Introduction
Various traumas on a body can cause gastrointestinal
tract erosion and mucosal epithelium damage which lead to
gastrointestinal tract bleeding and ulcer perforation and finally
aggravate the origin of disease. The gastrointestinal mucosal
epithelium is a fundamental barrier that provides protection
against the outside environment. It is important to protect the
mucosal epithelium from damage. The cytoprotective functions in
protecting gastrointestinal tract against ongoing damage may be
accomplished in both the early phase of epithelial repair known as
restitution and in the subsequent, protracted phase of epithelial
renewal (1–3). Restitution, the ability of epithelial
cells to spread and migrate across the basement membrane to cover
shallow defects, is the key initial step in repair of mucosal
injury and can achieve restoration of mucosal continuity over broad
areas of damage within hours (4,5). It
is well established that the integrity of the gastrointestinal
mucosa and other epithelium are maintained by a number of secreted
factors, but many studies suggest that one important mechanism
involves the secretion of the members of a protease-resistant
protein family known as the trefoil factor family (TFF) (6). TFF, a recently recognized family of
protease-resistant small peptides, is expressed in a regional
specific pattern throughout the normal gastrointestinal tract. This
family, comprising the intestinal trefoil factor (ITF) and the
gastric peptides SP and pS2, plays a critical role in epithelial
restitution and proliferation within the mammalian gastrointestinal
tract (7). The members of this
family share an array of structural features including, most
notably, a motif of six cysteine residues termed a trefoil or a P
domain, which is distinct from those found in other peptide
families. They are rapidly upregulated at the margins of mucosal
injury, and they are believed to promote epithelial cell
proliferation and migration (8,9). ITF
is a predominant factor of TFF and has been demonstrated in in
vitro and in vivo studies to play an important role in
mucosal homeostasis of the gastrointestinal mucosa (10,11).
Though ITF could maintain gastric mucosal integrity, the underlying
mechanisms controlling this process remain unclear. It is necessary
to explore the mechanisms of ITF that regulate the proliferation,
migration and maintenance integrity of gastric mucosal epithelial
cells.
It is well known that phosphatidylinositol 3′-kinase
(PI3K) is a vital regulatory protein responsible for maintaining
cell viability and cell survival. PI3K phosphorylates
phosphoinositides at their 3′-position in turn, activates
downstream effector molecules. Akt/protein kinase B is a primary
downstream target of PI3K intermediates and major component in cell
survival. Akt is activated by translocation to plasma membrane when
the PI3K-generated 3-phosphoinositides bind to its pleckstrin
homology domain. PI3K/Akt signaling pathway plays an essential role
in regulating cell proliferation, differentiation, apoptosis and
migration (12,13). In addition to its metabolic
actions, PI3K/Akt signaling pathway has been shown to preserve
epithelial integrity during inflammation (14). Numerous studies have indicated that
growth factors participate in many important physiological and
pathologic processes in gastrointestinal diseases via regulating
downstream signaling pathways which could mediate cell survival,
cell apoptosis, cell migration and immune response. Evidence has
shown that ITF (TFF3) as a growth factor could act on the epidermal
growth factor receptor (EGFR), which then activates several
downstream signaling pathways, including MAPK and PI3K/Akt
signaling pathway (15). The above
studies also demonstrated that ITF exerts antidepressant-like
effects that might be mediated by the PI3K/Akt signaling pathway in
the basolateral amygdala. These studies suggested that PI3K/Akt
signaling may participate in the regulation of ITF on the cell
physiological and pathologic processes. Based on these
observations, we hypothesized that ITF could activate the PI3K/Akt
signaling to regulate the proliferation, migration and maintain
epithelial integrity of GES-1 in vitro.
The present study examined the effects of ITF on the
proliferation, migration and epithelial integrity of GES-1 cells
in vitro. To investigate the underlying mechanisms, we
evaluated the role of the PI3K/Akt signaling pathway in these
physiological processes regulated by TFF3. Thus, we investigated
the protective effect of ITF on the gastric mucosa epithelium after
damage aiming to lay the foundations of clinical application of ITF
as a new type of gastric mucosal protective agent.
Materials and methods
Cell culture
GES-1 cells were obtained from the American Type
Culture Collection (ATCC, Rockville, MD, USA). Cells were cultured
in high glucose-Dulbecco’s modified Eagle’s medium (DMEM, Hyclone,
Thermo Scientific, San Jose, CA, USA) with 10% FBS (Gibco, Paisley,
UK; Invitrogen, Carlsbad, CA, USA), 1% L-glutamine, and a 1%
solution of penicillin and streptomycin seeded at a density of
2×106 cells/ml onto uncoated flasks, and cultured in a
humidified incubator at 37°C in 5% CO. When GES-1 cells reached 80%
confluence, they were routinely passaged using 0.25% trypsin and
were diluted 1:2 at each passage. Cells treated with appropriate
concentration of LPS, ITF and LY294002 were used in the following
experiments.
Cell viability assay
Cell viability was evaluated by the CCK-8 (Cell
Counting Kit-8) test. Cells were placed on a 6-well culture plate
at 2×106 cells/ml in 2 ml culture medium. After
incubation for 12 h, the wells were treated with ITF (100 ng/ml,
PeproTech, Inc., Rocky Hill, NJ, USA), LY294002 (15 μM, Cell
Signaling Technology, Inc., Danvers, MA, USA) or LPS (10 μg/ml,
Sigma-Aldrich, Inc., MO, USA) separately, then treated cells were
incubated at 37°C in an atmosphere of 95% air and 5% CO for 24, 48
and 72 h. After treatment for different periods, cells in each
group were plated on a 96-well culture plate (n=8) at
2×104 cells/well in 100 μl culture medium, after
incubated for 12 h, the culture medium was removed and 100 μl
serum-free medium was added with 10 μl CCK-8 solution to each well
of a culture plate. After incubation for 4 h, absorbance was
measured at optical density (OD) of 450 nm with a multi- detection
micro plate reader (VersaMax, USA).
Cell migration assay
The migration ability of GES-1 cells was determined
by their ability to cross the 8-μm pores of a migration chamber
that consists of trans-wells fitted with Millipore membranes
(6.5-mm filters; Costar). Cells were suspended in serum-free
culture medium at a concentration of 4×105 cells/ml and
then added to the upper chamber (at 4×104 cells/well).
Simultaneously, 0.5 ml of culture medium with 10% FBS containing
ITF (100 ng/ml) or LY294002 (15 μM) was added to the lower
compartment. The cells were allowed to migrate in a humidified CO
incubator at 37°C for 12 h. After incubation, cells that had
entered the lower surface of the filter membrane were fixed with
90% ethanol for 30 min at room temperature, washed three times with
distilled water, and stained with 0.1% crystal violet in 0.1 M
borate and 2% ethanol for 30 min at room temperature. Cells
remaining on the upper surface of the filter membrane were gently
scraped off with a cotton swab. Images of penetrated cells were
captured by a photomicroscope (BX51; Olympus). Cell migration
ability was quantified in a blinded manner by counting the number
of the penetrated cells on the lower surface of the membrane with
five fields (x100 magnification) per chamber. Experiments were
performed three times in duplicates.
