Introduction
Although melanoma only comprises <10% of all
forms of skin cancer; it is responsible for the majority of skin
cancer-related deaths (1–4). In 2013, it was estimated that 76,690
new cases of melanoma would be diagnosed in the United States, and
that 9,480 people would die of this disease (5). When diagnosed in the early stage and
when limited to the skin, melanoma is curable; however, in the case
of metastatic melanoma, the prognosis is very poor with a 5-year
survival rate of <20%. Recently, vemurafenib, a BRAF V600E
inhibitor, was found to be an attractive drug for melanoma patients
and to produce clinical responses in the majority of melanoma
patients (6); however, the
responses are variable and all patients eventually relapse. The
available medicines for melanoma patients are therefore not
significantly effective (7), and a
new strategy is required.
Resveratrol, a phytoalexin originating from plants
such as grapes, is an attractive compound for cancer treatment and
has a beneficial effect on various diseases, including
cardiovascular disease (8),
diabetes (9) and cancer (10). Subsequent preclinical and clinical
studies reported that resveratrol has both anti-proliferative and
anti-metastatic activity in leukemia, breast cancer, and colon
cancer (11). Even though
resveratrol is known to induce apoptosis in melanoma cells through
the suppression of anti-apoptotic proteins such as BCL-2, BCL-xL,
survivin (12–19), the responsible molecules and the
detailed mechanism have not been fully elucidated.
In the present investigation we showed that
resveratrol induces apoptosis in all human and murine melanoma
specimens used, even though they were genetically and
phenotypically different. Furthermore, we determined for the first
time that the transcriptional suppression of survivin is essential
for resveratrol-induced apoptosis. We also identified the
inhibition of both tumor growth and survivin expression in
vivo by resveratrol. Collectively, these results strongly
suggest that targeting survivin by reagents such as resveratrol
could provide an opportunity to treat genetically varied melanoma
patients.
Materials and methods
Reagents and plasmid preparation
Resveratrol (Tokyo Chemical Industry, Tokyo, Japan)
was dissolved in DMSO to make a 100-mM stock solution.
pcDNA3.1-polyP was a kind gift from Dr D.E. Fisher (Massachusetts
General Hospital, Boston, MA, USA). Survivin cDNA was subcloned
into the BamHI-XhoI site of pcDNA3.1 after RT-PCR
from cDNA of normal human melanocytes.
Cell culture
UACC257, SK-MEL-28, SK-MEL-2, UACC62, M14, MeWo,
SK-MEL-5 cell lines were kind gifts for Dr D.E. Fisher and B16-BL6
cell lines were from Dr I.J. Fidler (MD Anderson Cancer Center,
Houston, TX, USA). UACC257, UACC62, M14, MeWo cells were cultured
in RPMI-1640. SK-MEL-28, SK-MEL-2, and SK-MEL-5 were cultured in
Dulbecco’s modified Eagle’s medium (DMEM), and B16-BL6 cells were
cultured in Eagle’s minimal essential medium (EMEM). All of the
media were supplemented with 2 mM L-glutamine, 10% fetal bovine
serum, 100 U/ml penicillin, and 100 μg/ml streptomycin. The cells
were maintained at 37°C in a humidified atmosphere of 5%
CO2.
For the establishment of stable survivin-expressing
cells, SK-MEL-28 cells were transfected with pcDNA3.1-polyP as a
vector control, or pcDNA3.1-HA/survivin vector and selected with
500 μg/ml G418 over a period of 6 weeks.
WST-1 assay
Cell viability was quantified using the cell
proliferation reagent WST-1 (Dojindo, Kumamoto, Japan). Melanoma
cells were seeded and then incubated for 24 h.
Resveratrol-containing medium was added to the well at the
indicated concentrations. After the indicated incubation time,
WST-1 solution was added and absorbance was measured at 450 nm
using a microplate reader. Cells viability was determined as
percent viability compared with the control.
Western blot analysis
Whole cell lysates were prepared as described
previously (20). Primary
antibodies used were specific to caspase-3, poly (ADP-ribose)
polymerase (PARP), BCL-2, BCL-xL, XIAP, Survivin, BCL2A1, MCL-1,
STAT3, p-STAT3, β-catenin, p53 (Cell Signaling Technology, Beverly,
MA, USA), specific to hemagglutinin (HA) (Roche, Indianapolis, IN,
USA) and specific to β-actin (Santa Cruz Biotechnology, Santa Cruz,
CA, USA). All antibodies were used at ×2,000 dilution.
Annexin V and dead cell assay
Apoptotic cell number was determined using the MUSE
Annexin V and Dead Cell kit (Merck KGaA, Darmstadt, Germany)
according to the manufacturer’s instructions. Briefly, after cells
were treated with resveratrol, all cells were collected and diluted
with phosphate-bufferd saline (PBS) containing 1% bovine serum
albumin (BSA) as a dilution buffer to a concentration of
5×105 cells/ml. The cell suspension (100 ml) was then
added to 100 μl MUSE Annexin V and dead cell reagent (2× dilution),
incubated for 20 min at room temperature, and analyzed using the
MUSE Cell Analyzer.
