Introduction
Capillary morphogenesis gene 2 (CMG2) also
known as anthrax toxin receptor 2 (ANTXR2) has been
identified as a gene upregulated in endothelial cells during tubule
formation (1). CMG2 and tumour
endothelial marker-8 (TEM-8) are receptors of anthrax toxin
mediating the internalisation of the toxin (2,3).
CMG2 and TEM-8 are type I transmembrane proteins possessing an
extracellular integrin-like I domain and are members of the von
Willebrand factor A (vWA) domain-containing protein family
(4,5). The proteins share 40% overall amino
acid identity, with 60% identity within their I domains, including
a conserved metal ion dependent adhesion site (MIDAS) motif. The
vWA/I domain with the MIDAS region, existing in different isoforms,
allows the binding to protective antigen (PA) subunit of anthrax
toxin which mediates the internalisation of the toxin. In addition
to the binding with PA, the extracellular domain also interacts
with collagen IV, laminin and fibronectin (1). The CMG2 gene is located on chromosome
4q, and encodes a 489-amino acid (aa) protein. The full-length
protein has a putative signal peptide, extracellular, transmembrane
and cytoplasmic domains (2). Apart
from CMG2489, there are another three different natural
variants encoded by alternatively spliced mRNA transcripts.
CMG2488 has 12 different amino acids at the cytoplasmic
tail of the protein compared with CMG2489.
CMG2386 lacks amino acids 213–233 of the full length
protein. CMG2322 has been predicted to be a secreted
isoform due to the lack of the transmembrane domain (2). CMG2 is more widely expressed in
normal tissues except for brain and thymus (2), compared with TEM8 which is more
selectively overexpressed during tumour angiogenesis. The finding
of TEM8 as a specific tumour endothelial cell marker has led
researchers, including ourselves, to investigate their role in the
angiogenesis of malignancies. Since 2001, the host laboratory has
started an investigation of its role in tumour-related angiogenesis
and the interaction with other tumour-related cytokines and growth
factors. The relationships of TEM-8 expression with clinical
outcomes and the corresponding prognostic value have been evaluated
in colorectal cancer and breast cancer (6–9).
Elevated TEM8 expression in human colon cancer is associated with
lymphatic metastasis and disease progression (7). Although TEM-8 might not be a marker
specifically expressed in tumour-related endothelia, as it had been
previously claimed to be, it is still a useful marker for
identifying tumour associated micro-vessels and its elevated levels
are associated with disease progression of breast cancer (9).
Mutations of CMG2 gene have been identified in
juvenile hyaline fibromatosis (JHF) and infantile systemic
hyalinosis (ISH) which are autosomal recessive syndromes
characterized by multiple, recurring subcutaneous tumours, gingival
hypertrophy, joint contractures, osteolysis and osteoporosis
(10–14). The mutations result in low CMG2
mRNA and protein production due to the different cytosolic tails of
protein products which can direct the proteins to endoplasmic
reticulum (ER) associated degradation pathway (15). On the other hand, the mutation or
variants of CMG2 may also affect sensitivity to anthrax toxin and
lead to a reduced susceptibility to infection of Bacillus
anthracis (16). Mutations
like single nucleotide polymorphisms (SNPs) of CMG2 have also been
associated with ankylosing spondylitis (AS) risk in Caucasians
(17), but this was not evident in
a cohort of 309 AS patients in Chinese Han population (18,19).
CMG2 knockout female mice were unable to produce any offspring due
to a defect in parturition. This defect was caused by a diffuse
deposition of collagen within the myometrium. The deletion of CMG2
did not affect normal mouse embryonic development (20). A recent study showed that CMG2 and
TEM-8 were able to regulate the extracellular matrix via a
regulation of MT1-MMP and MMP2 (21). This evidence suggests that CMG2
functions as a collagen receptor to maintain collagen
homeostasis.
CMG2 has been shown to be able to regulate the
proliferation and tubule formation of endothelial cells, but not
the migration. This may have certain implication in tumour-related
angiogenesis (22). In the present
study we examined the impact of CMG2 on the angiogenic capacity of
vascular endothelial cells and the possibility of targeting CMG2
vWA domain to interfere with tumour-related angiogenesis.
Materials and methods
Materials and cell lines
HECV cells purchased from Interlab (Milan, Italy)
were maintained in Dulbecco’s modified Eagle’s medium (DMEM)
(Sigma-Aldrich, Poole, Dorset, UK) supplemented with
benzylpenicillin, amphotericin B, streptomysin and 10% foetal
bovine serum (Sigma-Aldrich). The cells were incubated at 37°C, 5%
CO2 and 95% humidity. Matrigel was purchased from
Collaborative Research Products (Bedford, MA, USA). Cell lines and
human tissue cDNA libraries were prepared and stored in the host
laboratory. Polyclonal goat anti-human-CMG2 was obtained from
R&D Systems (Minneapolis, MN, USA). Small polypeptides (as
shown in Table I) were customised
products synthesized by GeneCust Europe-Labbx (Luxembourg).
 | Table IAmino acid sequence of the small
peptides. |
Table I
Amino acid sequence of the small
peptides.
| Name | Sequence |
|---|
| LG20 |
LDGLVPSYAEKEAKISRSLG |
| LG64 |
LDGLVPSYAEKEAKISRSLGASVYCVGVLDFEQAQLERIADSKEQVFPVKGFQALKGINSIL |
| M1 |
GLVPSYAEKEAKISRSLG |
| M2 |
LVPSYAEKEAKISRSLG |
| M3 |
LDGLVPSYAEKEAKISRS |
| M4 |
LDGLVPSYAEKEAKIS |
| M5 |
LVPSYAEKEAKISLG |
| M7 |
VPSYAEKEAKISRSLG |
| M8 |
PSYAEKEAKISRSLG |
| M9 | VPSYAEKEAKISR |
| M10 | SYAEKEAKISRSLG |
RNA extraction and RT-PCR
RNA was extracted using total RNA isolation (TRI)
reagent and following the protocol provided (Sigma-Aldrich). RNA
was subsequently quantified using a spectrophotometer (WPA UV 1101,
Biotech Photometer, Cambridge, UK), at 500 ng of total RNA before
being converted to cDNA using an iScript cDNA synthesis kit
(Bio-Rad Laboratories, Hemel Hempstead, UK). The quality of cDNA
was verified using GAPDH primers (sense 5′-CAGGAGGTT GAAGGACTAAA
and antisense 5′-GGGATCAGTTTTCTT TGTCA). Conventional PCR was
performed with specific primers for CMG2 (sense
5′-CAAAATCAGTAAAGGCT TGG, and antisense 5′-CAAAGGTTCTTCTTCCTCCT).