Western blot analysis
The dose and time response of Akt activation by ITF
was determined. From our initial observations, Akt activation
occurred within minutes. On the basis of this, all further studies
evaluated Akt with time-points ranging between 5 and 240 min. GES-1
cells were first treated with increasing doses of ITF (0–500 ng/ml)
for 30 min. Next, the optimum dose (100 ng/ml) was used to
determine the time-dependent response of Akt activation. Cell
proteins were obtained from cultured or treated GES-1 cells in each
group. Western blot analysis of cellular lysates was performed as
previously described (22).
Briefly, the cells were washed with ice-cold PBS and lysed in a
modified RIPA buffer containing 1 mM DTT, 1 mM PMSF, complete
protease inhibitor cocktail (Roche, Indianapolis, IN, USA) for 30
min. The whole lysates were then centrifuged at 12,000 g for 30 min
at 4°C, and the protein concentration in the supernatant was
determined using BCA assays. Supernatants were mixed with an equal
volume of 2X loading buffer and boiled for 10 min. The isolated
protein samples were loaded at 30 μg on 12% SDS-polyacrylamide gel
to perform electrophoresis. The separated proteins were then
transferred to the polyvinylidene fluoride (PVDF) membranes using
standard procedures. For immunoblotting, the membranes were
incubated at 37°C for 1 h in blocking buffer (PBS, 0.1% Tween-20,
1% BSA and 5% non-fat milk). The primary antibodies were added to
the membranes and incubated at 4°C overnight. After three washes in
PBS, the membranes were incubated with 1:10,000 diluted secondary
antibodies (horseradish peroxidase-conjugated goat
anti-rabbit/mouse IgG, Boster, Wuhan, China) at 37°C for 1 h. After
additional washing with TBST, the target proteins on the blot
membrane were visualized using the ECL system. The Odyssey Scanning
System (LI-COR Inc., USA) was used for image capture. Equal loading
of proteins was confirmed by visualization of β-actin. Band
intensities were quantified by densitometry using Image J Software
(version 1.41). The primary antibodies were employed as follows:
rabbit anti-Akt, rabbit anti-pAkt (Cell Signaling Technology Inc.),
rabbit anti-occludin and mouse anti-β-actin (Abcam Inc., Cambridge,
MA, USA).
FDA and PI staining for morphologic
evaluation
The integrity of cell membrane was detected using
FDA/PI staining. Cells were placed on a 6-well culture plate at
1×106 cells/ml in 2 ml culture medium. After 12 h, cells
were treated with LPS (10 μg/ml), ITF (100 ng/ml) and LY294002 (15
μM) for 24 h, and then plated at a density of 2×105
cells/well onto 96-well plates, stained with 5 μg/ml PI and 4 μg/ml
FDA, and observed under a fluorescent microscope (Nikon Eclipse
TE2000-S, Japan).
Flow cytometric assay
Cell apoptosis was measured by Annexin V-FITC and
propidium iodide (PI) staining through flow cytometry
(Becton-Dickinson, San Jose, CA, USA). The cells were transplanted
to 6-well culture plates at a density of 2×106 cells/ml,
after 12 h, the cells were treated with LPS (10 μg/ml), ITF (100
ng/ml) and LY294002 (15 μM), then cells were incubated at 37°C in
an atmosphere of 95% air and 5% CO for 48 h. By centrifugation at
1,200 rpm for 6 min the cells were collected. After the cells were
washed twice with cold PBS, they were re-suspended in 500 μl
binding buffer at a concentration of 1×106 cells/ml.
Each cell sample was then stained with 5 μl Annexin V-FITC and 5 μl
PI according to the manufacturer’s instructions. Treated cells were
incubated in the dark at 37°C for 15 min. Samples were acquired on
a FACScan flow cytometer and analyzed.
Immunofluorescent staining
For studying the protective effect of ITF on
maintaining integrity of GES-1 cells and investigating the
regulation of Akt signaling pathway, 10 μg/ml LPS was added into
cultured GES-1 cells. After treatment for 4 h, 100 ng/ml ITF was
added, then treated GES-1 cells were cultured for 48 h.
Immunofluorescence analysis of GES-1 cells was performed as
described previously (16).
Briefly, cells were first fixed with 4% paraformaldehyde for 10
min. To block unspecific binding sites, the cells were incubated
with PBS containing 2% BSA for 1 h at 37°C. After blocking of the
non-specific staining, the cells were incubated with the primary
antibody (rabbit anti-occludin, Abcam, Inc., CA, USA) at a dilution
of 1:200 at 4°C overnight. After three washes with PBST (PBS with
0.2% Triton X-100), GES-1 cells were incubated with a secondary
antibody (goat anti-rabbit/mouse Alexa Fluor 594 or 488, Invitrogen
Life Technologies, Gaithersburg, MD, USA) at a 1:400 dilution in 2%
BSA for 1 h at 37°C in dark. Then cells were stained with 10 μg/ml
4′,6′-diamidino-2-phenylindole (DAPI, Biyuntian, Inc., Nantong,
Jiangsu, China) for 10 min to identify cellular nuclei. The images
were captured using a confocal fluorescence microscope (Olympus,
Tokyo, Japan).
Statistical analysis
Experimental results were expressed as mean ±
standard deviation (mean ± SD). Statistical analyses were performed
using one-way ANOVA techniques in Microsoft Excel 2003 using SPSS
software. This was followed by a Student Newman-Keuls’ post-test.
Differences between treatment groups were considered significant at
P<0.05, and highly at P<0.01.
