Introduction
The innate immune system plays an important role as
the first line of defense against bacteria, fungi, and viruses
before adaptive immunity to these potential pathogens is developed.
Mucosal cells in particular produce many types of antimicrobial
peptides that prevent invasion or growth of pathogens. The α- and
β-defensins are classes of cysteine-rich, cationic antimicrobial
peptides (1,2). To date, six human α-defensins and 28
human β-defensins (hBDs) have been identified (3,4).
Some hBDs are constitutively expressed in epithelial cells, whereas
expression of hBD-2 and hBD-3 is induced by microbial products or
inflammatory stimuli (5,6). Human BDs also have chemotactic
activity for memory T cells and immature dendritic cells through
binding to CCR6, thus bridging innate and adaptive immunity
(7,8).
In addition to their roles in the innate immune
system, several studies have suggested that hBDs are expressed by
and have certain roles in some cancer cells. For example,
Shestakova et al reported that lung cancer cells express
hBD-1, -2, and -4 (9,10). Arimura et al reported that
serum hBD levels are high in lung cancer patients and suggested
that hBD-1 could be a new diagnostic marker for lung cancer
(11). Markeeva et al
revealed that hBD-2 is overexpressed in gastric and cervical cancer
cells (12,13), although the level of expression is
not correlated with the differentiation grade or stage of the
cancer. Mburu et al reported that head and neck squamous
cell cancer cells secrete hBD-3. In addition, they found that hBD-3
induces CCR7 expression in these cells in an NF-κB-dependent manner
and provides migratory and pro-survival signals to cancer cells
(14).
In humans, the number of microbes is highest in the
colon, and as such, colon cancer cells would be constantly exposed
to a vast number of potential pathogens. Therefore, it is possible
that hBDs play specific roles in the development of colon cancer.
However, despite several reports suggesting that α-defensin is
expressed in colon cancer cells (15–17),
and that hBD-3, which is induced by microbial stimuli plays a
variety of roles in the pathogenesis and progression of head and
neck squamous cell and oral squamous cell cancer (OSCC) (14,18–20),
little is known about hBD expression in colon cancer cells. In this
study, we therefore examined the expression and role of hBD-3 in
colon cancer cells.
Materials and methods
Cell lines and reagents
The human colon cancer cell line COLO-320 (RCB1193)
was purchased from the Riken BRC Cell Bank (Tsukuba, Japan). The
SW480 (ATCC CCL-228), SW620 (ATCC CCL-227), LS180 (ATCC CL-187),
and HT29 (ATCC HTB-38) cell lines were purchased from the American
Type Culture Collection (Manassas, VA, USA), and the human
esophageal cell line KYSE30 (JCRB0188) was purchased from the
Health Science Research Resource Bank (Osaka, Japan). All cells
were cultured in a humidified atmosphere containing 5%
CO2 at 37°C in Dulbecco’s modified Eagle’s medium (DMEM)
(Life Technologies, Tokyo, Japan) supplemented with 1%
penicillin/streptomycin (Life Technologies) and 10% fetal calf
serum (FCS) (Life Technologies).
Human β-defensin 3 was purchased from the Peptide
Institute, Inc. (Osaka, Japan). Lipopolysaccharide (LPS) from E.
coli was purchased from Imgenex (San Diego, CA, USA), and LPS
from Sallmonella abortus equi was purchased from Enzo Life
Science (Farmingdale, NY, USA). Anti-hBD-3 polyclonal antibody was
purchased from Phoenix Pharmaceuticals, Inc. (Burlingame, CA,
USA).
Qualitative reverse
transcriptase-polymerase chain reaction (PCR)
The expression of hBD-3 mRNA in colon cancer cells
was analyzed by reverse transcriptase-PCR of total RNA. Total RNA
was extracted from ~107 cells of each cell line using an
RNeasy Mini kit (Qiagen, Tokyo, Japan), and cDNA was synthesized by
extension of oligo(dT) primers using PrimeScript reverse
transcriptase (Takara, Ohtsu, Japan). PCR of the cDNA was performed
using Ex Taq (Takara). The primer sets used for amplification of
hBD-3 and human GAPDH were as follows: hBD-3 forward, 5′-TTTTGGTGC
CTGTTCCAGGT-3′ and reverse, 5′-TTCTTCGGCAGCATT TTCGG-3′; human
GAPDH forward, 5′-TATAAATTGAGC CCGCAGCC-3′ and reverse,
5′-TTCCCGTTCTCAGCCTT GAC-3′.
Immunohistochemistry
Immunohistchemical staining for hBD-3 was performed
on surgically resected colon cancer tissues and tissue arrays
(Super Bio Chips, Seoul, Korea) using a Vecstain ABC kit (Vector
Laboratories, Burlingame, CA, USA). Deparaffinized sections were
heated for 5 min in citrate buffer at 100°C with a pressure cooker
to reactivate the antigen and then treated with 0.3%
H2O2 in methanol for 30 min to deactivate
endogenous peroxidases. Sections were blocked with 1% goat serum in
PBS, covered with primary antibody at 4°C overnight, covered with
second-step biotinylated antibody for 30 min, and then incubated
with peroxidase-labeled streptavidin for 30 min. After washing,
sections were incubated with 0.05% diaminobenzidene/0.15%
H2O2 and counterstained with 10% hematoxylin
(Wako, Osaka, Japan). This study was approved by the Institutional
Review Board of Mie University Hospital. Written informed consent
was obtained from each patient included in the study. The study
protocol conforms to the ethical guidelines of the 1975 Declaration
of Helsinki as reflected in a priori approval by the institution’s
human research committee.
