Introduction
Emerging evidence indicates that oral squamous cell
carcinoma (OSCC) harbors cancer initiating cells (1–6).
However, it is unclear what subpopulations initiate the growth of
OSCC in animals and clinical patients. Our recent studies indicate
that head and neck cancer including OSCC is characterized by the
expression of Id1 and NF-κB in the majority of clinical patients by
microarrays (7,8) and cancer cells, rich in these two
proteins are by nature aggressive and have poor clinical outcomes
(9–12).
Id1 has been shown to trigger the xenograft tumor
growth in nude mice via the regulation of the PI3K/Akt and
NF-κB/Survivin pathways (8). NF-κB
is also tumorigenic when combined with sv40 in keratinocytes
(13) or Id1 (8). Approximately 60% of head and neck
cancer specimens are positive for Id1 and NF-κB proteins. The in
vivo data from our previous studies indicate that both Id1 and
NF-κB are highly expressed in the edge of xenograft tumor lumps and
are highly proliferative in vitro (8). Biologically, Id1 is involved in the
immortalization of differentiated keratinoyctes (14), increases cellular proliferation
when associated with NF-κB (15),
yielding fast-growing keratinocyte spheres with naïve properties.
Therefore, Id1 and NF-κB are important oncogenic proteins in
keeping cells in a state of naiveness and proliferation and thought
to contribute to the generation of cancer initiating cells.
Cancer initiating cells are side populations with
the capability to initiate cancer growth in the body (16–18).
These naïve side populations are capable of differentiating into a
variety of cancer cell phenotypes and recapitulating the tumor
heterogeneity in animal models (19–21).
In general, naïve cancer cells are marked with stem cell markers
such as CD133 and are more resistant to radiation and chemotherapy
than other cancer cell populations (22,23).
CD133 is a glycoprotein, also known as Prominin 1
(PROM1), in humans and rodents (24,25).
It is the founding member of pentaspan transmembrane glycoproteins,
specifically associated with plasma membrane protrusions,
irrespective of the cell type. CD133 is expressed in hematopoietic
stem cells, neural stem cells, and some other cell types (26–30).
CD133+ cells are thought to be glioma initiating cells
in the brain, with its tumorigenicity much higher than than
CD133− cells (31,32).
Interestingly, CD133+ cells are extensively present in the clinical
head and neck squamous cell carcinoma specimens and possess stem
cell-like properties in vitro (33) and promote the initiation of head
and neck cancer (34).
BMI-1 is a key transcription factor that
immortalizes human mucosal keratinoyctes (35), which is a hallmark of cancer cells
(36). It is unclear whether BMI-1
is involved in the self renewal of OSCC. It has been observed that
BMI-1 is expressed in clinical OSCC specimens but not in normal
mucosal controls.
In this study, we hypothesized that
CD133+ and BMI-1+ keratinocytes via Id1 and
NF-κB subunit p65 in OSCC are capable of initiating xenograft
tumors in immunodeficient mice. To verify this hypothesis, we
isolated CD133+ and BMI-1+ keratinocytes from
fresh OSCC tissue cultures and CA9-22 cell cultures and then
injected them into SCID/Beige mice. It was found that
CD133+ and BMI-1+ cells, either from OSCC
tissues or CA9-22 cell cultures, initiated the xenograft tumor
growth in SCID/Beige mice whereas CD133− cells did not.
The xenograft tumors from these CD133+ and
BMI-1+ keratinocytes possessed similar phenotypes as
those of the OSCC clinical samples.
Materials and methods
Clinical specimens
The surgical specimens were collected from clinical
patients who underwent surgery at the Union Hospital, Fujian
Medical University, Fuzhou; Sun Yatsen Memorial Hospital,
University of Sun Yatsen, Guangzhou, China; and the Department of
Otolaryngology, University of Minnesota Hospital and Clinics,
Minneapolis, MN, USA. The expression of mRNA transcripts was
interrogated with the database of microarrays in terms of the genes
of interest in the present study. Control specimens were biopsies
of normal tissues close to the cancer site. Seven OSCC surgical
specimens and five normal tissues were used for western blot
analysis. An additional five OSCC fresh tissues were xenograft
tumor passages in SCID/Beige mice first and then harvested for
isolation of CD133+ and BMI-1+ cells and
subsequent cell cultures. All specimens and clinical data in this
study were procured, handled and maintained according to the
protocols approved by each Institutional Review Board (IRB), e.g.,
the University of Minnesota, the Fujian Medical University and the
Sun Yatsen University.
Cell cultures
Surgical OSCC tissues were obtained from clinical
patients at the University of Minnesota Hospitals and Clinics
(UMHC), Sun Yatsen University Hospital and the Union Hospital of
Fujian Medical University. Fresh tissues were first implanted into
the flank of mice to allow for tumor growth and then passed into
SCID/Beige mice generation after generation. Xenograft tumors were
dissected out and immersed into tissue culture media. Tissue cells
were dissociated by using a cell isolator (Gentle MACS, Miltenyi
Biotec, Auburn, CA, USA). CD133+ cells were then
isolated by MACS with CD133 monoclonal antibody beads cultured in
RPMI-1640 (Life Technologies, Invitrogen, Carlsbad, CA, USA) on
glass chamber slides and regular cell culture dishes. The
expression of BMI-1 was determined by
reverse-transcription-polymerase chain reaction (RT-PCR), western
blot analysis, and fluorescence activated cell sorting (FACS) from
these CD133+ cell cultures.
Construction of keratinocytes with stable
expression of both ID1 and NF-κB
HOK16B is a cell line derived from keratinocytes in
the oral cavity immortalized with human papillomavirus (37); CA9-22, a cell line established from
an oral cancer patient was maintained in RPMI-1640 (Life
Technologies, Invitrogen), HOK-16B was maintained in keratinocyte
basal medium (Lon2a) and the CD133 cDNA was prepared as previously
described (31). Efficacy of Id1
cDNA expression after transient transfection was determined by
inverted microscopy or flow cytometry.
Reverse transcription-polymerase chain
reaction (RT-PCR)
Total RNA was isolated from five clinical OSCC
tissues and controls using the RNA® Miniprep kit
(Stratagene, Santa Clara, CA, USA). Residual genomic DNA in total
RNA samples was digested with DNases according to the
manufacturer’s instruction. Specific primers for the human CD133
(5′-ctactatgaagc agggatta-3′/5′-aacgcctctttggtctcctt-3′) and BMI-1
(5′-cttggctcgc attcattttc-3′/5′-tcacctcctccttagatttc-3′) were used
for this study, using the method as described previously (26).
Immunohistochemistry
Cell cultures from clinical OSCC specimens were
performed as previously described. Cells were fixed in 100% alcohol
and incubated for 90 min with CD133 (AC133, Miltenyi Biotec) and
then incubated with secondary antibodies, FITC- or TRITC-conjugated
mouse anti-rabbit, goat anti-mouse IgG (Zymed, San Francisco, CA,
USA), as previously described (8).
