Introduction
Dendritic cells (DCs) are specialized
antigen-presenting cells (APCs) that play a critical role in the
induction of primary immune responses (1). Therefore, several strategies have
been developed to deliver tumor-associated antigens (TAAs) to
autologous monocyte-derived dendritic cells (MoDCs) for the
induction of efficient antigen-specific cytotoxic T lymphocytes
(CTLs). One of the strategies is the administration of fusion cells
generated from MoDCs and whole tumor cells (2). In MoDC/tumor fusions, a broad array
of TAAs, including known and unidentified molecules, are delivered
to MoDCs, processed, and presented to CD4+ and
CD8+ T cells in complex with MHC class I and II
molecules and in the context of co-stimulatory signals (3,4).
Moreover, MoDCs and tumor cells can be independently subjected to
manipulations for the acquisition of desired characteristics that
persist after fusion (4).
A major limitation to the use of MoDC/tumor fusions
is the availability of adequate amounts of autologous tumor cells,
which stems from the limited availability of viable tumor samples
and/or technical difficulties in cancer cell culture. Moreover,
MoDCs from advanced cancer patients may be defective in their
antigen-processing and presentation machinery due to the presence
of tumor-derived immune suppressive molecules or as a result of
chemotherapy (5). To circumvent
all of these issues, allogeneic DC and tumor cell lines can be used
instead of autologous cells. Cell lines that are well characterized
can be massively propagated in vitro under good
manufacturing practice (GMP) standards. Thus, unlimited amounts of
DC/tumor fusion cells can be readily available.
As APCs, plasmacytoid DCs (pDCs) have not been used
extensively in cancer vaccines thus far because they are more
difficult to isolate from human blood monocytes and to obtain in
sufficient quantities; however, they are more efficient than MoDCs
in triggering antitumor immune responses (6–8).
Moreover, pDCs differ from MoDCs in many aspects, such as TLR
expression, and are capable of antigen capture, processing and
presentation (9,10). A human leukemia pDC line (PMDC05)
was recently generated (11,12)
and tested for its capacity to induce effective antigen-specific
CTLs upon peptide pulsing (13,14).
However, little is known about whether antigen-specific CTLs can be
induced by pDC/tumor fusion cells.
Here, we show that fusions generated with a pDC line
and a pancreatic cancer cell line expressing MUC1 antigens induce
MUC1-specific CTLs in vitro. Moreover, significantly
augmented MUC1-specific CTLs are induced by lipopolysaccharide
(LPS)-stimulated pDC/tumor fusion cells in vitro compared
with unstimulated pDC/tumor fusion cells. By selecting cancer cell
lines that express the same TAAs as autologous tumor cells,
pDC/tumor fusions can be made from cells that are available in the
laboratory, without the use of any patient or donor materials and
avoiding the constraints of autologous cells.
Materials and methods
Cells and conditioned medium
PANC-1 (MUC1+, HLA-A2+,
HLA-A24−), MIA PaCa-2 (MUC1+,
HLA-A2−, HLA-A24+) and K562
(MUC1+, HLA-A2−, HLA-A24−) cells
were purchased from the American Type Culture Collection (ATCC,
Manassas, VA, USA) and maintained in Dulbecco’s modified Eagle’s
medium (DMEM) supplemented with 100 U/ml penicillin, 100 mg/ml
streptomycin and 10% fetal calf serum (FCS) (15). The leukemic pDC line PMDC05 was
kindly gifted from Dr Takahashi (Laboratory of Hematology and
Oncology, Graduate School of Health Sciences, Niigata University,
Niigata, Japan). The PMDC05 cells were cultured at a cell
concentration of 1×106/ml in Iscove’s modified
Dulbecco’s medium (IMDM) supplemented with 100 U/ml penicillin, 100
mg/ml streptomycin and 10% FCS.
Fusion of pDCs and tumor cells
We developed four types of pDC/tumor fusions by
alternating fusion cell partners and treating LPS as follows:
PMDC05 fused with PANC-1 (pDC/PANC-1), PMDC05 fused with MIA PaCa-2
(pDC/MIA PaCa-2), PMDC05 fused with PANC-1 in the presence of LPS
(LPS-pDC/PANC-1) and PMDC05 fused with MIA PaCa-2 in the presence
of LPS (LPS-pDC/MIA PaCa-2). Briefly, pancreatic cancer cells
(PANC-1 or MIA PaCa-2) were mixed with pDCs (PMDC05) at a ratio of
1:1, and fusion cells were generated using 50% polyethylene glycol
(PEG) (Sigma-Aldrich, St. Louis, MO) (3). The fusion cells were maintained in
DMEM with or without 0.1 g/ml LPS (Sigma-Aldrich). After 3 days of
culture, the fusion cell preparations were integrated to a single
entity and purified by gentle pipetting (16).
Phenotype analysis
Cells were incubated with FITC-conjugated monoclonal
antibodies (mAbs) against MUC1 (CD227 clone HMPV; BD Pharmingen,
San Jose, CA), MHC class I (W6/32), MHC class II (HLA-DR), B7-1
(CD80), B7-2 (CD86), CD83 (BD Pharmingen), HLA-A2 and HLA-A24 (One
Lambda, Canoga Park, CA) or matched isotype control IgG. The pDC
populations were gated based on their forward- vs. side-scatter
profile and then analyzed for their expression of HLA-ABC, HLA-DR,
CD80, CD86, CD83 and MUC1. For analysis of dual expression in the
fusion cell preparations, the cells were incubated with a
FITC-conjugated mAb against MUC1 and PE-conjugated mAbs against
HLA-DR and CD86. After the cell aggregates were gated out (16), the fused cells were identified as
MUC1 + HLA-DR+ or MUC1 + CD86+ using a
FACScan flow cytometer (Becton-Dickinson, Mountain View, CA) and
FlowJo analysis software (TreeStar, OR, USA).
T cell stimulation
The study protocol was reviewed and approved by the
ethics committee of the Institutional Review Board of the Jikei
University School of Medicine as well as the clinical study
committee of the Jikei University Kashiwa Hospital [No. 14–60
(3209)]. Peripheral blood mononuclear cells (PBMCs) from whole
blood (HLA-A2+ and HLA-A24+) were obtained
with written informed consent from each individual. Briefly, PBMCs
were prepared by Ficoll density gradient centrifugation and
incubated in tissue culture flasks at 37°C for 30 min in Roswell
Park Memorial Institute (RPMI) 1640 medium supplemented with 1%
heat-inactivated autologous serum. After incubation for 60 min at
37°C to allow for adherence, the non-adherent cells were cultured
with pDC/tumor fusion cells, pDCs or tumor cells. The number of
pDC/tumor fusion cells was determined based on the number of cells
that coexpressed HLA-DR and MUC1 in the fusion cell preparations.
