Introduction
Lung cancer is the most common cancer and the most
common cause of cancer-related death, representing 12.7% of all new
cancers and causing 18.2% of total cancer deaths worldwide
(1). At diagnosis, more than 50%
of patients have distant metastases and 25% have lymph node
metastases (2). Despite numerous
therapeutic advances in lung cancer treatment (3,4), the
prognosis remains dismal. The 5-year survival rate is 16% for all
stages of lung cancer, and is only 45% even when only considering
cases with lung-localized cancer (2,3).
Histopathologically, lung cancer is classified into small cell lung
cancer (SCLC) and non-small cell lung cancer (NSCLC), the latter
including adenocarcinoma (AD), squamous cell carcinoma (SCC) and
large cell carcinoma. AD is the most common histological subtype of
NSCLC (5). Molecular-targeted
therapies for AD have been introduced in clinical settings,
including epidermal growth factor receptor (EGFR)-tyrosine kinase
inhibitors (TKIs) and crizotinib, which is an oral TKI that targets
the oncogenic fusion product comprising echinoderm
microtubule-associated protein-like 4 (EML4) and anaplastic
lymphoma kinase (ALK) (4,6,7).
However, acquired resistances against these molecular-targeting
therapies are inevitable (8–10);
therefore, new treatment strategies are needed to improve the
prognosis of lung cancer patients.
Nestin is a class VI intermediate filament with a
short N-terminal extremity, which forms a heterodimer (11,12).
It is also known as a neuronal stem/progenitor cell marker
(13). High nestin expression
levels are observed in developing fetal tissues (14), as well as in various malignant
tumors, including glioblastoma (15), malignant melanoma (16), and pancreatic (17,18)
and prostate (19) cancers.
Previous studies of these malignant tumors have shown that nestin
expression correlates to worse prognosis, and that nestin
inhibition suppresses tumor cell migration, invasion, metastasis
and stemness (20). Nestin
expression has been also reported in lung cancers, with high nestin
expression reportedly correlated with poor prognosis (21–23),
lymphovascular invasion (21,24),
and stemness (25) in surgically
treated NSCLC cases. Nestin inhibition also reportedly reduces cell
growth and invasion in SCLC (26).
However, the detailed roles and molecular regulatory mechanisms of
nestin expression in lung cancers are not well understood.
In the present study, we determine the expression
patterns and roles of nestin in NSCLC. Our findings show that
nestin down-regulation induces anticancer effects, and that the
Akt/SRY-box containing protein 2 (Sox2) signaling pathway regulates
nestin expression in AD. These findings will enable us to develop
the therapeutic strategies that target nestin in lung cancers.
Materials and methods
Materials
Materials purchased: mouse monoclonal anti-nestin
antibody and recombinant human epidermal growth factor (EGF) from
R&D Systems, Inc. (Minneapolis, MN); Histofine Simple Stain MAX
PO (M) and (R) kits from Nichirei (Tokyo, Japan); High Capacity
cDNA Reverse Transcription Kit, TaqMan Fast Universal PCR master
mix, and TaqMan Gene Expression Assays for nestin (Hs00707120_s1)
and 18S rRNA (Hs99999901_s1), pre-designed siRNAs targeting human
nestin (s21143: siRNA-A and s21141: siRNA-B), negative control
siRNA (silencer select negative control no. 2: siControl), TaqMan
Fast Cells-to-CT kit, Alexa 488-labeled donkey anti-mouse IgG
antibody, Qubit Fluorometer Protein Assay Kit, and Geneticin from
Life Technologies (Carlsbad, CA); TransIT-si-QUEST transfection
reagent from Mirus Bio LLC (Madison, WI); pBAsi-hU6 Neo DNA vector
and FastPure RNA Kit from Takara Bio, Inc. (Shiga, Japan);
pAcGFP1-N1 from Clontech Laboratories (Mountain View, CA); FuGene
HD transfection reagent from Promega Corporation (Madison, WI);
Vectashield H-1200 containing 4′,6-diamidino-2-phenylindole-2HCl
(DAPI) from Vector Laboratories, Inc. (Burlingame, CA); WST-8 cell
counting kit from Wako Pure Chemical Industries (Osaka, Japan);
BioCoat Matrigel invasion chamber from BD Biosciences (Franklin
Lakes, NJ); Diff-Quick staining kit from Sysmex International
Reagents Co., Ltd. (Hyogo, Japan); recombinant human basic
fibroblast growth factor (bFGF) from ReproCell, Inc. (Kanagawa,
Japan); ultra-low attachment surface 24-well plate from Corning
(Corning, NY); Akt inhibitor IV (no. 124011; CAS no. 681281-88-9),
SB203580 (no. 559389; CAS no. 152121-47-6), and U0126 (no. 662005;
CAS no. 109511-58-2), and rabbit polyclonal anti-Sox2 antibody from
Merck KGaA (Darmstadt, Germany); Super Signal West Dura Extended
Duration chemiluminescent substrates from Thermo Scientific
(Waltham, MA); mouse monoclonal anti-glyceraldehyde 3-phosphate
dehydrogenase (GAPDH) antibody and HRP-labeled donkey anti-goat IgG
antibody from Santa Cruz Biotechnology, Inc. (Dallas, TX);
HRP-labeled goat anti-mouse IgG antibody from American Qualex
International, Inc. (San Clemente, CA); and mouse monoclonal
anti-Akt (pan) antibody and rabbit monoclonal anti-phosphorylated
Akt antibody from Cell Signaling Technology, Inc. (Danvers, MA).
SCADS inhibitor kit III was provided from the Screening Committee
of Anticancer Drugs. All other chemicals and reagents were
purchased from Sigma-Aldrich Corporation (St. Louis, MO).
Patients and tissue specimens
We retrospectively studied 95 patients who were
diagnosed with NSCLC and who had undergone surgery between 1994 and
2001 at Nippon Medical School Hospital. None of the included
surgical patients had received chemotherapy or radiation therapy
before surgery. All cases were classified according to the UICC 7th
TNM staging (27).
Paraffin-embedded specimens were prepared for immunohistochemical
analysis. The genetic profiles of driver oncogenes such as EGFR
mutations were not examined, because all cases had been treated
before the approval of EGFR-TKIs. The lung tissues from lung cancer
patients were used only for immunohistochemical analysis.
Immunohistochemical staining of the lung cancer tissue samples was
carried out in accordance with the principles embodied in the
Declaration of Helsinki, 2008. All included patients provided
written informed consent for the use of their tissue specimens for
medical research.
Immunohistochemistry
Paraffin-embedded tissue sections (3 μm) were
subjected to immunostaining using Histofine Simple Stain MAX PO (M)
or (R) kits, as previously reported (28,29).
After deparaffinization, antigen retrieval for phosphorylated Akt
antibody was performed at 121°C for 15 min in a sodium citrate
buffer solution (pH 6.0). For all antibodies, endogenous peroxidase
activity was blocked by incubating sections for 30 min with 0.3%
hydrogen peroxide in methanol. Sections were then incubated with
monoclonal anti-nestin antibody (1:200 dilution), monoclonal
anti-Akt (pan) antibody (1:100 dilution), monoclonal
anti-phosphorylated Akt (Ser 473) antibody (1:50 dilution), or
polyclonal anti-Sox2 antibody (1:100 dilution). Bound antibodies
were detected with Simple Stain MAX PO (M) or (R) reagent, using
diaminobenzidinetetrahydrochloride chromogen as the substrate.
Negative control studies were performed by omitting the primary
antibodies. In a blinded manner, two investigators (K.N. and Y.M.)
separately evaluated each specimen at x200 magnification. A sample
was considered positive if staining was present in the cytoplasm of
more than 30% of the tumor cells, regardless of staining intensity
(29).
Lung adenocarcinoma cell lines
The cell lines A549 and RERF-LC-KJ were obtained
from RIKEN BioResource Center (Ibaragi, Japan). PC-3 [the different
cell line from the American Type Culture Collection (ATCC;
Manassas, VA) CRL-1435] was obtained from the Health Science
Research Resources Bank (Osaka, Japan). The PC-9, PC-14, and LC2/ad
cell lines were obtained from Immuno-Biological Laboratories Co.,
Ltd. (Gunma, Japan). NCI-H1975 (no. CRL-5908, lot no. 3546434) and
HCC827 (no. CRL-2868, lot no. 59389082) were obtained from ATCC.
The cells were grown at 37°C in a humidified 5% CO2
atmosphere. The cancer cells were cultured in RPMI-1640 medium
containing 15% fetal bovine serum (FBS), except for H1975, which
was grown in RPMI-1640 containing 10% FBS.
Quantitative reverse
transcription-polymerase chain reaction (qRT-PCR)
A total of 2.5×105 cells were seeded in
60-mm dishes and incubated for 48 h. Total RNA was extracted from
cells with the FastPure RNA Kit, and 1 μg was used for
reverse transcription with the High Capacity cDNA Reverse
Transcription kit, following the manufacturer’s protocol. We
performed qRT-PCR for nestin and 18S rRNA (as an internal standard)
with the StepOnePlus Real-Time PCR system (Life Technologies) using
specific primers and a TaqMan probe, as previously reported
(30). Cycling conditions were as
follows: 20 sec at 95°C, and then 40 cycles of 1 sec at 95°C and 20
sec at 60°C. qRT-PCR results are expressed as the ratio of target
mRNA to 18S rRNA. Gene expression levels were measured in
triplicate.
Construction of expression vector for
nestin short hairpin RNA (nestin shRNA)
To construct expression vectors for human nestin
shRNA, we synthesized a DNA fragment that was flanked by the BamHI
and HindIII sites and contained the sense target sequence for
nestin (NM_006617; 5′-GAA-CAG-GAT-CAG-ATG-ACA-T-3′), the hairpin
loop sequence (5′-TAG-TGC-TCC-TGG-TTG-3′), and the corresponding
antisense sequence. This fragment was inserted into the pBAsi-hU6
Neo DNA vector (18). The
scrambled sequence (5′-TCT-TAA-TCG-CGT-ATA-AGG-C-3′) was used as a
negative control. Transfections of the nestin shRNA expression
vector (Sh) and scrambled sequence-expressing vector (Sc) were
performed using FuGene HD transfection reagent, according to the
manufacturer’s instructions. Briefly, H1975 cells (1×105
cells/well) were plated in 6-well plates and grown in 2 ml of
RPMI-1640 medium with 10% FBS. The vectors (5 μg) were
transfected into cells using 5 μl of FuGene HD, and the
cells were passaged and cultured with 1,000 μg/ml of
geneticin. Cell lysates were collected, and the decreased nestin
mRNA was confirmed using qRT-PCR.
