Introduction
Nasopharyngeal carcinoma (NPC) is particularly
prevalent in southern China and Southeast Asia (1). Radiotherapy is effective against
early stage NPC; however, over 70% of cases present with late stage
diseases and only 10–40% of these patients survive more than 5
years (2–4). Currently the mainstay of treatment
for locoregional advanced cases of NPC is concurrent
chemoradiotherapy. Unfortunately, undesirable complications
frequently occur after treatment because of the location of the
tumour at the base of the skull, closely surrounded by, and in
close proximity to, many vital structures that result in high
morbidity and poor quality of life (5). Unlike other head and neck cancers,
NPC is consistently associated with Epstein-Barr virus (EBV)
infection (6). EBV latent gene
expression in NPC is restricted to EBNA1, BARF1, variable
expression of LMP1 and LMP2A, and consistent expression of the
non-coding EBER1/2 RNAs and BARTs, a family of viral microRNAs. The
molecular events that drive the progression of NPC, including the
exact contribution of EBV to the pathogenesis of NPC, are still to
be elucidated.
The Wnt signalling pathways have historically been
divided into two classes: namely the canonical and non-canonical
pathways. The canonical signalling pathway induces the nuclear
accumulation and transcriptional activation of β-catenin. It is the
most intensively studied Wnt pathway implicated in cancer
development by promoting cancer cell proliferation and migration
(7). By contrast, the
non-canonical pathway is essentially an umbrella term for all
Wnt-activated cellular signalling pathways that do not promote
β-catenin-mediated transcription. The non-canonical and planar cell
polarity (PCP) pathways promote calcium mobilization and activate
downstream pathways involved in cell motility and metastasis.
However, emerging evidence suggests that these pathways are not as
autonomous as originally thought and there may be cross-talk
between these two pathways (8).
WNT5A is a representative of Wnt protein that activates
non-canonical Wnt signalling, it can, under certain circumstances,
signal through the canonical pathway (8). A number of studies indicates that
WNT5A has a tumour-suppressing effect with reduced expression being
reported in colorectal cancer (9,10),
neuroblastoma (11), ductal breast
cancer (12,13) and leukemias (14–16).
Conversely, some studies have reported overexpression of WNT5A in
melanoma (17), breast cancer
cells (18), gastric cancer
(19), pancreatic cancer (20), non-small-cell lung cancer (21) and prostate cancer (22), indicating that WNT5A may function
as an oncogene in these tumours. Collectively, these data suggest
that WNT5A can function as either a tumour suppressor gene or an
oncogene depending on the cancer type, and it is possible that
interactions between the canonical and non-canonical pathways may
explain these discrepancies.
Using expression microarrays, we have previously
identified WNT5A as one of the upregulated genes in EBV-positive
primary NPC tumours compared with cancer-free nasopharyngeal tissue
samples (23). This observation is
supported by evidence showing that WNT5A protein is overexpressed
in primary NPC tissues (24,25).
However, the functional role of WNT5A in NPC and the contribution
of EBV to its deregulation have not been investigated. In the
present study, the upregulation of WNT5A was further validated in
NPC tissues and a dramatic increase in WNT5A expression was
observed in LMP2A-expressing NPC cell lines, indicating LMP2A
contributes to the upregulation of WNT5A in NPC. Ectopic expression
of WNT5A in NPC cell lines significantly promotes cell
proliferation, migration and invasion. These data suggest that
WNT5A appears to have tumour-promoting activity in EBV-associated
NPC.
Materials and methods
Cell lines and tissue samples
The cell lines used in this study included: NP69 and
NP460, immortalised nasopharyngeal epithelial cell lines; eight
NPC-derived cell lines, of which seven were EBV negative (TW01,
TW04, HONE1, SUNE1, HK1, CNE1 and CNE2) and one of which was
EBV-positive (C666-1). NP69, NP460 and seven EBV-negative cell
lines were a kind gift from Professor S.W. Tsao (Department of
Anatomy, University of Hong Kong, Hong Kong, China), and C666-1 was
kindly provided by Professor K.W. Lo (Prince of Wales Hospital,
Hong Kong, China). NPC cell lines stably expressing LMP2A have been
described previously (26,27) and similar protocols were used to
generate cells expressing EBNA1 and LMP1 (28).
Snap-frozen nasopharyngeal biopsies from 16 patients
were included in the quantitative real-time PCR analysis: 14 with
undifferentiated EBER-positive NPC, and two with histologically
normal nasopharynx epithelial cells, with no evidence of malignancy
and EBER-negative. All tissue samples were collected from Tung Shin
Hospital, Kuala Lumpur, Malaysia. This study was approved by the
Medical Research Ethics Committee of the Ministry of Health,
Malaysia (KKM/NIHSEC/08/0804/MRG-IMR).
Quantitative real-time PCR and
semi-quantitative PCR
Total RNA was extracted using RNeasy mini kit
(Qiagen, Manchester, UK) and subjected to reverse transcription
using oligo (dT) primer and Superscript II (Invitrogen, Carlsbad,
CA, USA). Quantitative real-time PCR (Q-PCR) was performed in
triplicate using ABI Prism 7000 Sequence Detection System and
TaqMan Gene Expression Assays (WNT5A: Hs00998537_m1; Applied
Biosystems, Foster City, CA, USA). In parallel, GAPDH was amplified
in the same reaction to serve as an internal control for
normalization. Fold changes in gene expression between control and
samples were measured using the comparative threshold cycle method
(Delta;Delta;Ct). Semi-quantitative PCR was performed to examine
the WNT5A mRNA level in a panel of 16 human normal organs using the
Human MTC™ Panel I & II (Clontech, Mountain View, CA, USA). The
primers for WNT5A were 5′-CATTATGGGCTCAAATAGAAAGAAGA-3′ and
5′-AAAGAGCTAGGGTAGGCAACTAAAACT-3′.