Cell proliferation and viability
assays
MTT assay
Colon cancer cells were plated at a density of
1×104 cells per well in 96-well microtiter plates
(Corning Glass Works, Corning, NY, USA), and each plate was
incubated for 5 h at 37°C in a 5% CO2 atmosphere. Next,
50 μl of hBD-3 or control solution was added to each well, and the
plates were incubated for an additional 48 h. The live-cell count
was determined using a Cell Titer 96 Assay kit (Promega, Madison,
WI, USA) according to the manufacturer’s instructions. The
absorbance of the contents of each well was measured at 570 nm with
a microtiter plate reader (Bio-Rad Laboratories, Hercules, CA,
USA).
xCELLigence system
Cell proliferation and viability was also assessed
using an xCELLigence system (Roche Inc., Basel, Switzerland)
according to the manufacturer’s instructions. Briefly, each well of
each 16-well microtiter plate (E-Plate 16) was filled with 100 μl
of DMEM to equilibrate the well membrane, and the plates were then
incubated for 30 min at 37°C in a 5% CO2 atmosphere.
Colon cancer cells suspended in 50 μl of growth medium were seeded
at a density of 1×104 cells per well, and 50 μl of hBD-3
solution was added 6 h later. Cells were cultured for 48 h using a
Real-Time Cell Analyzer (RTCA) single plate (SP) instrument placed
in a standard incubator at 37°C in a 5% CO2 atmosphere.
Cell index values were monitored and recorded at 15-min
intervals.
Migration assay
Changes in the migration of SW480 and SW620 cells
were analyzed using a fibronectin-coated Oris Cell Migration Assay
kit (Platypus Technologies, Madison, WI, USA) according to the
manufacturer’s protocol. Briefly, cells were plated at a density of
1×104 cells per well and incubated at 37°C in a 5%
CO2 atmosphere. After 24 h, all the stoppers were
removed from the wells and the cells were washed once and the
medium was exchanged with medium containing various concentrations
of hBD-3 (0, 1 or 5 μM). After an additional 24 h of incubation,
the wells were photographed and cell migration was analyzed using
ImageJ software (US National Institutes of Health).
Real-time PCR array
Changes in the expression of genes related to the
migration and invasiveness of SW480 and SW620 cells following
exposure to hBD-3 (5 μM) were analyzed using an RT2 PCR
array (Human Extracellular Matrix and Adhesion Molecules)
(SABiosciences Corp., Frederick, MD, USA) according to the
manufacturer’s instructions. Observed changes in mRNA expression
were confirmed using quantitative real-time PCR.
Quantitative real-time PCR
cDNAs of colon cancer cell lines were synthesized
from 1 μg of total RNA. Quantitative real-time PCR (qRT-PCR) was
performed using an ABI PRISM 7300 Real-time PCR system (Applied
Biosystems, Foster City, CA, USA) with EagleTaq Master Mix kits
(Roche Molecular Systems, Branchburg, NJ, USA). The expression
levels of target genes were determined from triplicate reactions by
normalization of expression data to that of β-actin according to
the manufacturer’s instructions. The primer set and probe for
metastasis-associated 1 family, member 2 (MTA2) were as follows:
sense primer, 5′-CGCAGGGACATTTCTAGTAGC-3′; antisense primer,
5′-GCTGCTTTGATTCCTCTTCAAA-3′; probe, CAGCCTGG.
Statistical analysis
Cell proliferation, migration, and gene expression
data were compared using the two-tailed Student’s t-test. For
differences between rates, Fisher’s exact test was used. A P-value
<0.05 was considered statistically significant.
Results
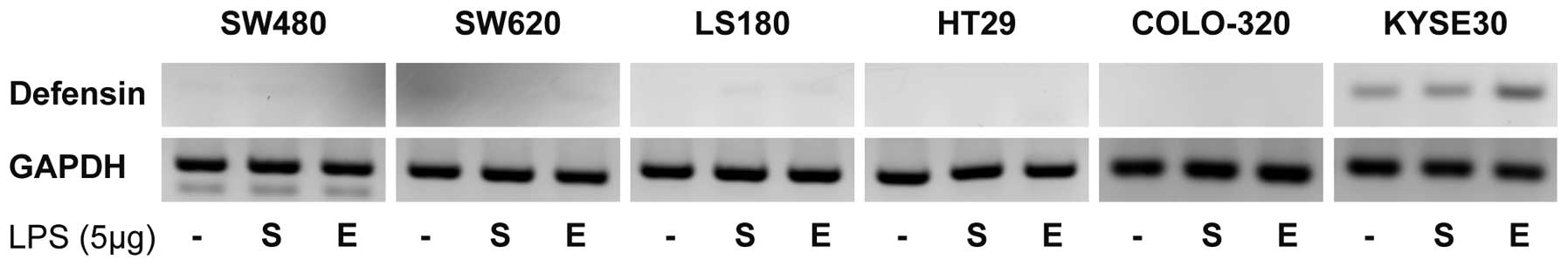
Human BD-3 mRNA is not expressed in colon
cancer cells
First, we examined hBD-3 mRNA expression in each
colon cancer cell line by qualitative reverse transcriptase-PCR. No
hBD-3 transcripts were detected in any of the colon cancer cell
lines examined (SW480, SW620, LS180, HT29 and COLO-320), and LPS
stimulation had no effect on hBD-3 mRNA expression in these cell
lines. In contrast, KYSE30 esophageal cancer cells did express
hBD-3 mRNA, the level of which increased with LPS stimulation
(Fig. 1).