Construction of nestin expression
vector
The full-length nestin cDNA fragment was ligated to
the 3′ end of the human cytomegalovirus early promoter/enhancer in
the eukaryotic expression vector pAcGFP1-N1 (18). Proper orientation of the insert was
verified by DNA sequencing. Transfections of the nestin expression
vector (Nes) and empty vector (EV) into PC-3 cells were performed
using FuGene HD transfection reagent, with the above-described
protocol for Sh and Sc vectors. To produce stably-transfected PC-3
cells, the cells were passaged and cultured with 1,000 μg/ml
of geneticin. Cell lysates were collected, and the increased nestin
mRNA levels were confirmed using qRT-PCR.
Transfection with nestin-targeting
small-interfering RNA (nestin siRNA)
Cells were seeded on 35-mm dishes, and then
transfected twice with the nestin siRNAs in serum-free RPMI-1640
medium using TransIT-si-QUEST transfection reagent (30). Briefly, each siRNA stock solution
was mixed with TransIT-si-QUEST (2.0 μl/well) in serum-free
medium, and incubated at room temperature for 15 min. This
siRNA-lipid complex was then added to cultured cells, which were
incubated for 24 h at 37°C. Next, the medium was replaced with
fresh medium containing FBS, siRNA was administered a second time,
and the treated cells were cultured for 48 h before being collected
for analyses. Prior to this experiment, we transfected cells with
the siRNAs once or twice at concentrations ranging from 5–20 nM,
and used qRT-PCR to determine that the appropriate siRNA
concentration was 10 nM.
Immunofluorescent staining and confocal
laser microscopy
The same anti-nestin antibody used for the
immunohistochemistry was employed for immunofluorescent staining.
H1975- and PC-3-derived cells (1×105 cells) were plated
in 35-mm glass-bottomed dishes and incubated for 72 h at 37°C in a
humidified 5% CO2 atmosphere. Cells were fixed with 4%
paraformaldehyde for 15 min. The fixed cells were treated with 50
mM glycine for 5 min, 0.1% Triton X-100 for 5 min, and 10% donkey
serum for 30 min at room temperature. The cells were then incubated
with the anti-nestin antibody (1:50 dilution) in phosphate-buffered
saline containing 1% bovine serum albumin overnight at 4°C. Next,
the cells were washed with phosphate-buffered saline, and incubated
with Alexa 488-conjugated goat anti-mouse IgG for 1 h. The cells
were mounted with Vectashield mounting medium containing DAPI.
Fluorescent images were acquired using a confocal laser scanning
microscope Digital Eclipse TE 2000-E (Nikon Insteck Co., Ltd.,
Tokyo, Japan), and were analyzed using the confocal microscope
Digital Eclipse C1 control software EZ-C1 version 2.30 (Nikon
Insteck Co., Ltd.).
Cell proliferation assay
The growth rates of lung AD cells were analyzed
using a non-radioactive cell proliferation assay (31). The H1975- and PC-3-derived cells
were seeded at a density of 5×103 in 96-well plates in
RPMI-1640 medium with 10 and 15% FBS, respectively. The cells were
incubated at 37°C in a humidified 5% CO2 atmosphere for
3 days for shRNA- or vector-transfected cells, and 5 days for
inhibitor-treated cells. Then, the cells were incubated with WST-8
cell counting reagent for 4 h at 37°C and the optical density of
the culture solution in the plate was measured at 450 nm using an
ELISA plate reader (Bio-Rad, Hercules, CA). Experiments were
performed in triplicate.
Time-lapse analysis
To confirm the effects of nestin on cell migration,
we performed a time-lapse assay of single cell movement. Briefly,
1,000 cells/well were seeded onto 4-well glass-bottom dishes and
grown for 48 h. Cell movement was then monitored for 24 h using a
Biostation IM (Nikon Insteck, Co., Ltd.), which photographed the
cells every 5 min. The total distances covered by individual cells
within 24 h were determined using MetaMorph software 7.6 (Universal
Image Corp., Ltd., Buckinghamshire, UK), as previously reported
(32). Images were taken from four
areas of each well at x200 magnification, and the movement of all
cells in each field was analyzed. Approximately 50–100 cells in
each well were analyzed for single-cell movement.
Cell invasion assays
To assess the effect of nestin on H1975 cell
invasion through Matrigel, we used a modified Boyden chamber
technique as previously reported (18). Briefly, cells were suspended in 500
μl serum-free medium, and 1.0×105 cells were
placed in the upper component of Matrigel-coated inserts. The lower
compartment was filled with 750 μl medium containing 10%
FBS, and the cells were incubated at 37°C in a humidified 5%
CO2 atmosphere. After 8 h, the cells on the bottom
surface of the filter were fixed and stained with a Diff-Quick
staining kit, and were counted under a light microscope. PC-3 cells
were not appropriate for this study due to their extremely low
motility.
Sphere-forming assays
To determine whether alteration of nestin expression
levels affected the cancer stem cell (CSC)-like characteristics of
AD cells, we performed sphere formation assays (16). PC-3 cells (1.0×104
cells/well) were plated in 24-well ultra-low attachment plates with
serum-free medium supplemented with bFGF (10 ng/ml) and EGF (20
ng/ml). After 7 days, the number of spheres in each well was
counted using a phase-contrast microscope (Eclipse TE2000-U; Nikon
Insteck, Co., Ltd.). Experiments were performed in triplicate.
Screening for nestin-related signaling
pathways using an inhibitor kit
We screened for molecules that regulated nestin
expression using the SCADS inhibitor kit III containing a total of
95 compounds in a 96-well plate. PC-3 cells (1.0×103
cells/well) were seeded in a 96-well plate on day 1, and each
inhibitor was added at 5 μM on day 3. On day 5, we examined
the altered levels of nestin mRNA by performing direct qRT-PCR
analysis of nestin using the TaqMan Fast Cells-to-CT kit.
Western blot analysis
Protein was extracted from cells using 2 M thiourea
buffer (33), and the lysates were
centrifuged twice for 10 min at 3,000 rpm and once for 30 min at
15,000 rpm to pellet cell debris. The resulting supernatants were
collected, and the protein concentration was measured using a Qubit
fluorometer (Life Technologies). Lysates were subjected to sodium
dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) using
reducing conditions for each antibody. Immunoblotting was performed
using mouse anti-nestin antibody (1:8,000 dilution), mouse anti-Akt
(pan) antibody (1:2,000 dilution), rabbit anti-phosphorylated Akt
(1:2,000 dilution), and rabbit anti-Sox2 antibody (1:1,000
dilution). To assess lane loading, the membranes were also blotted
with mouse anti-GAPDH antibody and mouse anti-β-actin antibody.
Statistical analysis
All quantitative data are presented as mean ±
standard deviation (SD), and were assessed using Student’s t-test
and the Tukey-Kramer test. The χ2 test and Fisher’s
exact test were used to analyze the correlations between nestin
expression and clinicopathological features. Cumulative survival
rates were calculated by the Kaplan-Meier method. The significance
of differences in survival rate was analyzed by the log-rank test,
with p<0.05 considered significant in all analyses. Computations
were performed using the StatView J version 4.5 software package
(SAS Institute, Inc., Cary, NC).
Results
Immunohistochemical analysis of nestin in
NSCLC specimens
Immunohistochemical analysis was performed to
examine nestin expression in surgically resected NSCLC tissues.
Positive nestin protein expression was detected in the cytoplasm of
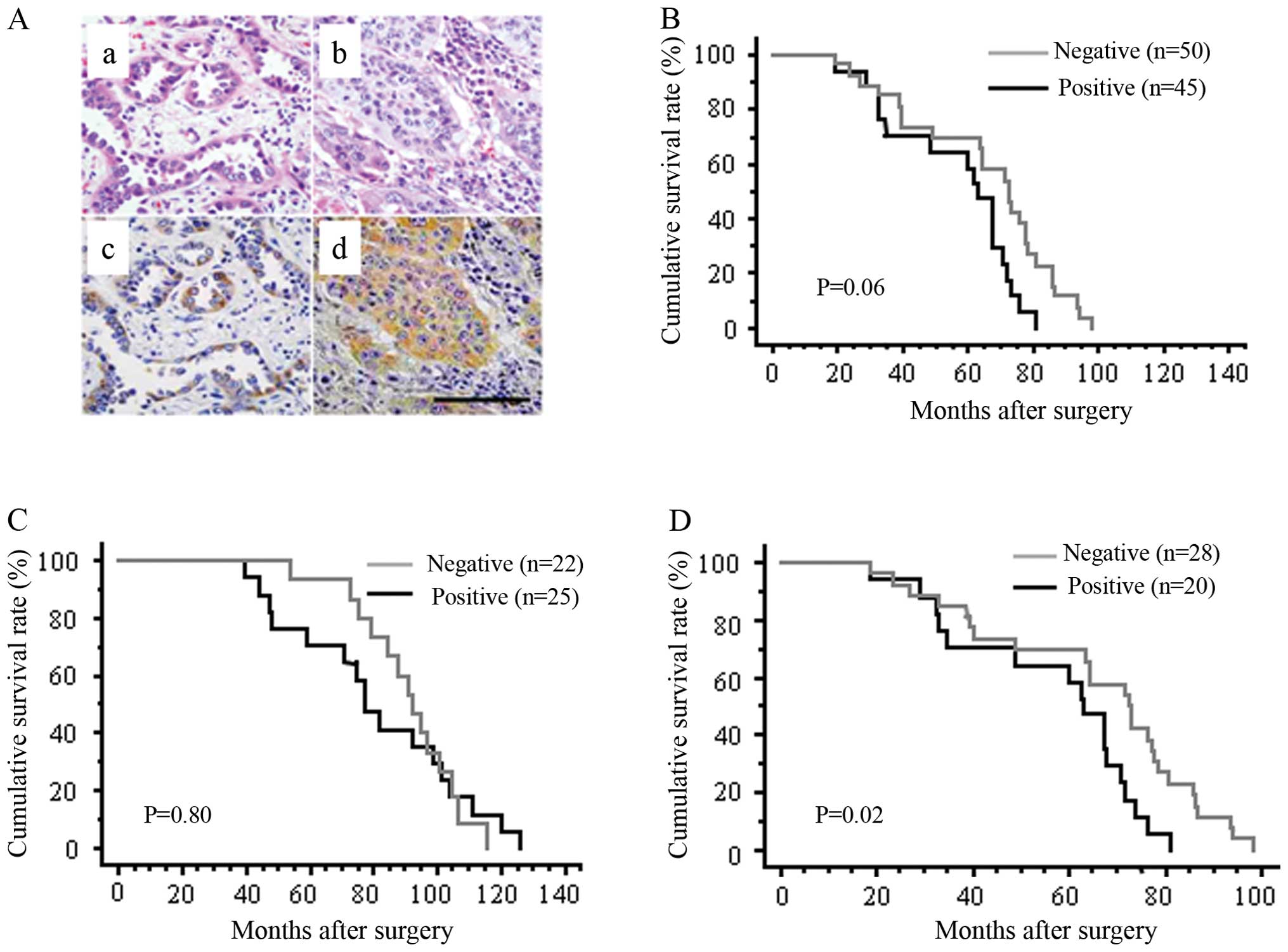
20 of 48 AD cases (41.7%) and 25 of 47 SCC cases (53.2%) (Fig. 1A, Table I). Nestin immunore-activity in
NSCLC correlated with tumor size and lymph node metastasis (p=
0.02, Table I). The
nestin-positive and nestin-negative groups did not significantly
differ in any other factors, including age, gender, tumor
differentiation, pathological staging, lymphovascular invasion,
vascular invasion, intrapulmonary metastasis and pleural invasion.