Western blot analysis
Cells were lysed in ice-cold RIPA buffer containing
protease inhibitor cocktail (Roche, Indianapolis, IN, USA) and the
extracted protein was subjected to western blot analysis. The
primary antibody used was WNT5A (1:500, R&D Systems,
Minneapolis, MN, USA) and the secondary antibody was HRP-conjugated
anti-goat IgG (1:5,000, Chemicon, Temecula, CA, USA). The protein
bands were detected with chemiluminescent reagent, Perkin Elmer
plus (Perkin-Elmer, Waltham, MA, USA) or West Femto (Pierce,
Rockford, IL, USA).
Retroviral transduction and establishment
of stable cell lines
The recombinant retroviral vectors pLNC-WNT5A or
pLNCX alone were used to establish TWO4 and HONE1 NPC cells stably
overexpressing WNT5A and the vector control. Briefly, the
retroviral vectors were transfected into retroviral packaging cell
lines, Phoenix Ampho using Lipofectamine 2000 (Invitrogen). At 48 h
post-transfection, the retroviral-containing supernatant was
centrifuged and filtered through 0.45 μm syringe filter
(Millipore, Billerica, MA, USA). The viral supernatant was then
added to plates cultured with TWO4 or HONE1 cells. Polybrene (0.8
μg/ml; Chemicon) was also added to the culture media. After
the transduction (24 h), cells were selected with 500 μg/ml
neomycin. Drug-resistant cells were then pooled and tested for
WNT5A expression by western blot analysis.
Cell proliferation assay
Cells (3–6×103) were seeded in a 96-well
plate in triplicate. Cell proliferation was determined daily by
adding 20 μl of MTT (thiazolyl blue tetrazolium bromide,
Sigma, St. Louis, MO, USA) stock solution (5 mg/ml) to each well
for 4 days. After 4 h of incubation, medium was removed carefully
and 100 μl DMSO was added to dissolve the blue crystals. The
absorbance was measured on an ELISA plate reader at a wavelength of
570 nm with reference wavelength of 630 nm.
Wound healing assay
Cells were grown as a monolayer in 6-well plates
coated with fibronectin (20 μg/ml) and serum starved
overnight. Cells were then treated with mitomycin C (25
μg/ml) for 2 h to inhibit cell proliferation. A sterile
pipette tip was used to scratch a wound along the centre of the
well. A demarcated area of the wound was photographed on an
inverted Olympus IX71 microscope at the time of wounding (0 h) and
at various time of wound healing.
Cell migration and invasion assays
Migration and invasion assays were carried out using
fibronectin-coated (10 μg/ml) and Matrigel-coated
polycarbonate filters (8-μm pore size, Transwell Costar
Corning; Corning, NY, USA), respectively, as described previously
(29). Cells were plated into the
upper chamber and allowed to migrate for 24 h (for migration assay)
or 48 h (for invasion assay). Cells migrating/invading to the lower
chamber were trypsinized and counted on a Casy 1 counter (Schärfe
System GmbH, Reutlingen, Germany). Statistical differences between
experimental groups were evaluated by Student’s t-test.
Results
Overexpression of WNT5A in EBV-positive
NPC
Using microarray analysis, we previously
demonstrated upregulation of WNT5A in 20/25 (80%) of EBV-positive
NPC tissue samples, while expression in normal nasopharyngeal
epithelium was low or absent (23). In agreement with the microarray
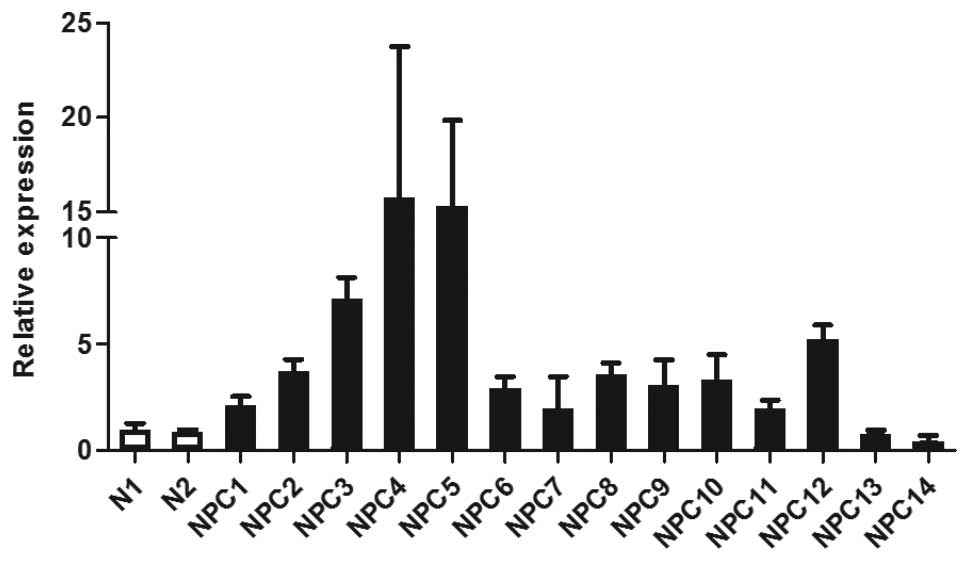
data, Q-PCR showed that WNT5A mRNA levels were elevated in 12 NPC
tissue samples available for analysis when compared to two
non-malignant controls (Fig. 1).
Two NPC samples (NPC13 and NPC14) that did not overexpress WNT5A in
the microarray analysis also did not show an elevated level of
WNT5A by Q-PCR, confirming the validity of the microarray data.