The conditions for the amplification were: 94°C for 5 min, followed
by 35 cycles of 94°C for 30 sec, 55°C for 30 sec and 72°C for 1
min, and the final extension for 7 min at 72°C. The products were
visualized on a 1.5% agarose gel after staining with ethidium
bromide.
Construction of plasmid vectors carrying
CMG2 sequences coding the full-length protein or vWA domain
fragments
Full length of human CMG2 coding sequence was
amplified from a cDNA library of human ovarian tissues stored at
the host lab using PCR. The PCR products were purified and cloned
into pEF/His TOPO TA plasmid vector (Invitrogen, Inc., Paisley,
UK). Following transformation into E. coli and analysis of
colonies, colonies carrying the correct inserts were amplified for
extraction of the constructed plasmid vectors. The constructed CMG2
expression vectors were verified by sequencing before being used
for the following experiments. The purified PCR products were also
used to amplify different fragments of the CMG2 vWA domain which
were then cloned into the same vector. The primer sequences are
listed in Table II.
 | Table IIPrimer sequences for PCR. |
Table II
Primer sequences for PCR.
| Forward | Reverse |
|---|
| CMG2 full
length |
ATGGTGGCGGAGCGGTCCCCGGCCCG |
AGCAGTTAGCTCTTTCTCAATA |
| CMG2B1 |
ATGGTGGCGGAGCGGTCCCCGGCCCG |
TCTCTGCAGAGCTGCTCT |
| CMG2B2 |
ATGGTGGCGGAGCGGTCCCCGGCCCG |
CTTGCATCTGTCAGAGCATAT |
| CMG2B3 |
ATGGTGGCGGAGCGGTCCCCGGCCCG |
TAGTATAGAATTAATTATTCCTTTAAG |
| CMG2B4 |
GCCTGATCTCTACTCGT |
TAGTATAGAATTAATTATTCCT |
| CMG2B5 |
GCCTGATCTCTACTTCGT |
CTTGCATCTGTCAGAGC |
| CMG2B6 |
GCCTGATCTCTACTCGT |
CCCAGTGACTGATATCTT |
| CMG2B7 |
TTGACGTCTGTGCAT |
TAGTATAGAATTATTATTCC |
Transfection of the constructed vectors
into human vascular endothelial cells
The constructed plasmid vectors carrying either
full-length CMG2 or fragments of the vWA domain were used to
transfect the HECV cells by way of electroporation. The empty
plasmid vectors were transfected into HECV cells and used as a
control for the following study. Following the transfection, the
cells were selected using blasticidin (5 μg/ml). The cells were
then cultured in DMEM with blasticidin at a lower concentration
(0.5 μg/ml) to maintain the expression level.
Growth assay
A standard procedure was used as previously
described (23,24). Cells were plated into a 96-well
plate (2,500 cells/well). Cell growth was assessed after 1, 3 and 5
days. Crystal violet was used to stain cells, and absorbance was
determined at a wavelength of 540 nm using a spectrophotometer
(BioTek, Elx800, UK).
Adhesion assay
This standard procedure was previously described
(25). Cells (40,000) were added
in each well of a 96-well plate, previously coated with the
Matrigel (5 μg/well). After 40 min of incubation, non-adherent
cells were washed off using BSS buffer. The number of adherent
cells was then counted after fixation and staining.
In vitro motility assay using Cytodex-2
beads
We followed a protocol previously described
(26,27). Approximate 1×106 cells
were incubated with 100 μl of Cytodex beads in 10 ml DMEM. After an
overnight incubation, the beads were washed twice in 5 ml DMEM to
remove dead cells, and then resuspended in 800 μl DMEM. A total of
100 μl of beads/cells was transferred into each well of a 24-well
plate in triplicate. After incubation for 4 h, the medium was
aspirated and cells were fixed with 4% formalin for 5 min. They
were then stained with 0.5% crystal violet (0.5% weight/volume in
distilled water) for 5 min. The cells were washed and allowed to
dry before counting.
Electric cell-substrate impedance sensing
(ECIS)
An ECIS instrument of 9600 model (Applied Biophysics
Inc., NJ, USA) was used for migration assay in the study, as
previous reported (28). 96W1E
arrays were used in this study. HECV cells were seeded at 40,000
cells per well in 200 μl DMEM medium. The resistance at 30 kHZ was
recorded for 10 h after an electrical wounding, and data were
analysed using ECIS-9600 software package.
Tubule formation
The processes used were modified from previously
published methods (29,30). Briefly, 96-well plates were coated
with 100 μl/well of Matrigel (diluted in a 1 to 1 ratio with serum
free medium) and incubated for 30 min to allow the gel to set. HECV
(4×104 cells per well) were seeded onto the Matrigel
layer. The cells were treated with the small peptides or medium
alone for 4–6 h to allow tubules to form.
Aorta ring assay
In this assay, angiogenic vessels grow from a
segment of the aorta, which was modified from previously described
methods (31,32). Briefly, mouse thoracic aorta was
dissected, the fat layer and adventitia were removed, and rings
approximately 1 mm in length were prepared. Individual rings were
embedded in Matrigel (2.5 mg/ml), cast inside individual wells of a
96-well plate. Small peptides were directly added into the medium
for culturing the aorta rings. Each group had three replicates and
two independent experiments were performed to assess the effect of
the small peptides. All aorta rings were cultured in DMEM
supplemented with 10% foetal bovine serum except for the negative
control group which used serum-free DMEM. Sprouting was observed by
inspection under a phase contrast microscope over a period of 6
days.
Mouse xenograft tumour model
Female athymic nude mice (4–8 weeks old; CD1;
Charles River Laboratories) were subcutaneously (s.c.) injected
with a mixture of cancer cells (5×105) and HECV cells
(5×105) in Matrigel (2.5 mg/ml). Small peptides were
given via intraperitoneal injection (i.p.). The mice were kept in
sterilised, filtered cages in 12-h dark/12-h light standardized
environmental conditions approved by Cardiff University Research
Ethics Committee (UREC). Tumours were measured twice a week using
digital callipers and calculated as tumour volume = 0.512 ×
width2 × length (mm3). The protocol and
procedure (project licence no. 30/2591) were approved by the Home
Office, UK.
Statistical analysis
Two sample t-tests were performed using the SPSS
statistical software (version 18, SPSS Inc. Chicago, IL, USA).
Differences were considered to be statistically significant at
p<0.05.