Real-time PCR
Expression of survivin mRNA was
quantitatively determined by real-time PCR on an ABI PRISM 7300
sequence detection system (Life Technologies Corp., Carlsbad, CA,
USA). Total RNAs were prepared using the RNeasy Plus Mini kit
(Qiagen, Hilden, Germany). Expression level of survivin mRNA
was normalized to the β-actin gene. The primers used were:
5′-TGC CTG GCA GCC CTT TC-3′ (sense) and 5′-CCT CCA AGA AGG GCC AGT
TC-3′ (antisense) for survivin mRNA and 5′-GCA CAG AGC CTC
GCC TT-3′ (sense) and 5′-GTT GTC GAC GAC GAG CG-3′ (antisense) for
β-actin mRNA.
Chromatin immunoprecipitation assay
(ChIP)
ChIP assays were performed as described previously
(21). The antibody used was
anti-polymerase II serine 2 phosphorylation (Abcam, Cambridge, MA,
USA). The primers were: 5′-GCA GTT CTG GTA ACG GTG ATA G-3′ (sense)
and 5′-GGG CAG AGA AGG GCA TTA TT-3′ (antisense) for the
survivin gene region, 5′-GAC ATT GAT GGA GAC GGT AAG G-3′
(sense) and 5′-ATA GCC AGG ACT AGA CAG AAG T-3′ (antisense) for the
THBS1 gene region, and 5′-CAT CCT CAC CCT GAA GTA CCC-3′
(sense) and 5′-TAG AAG GTG TGG TGC CAG ATT-3′ (antisense) for the
β-actin gene region.
Animal model
C57BL/6 mice (6-week-old) were purchased from Japan
SLC Inc. (Hamamatsu, Japan). The study was conducted in accordance
with the standards established by the Guidelines for the Care and
Use of Laboratory Animals and approved by the ethics committee of
the University of Toyama (permit no. A2012INM-6). Tumor inoculation
was performed under isoflurane anesthesia, and all efforts were
made to minimize suffering. The B16-BL6 cells were inoculated s.c
(2.5×105 cells/100 μl 50% Matrigel in PBS/mice) into the
flank of anesthetized mice. Mice in each group received resveratrol
in 0.5% carboxymethyl cellulose solution (100 mg/kg/day) or vehicle
by oral administration every day. The tumor volume was assessed
