Introduction
Breast cancer (BC) is the most frequently diagnosed
cancer and the leading cause of cancer death among females,
accounting for 23% (1.38 million) of the total new cancer cases and
14% (458,400) of all cancer deaths in 2008 (1). The last two decades have seen no
significant progress in extending the survival of patients with BC,
despite multiple trials of cytotoxic chemotherapeutic agents. Thus,
there is an urgent need to improve therapy in BC patients.
In recent years the expression and secretion of
peptides by tumors has gained increasing interest since these
substances have been shown to influence tumor proliferation and
progression (2–8). In this sense, many authors have
suggested that the substance P (SP)/neurokinin (NK)-1 receptor
system would play an important role in the development of cancer
(2–22). SP shows a widespread distribution
in both the central and peripheral nervous systems; it is released
from primary sensory nerve fibers, and the NK-1 receptor shows a
preferential affinity for SP (2–4). It
has been suggested that SP and the NK-1 receptor would be involved
in regional blockade and BC recurrence (5) and it has recently been demonstrated
that the NK-1 receptor is involved in the viability of tumor cells
(16,23,24).
It is also known that activation of the NK-1 receptor by SP induces
mitogenesis in tumor cells; that SP is a major mediator of the
growth of capillary vessels in vivo and of the proliferation
of cultured endothelial cells in vitro; that NK-1 receptor
agonists induce neoangiogenesis; that the migration of human breast
carcinoma cells (MDA-MB-468), a crucial requirement for invasion
and metastasis development, is regulated by SP; and that NK-1
receptor antagonists exert antitumor action (2,3,10,11,13,14,16–28).
Thus, NK-1 receptor antagonists represent an important opportunity
to further exploit compounds that are active against the NK-1
receptor as novel therapeutic agents in cancer. It has recently
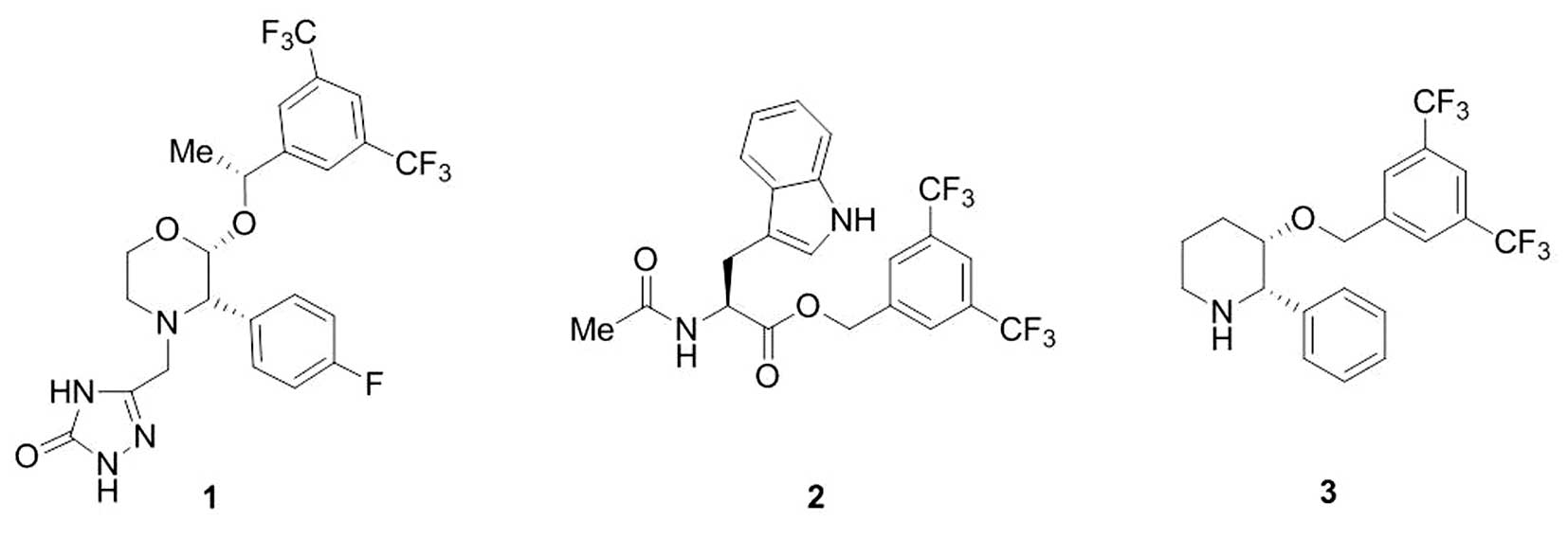
been demonstrated that, one of them, the drug 5-[[(2R,
3S)-2-[(1R)-1-[3,5-bis(trifluoromethyl) phenyl]
ethoxy]-3-(4-fluorophenyl) -4-morpholinyl]methyl]-1,2-dihydro-3
H-1,2,4-triazol-3-one (aprepitant, EMEND or MK-869), exerts
antitumor activity against many human cancer cell lines and that
aprepitant (Fig. 1) induces death
of tumor cells by apoptosis (20).
It has also been suggested that pretreatment with aprepitant prior
to surgical intervention in BC was able to inhibit the migration of
tumor cells (5). Thus, aprepitant
is an excellent candidate for testing in human trials since it has
already been tested (for activity and safety); it is currently used
in clinical practice for emesis, and it is well tolerated (29). In addition, the lack of toxicity of
aprepitant against normal cells (e.g., fibroblasts) has been
reported: the IC50 for these cells was found to be
~2-fold higher than the IC50 for tumor cells (8).
Currently, there are data suggesting the involvement
of the SP/NK-1 receptor system in BC. It has been described that BC
cell lines and samples overexpress NK-1 receptors; that these
receptors are present in the plasma membrane and/or cytoplasm of BC
cells; that the inhibition of SP with antibodies inhibit cell
growth and induce apoptosis in BC cell lines; that BC cell lines
and samples show increased expression of preprotachykinin-I; that
BC cells produce SP (demonstrated by ELISA tests); that SP
stimulates BC cell proliferation; that NK-1 receptor antagonists
(SR-140,333; CP-96,345, CP-99,994, MEN-11,467) reduce BC cell line
(e.g., T47D, BT-474, MDA-MB330, MDA-MB231, DU4475) proliferation;
that T47D cells die by apoptosis after the administration of
SR-140,333; that NK-1 receptor antagonists inhibit BC cell
migration, and that in vivo the NK-1 receptor antagonist
MEN-11,465 controls the growth of breast carcinoma (9–11,24,26).
However, in none of the studies reported above was the action of
the drug aprepitant studied. Thus, to date the antitumor action of
aprepitant on BC is unknown (20).
Moreover, there are several unknown issues in the involvement of
the SP/NK-1 receptor system in BC. For example, the presence or not
of NK-1 receptors in the nucleus of BC cells, the presence and
localization (e.g., in the nucleus) of SP in BC samples as
demonstrated by immunohistochemistry; the involvement of the NK-1
receptor in the viability of BC cells by using a small interfering
RNA gene silencing method, the antitumor action (total or partial)
of other NK-1 receptor antagonists hitherto not tested against BC
cells [e.g., 3, 5-bis (trifluoromethyl) benzyl ester (L-732,138)]
(Fig. 1). This NK-1 receptor
antagonist has been previously reported to totally inhibit tumor
cell proliferation (2,3).
Thus, to our knowledge, there remain many unknown
issues regarding the involvement of the SP/NK-1R system in BC and
no study has been carried out previously on the antitumor action of
the drug aprepitant against BC cells. Accordingly, in order to
answer all the above questions, we selected three BC cell lines
previously studied partially by other authors (BT-474, MCF-7 and
MDA-MB-468) and another (MT-3) in which the involvement of the
SP/NK-1 receptor system is studied here for the first time. The
aims of this study were to demonstrate in the four BC cell lines
mentioned: i) the presence of NK-1 receptors and their isoforms;
ii) mRNA expression for the NK-1 receptor; iii) the overexpression
of the NK-1 receptor; iv) the involvement of the NK-1 receptor in
cell viability; v) that SP induces BC cell proliferation; vi) that
NK-1 receptor antagonists not studied previously in BC (the drug
aprepitant and L-732,138) totally inhibit the growth of these BC
cell lines; vi) that this antitumor action occurs through the NK-1
receptor; and vii) to determine whether the three NK-1 receptor
antagonists produces apoptosis in the BC cell lines studied or not.
Moreover, the presence and distribution of NK-1 receptors and SP in
several human BC samples were studied. We report sufficient data to
suggest that the NK-1 receptor could be a promising target in human
BC treatment and that the NK-1 receptor antagonist, aprepitant,
should be designed as a novel therapeutic agent in BC.