Equal numbers of each type of pDC/tumor fusion cell
(HLA-A2+ and HLA-A24+) were cocultured with
the non-adherent PBMCs (HLA-A2+ and HLA-A24+)
at a ratio of 1:10 in the absence of recombinant human (rh)IL-2 for
3 days and then purified through nylon wool to remove the APCs. A
low dose of rhIL-2 (10 U/ml; Shionogi, Osaka, Japan) was added on
Day 4 and maintained until Day 8. pDCs, tumor cells and pDCs mixed
with tumor cells were used as controls.
Enzyme-linked immunosorbent assay
(ELISA)
pDC/tumor fusion cells (1×105
cells/ml/well) or pDCs (1×105 cells/ml/well) were
cultured for 48 h, and their supernatants were tested for IL-12p70
and IL-10 expression (R&D Systems, Minneapolis, MN). The
minimum detectable concentration of human IL-12p70 is typically
<0.5 pg/ml.
Proliferation assay
Stimulated T cells were harvested by nylon wool
separation and cultured in 96-well U-bottomed culture plates at
7×104 cells/well for 1 day. Dye solution was added to
each well and incubated for 4 h according to the protocol of the
Cell Titer 96 Non-radioactive Cell Proliferation Assay kit
(Promega, Madison, WI). For measurement of proliferating T cells,
we used a Microplate Imaging System (Bio-Rad, Hercules, CA) at an
OD of 550 nm.
IFN-γ-producing CD4+ and
CD8+ T cells
Stimulated T cells were harvested by nylon wool
separation, and their human IFN-γ production was analyzed using an
IFN-γ secretion assay kit (Miltenyi Biotec, Auburn, CA) according
to the manufacturer’s instructions. Briefly, the T cells were
incubated with IFN-γ catching reagent for 5 min at 4°C and then
cultured for 45 min. Next, the cells were stained with a
PE-conjugated anti-IFN-γ mAb and FITC-conjugated mAbs against CD4
and CD8 (BD Pharmingen), washed, fixed with 2% paraformalde-hyde
and analyzed by flow cytometry using FlowJo analysis software. The
T cell populations were gated based on their forward- vs.
side-scatter profile. The CD4+ and CD8+ T
cell populations were each gated, and then the percentages of
IFN-γ-positive CD4+ and CD8+ T cells among
the whole CD4+ and CD8+ T cell populations
were calculated.
MUC1 pentamer staining
Stimulated T cells were harvested by nylon wool
separation and then incubated with a PE-conjugated MUC1 pentamer
(HLA-A2, STAPPVHNV) (Proimmune, Oxford, UK) for 1 h at 4°C. After
washing, the T cells were stained with a FITC-conjugated mAb
against CD8 (BD Pharmingen), washed, fixed with 2%
paraformalde-hyde and analyzed by flow cytometry using FlowJo
analysis software. Complexes of PE-irrelevant pentamers were used
as controls. The T cell populations were gated based on their