Later on, cellular nuclei in the same slide were stained with
4′,6′-diamidino-2-phenylindole (DAPI) after the antibody stain
became faint. This allows cellular nuclei, CD133 and BMI-1 proteins
to be shown simultaneously by using Photoshop software to evaluate
the co-expression of both CD133 and BMI-1 in cultured OSCC
cells.
Western blot analysis
CD133 and BMI-1 proteins in OSCC tissues were
detected with an Enhanced Chemiluminescence Western Blotting
Detection Kit (Amersham Pharmacia Biotech, Piscataway, NJ, USA)
after binding of mouse anti-human CD133/1 (AC133) MAb (Miltenyi
Biotec) and goat anti-human BMI-1 (sc30943, Santa Cruz
Biotechnology, Santa Cruz, CA, USA). Horseradish peroxidase
(HPR)-conjugated anti-mouse and anti-goat secondary IgG antibodies
were used (Santa Cruz Biotechnology). Anti-CD44 antibody (ab41478,
Abcam, Cambridge, MA, USA), anti-CD1 (ab95587, Abcam), anti-Cdc2
(1:1,000 dilution, 9112S, Cell Signaling Technology, Danvers, MA,
USA), and anti-GAPDH antibody (ab9485, Novus Biologicals,
Littleton, CO, USA) were used for western blot analysis.
Cycloheximide at a concentration of 10 μM was used for
inhibition of protein synthesis in CA9-22 cell cultures. Catharidic
acid (CA, CAS#28874-45-5, 1 μM) and arginine vasopressin
(AVP, CAS#113-79-1, 10-6 mol) were used for inhibition of the Cdc2
protein and AVP protein, respectively, after cells were transfected
with Id1+p65. CH, CA and AVP were all purchased from Sigma-Aldrich
(St. Louis, MO, USA).
Fluorescence activated cell sorting
(FACS) analysis
Briefly, cells were washed with phosphate-buffered
saline (PBS), harvested by trypsinization, and incubated with
PE-conjugated anti-human CD133 (130-080-901, Miltenyi Biotec),
APC-conjugated anti-human CD44 (559942, BD Biosciences Pharmingen,
San Diego, CA, USA), and FITC-conjugated anti-human CD24 (555427,
BD Biosciences Pharmingen) antibodies. For BMI-1 analysis, cells
were washed with PBS, harvested by trypsinization, pre-incubated
with 0.3% saponin in PBS for 10 min, and then incubated with goat
anti-human BMI-1 (CAT#sc-30934, Santa Cruz Biotechnology), followed
by secondary mouse anti-goat antibody (FITC conjugated). In a
similar way, cells were stained with anti-Id1 (sc-488, Santa Cruz
Biotechnology) and anti-BMI-1 (sc-30943, Santa Cruz Biotechnology)
antibodies. Cells were resuspended in PBS and analyzed on
FACSCalibur using CellQuest Pro (BD Biosciences) and FlowJo 7.6
(Tree Star, Inc., Ashland, OR, USA). Similarly, Id1+ and
BrdU+ cells were analyzed with flow cytometry as
above.
Cell sorting
The dissociated cells were incubated with
PE-conjugated anti-human CD133 at 20 μl per 106
cells for 20 min, at 4˚C, in the dark and washed three times with
excessive PBS. In a final labelling step, 80 μl MACS buffer
(PBS supplemented with 0.5% BSA and 2 mM EDTA) per 107
cells and 20 μl anti-PE MACS Microbeads®
(Miltenyi Biotec) per 107 cells were added to the cell
pellet. After mixing, the cells were incubated in the dark for 15
min at 4˚C. The cells were washed with excess MACS buffer and
resuspended in 500 μl buffer per 107 cells. The
magnetic separation of the cells was performed using an LS-column
(Miltenyi Biotec) that was placed in a VarioMACS separator
(Miltenyi Biotec), as described by the manufacturer’s protocol. The
positive selected cells were finally suspended in 1 ml of MACS
buffer per 4×106 total cells. Id1+ cell
subpopulation was sorted out by using the same protocol as above.
Id1 antibody was conjugated with FITC.
Trypan blue exclusion
Briefly, cells were transfected in transfection
medium with Id1+p65, p65, Id1, and empty vector at 1.4 μg/ml
for 16 h, recovered in cell culture medium for 24 h, and then
harvested for evaluation of cell numbers after staining with a
(trypan blue) dye. Cells were washed first in PBS and incubated in
0.3 ml of 0.05% trypsin-EDTA solution for 10 min. Trypsin solution
(5 μl) was mixed with 5 μl of trypan blue and
transferred to a hemacytometer for cell counting. Results are
presented as 104 viable cells.
Cell cycle progression
For cell cycle progression analysis, cells were
cultured in 6-well plates till 60% confluence, transfected with
empty vector, Id1, p65 and ID1+p65 at 1.4 μg/ml for 16 h,
and then recovered in full culture media for 24 h, washed with PBS,
dissociated with trypsin-EDTA, washed with ice cold PBS, fixed with
100% ethanol, and stored at −20˚C until analysis. Approximately
5×104 cells were resuspended in 100 μl of 40
μg/ml DNase-free RNase A with 100 μl of propidium
iodide (stock solution 200 μg/ml) and incubated at room
temperature for 30 min. The fluorescence excited by FL-2 was
measured on a FACSCalibur (BD Biosciences). The cell cycle
progression of approximately 104 CA9-22 cells was
analyzed on CellQuest Pro (software version 4.0 Becton-Dickinson,
Franklin Lakes, NJ, USA). The experiment was performed in
triplicate. Data are presented as means ± SD (n=3).
Xenograft tumor growth in SCID/Beige
mice
SCID/Beige mice were used in this study.
CD133+ and BMI-1+ keratinocytes from both
fresh OSCC tissue cultures and CA9-22 cell cultures were,
respectively, injected into 8 SCID/Beige mice in the flank back
skin subcutaneously with 100, 1,000, 10,000, and 100,000 viable
cells per injection site, namely, groups I, II, III and IV with the
above cell numbers being injected. After injection, tumor volume in
SCID/Beige mice was measured routinely by width, length and height
of xenograft tumors in a three-dimension manner on a biweekly basis
up to two months. Tumor weight was measured when animals were
sacrificed for harvesting xenograft tissues.
Statistical analysis
The Student’s t-test was used for evaluation of
differences between controls and experiments in vitro.
P-values <0.05 were considered to indicate a statistically
significant difference.
