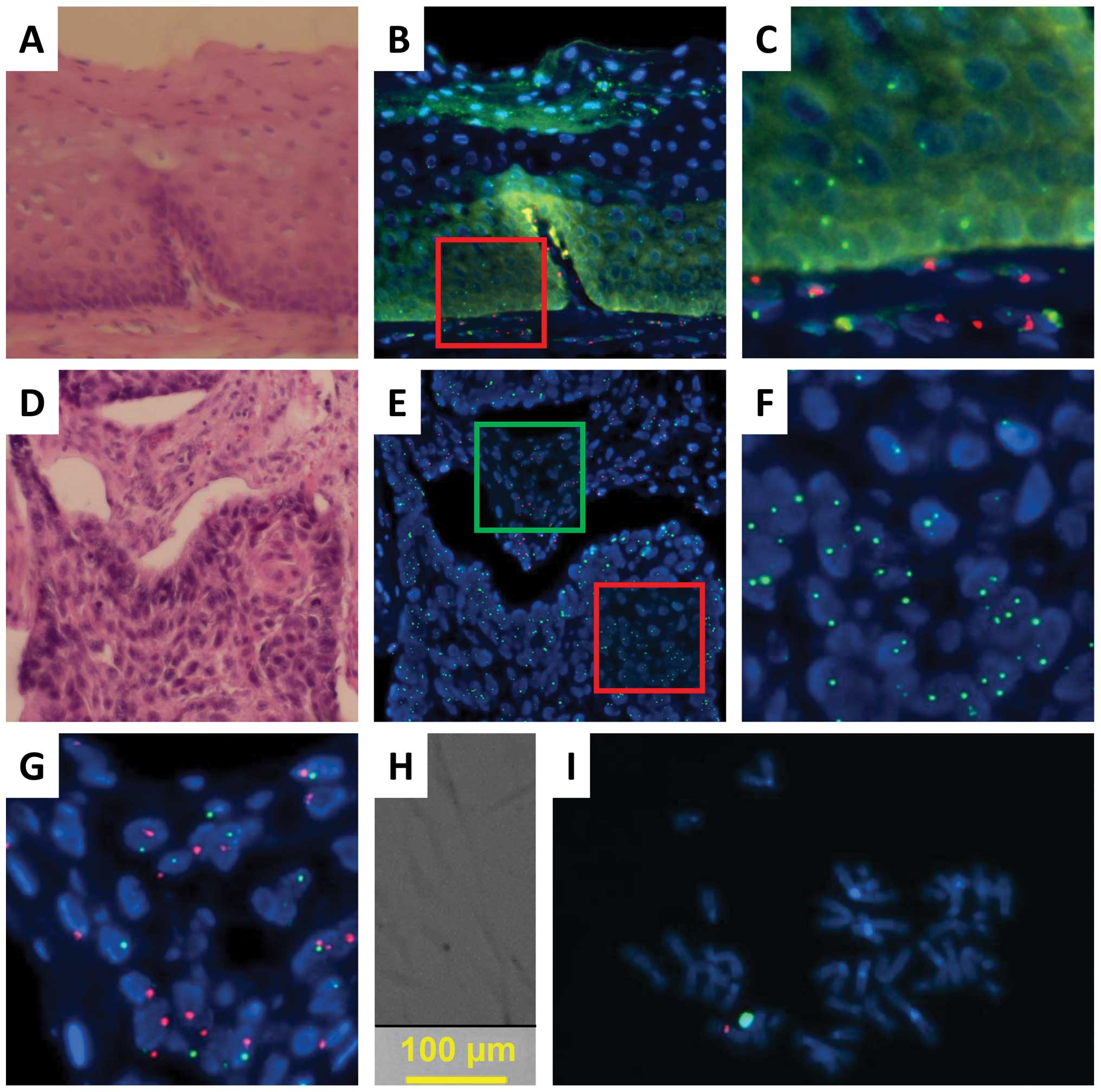
We encountered a patient who developed oral SCC on
different tongue regions 150 (case 4-1) and 163 months (case 4-2)
after single HSCT for chronic myeloid leukemia. The recipient was a
female and the donor was a male. FISH and histopathological
analyses showed that all infiltrating lymphocytes displayed red (Y)
signals, indicating that these lymphocytes were replaced after
transplantation and compatibility with GVHD (Cy5 for the X
chromosome; SpectrumRed for the Y chromosome; Fig. 4). An analysis of normal epithelial
cells and carcinoma cells revealed the expression of green (X)
signals but not red (Y) signals (Figs.