every two days starting from day 9. The primary tumor was measured
using a caliper square along the longer (a) and shorter (b) axis,
and tumor volume was calculated by the following formula: tumor
volume (mm3) = ab2/2.
Statistical analysis
The results are expressed as the mean ± standard
deviation. Statistical significance (p<0.05) was evaluated by
either Student’s two-tailed t-test or one-way ANOVA followed by the
Bonferroni post-hoc test, comparing the results to the control.
Results
Resveratrol induces apoptosis in melanoma
cells
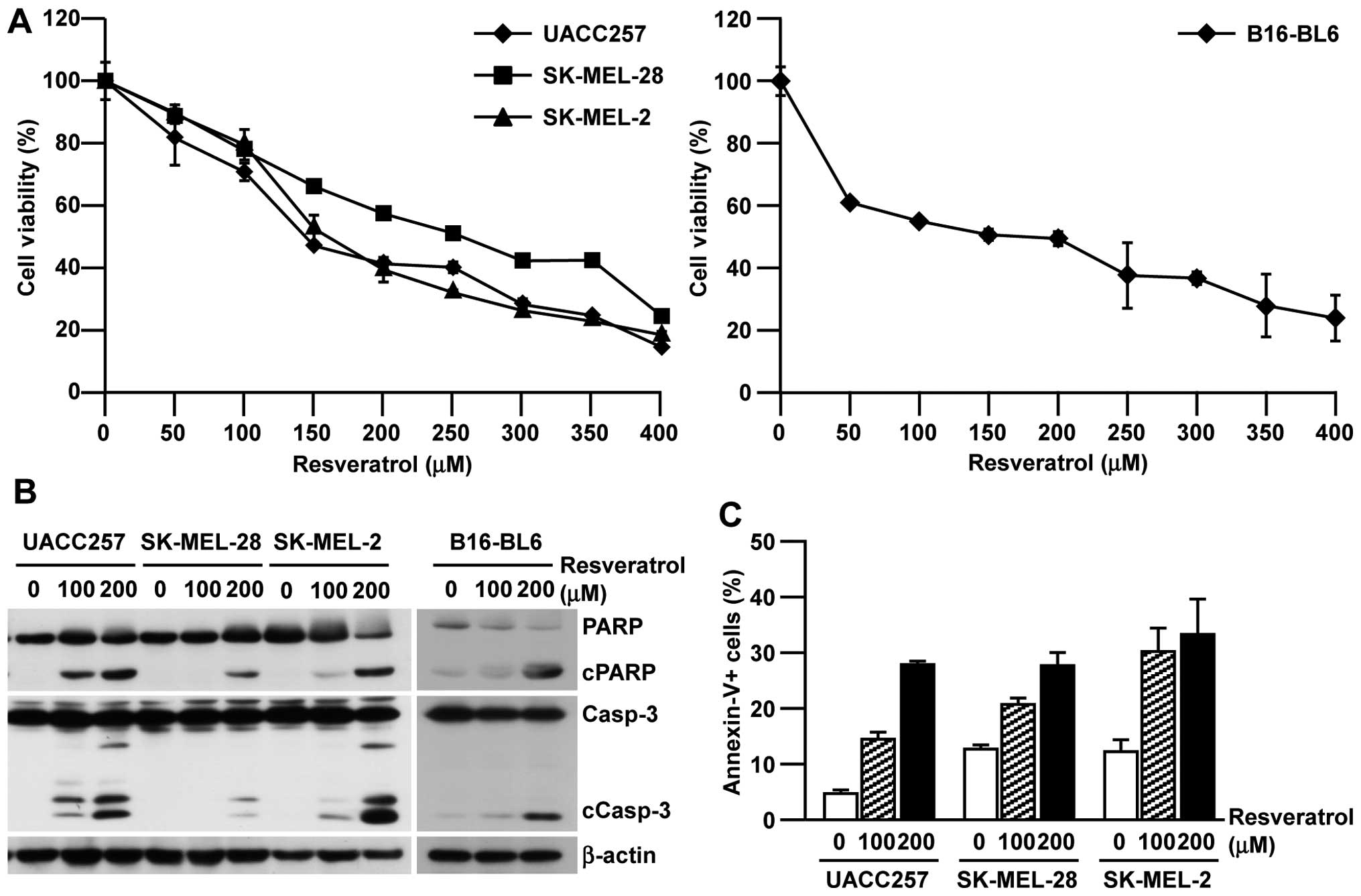
To examine the cytotoxicity of resveratrol against
melanoma, seven human melanoma cell lines with various genetic
backgrounds (Table I) were treated
with resveratrol. As summarized in Table I, all the human melanoma cell lines
tested showed a similar IC50 range (120.4–257.0 μM) even
with different genetic backgrounds; therefore, there seemed to be
no specific genetic requirement for the efficacy of resveratrol in
melanoma cell lines. Because of their different backgrounds,
further experiments were performed using UACC257, SK-MEL-28, and
SK-MEL-2 cells. The cytotoxicity of resveratrol against human
melanoma cell lines was also observed in a dose-dependent manner
and a similar result was confirmed in murine B16-BL6 cells
(Fig. 1A). To determine whether
the cytotoxicity is mediated through canonical apoptotic pathways,
we next assessed the biomarkers for apoptotic cell death. As shown
in Fig. 1B and C, resveratrol
induced the cleavage of caspase-3 and PARP in both human and murine
melanoma cell lines and subsequently induced Annexin-V-positive
apoptotic cells in a dose-dependent manner. Collectively, these
results indicate that resveratrol showed cytotoxicity in human and
murine melanoma cells through the induction of canonical apoptotic