LMP2A upregulates WNT5A expression
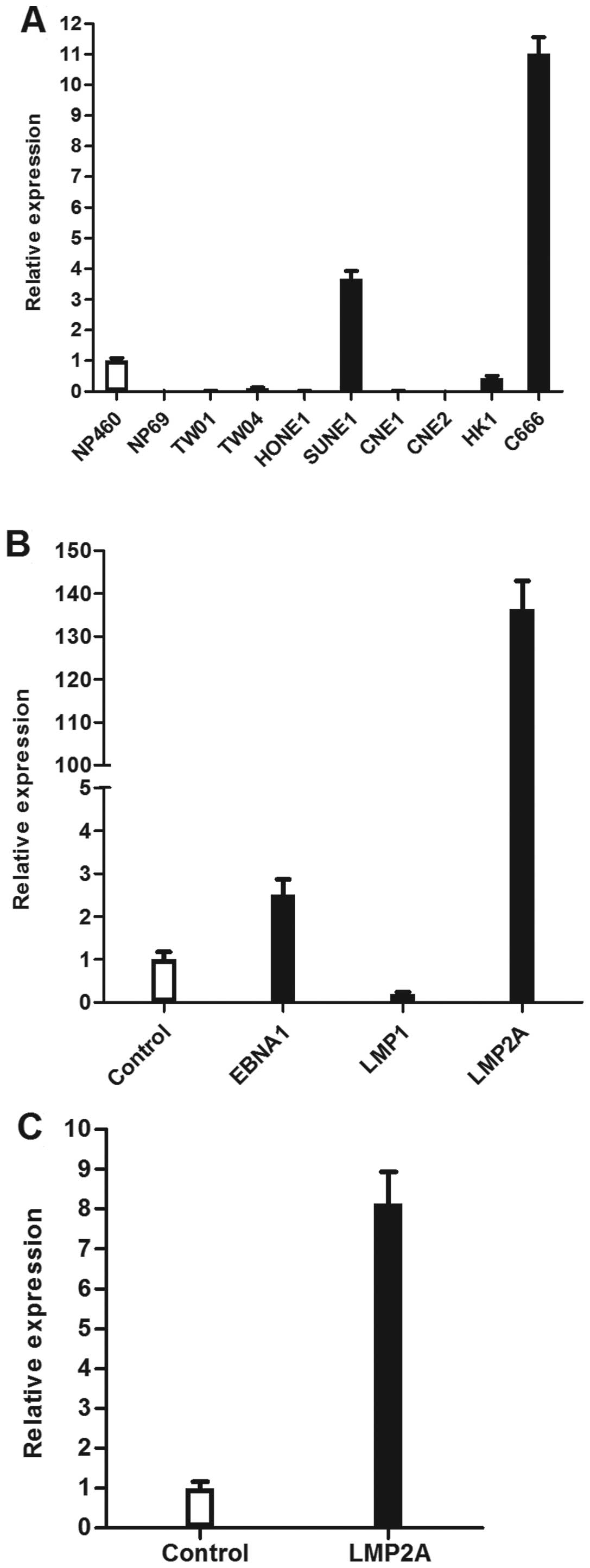
The expression of WNT5A was heterogeneous in NPC
cell lines. Nonetheless, particularly high levels of WNT5A
expression were observed in the only EBV-positive cell line,
C666-1, compared to the a panel of EBV-negative cell lines, which
included seven NPC cell lines (CNE1, CNE2, HONE1, HK1, SUNE1, TW01,
TW04) and two immortalised nasopharyngeal epithelial cell lines
(NP460, NP69; Fig. 2A).
To further investigate which EBV gene is responsible
for the upregulation of WNT5A, the expression of WNT5A was examined
in HONE1 cells independently transfected with three EBV latent
genes (EBNA1, LMP1 and LMP2A). The results showed that levels of
WNT5A mRNA were significantly upregulated in cells transfected with
EBNA1 and LMP2A (p<0.01; Fig.
2B). Due to the robust upregulation of WNT5A in
LMP2A-expressing cells, we further examined the expression of WNT5A
in another NPC cell line, CNE2, transfected with LMP2A. We
confirmed that LMP2A stimulated WNT5A expression in NPC cells
(p<0.01; Fig. 2C).