Materials and methods
Cell cultures
We used the human BC cell lines MT-3, MCF-7,
MDA-MB-468 and BT-474 (DSMZ - Deutsche Sammlung von Mikroorganismen
und Zellkulturen, Braunschweig, Germany and ICLC - Interlab Cell
Line Collection, Genova, Italy). These cell lines were maintained
in RPMI-1640 or D-MEM (Gibco, Barcelona, Spain) supplemented with
10 or 20% heat-inactivated fetal bovine serum according to the
culture conditions suggested by DSMZ and ICLC. The immortalized
breast epithelium MCF-10A and MCF-12A cell lines were obtained from
American Type Culture Collection (Rockville, MD, USA) and cultured
according to their instructions. Cell lines were seeded in 75
cm2 tissue culture flasks (Falcon, Heidelberg, Germany).
The medium was renewed every 2 days and the cells were harvested by
treatment with trypsin (0.05 and 0.02% EDTA without Ca2+
and Mg2+, Sigma-Aldrich, Madrid, Spain) on the sixth day
after seeding. Cells were incubated at 37°C in a humidified
atmosphere of 95% air/5% CO2.
Drug treatments
Three different NK-1 receptor antagonists were used
in this study: L-733,060 (MW 438.9, Sigma-Aldrich; L-732,138 (MW
472.39, Sigma-Aldrich); and aprepitant (MW 534.43, the drug was
kindly supplied by Merck Research Laboratories, Madrid, Spain).
L-732,138 and L-733,060 were dissolved in distilled water
containing 0.2% dimethylsulphoxide (DMSO) and aprepitant was
dissolved in distilled water containing acetonitrile before sample
treatment. In order to determine the IC50, different
concentrations (2.5–30 μM) of L-733,060; (5–60 μM) of L-732,138 and
(10–60 μM) of aprepitant were evaluated for BC cell lines. SP,
acetate salt (Sigma-Aldrich), was dissolved in distilled water and
different concentrations (1, 5, 10 and 100 nM) were used. The most
mitogenic nanomolar SP concentration for each cell line was
incubated for 1 h before the addition of each NK-1 receptor
antagonist.
Proliferation assays
Cell proliferation was evaluated using the
tetrazolium compound 3-(4, 5-dimethylthiazol-2-yl)-5-
(3-carboxymethoxyphenyl)2-(4-sulfophenyl)-2H-tetrazolium, inner
salt (MTS), according to the manufacturer’s instructions (CellTiter
96 Aqueous One-Solution Cell Proliferation Assay, Promega Corp.,
Madison, WI, USA). Cell numbers were quantified using a Coulter
counter. The plate included blank wells (0 cells/0.1 ml), control
wells (104 cells/0.1 ml), control wells with DMSO or
acetonitrile, control wells treated with SP, control wells treated
with the NK-1 receptor antagonist, and control wells treated with
the most mitogenic exogenous at SP nM concentration and the NK-1
receptor antagonist [50% μM inhibition concentration
(IC50) of antagonist for their first doubling times].
For the proliferation assay, 20 μl of the MTS reagent was added to
each well 90 min before reading the samples on a multiscanner
microplate reader (Tecan Spectra classic, Barcelona, Spain) at 492
nm. Each experimental condition (blank wells, control wells, and
control wells treated with the different concentrations of each
antagonist and/or SP) was assayed in duplicate and all experiments
were performed at least three times. The IC50 of the
NK-1 receptor antagonists was calculated using the regression
straight line function based on the least squares technique.
Statistical analyses
Data are expressed as the means ± SD. Statistical
analysis was performed with SPPS statistical software for Microsoft
Windows, release 14.0 (Professional Statistic, Chicago, IL, USA).
The homogeneity of variance was tested using the Levene test. If
the variances were homogeneous, the data were analyzed using the
one-way ANOVA test with Bonferroni’s correction for multiple
comparisons. For data sets with non-homogeneous variances, the
ANOVA test with T3 Dunnett post-hoc analysis was applied. The
criterion for significance was p<0.05 for all comparisons.
Western blot analyses
As previously reported (30), total protein was prepared from
subconfluent MT-3, MCF-7, BT-474, MDA-MB-468, MCF-10A and MCF-12A
human cell cultures. Protein concentrations were determined using
the protein assay kit from Bio-Rad according to the manufacturer’s
instructions.
From each sample, 50 μg of protein was separated by
electrophoresis on 10% SDS-polyacrylamide gels and electroblotted
onto PVDF membranes. Blots were incubated in blocking solution [5%
non-fat milk in phosphate-buffered saline (PBS), 0.1% Tween-20
(PBS-T)], followed by overnight incubation with antibodies against
the KTMTESSSFYSNMLA conserved domain, corresponding to the
C-terminus of the NK-1 receptor (product no. S8305, Sigma-Aldrich)
(1:4,000 dilution), against poly-(ADP)-ribose polymerase (PARP)
(product no. 118352380001, Roche) (1:1,000 dilution), against
cleaved caspase-3 (Asp175) (product no. 9664, Cell Signaling)
(1:1,000 dilution), and against β-actin (product no. A2066, Sigma)
(1:1,000 dilution) that was used as endogenous control to ensure
the equal amount of protein loading. Membranes were then washed
with PBS-T and incubated with a horseradish peroxidase-conjugated
goat anti-rabbit IgG antibody for 2 h at room temperature (1:10,000
dilution). Antibody detection was performed with an enhanced
chemiluminescence reaction (ECL Western blotting detection;
Amersham Life Science, UK). Chemiluminescence on membranes was
detected after ECL treatment (Amersham, Piscataway, NJ, USA) and
image capture using a Fujifilm LAS3000 imaging system. Image Gauge
software was used to perform the densitometric quantification of
each protein.
Immunohistochemical staining for NK-1
receptors and SP
Twelve breast human carcinomas specimens were
retrieved from the archives of the Department of Pathology of the
Virgen del Rocío University Hospital, Sevilla, Spain, corresponding
to infiltrating ductal breast carcinoma. Age at diagnosis ranged
between 36 and 82 years. The experimental design, protocols, and
procedures of this work were performed under the guidelines of the
ethics and legal recommendations of Spanish and European law, as
well as in accordance with the Declaration of Helsinki.
Paraffin-embedded breast human carcinoma tissues cut
at 5 mm and dried overnight at 60°C were used. The sections were
deparaffinized with xylene, hydrated through a series of solutions
containing decreasing concentrations of ethanol and immersed in
distilled water. After pressure-cooker antigen retrieval in citrate
buffer, pH 6.0, slides were allowed to cool at room temperature for
10 min. Endogenous peroxidase activity was blocked with 3% hydrogen
peroxide for 30 min at room temperature. After washing in 0.05 M
Tris, sections were incubated with 10% non-immune pig serum for 30
min at room temperature. Subsequently, they were incubated
overnight at 4°C with 1:1,000 diluted anti-NK-1 receptor antibody
(Sigma-Aldrich) or 1:3,000 diluted anti-SP antibody
(Sigma-Aldrich). Sections were then washed in 0.05 M Tris at room
temperature. Sections were incubated in the Envision + System-HRP
(Dako) reagents for 30 min at room temperature. The slides were
rinsed in 0.05 M Tris, and the immunoreactivity was visualized
using a 3, 3′-diaminobenzidine chromogen solution (DAB+,
Dako). Cell nuclei were lightly counterstained with hematoxylin.
Finally, as previously reported (15,30,31),
in order to determine the specificity of the immunostaining, human
gliomas were used as a positive control, while as a negative
control the primary antibody was omitted, being replaced by
non-immune serum. In both cases, the results obtained confirmed the
specificity of the anti-NK-1 receptor antibody used. Moreover,
preabsorption of the primary antibodies with the corresponding
synthetic peptides (100 μg per ml of diluted antiserum) were
carried out. In all the cases, the results obtained confirmed the
specificity of the anti-NK-1 receptor and anti-SP antibodies used.
All slides were evaluated by two independent pathologists. In each
slide, 10 representative high-power microscopic fields were
evaluated using a 40× objective. The presence or absence of
staining and the intensity of the immunoreactivity were noted, as
well as the number of cells showing a brown staining and whether or
not the staining was localized in the tumor cells and/or in the
plasma membrane. Tumors were recorded as positive when they showed
cellular and/or plasma membrane staining ranging from moderate to
strong in >10% of the tumor cells. By consensus among the
pathologists, the intensity of the immunoreactivity was evaluated
as ‘acceptable’ only if the tumor cells showed a clear brown
staining localized in the cytoplasm, nucleus and/or plasma membrane
of the tumor cells. The number of immunoreactive cells acceptable
was quantified as follows: negative (0), if the immunoreactivity
was observed in <10% of the tumor cells; positive (+), if the
immunoreactivity was found to be between 11 and 50% of the tumor
cells and strongly positive (++) when present in >50% of the
tumor cells.