Results
CD133 and BMI-1 proteins are co-expressed
in OSCC tissue cultures
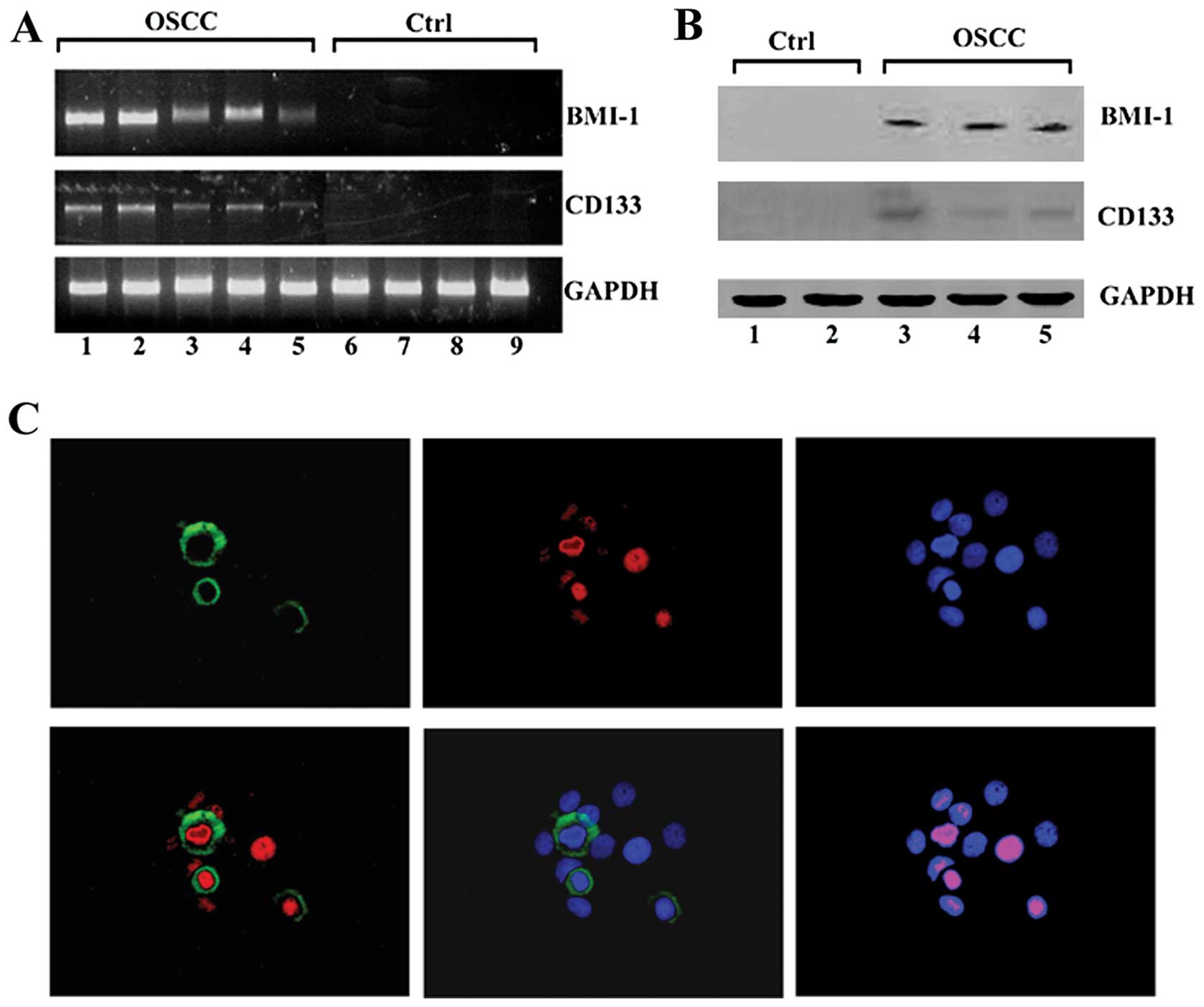
RT-PCR demonstrated the expression of both CD133 and
BMI-1 mRNA transcripts in OSCC clinical specimens (Fig. 1A). Western blot analysis verified
the expression of BMI-1 in the surgical specimens of OSCC (Fig. 1B). Immunohistochemistry
demonstrated the co-expression of the both CD133 and BMI-1 proteins
in cultured cells from clinical OSCC tissues (Fig. 1C).
CD133 and BMI-1 increase in CA9-22 cell
cultures transfected with Id1 and NF-κB
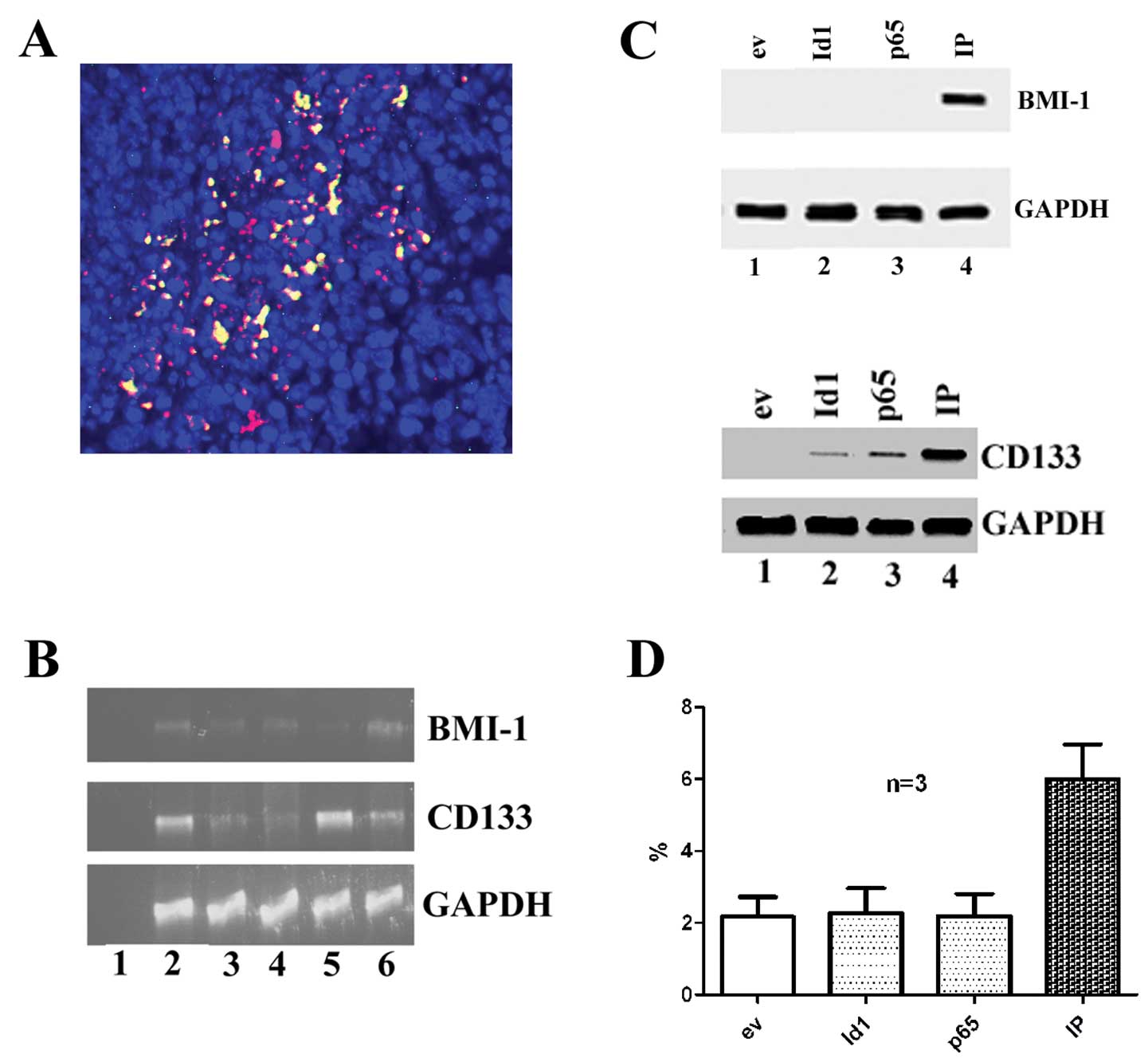
Id1 and NF-κB subunit p65 were detected in OSCC
specimens (Fig. 2A). It is noted
that some OSCC cells express both Id1 and NF-κB subunit p65 (yellow
color indicating the co-expression of both Id1 and p65, red
indicating Id1 positive cells and green p65 positive cells). The
CD133 and BMI-1 mRNA transcripts were detected in OSCC tissue
cultures by RT-PCR (Fig. 2B). In
the cell cultures of CA9-22, the expression of BMI1 protein was not
detected in Id1 or p65 transfected cells alone but detected in
cells with the transfection of both Id1 and p65 in combination.