To explore the therapeutic potential of targeting nestin,
postoperative survival was evaluated, although the number of
patients was small. Kaplan-Meier analysis showed no statistical
differences in overall survival rates between nestin-positive and
nestin-negative groups among all NSCLC (Fig. 1B) or within the SCC subpopulation
(Fig. 1C). However, within the AD
subpopulation, the overall survival rates significantly differed
between nestin-positive and nestin-negative groups (p= 0.02;
Fig. 1D), indicating that nestin
expression might correlate with poorer survival in AD.
 | Table I.Immunohistochemistry of nestin in
surgically treated non-small cell lung cancer cases (n=95). |
Table I.
Immunohistochemistry of nestin in
surgically treated non-small cell lung cancer cases (n=95).
| Nestin expression
| Total | p-value |
|---|
| Positive
(n=45) | Negative
(n=50) |
|---|
| Age, years | | | | 0.21 |
| <65 | 15 | 23 | 38 | |
| ≥65 | 30 | 27 | 57 | |
| Gender | | | | 0.61 |
| Men | 37 | 39 | 76 | |
| Women | 8 | 11 | 19 | |
| Histological
type | | | | 0.26 |
|
Adenocarcinoma | 20 | 28 | 48 | |
| Squamous cell
carcinoma | 25 | 22 | 47 | |
| Tumor
differentiation | | | | 0.22 |
| Well or
moderate | 28 | 37 | 65 | |
| Poor | 17 | 13 | 30 | |
| Pathological TNM
staging | | | | 0.02a |
| Tis, 1 | 10 | 23 | 33 | |
| T, >1 | 35 | 27 | 62 | |
| N0 | 31 | 44 | 75 | 0.02a |
| N, >0 | 14 | 6 | 20 | |
| Pathological
staging | | | | 0.22 |
| 0, I | 30 | 39 | 69 | |
| II, III | 15 | 11 | 26 | |
| Lymph duct
invasion | | | | 0.06 |
| Positive | 22 | 15 | 37 | |
| Negative | 23 | 35 | 58 | |
| Vascular
invasion | | | | 0.49 |
| Positive | 23 | 22 | 45 | |
| Negative | 22 | 28 | 50 | |
| Intrapulmonary
metastasis | | | | 0.34 |
| Positive | 0 | 1 | 1 | |
| Negative | 45 | 49 | 94 | |
| Pleural
invasion | | | | 0.20 |
| Positive | 22 | 18 | 40 | |
| Negative | 23 | 32 | 55 | |
Nestin expression in AD cell lines and
shRNA transfection
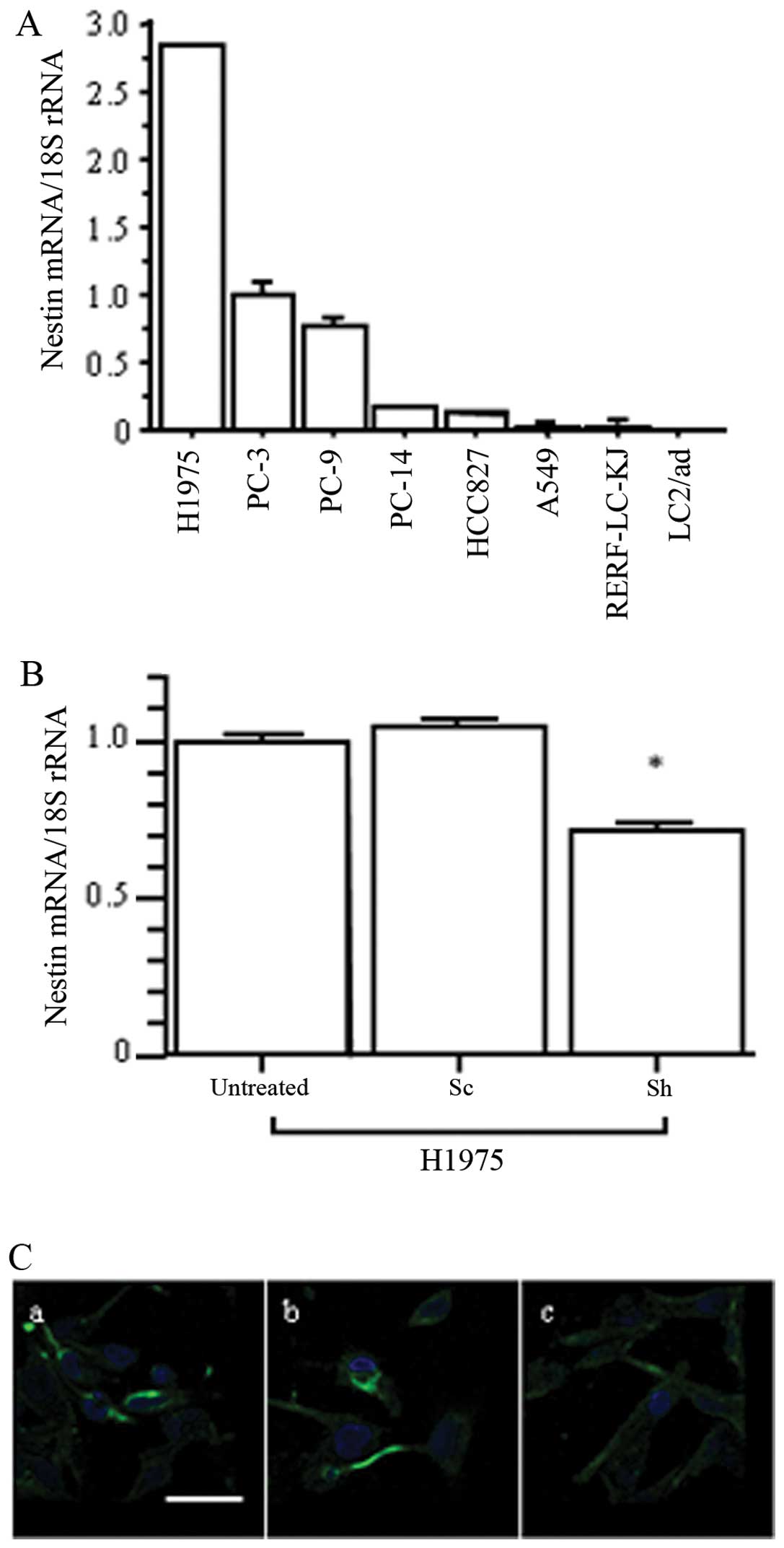
We next confirmed nestin expression levels in human
lung AD cell lines by qRT-PCR (Fig.
2A). H1975 cells showed the highest levels of nestin mRNA, and
PC-3 expressed moderate nestin levels. To examine the effects of
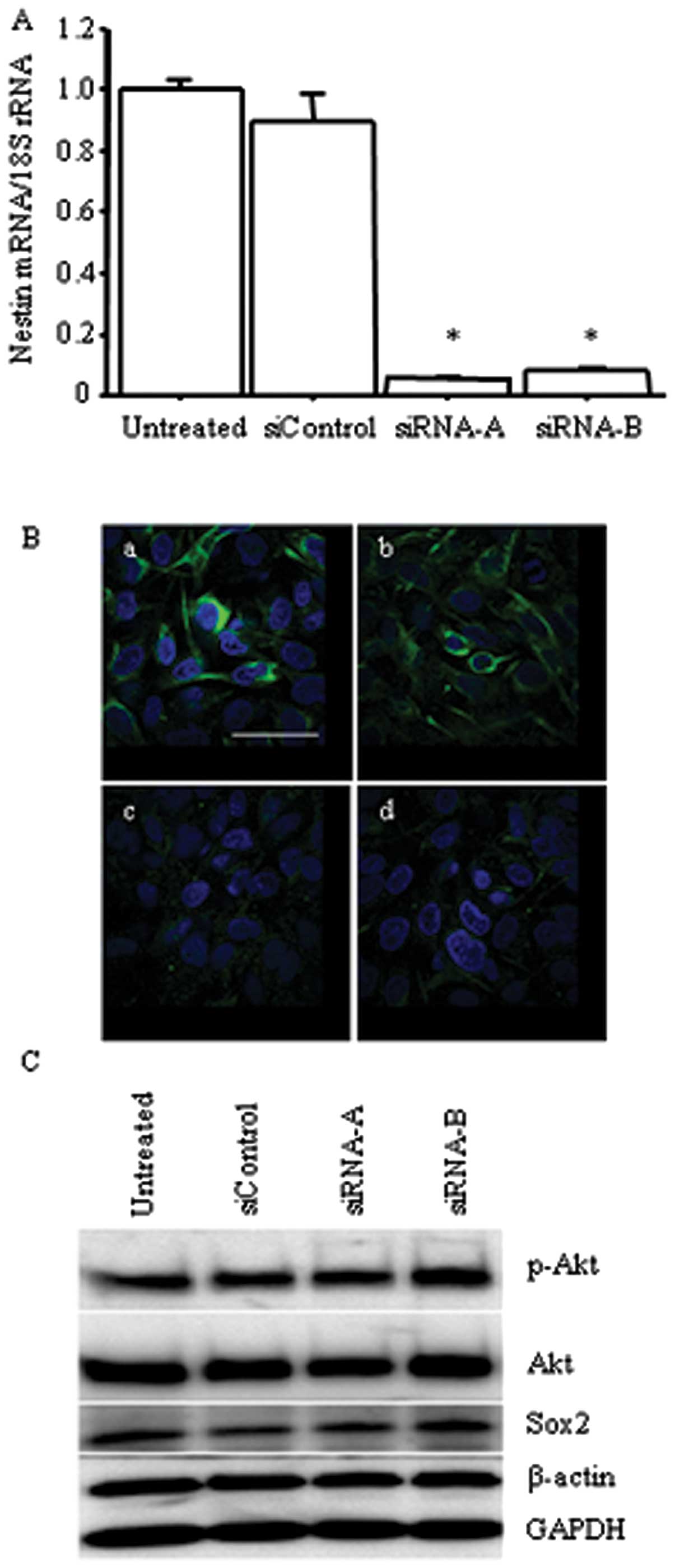
nestin inhibition in AD cells, we established nestin
shRNA-transfected H1975 (Sh) cells. qRT-PCR showed that nestin mRNA
expression was significantly decreased in Sh cells compared to in
untreated and scrambled sequence-expressing vector-transfected (Sc)
cells (p<0.05; Fig. 2B), but
the Sh cells exhibited no characteristic morphological changes.