pathways regardless of their genetic background.
 | Table IOncogenic mutation and IC50
of resveratrol in human melanoma cells. |
Table I
Oncogenic mutation and IC50
of resveratrol in human melanoma cells.
| Melanoma cells | BRAF | NRAS | p53 | PTEN | IC50
(μM) |
|---|
| UACC257 | V600E | wt | wt | wt | 143.4 |
| SK-MEL-28 | V600E | wt | L145R | wt | 257.0 |
| SK-MEL-2 | wt | Q61R | G245S | wt | 159.5 |
| M14 | V600E | wt | G266E | wt | 232.8 |
| MeWo | wt | wt | Q317X/E258K | wt | 120.4 |
| SK-MEL-5 | V600E | wt | wt | wt | 158.8 |
| UACC62 | V600E | wt | wt | p245fs5 | 218.8 |
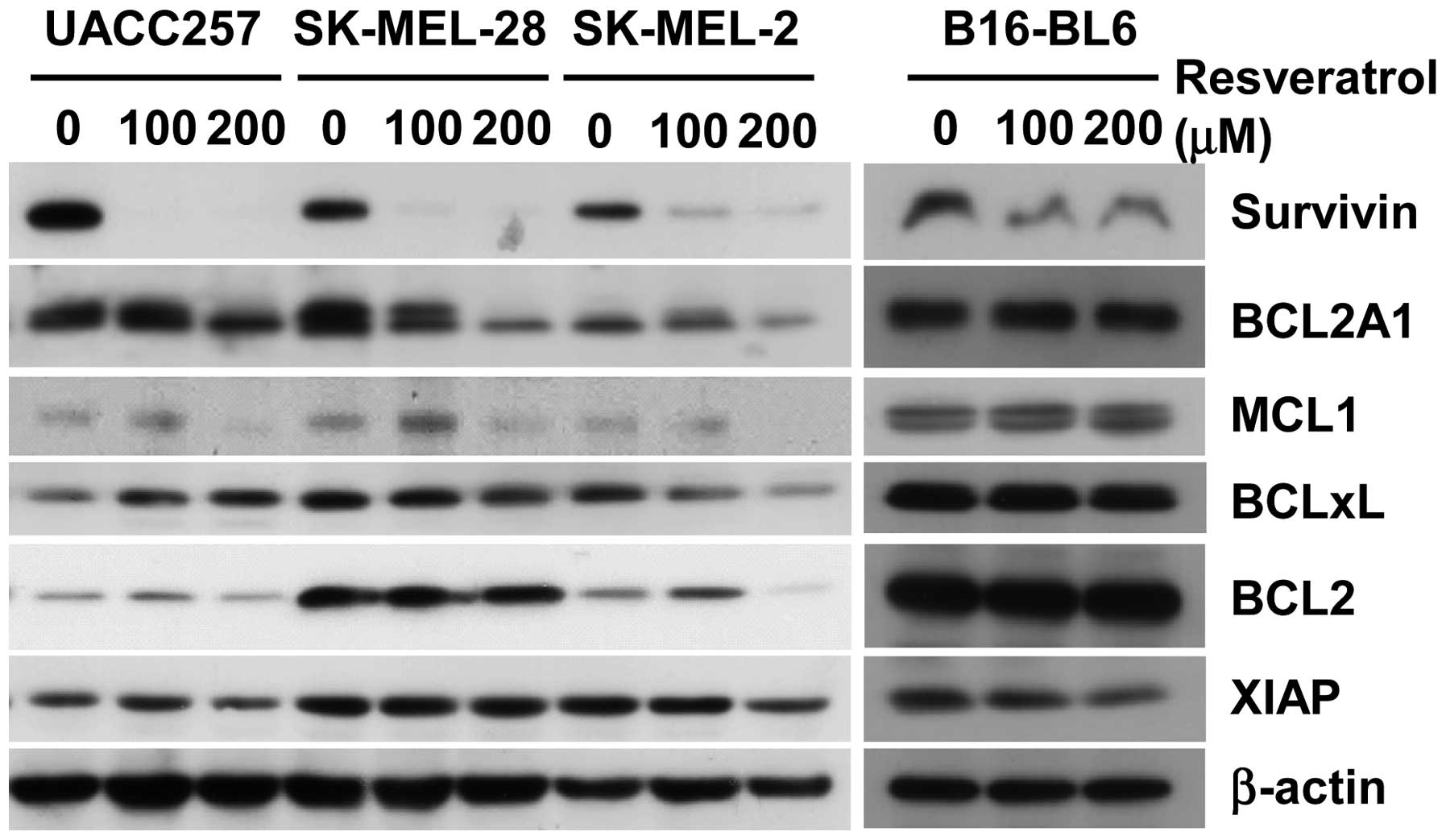
Resveratrol suppresses survivin
expression in melanoma cells
In order to determine whether apoptosis by
resveratrol could be mediated via the suppression of anti-apoptotic
proteins, the expression levels of BCL-2, BCL-xL, XIAP, survivin,
BCL2A1, and MCL-1 were determined after treatment with resveratrol
(Fig. 2). While suppression of
BCL-2, XIAP, and BCL-xL was observed only in SK-MEL-2 cells, not in
SK-MEL-28 and UACC257 cells, the expressions of survivin, BCL2A1
and MCL-1 were strongly suppressed in all three human melanoma cell
lines. Since only survivin was consistently downregulated in murine
B16-BL6 melanoma cells as well as human melanoma cell lines, we
decided to further focus on the role of survivin in the anticancer
efficacy of resveratrol in melanoma cells as a conserved mechanism
in both humans and mice.
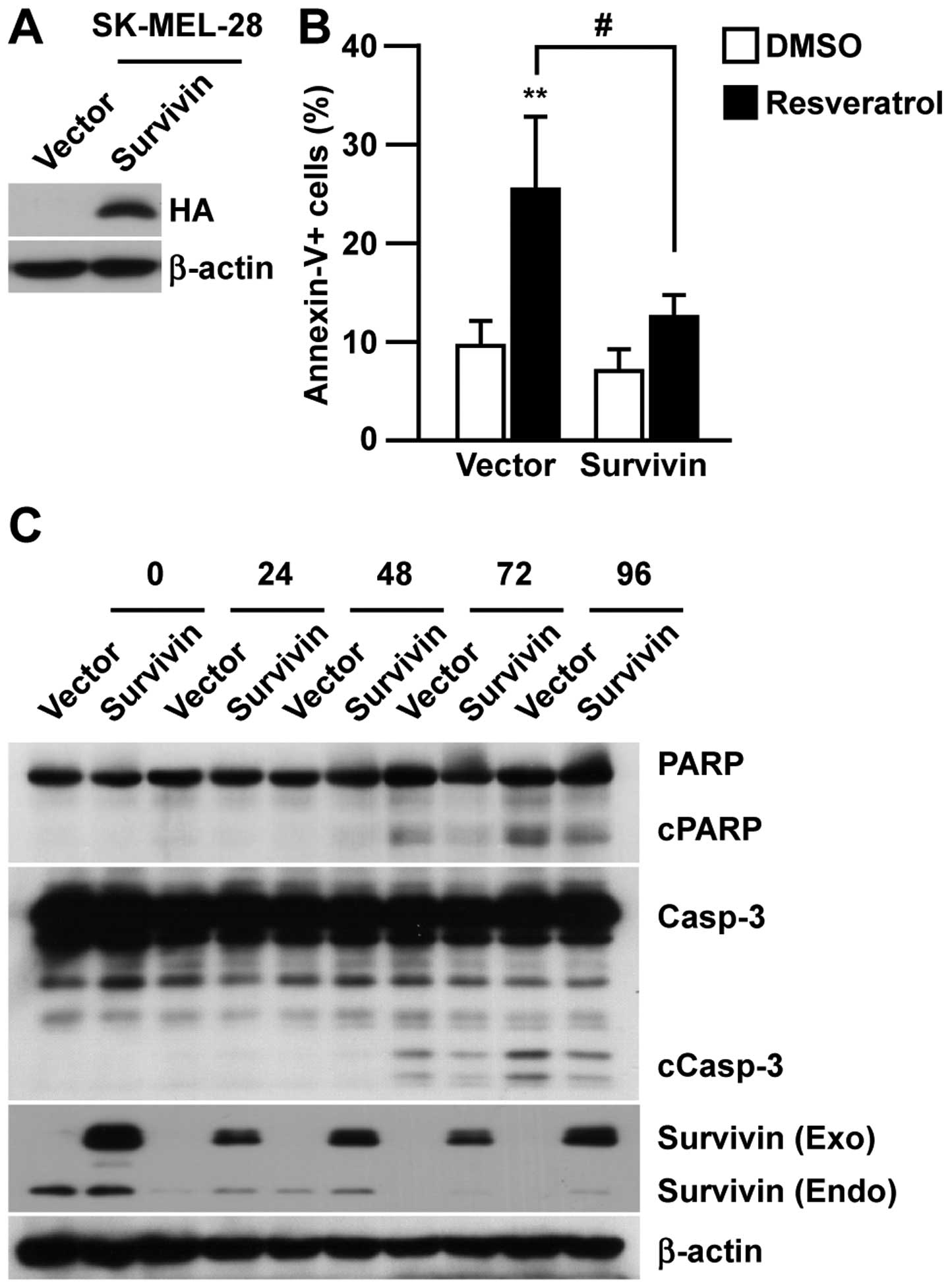
Requirement of survivin suppression for
resveratrol-induced apoptosis
To investigate the requirement of survivin for
resveratrol-induced apoptosis in melanoma, we established
hemagglutinin (HA)-tagged survivin-overexpressing cells
(SK-MEL-28/Survivin) and control cells (SK-MEL-28/Vector, Fig. 3A) to test the pro-apoptotic effect
of resveratrol. As shown in Fig.