Results
ITF promotes GES-1 cell proliferation and
migration
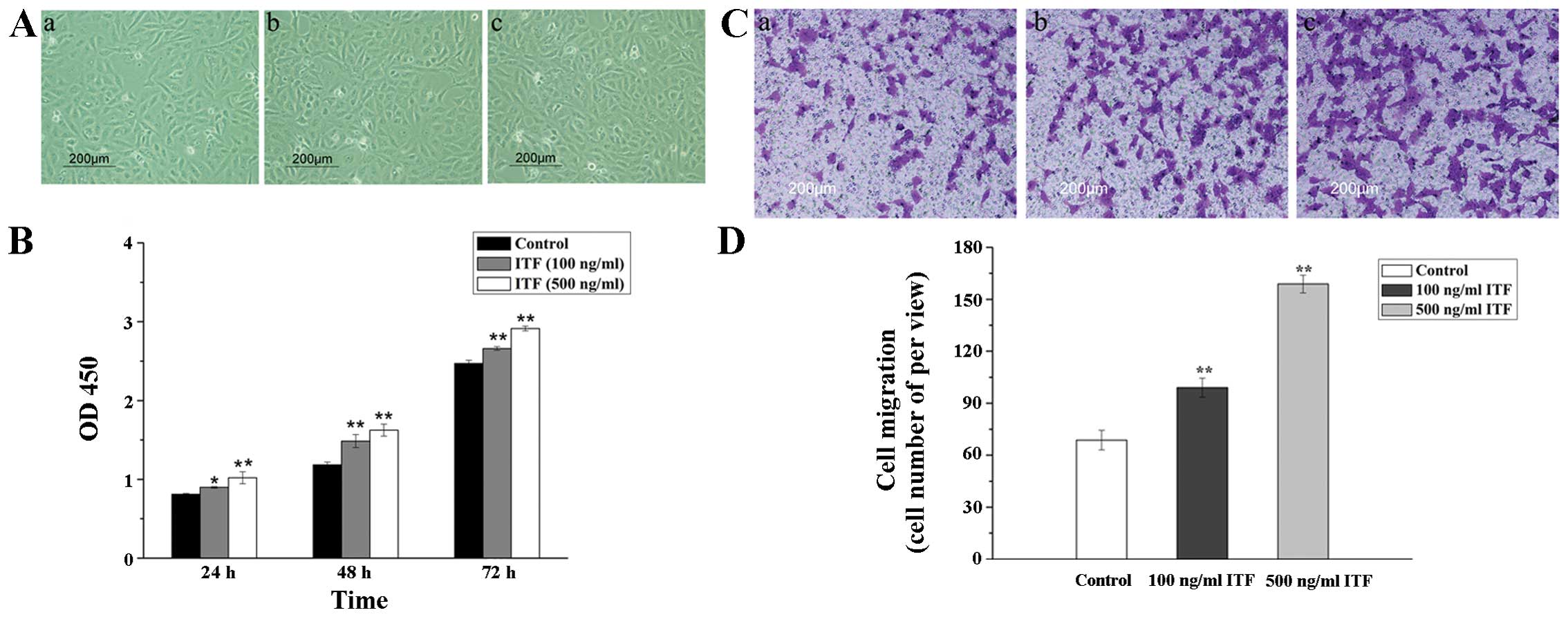
In this study, we first used 100 and 500 ng/ml ITF
to treat the cultured GES-1 cells in vitro. The
microphotographs of GES-1 cells after treatment with ITF are
presented in Fig. 1A. We found
that different concentration of ITF promoted the proliferation of
GES-1 cells after treatment for 48 h. Cell viability of GES-1 cells
treated with 100 and 500 ng/ml ITF for 24, 48 and 72 h was then
detected by CCK-8 assay (Fig. 1B).
The results of CCK-8 indicated that cell viability was
significantly increased in GES-1 cells treated with ITF for 48 or
72 h (P<0.01) compared with control. Transwell migration assay
was used to investigate the effect of ITF on the migration of GES-1
cells after treatment with different concentrations of ITF. As
displayed in Fig. 1C and D, ITF
improved the migration of treated GES-1 cells compared with control
(P<0.01). Furthermore, 500 and 100 ng/ml ITF promoted the cell
migration by 2.3-fold (P<0.01) and 1.6-fold (P<0.01) as
compared to the control. These results indicated that ITF could
promote the proliferation and migration of GES-1 cells after
treatment with ITF in vitro.
ITF activates the Akt signaling
pathway
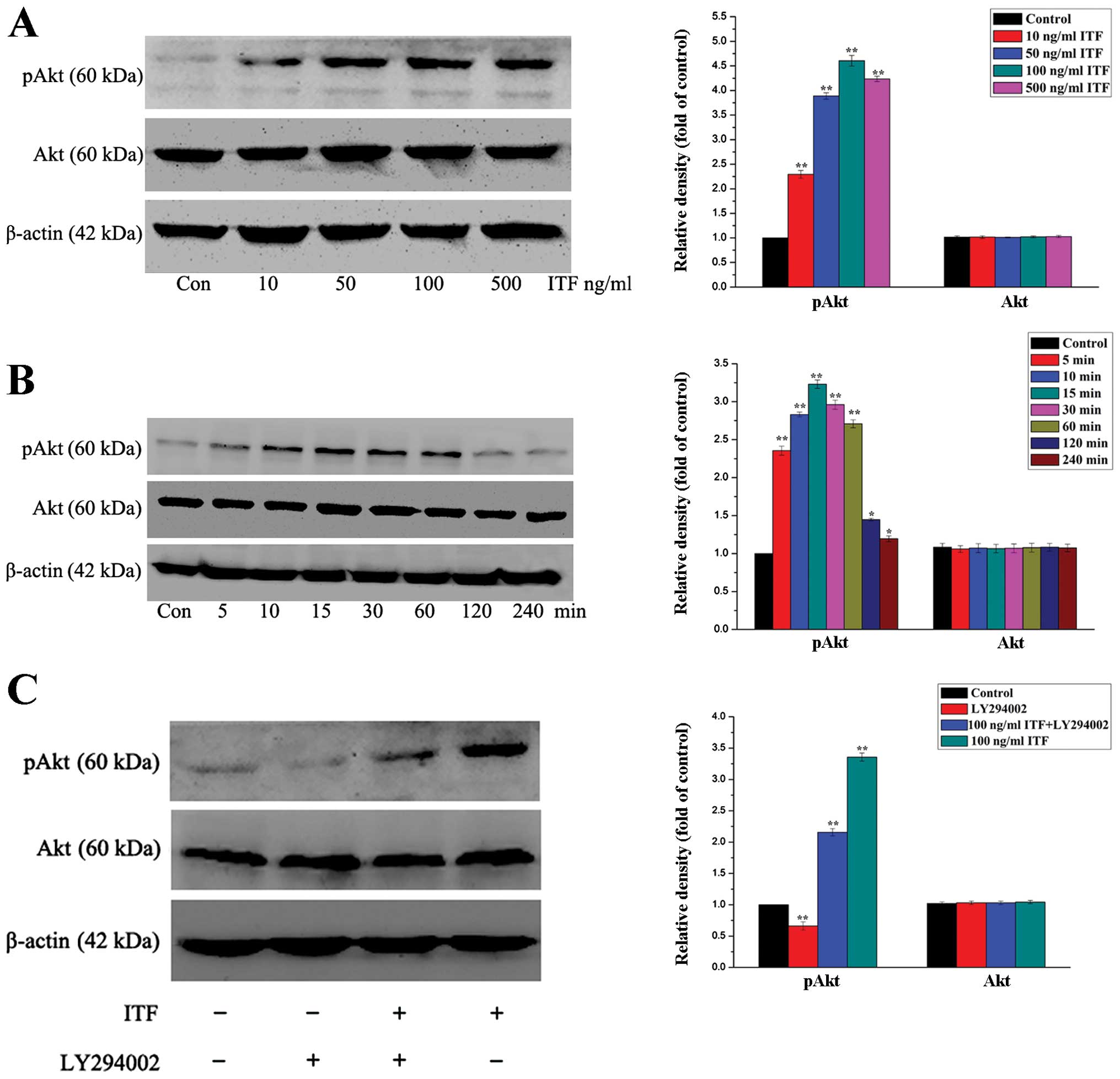
To investigate the underlying mechanisms of ITF in
the processes of cell proliferation and migration, we next
investigated the functional role of Akt signaling pathway in these
regulation processes of ITF. Akt as a vital regulatory factor
responsible for maintaining cell viability and cell survival, we
first measured the pAkt expression level in GES-1 cells in response
to increasing doses of ITF. Western blot illustrated that treatment
of GES-1 cells with ITF resulted in an increase in protein
expression of pAkt (Fig. 2A).