Immunofluorescent staining confirmed decreased levels of nestin
protein expression in Sh cells compared to untreated and Sc cells
(Fig. 2C). Thus, H1975 cells were
used in the following experiments to examine the inhibitory effects
of nestin on AD cell behavior.
Cell growth, migration, invasion and
sphere formation assays in nestin shRNA-transfected H1975
cells
In order to clarify the effects of nestin
downregulation on cell growth, migration, invasion and stemness of
lung adenocarcinoma cells, we used H1975 untreated, Sc and Sh
cells. Compared to untreated and Sc cells, Sh cells exhibited
significantly decreased cell proliferation (p<0.05; Fig. 3A) and cell migration in the
time-lapse assay (p<0.05; Fig.
3B). The Boyden chamber assay showed that Sh cell invasion
through Matrigel was markedly inhibited (p<0.05 vs. untreated
and Sc; Fig. 3C). We also analyzed
sphere formation of H1975 to determine CSC properties. In the
ultra-low attachment plates, Sh cells exhibited remarkably
decreased sphere size and number compared with in untreated and
Sc-transfected H1975 cells (p<0.05; Fig. 3D). Overall, our results showed that
inhibition of nestin in H1975 cells resulted in reduced cell
proliferation, migration, invasion and sphere-forming ability.
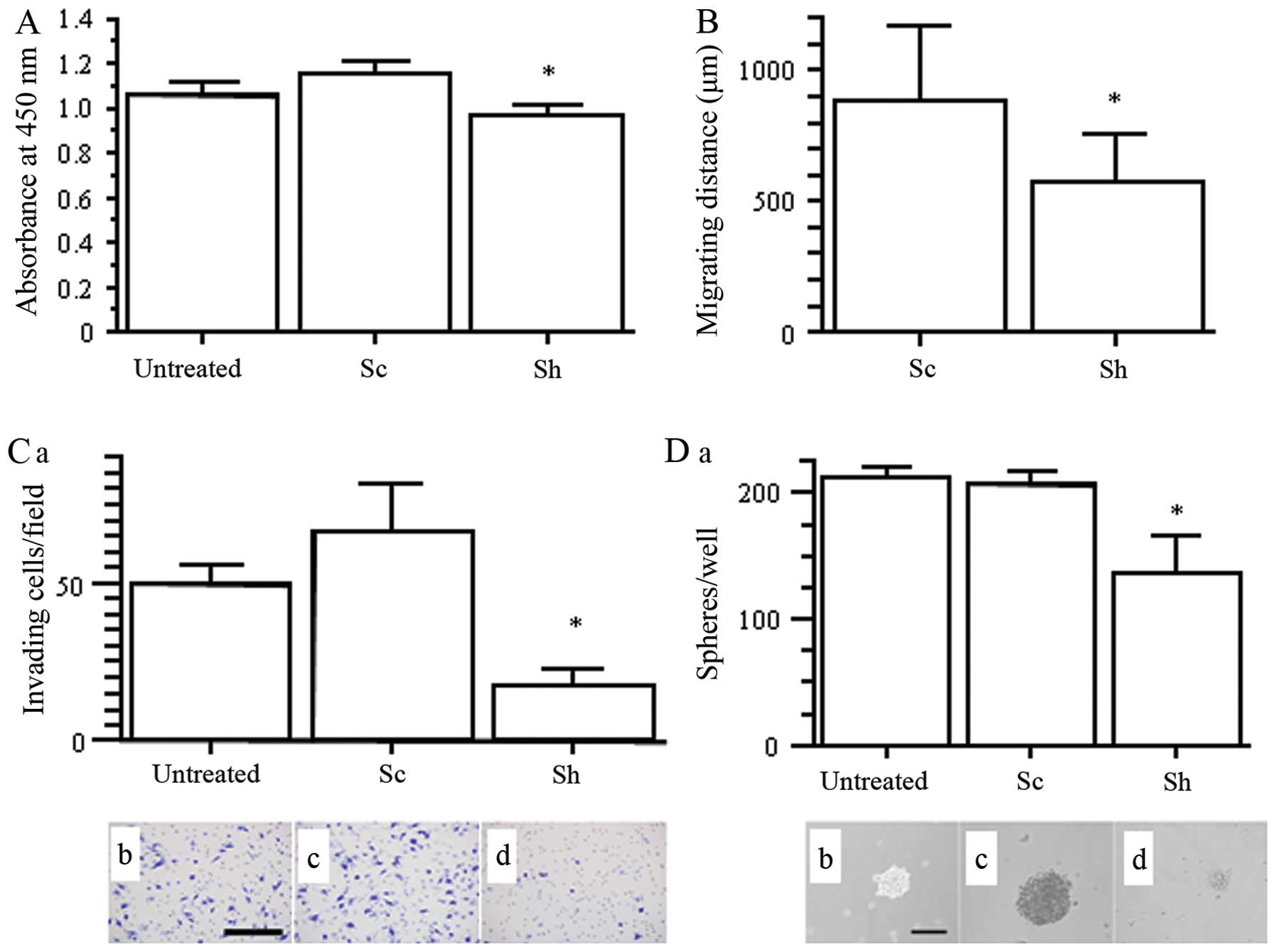 | Figure 3.Effects of nestin shRNA on cancer
cell growth, migration, invasion, and sphere formation in H1975
cells. (A) WST-8 cell growth assay. *p<0.05 vs.
untreated and Sc cells. (B) Migration distance over 24 h determined
by time-lapse analysis. *p<0.05 vs. Sc cell. (C) Cell
invasion using Matrigel-coated Boyden chamber assay. (a) The number
of invading cells per field. *p<0.05 vs. untreated
and Sc cells. (b, c and d) Diff-Quick staining for untreated, Sc
and Sh cells, respectively. Scale bar, 100 μm. (D) (a) The
number of spheres per well. *p<0.05 vs. untreated and
Sc cells. (b, c and d) Phase contrast images of the spheres in
untreated, Sc and Sh cells, respectively. Scale bar, 100
μm. |
Cell growth, migration and sphere
formation assays in nestin gene-transfected PC-3 cells
We also established nestin expression
vector-transfected PC-3 (PC-Nes) cells to examine the effects of
upregulation of nestin on AD cell behavior. Transfection with
PC-Nes significantly enhanced nestin expression at both the mRNA
and protein levels compared to the untreated and empty
vector-transfected PC-3 (EV) cells (p<0.05; Fig. 4A and B). Stable transfection of the
nestin gene resulted in increased PC-3 cell proliferation and
migration (p<0.05; Fig. 4C and
D). PC-Nes cells also showed higher sphere formation capability
regarding the number of spheres (p<0.05 vs. untreated and EV;
Fig. 4E). Overall, increased
nestin expression in PC-3 cells resulted in the opposite effects on
cell behavior, when compared to the effects of decreased nestin
expression in H1975 cells.
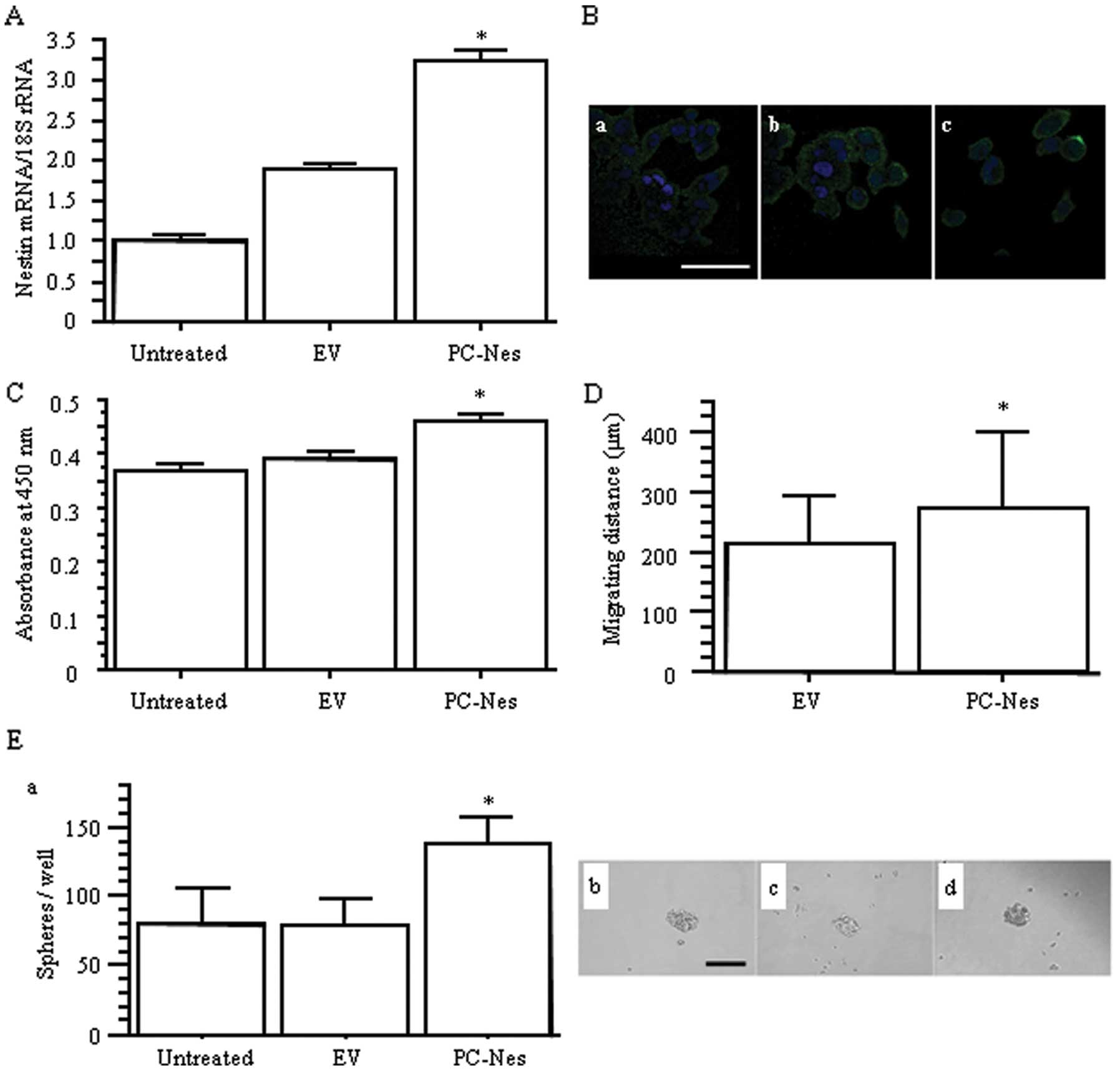 | Figure 4.The effects of nestin gene
transfection on proliferation, migration, and sphere formation in
PC-3 cells. (A) Relative nestin mRNA expression levels in nestin
expression vector (PC-Nes)- and empty vector (EV)-transfected
cells. *p<0.05 vs. untreated and EV cells. (B)
Immunofluorescent staining for nestin in (a) untreated, (b) EV and
(c) PC-Nes cells, respectively. Green, nestin; blue, DAPI. Scale
bar, 50 μm. (C) WST-8 cell growth assay.