3B, the induction of Annexin-V-positive apoptotic cells was
significantly suppressed by overexpressing survivin in SK-MEL-28.
Consistent with the reduction in Annexin-V-positive cells, the
overexpression of survivin in SK-MEL-28 reduced the cleavage of
PARP and caspase-3 upon resveratrol treatment (Fig. 3C). This indicated the direct
involvement of survivin suppression in resveratrol-induced
apoptosis.
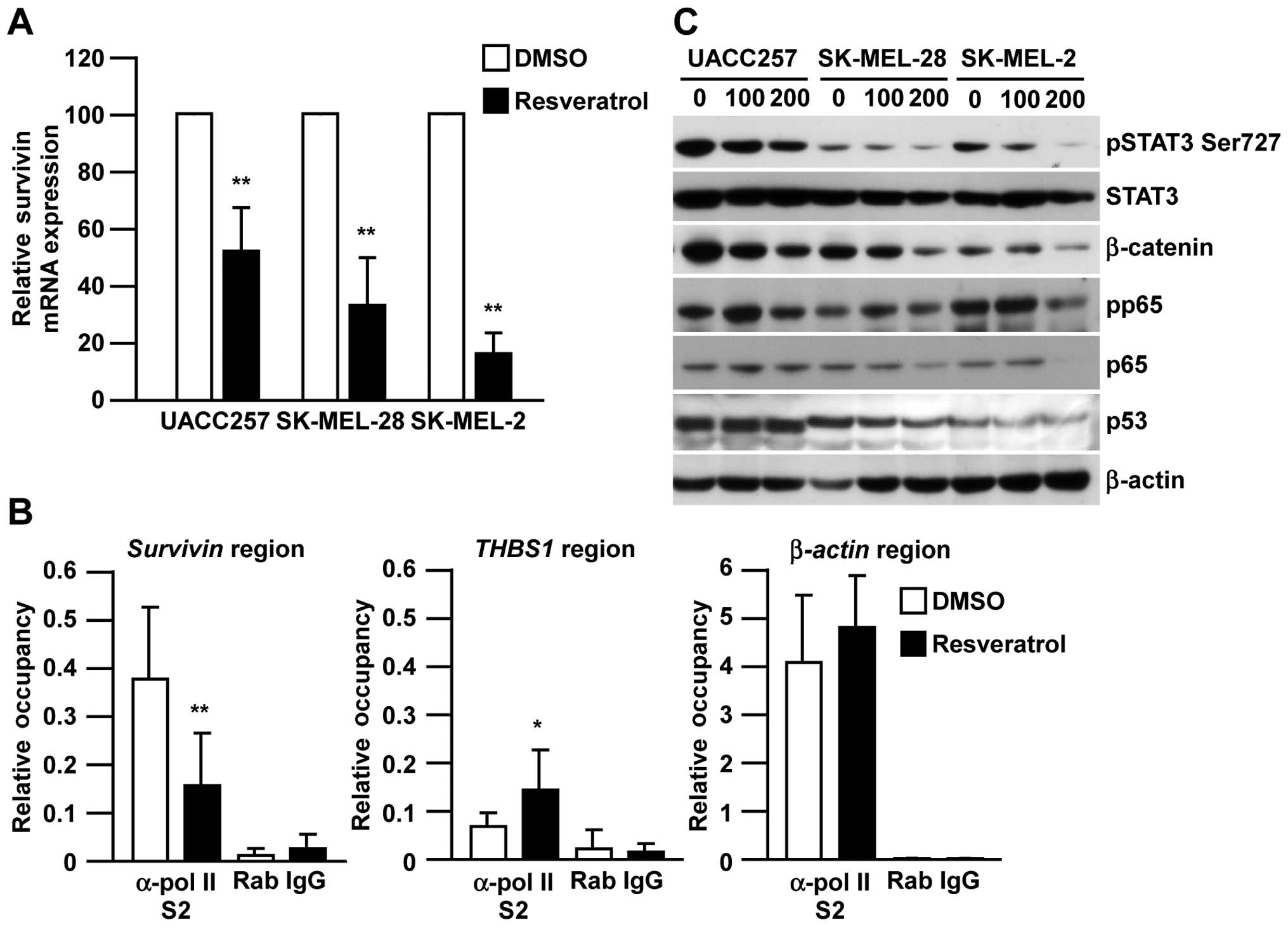
Transcriptional regulation of survivin by
resveratrol in melanoma cells
In order to determine the molecular mechanism by
which resveratrol inhibits survivin expression, we next examined
the effect of resveratrol on survivin mRNA expression using
real-time RT-PCR and the transcriptional levels of survivin by
chromatin immunoprecipitation assay. As shown in Fig. 4A, the expression of survivin
mRNA was decreased in all three human melanoma cell lines after
resveratrol treatment. Consistently, the occupancy of pol-II S2 in
the survivin gene region specifically decreased after
resveratrol treatment compared with the β-actin region or
THBS1 region, in which THBS1 expression is known to be
increased by resveratrol treatment (Fig. 4B) (22). To further investigate which
transcription factor is responsible for the suppression of
survivin transcription, we next determined the expression of
STAT3, β-catenin, p65 and p53, which are known to be involved in
survivin transcription (23). Interestingly, resveratrol strongly
inhibited β-catenin expression and STAT3 phosphorylation in all
three melanoma cell lines, but did not show a significant effect on
p53 expression (Fig. 4C).