While the expression of CD133 protein was not detected in empty
vector transfected cells, but it was detected in cells with the
transfection of Id1, p65 or Id1+p65 (Fig. 2C). The BMI-1 protein amount
increased and became detected when cells were transfected with both
Id1+p65, but were not detected with Id1 and p65 alone in OSCC
tissue cultures (Fig. 2D).
Id1 and BMI-1 are co-expressed in
proliferating OSCC cells
Id1 is known to antagonize the differentiation of
cells and make cells naïve whereas BMI-1 is involved in the
self-renewal of keratinocytes. To study whether Id1 and BMI-1 are
actually expressed in the same cell subpopulation, we performed
FACS analyses after staining cells with both Id1 and BMI-1 specific
antibodies. It was found that 3.4% of total cells were
Id1+ and 96.6% of total cells were Id1−
(Fig. 3A). Among the
Id1− cells, there were almost 99.9% BMI-1−
(Fig. 3B). While among the
Id1+ cells, there were approximately 99.8%
BMI-1+ (Fig. 3C). In a
similar way, approximately 3% of total cells were BMI-1+
(Fig. 3D). Among the
BMI-1− cells, there were nearly 100% Id1−
(Fig. 3E). While among the
BMI-1+ cells, there were 81.3% of Id1+ cells
(Fig. 3F). To study whether Id1 is
involved in cell proliferation, we examined BrdU incorporation on
the Id1+ subpopulation. It was found that of these
Id1+ cells following cell cultivation for 24 h, 82.8% of
cells remained Id1+ and 18.2% of cells became
Id1− (Fig. 3G). Among
the Id1− cells, there were 52.2% of BrdU+
cells (Fig. 3H). Among these
Id1+ cells, 98.6% of cells were BrdU+
(Fig. 3I). In the Id1+
cells following cell cultivation for 24 h, there were 3.7% of cells
remaining BrdU− and 96.3% of cells were BrdU+
(Fig. 3J). Among the
BrdU− cells, there were 8.1% of BrdU+ cells
(Fig. 3K). Among these
BrdU+ cells, there were 79.0% of Id1+ cells
(Fig. 3L). The effect of both Id1
and p65 on cell cycle progression and cell counts of CA9-22 cells
was studied. It was found that Id1 and p65 increased the cell cycle
progression (Fig. 3M), cell counts
(Fig. 3N), and BrdU incorporation
(Fig. 3O) of CA9-22 cell cultures.
FACS data verified that both Id1 and BMI-1 were co-expressed in
OSCC cells and Id1 increases BrdU+ cells.
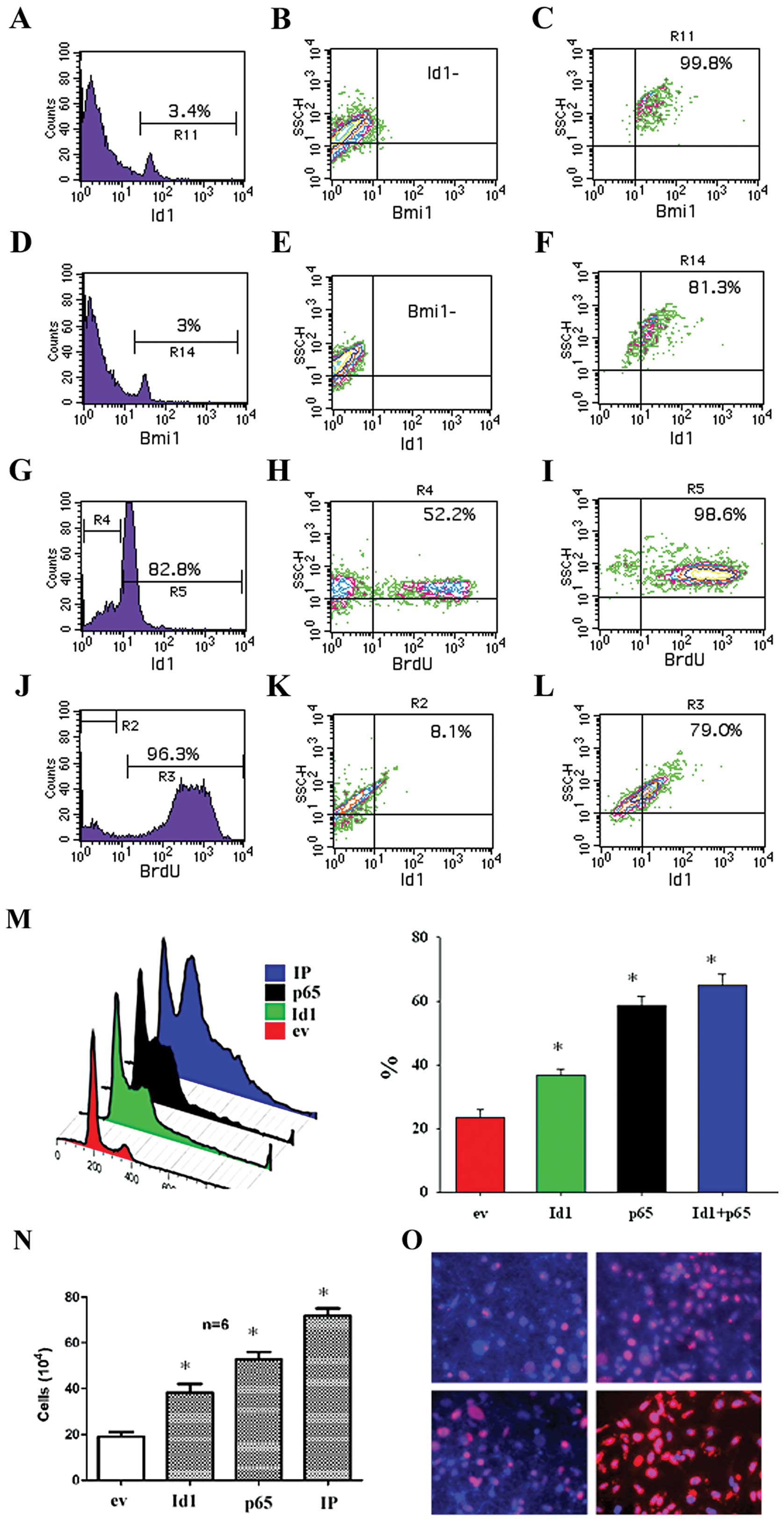 | Figure 3.The relationship between
Id1+ and BMI-1+ cells as well as
Id1+ and BrdU+ in OSCC cells. Cells were stained with
both Id1 and BMI-1 antibodies and examined by FACS. (A) In OSCC
cells, as indicated by marker R11, there were 3.4% of
Id1+ cells. (B) Approximately 97.6% of total cells were
Id1− and these cells were also BMI-1−. (C)
Among Id1+ cells, 99.8% were BMI-1+. (D) In a
similar way, as measured by marker R14, there were approximately 3%
of BMI-1+ cells. (E) Among BMI-1− cells,
accounting for 97% of total cells, were also Id1−. (F)
Among the Id1+ cells, they were 81.3% BMI-1+.