*p<0.05 vs. untreated and EV cells. (D) Migration
distance over 24 h determined by time-lapse analysis.
*p<0.05 vs. EV cells. (E) (a) The number of spheres
per well. *p<0.05 vs. untreated and EV cells. (b, c
and d) Phase contrast images of spheres in (b) untreated, (c) EV
and (d) PC-NES cells. Scale bar, 100 μm. |
Screening analysis using chemical
compounds to clarify the candidate molecules of signaling pathway
that regulate nestin expression in lung adenocarcinoma cells
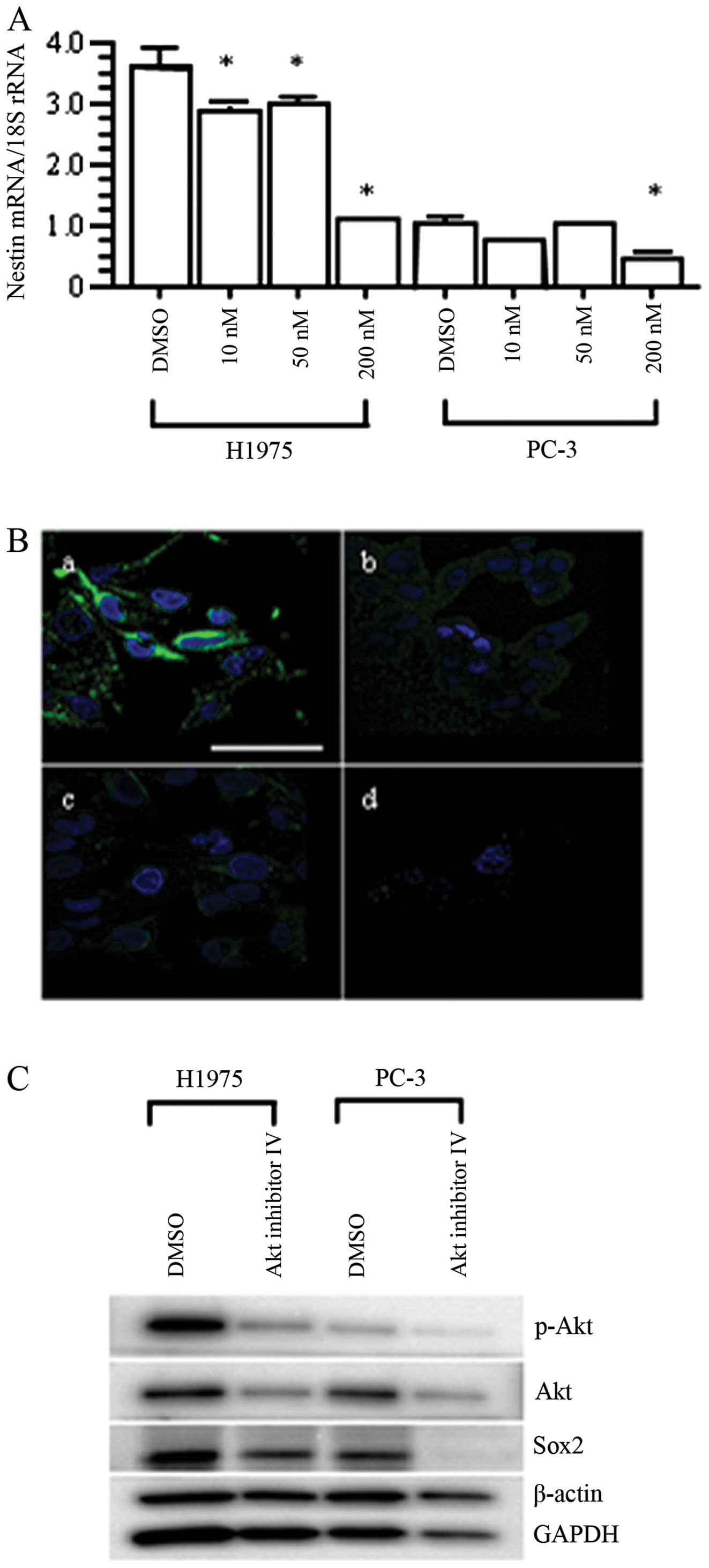
Next, we searched for upstream molecules of nestin
using the SCADS Inhibitor Kit III, which included 95 chemical
compounds that each inhibited a specific molecular target (34). In PC-3 cells, several compounds
suppressed nestin mRNA expression, including cyclin-dependent
kinase (CDK) inhibitors, as expected from previous reports
(35,36). Akt inhibitor IV led to a 72%
reduction of nestin expression compared to in the DMSO-treated
negative control. Akt is located downstream of the EGFR pathway
(37,38), and Akt regulates Sox2 expression
and the self-renewal of CSC-like cells in NSCLC (39). Sox2 is also known to be a
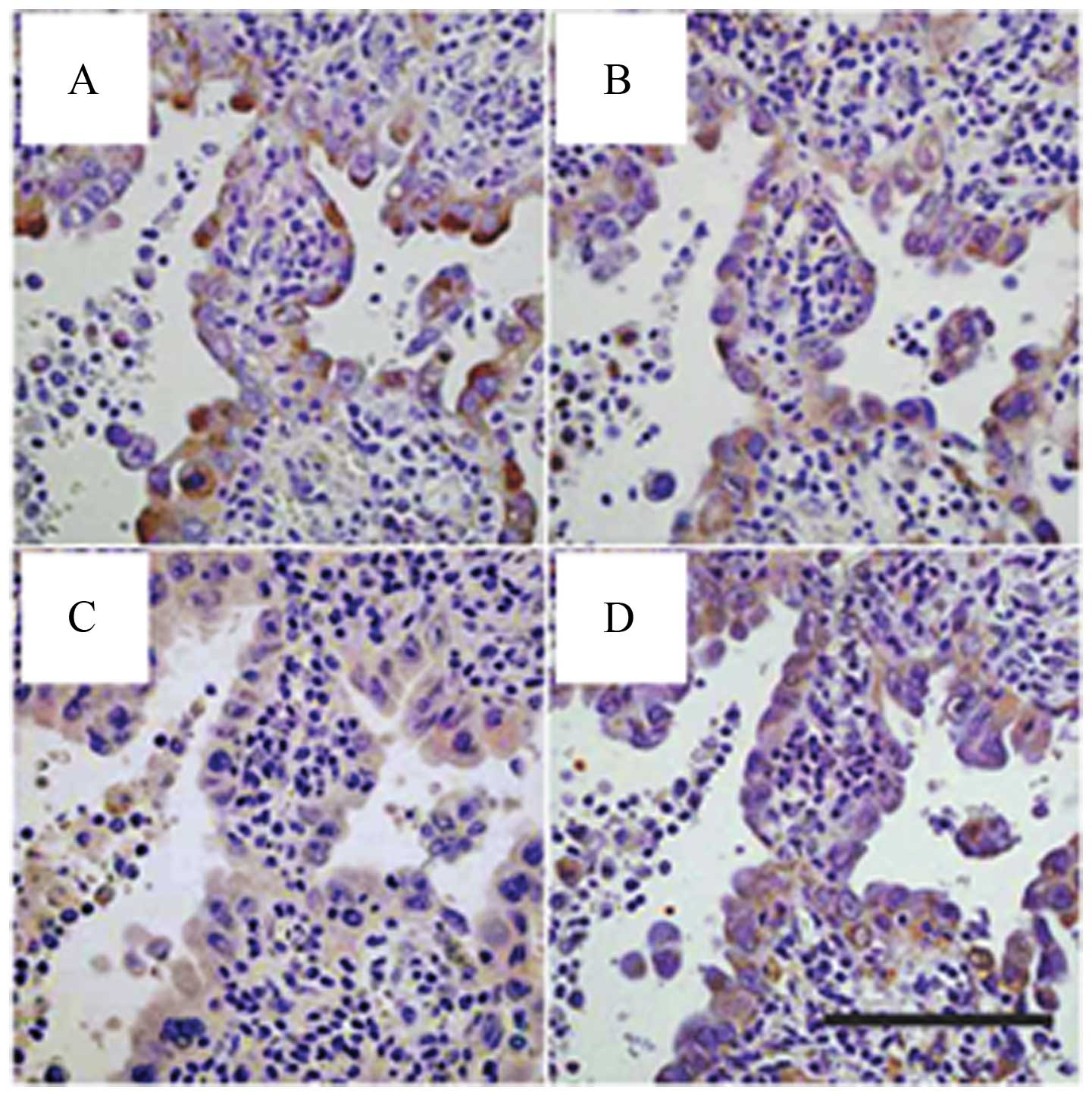
transcriptional factor for nestin (40). We confirmed co-expression of
nestin, Akt, phosphorylated Akt (p-Akt), and Sox2 in serial tissue
sections from AD cases (Fig. 5).
Thus, we focused further experiments on Akt inhibitor IV to clarify
the relationship between Akt, Sox2 and nestin in AD cells.
Nestin expression is regulated via
Akt/Sox2 signaling
Concordant with the results of screening analysis of
nestin regulatory pathway using PC-3 cells with chemical compounds,
qRT-PCR analysis and immunofluorescent staining confirmed the
effective inhibition of nestin mRNA and protein expression by Akt
inhibitor IV in H1975 and PC-3 cells (p<0.05 vs. DMSO-treated
cells; Fig. 6A and B). Then we
investigated changes in the expression of signaling pathway
molecules, including p-Akt, Akt and Sox2. Akt inhibitor IV
decreased the expression levels of Akt, p-Akt, and Sox2 in both
H1975 and PC-3 cells (Fig. 6C). On
the other hand, transfection of nestin-targeting siRNAs (siRNA-A
and siRNA-B) into H1975 cells resulted in dramatically decreased
expression levels of nestin mRNA and protein compared with the
untreated and negative control siRNA (siControl)-transfected H1975
cells (p<0.05; Fig. 7A and B).
In contrast, nestin siRNAs did not decrease the expression levels
of p-Akt, Akt or Sox2 (Fig. 7C).
These results indicate that Akt is located upstream of nestin, and
that Akt/Sox2 signaling regulates nestin expression.