forward- vs. side-scatter profile. The CD8+ T cell
populations were gated, and then the percentage of MUC1
pentamer-positive CD8+ T cells among the whole
CD8+ T cell population was calculated.
Cytotoxicity assays
The cytotoxicity assays were performed by flow
cytometric analysis using Active Caspase-3 Apoptosis kit I (BD
Pharmingen), which measures CTL-induced caspase-3 activation in
target cells by detecting the specific cleavage of fluorogenic
caspase-3 (17). Briefly, the
target cells were labeled with PKH-26 (Sigma-Aldrich), washed,
cultured with stimulated T cells for 2 h at 37°C in 96-well
V-bottomed plates at the indicated effector cell:T cell (E:T)
ratios. The cells were then fixed with Cytofix/Cytoperm Solution
(BD Pharmingen), washed with Perm/Wash Buffer (BD Pharmingen) and
incubated with a FITC-conjugated mAb against human active caspase-3
(BD Pharmingen) for 30 min at room temperature, followed by two
washes with Perm/Wash buffer. In certain experiments, the tumor
target cells were preincubated with anti-HLA-ABC mAb (W6/32; 1:100
dilution) or control IgG for 30 min at 37°C before adding the
effector cells. The percentage of cytotoxicity (mean ± SD of three
replicates) was determined using the following equation: percentage
of caspase-3 staining =
(caspase-3+PKH-26+cells)/(caspase-3+PKH-26+cells +
caspase-3-PKH-26+cells) ×100.
Statistical analysis
The results are expressed as means ± SD, as
indicated in the legends. One-way analysis of variance was used to
determine significance. When the P-values ≤0.05, the differences
were considered to be statistically significant.
Results
Characterization of the cell lines used
for fusion
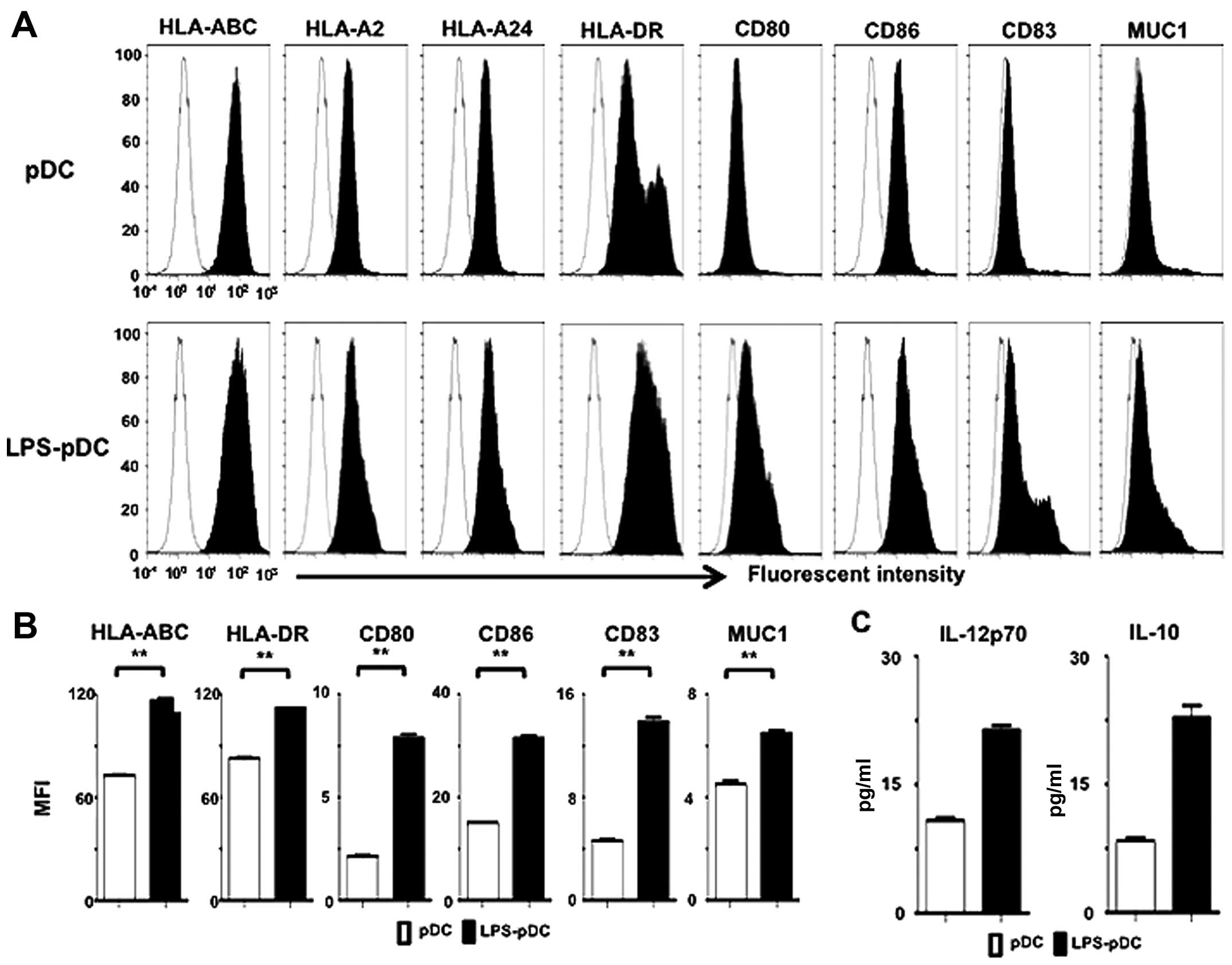
The pDC line PMDC05 displayed a characteristic
phenotype, with easily detectable levels of HLA-ABC, HLA-DR, CD80
and CD86 but low levels of CD83 and very low levels of MUC1 (CD227)
(Fig. 1A). Stimulation of this pDC
line with LPS (LPS-pDC) resulted in the upregulated expression of
HLA-ABC, HLA-DR, CD80, CD86, CD83 and MUC1 (CD227) compared with
unstimulated pDCs (Fig. 1A and B).
Moreover, the LPS-pDCs exhibited increased levels of IL-12p70 and
IL-10 compared with unstimulated pDCs (Fig. 1C). These results suggest that LPS
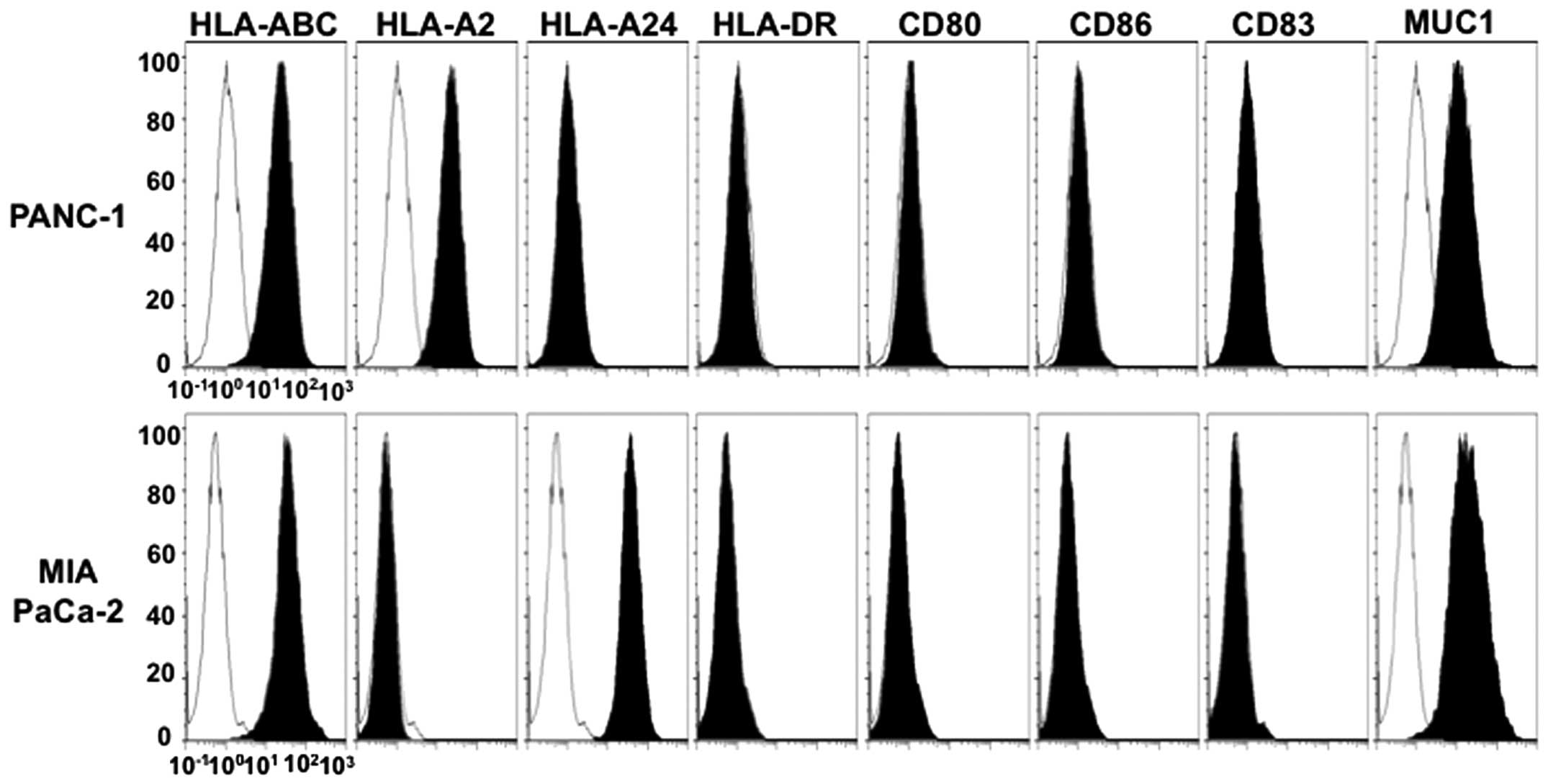
activates pDCs. The pancreatic cancer cell lines used in this
study, PANC-1 and MIA PaCa-2, expressed high levels of HLA-ABC and
MUC1 but did not express HLA-DR, CD80, CD86 or CD83 (Fig. 2). Moreover, the PANC-1 cells
expressed HLA-A2 but not HLA-A24, and conversely, the MIA PaCa-2
cells expressed HLA-A24 but not HLA-A2 (Fig. 2).