Increasing concentrations of ITF proportionally induced the
expression level of pAkt with maximum stimulation at 4.5-fold
(P<0.01) as compared to the control at a concentration of 100
ng/ml (Fig. 2A). Next, we detected
and evaluated the time course of pAkt induction by ITF. As
illustrated in Fig. 2B, using a
concentration of 100 ng/ml ITF, we found that ITF induced a peak in
pAkt expression level within 30 min of exposure. The protein
expression level of pAkt in treated GES-1 cells reached a peak at
15 min and increased 3.3-fold (P<0.01) compared to control. The
pAkt expression level in treated GES-1 cells could be maintained
for 60 min, and we did not find additional modulation in Akt kinase
function following evaluation for extended time-points (Fig. 2B). The results of western blotting
in Fig. 2A and B show that ITF was
able to induce a dose- and time-dependent increase in Akt kinase
activity.
We expected that ITF-mediated PI3K activity would
result in the activation of Akt kinase activity. To confirm this,
we evaluated the ability of ITF to induce pAkt expression in the
presence of the PI3K inhibitor LY-249002. As shown in Fig. 2C, inhibition of PI3K by LY-294002
resulted in decreased induction of pAkt expression level following
treatment with ITF in GES-1 cells compared with control. All the
western blot results indicated that ITF activated the Akt signaling
pathway to regulate the physiological process of GES-1 cells. In
the following experiments, we used LY294002 to regulate the Akt
signaling pathway to evaluate the cell proliferation and migration
of GES-1 cells.
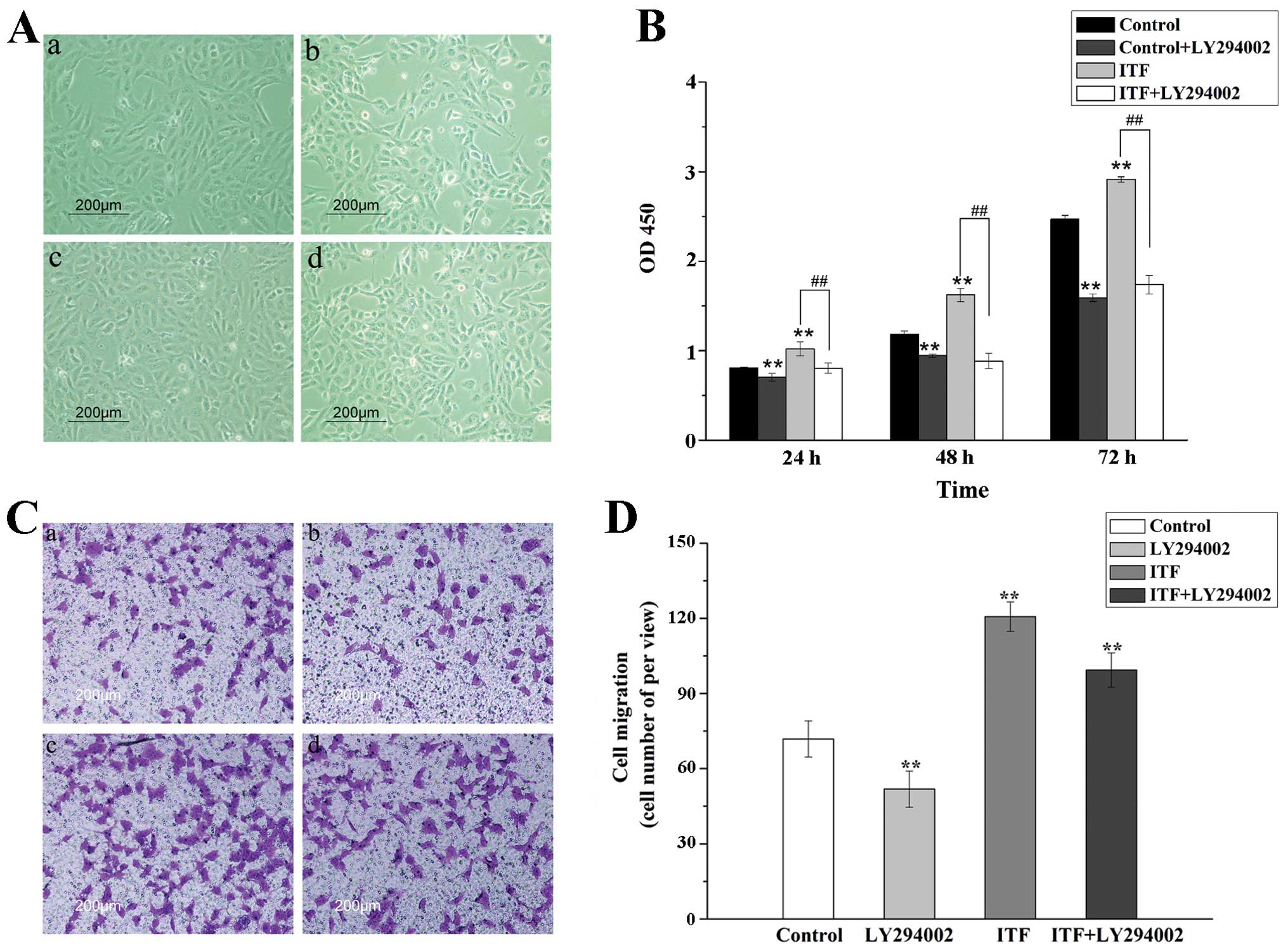
Activated Akt signaling is required for
ITF-induced GES-1 cell proliferation and migration
ITF have been reported to promote cell proliferation
and migration of epithelial cells in some studies (7,17,18),
and our experiments indicated that ITF promote proliferation and
migration of GES-1 cell in vitro. To verify whether Akt
signaling pathway participated in this stimulatory effect, CCK-8
assays and transwell migration assays were performed. As shown in
Fig. 3A and B, the results
illustrated that ITF promote the proliferation of GES-1 cells,
which is decreased significantly compared with ITF group
(P<0.01) by LY294002 inhibiting Akt signaling pathway. LY24002
inhibited Akt signaling to reduce the cell viability induced by
ITF, which suggested that Akt signaling might be an essential
downstream mediator in ITF-regulated cell proliferation.
The results of transwell migration assays showed
that ITF (100 ng/ml) promoted the cell migration by 1.7-fold
(P<0.01) as compared to the control. In contrast, the motility
was inhibited by LY29002 significantly (P<0.01) as compared to
ITF group (Fig. 3C and D). This
result illustrated that the cell migration induced by ITF was
dramatically attenuated because of Akt signaling blockade.
Therefore, the results of cell proliferation and migration
demonstrated that ITF regulated the cell viability of GES-1 cells
via activating the Akt signaling pathway.