WNT5A promotes cell growth
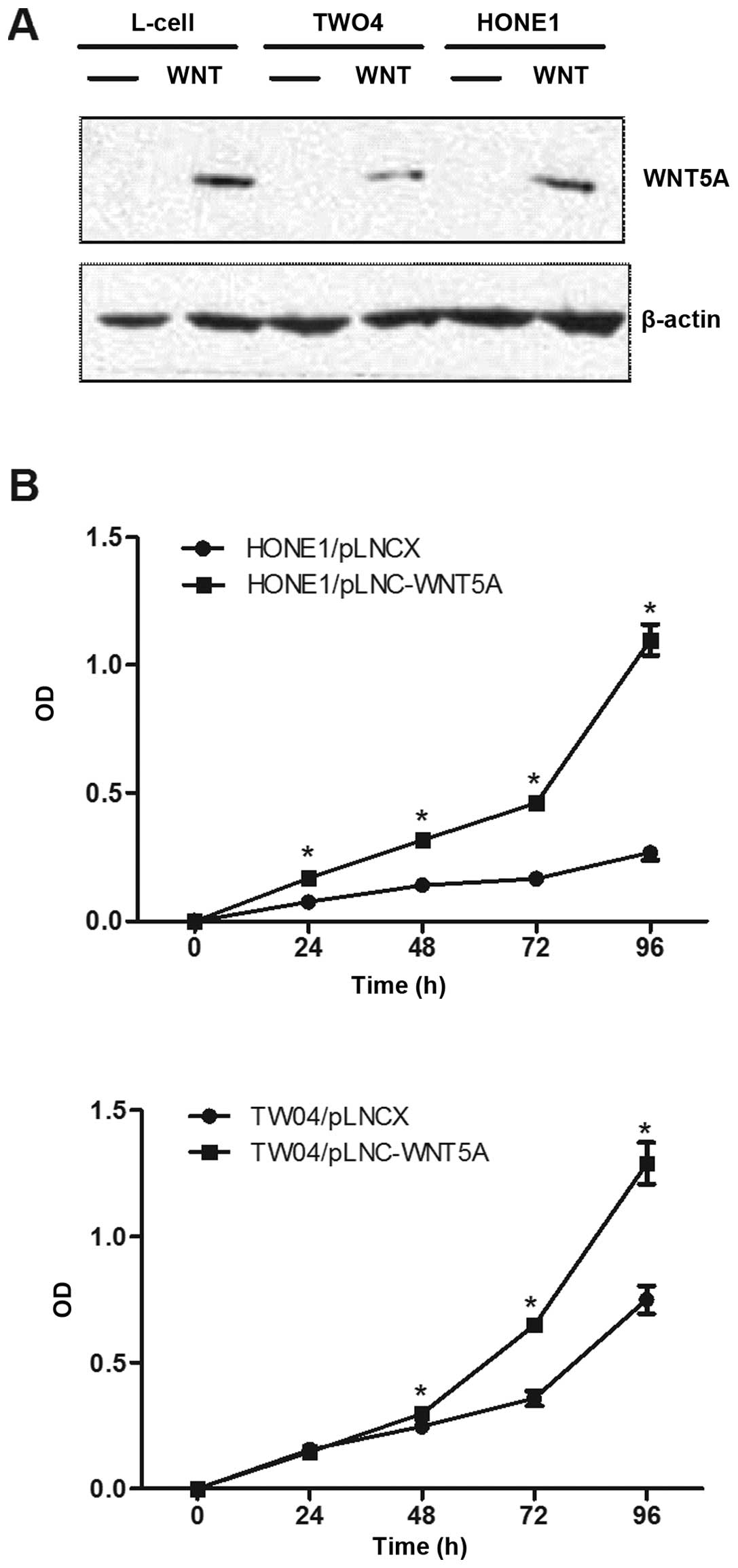
To investigate the functional role of WNT5A in NPC,
two NPC cell lines which expressed low levels of endogenous WNT5A,
HONE1 and TW04, were stably transduced with recombinant retroviral
vector pLNCX containing the WNT5A cDNA (designated pLNC-WNT5A) or
with the vector alone (designated pLNC). The expression of WNT5A
protein in these two cell lines was confirmed by western blot
analysis (Fig. 3A).
We next examined the effect of ectopic expression of
WNT5A on the growth of NPC cells using MTT assays. As shown in
Fig. 3B, HONE1 and TW04 cells
stably expressing WNT5A grew significantly faster than the vector
control cells (p<0.01), indicating that the WNT5A promotes cell
proliferation.
WNT5A promotes cell migration and
invasion
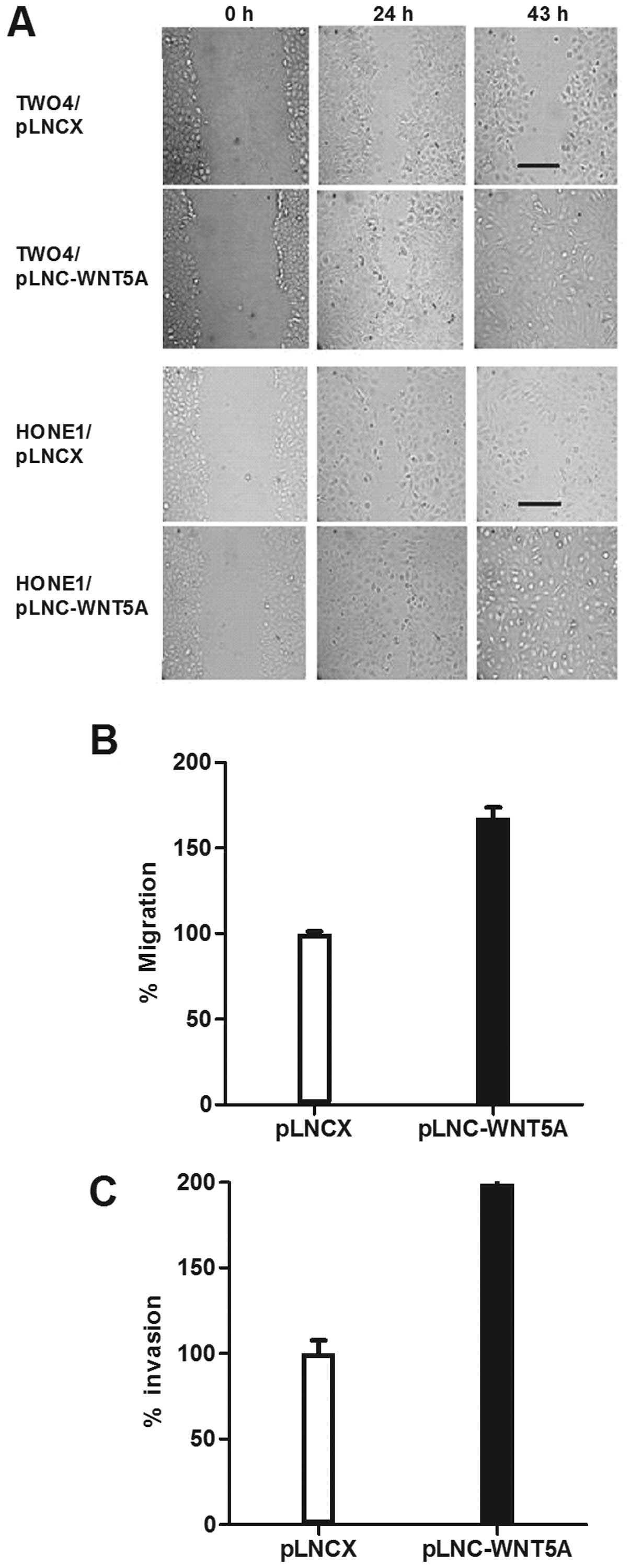
To investigate whether WNT5A functions to promote
cell migration in NPC, a wound-healing assay was performed using
HONE1 and TW04 transfected cells. Compared to the vector controls,
an increase in cell motility was observed in cells expressing WNT5A
(Fig. 4A). This result was further
confirmed using standard Transwell assays which showed that
migration of HONE1 cells expressing WNT5A was significantly
enhanced (p<0.01; Fig. 4B).