Results
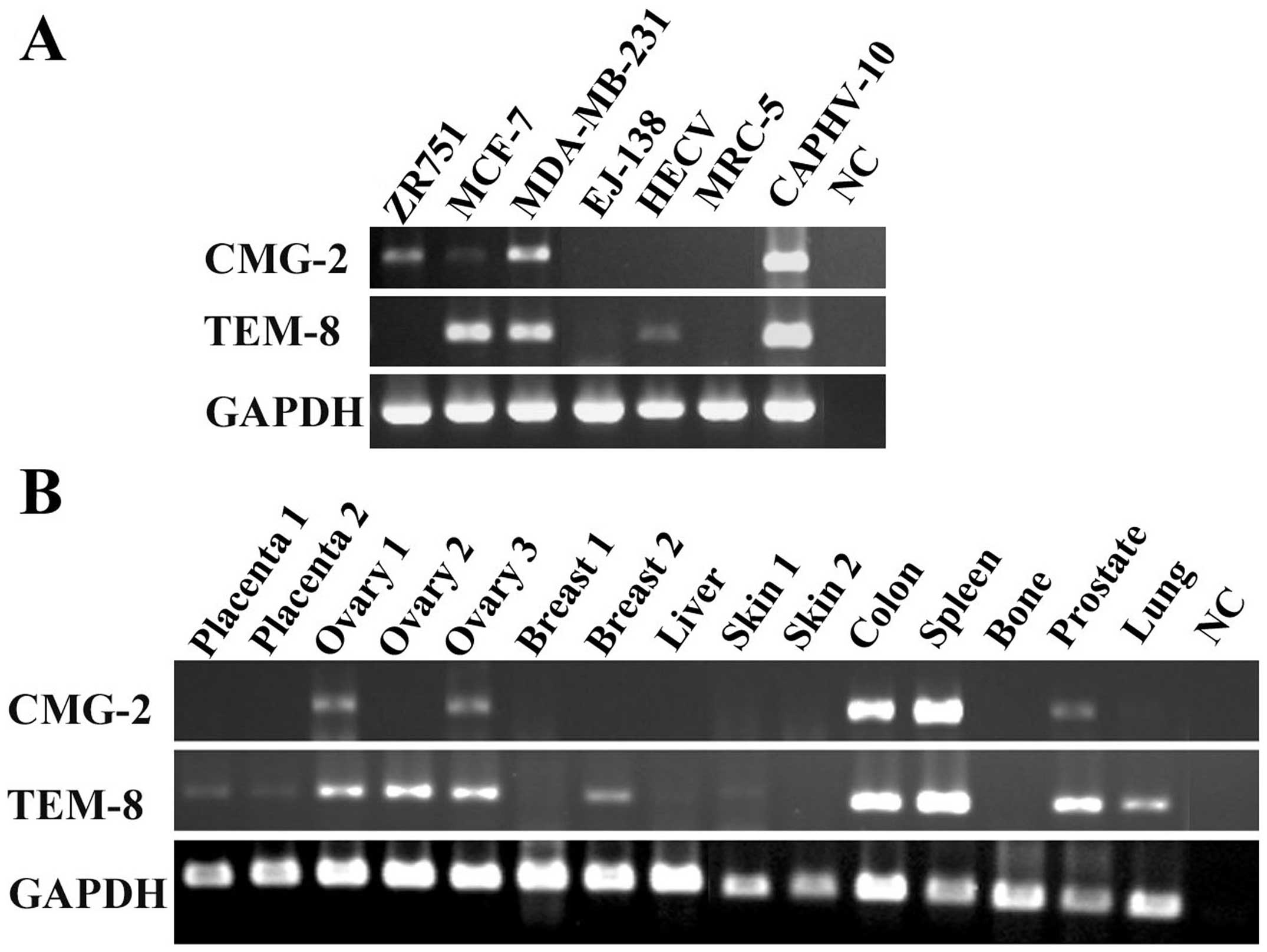
The expression of CMG2 and TEM-8 in cell
lines and human tissues
The expression of CMG2 was examined in human
vascular endothelial cells (HECV), bladder cancer cell line
(EJ-138), fibroblast cells (MRC-5), prostate cancer cell line
(CAHPV-10) and breast cancer cell lines (ZR751, MCF-7 and
MDA-MB-231) using RT-PCR (Fig.
1A). CMG2 transcripts are expressed in breast cancer cell
lines, including ZR751, MCF-7 and MDA-MB-231, in which MCF-7 has a
much lower level compared with the others. CMG-2 is not detectable
in HECV cells, which do express TEM-8. Both CMG-2 and TEM-8 are
expressed by CAHPV-10 cells. This provided information to plan both
in vitro and in vivo experimental models for further
investigations of the role played by CMG2 in cancer and
angiogenesis.
The expression of both CMG2 and TEM-8 was also
examined in various human tissues (Fig. 1B). CMG2 was expressed in the two of
the three ovarian tissues, and is highly expressed in colon and
spleen tissues. It is also detectable in prostate tissue. Compared
with CMG2, TEM-8 is more ubiquitously expressed in the tissues we
examined.
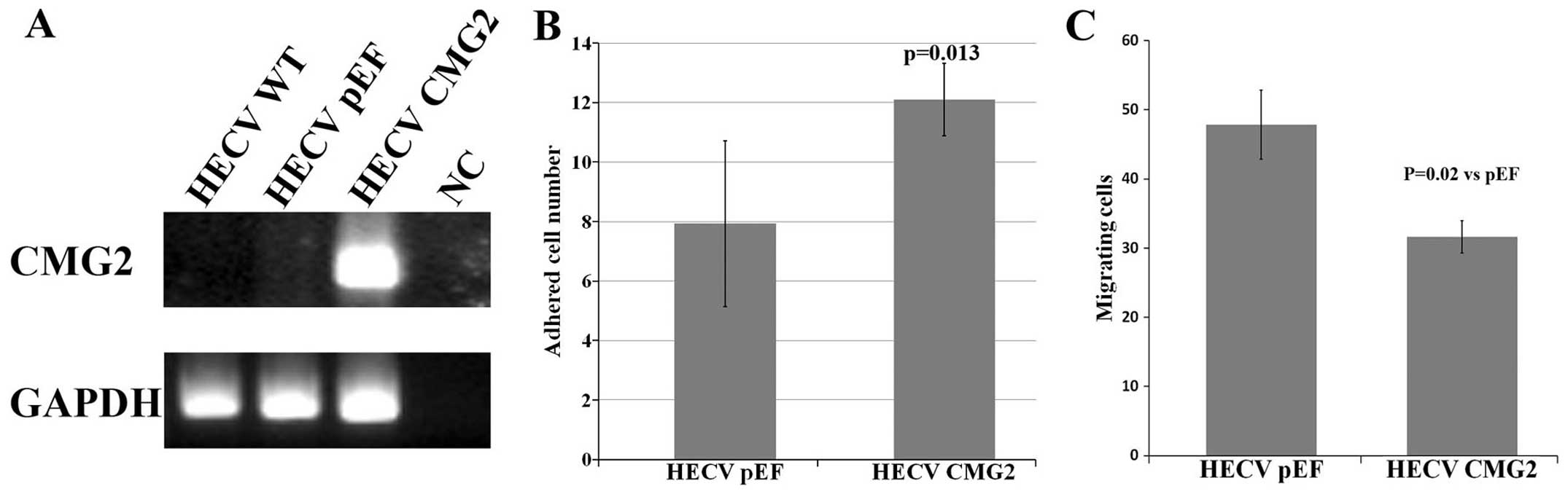
Overexpression of CMG2 in HECV cells and
the effect on cell adhesion and migration
To examine the effect of CMG2 on functions of
vascular endothelial cells, we transfected the HECV cells with the
constructed plasmid vector carrying full-length of CMG2. An
overexpression of CMG2 was confirmed in the transfected cells
compared with the control cells (Fig.