4 and 5; Tables II and III). Red (Y) signals were detected only
in cells with relatively small nuclei compared with those of the
epithelial cells, indicating lymphocyte infiltration and
compatibility with GVHD (Figs. 4C,
5C and F).
Discussion
In this study, we utilized a highly sensitive
combination of FISH using gender chromosome-specific probes and
histopathological analyses to clearly demonstrate that human
epithelial cancer of the head and neck can arise from donor cells
after gender-mismatched hematopoietic transplantation (Tables II and III). In a case of esophageal cancer (case
1), almost all cancer cells were donor-derived in the regions
examined, whereas a dysplastic lesion was composed exclusively of
donor cells in case 2. Our observations indicated that donor BM
contains various cells, including mesenchymal cells, and the
collection of hematopoietic cells after HSCT caused those cells to
be transferred to the recipient and contributed to the formation of
epithelial structures (7,8). Alternatively, some epithelial cell
types, even though normal, can free themselves from epithelial
structures and migrate into the blood stream through the well-known
phenomenon of epithelial-mesenchymal transition, as occurs during
the metastasis of solid tumors (10). Although the release frequency of
normal epithelial cells from tissues under physiological conditions
is uncertain, patients with solid tumors reportedly possess
circulating epithelial cancer cells in the peripheral blood and BM
(10). In the cases we examined,
none of the donors developed late-onset malignancies during the
observation period, which reinforced the fact that BM-derived or
circulating mesenchymal stem cells, rather than apparent epithelial
cells, in donors may change their phenotypes in recipient organs.
This notion is compatible with recent studies in which circulating
donor-derived BMSCs differentiated into epithelial cells and tumor
cells after HSCT in mice and humans (11–30).
Nevertheless, a mixture of recipient- and donor-derived origins was
involved in cases 2 and 3; therefore, we hypothesized that the
initiation and progression of esophageal carcinogenesis may occur
in either recipient- or donor-derived single cells in the
epithelium and that the adjacent cells may be recruited or
accumulated against a background of inflammation and exposure to an
immunosuppressant. However, we cannot exclude the possibility of
simultaneous transformation in both recipient- and donor-derived
cells.
BMSCs are known to differentiate into many types of
cells. In the mouse, circulating BMSCs have been reported to
differentiate into gastric mucosal cells, lung epithelial cells,
renal epithelial cells, keratinocytes, hepatocytes, duct cells,
astrocytes and neurons (3–7). In humans, it has been observed that
BMSCs differentiate into buccal epithelial cells, keratinocytes,
gastrointestinal tract cells, lung epithelial cells, hepatocytes,
duct cells, astrocytes and neurons (8–11).
In our cases of secondary epithelial cancers subsequent to HSCT,
GVHD and exposure to immunosuppressants may have elicited the
recruitment and accumulation of BMSC-derived epithelial cells in
the tissues (5). The present study
suggested the involvement of at least three components in the
maintenance and carcinogenesis of head and neck tissues: tissue
stem cells in the epithelial layer, circulating stem cells and
HSCs.
In mice, BMSCs are reportedly involved in various
types of solid tumor formation, including tumors of the epithelium,
neural and muscle tissues, fibroblasts and blood vessel endothelium
(12,14–25).