Cell growth, migration and invasion
assays under Akt inhibitor IV administration
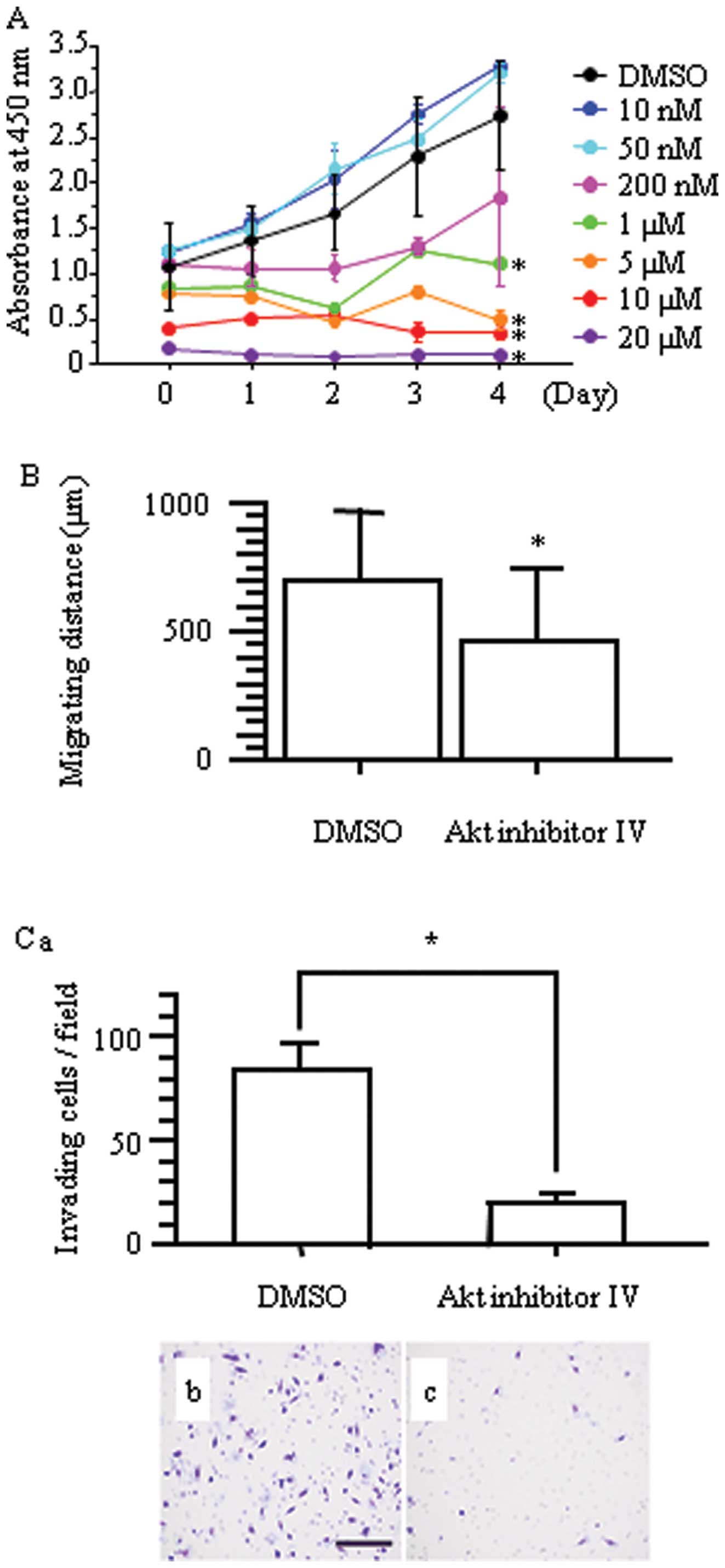
Concordant with the result of nestin downregulation,
Akt inhibitor IV inhibited cell proliferation of H1975 cells
(*p<0.05 vs. DMSO-treated cells; Fig. 8A). Inhibition of Akt significantly
inhibited the migration of H1975 cells compared with DMSO-treated
cells (p<0.05; Fig. 8B), and
Boyden chamber assays confirmed that Akt inhibitor IV decreased
invasion of H1975 cells (Fig.
8C).
Inhibitory effects of Akt inhibitor IV on
nestin expression in nestin gene-transfected PC-3
Comparative studies showed that among the three
tested cell signaling inhibitors, Akt inhibitor IV, p38 inhibitor
(SB203580), and MEK 1/2 inhibitor (U0126), only Akt inhibitor IV
significantly inhibited nestin expression in H1975 and PC-3 cells
(p<0.05 vs. untreated and DMSO-treated groups; Fig. 9A and B). Next, we evaluated the
effects of these inhibitors on nestin gene-transfected PC-3 cells
(Fig. 9C). Stably nestin
gene-transfected PC-Nes cells showed significantly increased nestin
expression compared with the untreated and EV cells
(*p<0.05; Fig. 9C).
In PC-Nes cells, only Akt inhibition resulted in nestin
downregulation (§p<0.05 vs. PC-Nes+/−DMSO; Fig. 9C).
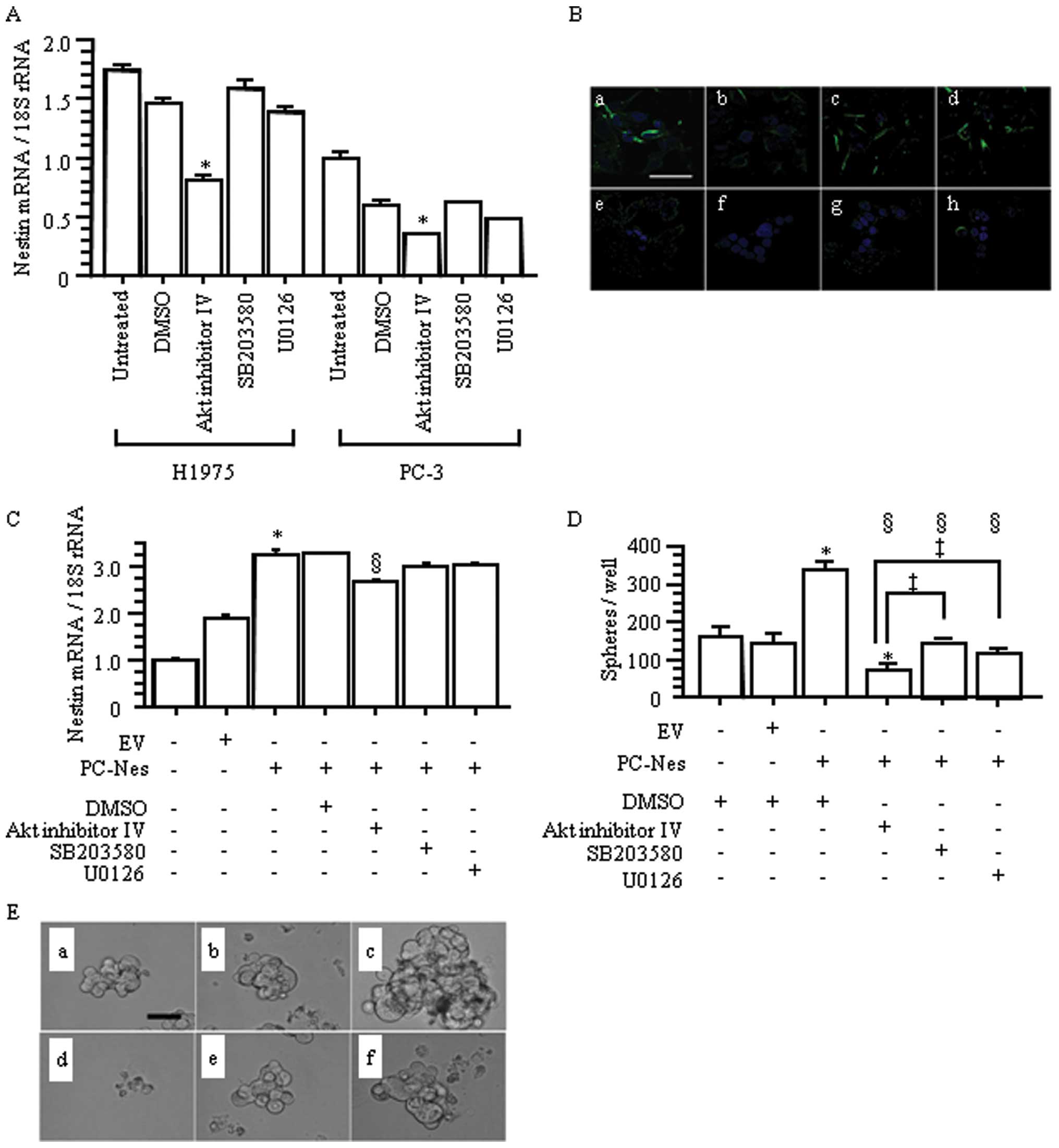 | Figure 9.Effects of Akt, p38 MAP kinase and
MEK 1/2 inhibitors in lung adenocarcinoma cells. (A) Relative
nestin mRNA expression levels in the groups that were treated with
200 nM of Akt inhibitor IV, p38 MAP kinase inhibitor (SB203580),
and MEK 1/2 inhibitor (U0126). *p<0.05 vs. untreated
and DMSO-treated control cells. (B) Inhibitory effects of Akt
inhibitor IV, p38 MAP kinase inhibitor (SB203580), and MEK 1/2
inhibitor (U0126) on nestin expression were determined by
immunofluorescent staining, respectively. (a) DMSO-treated H1975;
(b) Akt inhibitor IV-treated H1975; (c) SB203580-treated H1975; (d)
U0126-treated H1975; (e) DMSO-treated PC-3; (f) Akt inhibitor
IV-treated PC-3; (g) SB203580-treated PC-3; and (h) U0126-treated
PC-3. Green, nestin; blue, DAPI. Scale bar, 50 μm. (C)
Nestin expression vector-transfected PC-Nes cells were treated with
200 nM of Akt inhibitor IV, SB203580 and U0126. Empty vector
(EV)-transfected cells were used as a control.
*p<0.05 vs. EV−PC-Nes− and
EV+PC-Nes− cells without any treatment;
§p<0.05 vs. EV−PC-Nes+ cells
with or without DMSO. (D) PC-Nes cells exhibited significantly
increased number of spheres, compared to untreated and EV cells.
*p<0.05. Treatment of PC-Nes cells with inhibitors
significantly decreased sphere formation. §p<0.05 vs.
EV−PC-Nes+ cells with DMSO. Akt inhibitor IV
most effectively suppressed sphere formation of PC-Nes cells
compared with SB203580 and U0126 (‡p<0.05 vs.
EV−PC-Nes+ cells with SB203580 or U0126). The
inhibitory effect of Akt inhibitor IV on sphere formation was
strong enough that it reduced sphere formation of PC-Nes cells to
below that of untreated and EV+ cells (p<0.05 vs.