2A). Following the verification, the effect on in vitro
cell proliferation was determined using an in vitro cell
growth assay. No obvious effect on cell growth was seen in the CMG2
overexpression cells (data not shown). The overexpression of CMG2
resulted in an enhanced cell-matrix adhesion of HECV cells
(Fig. 2B). An opposite effect was
seen in the cell motility which was determined using an in
vitro beads assay (Fig.
2C).
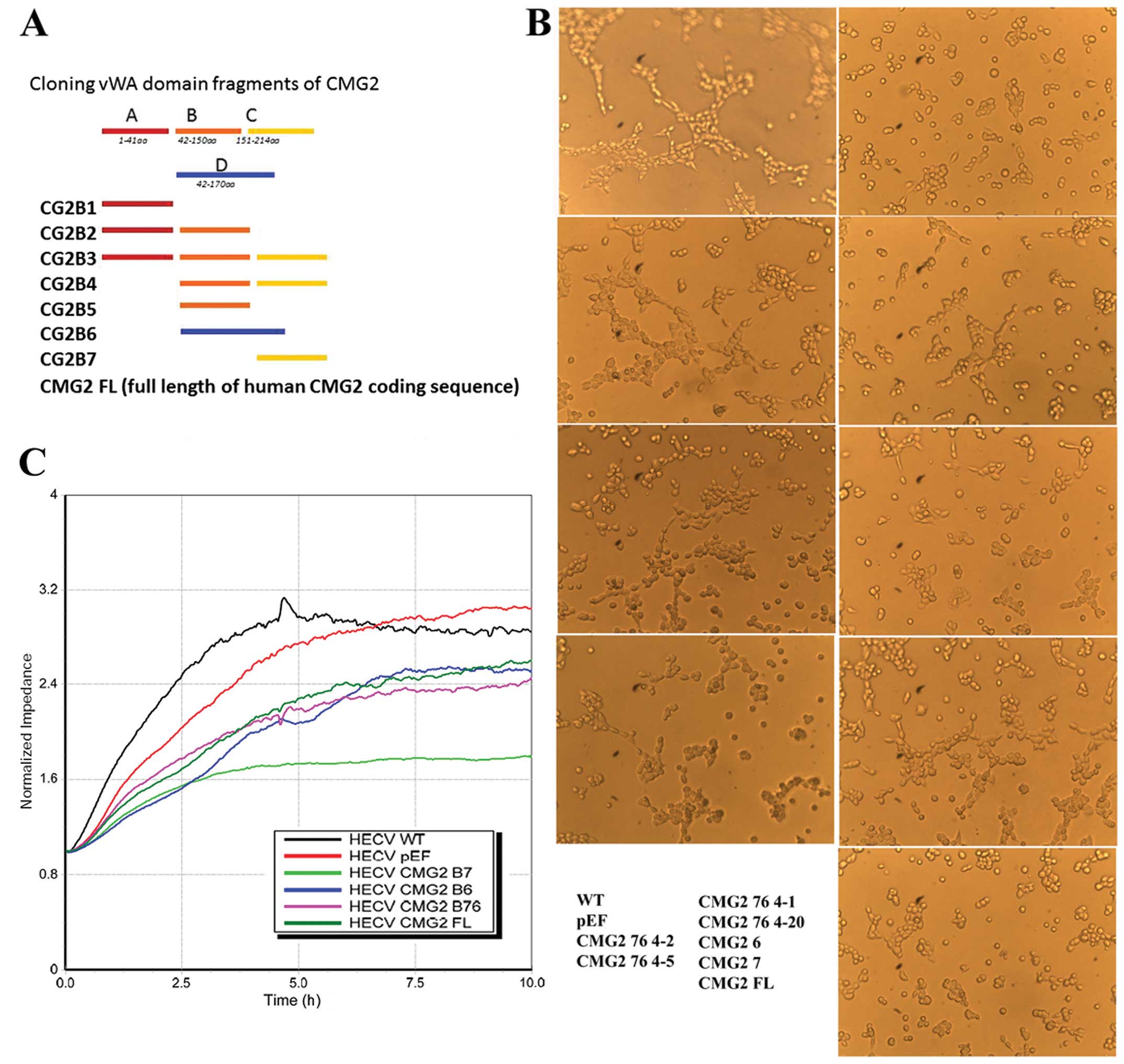
Targeting extracellular vWA domain of
CMG2 to interfere with angiogenesis
vWA domain has been identified as a pivotal domain
in regulation of cell functions, such as cell-matrix adhesion and
cell motility. The vWA domain of TEM-8 and CMG2 has been indicated
as a key domain in control of adhesion and in vitro tubule
formation of endothelial cells. Coding sequence of the vWA domain
was divided into 6 fragments. A set of primers were designed to
amplify 6 different products including these 6 fragments (Fig. 3A). Different fragmental sequences
of the vWA domain were amplified from human ovarian tissue cDNA
library, and cloned into plasmid vectors, respectively. The
recombinant plasmid constructs and empty control plasmid vectors
were then transfected into human vascular endothelial cells (HECV).
The influence on cell function was examined using a series of in
vitro functional assays, including cell growth, cell-matrix
adhesion, motility and tubule formation.
The influence on tubule formation of HECV cells by
overexpression of the full-length and different fragments of CMG2
was determined using the tubule formation assay. After the first
stage of identification, the sequences of B6 and B7 and the common
part of these two fragments were deduced to be potential candidates
for further investigation. The overlapping part of CMG2-B6 and
CMG2-B7 sequences was then amplified and cloned into the
aforementioned plasmid vector. This fragment was named as CMG2 76.