Characterization of the pDC/tumor fusion
cells
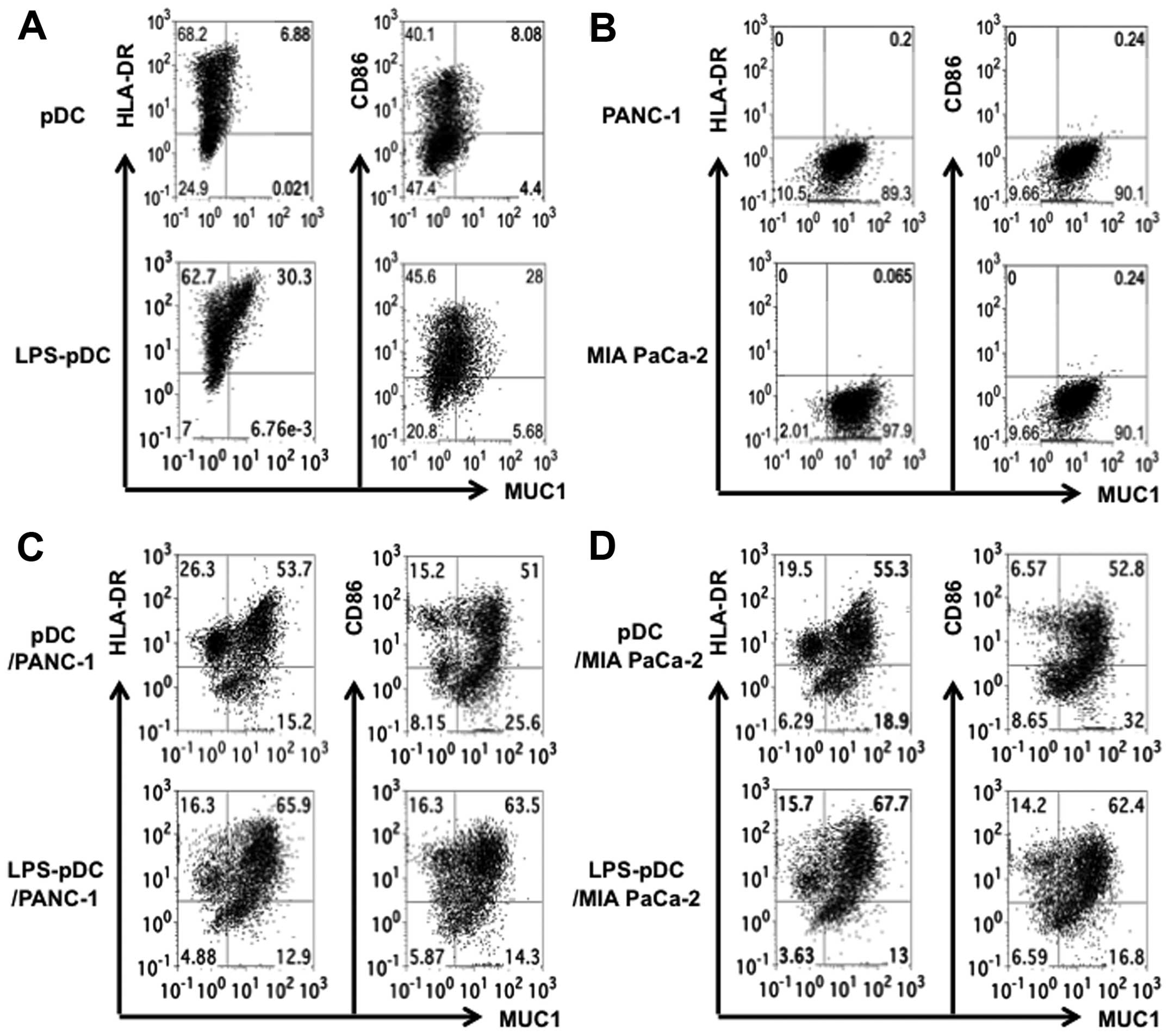
To assess the capacity of the pDC/tumor fusion cells
to induce antigen-specific CTL responses in vitro, we
developed four types of fusion cell preparations by alternating
fusion partners and treating with LPS. PANC-1 and MIA PaCa-2 cells
were each successfully fused with pDCs with or without LPS
stimulation (Fig. 3). The fusion
efficiency was determined using the percentage of MUC1 and HLA-DR
or CD86 double-stained cells (Fig.
3). Analysis of the fusion cells created from the pancreatic
cancer cells and the pDCs demonstrated that about 50% of the popul
ation expressed both MUC1 and HLA-DR or CD86 (Fig. 3C and D). Interestingly, the fusions
generated in the presence of LPS exhibited higher double-positive
cells that expressed MUC1 and HLA-DR or CD86 than those generated
with the unstimulated pDCs (Fig. 3C
and D).
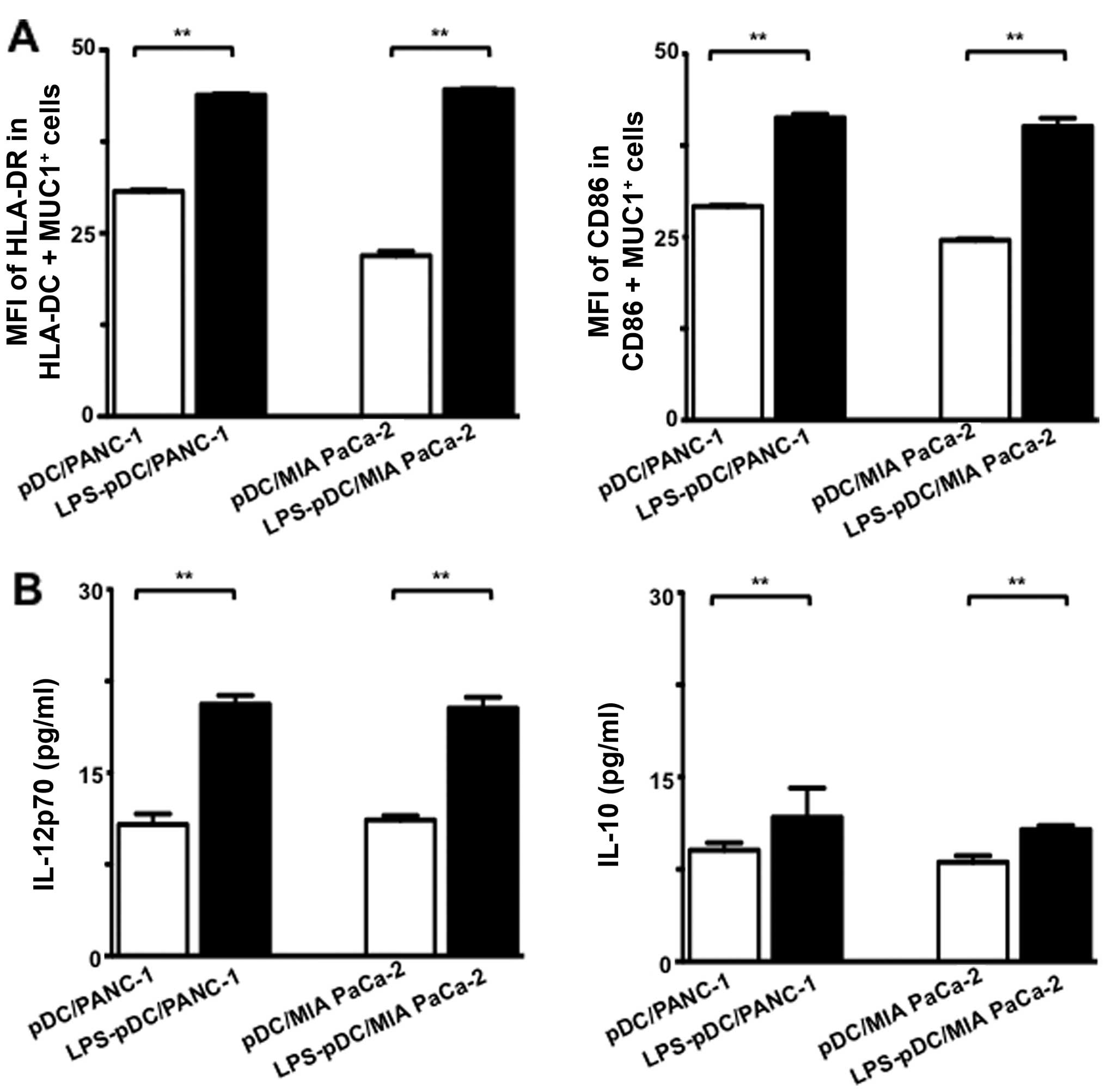
Next, to attain a detailed phenotypic
characterization of the DC/tumor fusion cells, the mean
fluorescence intensity (MFI) of HLA-DR and CD86 expression was
determined by FACS analysis, where the fused cells were identified
as MUC1 + HLA-DR+ or MUC1 + CD86+. Although
the pDC/PANC-1 and pDC/MIA PaCa-2 cells displayed high MFI values
for HLA-DR and CD86, the LPS-DC/PANC-1 and LPS-DC/MIA PaCa-2 cells