EV−PC-NES− and
EV+PC-Nes− cells with DMSO). (E) Phase
contrast images of spheres. Akt inhibitor IV strongly reduced
sphere formation of PC-Nes cells. (a)
EV−PC-NES− cells with DMSO; (b)
EV+PC-NES− cells with DMSO; (c)
EV−PC-NES+ with DMSO; (d)
EV−PC-NES+ with Akt inhibitor IV; (f)
EV−PC-NES+ with SB203580; and (g)
EV−PC-NES+ with U0126. Scale bar for upper
panels, 100 μm. |
We also evaluated sphere formation in PC-Nes cells
following treatment with each inhibitor. PC-Nes cells exhibited
significantly enhanced sphere formation (in both size and number)
compared with DMSO-treated and EV cells (*p<0.05;
Fig. 9D and E). Compared to
SB203580 and U0126, Akt inhibitor IV more markedly suppressed this
increased sphere formation in PC-Nes cells (‡p<0.05
vs. PC-Nes cells with SB203580 or U0126; Fig. 9D and E). Akt inhibitor IV-treated
PC-Nes cells also exhibited reduced sphere formation compared to
DMSO-treated cells and EV cells (*p<0.05; Fig. 9D and E). These findings indicated
that Akt inhibitor IV effectively overcame the enhanced sphere
formation induced by nestin upregulation. Overall, the
Akt-Sox2-nestin pathway regulates cell proliferation, migration,
invasion and sphere formation in lung AD.
Discussion
Our present results showed that nestin regulates
growth, migration, invasion and stemness of lung AD, and that the
Akt/Sox2 pathway regulates nestin expression and functions. To the
best of our knowledge, this is the first report to elucidate the
detailed functions and signaling regulation mechanism of nestin in
lung cancer. These findings will help develop new therapies to
target nestin in lung cancers.
Akt is located downstream of EGFR, a well-known
molecular target in AD. Previous immunohistochemical analysis of
NSCLC biopsy specimens revealed Akt overexpression in 62% and
phosphorylated Akt in 44% (41),
and constitutional Akt activation in NSCLC cells promotes survival
and resistance to chemotherapy and radiation (37). Akt also reportedly regulates Sox2
expression and self-renewal of CSC-like cell sub-populations in
NSCLC (39). Sox2 is a key
transcription factor in both embryonic stem cells and induced
pluripotent stem cells, and is also expressed in various cancers
(42). Sox2 overexpression
reportedly correlates with stemness, augmentation of
tumorigenicity, and poorer prognosis in lung AD (43,44).
Moreover, the enhancer region of the nestin gene has been shown to
contain a Sox2 binding site (40).
Our study demonstrated that inhibition of Akt and p-Akt by Akt
inhibitor IV resulted in downregulation of Sox2 and nestin, and
that siRNA targeting nestin did not affect the expression levels of
Akt, p-Akt or Sox2. These observations clarified the nestin
regulating pathway via Akt/Sox2 and the therapeutic potential of
targeting nestin. Together, these findings highlight and explain
the significance of upstream nestin-regulating mechanisms in lung
AD. In the present study, we used two types of lung AD cell lines
that each have a different EGFR gene status: PC-3 has no activation
of EGFR signaling (45), whereas
H1975 harbors exon 19 deletion and T790M mutation in EGFR (46). We did not observe any conflicting
cell behavior between these two types of AD cells when nestin
expression was increased or decreased; thus, we suggest that EGFR
mutation status does not affect the roles of nestin in AD cells.
However, the relationship between nestin expression status and
resistance to EGFR-TKIs requires further investigation.
Several groups have confirmed that nestin correlates
with poorer postoperative prognosis in patients diagnosed with
NSCLC, including AD, SCC and large cell carcinoma (21–24).
Here we confirmed that nestin might be related to shorter
postoperative survival in patients diagnosed with AD. Nestin
expression was also correlated to tumor size and LN metastasis.
Moreover, we found that nestin inhibition induced anticancer
effects (including anti-proliferation, migration and invasion) as
well as suppressed sphere formation. Sphere formation ability
represents the CSC property in various cancers, including NSCLC
(47). CSCs, defined as a
subpopulation that can self-renew and maintain the tumor presence
(48), are also characterized by
resistance to radiation and chemotherapy (49,50).
Several markers have been proposed to identify CSCs in NSCLC,
including CD133 (49), ATP-binding
cassette subfamily G member-2 (ABCG2) (51), aldehyde dehydrogenase-1 (ALDH-1)
(52) and Hoechst side population
(51). However, these markers are
not sufficiently sensitive and specific to CSCs (53), possibly reflecting the
heterogeneity of each tumor, histological subtype and individual
(54). Here we showed that nestin
upregulation induced increased sphere formation, while nestin
downregulation (by both shRNA and Akt inhibition) resulted in
decreased sphere forming ability. These findings indicate that
nestin is a key regulator of CSCs in AD. Recent therapeutic
advances have led to the molecular targeting therapies that inhibit
driver oncogenes; however, these therapies inevitably face acquired
resistance, resulting in unfavorable outcomes in AD.
Nestin-targeting therapy may eradicate CSCs in AD; thus,
suppressing relapse and resistance against chemotherapy and
molecular-directed therapy targeting driver oncogenes.
The results of our screening assay confirmed that
nestin expression was decreased by some chemical compounds that
target specific signaling molecules, including several compounds
that specifically inhibit Akt. The chemical agent that strongly
decreased nestin expression was Akt inhibitor IV, a cell-permeable
compound that inhibits Akt phosphorylation/activation. Akt
inhibitor IV presumably targets the ATP binding site of a kinase
that is upstream of Akt but downstream of phosphatidylinositol-3
kinase (PI3K), resulting in blockade of the Akt-mediated nuclear
export of forkhead box O1 (FOXO1) to the cytoplasm (55). FOXO1 is a transcriptional regulator
of the G1/S checkpoint and of apoptosis. Loss of FOXO1 activity
that is induced by phosphorylation via Akt signaling impairs cell
cycle arrest, resulting in unlimited proliferation (56). EGFR-TKI resistance is driven by Akt
activation, and can be overcome by decreasing Akt signaling with
Akt inhibitors, resulting in FOXO1 reactivation or expression
(57). FOXO1 is reported to
control Sox2 expression (58).
Therefore, inhibition of FOXO1 by Akt inhibitor IV might also
promote nestin downregulation via suppression of Sox2
signaling.
In our screening assay using chemical compounds, we
also found that nestin expression was strongly inhibited by Akt
inhibitor VIII, the activity of which is reportedly pleckstrin
homology (PH) domain-dependent (59). In contrast, nestin mRNA level was
only slightly decreased by Akt inhibitor XI, which directly affects
Akt but not the level of p-Akt. The relationship between the
inhibitory effect on nestin and the mechanism of Akt inhibition for
each Akt inhibitor remains unclear, but further investigation of
the signaling pathway that connects Akt with nestin might reveal an
appropriate approach to Akt inhibition that enhances nestin
downregulation. We have also confirmed the nestin-inhibiting
ability of compounds that inhibit cell cycle and mitosis, such as
cdk1/2 inhibitor III and cdk2/9 inhibitor. Thus, nestin is thought
to play important roles in the cell cycle of tumor cells, and cell
cycle-targeted therapies via downregulation of nestin and its
upstream molecules are promising for the development of new
anticancer drugs.
In this study, we confirmed the expression of nestin
in NSCLC and its correlation with poor survival in surgical
patients with AD. Nestin inhibition in AD cell lines resulted in
decreased proliferation, migration, invasion and sphere formation.
Correspondingly, nestin upregulation increased proliferation,
migration, and sphere formation. Akt inhibitor IV effectively
decreased the expression of nestin mRNA and protein via Sox2
downregulation, and decreased proliferation, migration, invasion
and sphere formation in AD. The effects of Akt inhibitor IV were
strong enough to overcome the enhanced sphere formation induced by
nestin upregulation. In conclusion, nestin regulates proliferation,
migration, invasion and stemness of lung AD, and thus nestin is
considered a promising novel molecular target in AD.
Acknowledgements
For kindly providing the SCADS
Inhibitor Kit III, we deeply thank the Screening Committee of
Anticancer Drugs, which is supported by a Grant-in-Aid for
Scientific Research on Innovative Areas, Scientific Support
Programs for Cancer Research, from The Ministry of Education,
Culture, Sports, Science and Technology, Japan. We also express our
appreciation to Ms. Taeko Suzuki, Ms. Yoko Kawamoto, and Ms. Kiyoko
Kawahara for technical assistance (Departments of Pathology and
Integrative Oncological Pathology). We thank Dr Shin-ichi Tsuchiya
(Division of Diagnostic Pathology, Nippon Medical School Hospital),
Dr Yuki Nakajima, Dr Kiyoshi Koizumi, and Dr Jitsuo Usuda
(Department of Surgery, Division of Thoracic Surgery, Nippon
Medical School) for preparing tissue samples. This study was
supported in part by Grants-in-Aid from the Japan Society for the
Promotion of Science to Y.M. and T.I. (C, nos. 25462127 and
25461027), and Clinical Rebiopsy Bank Project for Comprehensive
Cancer Therapy Development to Z.N., S.M. and G.A.
References
|
1.
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Husain AN: Lung tumors. Robbins Basic
Pathology. Kumar V, Abbas AK and Aster JC: Elsevier Saunders;
Philadelphia, PA: pp. 505–510. 2013
|
|
3.
|
Non-small Cell Lung Cancer Collaborative
Group: Chemotherapy in non-small cell lung cancer: a meta-analysis
using updated data on individual patients from 52 randomised
clinical trials. BMJ. 311:899–909. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Maemondo M, Inoue A, Kobayashi K, et al:
Gefitinib or chemotherapy for non-small-cell lung cancer with
mutated EGFR. N Engl J Med. 362:2380–2388. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Devesa SS, Bray F, Vizcaino AP and Parkin
DM: International lung cancer trends by histologic type:
male:female differences diminishing and adenocarcinoma rates
rising. Int J Cancer. 117:294–299. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Mitsudomi T, Morita S, Yatabe Y, et al:
Gefitinib versus cisplatin plus docetaxel in patients with
non-small-cell lung cancer harbouring mutations of the epidermal
growth factor receptor (WJTOG3405): an open label, randomised phase
3 trial. Lancet Oncol. 11:121–128. 2010.
|
|
7.
|
Shaw AT, Kim DW, Nakagawa K, et al:
Crizotinib versus chemotherapy in advanced ALK-positive lung
cancer. N Engl J Med. 368:2385–2394. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Choi YL, Soda M, Yamashita Y, et al:
EML4-ALK mutations in lung cancer that confer resistance to ALK
inhibitors. N Engl J Med. 363:1734–1739. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Kobayashi S, Boggon TJ, Dayaram T, et al:
EGFR mutation and resistance of non-small-cell lung cancer to
gefitinib. N Engl J Med. 352:786–792. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Engelman JA, Zejnullahu K, Mitsudomi T, et
al: MET amplification leads to gefitinib resistance in lung cancer
by activating ERBB3 signaling. Science. 316:1039–1043. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Marvin MJ, Dahlstrand J, Lendahl U and
McKay RD: A rod end deletion in the intermediate filament protein
nestin alters its subcellular localization in neuroepithelial cells
of transgenic mice. J Cell Sci. 111:1951–1961. 1998.PubMed/NCBI
|
|
12.
|
Sjöberg G, Jiang WQ, Ringertz NR, Lendahl
U and Sejersen T: Colocalization of nestin and vimentin/desmin in
skeletal muscle cells demonstrated by three-dimensional
fluorescence digital imaging microscopy. Exp Cell Res. 214:447–458.