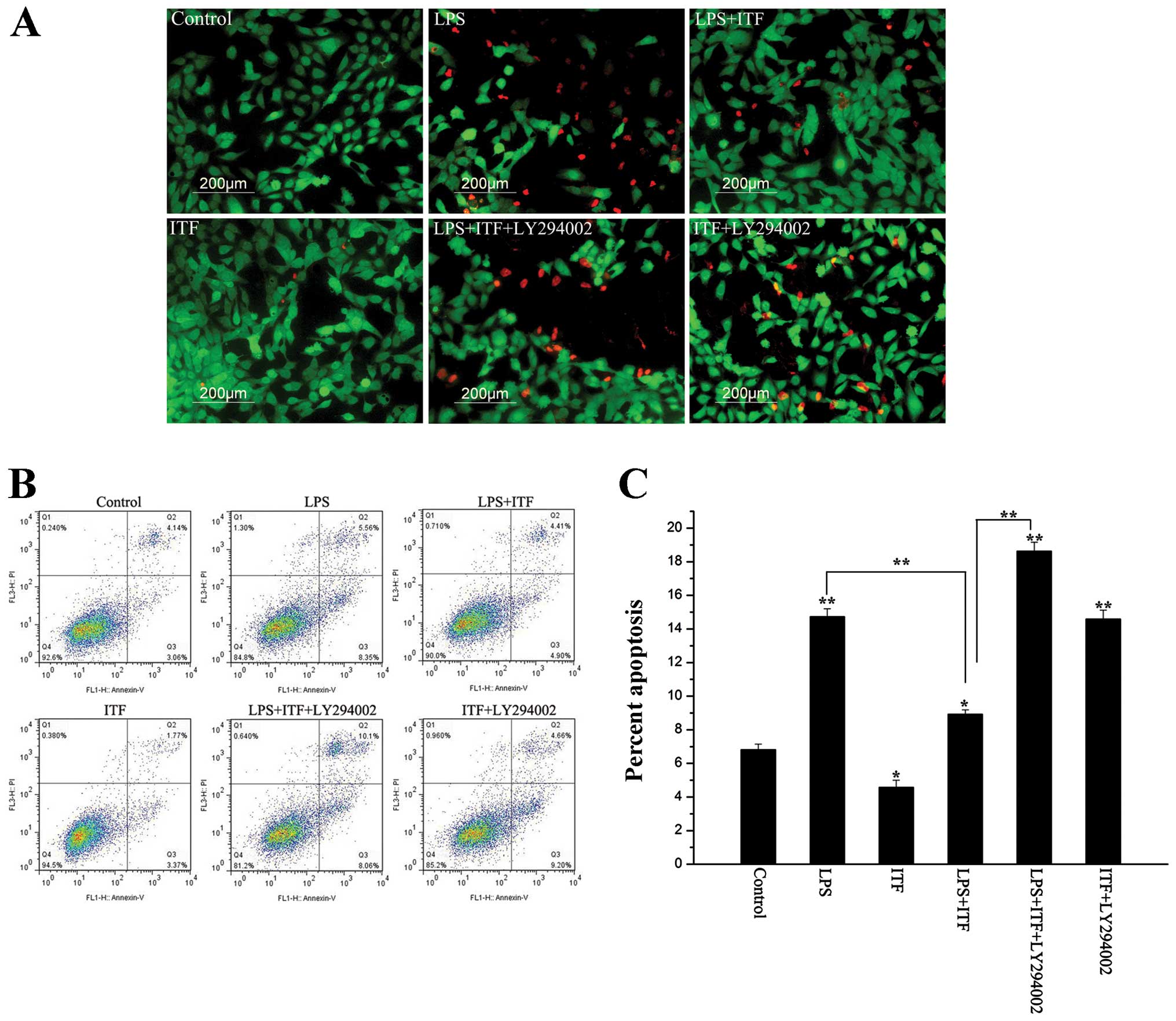
ITF activates the Akt signaling pathway
to protect GES-1 cells from LPS induced epithelium injury
In order to investigate the protective effects of
ITF on the cell survival and epithelium integrity in GES-1 cells,
10 μg/ml LPS was used to induce epithelium injury of GES-1 cells.
GES-1 cells exposed to 10 μg/ml LPS for 24 h were stained with FDA
(green) and PI (red). As illustrated in Fig. 4A, necrotic cells were obviously
increased after LPS treated compared with control. In contrast, ITF
decreased the number of necrotic cells in LPS+ITF group, however,
LY294002 inhibited the protection of ITF and increased the number
of necrotic cells in LPS+ITF+ly294002 group. Apoptosis was detected
in cultured GES-1 cells after 48-h exposure to LPS (Fig. 4B and C). As shown in Fig. 4B and C, apoptosis of GES-1 cells
was significantly increased (P<0.01) after LPS treatment.
Treatment with ITF after the addition of LPS significantly
decreased (P<0.01) the incidence of apoptosis by approximately
one-half (8.9±1.2%). Addition of the PI3K inhibitor LY-249002 after
LPS treatment increased the number of apoptotic cells to 18% of the
total population, even in the presence of ITF (18.6±2.2%). On the
basis of these results, we demonstrated that the PI3K/Akt signaling
pathway contributes to ITF protection against LPS-induced necrosis
and apoptosis.
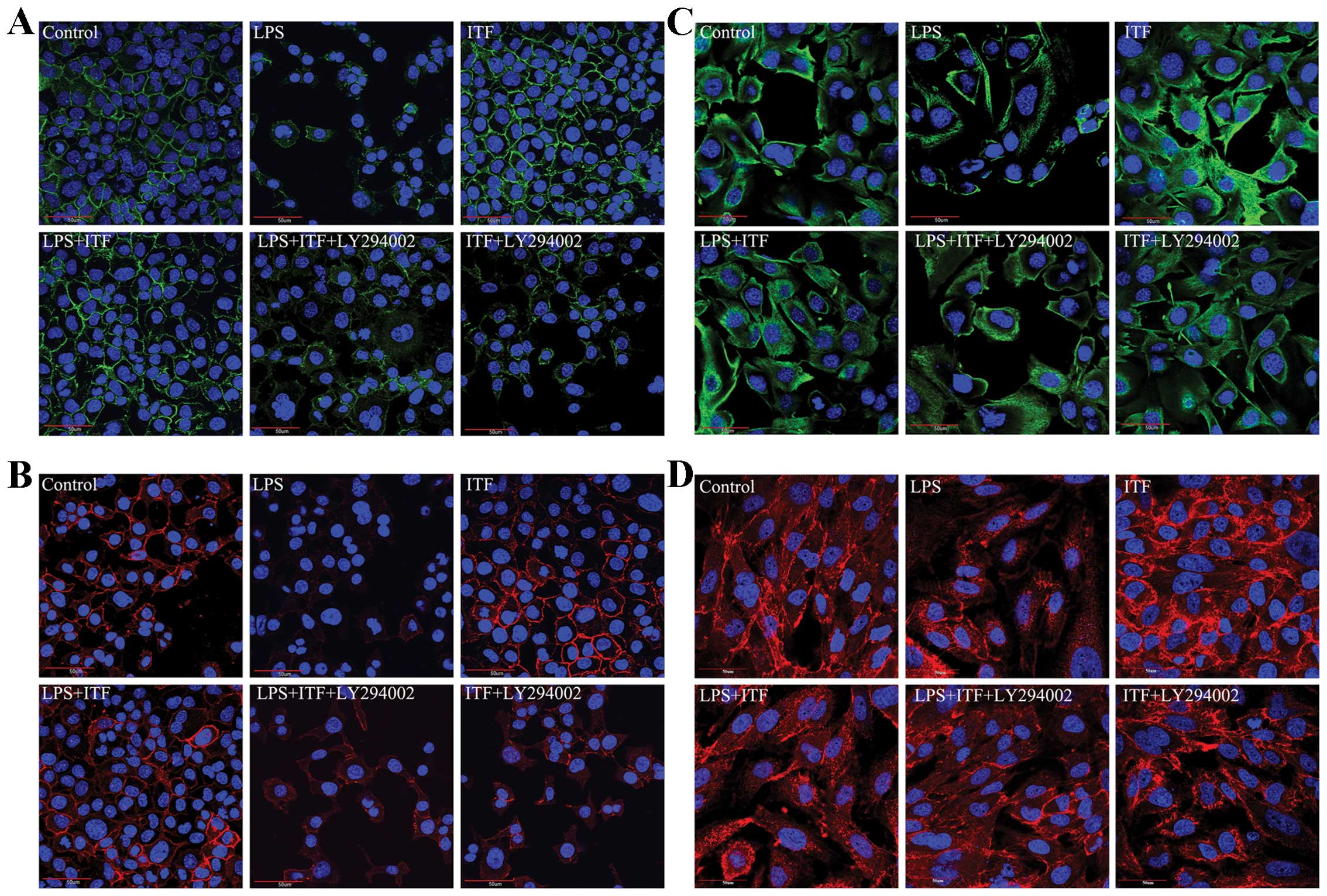
ITF is a potent protection factor specific to the
epithelium that promotes mucosal epithelial cell survival,
accelerates wound closure, and maintains epithelium integrity. In
the following experiments, we detected the epithelial tight
junction (occludin and ZO-1) and specific epithelium markers (CK-18
and CK-19) in GES-1 cells after treatment with LPS. The
immunofluorescence results (as shown in Fig. 5) indicated that LPS induced mucosal
epithelium injury and tight junction damage, which led the
expression of occludin (Fig. 5A)
and ZO-1 (Fig. 5B) decreased. The
expression level of specific epithelium markers CK-18 (Fig. 5C) and CK-19 (Fig. 5D) was also decreased in GES-1
cells. On the contrary, ITF prevented the epithelium injury from
LPS and preserved epithelial integrity of GES-1 cells. However,
addition of the PI3K/Akt signaling inhibitor LY294002 resulted in
the loss of protective effects of ITF on epithelium integrity.