exhibited higher MFI values on a per-fusion-cell basis (Fig. 4A). Therefore, fusions generated in
the presence of LPS may have a more active phenotype compared to
those generated with unstimulated pDCs.
Furthermore, we assessed the production of IL-12p70
and IL-10 in the supernatants from the fusion cell preparations.
About 2-fold higher levels of IL-12p70 production were observed for
the LPS-pDC/PANC-1 and LPS-pDC/MIA PaCa-2 cells compared with the
pDC/PANC-1 and pDC/MIA PaCa-2 cells (Fig. 4B). Moreover, IL-10 production was
also increased in the LPS-pDC/PANC-1 and LPS-pDC/MIA PaCa-2 cells
but to a lesser extent than that observed for IL-12p70 (Fig. 4B). Collectively, these results
suggest that the upregulated production of IL-12p70 and the active
phenotype of the fusion cells generated in the presence of LPS
increase their immunogenicity.
Stimulation of T cells by the pDC/tumor
fusions
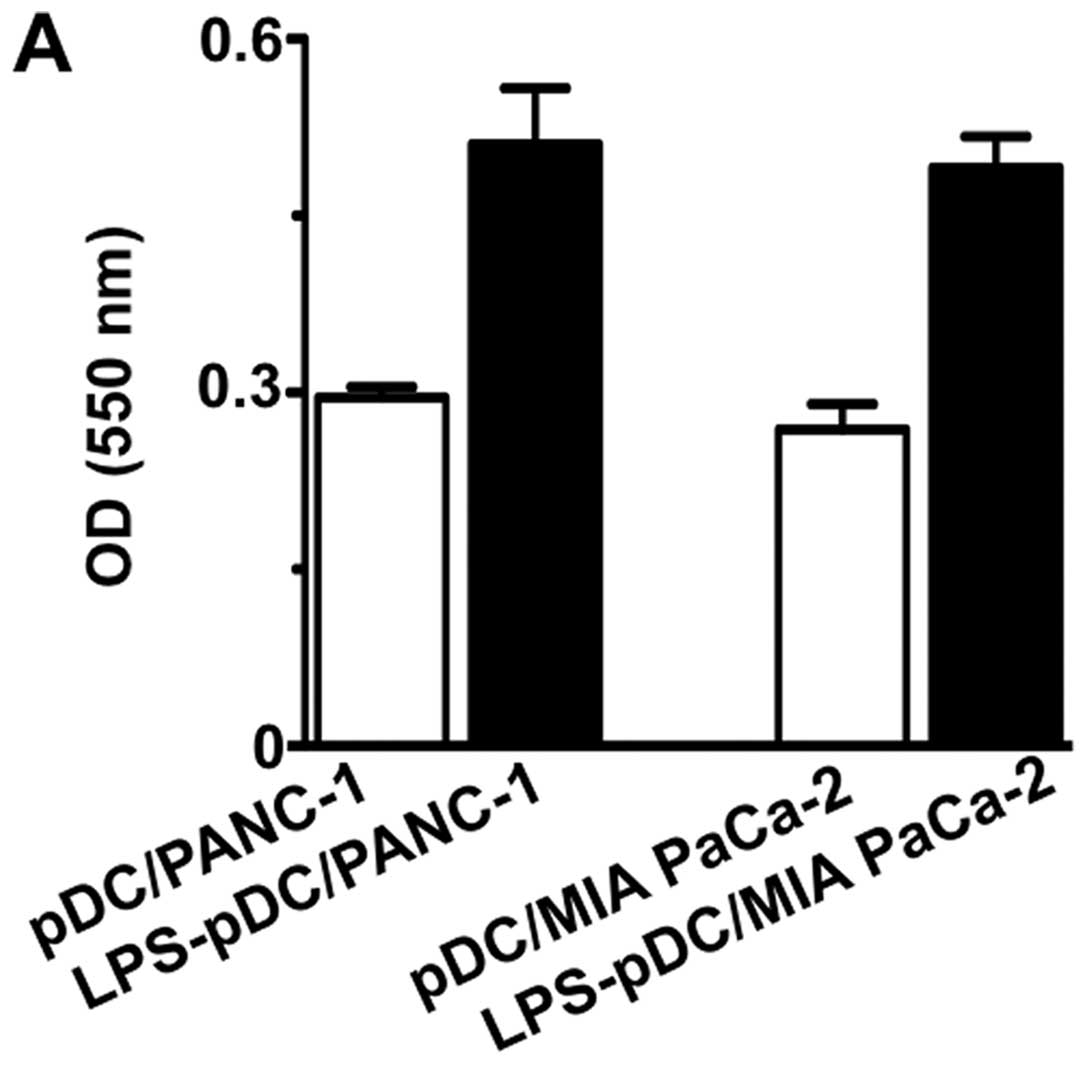
Although all four types of fusions affected T cell
proliferation, the LPS-pDC/tumor fusion cells showed the most
significant stimulation of T cell proliferation (Fig. 5A). In addition, an unfused mixture
of tumor cells and pDCs or LPS-DCs had no effect on T cell
proliferation (data not shown). Moreover, both the pDC/tumor and
LPS-pDC/tumor fusions stimulated IFN-γ-producing CD4+
and CD8+ T cells (Fig.