Suppression of p65 phosphorylation was only seen in SK-MEL-2 cells,
not in SK-MEL-28 or UACC257 cells. Collectively, these results
indicate that resveratrol inhibits survivin expression at the
transcriptional level through the regulation of β-catenin and STAT3
pathways.
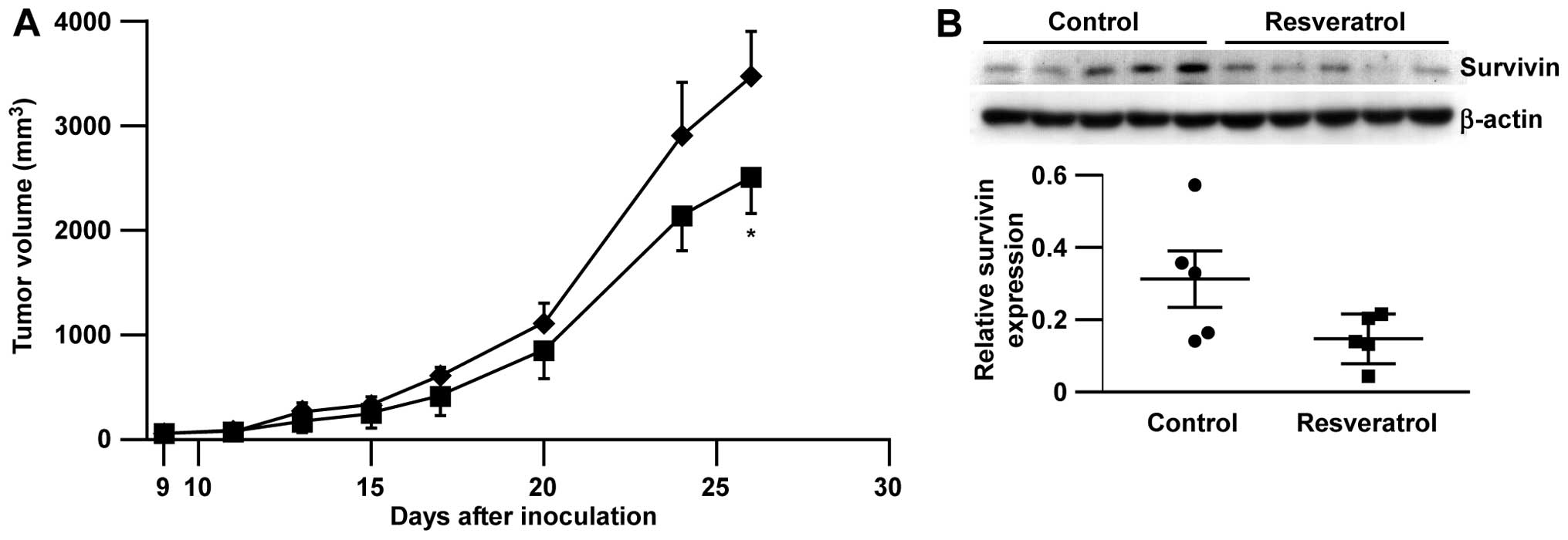
In vivo antitumor effect of resveratrol
in B16-BL6 melanoma model
Finally, we tested the efficacy of resveratrol
against highly malignant B16-BL6 melanoma in syngenic
immunocompetent C57BL/6 mice. Consistent with our in vitro
data, we observed that the administration of resveratrol
significantly inhibited B16-BL6 melanoma growth compared to the
vehicle control without any body weight loss (Fig. 5A and data not shown) and further,
the reduction of survivin expression in tumors after consecutive
treatment with resveratrol (14 days, Fig. 5B).
Discussion
In this study, we showed that resveratrol induces
apoptosis through survivin in various melanoma cell lines in
vitro without any genetic requirement and further demonstrated
the efficacy of resveratrol against melanoma tumor growth in a
mouse model. The transcriptional suppression of survivin by
resveratrol was mediated through either the β-catenin or STAT3
pathway.
Although we concluded that survivin was critically
involved in resveratrol-induced apoptosis, a substantial level of
apoptosis (Fig. 3C) and in
vitro growth inhibition (Fig.