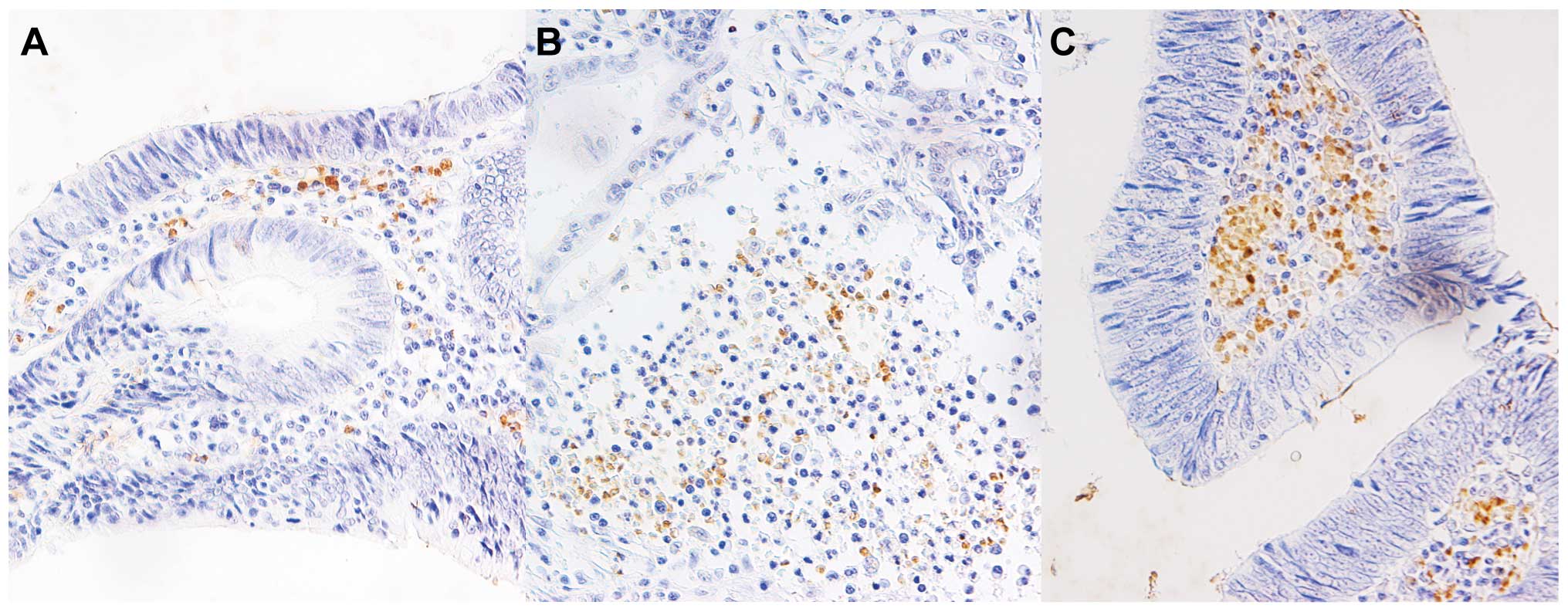
Human BD-3 is expressed in infiltrating
monocytes of colon cancer tissues
Next, we performed immunohistochemical staining for
hBD-3 in surgically resected colon cancer tissues. No hBD-3
expression was detected in the colon cancer cells; however, many
tissue samples stained positive for hBD-3 in infiltrating monocytes
surrounding the cancer cells (Fig.
2). Similar results were obtained by immunohistochemical
staining of tissue array samples. Results of the
immunohistochemistry analyses are summarized in Table I. The level of hBD-3 expression was
analyzed in each group, and the percentage of positive-staining
stromal monocytes was divided into three categories (0–33, 34–66
and 67–100%). No correlation was found between the degree of
positive staining for hBD-3 and the degree of cancer cell
differentiation, possibly due to the small number of well
differentiated and poorly differentiated colon cancer tissues.
 | Table ISummary of immunohistochemical
staining results. |
Table I
Summary of immunohistochemical
staining results.