5B). However, the LPS-pDC/tumor fusion cells more strongly
induced the proliferation of both CD4+ and
CD8+ T cells that were capable of producing high levels
of IFN-γ compared to the pDC/tumor fusion cells (Fig. 5B). In contrast, very low levels or
no IFN-γ-producing cells were detected in the CD4+ and
CD8+ T cell populations stimulated by an unfused mixture
of DCs and tumor cells (data not shown). These results suggest that
pDC/tumor fusion cells stimulated with LPS have a more potent
capacity to induce CTL responses compared to unstimulated pDC/tumor
fusion cells.
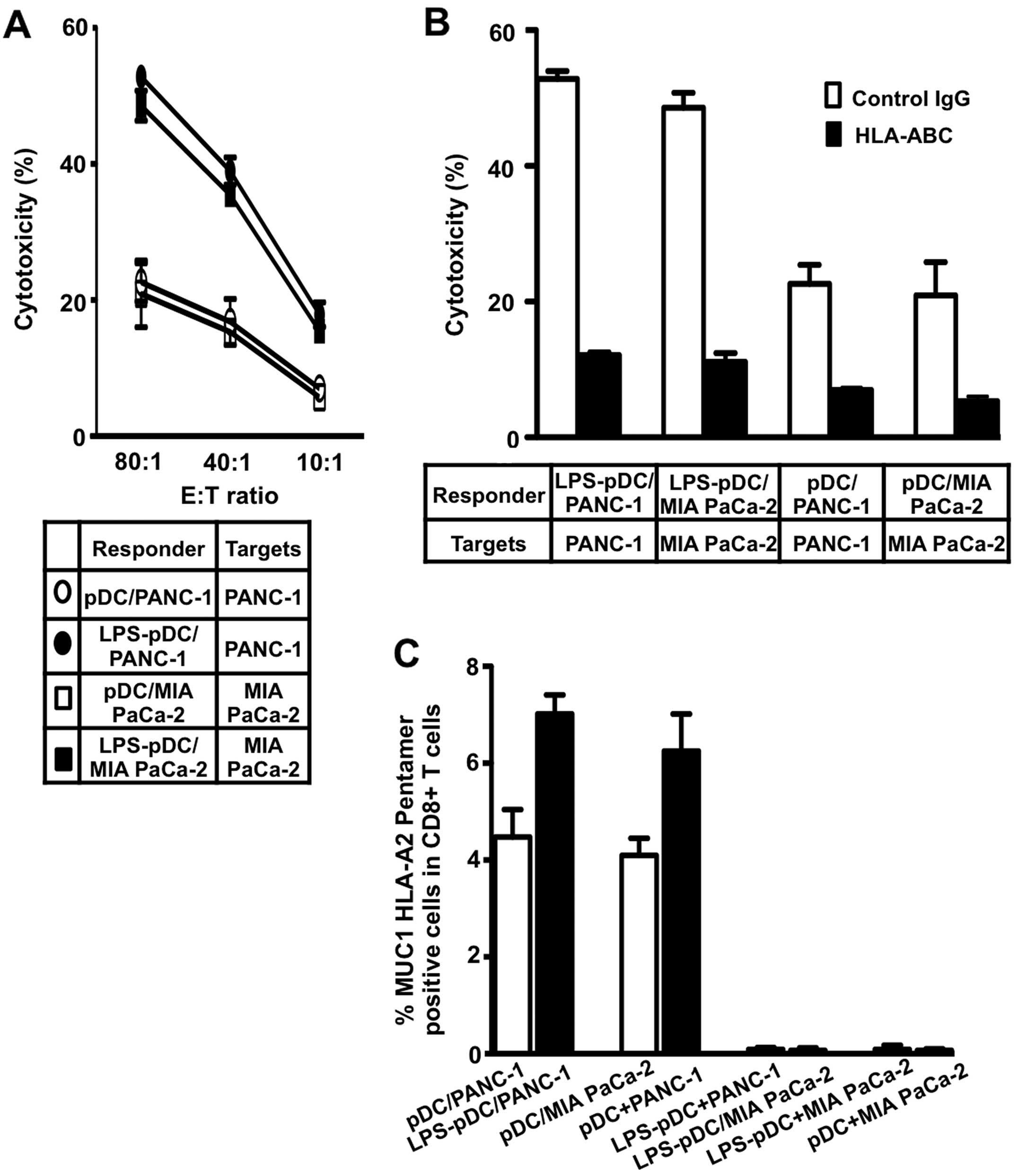
MUC1-specific CTL responses induced by
the pDC/tumor fusions
The CTLs induced by all four types of fusions lysed
the tumor target cells used for fusion (Fig. 6A and B) but not K562 cells (data
not shown). Moreover, the lytic activity induced by the
LPS-stimulated pDC/tumor fusions was significantly higher than that
induced by the unstimulated pDC/tumor fusions (Fig. 6A and B), suggesting that LPS
increases the immunogenicity of the pDC/tumor fusion cells to
induce efficient CTL responses. In addition, preincubation of the
target cells with an anti-HL-ABC mAb inhibited their lysis,
indicating restriction by MHC class I molecules (Fig. 6B). Interestingly, an increased
percentage of HLA-A2-restricted, MUC1-specific CD8+ T
cells in the whole CD8+ T cell population was observed
for the LPS-pDC/tumor fusions (HLA-A2+) compared with
the pDC/tumor fusions (HLA-A2+) (Fig. 6C). In addition, CTLs specific for
MUC1 were not detected in a population of T cells stimulated by an
unfused mixture of tumor cells and pDCs or LPS-pDCs (Fig. 6D). Together, these findings
indicate that HLA-A2-restrictive, MUC1-specific CTLs are
efficiently induced by LPS-pDC/tumor fusions in vitro.
Discussion
The data presented herein show that DC/tumor fusion
cells generated with a pDC line (HLA-A2+) and a
pancreatic cancer cell line expressing MUC1 antigens induce
HLA-A2-restricted, MUC1-specific CTLs in vitro. Moreover,
LPS-stimulated pDC/tumor fusion cells efficiently induce augmented
CTL responses.