We next examined the effect of WNT5A on the
invasiveness of NPC cells using Matrigel invasion assays.
WNT5A-transfected HONE1 cells were 2 times more invasive than the
vector controls (p=0.01; Fig.
4C).
Discussion
NPC is the most common cancer arising in
nasopharynx. Despite recent advances in treatment regimens, NPC
patients continue to have generally poor prognoses due to unwanted
side-effects and a subset of tumours is resistant to radiotherapy
and chemotherapy. The identification of genes differentially
expressed between normal and malignant cells may lead to the
identification of biomarkers and novel therapeutic targets for this
disease. We have previously shown that WNT5A is overexpressed in
NPC relative to cancer-free controls (23). WNT5A is one of the most highly
investigated Wnt proteins that activate non-canonical Wnt
signalling. At present, the role of WNT5A in human cancer is
controversial and the discrepancy may be attributable to
differences in receptor context or cell context of cancer cells.
Although aberrant Wnt signalling has been implicated in the
development of NPC, these early studies mainly focused on the
canonical Wnt/β-catenin pathway which is often activated in human
cancers (30). Recently,
immunohistochemical data have shown that the WNT5A protein is
overexpressed in primary NPC tissues (24,25),
suggesting a dysregulation of non-canonical pathway in NPC.
However, information regarding the biological role of WNT5A in the
pathogenesis of NPC or the mechanisms of its overexpression has not
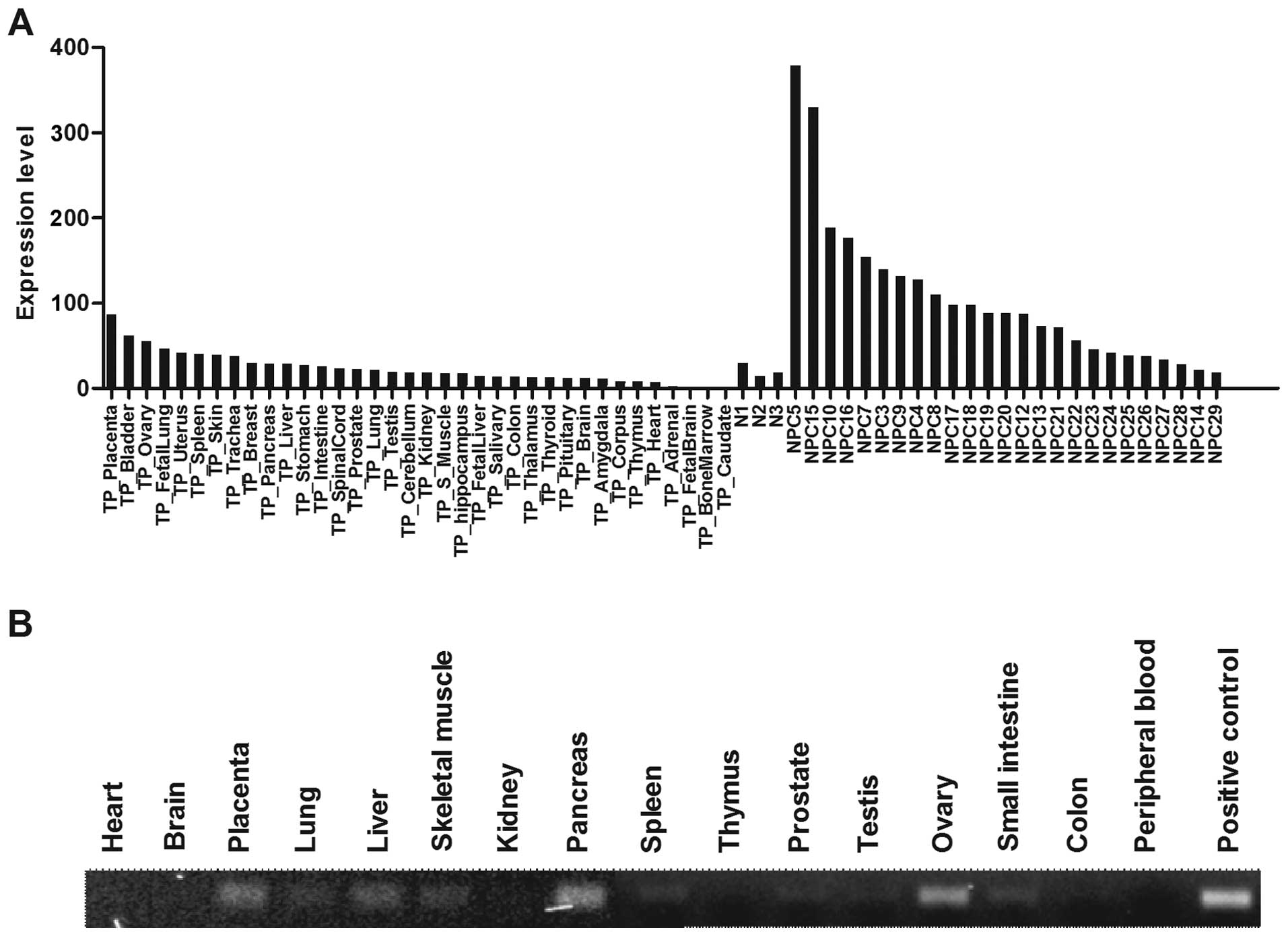
been explored. It should be noted that a comparison between our NPC
microarray data and a published microarray study using 36 normal
human organs (31) revealed that
the levels of WNT5A expression in NPC was higher than that observed
in a wide range of normal organs, with exception of placenta, ovary
and bladder (Fig. 5A). Similarly,
we showed that the expression of WNT5A was low in various normal
human organs using Multiple cDNA Panels (BD BioSciences, Franklin
Lakes, NJ, USA) by PCR analysis (Fig.