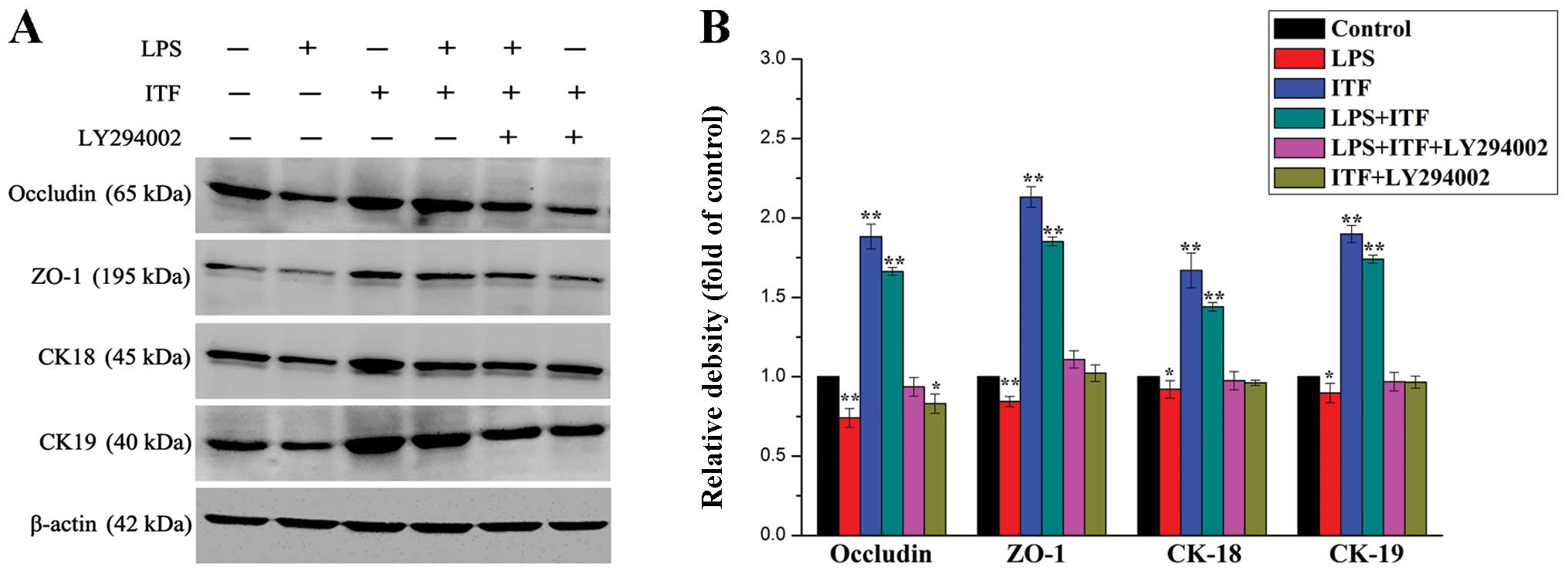
Western blot analysis was performed for quantitative
analysis of tight junction and expression of specific epithelium
markers in treated GES-1 cells for 48 h. The results showed a
similar result as immunofluorescence data supporting the protection
of ITF on preserving epithelium integrity in GES-1 cells (Fig. 6) via activating the Akt signaling
pathway. As illustrated in Fig. 6,
the western blot results indicated that LPS decreased the
expression of occludin, ZO-1, CK-18 and CK-19 in LPS group
significantly (P<0.01), which is reversed via ITF treatment in
LPS+ITF group. However, LY294002 decreased the expression levels of
these proteins in LPS+ITF+LY294002 group via inhibiting the Akt
signaling even in the presence of ITF. Therefore,
immunofluorescence staining and western blot data confirmed that
ITF maintained mucosal epithelium integrity against LPS-induced
injury via activating the Akt signaling. It indicated that Akt
signaling pathway plays a critical role in the physiological and
pathological process of gastric mucosal epithelium regulated by
ITF.
Discussion
The trefoil factor family (TFF) peptides are small
regulatory proteins consisting of three members. ITF (TFF3) is a
major component in the goblet cells in small and large intestines
(19), especially a typical
secretary peptide of the normal human antral and pyloric gastric
mucosa. Recent studies have demonstrated that ITF was able to
attenuate the gastrointestinal mucosal injury caused by various
injury factors and promotes the repair of damaged mucosa
epithelium. ITF plays an essential role in inducing mucosal
epithelial healing (7,20), cell migration (21) and maintaining normal
gastrointestinal mucosal epithelium integrity (22,23).
However, we know little as yet about the detailed mechanism
underlying the regulation of ITF on the physiological and
pathological process of gastric mucosal epithelium.
Rapid proliferation and migration of mucosal
epithelial cells is the key mechanism for resurfacing epithelial
defects after various forms of mucosal epithelium injury. In the
present study, we explored the effects of ITF on the proliferation
and migration of GES-1 cells. Firstly, we used ITF to treat the
cultured GES-1 cells to detect the cell viability and migration
in vitro. After GES-1 cells were treated with different
concentration of TTF, we found that different concentration of ITF
is sufficient to promote the proliferation and migration of GES-1
cells. The higher the concentration of ITF, the more cells
proliferated and migrated. These results of ITF effects on the cell
proliferation and migration were similar with previous studies
(24–26). In our present study, we
additionally found that ITF could preserve mucosal integrity
resistance to LPS-induced injury.