We attempted to prepare immunogenic DC/tumor fusion
cells using a DC line and a pancreatic cancer cell line. We used
the plasmacytoid DC line PMDC05, a leukemic blast line that was
isolated from a patient with acute leukemia (11,12)
and has been reported to have the capacity to induce effective
antigen-specific CTLs (13,14).
This pDC line has been pulsed with peptide to induce CTL responses;
however, little is known about its utility in cancer vaccines if
used to generate pDC/tumor fusion cells. Cell lines that are well
characterized can be massively propagated in vitro adhering
to GMP. Thus, unlimited amounts of DC/tumor fusion cells can be
readily available to induce antigen-specific CTLs for adoptive
immunotherapy. Therefore, one important aspect of our work is its
potential clinical relevance.
The binding of the pathogen-associated microbial
pattern molecule LPS to Toll-like receptor (TLR) 4 on human MoDCs
signals danger, which induces a potent immune stimulatory phenotype
that is characterized by the release of IL-12p70 (18,19).
Our finding that a pDC line activated with LPS is more active
compared to unstimulated pDCs suggests that this TLR4 agonist plays
a role in the activation of pDC functions (11). Moreover, LPS stimulation resulted
in increased production of both IL-12p70 and IL-10 by the pDCs. The
surface phenotype and cytokine production response pattern of the
pDCs in this study was similar to that of human MoDCs (20), which implies that this pDC line
possesses characteristics of MoDCs (11). Moreover, stimulation of the pDC
line with LPS resulted in considerably increased expression of MUC1
(CD227) on the cell surface. MUC1 (CD227) is considered to be an
epithelial mucin that is expressed extensively in pancreatic and
other cancer types; thus, MUC1 is a target for immunotherapy in a
variety of cancers (21). This
molecule is also expressed by a wide variety of hemopoietic cells,
from early differentiating bone marrow mononuclear cells to mature
cell types (22). It is also known
that MUC1 (CD227) is expressed by activated DCs and T cells
(23). Therefore, LPS-stimulated
pDCs, which express increased levels of MUC1 (CD227), HLA-ABC, -DR,
CD80, CD86, CD83 and IL-12p70, may be suitable for cancer vaccines.
Therefore, we speculated that fusion cells generated with a pDC
line and a tumor cell line in the presence of a TLR4 agonist would
be immunogenic and induce more effective MUC1-specific CTLs than
their unstimulated counterparts. We successfully fused a pDC line
with two different tumor cell lines with or without LPS
stimulation. The pDC/tumor fusion cells were identified as MUC1 +
HLA-DR+ or MUC1 + CD86+. The characteristic
phenotype of the LPS-stimulated pDCs was associated with an
increased percentage of double-positive cells (MUC1 +
HLA-DR+ or MUC1 + CD86+) in the pDC/tumor
fusion cell preparations (data not shown). Moreover, the cells that
were double positive for MUC1 and HLA-DR or CD86 in the
LPS-stimulated pDC/tumor fusion cell preparations had high MFI
values for HLA-DR and CD86 on a per-fusion-cell basis, indicating
that the fusions were more immunogenic compared to their
unstimulated counterparts. Our previous report demonstrated that
efficient CTL induction is closely correlated to fusion efficiency
for fusion cells generated with MoDCs (24). LPS might provide the costimulation
required during the fusion process and might be involved in
polarizing the T cell responses to a Th1-dominant state. Therefore,
the efficient activation of the pDC/tumor fusion cells by LPS led
us to speculate that the MUC1-specific CTLs induced by these
activated fusion cells would be more effective than conventional
unactivated fusion cells.
We previously reported that the tumor antigens
delivered to MoDCs by fusion cells were processed and presented in
the context of MHC class I and II molecules of MoDC origin of
fusion cells (15,25). Therefore, the HLA typing of the
MoDCs and allogeneic tumor cell lines does not need to match
(26). HLA-A2-restricted,
MUC1-specific CTLs were efficiently generated with the fusion cells
generated from allogeneic pDC (HLA-A2+) and MIA PaCa-2
(HLA-A−), suggesting that the MUC1 antigens from the MIA
PaCa-2 cells were also processed and presented by HLA-A2 on the pDC
part of pDC/tumor fusion cells. Although the LPS-pDC/tumor and
pDC/tumor fusions stimulated IFN-γ-producing CD4+ and
CD8+ T cells that lyse the tumor target cells used for
fusion, the LPS-pDC/tumor fusions more strongly induced T cell
activation, indicating that LPS stimulation is effective for
pDC/tumor fusion cell vaccines. Moreover, the MUC1-specific CTLs
were more effectively augmented by the LPS-pDC/tumor fusion cells
compared to the pDC/tumor fusion cells. These results may be
associated with the active function of LPS-DCs as PACs, as
demonstrated by their mature phenotype, IL-12p70 production and
increased MUC1 expression. In patients with melanoma or renal cell
carcinoma, vaccines using fusions of allogeneic MoDCs and
autologous tumor cells have been shown to induce efficient
antitumor immune responses and clinical outcomes (27,28).
Moreover, allogeneic tumor cell lines have been used in fusion cell
vaccines in both preclinical (25,29,30)
and clinical studies (31) and a
MoDC/tumor fusion cell vaccine with fully allogeneic components has
been demonstrated to induce clinical responses (31). Therefore, DC/tumor fusions
generated with fully syngeneic, semi-allogeneic or fully allogeneic
components are effective in inducing antigen-specific, long-lasting
antitumor immunity (32).
In conclusion, our results indicate that fusion cell
vaccines generated with a plasmacytoid DC line and tumor cell line
can induce antigen-specific CTL responses in vitro. Our
findings introduce the possibility of using defined allogeneic
plasmacytoid DC and tumor lines to simplify CTL manufacturing for
adoptive immunotherapy.
Acknowledgements
This study was supported by
Grants-in-Aid for Scientific Research (C) from the Ministry of
Education, Cultures, Sports, Science and Technology of Japan, the
Foundation for Promotion of Cancer Research, the Mitsui Life Social
Welfare Foundation, and a Grant-in-Aid from the Japan Medical
Association. The funders had no role in the study design, data
collection or analysis, decision to publish or manuscript
preparation.
References
|
1.
|
Steinman RM: The dendritic cell system and
its role in immunogenicity. Annu Rev Immunol. 9:271–296. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Gong J, Chen D, Kashiwaba M and Kufe D:
Induction of antitumor activity by immunization with fusions of
dendritic and carcinoma cells. Nat Med. 3:558–561. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Gong J, Koido S and Calderwood SK: Cell
fusion: from hybridoma to dendritic cell-based vaccine. Expert Rev
Vaccines. 7:1055–1068. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Koido S, Homma S, Okamoto M, et al:
Fusions between dendritic cells and whole tumor cells as anticancer
vaccines. Oncoimmunology. 2:e244372013. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Yanagimoto H, Takai S, Satoi S, et al:
Impaired function of circulating dendritic cells in patients with
pancreatic cancer. Clin Immunol. 114:52–60. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Colonna M, Trinchieri G and Liu YJ:
Plasmacytoid dendritic cells in immunity. Nat Immunol. 5:1219–1226.