5B). These observations suggest that WNT5A or its downstream
signalling events could be potentially useful targets for treatment
of NPC.
In this study, we further confirmed the upregulation
of WNT5A mRNA in primary NPC tissue samples by QPCR, supporting the
hypothesis that WNT5A exhibits an oncogenic effect in NPC. The
strong etiological link between EBV infection and NPC is well
recognised (6). Most, if not all,
NPC cases in endemic regions, such as Malaysia, are associated with
EBV infection (23,32). Interestingly, in the present study,
very high levels of WNT5A expression were observed in the only
EBV-positive NPC cell line, C666.1, compared to a panel of
EBV-negative epithelial cells. Further, we found that the levels of
WNT5A were dramatically increased in HONE1 and CNE2 cells stably
expressing the EBV-encoded oncogene LMP2A. LMP2A mRNA is regularly
detected in NPC and when expressed in certain immortalized
epithelial cell lines, it can induce anchorage-independent growth,
enhance cell adhesion and cell motility, and inhibit epithelial
cell differentiation (33).
Previous studies indicate that LMP2A can modulate the canonical Wnt
pathway in EBV-infected epithelial cells (34–36).
Here, we report, for the first time, that EBV infection,
particularly LMP2A, may also play a major role in inducing an
aberrant non-canonical Wnt signalling in NPC.
Uncontrolled cell growth and tissue
invasion/metastasis are hallmarks of cancer cells, and it is well
established that EBV-positive NPC is a highly metastatic cancer
(37). In tumours that express
elevated levels of WNT5A, WNT5A functions as an oncogene by
promoting cell proliferation and migration/invasion (20,38,39).
Similarly, we showed that WNT5A promotes cell growth and
migration/invasion of NPC cells, contributing to the acquisition of
a highly motile and invasive phenotype, a phenotype that is
consistent with the highly aggressive behaviour of NPC cells. In
melanoma, WNT5A exerted its pro-migratory and invasion effects by
activating PKC (39). This data
was supported by a subsequent study in gastric cancer in which
WNT5A was found to promote migration of cancer cells by stimulating
focal adhesion kinase (FAK) and Rac through the activation of PKC
and JNK (19). A recent study also
showed that WNT5A activated JNK through PKD to promote
aggressiveness of prostate cancer (40). These studies also demonstrated that
the expression of WNT5A and β-catenin in cancer cells was mutually
exclusive, suggesting WNT5A primarily functions through the
non-canonical planar cell polarity and Wnt calcium signalling
pathways.
Although WNT5A may inhibit the activation of
β-catenin-mediated transcription, there is evidence to suggest that
in the presence of specific forms of receptors, WNT5A could
stimulate cancer-promoting canonical Wnt signalling pathway in
certain cell types (41,42). This phenomenon has been shown in
pancreatic cancer that WNT5A mediated its pro-invasive effects
through β-catenin/TCF-dependent pathway (20). We have recently generated a
compendium of potential biomarkers for NPC by systematically
comparing the genes that are differentially expressed between NPC
and cancer-free controls from published microarray studies
(23,25,43–45).
In concordance with the previous studies reporting the
dysregulation of Wnt/β-catenin signalling in NPC, overexpression of
a number of genes involved in this pathway were commonly shown in
independent datasets (Table I).
These data suggest that further studies are warranted to elucidate
the signalling events downstream WNT5A in NPC.
 | Table I.Genes involved in the Wnt/β-catenin
signalling pathway commonly identified to be upregulated in primary
NPC tissues in microarray studies. |
Table I.
Genes involved in the Wnt/β-catenin
signalling pathway commonly identified to be upregulated in primary
NPC tissues in microarray studies.
| Gene symbol | Gene name |
|---|
| AKT3 | v-akt murine
thymoma viral oncogene homolog 3 (protein kinase B, gamma) |
| BIRC5 | Baculoviral IAP
repeat-containing 5 |
| CCND2 | Cyclin D2 |
| CD44 | CD44 molecule
(Indian blood group) |
| CDC42EP3 | CDC42 effector
protein (Rho GTPase binding) 3 |
| CLDN1 | Claudin 1 |
| DKK1 | Dickkopf homolog 1
(Xenopus laevis) |
| DVL3 | Dishevelled, dsh
homolog 3 (Drosophila) |
| EPHB1 | EPH receptor
B1 |
| FZD5 | Frizzled homolog 5
(Drosophila) |
| FZD6 | Frizzled homolog 6
(Drosophila) |
| FZD7 | Frizzled homolog 7
(Drosophila) |
| GREM1 | Gremlin 1 |
| JAG2 | Jagged 2 |
| JUN | Jun
proto-oncogene |
| LGR5 | Leucine-rich
repeat-containing G protein-coupled receptor 5 |
| LEF1 | Lymphoid
enhancer-binding factor 1 |
| LRP4 | Low density
lipoprotein receptor-related protein 4 |
| LRPPRC | Leucine-rich
PPR-motif containing |
| MMP9 | Matrix
metallopeptidase 9 |
| PRKAB2 | Protein kinase,
AMP-activated, beta 2 non-catalytic subunit |
| PRKCI | Protein kinase C,
iota |
| PTTG1 | Pituitary
tumor-transforming 1 |
| SOX2 | SRY (sex
determining region Y)-box 2 |
| TCF12 | Transcription
factor 12 |
| TCF3 | Transcription
factor 3 |
| TCF7L2 | Transcription
factor 7-like 2 |
| VCAN | Versican |
| VEGFA | Vascular
endothelial growth factor A |
In summary, we report that WNT5A is overexpressed in
primary NPC tissues, where it may function to promote tumour
growth, migration and invasion. Furthermore, we show that LMP2A
induces the transcription of WNT5A. Taken together, these results
not only demonstrate pro-tumorigenic effects of WNT5A in NPC, but
also that patients with EBV-associated NPC could potentially
benefit from the therapeutic targeting of this molecule.