Although some studies have demonstrated that TFF
family including ITF, regulate cell migration and cell survival via
TGF-β signaling pathway, MAPK/ERK signaling pathway, β-catenin
signaling pathway or EGF signaling pathway (8,17,27–31)
in some cell types, the defined underlying mechanism of protective
effects of ITF on the physiological and pathological process of
GES-1 cells needs to be clarified.
Many studies have indicated that ITF regulates
multiple downstream pathways in remodeling of gastrointestinal
mucosal tissues. The effects of ITF are transmitted to signaling
cascades by still unknown adaptor proteins. Despite the absence of
an identified cell surface receptor for ITF, ITF can act through
the EGFR to activate several downstream effector pathways,
including ERK1/2, Jun N-terminal kinase, and PI3K signal (15,32).
In the present study, the results of western blotting (Fig. 2) suggested that the expression of
pAk which is the essential components of PI3K/Akt signaling was
upregulated in GES-1 cells treated with ITF. In addition, these
data indicated that ITF induces a dose- and time-dependent increase
in Akt kinase activity of treated GES-1 cells. The downstream
regulatory signaling pathway of ITF, the PI3K/Akt signaling pathway
as an intracellular signal transducer plays a vital role in
regulating cell survival, proliferation, migration and apoptosis
(12,13,32–36).
In this study, the results indicated that PI3K/Akt signaling
pathway participate in the regulation of ITF in GES-1 cell
proliferation and migration in vitro. Using LY294002, the
inhibitor of the PI3K/Akt signaling pathway, we found that the cell
proliferation and migration induced by ITF was attenuated
significantly. LY294002 inhibited the PI3K/Akt signaling to reduce
GES-1 cell proliferation and migration, which suggested that Akt
signaling as an essential signal regulated the effects of ITF on
the proliferation and migration processes in vitro.
The gastrointestinal mucosal epithelium is a
fundamental barrier providing protection against the outside stress
environment. Interestingly, several in vitro and in
vivo studies have demonstrated that trefoil peptides can also
protect the intestinal epithelium from a variety of noxious agents,
including bacterial toxins, chemicals, and drugs (10,37–40).
It is unknown whether ITF could act to exert cytoprotective effects
on the gastric epithelium integrity. Based on the above results, we
used LPS to act as a damage factor to induce the GES-1 cell injury.
After LPS treatment, we found that LPS induced necrosis and
apoptosis in GES-1 cells. However, the addition of ITF as a
protective factor protected the cells from necrosis and apoptosis.
In contrast, LY294002 inhibited the protective effects via
inhibiting the PI3K/Akt signaling pathway. Our results obtained
from GES-1 cells indicated that ITF provided protection but only
when PI3K/Akt signaling is active. In the presence of PI3K
inhibition, ITF lost the ability to protect cells from necrosis and
apoptosis. The results indicated that Akt signaling provides an
essential function in the GES-1 cells as a central survival pathway
during the ITF protection process. We also detected the epithelium
tight junction (occludin and ZO-1) and epithelium marker (CK-18 and
CK-19) expression in treated GES-1 cells using immunofluorescence
and western blot analysis. Our results demonstrated that LPS led to
epithelium tight junction damage and reduced the epithelium marker
expression. Using ITF treatment, we found that ITF prevents ongoing
damage induced by LPS. However, LY294002 inhibited the protection
of ITF in GES-1 cells. Our results suggested that ITF is a potent
survival factor for the gastric mucosal epithelium integrity and
provides protection against many external stimuli. On the basis of
our above investigation, we believe that protection afforded by ITF
is at least in part related to activation of the PI3K/Akt signaling
pathway and possibly others. Activation of this survival pathway
helps to protect gastric mucosal epithelium barrier function during
inflammatory stress and facilitates wound repair when the barrier
is compromised.
The results in this study suggested that Akt
signaling is a critical downstream effector of ITF in the
protection of gastric mucosal epithelium. It is supported by the
following observations: i) Akt kinase activity was upregulated by
ITF in the GES-1 cell line. ii) ITF-induced cell proliferation and
migration was abolished when Akt signaling was inhibited. iii) ITF
maintained mucosal epithelium integrity via activating the Akt
signaling.
In conclusion, our results revealed that ITF
regulated GES-1 cell proliferation, migration and maintained
epithelium integrity via triggering the PI3K/Akt response. As a
downstream mediator of ITF, activated Akt signaling pathway
contributed to the protection process of ITF. The present study
supports the notion that ITF can promote cell proliferation,
migration and preserve epithelium integrity. In addition, it
suggests that protection of ITF in gastric mucosal epithelium is
mediated in part through activation of the PI3K/Akt signaling
pathway. Therefore, the PI3K/Akt axis may serve as a therapeutic
target to preserve mucosal epithelial integrity and provide a
useful strategy to block damage thereby limiting gastric disease
such as gastric ulcer in humans.
Acknowledgements
This study was supported by a grant from the Major
Projects Foundation of Nanjing Military Region (12Z32), the Medical
Science Foundation for young cultivation project of PLA (13QNP038)
and the Natural Science Foundation of Jinling Hospital (2013023 and
2014004).
References
|
1
|
Podolsky DK: Mucosal immunity and
inflammation. V Innate mechanisms of mucosal defense and repair:
the best offense is a good defense. Am J Physiol. 277:G495–G499.
1999.PubMed/NCBI
|
|
2
|
Wright NA: Aspects of the biology of
regeneration and repair in the human gastrointestinal tract. Philos
Trans R Soc Lond B Biol Sci. 353:925–933. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Podolsky DK: Healing the epithelium:
solving the problem from two sides. J Gastroenterol. 32:122–126.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Santos MF, McCormack SA, Guo Z, et al: Rho
proteins play a critical role in cell migration during the early
phase of mucosal restitution. J Clin Invest. 100:216–225. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dieckgraefe BK, Santoro SA and Alpers DH:
Immunolocalization of alpha-integrin subunits and extracellular
matrix components during human colonic organogenesis.
Gastroenterology. 110:58–71. 1996. View Article : Google Scholar
|
|
6
|
Wright NA, Hoffmann W, Otto WR, Rio MC and
Thim L: Rolling in the clover: trefoil factor family (TFF)-domain
peptides, cell migration and cancer. FEBS Lett. 408:121–123. 1997.
View Article : Google Scholar
|
|
7
|
Taupin D and Podolsky DK: Trefoil factors:
initiators of mucosal healing. Nat Rev Mol Cell Biol. 4:721–732.
2003. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dignass A, Lynch-Devaney K, Kindon H, Thim
L and Podolsky DK: Trefoil peptides promote epithelial migration
through a transforming growth factor beta-independent pathway. J
Clin Invest. 94:376–383. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Potten CS, Merritt A, Hickman J, Hall P
and Faranda A: Characterization of radiation-induced apoptosis in
the small intestine and its biological implications. Int J Radiat
Biol. 65:71–78. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Babyatsky MW, deBeaumont M, Thim L and
Podolsky DK: Oral trefoil peptides protect against ethanol- and
indomethacin-induced gastric injury in rats. Gastroenterology.