| | Extent of positive
staining |
|---|
| |
|
|---|
| Cases (n) | 0–33% | 34–66% | 67–100% |
|---|
| Total | 39 | 7 (17.9%) | 24 (61.5%) | 8 (20.1%) |
| Well
differentiateda | 9 | 2 (22.2%) | 4 (44.4%) | 3 (33.3%) |
| Moderately
differentiateda | 26 | 4 (15.4%) | 17 (65.4%) | 5 (19.2%) |
| Poorly
differentiateda | 1 | 1 (100%) | 0 (0%) | 0 (0%) |
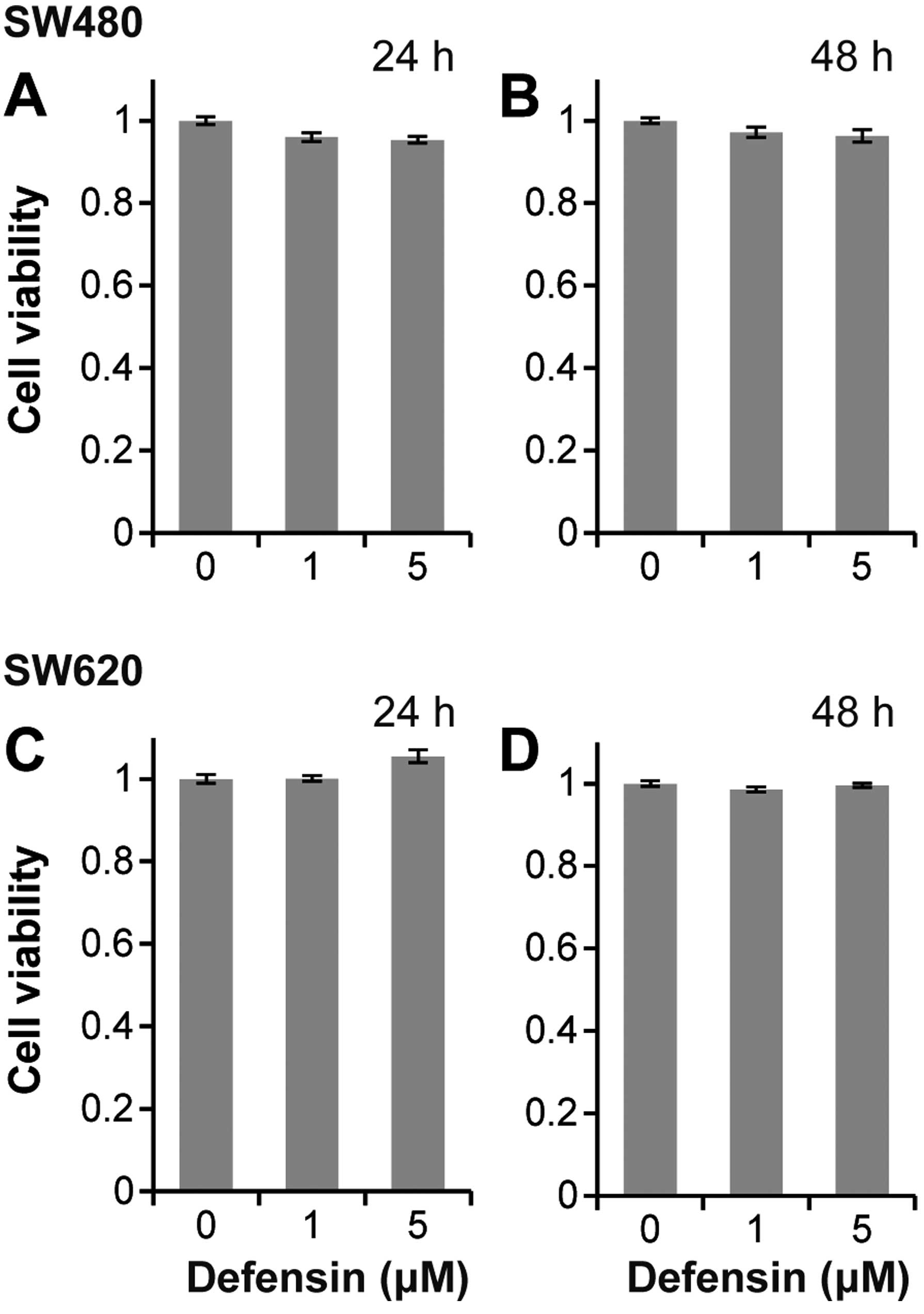
Human BD-3 does not affect the
proliferation of colon cancer cells
To assess how hBD-3 affects colon cancer cells, we
first examined the effect of hBD-3 on cancer cell proliferation
using an MTT assay and the xCELLigence system. Fig. 3 shows the results of the MTT assay
after 24 and 48 h. Proliferation of SW480 and SW620 cells was not
affected by exposure to hBD-3 at concentrations of 1 and 5 μM.
Similar results were obtained with the xCELLigence system (data not
shown).