Acknowledgements
This study is supported by the
University of Malaya (UM.C/625/1/HIR/MOHE/DENT/23), Ministry of
Health, Malaysia (JPP-IMR 06-056) and Cancer Research Initiatives
Foundation (06-061). We thank the Director General of Health of
Malaysia for his permission to publish this article and the
Director of the Institute for Medical Research, Kuala Lumpur, for
her support. We wish to thank P. Siti Rohana, A.S. Roslinda and
other staff of the Molecular Pathology Unit, IMR for their
assistance. We also wish to thank A.M.C. Brown (Cornell University)
for the pLNC-WNT5A.
References
|
1.
|
Yu MC and Yuan JM: Epidemiology of
nasopharyngeal carcinoma. Semin Cancer Biol. 12:421–429. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Qin DX, Hu YH, Yan JH, et al: Analysis of
1379 patients with nasopharyngeal carcinoma treated by radiation.
Cancer. 61:1117–1124. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Pua KC, Khoo AS, Yap YY, et al:
Nasopharyngeal Carcinoma Database. Med J Malaysia. 63(Suppl C):
59–62. 2008.
|
|
4.
|
Lee AW, Poon YF, Foo W, et al:
Retrospective analysis of 5037 patients with nasopharyngeal
carcinoma treated during 1976–1985: overall survival and patterns
of failure. Int J Radiat Oncol Biol Phys. 23:261–270.
1992.PubMed/NCBI
|
|
5.
|
Agulnik M and Epstein JB: Nasopharyngeal
carcinoma: current management, future directions and dental
implications. Oral Oncol. 44:617–627. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Pathmanathan R, Prasad U, Sadler R, Flynn
K and Raab-Traub N: Clonal proliferations of cells infected with
Epstein-Barr virus in preinvasive lesions related to nasopharyngeal
carcinoma. N Engl J Med. 333:693–698. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Giles RH, van Es JH and Clevers H: Caught
up in a Wnt storm: Wnt signaling in cancer. Biochim Biophys Acta.
1653:1–24. 2003.PubMed/NCBI
|
|
8.
|
McDonald SL and Silver A: The opposing
roles of Wnt-5a in cancer. Br J Cancer. 101:209–214. 2009.
View Article : Google Scholar
|
|
9.
|
Ying J, Li H, Yu J, et al: WNT5A exhibits
tumor-suppressive activity through antagonizing the
Wnt/beta-catenin signaling, and is frequently methylated in
colorectal cancer. Clin Cancer Res. 14:55–61. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Dejmek J, Dejmek A, Safholm A, Sjolander A
and Andersson T: Wnt-5a protein expression in primary dukes B colon
cancers identifies a subgroup of patients with good prognosis.
Cancer Res. 65:9142–9146. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Blanc E, Roux GL, Benard J and Raguenez G:
Low expression of Wnt-5a gene is associated with high-risk
neuroblastoma. Oncogene. 24:1277–1283. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Jonsson M, Dejmek J, Bendahl PO and
Andersson T: Loss of Wnt-5a protein is associated with early
relapse in invasive ductal breast carcinomas. Cancer Res.
62:409–416. 2002.PubMed/NCBI
|
|
13.
|
Dejmek J, Leandersson K, Manjer J, et al:
Expression and signaling activity of Wnt-5a/discoidin domain
receptor-1 and Syk plays distinct but decisive roles in breast
cancer patient survival. Clin Cancer Res. 11:520–528.
2005.PubMed/NCBI
|
|
14.
|
Ying J, Li H, Chen YW, Srivastava G, Gao Z
and Tao Q: WNT5A is epigenetically silenced in hematologic
malignancies and inhibits leukemia cell growth as a tumor
suppressor. Blood. 110:4130–4132. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Roman-Gomez J, Jimenez-Velasco A, Cordeu
L, et al: WNT5A, a putative tumour suppressor of lymphoid
malignancies, is inactivated by aberrant methylation in acute
lymphoblastic leukaemia. Eur J Cancer. 43:2736–2746. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Liang H, Chen Q, Coles AH, et al: Wnt5a
inhibits B cell proliferation and functions as a tumor suppressor
in hematopoietic tissue. Cancer Cell. 4:349–360. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Da Forno PD, Pringle JH, Hutchinson P, et
al: WNT5A expression increases during melanoma progression and
correlates with outcome. Clin Cancer Res. 14:5825–5832.
2008.PubMed/NCBI
|
|
18.
|
Fernandez-Cobo M, Zammarchi F, Mandeli J,
Holland JF and Pogo BG: Expression of Wnt5A and Wnt10B in
non-immortalized breast cancer cells. Oncol Rep. 17:903–907.
2007.PubMed/NCBI
|
|
19.
|
Kurayoshi M, Oue N, Yamamoto H, et al:
Expression of Wnt-5a is correlated with aggressiveness of gastric
cancer by stimulating cell migration and invasion. Cancer Res.