DAPI staining
In order to determine whether the NK-1 receptor
antagonists studied induced apoptosis, DAPI staining was performed.
After treatment with NK-1 receptor antagonists for their
approximate first doubling times or after the application of the
knockdown method (see below), the cells were fixed in 4%
paraformaldehyde. Following a second wash in PBS, cells were
incubated in DAPI solution (Sigma-Aldrich) at a dilution of 1/1,000
(1 μg/ml) for 30 min in the dark. Cells were then observed through
a fluorescence microscope (Zeiss, Oberkochen, Germany). Apoptotic
cells were defined by chromatin condensation and nuclear
fragmentation. We counted the number of apoptotic cells, repeating
the counts on three different slides. Finally, on each slide, we
counted the number of apoptotic cells located in five different
sequential fields.
Polymerase chain reaction (PCR)
From cultured cells (BT-474, MCF-7, MDA-MB-468,
MT-3, MCF-10A and MCF-12A), total RNA isolation was achieved with
the NucleoSpin RNA II kit (Macherey-Nagel), allowing the
purification of ~5×106 cultured cells. Final RNA was
dissolved in RNase-free water. The purity and quality of the RNA
purified were also checked. Reverse transcription with elimination
of genomic DNA was performed according to the manufacturer’s
instructions (QuantiTect Reverse transcription Handbook, Qiagen).
All reactions were carried out on ice in order to minimize the risk
of RNA degradation. The cDNA obtained was kept at −80°C.
From the cDNA preparation, 4 μl was used in PCR with
specifics primers according to the modified method of Bigioni et
al (11) based on the common
sequence of the TAC1R human isoforms (NM 001058, NM 015727)
TAC1R-forward (CTG CTG GTG ATT GGC TAT GC) and
TAC1R-reverse (AGG AGG AAG AAG ATG TGG AAG G), which yielded
a 186-bp fragment. The amplification of the specimen was performed
in a final reaction volume of 20 μl, and was incubated at 95°C for
7 min, subjected to 40 cycles of 95°C for 30 sec, 62°C for 40 sec
and 72°C 30 sec, followed by a final extension cycle at 72°C for 7
min. The amplification products were visualized by electrophoresis
on 2% agarose gel stained with ethidium bromide.
Real-time quantitative RT-PCR
Real-time quantitative RT-PCR was performed as
described previously (16).
Reverse transcription with elimination of genomic DNA was performed
according to the manufacturer’s instructions (QuantiTect Reverse
transcription Handbook, Qiagen). Real-time quantitative RT-PCR
analysis was performed using a de Roche Light Cycler with a
fluorogenic detection system (SYBR green). The β-actin gene was
chosen as the housekeeping gene for normalization. The sequences of
primers for the human NK-1 receptor were based on the common
sequence of the TAC1R human isoforms (NM 001058, NM 015727),
TAC1R-foward (CTG CTG GTG ATT GGC TAT GC) and
TAC1R-reverse (AGG AGG AAG AAG ATG TGG AAG G), which yielded
a 186-bp fragment and for β-actin gene were used forward primer
CGGCATCGTCACCAACTG, and the reverse primer CACGCAGCTCATTGTAGAAGGT,
yielding a 70-bp fragment. All amplification reactions were
performed in a final volume of 20 μl containing 2 μl of the Master
Mix PCR and 2 μl (1 μg) of cDNA. The primer concentrations were
optimized as follows: 500 nM for the NK-1 receptor forward primer
and reverse primer; 300 nM for β-actin forward primer and reverse
primer. PCR for the NK-1 receptor included 40 cycles. In each
cycle, the temperature reached 92°C for 10 sec; 62°C for 15 sec,
and 72°C for 10 sec, while the conditions for PCR for the β-actin
were 95°C for 5 sec; 58°C for 15 sec and 72°C for 10 sec. Data were
analyzed using the relative standard-curve method, as reported
previously (16). Experiments were
performed in duplicate for each data point. Each PCR run included
five standard samples, two negative controls, and the experimental
samples. Standard curves for both NK-1 receptor and β-actin were
generated using cDNAs from MT-3, MCF-7, BT-474 and MDA-MB-468 BC
and HEK-293, MCF-12A and MCF-10A non-tumor cell lines. For each
experimental sample, the relative amounts (copy number) of NK-1
receptor mRNA and β-actin mRNA were respectively determined from
the standard curve. The normalized amount of NK-1 receptor was
determined by dividing the amount of NK-1 receptor mRNA by the
amount of β-actin mRNA for each sample. For MT-3, MCF-7, BT-474 and
MDA-MB-468 BC cell lines and the HEK-293 non-tumor cell line and
MCF-12A and MCF-10A epithelial breast cell lines, NK-1 receptor
mRNA and β-actin mRNA analysis was repeated four times in
duplicate.
Small interfering RNA (siRNA) gene
silencing method
We carried out this method according to the
manufacturer’s instructions (Invitrogen, Madrid, Spain) and
according to a previous study (16,23,32).
This procedure was carried out three times. One day before
transfection, 2×104 cells per well from the BT-474,
MCF-7, MDA-MB-468 and MT-3 human BC cell lines were seeded in
6-wells plates containing 2 ml of normal growth medium. Total cell
number was determined the day before the transfection. Cells were
incubated at 37°C in a CO2 incubator for 17–20 h for
normalization. Then, the normal growth medium was removed and an
antibiotic-free growth medium (Opti-MEM; Gibco) was added and
incubated for 1 h. The latter medium was removed and then the
transfection mixture was added, which contained the siRNA
TAC1R (Tachykinin 1 gene) (Invitrogen) and the diluted
transfection reagent medium (Hiperfect, Qiagen); the latter two
were previously incubated for 30 min. Following this, the final
volume obtained was 200 μl. In the same way, this method was
applied to siRNA-negative control solution. For each transfection,
200 ml siRNA transfection reagent mixture was added to each well
containing 800 ml of the antibiotic-free growth medium. The final
siRNA concentration used for each transfection was 20 nM. It was
incubated for 4–5 h at 37°C in a CO2 incubator. Finally,
2 ml of normal growth medium was added for an additional 72-h
incubation.
Results
Breast cancer and breast epithelial cell
lines express NK-1 receptors
We carried out western blot analyses in order to
test the presence of the NK-1 receptor in the human MT-3, MCF-7,
BT-474 and MDA-MB-468 BC cell lines and in the human MCF-10A and
MCF-12A breast epithelial cell lines. Incubation of the membranes
with the anti-NK-1 receptor antibody revealed the presence of
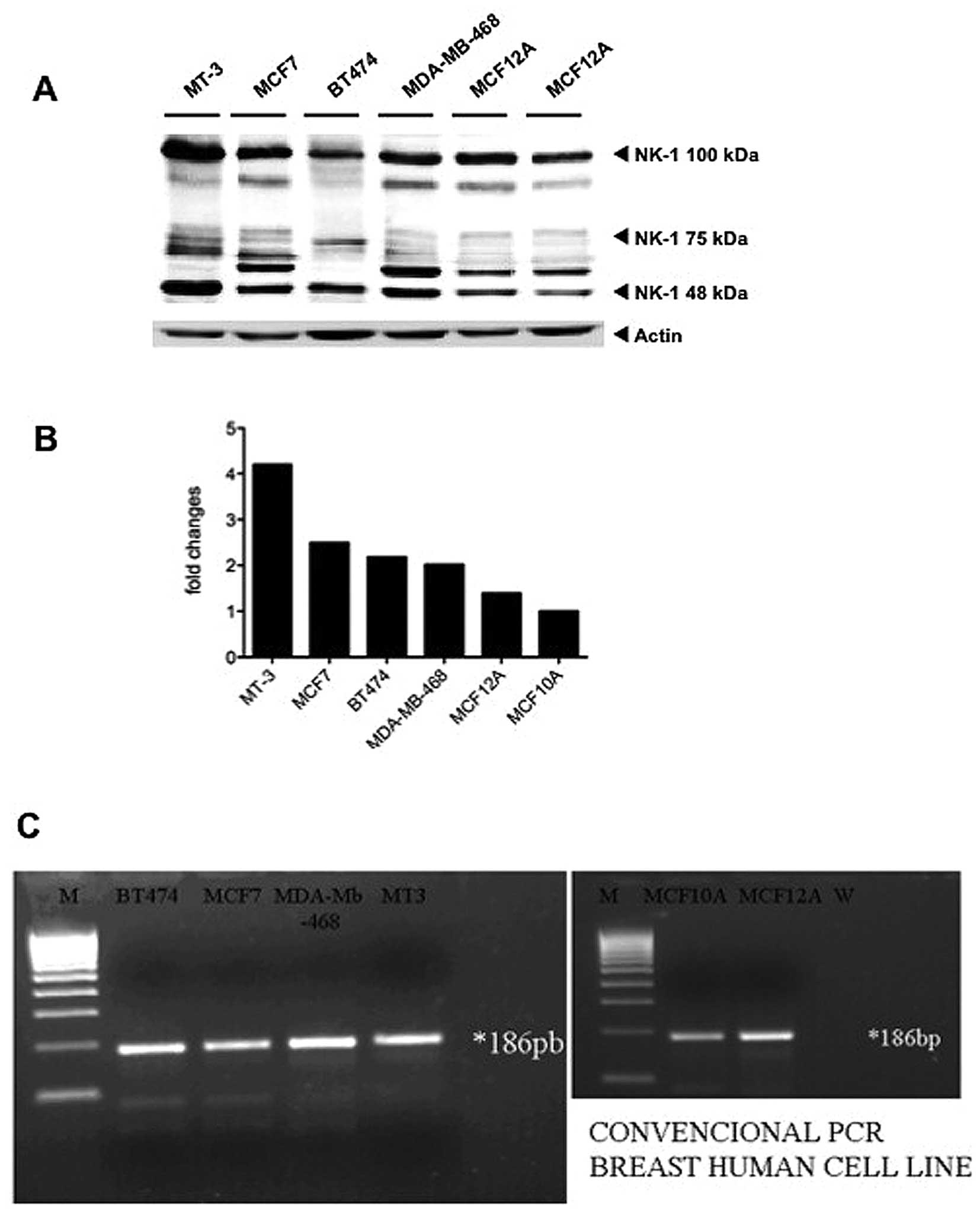
different isoforms of the NK-1 receptor (Fig. 2A), corresponding to the different
N-glycosylation levels that this receptor undergoes after synthesis
(33). The quantification for the
band corresponding to ~50 kDa in each cell line is shown (Fig. 2B). No bands were detected when
incubation was performed with the secondary antibody alone and in
addition as a positive control (not shown), a protein extract from
a glioma cell line was included (30).