In the Id1+ subpopulation, there were 82.8% of cells
remaining Id1+ after culture for 24 h (G). In the
Id− cells, 52.2% of cells remained BrdU+ (H)
whereas in the Id1+ cells 98.6% of cells remained
BrdU+ (I). Accordingly, in the Id1+ cell
cultures, there were 96.3% of BrdU+ cells (J). In the
BrdU− cells 8.1% of cells were Id1+ (K)
whereas in the BrdU+ cells 79.0% of cells were
Id1+ (L). Transfection with ev, Id1, p65 and IP
indicated that Id1, p65, and IP significantly increased the cell
cycle progression (M), cell counts (N), and BrdU incorporation (O)
of CA9-22 cells. *p<0.05. |
Id1 and p65 synergistically induce the
expression of Bmi-1 in association with cyclin D1 and Cdc2
proteins
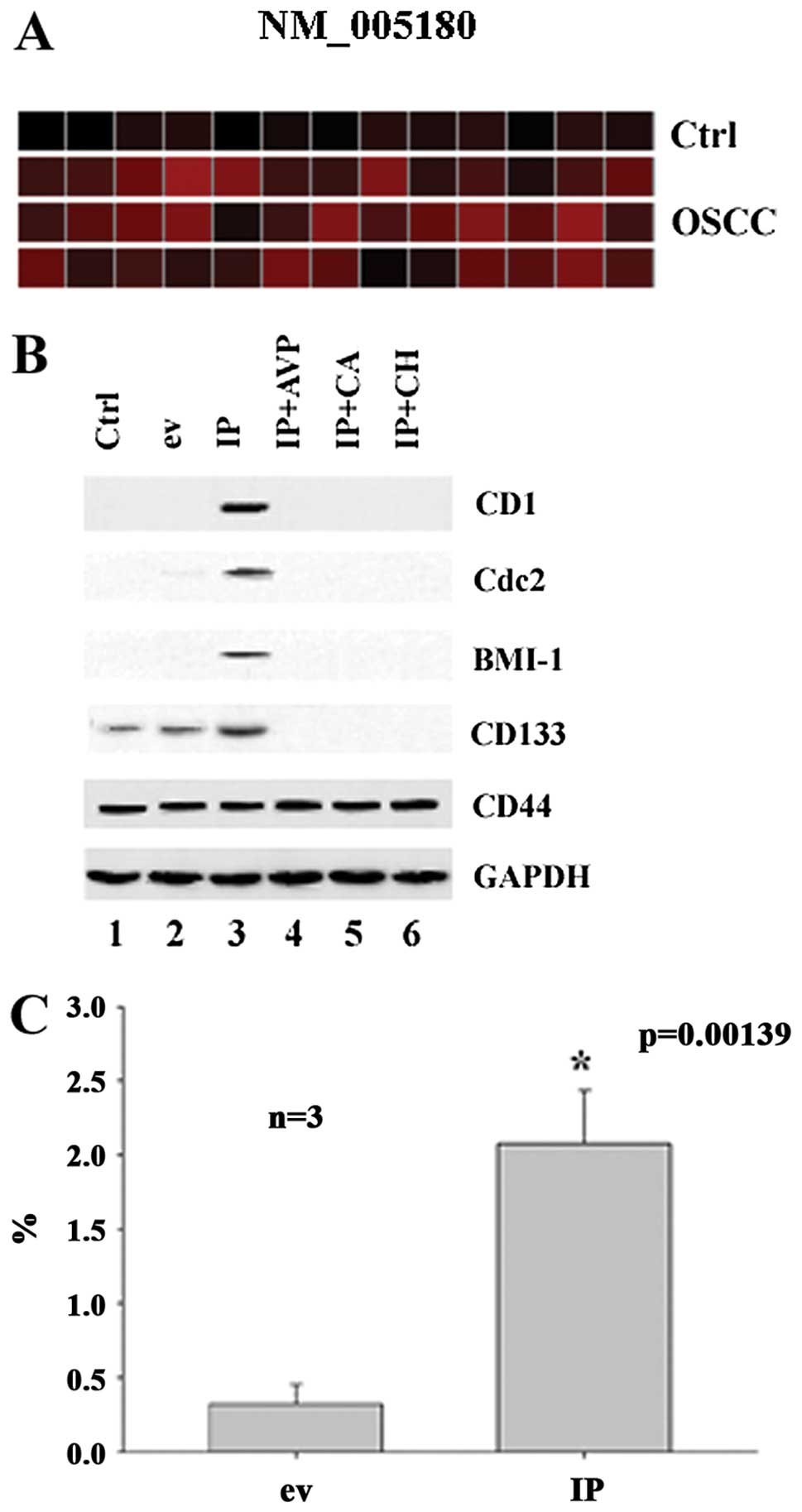
As shown in Fig.
4A, BMI-1 was detected in the majority of the 39 OSCC specimens
but not in 13 OSCC control tissues. To study whether Id1 and p65
are involved in the regulation of BMI-1, CD1 and Cdc2, we studied
the expression of BMI-1, CD1 and Cdc2 in CA9-22 cells using
molecular biological techniques. It was found that Id1+p65
synergistically induced the expression of Bmi-1, CD1, Cdc2 in
CA9-22 cells (Fig. 4B). For
verification of this finding, we transfected the cells with Id1 and
p65 and examined the expression of Bmi-1. It was found that Id1+p65
significantly upregulated the expression of BMI-1 in CA9-22 cells
by FACS (Fig. 4C). This indicates
that Id1 and p65 synergistically regulate the expression of BMI-1
probably in association with the protein of CD1 and Cdc2 in OSCC
cell cultures (CA9-22).