In human oral SCC, BMSCs have been implicated in mucoepidermoid
carcinoma of the parotid glands, invasive ductal carcinoma in the
breast, papillary thyroid carcinoma, cervical carcinoma, Kaposi’s
sarcoma, lung adenocarcinoma, skin SCC, glioblastoma multiforme and
pharyngeal SCC (26–31). Although five previous cases
suggested donor-derived tumor tissues (29,31),
in the present study, we performed highly sensitive FISH using
gender chromosome-specific probes and histopathological analyses in
cases of head and neck tumors to clearly demonstrate the occurrence
of donor-derived human esophageal cancer and dysplasia, a
precancerous lesion. This is the first and definite examination to
the best of our knowledge. We conclude that BMDCs can contribute to
the constitution of epithelial tissues and further the occurrence
of carcinogenesis stimulated by chromic inflammation and
immunosuppressive conditions.
Acknowledgements
This study was supported in part by a
Grant-in-Aid for Scientific Research from the Ministry of
Education, Culture, Sports, Science and Technology (H.I., M.M.); a
Grant-in-Aid from the Third Comprehensive 10-year Strategy for
Cancer Control, Ministry of Health, Labor and Welfare (H.I., M.M.);
a grant from the Kobayashi Cancer Research Foundation (H.I.); and a
grant from the Princess Takamatsu Cancer Research Fund, Japan
(H.I.). M.K., Toshihiro Kudo, Daisuke Sakai, Taroh Satoh and H.I.
received partial support from Chugai Co. Ltd. and Yakult Honsha Co.
Ltd. through institutional endowments.
References
|
1.
|
Konno M, Hamabe A, Hasegawa O, et al:
Adipose-derived mesenchymal stem cells and regenerative medicine.
Dev Growth Differ. 55:309–318. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Puetzer JL, Petitte JN and Loboa EG:
Comparative review of growth factors for induction of
three-dimensional in vitro chondrogenesis in human mesenchymal stem
cells isolated from bone marrow and adipose tissue. Tissue Eng Part
B Rev. 16:435–444. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Varon C, Dubus P, Mazurier F, et al:
Helicobacter pylori infection recruits bone marrow-derived
cells that participate in gastric preneoplasia in mice.
Gastroenterology. 142:281–291. 2012. View Article : Google Scholar
|
|
4.
|
Sung AD and Chao NJ: Concise review: acute
graft-versus-host disease: immunobiology, prevention, and
treatment. Stem Cells Transl Med. 2:25–32. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Ohtani N and Hara E: Roles and mechanisms
of cellular senescence in regulation of tissue homeostasis. Cancer
Sci. 104:525–530. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Du K, Hu Y, Wu K and Huang H: Long-term
outcomes of antithymocyte globulin in patients with hematological
malignancies undergoing myeloablative allogeneic hematopoietic cell
transplantation: a systematic review and meta-analysis. Clin
Transplant. 27:E91–E100. 2013. View Article : Google Scholar
|
|
7.
|
Chao YH, Wu HP, Chan CK, et al: Umbilical
cord-derived mesenchymal stem cells for hematopoietic stem cell
transplantation. J Biomed Biotechnol. 2012:7595032012.PubMed/NCBI
|
|
8.
|
Bernardo ME, Cometa AM and Locatelli F:
Mesenchymal stromal cells: a novel and effective strategy for
facilitating engraftment and accelerating hematopoietic recovery
after transplantation? Bone Marrow Transplant. 47:323–329. 2012.
View Article : Google Scholar
|
|
9.
|
Majhail NS: Secondary cancers following
allogeneic haematopoietic cell transplantation in adults. Br J
Haematol. 154:301–310. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Arwert EN, Hoste E and Watt FM: Epithelial
stem cells, wound healing and cancer. Nat Rev Cancer. 12:170–180.
2012. View
Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Houghton J, Stoicov C, Nomura S, et al:
Gastric cancer originating from bone marrow-derived cells. Science.