From the PCR analyses, we also observed that the
BT-474, MCF-7, MDA-MB-468, MT-3 and MCF-10A and MCF-12A human cell
lines expressed mRNA for the tachykinin NK-1 receptor (Fig. 2C). NK-1 mRNA expression was
detectable in these cell lines as a product of the expected size of
186 bp, corresponding to only one band because the primers used in
our PCR were designed for both short and long isoforms.
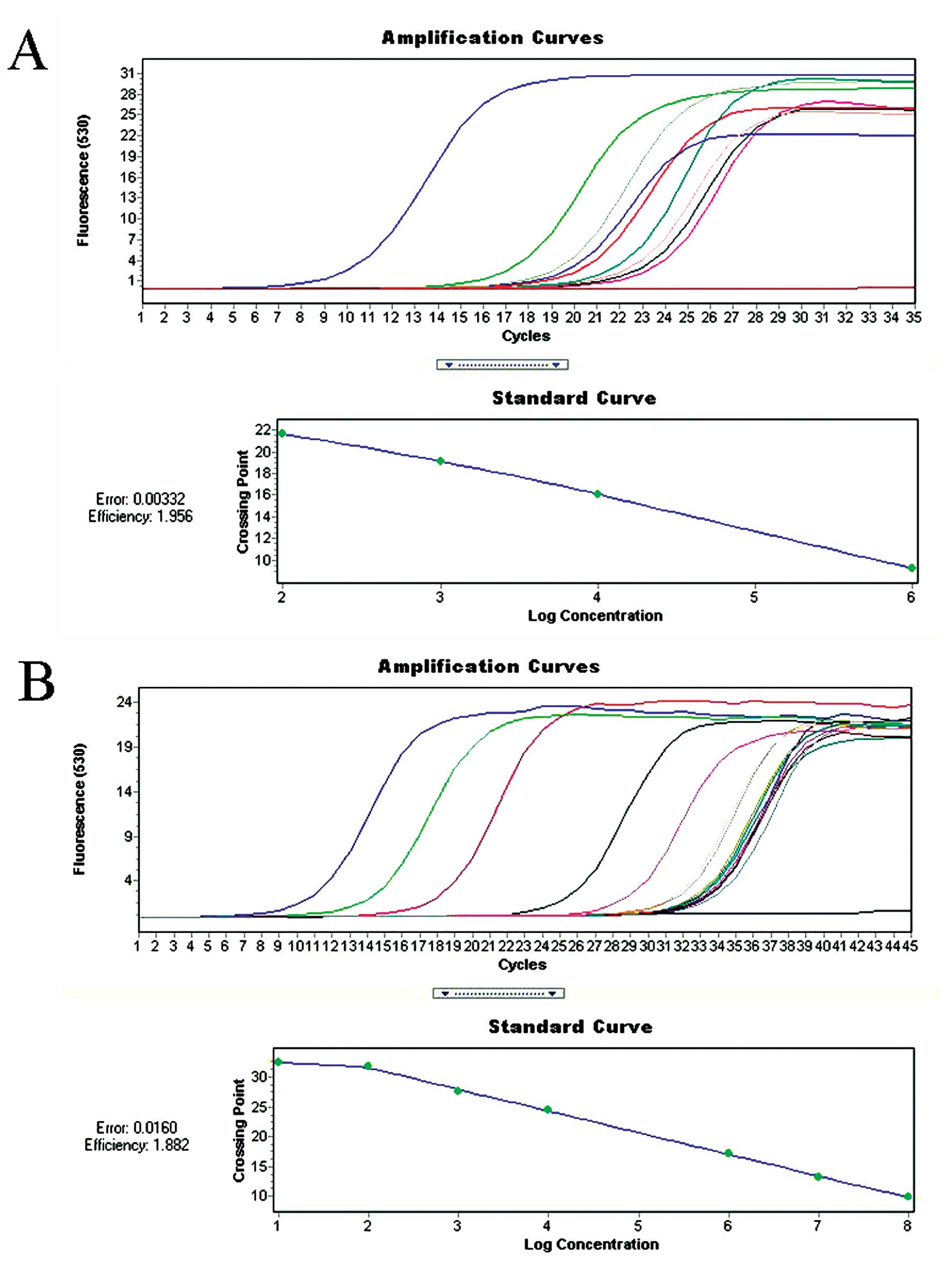
Real-time quantitative RT-PCR was performed to
analyze NK-1 receptor expression using β-actin as a control
(Fig. 3). The mean NK-1
receptor/β-actin ratio was 45±1.2, 35±3.1, 23±1.08, 63±1.27 for
BT-474, MCF-7, MDA-MB-468 and MT-3 human BC cell lines. However,
the ratio was 1.6±3.9 for HEK-293 non-tumor cell line and 1.8±1.92
for MCF-12A and 2.1±1.96 for MCF-10A epithelial breast cell lines
(Table I). Thus, the NK-1 receptor
mRNA level was ~20–60-fold higher in BC cell lines than in the
normal cell line (Table I).
 | Table IReal-time quantitative RT-PCR in BC
and normal cell lines. |
Table I
Real-time quantitative RT-PCR in BC
and normal cell lines.