5A) were observed in SK-MEL-28/Survivin cells treated with
resveratrol. These results may imply some additional mechanisms of
resveratrol in melanoma growth inhibition other than the
transcriptional suppression of survivin. Indeed, there are several
reports regarding the downstream targets of β-catenin or STAT3
other than survivin that might be related to resveratrol-induced
apoptosis. In our results, BCL2A1, MCL-1, and BCL-xL were not
consistently regulated by resveratrol in some melanoma cells
(Fig. 2). In addition,
resveratrol-induced apoptosis was not rescued by overexpression of
BCL2A1 in melanoma cells (data not shown), suggesting that BCL2A1,
MCL-1 and BCL-xL are unlikely to be responsible for
resveratrol-induced apoptosis. The mTOR pathway is also known as an
important target of resveratrol in certain cancers (24,25)
and is a potential repressor of survivin translation (26). Consistent with previous reports
(24,25), we observed the suppression of p70
S6K phosphorylation by resveratrol, one of the downstream molecules
in the mTOR pathway in some melanoma cell lines (data not shown);
therefore, inhibition of the mTOR pathway might be an alternative
mechanism of resveratrol-induced apoptosis in melanoma.
Considering that survivin is known as a therapeutic
target for various cancer types such as leukemia, prostate, breast,
bladder, colon and melanoma (27–31)
and that resveratrol reduced cell viability in all melanoma cell
lines regardless of their genetic background in BRAF/NRAS/p53/PTEN
(Fig. 1 and Table I), the utility of resveratrol in
cancer treatment is feasible. Importantly, survivin expression was
higher in melanoma than in nevus and normal skin when we
re-analyzed the published microarray data set (32) (data not shown) and survivin
expression was also very limited in normal adult tissues (33). In addition, resveratrol could
suppress UV damage in normal human keratinocytes, resulting in
adverse effects of the prevention of photocarcinogenesis (34). Considering that we did not observe
any weight loss in mice with resveratrol in vivo (data not
shown), survivin inhibitors including resveratrol could be
attractive reagents for cancer therapy regardless of the cancer
type. Of note, resveratrol even reduced the cell viability of
SK-MEL-5 cell line, which is known to have high β-catenin activity
(35), suggesting that resveratrol
might be effective against various target cancer cells by
overcoming the high transcriptional activity of β-catenin or
STAT3.
Collectively, these results strongly suggest that
targeting survivin with reagents such as resveratrol could provide
a new opportunity for melanoma therapy, regardless of the genetic
background.
Acknowledgements
We thank Dr D.E. Fisher (MGH, Boston, MA, USA) for
the kind gifts of melanoma cell lines and plasmids, and all members
of the Saiki laboratory for discussions and suggestions. This study
was supported in part by a Grant-in-Aid for Young Scientists (B)
(no. 24700971) (S.Y.), by a Grant-in-aid for Challenging
Exploratory Research (24659348) (I.S.) from the Ministry of
Education, Culture, Sports, Science, and Technology (Japan), by
Kato Memorial Bioscience Foundation (Japan) (S.Y.), and by a
Grant-in-Aid for the Cooperative Research Project (I.S.) from the
Joint Usage/Research Center (Joint Usage/Research Center for
Science-Based Natural Medicine), Institute of Natural Medicine,
University of Toyama, in 2013.
References
|
1
|
Chudnovsky Y, Khavari PA and Adams AE:
Melanoma genetics and the development of rational therapeutics. J
Clin Invest. 115:813–824. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Devesa SS, Fears TR and Hartge P:
Cancer surveillance series: changing patterns of cutaneous
malignant melanoma mortality rates among whites in the United
States. J Natl Cancer Inst. 92:811–818. 2000. View Article : Google Scholar
|
|
3
|
Jemal A, Devesa SS, Hartge P and Tucker
MA: Recent trends in cutaneous melanoma incidence among whites in
the United States. J Natl Cancer Inst. 93:678–683. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J and
Thun MJ: Cancer statistics, 2009. CA Cancer J Clin. 59:225–249.
2009. View Article : Google Scholar
|
|
5
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar
|
|
6
|
Flaherty KT, Puzanov I, Kim KB, et al:
Inhibition of mutated, activated BRAF in metastatic melanoma. N
Engl J Med. 363:809–819. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nashan D, Muller ML, Grabbe S, Wustlich S
and Enk A: Systemic therapy of disseminated malignant melanoma: an
evidence-based overview of the state-of-the-art in daily routine. J
Eur Acad Dermatol Venereol. 21:1305–1318. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Petrovski G, Gurusamy N and Das DK:
Resveratrol in cardiovascular health and disease. Ann NY Acad Sci.
1215:22–33. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Szkudelska K and Szkudelski T:
Resveratrol, obesity and diabetes. Eur J Pharmacol. 635:1–8. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Signorelli P and Ghidoni R: Resveratrol as
an anticancer nutrient: molecular basis, open questions and
promises. J Nutr Biochem. 16:449–466. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Aggarwal BB, Bhardwaj A, Aggarwal RS,
Seeram NP, Shishodia S and Takada Y: Role of resveratrol in
prevention and therapy of cancer: preclinical and clinical studies.