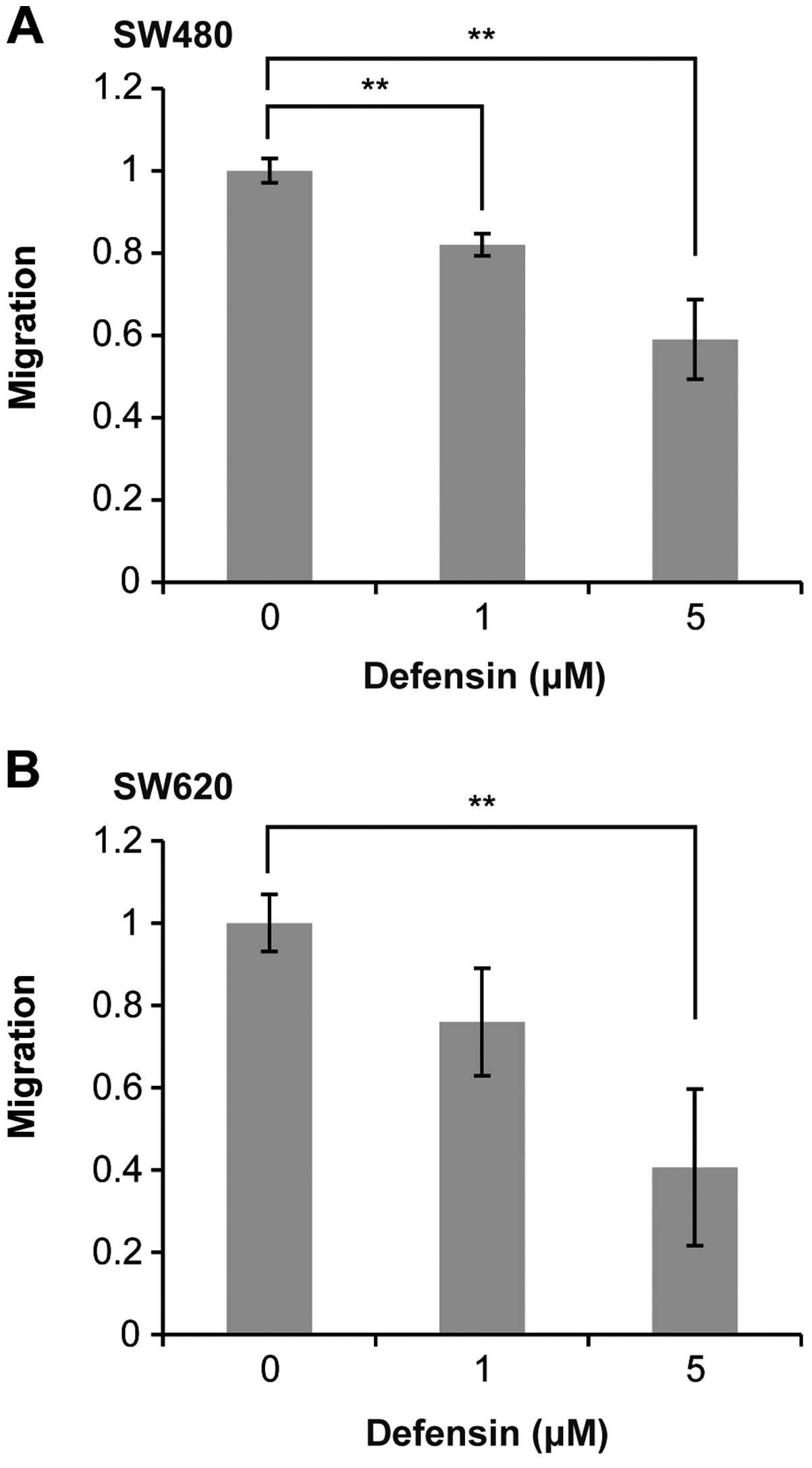
Human BD-3 inhibits the migration of
colon cancer cells
Next, we examined the effect of hBD-3 on colon
cancer cell migration. Interestingly, as shown in Fig. 4A, co-culture with hBD-3 (1 or 5 μM)
significantly inhibited the migration of SW480 cells after 24 h in
a dose-dependent manner. A similar inhibition of migration
following exposure to hBD-3 was observed in SW620 cells (Fig. 4B).
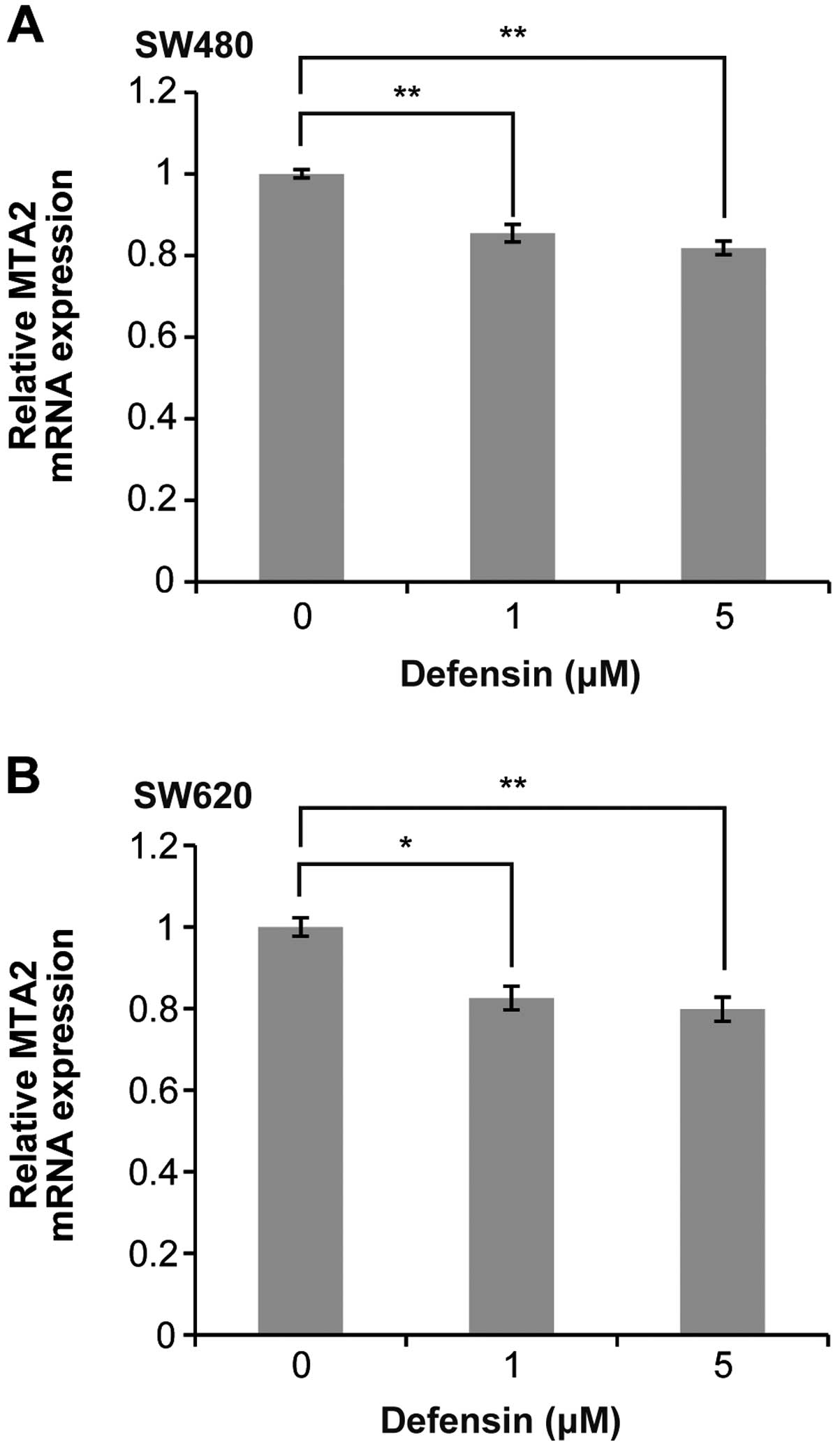
Human BD-3 reduces MTA2 mRNA expression
in colon cancer cells
To elucidate the mechanism through which hBD-3
suppresses the migration of colon cancer cells, we compared the
expression of MTA2 mRNA in SW480 cells co-cultured with 5 μM hBD-3
and control cells using a real-time PCR array. This experiment was
repeated twice, and the results of both experiments showed that the
expression of MTA2 was reduced by addition of hBD-3 to the culture
medium (data not shown). To confirm these results, we examined
hBD-3-induced changes in the level of MTA2 mRNA expression in colon
cancer cells using qRT-PCR. As shown in Fig. 5, the level of MTA2 mRNA expression
was significantly reduced in both SW480 and SW620 cells in a
dose-dependent manner following exposure to hBD-3.
Discussion
In the present study, we demonstrated that hBD-3 is
not expressed in colon cancer cells but is expressed in
tumor-infiltrating monocytes. Migration of cells of various colon
cancer lines was significantly inhibited by exposure to hBD-3,
although hBD-3 had no effect on proliferation of these cells.
Moreover, reduced expression of MTA2 mRNA in colon cancer cells was
associated with exposure to hBD-3.
Accumulating evidence indicates that several types
of epithelial and cancer cells produce hBD-3. It was reported that
hBD-3 is expressed in the skin, oral cavity, esophagus, trachea and
placenta (5,21). Moreover, gastric and colon
epithelial cells produce a large amount of hBD-3 in response to
chronic inflammation (22,23). Expression of hBDs has been reported
in lung cancer, head and neck cancer, and OSCC (9,10,14,18–20).
However, the significance of the expression of hBDs in cancer cells
is still controversial; for example, the expression of hBD-1 and
hBD-3 is lost at a high frequency in prostate cancer, renal cell
carcinoma and OSCC (24–28). Initially, we hypothesized that
colon cancer cells themselves produce hBD-3 because these cells are
constantly exposed to many microorganisms. However, our results
demonstrate that hBD-3 is not expressed in cells of various colon
cancer lines, nor is it expressed in surgically resected colon