CD133+ and BMI-1+
cells initiate xenograft tumors in SCID mice
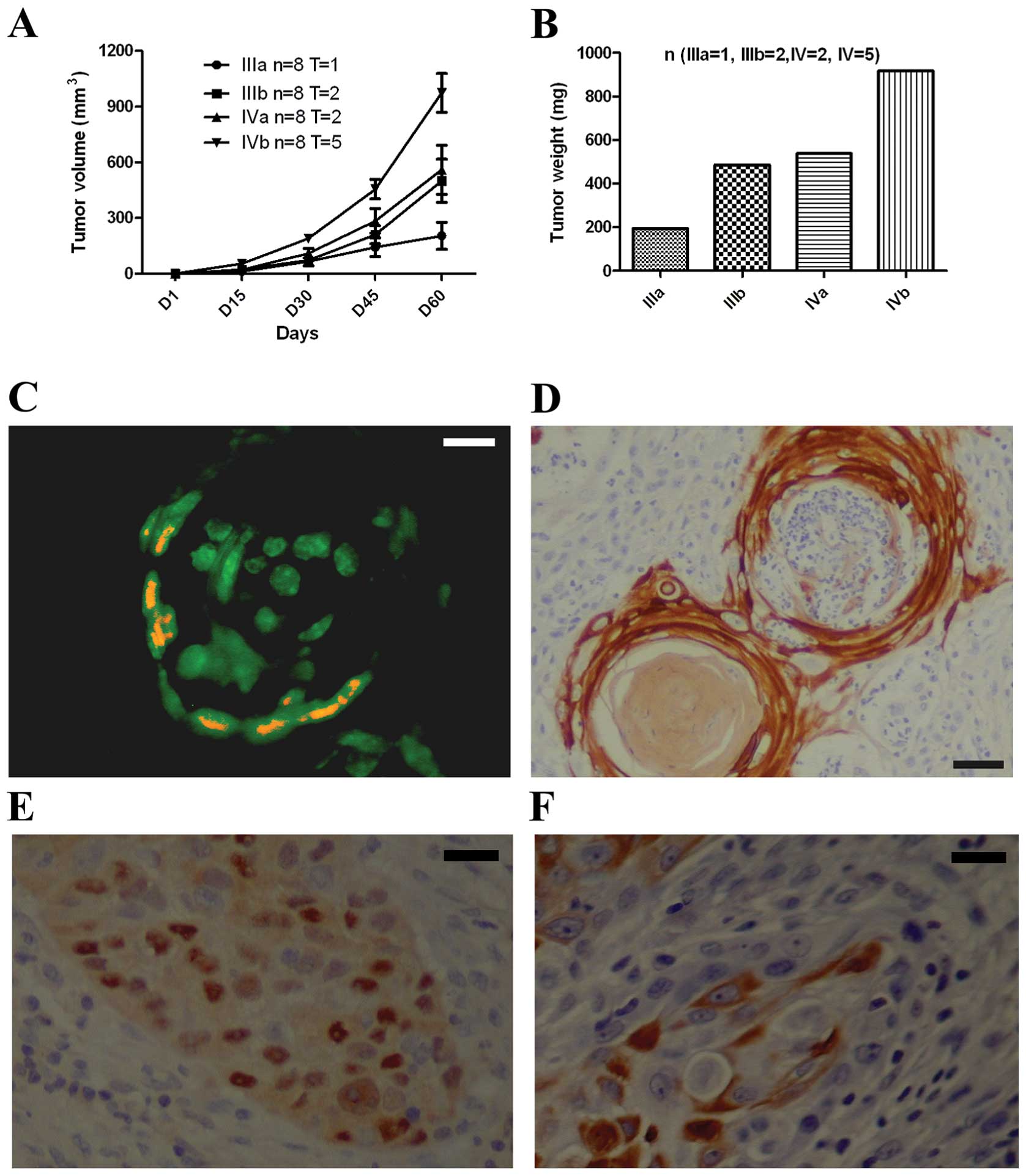
To study the tumorigenic potential of both
CD133+ and BMI-1+ cells, we sorted out
CD133+ cells from OSCC and CA9-22 cell cultures and then
injected into SCID/Beige mice. These CD133+ cells are
also BMI-1+ by FACS. At 100 and 1,000 cells, namely,
groups I and II, there were no xenograft tumors in animals. At
10,000 CD133+ and BMI-1+ keratinocytes of
CA9-22 and OSCC cells, there were one and two xenograft tumors,
respectively, from CA9-22- and OSCC-injected animals. At 100,000
CD133+ and BMI-1+ keratinoyctes, there were
two and five xenograft tumors, respectively, from CA9-22- and
OSCC-injected animals (Fig. 5A and
B). No xenograft tumors were found in animals with injection of
CD133− and BMI-1− cells. Tissue sections were
then stained with antibodies against Id1, NF-κB, CD133 and BMI-1.
It was found that Id1 and NF-κB were co-expressed in the edge of
xenograft tumors (Fig. 5C and D).
It is noted that Id1 is expressed in the entire xenograft tumor
lump whereas NF-κB is in the edge of xenograft tumors. BMI-1 was
found in the nuclei of xenograft tumors (Fig. 5E) whereas CD133 was found in the
membrane of xenograft tumors (Fig.
5F).
Discussion
CD133+ and BMI-1+
keratinocytes exist in OSCC clinical specimens, tissue cultures,
and OSCC cell lines such as CA9-22 as shown in this study. The
CD133 positive cells are few so that the protein is not abundant
(faint bands) by western blot analysis but clearly observed in
tissue cultures with dual immunohistochemistry staining.
CD133+ BMI-1+ keratinoyctes from both OSCC
tissue cultures and CA9-22 cell cultures are capable of growing
xenograft tumors in SCID/Beige mice. This confirms the importance
of CD133+ and BMI-1+ subpopulation in the
carcinogenesis of OSCC. It is becoming clear that there is a stem
cell like subpopulation, that is, the CD133+ and
BMI-1+ cells in OSCC specimens, OSCC tissue cultures,
and OSCC cell lines, which is responsible for the initiation of
OSCC.
This subpopulation is relevant to both Id1 and NF-κB
subunit p65 which are frequently expressed in head and neck cancer
including OSCC (8). In this study,
we particularly examined the co-expression of both Id1 and p65 and
it was found that both proteins are expressed in the OSCC tissue
specimens (Fig. 2A). The in
vitro studies indicate the synergy of both the Id1 and p65
proteins in OSCC to regulate the expression of BMI-1 (Fig. 2D). Id1 and p65 alone upregulated
the expression of CD133 but was not sufficient for the upregulation
of BMI-1. In the literature, the expression of the BMI-1 gene is
related to the generation of naïve keratinocytes (38) and is also key to generation of stem
cells in normal tissues (38–40).