| Ratio
TAC1R/β-actin |
|---|
| BT-474 | 45 |
| MCF-7 | 35 |
| MDA-MB-468 | 23 |
| MT-3 | 63 |
| HEK-293 | 1.6 |
| MCF-12A | 1.8 |
| MCF-10A | 2.1 |
Antitumor action of NK-1 receptor
antagonists (1, 2 and (2S, 3S) 3-([3, 5-Bis
(trifluoromethyl)phenyl]methoxy)-2-phenylpiperidine
(L-733,060))
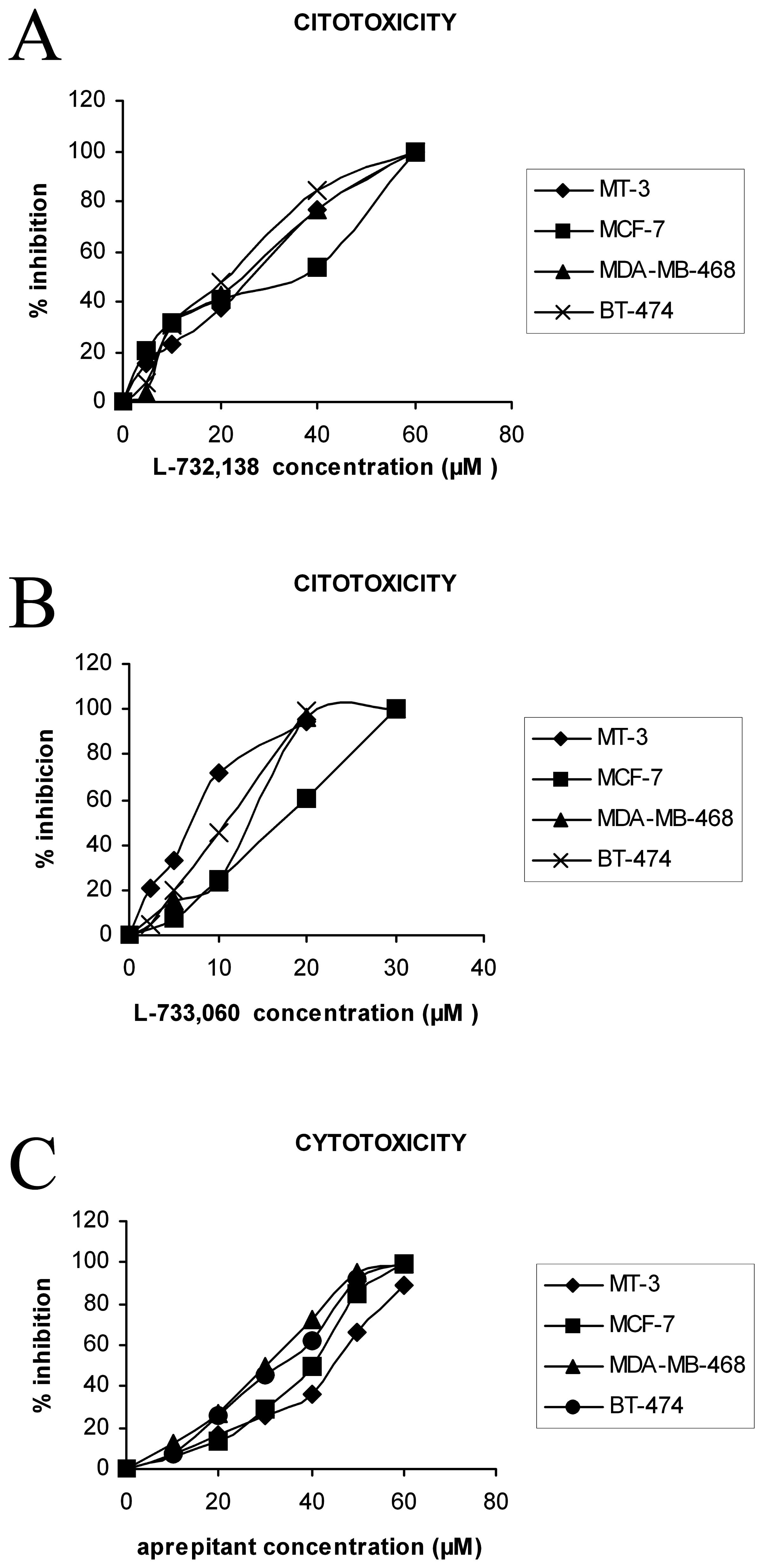
Growth inhibition of the BT-474, MCF-7, MDA-MB-468
and MT-3 human BC cell lines by different NK-1 receptor antagonists
was observed after the addition of increasing concentrations of
these antagonists (Fig. 4).
Moreover, treatment of both cell lines with the three antagonists
resulted in a concentration-dependent cytotoxicity. From Fig. 4, the IC50 (50%
inhibitory concentration) at the first doubling times can be
calculated. Thus, the concentrations required for a 50% reduction
in optical density (IC50) observed in the controls
treated with L-733,060 (L-733,060) (Fig. 1) were 10.6 μM for BT-474, 16.4 μM
for MCF-7, 13.8 μM for MDA-MB-468 and 8.4 μM for MT-3; with
L-732,138 they were 25.4 μM for BT-474, 28.8 μM for MCF-7, 27.1 μM
for MDA-MB-468 and 27.3 μM for MT-3, and with aprepitant they were
31.4 μM for BT-474, 35.6 μM for MCF-7, 29.5 μM for MDA-MB-468 and
40.8 μM for MT-3 (Table II).
Maximum inhibition was observed when the drug was present at a
concentration of 20.4 μM of L-733,060, 58 μM of L-732,138 or 59.1
μM of aprepitant (BT-474); 31 μM of L-733,060, 64.1 μM of L-732,138
or 64 μM of aprepitant (MCF-7); 27.1 μM of L-733,060, 56.8 μM of
L-732,138 or 57 μM of aprepitant (MDA-MB-468), and 18.8 μM of
733,060, 57.7 μM of L-732,138 or 75.3 μM of aprepitant (MT-3)
during the culture periods. At the first doubling time, a strong
decrease in the number of the four cell lines was found at
intermediate concentrations and with the maximum concentration no
remaining living cells were observed. A lower inhibition of growth
of the four cell lines was observed in the presence of low doses of
each antagonist. In Table II, the
IC50 and IC100 values for BC cells are
indicated.
 | Table IIHalf inhibition (IC50) and
maximum inhibition (IC100) experiments in BC cell lines
following the administration of NK-1 receptor antagonists. |
Table II
Half inhibition (IC50) and
maximum inhibition (IC100) experiments in BC cell lines
following the administration of NK-1 receptor antagonists.
| L-733,060 | L-732,138 | Aprepitant |
|---|
|
|
|
|
|---|
| IC50
(μM) | IC100
(μM) | IC50
(μM) | IC100
(μM) | IC50
(μM) | IC100
(μM) |
|---|
| BT-474 | 10.6 | 20.4 | 25.4 | 58 | 31.4 | 59.1 |
| MCF-7 | 16.4 | 31 | 28.8 | 64.1 | 35.6 | 64 |
| MDA-MB-468 | 13.8 | 27.1 | 27.1 | 56.8 | 29.5 | 57 |
| MT-3 | 8.4 | 18.8 | 27.3 | 57.7 | 40.8 | 75.3 |
| HEK-293 | 25 | 75 | 60 | 114 | 86.3 | 187.4 |
After administration of aprepitant the
IC50 for MCF-10A and MCF-12A human breast epithelial
cell lines was, in both cases, >90 μM (data not shown in
Table II).
NK-1 receptor antagonists block
SP-induced mitogen stimulation
Growth of the BT-474, MCF-7, MDA-MB-468 and MT-3
human BC cell lines was observed after the addition of SP, and
nanomolar concentrations of SP induced cell proliferation as
compared to the controls (Fig. 5
and Table III). SP stimulation
was evident at 5 nM and the maximum level was reached at 10 nM for
BT-474, MCF-7 and MT-3 and 5 nM for MDA-MB-468 (Fig. 5). This indicates that the
activation of SP receptors leads to mitogenesis in the BT-474,
MCF-7, MDA-MB-468 and MT-3 human BC cell lines. Thus, the
percentage of cell proliferation increased from 7.5 to 17.3%,
depending on the dose of SP administered (Table III). Treatment with L-733,060 (5
μM), L-732,138 (10 μM) or aprepitant (30 μM) partially inhibited
the growth of cell lines (Fig. 5).
In order to examine whether NK-1 receptor antagonists inhibited
cell proliferation via an interaction with the NK-1 receptor, we
used the specific NK-1 receptor agonist SP in competition
experiments (Table III). Thus,
the cellular concentration at 5 μM of L-733,060, 10 μM of L-732,138
or 30 μM of aprepitant and 5–10 nM of SP was higher than that
observed with NK-1 receptor antagonist alone (Fig. 5). These results indicate that
L-733,060, L-732,138 and aprepitant block SP mitogen stimulation,
since NK-1 receptor antagonist-induced growth inhibition was
partially reversed by the administration of a nanomolar dose of
exogenous SP. This indicates the specificity of tachykinin NK-1
receptor activation in the growth of the human BC cell lines, since
an increase in the cellular concentration was observed,
respectively, in BT-474, MCF-7, MDA-MB-468 and MT-3 human BC cell
lines with respect to the values found when the antagonist was
administered alone (Fig. 5). There
were no significant differences between the control and the
control-DMSO/acetonitrile (data not shown).