cancer cells. Furthermore, we found that hBD-3 mRNA expression is
not affected by the presence of bacterial LPS, which was reported
to upregulate hBD-3 expression in OSCC (29).
Although our results indicate that colon cancer
cells do not produce hBD-3, immunohistochemical staining showed
that tumor-infiltrating monocytes express high levels of hBD-3. As
we supposed that colon cancer cells are exposed to hBD-3 excreted
by monocytes, we next investigated the effects of hBD-3 on colon
cancer progression. The results of our functional assays showed
that hBD-3 significantly suppresses the migration of colon cancer
cells. An increasing number of studies have suggested that
defensins play important roles in modulating the development and
progression of several kinds of cancers. Xu et al showed
that intratumoral administration of α-defensin-1 inhibits the
growth of human lung adenocarcinoma xenografts in nude mice,
inducing apoptosis of these cells (30). Several studies have suggested that
hBD-3 enhances progression of oral and head squamous carcinomas
(14,20,29).
However, Wang et al reported that hBD-3 inhibits the
migration of head and neck cancer cells (18), and our results agree with their
data.
The addition of hBD-3 to the culture medium of colon
cancer cell lines significantly reduced the expression of MTA2 mRNA
in the current study. MTA2 is a member of the metastasis-associated
family of proteins, which includes MTA1, MTA2, and MTA3. MTA1 was
the first of these proteins to be identified, and there have been
many reports demonstrating that MTA1 overexpression is closely
correlated with carcinogenesis and the progression of a wide range
of solid human cancers, including breast, liver, lung, gastric and
colorectal (31–34). MTA2 is highly homologous to MTA1
and is a component of the nucleosome remodeling and histone
deacetylase (NuRD) complex (35,36).
Several studies have also reported that there is a relationship
between MTA2 expression and a higher degree of malignant potential
for various cancers (35,37–41).
All of these reports indicate that MTA2 drives carcinogenesis and
enhances tumor invasiveness. Recent studies have provided insight
into how MTA proteins may enhance cancer progression. Cui et
al reported that the NuRD complex represses the transactivation
function of estrogen receptor-α, causing breast cancer cells to
become more aggressive (42). Luo
et al found that the NuRD complex also deacetylates and thus
inactivates p53, a protein that mediates cell growth arrest and
apoptosis (43). Our findings
suggest that hBD-3 inhibits the migration of colon cancer cells by
downregulating MTA2 expression.