Its effect on tubule formation was assessed in the HECV cells which
had this fragment overexpressed. Inhibition of tubule formation was
seen in the cells overexpressed in the CMG2vWA domain fragments B3,
B6, B7, B76 and the full-length CMG2 (Fig. 3B). Fragment B76 is an overlap
sequence shared by the B6 and B7 fragments. The forced expression
of these fragments and full-length of CMG2 suppressed the migration
of endothelial cells in vitro (Fig. 3C). The B76 fragment exhibited a
potent inhibitory effect on both migration and tubule formation of
the endothelial cells.
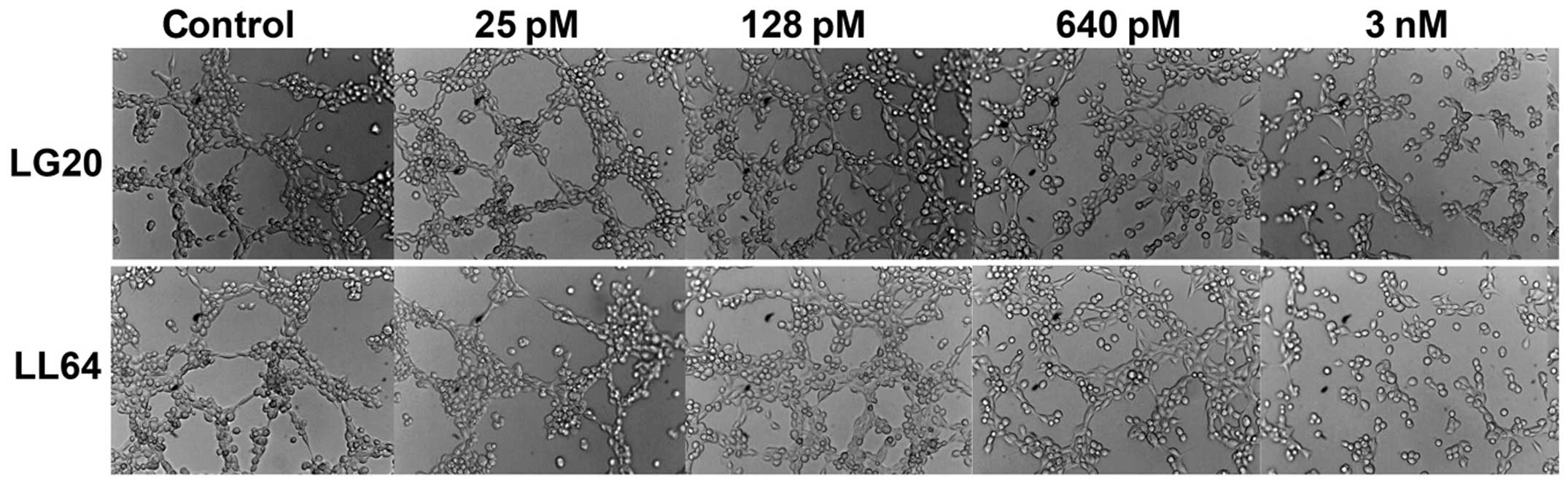
Anti-angiogenic potential of small
peptides based on the vWA domain of CMG2
Eleven polypeptides were then synthesised based on
the amino acid sequence of CMG2 vWA domain fragment B76. The amino
acid sequences of the polypeptides are provided in Table I. The influence on angiogenesis was
then assessed using both in vitro tubule formation of HECV
cells and ex vivo aorta assay. The cellular toxicity of
these peptides was tested using in vitro cell growth assay,
which indicated that these peptides are safe to human vascular
endothelial cells over a range of concentration, from 60 pM to 20
μM (data not shown).
The effect on tubule formation of HECV cells was
then assessed using the aforementioned method. In the experiments,
LG20 and LL64 demonstrated better inhibitory effect on the in
vitro tubule formation. The inhibition was seen in the
endothelial cells exposed to a range of concentration from 640 pM
to 3 nM, the most obvious inhibition was seen at a concentration of
3 nM (Fig. 4). The anti-angiogenic
effect of these polypeptides was also assessed using the aorta ring
assay, in which marked inhibition of angiogenesis was seen in the
aorta rings exposed to LG20, M3 and M10 compared with the control
and other polypeptides (Fig.
5).
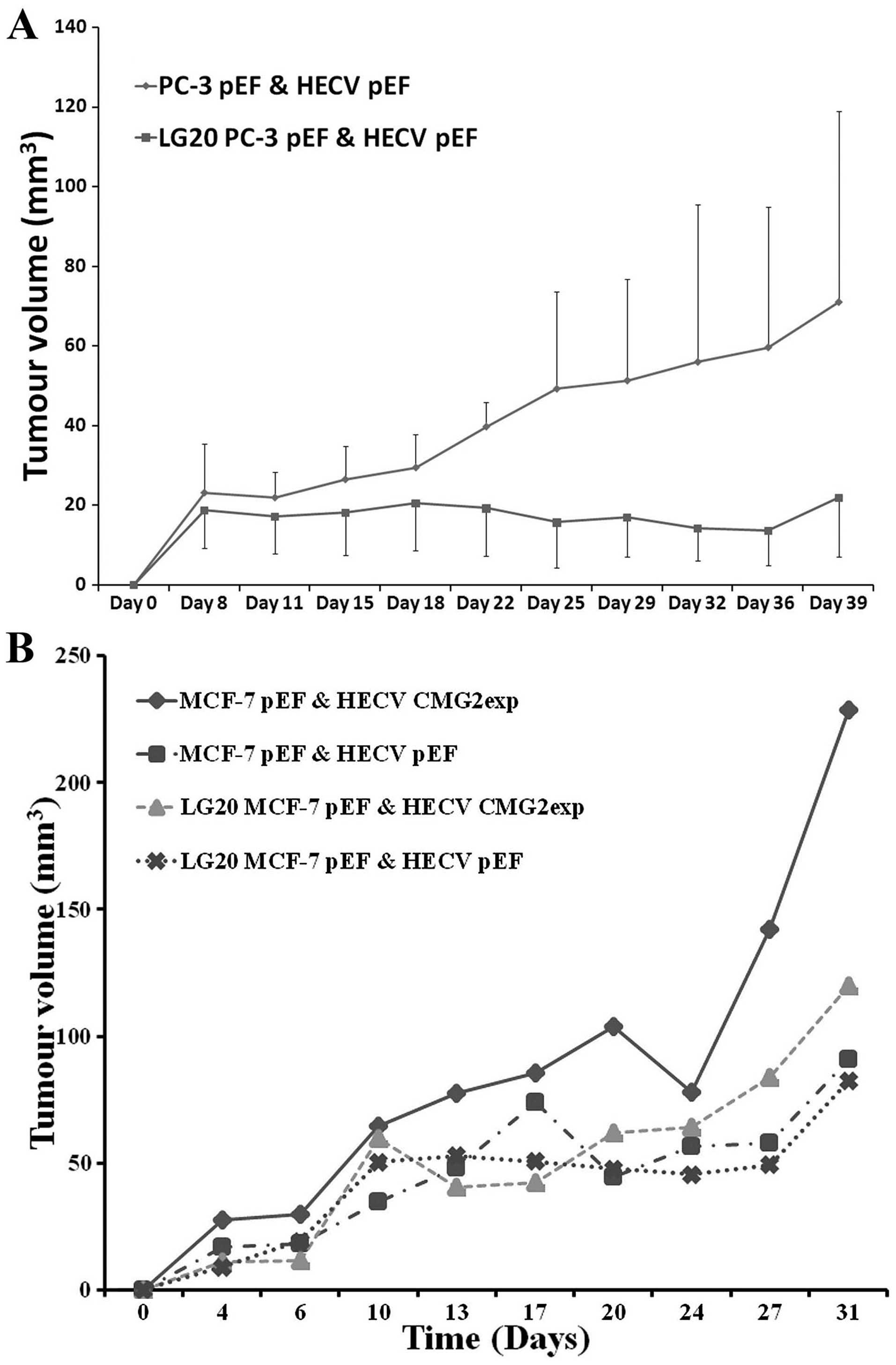
The effect of LG20 on the in vivo tumour
growth was then assessed. A prostate cancer cell line PC-3 was used
to examine the effect on in vivo growth of prostate cancer
cells. PC-3 cells and endothelia (HECV) were inoculated
subcutaneously in nude mice. LG20 and control buffer (BSS) were
injected via i.p. three times a week. A reduced tumour growth was
seen in the mice receiving the LG20 treatment (Fig. 6A). After this study, we further
assessed the therapeutic potential of LG20 in breast cancer. Breast
cancer cells co-implanted with HECV cells and treated with LG20,
did not affect the in vivo tumour growth as seen in the
prostate cancer cells. However, when co-implanting the MCF-7 cells
with HECV which overexpressed CMG2, a remarkable increase of tumour
growth was seen. This enhanced tumour growth was diminished by the
LG20 treatment (Fig. 6B).
Discussion
CMG2 is a type I transmembrane protein possessing an
extra cellular integrin-like I domain, a member of the larger
family of von Willebrand factor A domains (4,5). The
CMG2 and TEM-8 proteins share 40% overall amino acid identity, with
60% identity within their I domains, including a perfectly
conserved metal ion dependent adhesion site (MIDAS) motif. CMG2 is
widely expressed in normal tissues (2), whereas TEM-8 was reported to be
selectively overexpressed during tumour angiogenesis. In the
current study, we examined the expression of CMG2 in cDNAs of cell
lines and tissues. Three breast cancer cell lines (ZR751, MCF7 and
MDA-MB-231) and prostate cancer cell line (CAPHV10) are positive
for the CMG2 expression. However, a vascular endothelial cell line
(HECV) appears to be negative while TEM-8 appears to be positive in
this endothelial cell line. CMG2 was also not detectable in the two
placenta tissues which comprised of abundant new vasculature.
Similarly, this absence was also evident in other tissues with