In OSCC, BMI-1 induction requires the synergy of both the Id1 and
NF-κB subunit p65. In addition, the expression of BMI-1 is related
to human papillomavirus (HPV) infection of the upper respiratory
tract (35,41). HPV E6/E7 oncogenes correlate with
the expression of Id1 (42). It
appears that both Id1 and NF-κB are related to chronic inflammation
in the upper respiratory tract.
The importance of this subpopulation lies in its
potential to initiate the growth of xenograft tumors in SCID/Beige
mice. In comparison with other subpopulations such as
CD44+ subpopulation, CD133+ cells are indeed
a minority cell subpopulation, accounting for less than 3% in total
cell population, and more aggressive than others. With 1,000 cells,
CD133+ and BMI-1+ subpopulations fail to grow
any xenograft tumors in SCID/Beige mice whereas CD133+
and BMI-1+ cells at 100,000 succeeded in growing
xenograft tumors in 62.5% of SCID/Beige mice. CD133+ and
BMI-1+ keratinoyctes from the OSCC tissue cultures and
CA9-22 cell cultures are linked with Id1 and p65 as well as cell
proliferation and renewal.
Carcinogenesis of OSCC involves multiple signaling
pathways. One is the Id1-CD133 pathway in which Id1 plays an
important role in the naïve behavior of OSCC. In our previous study
(8), we demonstrated that Id1 is
involved in the upregulation of the PI3K/Akt and survivin pathways
which promote the survival of cancer cells. In addition to cell
survival, we now learn that Id1 also makes cancer cells naïve by
increasing the expression of CD133. Apparently, CD133 is associated
with BMI-1. These cell markers indicate their stemness of the
CD133+ and BMI-1+ subpopulation when it is
linked to the above signaling pathways (Id1 and NF-κB pathways)
which are relevant to chronic inflammation.
Furthermore, the synergy of both Id1 and p65 in
immortalized mucosal keratinocytes (HOK16B) resulted in the
upregulation of BMI-1. This signaling pathway plays an important
role in the self-renewal of immortalized mucosal keratinocytes.
This may promote cells to a malignant level of keratinocytes,
associated with chronic infections such as HPV in the larynx.
Similar situation is also observed in nasopharyngeal carcinoma, as
shown in our recent study, Id1 and NF-κB subunit p65 in
nasopharyngeal cancer are important factors for prediction of
clinically poor outcomes (9).
Recently, CD133 has been shown to be present in
stem-like cells in head and neck squemous cell carcinoma (34). In addition, we have observed in our
laboratory that Id1 regulates the expression of CD44 (data not
shown). CD44 in head and neck cancer has been shown to be present
in cancer stem-like cells in head and neck cancer (1). These data collectively suggest that
Id1 is an important transcription factor for the carcinogenesis of
OSCC by blocking the differentiation of mucosal keratinocytes and
promoting the generation of naïve keratinocytes. NF-κB also
contributes to this carcinogenesis in conjunction with Id1. Id1
usually potentiates the activity of NF-κB in keratinocytes
(15,43). We have shown in our previous study
that the Id1 and NF-κB genes are upregulated in approximately
60–70% of head and neck cancer specimens by microarrays and
immunohistochemistry analyses (8).
In this study, we found that the CD133+
and BMI-1+ cells are upregulated in OSCC tissues, and
act like cancer stem cells. These cells initiate the growth of
xenograft tumors derived from OSCC specimens and CA9-22 cell
cultures as well as HOK16B when transfected with Id1 and p65. Since
the potential of this subpopulation is not as high as genuine
cancer stem cells, it is called cancer stem-like cells. The
capability to initiate the growth of xenograft tumors in SCID/Beige
mice is certain, approximately 10,000 cells are required to grow
xenograft tumors in SCID/Beige mice. We note that there are 1–3% of
total cells in OSCC cell cultures positive for CD133 and BMI-1.
Consistent with this study, CD133+ cells are estimated
to be approximately 3-5% of the total cancer cell population in the
clinical head and neck cancer specimens and several head and neck
cancer cell lines of a referenced study (33).
Acknowledgements
This study was financially supported
by the NCI grants P01 CA055791 (M.A.B.), the Fujian Provincial Key
Project of Science and Technical Collaboration 2012I0003 grant no.
2060801 (L.J.H.), R03 CA107989 (J.L.), and the Lions grants (J.L.).
This study was performed in accordance with the PHS Policy on Human
Care and Use of Laboratory Animals, the NIH guide for the Care and
Use of Laboratory Animals, and the Animal Welfare Act (7 U.S.C. et
seq.); the animal use protocol was approved by the Institutional
Animal Care and Use Committee (IACUC) of the University of
Minnesota.
References
|
1.
|
Prince ME, Sivanandan R, Kaczorowski A, et
al: Identification of a subpopulation of cells with cancer stem
cell properties in head and neck squamous cell carcinoma. Proc Natl
Acad Sci USA. 104:973–978. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Song J, Chang I, Chen Z, Kang M and Wang
CY: Characterization of side populations in HNSCC: highly invasive,
chemoresistant and abnormal Wnt signaling. PLoS One. 5:e114562010.
View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Krishnamurthy S and Nor JE: Orosphere
assay: a method for propagation of head and neck cancer stem cells.
Head Neck. 35:1015–1021. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Campos MS, Neiva KG, Meyers KA,
Krishnamurthy S and Nor JE: Endothelial derived factors inhibit
anoikis of head and neck cancer stem cells. Oral Oncol. 48:26–32.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Krishnamurthy S and Nor JE: Head and neck
cancer stem cells. J Dent Res. 91:334–340. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Zhang Z, Filho MS and Nor JE: The biology
of head and neck cancer stem cells. Oral Oncol. 48:1–9. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Ginos MA, Page GP, Michalowicz BS, et al:
Identification of a gene expression signature associated with
recurrent disease in squamous cell carcinoma of the head and neck.
Cancer Res. 64:55–63. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Lin J, Guan Z, Wang C, et al: Id1
regulates the survival of HNSCC via the NF-κB/survivin and PI3K/Akt
signaling pathways. Clin Cancer Res. 16:77–87. 2010.
|
|
9.
|
Sun W, Guo MM, Han P, et al: Id-1 and the
p65 subunit of NF-kappaB promote migration of nasopharyngeal
carcinoma cells and are correlated with poor prognosis.