Anticancer Res. 24:2783–2840. 2004.PubMed/NCBI
|
|
12
|
Niles RM, McFarland M, Weimer MB, Redkar
A, Fu YM and Meadows GG: Resveratrol is a potent inducer of
apoptosis in human melanoma cells. Cancer Lett. 190:157–163. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fulda S and Debatin KM: Sensitization for
anticancer drug-induced apoptosis by the chemopreventive agent
resveratrol. Oncogene. 23:6702–6711. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ivanov VN, Partridge MA, Johnson GE, Huang
SX, Zhou H and Hei TK: Resveratrol sensitizes melanomas to TRAIL
through modulation of antiapoptotic gene expression. Exp Cell Res.
314:1163–1176. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fulda S and Debatin KM: Sensitization for
tumor necrosis factor-related apoptosis-inducing ligand-induced
apoptosis by the chemopreventive agent resveratrol. Cancer Res.
64:337–346. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fang Y, Bradley MJ, Cook KM, Herrick EJ
and Nicholl MB: A potential role for resveratrol as a radiation
sensitizer for melanoma treatment. J Surg Res. 183:645–653. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim MY: Nitric oxide triggers apoptosis in
A375 human melanoma cells treated with capsaicin and resveratrol.
Mol Med Rep. 5:585–591. 2012.PubMed/NCBI
|
|
18
|
Osmond GW, Augustine CK, Zipfel PA,
Padussis J and Tyler DS: Enhancing melanoma treatment with
resveratrol. J Surg Res. 172:109–115. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tak JK, Lee JH and Park JW: Resveratrol
and piperine enhance radiosensitivity of tumor cells. BMB Rep.
45:242–246. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sakurai H, Suzuki S, Kawasaki N, et al:
Tumor necrosis factor-alpha-induced IKK phosphorylation of
NF-kappaB p65 on serine 536 is mediated through the TRAF2, TRAF5,
and TAK1 signaling pathway. J Biol Chem. 278:36916–36923. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Haq R, Yokoyama S, Hawryluk EB, et al:
BCL2A1 is a lineage-specific antiapoptotic melanoma oncogene that
confers resistance to BRAF inhibition. Proc Natl Acad Sci USA.
110:4321–4326. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Trapp V, Parmakhtiar B, Papazian V,
Willmott L and Fruehauf JP: Anti-angiogenic effects of resveratrol
mediated by decreased VEGF and increased TSP1 expression in
melanoma-endothelial cell co-culture. Angiogenesis. 13:305–315.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Altieri DC: Survivin, cancer networks and
pathway-directed drug discovery. Nat Rev Cancer. 8:61–70. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang J, Chiu J, Zhang H, et al:
Autophagic cell death induced by resveratrol depends on the
Ca(2+)/AMPK/mTOR pathway in A549 cells. Biochem Pharmacol.
86:317–328. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jiang H, Shang X, Wu H, et al: Resveratrol
downregulates PI3K/Akt/mTOR signaling pathways in human U251 glioma
cells. J Exp Ther Oncol. 8:25–33. 2009.PubMed/NCBI
|
|
26
|
Vaira V, Lee CW, Goel HL, Bosari S,
Languino LR and Altieri DC: Regulation of survivin expression by
IGF-1/mTOR signaling. Oncogene. 26:2678–2684. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhao W, Bao P, Qi H and You H: Resveratrol
down-regulates survivin and induces apoptosis in human
multidrug-resistant SPC-A-1/CDDP cells. Oncol Rep. 23:279–286.
2010.PubMed/NCBI
|
|
28
|
Hayashibara T, Yamada Y, Nakayama S, et
al: Resveratrol induces downregulation in survivin expression and
apoptosis in HTLV-1-infected cell lines: a prospective agent for
adult T cell leukemia chemotherapy. Nutr Cancer. 44:193–201. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shankar S, Siddiqui I and Srivastava RK:
Molecular mechanisms of resveratrol
(3,4,5-trihydroxy-trans-stilbene) and its interaction with
TNF-related apoptosis inducing ligand (TRAIL) in
androgen-insensitive prostate cancer cells. Mol Cell Biochem.
304:273–285. 2007. View Article : Google Scholar
|
|
30
|
Singh SK, Moretta D, Almaguel F, Wall NR,
De Leon M and De Leon D: Differential effect of proIGF-II and
IGF-II on resveratrol induced cell death by regulating survivin
cellular localization and mitochondrial depolarization in breast
cancer cells. Growth Factors. 25:363–372. 2007. View Article : Google Scholar
|
|
31
|
Singh RP, Tyagi A, Sharma G, Mohan S and
Agarwal R: Oral silibinin inhibits in vivo human bladder tumor
xenograft growth involving down-regulation of survivin. Clin Cancer
Res. 14:300–308. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Talantov D, Mazumder A, Yu JX, et al:
Novel genes associated with malignant melanoma but not benign
melanocytic lesions. Clin Cancer Res. 11:7234–7242. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Velculescu VE, Madden SL, Zhang L, et al:
Analysis of human transcriptomes. Nat Genet. 23:387–388. 1999.
View Article : Google Scholar
|
|
34
|
Adhami VM, Afaq F and Ahmad N: Suppression
of ultraviolet B exposure-mediated activation of NF-kappaB in
normal human keratinocytes by resveratrol. Neoplasia. 5:74–82.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Widlund HR, Horstmann MA, Price ER, et al:
Beta-catenin-induced melanoma growth requires the downstream target
Microphthalmia-associated transcription factor. J Cell Biol.
158:1079–1087. 2002. View Article : Google Scholar
|