abundant blood vessels such as the liver. However, highly positive
expression of both CMG2 and TEM-8 was seen in both spleen and colon
tissues. This suggests CMG2 expression in the endothelial cells may
have spatial-temporal variations during the angiogenic process and
maturation of the blood vasculature. This requires further
investigation into the expression of CMG2 and its functions in
different tissues and endothelial cells at various phases according
to the angiogenic process.
The discovery of TEM-8 as a specific tumour cell
marker has raised great interest for researchers to develop
anti-angiogenesis approaches. The potential and recent development
of anti-angiogenesis therapy targeting TEM-8 and CMG2, such as
modified anthrax toxin and anti-TEM-8 antibodies have been reviewed
recently (33). CMG2 has been
demonstrated as the major receptor of anthrax toxin mediating
direct lethality (34). A binding
of the CMG2 to domain 2 and 4 of PA may act as receptor-based
molecular switch that controls anthrax toxin entry into cells
(35). The binding to the domain 2
is weakened prior to pore-to-pore conversion whilst the binding to
domain 4 remains the same during the conversion (35). Protective antigen (PA) is a
non-pathogenic component of anthrax toxin and can also inhibit
angiogenesis by interacting with CMG2 and TEM-8. For example, a
form of modified PA with three mutated amino acids, PA-SSSR, can
inhibit migration of endothelial cells and also angiogenesis in
vivo (36). PA-SSSR can
suppress VEGF and serum induced migration of microvascular
endothelial cells (HMVEC) with no effect on their proliferation.
PA-SSSR also inhibits angiogenesis in a corneal angiogenesis assay
and growth of lung cancer cells. The modified PA, without the
assistance of the lethal factor and oedema factor of anthrax toxin,
suggests the binding of PA to its receptor CMG2 and TEM-8 can
directly influence cellular functions via machinery yet to be
investigated.
Research to develop a therapeutic approach targeting
TEM-8 has been underway in the host laboratory since 2002.
Hammer-head ribozyme transgenes targeting TEM-8 could reduce the
expression of TEM-8 in human vascular endothelial cells (HECV), and
therefore prevent their in vitro tubule formation. On the
other hand overexpression of different domains of TEM-8 has
revealed that vWA domain played a key role in control of in
vitro tubule formation, as well as the extracellular domain
with transmembrane domain (37).
This suggests a potential for targeting at the vWA domain of
TEM-8.
CMG2 is upregulated in vascular endothelial cells
during formation of new capillaries (1). A recent study showed that CMG2
inhibits the growth of vascular endothelial cells (HUVEC) leading
to inhibition of angiogenic capacity of the endothelia with no
obvious effect on cell migration. In the current study, the
overexpression of CMG2 in HECV cells enhanced the adhesion to
extracellular matrix, but was negatively associated with cell
migration. Overexpression of certain fragments (extracellular
domains) inhibited the tubule formation and migration of
endothelial cells. Our data suggest a negative role played by CMG2
for the angiogenic capacity of HECV cells. The controversial
findings indicate diverse functions possibly played by CMG2 at
different stages of the angiogenic process. Differences in the
endothelial cells examined in the studies may be a possible reason
for the different findings, which may reflect the nature of
endothelial cells from different collections.
CMG2 contains a signal peptide, an extracellular von
Willebrand factor A (vWA) domain, a single-pass transmembrane
region (TM) for plasma membrane anchoring, and a cytosolic tail
that might be involved in cytoskeleton interaction and is subject
to certain post-translational modifications (38,39).