1994.PubMed/NCBI
|
|
13.
|
Lendahl U, Zimmerman LB and McKay RD: CNS
stem cells express a new class of intermediate filament protein.
Cell. 60:585–595. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Esni F, Stoffers DA, Takeuchi T and Leach
SD: Origin of exocrine pancreatic cells from nestin-positive
precursors in developing mouse pancreas. Mech Dev. 121:15–25. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Ishiwata T, Teduka K, Yamamoto T, Kawahara
K, Matsuda Y and Naito Z: Neuroepithelial stem cell marker nestin
regulates the migration, invasion and growth of human gliomas.
Oncol Rep. 26:91–99. 2011.PubMed/NCBI
|
|
16.
|
Akiyama M, Matsuda Y, Ishiwata T, Naito Z
and Kawana S: Inhibition of the stem cell marker nestin reduces
tumor growth and invasion of malignant melanoma. J Invest Dermatol.
133:1384–1387. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Kawamoto M, Ishiwata T, Cho K, et al:
Nestin expression correlates with nerve and retroperitoneal tissue
invasion in pancreatic cancer. Hum Pathol. 40:189–198. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Matsuda Y, Naito Z, Kawahara K, Nakazawa
N, Korc M and Ishiwata T: Nestin is a novel target for suppressing
pancreatic cancer cell migration, invasion and metastasis. Cancer
Biol Ther. 11:512–523. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Kleeberger W, Bova GS, Nielsen ME, et al:
Roles for the stem cell associated intermediate filament Nestin in
prostate cancer migration and metastasis. Cancer Res. 67:9199–9206.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Ishiwata T, Matsuda Y and Naito Z: Nestin
in gastrointestinal and other cancers: effects on cells and tumor
angiogenesis. World J Gastroenterol. 17:409–418. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Ryuge S, Sato Y, Wang GQ, et al:
Prognostic significance of nestin expression in resected non-small
cell lung cancer. Chest. 139:862–869. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Ryuge S, Sato Y, Jiang SX, et al:
Prognostic impact of nestin expression in resected large cell
neuroendocrine carcinoma of the lung. Lung Cancer. 77:415–420.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Skarda J, Kolar Z, Janikova M, et al:
Analysis of the prognostic impact of nestin expression in non-small
cell lung cancer. Biomed Pap Med Fac, Univ Palacky Olomouc, Czech
Repub. 156:135–142. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Chen Z, Wang T, Luo H, et al: Expression
of nestin in lymph node metastasis and lymphangiogenesis in
non-small cell lung cancer patients. Hum Pathol. 41:737–744. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Janikova M, Skarda J, Dziechciarkova M, et
al: Identification of CD133+/nestin+ putative
cancer stem cells in non-small cell lung cancer. Biomed Pap Med Fac
Univ Palacky Olomouc Czech Repub. 154:321–326. 2010.
|
|
26.
|
Takakuwa O, Maeno K, Kunii E, et al:
Involvement of intermediate filament nestin in cell growth of
small-cell lung cancer. Lung Cancer. 81:174–179. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Goldstraw P, Crowley J, Chansky K, et al:
The IASLC Lung Cancer Staging Project: proposals for the revision
of the TNM stage groupings in the forthcoming (seventh) edition of
the TNM Classification of malignant tumours. J Thorac Oncol.
2:706–714. 2007. View Article : Google Scholar
|
|
28.
|
Ishiwata T, Matsuda Y, Yamamoto T, Uchida
E, Korc M and Naito Z: Enhanced expression of fibroblast growth
factor receptor 2 IIIc promotes human pancreatic cancer cell
proliferation. Am J Pathol. 180:1928–1941. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Matsuda Y, Ishiwata T, Yamahatsu K, et al:
Overexpressed fibroblast growth factor receptor 2 in the invasive
front of colorectal cancer: a potential therapeutic target in
colorectal cancer. Cancer Lett. 309:209–219. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Yamahatsu K, Matsuda Y, Ishiwata T, Uchida
E and Naito Z: Nestin as a novel therapeutic target for pancreatic
cancer via tumor angiogenesis. Int J Oncol. 40:1345–1357.
2012.PubMed/NCBI
|
|
31.
|
Matsuda Y, Hagio M, Seya T and Ishiwata T:
Fibroblast growth factor receptor 2 IIIc as a therapeutic target
for colorectal cancer cells. Mol Cancer Ther. 11:2010–2020. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Kawase R, Ishiwata T, Matsuda Y, et al:
Expression of fibroblast growth factor receptor 2 IIIc in human
uterine cervical intraepithelial neoplasia and cervical cancer. Int
J Oncol. 36:331–340. 2010.PubMed/NCBI
|
|
33.
|
Arai K, Sakamoto R, Kubota D and Kondo T:
Proteomic approach toward molecular backgrounds of drug resistance
of osteosarcoma cells in spheroid culture system. Proteomics.
13:2351–2360. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Ushijima H and Maeda M: cAMP-dependent
proteolysis of GATA-6 is linked to JNK-signaling pathway. Biochem
Biophys Res Commun. 423:679–683. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Huang YL, Wu CM, Shi GY, et al: Nestin
serves as a prosurvival determinant that is linked to the
cytoprotective effect of epidermal growth factor in rat vascular
smooth muscle cells. J Biochem. 146:307–315. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Xue XJ and Yuan XB: Nestin is essential
for mitogen-stimulated proliferation of neural progenitor cells.
Mol Cell Neurosci. 45:26–36. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Brognard J, Clark AS, Ni Y and Dennis PA:
Akt/protein kinase B is constitutively active in non-small cell
lung cancer cells and promotes cellular survival and resistance to
chemotherapy and radiation. Cancer Res. 61:3986–3997.
2001.PubMed/NCBI
|
|
38.
|
Guo Y, Du J and Kwiatkowski DJ: Molecular
dissection of AKT activation in lung cancer cell lines. Mol Cancer
Res. 11:282–293. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39.
|
Singh S, Trevino J, Bora-Singhal N, et al:
EGFR/Src/Akt signaling modulates Sox2 expression and self-renewal
of stem-like side-population cells in non-small cell lung cancer.
Mol Cancer. 11:732012. View Article : Google Scholar : PubMed/NCBI
|
|
40.
|
Tanaka S, Kamachi Y, Tanouchi A, Hamada H,
Jing N and Kondoh H: Interplay of SOX and POU factors in regulation
of the Nestin gene in neural primordial cells. Mol Cell Biol.
24:8834–8846. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
41.
|
Dobashi Y, Kimura M, Matsubara H, Endo S,
Inazawa J and Ooi A: Molecular alterations in AKT and its protein
activation in human lung carcinomas. Hum Pathol. 43:2229–2240.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
42.
|
Liu K, Lin B, Zhao M, et al: The multiple
roles for Sox2 in stem cell maintenance and tumorigenesis. Cell
Signal. 25:1264–1271. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43.
|
Nakatsugawa M, Takahashi A, Hirohashi Y,
et al: SOX2 is overexpressed in stem-like cells of human lung
adenocarcinoma and augments the tumorigenicity. Lab Invest.
91:1796–1804. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44.
|
Sholl LM, Barletta JA, Yeap BY, Chirieac
LR and Hornick JL: Sox2 protein expression is an independent poor
prognostic indicator in stage I lung adenocarcinoma. Am J Surg
Pathol. 34:1193–1198. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45.
|
Noro R, Gemma A, Kosaihira S, et al:
Gefitinib (IRESSA) sensitive lung cancer cell lines show
phosphorylation of Akt without ligand stimulation. BMC Cancer.
6:2772006. View Article : Google Scholar : PubMed/NCBI
|
|
46.
|
Pao W, Miller VA, Politi KA, et al:
Acquired resistance of lung adenocarcinomas to gefitinib or
erlotinib is associated with a second mutation in the EGFR kinase
domain. PLoS Med. 2:e732005. View Article : Google Scholar : PubMed/NCBI
|
|
47.
|
Nolte SM, Venugopal C, McFarlane N, et al:
A cancer stem cell model for studying brain metastases from primary
lung cancer. J Natl Cancer Inst. 105:551–562. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48.
|
Clarke MF, Dick JE, Dirks PB, et al:
Cancer stem cells - perspectives on current status and future
directions: AACR Workshop on cancer stem cells. Cancer Res.
66:9339–9344. 2006. View Article : Google Scholar
|
|
49.
|
Eramo A, Lotti F, Sette G, et al:
Identification and expansion of the tumorigenic lung cancer stem
cell population. Cell Death Differ. 15:504–514. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
50.
|
Reya T, Morrison SJ, Clarke MF and
Weissman IL: Stem cells, cancer, and cancer stem cells. Nature.
414:105–111. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
51.
|
Ho MM, Ng AV, Lam S and Hung JY: Side
population in human lung cancer cell lines and tumors is enriched
with stem-like cancer cells. Cancer Res. 67:4827–4833. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
52.
|
Liang D and Shi Y: Aldehyde
dehydrogenase-1 is a specific marker for stem cells in human lung
adenocarcinoma. Med Oncol. 29:633–639. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
53.
|
Alamgeer M, Peacock CD, Matsui W, Ganju V
and Watkins DN: Cancer stem cells in lung cancer: Evidence and
controversies. Respirology. 18:757–764. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
54.
|
Bombí JA, Martínez A, Ramírez J, et al:
Ultrastructural and molecular heterogeneity in non-small cell lung
carcinomas: study of 110 cases and review of the literature.
Ultrastruct Pathol. 26:211–218. 2002.PubMed/NCBI
|
|
55.
|
Kau TR, Schroeder F, Ramaswamy S, et al: A
chemical genetic screen identifies inhibitors of regulated nuclear
export of a Forkhead transcription factor in PTEN-deficient tumor
cells. Cancer Cell. 4:463–476. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
56.
|
Lam EW, Francis RE and Petkovic M: FOXO
transcription factors: key regulators of cell fate. Biochem Soc
Trans. 34:722–726. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
57.
|
Sangodkar J, Dhawan NS, Melville H, et al:
Targeting the FOXO1/KLF6 axis regulates EGFR signaling and
treatment response. J Clin Invest. 122:2637–2651. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
58.
|
Zhang X, Yalcin S, Lee DF, et al: FOXO1 is
an essential regulator of pluripotency in human embryonic stem
cells. Nat Cell Biol. 13:1092–1099. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
59.
|
DeFeo-Jones D, Barnett SF, Fu S, et al:
Tumor cell sensitization to apoptotic stimuli by selective
inhibition of specific Akt/PKB family members. Mol Cancer Ther.
4:271–279. 2005.PubMed/NCBI
|