2004. View
Article : Google Scholar
|
|
7.
|
Liu YJ: IPC: professional type 1
interferon-producing cells and plasmacytoid dendritic cell
precursors. Annu Rev Immunol. 23:275–306. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Kim R, Emi M, Tanabe K and Arihiro K:
Potential functional role of plasmacytoid dendritic cells in cancer
immunity. Immunology. 121:149–157. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Segura E, Kapp E, Gupta N, et al:
Differential expression of pathogen-recognition molecules between
dendritic cell subsets revealed by plasma membrane proteomic
analysis. Mol Immunol. 47:1765–1773. 2010. View Article : Google Scholar
|
|
10.
|
Mouries J, Moron G, Schlecht G, Escriou N,
Dadaglio G and Leclerc C: Plasmacytoid dendritic cells efficiently
cross-prime naive T cells in vivo after TLR activation. Blood.
112:3713–3722. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Narita M, Watanabe N, Yamahira A, et al: A
leukemic plasmacytoid dendritic cell line, PMDC05, with the ability
to secrete IFN-alpha by stimulation via Toll-like receptors and
present antigens to naive T cells. Leuk Res. 33:1224–1232. 2009.
View Article : Google Scholar
|
|
12.
|
Watanabe N, Narita M, Yamahira A, et al:
Transformation of dendritic cells from plasmacytoid to myeloid in a
leukemic plasmacytoid dendritic cell line (PMDC05). Leuk Res.
34:1517–1524. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Yamahira A, Narita M, Nakamura T, et al:
Generation of antigen-specific cytotoxic T lymphocytes using a
leukemic plasmacytoid dendritic cell line as antigen presenting
cells. Leuk Res. 35:793–799. 2011. View Article : Google Scholar
|
|
14.
|
Yamahira A, Narita M, Ishii K, et al:
Enhancement of antigen presenting ability in the leukemic
plasmacytoid dendritic cell line (PMDC05) by lentiviral
vector-mediated transduction of CD80 gene. Leuk Res. 36:1541–1546.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Koido S, Hara E, Homma S, et al:
Dendritic/pancreatic carcinoma fusions for clinical use:
comparative functional analysis of healthy-versus patient-derived
fusions. Clin Immunol. 135:384–400. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Koido S and Gong J: Characterization of
structure and direct antigen presentation by dendritic/tumor-fused
cells as cancer vaccines. Anticancer Res. 33:347–354.
2013.PubMed/NCBI
|
|
17.
|
Liu L, Chahroudi A, Silvestri G, et al:
Visualization and quantification of T cell-mediated cytotoxicity
using cell-permeable fluorogenic caspase substrates. Nat Med.
8:185–189. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Lapteva N, Seethammagari MR, Hanks BA, et
al: Enhanced activ ation of human dendritic cells by inducible CD40
and Toll-like receptor-4 ligation. Cancer Res. 67:10528–10537.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Luger R, Valookaran S, Knapp N,
Vizzardelli C, Dohnal AM and Felzmann T: Toll-like receptor 4
engagement drives differentiation of human and murine dendritic
cells from a pro- into an anti-inflammatory mode. PLoS One.
8:e548792013. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Koido S, Homma S, Okamoto M, et al:
Combined TLR2/4-activated dendritic/tumor cell fusions induce
augmented cytotoxic T lymphocytes. PLoS One. 8:e592802013.
View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Kimura T and Finn OJ: MUC1 immunotherapy
is here to stay. Expert Opin Biol Ther. 13:35–49. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Brugger W, Buhring HJ, Grunebach F, et al:
Expression of MUC-1 epitopes on normal bone marrow: implications
for the detection of micrometastatic tumor cells. J Clin Oncol.
17:1535–1544. 1999.PubMed/NCBI
|
|
23.
|
Wykes M, MacDonald KP, Tran M, et al: MUC1
epithelial mucin (CD227) is expressed by activated dendritic cells.
J Leukoc Biol. 72:692–701. 2002.PubMed/NCBI
|
|
24.
|
Koido S, Hara E, Homma S, et al:
Streptococcal preparation OK-432 promotes fusion efficiency and
enhances induction of antigen-specific CTL by fusions of dendritic
cells and colorectal cancer cells. J Immunol. 178:613–622. 2007.
View Article : Google Scholar
|
|
25.
|
Koido S, Hara E, Homma S, et al: Dendritic
cells fused with allogeneic colorectal cancer cell line present
multiple colorectal cancer-specific antigens and induce antitumor
immunity against autologous tumor cells. Clin Cancer Res.
11:7891–7900. 2005. View Article : Google Scholar
|
|
26.
|
Koido S, Hara E, Homma S, Ohkusa T, Gong J
and Tajiri H: Cancer immunotherapy by fusions of dendritic cells
and tumor cells. Immunotherapy. 1:49–62. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Haenssle HA, Krause SW, Emmert S, et al:
Hybrid cell vaccination in metastatic melanoma: clinical and
immunologic results of a phase I/II study. J Immunother.
27:147–155. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Trefzer U, Herberth G, Wohlan K, et al:
Tumour-dendritic hybrid cell vaccination for the treatment of
patients with malignant melanoma: immunological effects and
clinical results. Vaccine. 23:2367–2373. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Lundqvist A, Palmborg A, Bidla G, Whelan
M, Pandha H and Pisa P: Allogeneic tumor-dendritic cell fusion
vaccines for generation of broad prostate cancer T-cell responses.
Med Oncol. 21:155–165. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Matsumoto S, Saito H, Tsujitani S and
Ikeguchi M: Allogeneic gastric cancer cell-dendritic cell hybrids
induce tumor antigen (carcinoembryonic antigen) specific CD8(+) T
cells. Cancer Immunol Immunother. 55:131–139. 2006.PubMed/NCBI
|
|
31.
|
Marten A, Renoth S, Heinicke T, et al:
Allogeneic dendritic cells fused with tumor cells: preclinical
results and outcome of a clinical phase I/II trial in patients with
metastatic renal cell carcinoma. Hum Gene Ther. 14:483–494. 2003.
View Article : Google Scholar
|
|
32.
|
Siders WM, Garron C, Shields J and Kaplan
JM: Induction of antitumor immunity by semi-allogeneic and fully
allogeneic electrofusion products of tumor cells and dendritic
cells. Clin Transl Sci. 2:75–79. 2009. View Article : Google Scholar : PubMed/NCBI
|