CMG2 and TEM-8 share 60% sequence identity in their vWA domains,
which contain a typical metal ion-dependent adhesion site (MIDAS)
motif responsible for PA binding (39). The cytosolic tail of the TEM-8 can
bind to filamentous actin which leads to a reduced association of
the extracellular domain with PA (40).
The current study was carried out to examine the
role played by CMG2 in tumour-related angiogenesis, and to develop
an approach for anti-angiogenesis targeting CMG2. We examined the
function of different fragments within CMG2 vWA domain by
overexpressing these fragments in vascular endothelial cells.
Experimental data showed an anti-angiogenesis effect by a specific
fragment. A few polypeptides have been designed and synthesised
based on this fragment. The effect against angiogenesis has been
examined using in vitro and ex vivo angiogenesis
models. Small peptides mimicking the amino acid sequence of the
fragments potently inhibit the in vitro tubule formation and
ex vivo angiogenesis. Tests of certain small peptides showed
an inhibitory effect on in vivo tumour growth of cancer
cells which we have examined. In addition to its crucial role in
angiogenesis, our recent studies have also demonstrated direct
impact of this molecule on cancer cells in prostate and breast
cancer (unpublished data). Other recent studies have revealed an
important role of the vWA domain for the functions of CMG2 protein,
and great potential for antiangiogenesis by targeting this domain.
For example, TEM-8 and CMG2 extracellular domain, particularly vWA
domain based decoys can be used as anthrax toxin inhibitors
(41).
In addition to the above approaches, natural
molecules targeting CMG2 have been assessed for their
anti-angiogenic potential. For example, PGG
(1,2,3,4,6-penta-O-galloyl-β-D-glucopyranose) is a gallotannin
produced by a variety of medicinal plants and has antitumour
effects. It has been recently shown as a CMG2 inhibitor with
anti-angiogenic activity using a high-throughput fluorescence
resonance energy transfer (FRET) based screening assay (42). PGG can inhibit migration of human
dermal microvascular endothelial cells which together with its
other antitumour activities may contribute to the inhibition of
in vivo tumour growth. The same research team has also
identified some other inhibitors which may target CMG2 and can be
used for anti-angiogenic therapy. These inhibitors include
compounds from CR252M and CR1207B. The CR252M is an endophytic
fungus Coccomyces proteae collected from a Costa Rican
rainforest, and the CR1207B is Aurapex penicillata (43). The first inhibitor identified using
the FRET was tannic acid which can interact with CMG2 and
suppressed angiogenesis (44).
The present data have demonstrated great therapeutic
potential of the CMG2 vWA fragment for tumour-related angiogenesis.
However, further investigations are required to elucidate the
mechanisms underlying their anticancer and anti-angiogenesis
effects, and examine the safety of novel therapeutic reagents and
improve their efficiency and specificity towards a real drug
against tumour-associated angiogenesis.
In summary, CMG2 is a potential target of
anti-angiogenic therapy. Small peptides based on the extracellular
vWA domain of CMG2 can potently inhibit angiogenesis in
vitro and ex vivo, which may contribute to its
inhibitory effect on the in vivo tumour growth. Further
investigations will shed light on the underlying mechanisms and
help to fully establish the therapeutic potential of targeting CMG2
vWA domain to prevent tumour-related angiogenesis.
Acknowledgements
The authors thank Cancer Research Wales for the
great support to this study.
References
|
1
|
Bell SE, Mavila A, Salazar R, et al:
Differential gene expression during capillary morphogenesis in 3D
collagen matrices: regulated expression of genes involved in
basement membrane matrix assembly, cell cycle progression, cellular
differentiation and G-protein signaling. J Cell Sci. 114:2755–2773.
2001.
|
|
2
|
Scobie HM, Rainey GJ, Bradley KA and Young
JA: Human capillary morphogenesis protein 2 functions as an anthrax
toxin receptor. Proc Natl Acad Sci USA. 100:5170–5174. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bradley KA, Mogridge J, Mourez M, Collier
RJ and Young JA: Identification of the cellular receptor for
anthrax toxin. Nature. 414:225–229. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Carson-Walter EB, Watkins DN, Nanda A,
Vogelstein B, Kinzler KW and St Croix B: Cell surface tumor
endothelial markers are conserved in mice and humans. Cancer Res.
61:6649–6655. 2001.PubMed/NCBI
|
|
5
|
Emsley J, King SL, Bergelson JM and
Liddington RC: Crystal structure of the I domain from integrin
alpha2beta1. J Biol Chem. 272:28512–28517. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Davies G, Cunnick GH, Mansel RE, Mason MD
and Jiang WG: Levels of expression of endothelial markers specific
to tumour-associated endothelial cells and their correlation with
prognosis in patients with breast cancer. Clin Exp Metastasis.
21:31–37. 2004. View Article : Google Scholar
|
|
7
|
Rmali KA, Watkins G, Harrison G, Parr C,
Puntis MC and Jiang WG: Tumour endothelial marker 8 (TEM-8) in
human colon cancer and its association with tumour progression. Eur
J Surg Oncol. 30:948–953. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rmali KA, Puntis MC and Jiang WG:
Prognostic values of tumor endothelial markers in patients with
colorectal cancer. World J Gastroenterol. 11:1283–1286. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Davies G, Rmali KA, Watkins G, Mansel RE,
Mason MD and Jiang WG: Elevated levels of tumour endothelial
marker-8 in human breast cancer and its clinical significance. Int
J Oncol. 29:1311–1317. 2006.PubMed/NCBI
|
|
10
|
Hanks S, Adams S, Douglas J, et al:
Mutations in the gene encoding capillary morphogenesis protein 2
cause juvenile hyaline fibromatosis and infantile systemic
hyalinosis. Am J Hum Genet. 73:791–800. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dowling O, Difeo A, Ramirez MC, et al:
Mutations in capillary morphogenesis gene-2 result in the allelic
disorders juvenile hyaline fibromatosis and infantile systemic
hyalinosis. Am J Hum Genet. 73:957–966. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tumer L, Kasapkara C, Fong K, Serdaroglu A
and McGrath JA: Hyaline fibromatosis syndrome resulting from a new
homozygous missense mutation, p. Gly116Val, in ANTXR2. J Dermatol.
40:677–678. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Al Sinani S, Al Murshedy F and Abdwani R:
Infantile systemic hyalinosis: a case report with a novel mutation.