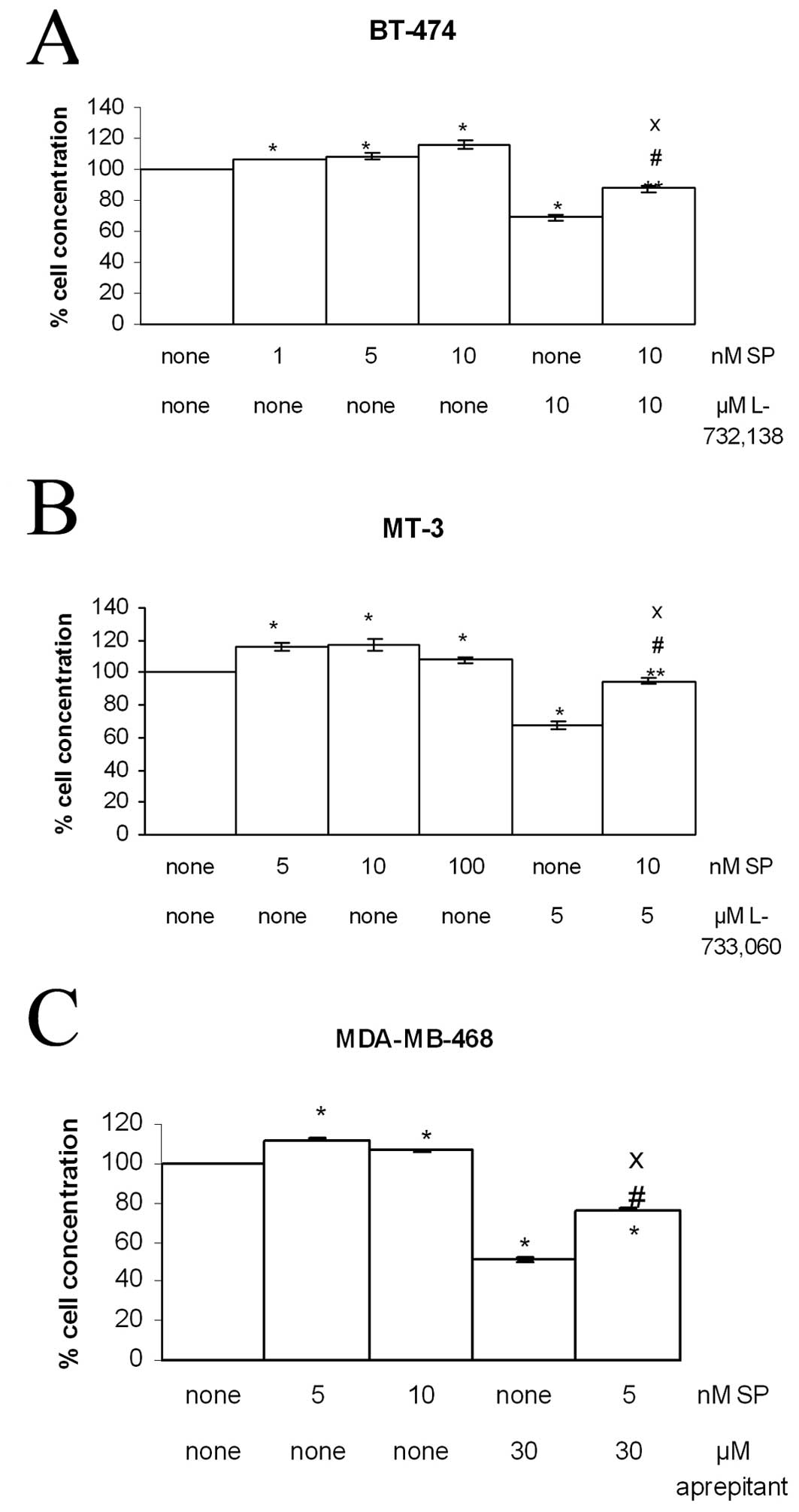 | Figure 5Induction of cell proliferation of
human BT-474, MDA-MB-468 and MT-3 BC cell lines by SP at several
nanomolar concentrations (1, 5, 10 and 100 nM). NK-1 receptor
antagonists L-733,060, L-732,138 or aprepitant were added (5, 10
and 30 μM respectively) in the presence (5 or 10 nM) or absence
(none) of SP for their first doubling time. In all cases, each NK-1
receptor antagonist inhibited human BC cell proliferation. Using
the ANOVA test, a significant difference between each group and the
control group (none-none) was found. Level of significance:
*p≤0.01. **p≤0.05. #Value of
significance of IC50 - most mitogenic SP concentration
vs. IC50 - none p<0.05; XIC50 -
most mitogenic SP concentration vs. none-most mitogenic SP
concentration, p≤0.01. Vertical bars indicate SD. |
 | Table IIIBC cell proliferation by SP and
SP/NK-1 receptor antagonist competition experiments. |
Table III
BC cell proliferation by SP and
SP/NK-1 receptor antagonist competition experiments.
| BT-474 | MCF-7 | MDA-MB-468 | MT-3 |
|---|
| Most mitogenic dose
of SP ( nM) | 10 | 10 | 5 | 10 |
| %
proliferation | 115.7 | 107.5 | 112.1 | 117.3 |
|
| BT-474 | MCF-7 | MDA-MB-468 | MT-3 |
|
| %
proliferation | 94.77 | 87.3 | 87.6 | 94.3 |
| SP/L-733,060 | (10 nM/5 μM) | (10 nM/10 μM) | (5 nM/10 μM) | (10 nM/10 μM) |
| %
proliferation | 87.5 | 95.1 | 86.3 | 93.6 |
| SP/L-732,138 | (10 nM/10 μM) | (10 nM/10 μM) | (5 nM/10 μM) | (10 nM/10 μM) |
| %
proliferation | 90.7 | 85.9 | 76.3 | 89.2 |
| SP/aprepitant | (10 nM/30 μM) | (10 nM/40 μM) | (5 nM/30 μM) | (10 nM/40 μM) |
Apoptosis
To investigate the activation of apoptotic
mechanisms, we carried out DAPI staining to assess for nuclear
morphology and percentage of death cells. After administration of
the NK-1 receptor antagonists many apoptotic cells were found in
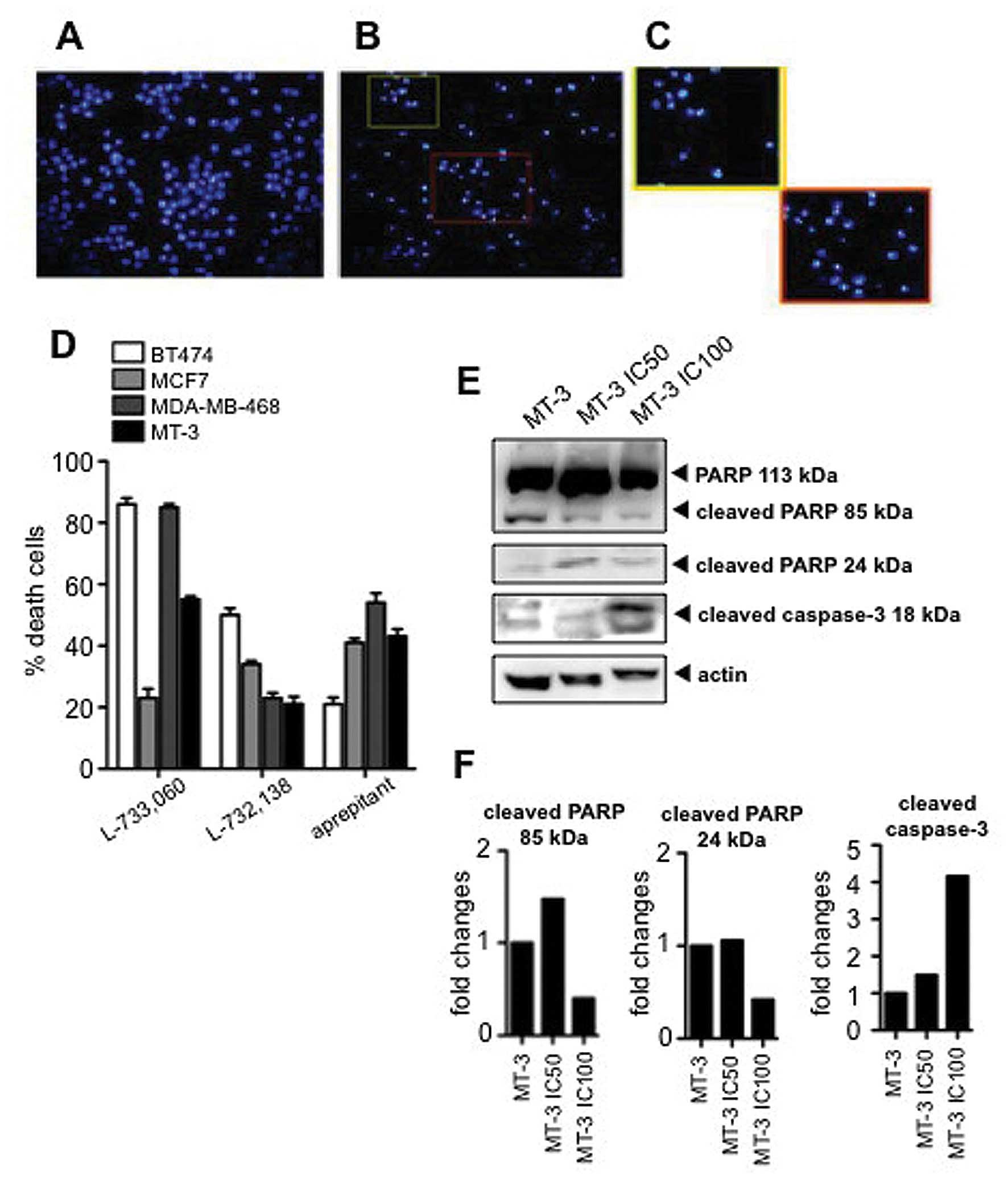
the BT-474, MCF-7, MDA-MB-468 and MT3 human BC cell lines (Fig. 6A–C). The percentage of apoptosis
after treatment was estimated by counting the number of cells with
aberrant chromatin condensation in DAPI-stained cultures. We
observed an 86±2.0, 23±3.1, 85±1.1 and 55±1.02% SD of apoptotic
cells, respectively, in BT-474, MCF-7, MDA-MB-468 and MT-3 human BC
cell lines after administration of IC100 L-733,060; and
50±2,2, 34±1.15, 23±1,7 and 21±2.4% SD of apoptotic cells in the
BT-474, MCF-7, MDA-MB-468 and MT-3 human BC cell lines
respectively, after administration of IC100 L-732,138.