110:489–497. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sun Y, Zhu Y, Wang L, Mao X, Peng X and
Peng Y: Recombinant adenovirus-mediated intestinal trefoil factor
gene therapy for burn-induced intestinal mucosal injury. PLoS One.
8:e624292013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cantley LC: The phosphoinositide 3-kinase
pathway. Science. 296:1655–1657. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li Q and Zhu GD: Targeting
serine/threonine protein kinase B/Akt and cell-cycle checkpoint
kinases for treating cancer. Curr Top Med Chem. 2:939–971. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bao S, Wang Y, Sweeney P, et al:
Keratinocyte growth factor induces Akt kinase activity and inhibits
Fas-mediated apoptosis in A549 lung epithelial cells. Am J Physiol
Lung Cell Mol Physiol. 288:L36–L42. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Baus-Loncar M and Giraud AS: Multiple
regulatory pathways for trefoil factor (TFF) genes. Cell Mol Life
Sci. 62:2921–2931. 2005.PubMed/NCBI
|
|
16
|
Sun Z, Wang Y, Gong X, Su H and Han X:
Secretion of rat tracheal epithelial cells induces mesenchymal stem
cells to differentiate into epithelial cells. Cell Biol Int.
36:169–175. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Storesund T, Hayashi K, Kolltveit KM,
Bryne M and Schenck K: Salivary trefoil factor 3 enhances migration
of oral keratinocytes. Eur J Oral Sci. 116:135–140. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yee DS, Tang Y, Li X, et al: The Wnt
inhibitory factor 1 restoration in prostate cancer cells was
associated with reduced tumor growth, decreased capacity of cell
migration and invasion and a reversal of epithelial to mesenchymal
transition. Mol Cancer. 9:1622010. View Article : Google Scholar
|
|
19
|
Madsen J, Nielsen O, Tornoe I, Thim L and
Holmskov U: Tissue localization of human trefoil factors 1, 2, and
3. J Histochem Cytochem. 55:505–513. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kouznetsova I, Peitz U, Vieth M, et al: A
gradient of TFF3 (trefoil factor family 3) peptide synthesis within
the normal human gastric mucosa. Cell Tissue Res. 316:155–165.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Durer U, Hartig R, Bang S, Thim L and
Hoffmann W: TFF3 and EGF induce different migration patterns of
intestinal epithelial cells in vitro and trigger increased
internalization of E-cadherin. Cell Physiol Biochem. 20:329–346.
2007. View Article : Google Scholar
|
|
22
|
Podolsky DK: Mechanisms of regulatory
peptide action in the gastrointestinal tract: trefoil peptides. J
Gastroenterol. 35(Suppl 12): 69–74. 2000.PubMed/NCBI
|
|
23
|
Lin N, Xu LF and Sun M: The protective
effect of trefoil factor 3 on the intestinal tight junction barrier
is mediated by toll-like receptor 2 via a PI3K/Akt dependent
mechanism. Biochem Biophys Res Commun. 440:143–149. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zheng Q, Gao J, Li H, et al: Trefoil
factor 3 peptide regulates migration via a Twist-dependent pathway
in gastric cell. Biochem Biophys Res Commun. 438:6–12. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Qu Y, Yang Y, Ma D and Xiao W: Increased
trefoil factor 3 levels in the serum of patients with three major
histological subtypes of lung cancer. Oncol Rep. 27:1277–1283.
2012.PubMed/NCBI
|
|
26
|
Hernandez C, Santamatilde E, McCreath KJ,
et al: Induction of trefoil factor (TFF)1, TFF2 and TFF3 by hypoxia
is mediated by hypoxia inducible factor-1: implications for gastric
mucosal healing. Br J Pharmacol. 156:262–272. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu D, el-Hariry I, Karayiannakis AJ, et
al: Phosphorylation of beta-catenin and epidermal growth factor
receptor by intestinal trefoil factor. Lab Invest. 77:557–563.
1997.PubMed/NCBI
|
|
28
|
Gibson S, Tu S, Oyer R, Anderson SM and
Johnson GL: Epidermal growth factor protects epithelial cells
against Fas-induced apoptosis. Requirement for Akt activation. J
Biol Chem. 274:17612–17618. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Moro L, Venturino M, Bozzo C, et al:
Integrins induce activation of EGF receptor: role in MAP kinase
induction and adhesion-dependent cell survival. EMBO J.
17:6622–6632. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Walker F, Kato A, Gonez LJ, et al:
Activation of the Ras/mitogen-activated protein kinase pathway by
kinase-defective epidermal growth factor receptors results in cell
survival but not proliferation. Mol Cell Biol. 18:7192–7204.
1998.
|
|
31
|
Graness A, Chwieralski CE, Reinhold D,
Thim L and Hoffmann W: Protein kinase C and ERK activation are
required for TFF-peptide-stimulated bronchial epithelial cell
migration and tumor necrosis factor-alpha-induced interleukin-6
(IL-6) and IL-8 secretion. J Biol Chem. 277:18440–18446. 2002.
View Article : Google Scholar
|
|
32
|
Shi HS, Zhu WL, Liu JF, et al: PI3K/Akt
signaling pathway in the basolateral amygdala mediates the rapid
antidepressant-like effects of trefoil factor 3.
Neuropsychopharmacology. 37:2671–2683. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Astle MV, Ooms LM, Cole AR, et al:
Identification of a proline-rich inositol polyphosphate
5-phosphatase (PIPP)*collapsin response mediator protein
2 (CRMP2) complex that regulates neurite elongation. J Biol Chem.
286:23407–23418. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chan CB, Liu X, Pradoldej S, et al:
Phosphoinositide 3-kinase enhancer regulates neuronal
dendritogenesis and survival in neocortex. J Neurosci.
31:8083–8092. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nedachi T, Kawai T, Matsuwaki T,
Yamanouchi K and Nishihara M: Progranulin enhances neural
progenitor cell proliferation through glycogen synthase kinase
3beta phosphorylation. Neuroscience. 185:106–115. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Pugazhenthi S, Nesterova A, Sable C, et
al: Akt/protein kinase B up-regulates Bcl-2 expression through
cAMP-response element-binding protein. J Biol Chem.
275:10761–10766. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mashimo H, Wu DC, Podolsky DK and Fishman
MC: Impaired defense of intestinal mucosa in mice lacking
intestinal trefoil factor. Science. 274:262–265. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kindon H, Pothoulakis C, Thim L,
Lynch-Devaney K and Podolsky DK: Trefoil peptide protection of
intestinal epithelial barrier function: cooperative interaction
with mucin glycoprotein. Gastroenterology. 109:516–523. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Taupin DR, Kinoshita K and Podolsky DK:
Intestinal trefoil factor confers colonic epithelial resistance to
apoptosis. Proc Natl Acad Sci USA. 97:799–804. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Playford RJ, Marchbank T, Goodlad RA,
Chinery RA, Poulsom R and Hanby AM: Transgenic mice that
overexpress the human trefoil peptide pS2 have an increased
resistance to intestinal damage. Proc Natl Acad Sci USA.
93:2137–2142. 1996. View Article : Google Scholar : PubMed/NCBI
|