Oman Med J. 28:53–55. 2013.PubMed/NCBI
|
|
14
|
Wang YY, Wen CQ, Wei Z and Jin X: A novel
splice site mutation in ANTXR2 (CMG2) gene results in systemic
hyalinosis. J Pediatr Hematol Oncol. 33:e355–e357. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yan SE, Lemmin T, Salvi S, et al: In-depth
analysis of hyaline fibromatosis syndrome frameshift mutations at
the same site reveal the necessity of personalized therapy. Hum
Mutat. 34:1005–1017. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Martchenko M, Candille SI, Tang H and
Cohen SN: Human genetic variation altering anthrax toxin
sensitivity. Proc Natl Acad Sci USA. 109:2972–2977. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Reveille JD, Sims AM, Danoy P, et al:
Genome-wide association study of ankylosing spondylitis identifies
non-MHC susceptibility loci. Nat Genet. 42:123–127. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Guo C, Xia Y, Yang Q, Qiu R, Zhao H and
Liu Q: Association of the ANTXR2 gene polymorphism and ankylosing
spondylitis in Chinese Han. Scand J Rheumatol. 41:29–32. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen C, Zhang X and Wang Y: ANTXR2 and
IL-1R2 polymorphisms are not associated with ankylosing spondylitis
in Chinese Han population. Rheumatol Int. 32:15–19. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Peters DE, Zhang Y, Molinolo AA, et al:
Capillary morphogenesis protein-2 is required for mouse parturition
by maintaining uterine collagen homeostasis. Biochem Bioph Res Co.
422:393–397. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Reeves CV, Wang X, Charles-Horvath PC, et
al: Anthrax toxin receptor 2 functions in ECM homeostasis of the
murine reproductive tract and promotes MMP activity. PLoS One.
7:e348622012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Reeves CV, Dufraine J, Young JA and
Kitajewski J: Anthrax toxin receptor 2 is expressed in murine and
tumor vasculature and functions in endothelial proliferation and
morphogenesis. Oncogene. 29:789–801. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bonnekoh B, Wevers A, Jugert F, Merk H and
Mahrle G: Colorimetric growth assay for epidermal cell cultures by
their crystal violet binding capacity. Arch Dermatol Res.
281:487–490. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jiang WG, Davies G, Martin TA, et al:
Targeting matrilysin and its impact on tumor growth in vivo: the
potential implications in breast cancer therapy. Clin Cancer Res.
11:6012–6019. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jiang WG, Hiscox S, Hallett MB, Scott C,
Horrobin DF and Puntis MC: Inhibition of hepatocyte growth
factor-induced motility and in vitro invasion of human colon cancer
cells by gamma-linolenic acid. Br J Cancer. 71:744–752. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rosen EM, Meromsky L, Setter E, Vinter DW
and Goldberg ID: Smooth muscle-derived factor stimulates mobility
of human tumor cells. Invasion Metastasis. 10:49–64.
1990.PubMed/NCBI
|
|
27
|
Jiang WG, Hiscox S, Singhrao SK, Nakamura
T, Puntis MC and Hallett MB: Inhibition of HGF/SF-induced membrane
ruffling and cell motility by transient elevation of cytosolic free
Ca2+. Exp Cell Res. 220:424–433. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jiang WG, Martin TA, Lewis-Russell JM,
Douglas-Jones A, Ye L and Mansel RE: Eplin-alpha expression in
human breast cancer, the impact on cellular migration and clinical
outcome. Mol Cancer. 7:712008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cai J, Jiang WG and Mansel RE: Inhibition
of the expression of VE-cadherin/catenin complex by gamma linolenic
acid in human vascular endothelial cells, and its impact on
angiogenesis. Biochem Biophys Res Commun. 258:113–118. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Grant DS, Tashiro K, Segui-Real B, Yamada
Y, Martin GR and Kleinman HK: Two different laminin domains mediate
the differentiation of human endothelial cells into capillary-like
structures in vitro. Cell. 58:933–943. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cai J, Jiang WG and Mansel RE: Inhibition
of angiogenic factor- and tumour-induced angiogenesis by gamma
linolenic acid. Prostaglandins Leukot Essent Fatty Acids. 60:21–29.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jiang WG and Harding KG: Enhancement of
wound tissue expansion and angiogenesis by matrix-embedded
fibroblast (dermagraft), a role of hepatocyte growth factor/scatter
factor. Int J Mol Med. 2:203–210. 1998.PubMed/NCBI
|
|
33
|
Chaudhary A and St Croix B: Selective
blockade of tumor angiogenesis. Cell Cycle. 11:2253–2259. 2012.
View Article : Google Scholar
|
|
34
|
Liu S, Zhang Y, Hoover B and Leppla SH:
The receptors that mediate the direct lethality of anthrax toxin.
Toxins (Basel). 5:1–8. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Pilpa RM, Bayrhuber M, Marlett JM, Riek R
and Young JA: A receptor-based switch that regulates anthrax toxin
pore formation. PLoS Pathog. 7:e10023542011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Rogers MS, Christensen KA, Birsner AE, et
al: Mutant anthrax toxin B moiety (protective antigen) inhibits
angiogenesis and tumor growth. Cancer Res. 67:9980–9985. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Rmali KA, Puntis MC and Jiang WG: TEM-8
and tubule formation in endothelial cells, its potential role of
its vW/TM domains. Biochem Biophys Res Commun. 334:231–238. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Abrami L, Leppla SH and van der Goot FG:
Receptor palmitoylation and ubiquitination regulate anthrax toxin
endocytosis. J Cell Biol. 172:309–320. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liu S, Leung HJ and Leppla SH:
Characterization of the interaction between anthrax toxin and its
cellular receptors. Cell Microbiol. 9:977–987. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Garlick KM, Batty S and Mogridge J:
Binding of filamentous actin to anthrax toxin receptor 1 decreases
its association with protective antigen. Biochemistry.
51:1249–1256. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Cai C, Che J, Xu L, et al: Tumor
endothelium marker-8 based decoys exhibit superiority over
capillary morphogenesis protein-2 based decoys as anthrax toxin
inhibitors. PLoS One. 6:e206462011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Cryan LM, Bazinet L, Habeshian KA, et al:
1,2,3,4,6-Penta-O-galloyl-beta-D-glucopyranose inhibits
angiogenesis via inhibition of capillary morphogenesis gene 2. J
Med Chem. 56:1940–1945. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Cao S, Cryan L, Habeshian KA, et al:
Phenolic compounds as antiangiogenic CMG2 inhibitors from Costa
Rican endophytic fungi. Bioorg Med Chem Lett. 22:5885–5888. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Rogers MS, Cryan LM, Habeshian KA, et al:
A FRET-based high throughput screening assay to identify inhibitors
of anthrax protective antigen binding to capillary morphogenesis
gene 2 protein. PLoS One. 7:e399112012. View Article : Google Scholar : PubMed/NCBI
|