66:10439–10448. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Ripka S, Konig A, Buchholz M, et al: WNT5A
- target of CUTL1 and potent modulator of tumor cell migration and
invasion in pancreatic cancer. Carcinogenesis. 28:1178–1187. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Huang CL, Liu D, Nakano J, et al: Wnt5a
expression is associated with the tumor proliferation and the
stromal vascular endothelial growth factor - an expression in
non-small-cell lung cancer. J Clin Oncol. 23:8765–8773. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Wang Q, Williamson M, Bott S, et al:
Hypomethylation of WNT5A, CRIP1 and S100P in prostate cancer.
Oncogene. 26:6560–6565. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Bose S, Yap LF, Fung M, et al: The ATM
tumour suppressor gene is down-regulated in EBV-associated
nasopharyngeal carcinoma. J Pathol. 217:345–352. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Zeng ZY, Zhou YH, Zhang WL, et al: Gene
expression profiling of nasopharyngeal carcinoma reveals the
abnormally regulated Wnt signaling pathway. Hum Pathol. 38:120–133.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Hu C, Wei W, Chen X, et al: A global view
of the oncogenic landscape in nasopharyngeal carcinoma: an
integrated analysis at the genetic and expression levels. PLoS One.
7:e410552012. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Moody CA, Scott RS, Amirghahari N, et al:
Modulation of the cell growth regulator mTOR by Epstein-Barr
virus-encoded LMP2A. J Virol. 79:5499–5506. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Shah KM, Stewart SE, Wei W, et al: The
EBV-encoded latent membrane proteins, LMP2A and LMP2B, limit the
actions of interferon by targeting interferon receptors for
degradation. Oncogene. 28:3903–3914. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Allen MD, Young LS and Dawson CW: The
Epstein-Barr virus-encoded LMP2A and LMP2B proteins promote
epithelial cell spreading and motility. J Virol. 79:1789–1802.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Yap LF, Jenei V, Robinson CM, et al:
Upregulation of Eps8 in oral squamous cell carcinoma promotes cell
migration and invasion through integrin-dependent Rac1 activation.
Oncogene. 28:2524–2534. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Tulalamba W and Janvilisri T:
Nasopharyngeal carcinoma signaling pathway: an update on molecular
biomarkers. Int J Cell Biol. 2012.5946812012.PubMed/NCBI
|
|
31.
|
Ge X, Yamamoto S, Tsutsumi S, et al:
Interpreting expression profiles of cancers by genome-wide survey
of breadth of expression in normal tissues. Genomics. 86:127–141.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Yap YY, Hassan S, Chan M, Choo PK and
Ravichandran M: Epstein-Barr virus DNA detection in the diagnosis
of nasopharyngeal carcinoma. Otolaryngol Head Neck Surg.
136:986–991. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Dawson CW, Port RJ and Young LS: The role
of the EBV-encoded latent membrane proteins LMP1 and LMP2 in the
pathogenesis of nasopharyngeal carcinoma (NPC). Semin Cancer Biol.
22:144–153. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Morrison JA and Raab-Traub N: Roles of the
ITAM and PY motifs of Epstein-Barr virus latent membrane protein 2A
in the inhibition of epithelial cell differentiation and activation
of {beta}-catenin signaling. J Virol. 79:2375–2382. 2005.PubMed/NCBI
|
|
35.
|
Morrison JA, Klingelhutz AJ and Raab-Traub
N: Epstein-Barr virus latent membrane protein 2A activates
beta-catenin signaling in epithelial cells. J Virol.
77:12276–12284. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Morrison JA, Gulley ML, Pathmanathan R and
Raab-Traub N: Differential signaling pathways are activated in the
Epstein-Barr virus-associated malignancies nasopharyngeal carcinoma
and Hodgkin lymphoma. Cancer Res. 64:5251–5260. 2004. View Article : Google Scholar
|
|
37.
|
Hanahan D and Weinberg RA: The hallmarks
of cancer. Cell. 100:57–70. 2000. View Article : Google Scholar
|
|
38.
|
Yu JM, Jun ES, Jung JS, et al: Role of
Wnt5a in the proliferation of human glioblastoma cells. Cancer
Lett. 257:172–181. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39.
|
Weeraratna AT, Jiang Y, Hostetter G, et
al: Wnt5a signaling directly affects cell motility and invasion of
metastatic melanoma. Cancer Cell. 1:279–288. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
40.
|
Yamamoto H, Oue N, Sato A, et al: Wnt5a
signaling is involved in the aggressiveness of prostate cancer and
expression of metalloproteinase. Oncogene. 29:2036–2046. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
41.
|
Torii K, Nishizawa K, Kawasaki A, et al:
Anti-apoptotic action of Wnt5a in dermal fibroblasts is mediated by
the PKA signaling pathways. Cell Signal. 20:1256–1266. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
42.
|
Mikels AJ and Nusse R: Purified Wnt5a
protein activates or inhibits beta-catenin-TCF signaling depending
on receptor context. PLoS Biol. 4:e1152006. View Article : Google Scholar : PubMed/NCBI
|
|
43.
|
Fang W, Li X, Jiang Q, et al:
Transcriptional patterns, biomarkers and pathways characterizing
nasopharyngeal carcinoma of Southern China. J Transl Med. 6:322008.
View Article : Google Scholar : PubMed/NCBI
|
|
44.
|
Shi W, Bastianutto C, Li A, et al:
Multiple dysregulated pathways in nasopharyngeal carcinoma revealed
by gene expression profiling. Int J Cancer. 119:2467–2475. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
45.
|
Sriuranpong V, Mutirangura A, Gillespie
JW, et al: Global gene expression profile of nasopharyngeal
carcinoma by laser capture microdissection and complementary DNA
microarrays. Clin Cancer Res. 10:4944–4958. 2004. View Article : Google Scholar : PubMed/NCBI
|