306:1568–1571. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Jenkins RB, Qian J, Lieber MM, et al:
Detection of c-myc oncogene amplification and chromosomal anomalies
in metastatic prostatic carcinoma by fluorescence in situ
hybridization. Cancer Res. 57:524–531. 1997.PubMed/NCBI
|
|
13.
|
Krause DS, Theise ND, Collector MI, et al:
Multi-organ, multi-lineage engraftment by a single bone
marrow-derived stem cell. Cell. 105:369–377. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Rizvi AZ, Swain JR, Davies PS, et al: Bone
marrow-derived cells fuse with normal and transformed intestinal
stem cells. Proc Natl Acad Sci USA. 103:6321–6325. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Inokuma D, Abe R, Fujita Y, et al:
CTACK/CCL27 accelerates skin regeneration via accumulation of bone
marrow-derived keratinocytes. Stem Cells. 24:2810–2816. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Okumura T, Wang SS, Takaishi S, et al:
Identification of a bone marrow-derived mesenchymal progenitor cell
subset that can contribute to the gastric epithelium. Lab Invest.
89:1410–1422. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Scarlett CJ, Colvin EK, Pinese M, et al:
Recruitment and activation of pancreatic stellate cells from the
bone marrow in pancreatic cancer: a model of tumor-host
interaction. PLoS One. 6:e260882011. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Matsumoto T, Okamoto R, Yajima T, et al:
Increase of bone marrow-derived secretory lineage epithelial cells
during regeneration in the human intestine. Gastroenterology.
128:1851–1867. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Tran SD, Pillemer SR, Dutra A, et al:
Differentiation of human bone marrow-derived cells into buccal
epithelial cells in vivo: a molecular analytical study. Lancet.
361:1084–1088. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Mattsson J, Jansson M, Wernerson A, et al:
Lung epithelial cells and type II pneumocytes of donor origin after
allogeneic hematopoietic stem cell transplantation.
Transplantation. 78:154–157. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Körbling M, Katz RL, Khanna A, et al:
Hepatocytes and epithelial cells of donor origin in recipients of
peripheral-blood stem cells. N Engl J Med. 346:738–746.
2002.PubMed/NCBI
|
|
22.
|
Miura M, Miura Y, Padilla-Nash HM, et al:
Accumulated chromosomal instability in murine bone marrow
mesenchymal stem cells leads to malignant transformation. Stem
Cells. 24:1095–1103. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Li HC, Stoicov C, Rogers AB and Houghton
J: Stem cells and cancer: evidence for bone marrow stem cells in
epithelial cancers. World J Gastroenterol. 12:363–371.
2006.PubMed/NCBI
|
|
24.
|
Liu C, Chen Z, Chen Z, et al: Multiple
tumor types may originate from bone marrow-derived cells.
Neoplasia. 8:716–724. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Guest I, Ilic Z, Ma J, Grant D, et al:
Direct and indirect contribution of bone marrow-derived cells to
cancer. Int J Cancer. 126:2308–2318. 2010.PubMed/NCBI
|
|
26.
|
Cogle CR, Theise ND, Fu D, et al: Bone
marrow contributes to epithelial cancers in mice and humans as
developmental mimicry. Stem Cells. 25:1881–1887. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Avital I, Moreira AL, Klimstra DS, et al:
Donor-derived human bone marrow cells contribute to solid organ
cancers developing after bone marrow transplantation. Stem Cells.
25:2903–2909. 2007. View Article : Google Scholar
|
|
28.
|
Soldini D, Moreno E, Martin V, et al:
BM-derived cells randomly contribute to neoplastic and
non-neoplastic epithelial tissues at low rates. Bone Marrow
Transplant. 42:749–755. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Janin A, Murata H, Leboeuf C, et al:
Donor-derived oral squamous cell carcinoma after allogeneic bone
marrow transplantation. Blood. 113:1834–1840. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Hutchinson L, Stenstrom B, Chen D, et al:
Human Barrett’s adenocarcinoma of the esophagus, associated
myofibroblasts, and endothelium can arise from bone marrow-derived
cells after allogeneic stem cell transplant. Stem Cells Dev.
20:11–17. 2011.
|
|
31.
|
Munakata W, Nomoto J, Takahashi N, et al:
Carcinoma of donor origin after allogeneic peripheral blood stem
cell transplantation. Am J Surg Pathol. 36:1376–1384. 2012.
View Article : Google Scholar : PubMed/NCBI
|