Furthermore, we observed 21±2.2, 41±1.4, 54.3±3.1 and 34.3±2.34% SD
of apoptotic cells in the BT-474, MCF-7, MDA-MB-468 and MT-3 human
BC cell lines respectively after administration of IC100
aprepitant (Fig. 6D).
To further investigate whether aprepitant was able
to induce cell death by activating apoptotic mechanisms, we
determined the cleavage of PARP and the presence of cleaved
caspase-3 by western blot analysis. We observed that the treatment
with aprepitant induced an increase in the cleaved forms of PARP
(88 kDa) and an increase in the cleaved caspase-3 form (18 kDa)
(Fig. 6E and F), indicative of the
activation of apoptosis.
Knockdown gene silencing method
(siRNA)
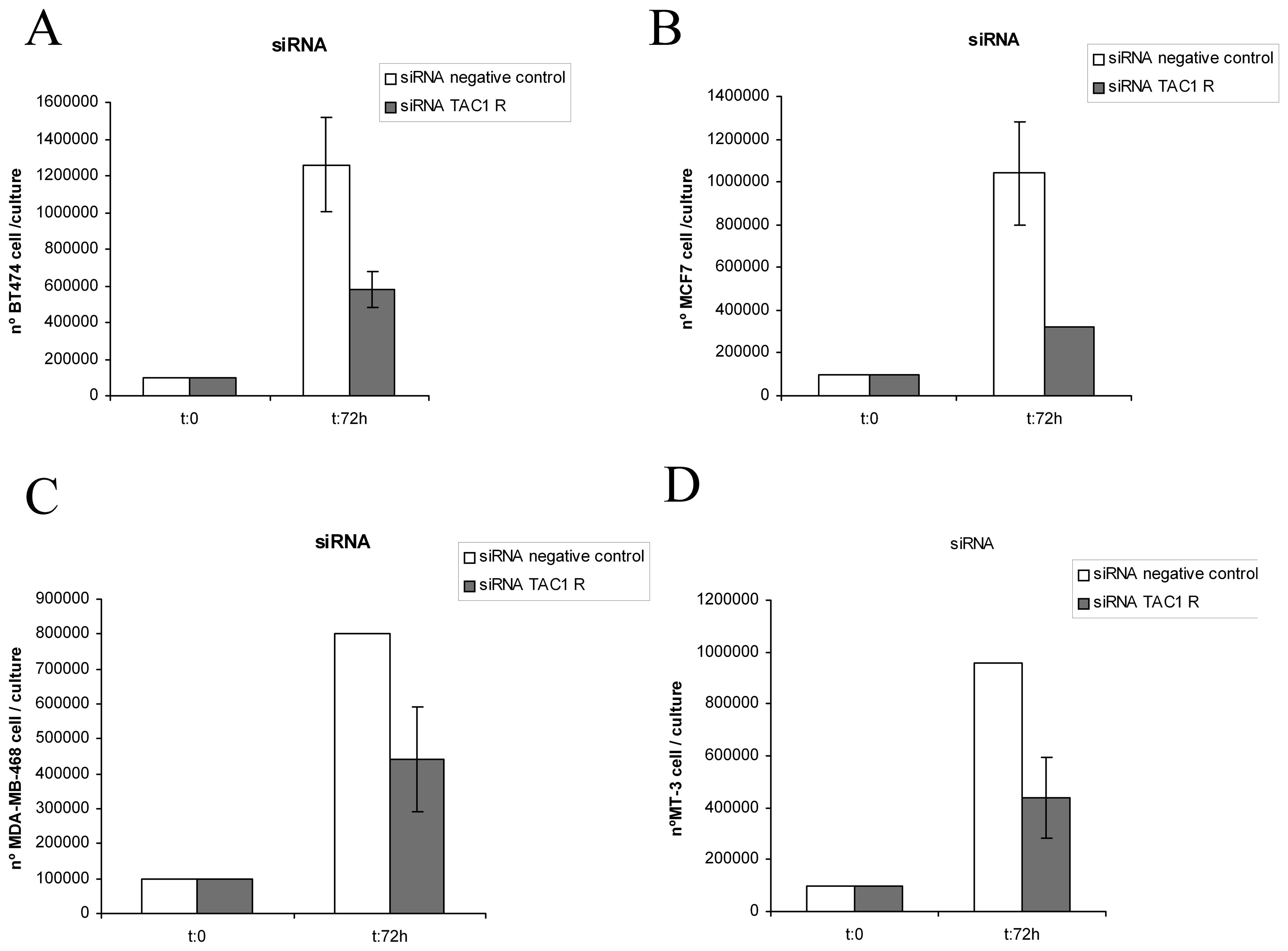
The knockdown method was carried out in BC cell
lines. Thus, after 72 h we, respectively, found
1.26×106, 1.04×106, 8×105,
9.6×105 siRNA-negative control BT-474, MCF-7, MDA-MB-468
and MT-3 BC cells and 5.8×105, 3.2×105,
4.4×105, 4.4×105 siRNA TAC1R BT-474,
MCF-7, MDA-MB-468 and MT-3 BC cells (Fig. 7). Moreover, after the
administration of siRNA TAC1R to cultured cell lines, many
apoptotic cells were found in all the wells studied (Table IV). In the DAPI-stained cultures
the mean was 39.1±1.6% (SD) of apoptotic cells. However, in the
siRNA-negative control cells, we observed means of 7.4±1.05% (SD)
(Table IV). All these data show
that NK-1 receptors play an important role in the viability of such
tumor cells.
 | Table IVMeans of apoptotic cells after the
application of the knockdown gene-silencing method. |
Table IV
Means of apoptotic cells after the
application of the knockdown gene-silencing method.