Probably one of the most important findings of this
study is that hBD-3 has a suppressive effect on colon cancer.
However, we acknowledge that our experimental data were obtained
from cultured cell lines, and we did not clarify whether any
relationship exists between prognosis and hBD-3 expression in
either cancer cells or stromal monocytes in colon cancer tissues;
therefore, further investigation is required. Also, the precise
mechanism through which hBD-3 downregulates MTA2 expression remains
to be elucidated.
In conclusion, our present study demonstrates that
hBD-3 inhibits the progression of colon cancer in a paracrine
fashion. We believe that hBD-3 will prove to be a potent new
addition to the therapeutic arsenal for treating colon cancer.
References
|
1
|
Raj PA and Dentino AR: Current status of
defensins and their role in innate and adaptive immunity. FEMS
Microbiol Lett. 206:9–18. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ganz T: Defensins: antimicrobial peptides
of innate immunity. Nat Rev Immunol. 3:710–720. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yang D, Biragyn A, Hoover DM, Lubkowski J
and Oppenheim JJ: Multiple roles of antimicrobial defensins,
cathelicidins, and eosinophil-derived neurotoxin in host defense.
Annu Rev Immunol. 22:181–215. 2004. View Article : Google Scholar
|
|
4
|
Schutte BC, Mitros JP, Bartlett JA, et al:
Discovery of five conserved beta-defensin gene clusters using a
computational search strategy. Proc Natl Acad Sci USA.
99:2129–2133. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jia HP, Schutte BC, Schudy A, et al:
Discovery of new human beta-defensins using a genomics-based
approach. Gene. 263:211–218. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sorensen OE, Thapa DR, Rosenthal A, Liu L,
Roberts AA and Ganz T: Differential regulation of beta-defensin
expression in human skin by microbial stimuli. J Immunol.
174:4870–4879. 2005. View Article : Google Scholar
|
|
7
|
Zlotnik A and Yoshie O: Chemokines: a new
classification system and their role in immunity. Immunity.
12:121–127. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wu Z, Hoover DM, Yang D, et al:
Engineering disulfide bridges to dissect antimicrobial and
chemotactic activities of human beta-defensin 3. Proc Natl Acad Sci
USA. 100:8880–8885. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shestakova T, Zhuravel E, Bolgova L,
Alekseenko O, Soldatkina M and Pogrebnoy P: Expression of human
beta-defensins-1, 2 and 4 mRNA in human lung tumor tissue: a pilot
study. Exp Oncol. 30:153–156. 2008.PubMed/NCBI
|
|
10
|
Shestakova T, Zhuravel E, Bolgova L, et
al: Immunohistochemical analysis of beta-defensin-2 expression in
human lung tumors. Exp Oncol. 32:273–276. 2010.PubMed/NCBI
|
|
11
|
Arimura Y, Ashitani J, Yanagi S, et al:
Elevated serum beta-defensins concentrations in patients with lung
cancer. Anticancer Res. 24:4051–4057. 2004.PubMed/NCBI
|
|
12
|
Markeeva N, Lisovskiy I, Lyzogubov V, et
al: Expression of beta-defensin-2 in human gastric tumors: a pilot
study. Exp Oncol. 27:130–135. 2005.PubMed/NCBI
|
|
13
|
Markeeva N, Lysovskiy I, Zhuravel E, et
al: Involvement of human beta-defensin-2 in proliferation of
transformed cells of human cervix. Exp Oncol. 27:308–313.
2005.PubMed/NCBI
|
|
14
|
Mburu YK, Abe K, Ferris LK, Sarkar SN and
Ferris RL: Human beta-defensin 3 promotes NF-kappaB-mediated CCR7
expression and anti-apoptotic signals in squamous cell carcinoma of
the head and neck. Carcinogenesis. 32:168–174. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Melle C, Ernst G, Schimmel B, et al:
Discovery and identification of alpha-defensins as low abundant,
tumor-derived serum markers in colorectal cancer. Gastroenterology.
129:66–73. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Albrethsen J, Bogebo R, Gammeltoft S,
Olsen J, Winther B and Raskov H: Upregulated expression of human
neutrophil peptides 1, 2 and 3 (HNP 1–3) in colon cancer serum and
tumours: a biomarker study. BMC Cancer. 5:82005.
|
|
17
|
Albrethsen J, Moller CH, Olsen J, Raskov H
and Gammeltoft S: Human neutrophil peptides 1, 2 and 3 are
biochemical markers for metastatic colorectal cancer. Eur J Cancer.
42:3057–3064. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang K, Wang JH, Baskaran H, Wang R and
Jurevic R: Effect of human beta-defensin-3 on head and neck cancer
cell migration using micro-fabricated cell islands. Head Neck
Oncol. 4:412012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kawsar HI, Weinberg A, Hirsch SA, et al:
Overexpression of human beta-defensin-3 in oral dysplasia:
potential role in macrophage trafficking. Oral Oncol. 45:696–702.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Winter J, Pantelis A, Reich R, et al:
Human beta-defensin-1, -2, and -3 exhibit opposite effects on oral
squamous cell carcinoma cell proliferation. Cancer Invest.