Carcinogenesis. 33:810–817. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Yuen HF, Chan YP, Chan KK, et al: Id-1 and
Id-2 are markers for metastasis and prognosis in oesophageal
squamous cell carcinoma. Br J Cancer. 97:1409–1415. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Ling MT, Wang X, Ouyang XS, Xu K, Tsao SW
and Wong YC: Id-1 expression promotes cell survival through
activation of NF-kappaB signalling pathway in prostate cancer
cells. Oncogene. 22:4498–4508. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Ling MT, Lau TC, Zhou C, et al:
Overexpression of Id-1 in prostate cancer cells promotes
angiogenesis through the activation of vascular endothelial growth
factor (VEGF). Carcinogenesis. 26:1668–1676. 2005. View Article : Google Scholar
|
|
13.
|
Caicedo-Granados EE, Wuertz BR and Ondrey
FG: Enforced expression of nuclear factor kappa B in p53 deficient
keratinocytes induces cell cycle, angiogenic potential and
tumorigenesis. Oral Oncol. 48:303–310. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Alani RM, Hasskarl J, Grace M, Hernandez
MC, Israel MA and Munger K: Immortalization of primary human
keratinocytes by the helix-loop-helix protein, Id-1. Proc Natl Acad
Sci USA. 96:9637–9641. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Hamajima Y, Komori M, Preciado DA, et al:
Id1 induces proliferation of keratinocytes via the NF-kB/cyclin D1
pathway: the pathological basis for cholesteatoma. Cell
Proliferation. 43:457–463. 2010.PubMed/NCBI
|
|
16.
|
Jordan CT, Guzman ML and Noble M: Cancer
stem cells. N Engl J Med. 355:1253–1261. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
O’Brien CA, Pollett A, Gallinger S and
Dick JE: A human colon cancer cell capable of initiating tumour
growth in immunodeficient mice. Nature. 445:106–110.
2007.PubMed/NCBI
|
|
18.
|
Ricci-Vitiani L, Lombardi DG, Pilozzi E,
et al: Identification and expansion of human
colon-cancer-initiating cells. Nature. 445:111–115. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Singh J, Ling LE, Sawyer JS, Lee WC, Zhang
F and Yingling JM: Transforming the TGFbeta pathway: convergence of
distinct lead generation strategies on a novel kinase pharmacophore
for TbetaRI (ALK5). Curr Opin Drug Discov Devel. 7:437–445.
2004.PubMed/NCBI
|
|
20.
|
Chang CC, Shieh GS, Wu P, Lin CC, Shiau AL
and Wu CL: Oct-3/4 expression reflects tumor progression and
regulates motility of bladder cancer cells. Cancer Res.
68:6281–6291. 2008. View Article : Google Scholar
|
|
21.
|
Chiou SH, Yu CC, Huang CY, et al: Positive
correlations of Oct-4 and Nanog in oral cancer stem-like cells and
high-grade oral squamous cell carcinoma. Clin Cancer Res.
14:4085–4095. 2008. View Article : Google Scholar
|
|
22.
|
Bao S, Wu Q, Sathornsumetee S, et al: Stem
cell-like glioma cells promote tumor angiogenesis through vascular
endothelial growth factor. Cancer Res. 66:7843–7848. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Diehn M, Cho RW and Clarke MF: Therapeutic
implications of the cancer stem cell hypothesis. Semin Radiat
Oncol. 19:78–86. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Corbeil D, Fargeas CA and Huttner WB: Rat
prominin, like its mouse and human orthologues, is a pentaspan
membrane glycoprotein. Biochem Biophys Res Commun. 285:939–944.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Corbeil D, Roper K, Fargeas CA, Joester A
and Huttner WB: Prominin: a story of cholesterol, plasma membrane
protrusions and human pathology. Traffic. 2:82–91. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Yin AH, Miraglia S, Zanjani ED, et al:
AC133, a novel marker for human hematopoietic stem and progenitor
cells. Blood. 90:5002–5012. 1997.
|
|
27.
|
Corbeil D, Roper K, Hellwig A, et al: The
human AC133 hematopoietic stem cell antigen is also expressed in
epithelial cells and targeted to plasma membrane protrusions. J
Biol Chem. 275:5512–5520. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Shmelkov SV, St Clair R, Lyden D and Rafii
S: AC133/CD133/Prominin-1. Int J Biochem Cell Biol. 37:715–719.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Salven P, Mustjoki S, Alitalo R, Alitalo K
and Rafii S: VEGFR-3 and CD133 identify a population of
CD34+lymphatic/vascular endothelial precursor cells.
Blood. 101:168–172. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Wu A, Oh S, Wiesner SM, et al: Persistence
of CD133+cells in human and mouse glioma cell lines:
detailed characterization of GL261 glioma cells with cancer stem
cell-like properties. Stem Cells Dev. 17:173–184. 2008.
|
|
31.
|
Wu A, Wiesner S, Xiao J, et al: Expression
of MHC I and NK ligands on human CD133+glioma cells:
possible targets of immunotherapy. J Neurooncol. 83:121–131. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Wu CJ: Immunologic targeting of the cancer
stem cell. StemBook (Internet). Cambridge, MA: Harvard Stem Cell
Institute; Dec 15–2008
|
|
33.
|
Damek-Poprawa M, Volgina A, Korostoff J,
et al: Targeted inhibition of CD133+cells in oral cancer
cell lines. J Dent Res. 90:638–645. 2011. View Article : Google Scholar
|
|
34.
|
Chen YS, Wu MJ, Huang CY, et al: CD133/Src
axis mediates tumor initiating property and epithelial-mesenchymal
transition of head and neck cancer. PLoS One. 6:e280532011.
View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Miller J, Dakic A, Chen R, et al: HPV16 E7
protein and hTERT proteins defective for telomere maintenance
cooperate to immortalize human keratinocytes. PLoS Pathog.
9:e10032842013. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: the next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Park NH, Min BM, Li SL, Huang MZ, Cherick
HM and Doniger J: Immortalization of normal human oral
keratinocytes with type 16 human papillomavirus. Carcinogenesis.
12:1627–1631. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Cordisco S, Maurelli R, Bondanza S, et al:
Bmi-1 reduction plays a key role in physiological and premature
aging of primary human keratinocytes. J Invest Dermatol.
130:1048–1062. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39.
|
Lessard J and Sauvageau G: Bmi-1
determines the proliferative capacity of normal and leukaemic stem
cells. Nature. 423:255–260. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
40.
|
Molofsky AV, Pardal R, Iwashita T, Park
IK, Clarke MF and Morrison SJ: Bmi-1 dependence distinguishes
neural stem cell self-renewal from progenitor proliferation.
Nature. 425:962–967. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
41.
|
Itakura M, Mori S, Park NH and Bonavida B:
Both HPV and carcinogen contribute to the development of resistance
to apoptosis during oral carcinogenesis. Int J Oncol. 16:591–597.
2000.PubMed/NCBI
|
|
42.
|
Yasmeen A, Bismar TA, Kandouz M, Foulkes
WD, Desprez PY and Al Moustafa AE: E6/E7 of HPV type 16 promotes
cell invasion and metastasis of human breast cancer cells. Cell
Cycle. 6:2038–2042. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43.
|
Yang Y, Liou HC and Sun XH: Id1
potentiates NF-kappaB activation upon T cell receptor signaling. J
Biol Chem. 281:34989–34996. 2006. View Article : Google Scholar : PubMed/NCBI
|