29:196–201. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Harder J, Bartels J, Christophers E and
Schroder JM: Isolation and characterization of human
beta-defensin-3, a novel human inducible peptide antibiotic. J Biol
Chem. 276:5707–5713. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kawauchi K, Yagihashi A, Tsuji N, et al:
Human beta-defensin-3 induction in H. pylori-infected
gastric mucosal tissues. World J Gastroenterol. 12:5793–5797.
2006.PubMed/NCBI
|
|
23
|
Fahlgren A, Hammarstrom S, Danielsson A
and Hammarstrom ML: beta-Defensin-3 and -4 in intestinal epithelial
cells display increased mRNA expression in ulcerative colitis. Clin
Exp Immunol. 137:379–385. 2004. View Article : Google Scholar
|
|
24
|
Donald CD, Sun CQ, Lim SD, et al:
Cancer-specific loss of beta-defensin 1 in renal and prostatic
carcinomas. Lab Invest. 83:501–505. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Young AN, de Oliveira Salles PG, Lim SD,
et al: Beta defensin-1, parvalbumin, and vimentin: a panel of
diagnostic immunohistochemical markers for renal tumors derived
from gene expression profiling studies using cDNA microarrays. Am J
Surg Pathol. 27:199–205. 2003. View Article : Google Scholar
|
|
26
|
Bullard RS, Gibson W, Bose SK, et al:
Functional analysis of the host defense peptide Human Beta
Defensin-1: new insight into its potential role in cancer. Mol
Immunol. 45:839–848. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Joly S, Compton LM, Pujol C, Kurago ZB and
Guthmiller JM: Loss of human beta-defensin 1, 2, and 3 expression
in oral squamous cell carcinoma. Oral Microbiol Immunol.
24:353–360. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yoshimoto T, Yamaai T, Mizukawa N, et al:
Different expression patterns of beta-defensins in human squamous
cell carcinomas. Anticancer Res. 23:4629–4633. 2003.PubMed/NCBI
|
|
29
|
Shuyi Y, Feng W, Jing T, et al: Human
beta-defensin-3 (hBD-3) upregulated by LPS via epidermal growth
factor receptor (EGFR) signaling pathways to enhance lymphatic
invasion of oral squamous cell carcinoma. Oral Surg Oral Med Oral
Pathol Oral Radiol Endod. 112:616–625. 2011. View Article : Google Scholar
|
|
30
|
Xu N, Wang YS, Pan WB, et al: Human
alpha-defensin-1 inhibits growth of human lung adenocarcinoma
xenograft in nude mice. Mol Cancer Ther. 7:1588–1597. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Toh Y, Oki E, Oda S, et al: Overexpression
of the MTA1 gene in gastrointestinal carcinomas: correlation with
invasion and metastasis. Int J Cancer. 74:459–463. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Martin MD, Fischbach K, Osborne CK, Mohsin
SK, Allred DC and O’Connell P: Loss of heterozygosity events
impeding breast cancer metastasis contain the MTA1 gene. Cancer
Res. 61:3578–3580. 2001.PubMed/NCBI
|
|
33
|
Hamatsu T, Rikimaru T, Yamashita Y, et al:
The role of MTA1 gene expression in human hepatocellular carcinoma.
Oncol Rep. 10:599–604. 2003.PubMed/NCBI
|
|
34
|
Sasaki H, Moriyama S, Nakashima Y, et al:
Expression of the MTA1 mRNA in advanced lung cancer. Lung Cancer.
35:149–154. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang Y, Ng HH, Erdjument-Bromage H,
Tempst P, Bird A and Reinberg D: Analysis of the NuRD subunits
reveals a histone deacetylase core complex and a connection with
DNA methylation. Genes Dev. 13:1924–1935. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Toh Y and Nicolson GL: The role of the MTA
family and their encoded proteins in human cancers: molecular
functions and clinical implications. Clin Exp Metastasis.
26:215–227. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ji Y, Zhang P, Lu Y and Ma D: Expression
of MTA2 gene in ovarian epithelial cancer and its clinical
implication. J Huazhong Univ Sci Technolog Med Sci. 26:359–362.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lee H, Ryu SH, Hong SS, et al:
Overexpression of metastasis-associated protein 2 is associated
with hepatocellular carcinoma size and differentiation. J
Gastroenterol Hepatol. 24:1445–1450. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liu SL, Han Y, Zhang Y, et al: Expression
of metastasis-associated protein 2 (MTA2) might predict
proliferation in non-small cell lung cancer. Target Oncol.
7:135–143. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Covington KR, Brusco L, Barone I, et al:
Metastasis tumor-associated protein 2 enhances metastatic behavior
and is associated with poor outcomes in estrogen receptor-negative
breast cancer. Breast Cancer Res Treat. 141:375–384. 2013.
View Article : Google Scholar
|
|
41
|
Zhou C, Ji J, Cai Q, et al: MTA2 promotes
gastric cancer cells invasion and is transcriptionally regulated by
Sp1. Mol Cancer. 12:1022013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Cui Y, Niu A, Pestell R, et al:
Metastasis-associated protein 2 is a repressor of estrogen receptor
alpha whose overexpression leads to estrogen-independent growth of
human breast cancer cells. Mol Endocrinol. 20:2020–2035. 2006.
View Article : Google Scholar
|
|
43
|
Luo J, Su F, Chen D, Shiloh A and Gu W:
Deacetylation of p53 modulates its effect on cell growth and
apoptosis. Nature. 408:377–381. 2000. View Article : Google Scholar : PubMed/NCBI
|