| % apoptosis
siRNA-negative | SD | % apoptosis siRNA
TAC1R | SD |
|---|
| BT-474 | 3 | 1.3 | 36 | 2.9 |
| MCF-7 | 8 | 0.6 | 37 | 1.2 |
| MDA-MB-468 | 7.6 | 1 | 31 | 1.2 |
| MT-3 | 11 | 1.3 | 52.4 | 1.3 |
NK-1 receptor- and SP-immunoreactivity in
BC
Using an immunhistochemical technique, twelve
infiltrating ductal breast carcinomas were examined for the
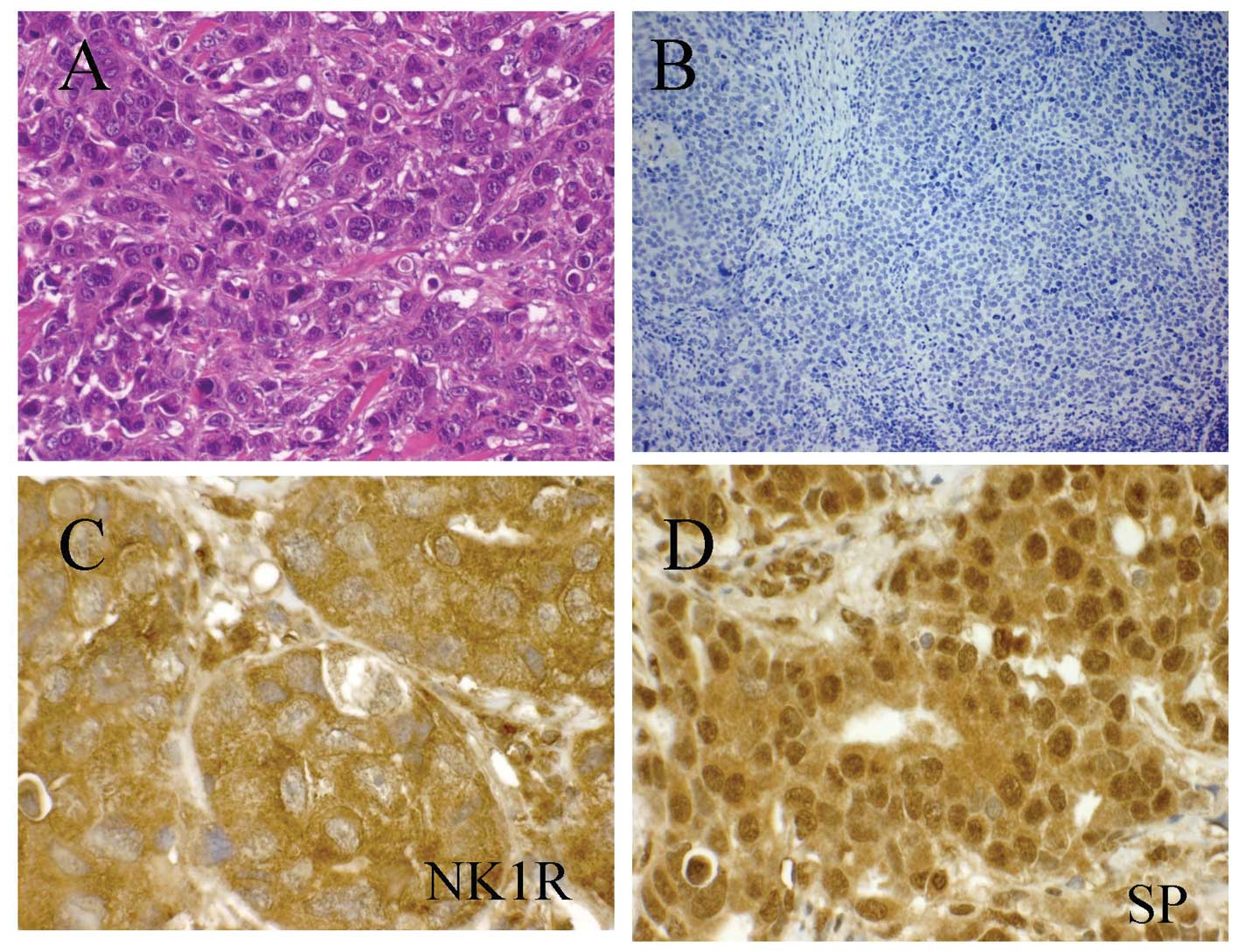
presence of NK-1 receptors and SP. The expression of NK-1 receptors
and SP was observed in all the breast carcinoma samples studied
(12/12). The immunoreactivity for the NK-1 receptor was mainly
found in the cytoplasm (Fig. 8C)
and occasionally in the nuclei of tumor cells (Table V), whereas SP-immunoreactivity was
predominantly located in the nucleus showing a strong staining
(Fig. 8D). Moreover, the
immunoreactivity for NK-1 receptors was observed in the peritumor
area, in macrophages and in plasma cells. Immunoreactivity for SP
was also observed in the nucleus of smooth muscle cells and in the
endothelial cells of the small- and medium-calibre blood vessels,
in the nucleus of macrophages located in the peritumor area and in
the nucleus and the cytoplasm of plasma cells. The clinical data of
patients included in this study, together with the results of the
evaluation of the expression of the NK-1 receptors and SP in tumor
cells are shown in Table V.
 | Table VInmunolocalization of NK-1 receptors
and SP in BC samples. |
Table V
Inmunolocalization of NK-1 receptors
and SP in BC samples.
| NK-1 receptor | SP | | |
|---|
|
|
| | |
|---|
| Cases | Nucleus | Cytoplasm | Nucleus | Cytoplasm | | Observations (tumor
stage) |
|---|
| 1 | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | I | Grade IIB tumor in
peritumor area, and III in situ, lymph node metastases |
| 20a | - | - | - | 40b | 50 | - | 40 | 40 | 60 | 20 | - | % |
| 2 | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | I | High-grade tumor
metastases in the majority of lymph nodes |
| 30a | - | - | - | 40 | 55 | 10 | 50 | 30 | 50 | 20 | - | % |
| 3 | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | I | Grade IIB tumor,
lymph node metastasis without invading adipose tissue |
| 10 | - | - | - | 70 | 10 | 30 | 30 | 5 | 70 | 10 | - | % |
| 4 | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | I | Grade III tumor,
without metastases |
| - | - | - | - | 70 | 10 | 30 | 40 | | 50 | 20 | - | % |
| 5 | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | I | High-grade tumor,
undifferentiated cells, metastasis (6 months pregnant) |
| 10a | - | - | 20 | 40 | 10 | 20 | 30 | 20 | 60 | 20 | - | % |
| 6 | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | I | Infiltrating ductal
carcinoma recurrence recurrence |
| - | - | - | 10 | 40 | 20 | 1 | 1 | - | 20 | 10 | - | % |
| 7 | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | I | Grade I tumor,
absence of metastasis |
| 20 | 10 | - | 20 | 50 | 10 | 30 | - | 10 | - | - | - | % |
| 8 | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | I | Grade III tumor,
plus tumor tissue biopsy |
| 30 | - | - | 20 | 60 | 10 | 10 | 30 | 10 | 20 | 20 | - | % |
| 9 | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | I | Grade II tumor,
rare foci of metastasis |
| 30a | 10 | - | 40 | 40 | 10b | 30 | 20 | - | - | 20 | 20 | % |
| 10 | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | I | Grade III tumor,
node metastases |
| 20a | 10 | - | - | 40 | 40 | - | 30 | 10 | 20 | 5 | - | % |
| 11 | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | I | Grade IIA tumor,
appearance of two separate tumors of 2 cm, no lymph node
metastases |
| - | - | - | 10 | 10 | - | - | 20 | 10 | 20 | 10 | - | % |
| 12 | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | I | Grade I tumor,
sentinel node metastases |
| - | - | - | 40 | - | - | - | 50 | 10 | 30 | - | - | % |
Discussion
General considerations
This study confirms and complements several aspects
previously published about the involvement of the SP/NK-1 receptor
system in BC (e.g., the presence of NK-1 receptors, the fact that
SP stimulates the proliferation of MDA-MB-231 cells, and the
overexpression of the NK-1 receptor) (9–11,24)
and also reports the following in BC for the first time: i) the
presence of NK-1 receptors in the nuclei of BC cells; ii) mRNA
expression for the NK-1 receptor and the overexpression of the NK-1
receptor in MT-3 human BC line; iii) the presence of SP in the
nuclei of BC cells; iv) the involvement of the NK-1 receptor in the
viability of BC cells after using a knockdown method; v) the
mitogenic action of SP on MT-3 human BC line; vi) the total
inhibition of BC cell growth after treatment with NK-1 receptor
antagonists (aprepitant and L-732,138), this action being due to
the apoptosis of BC cells; and vii) that the antitumor action
exerted by the two antagonists against BC cells occurs through the
NK-1 receptor concentration-dependently.
NK-1 receptor and BC
We report the presence of NK-1 receptors in BC
samples and the presence of NK-1 receptors with different molecular
weights in the BC cell lines corresponding to the different
N-glycosylation process that the receptor undergoes after synthesis
(33). Moreover, we demonstrate
that NK-1 receptors are involved in the viability of BC cell lines,
that these cell lines express mRNA for the NK-1 receptor, that
TAC1R cDNA is present in the BC cell lines studied and that
the NK-1 receptor is overexpressed in these cell lines.
Our data are partially in agreement with previous
studies in which the presence of NK-1 receptors has been reported
by immunocytochemistry in a BC cell line (T47D) and in BC samples
(10). The authors demonstrated
the presence of NK-1 receptors in the plasma membrane and/or the
cytoplasm of cancer cells. However, we observed NK-1
receptor-immunoreactivity in the cytoplasm and in the nucleus of BC
cells, but not in the plasma membrane. To date, the functional
significance of the presence of NK-1 receptors in the nucleus of BC
cells is unknown. It should be noted that we used the same
anti-NK-1 receptor antibody and methodology as that employed in
previous studies (15,23,31,34)
in which the presence of NK-1 receptors in the nuclei of tumors
cells was not observed. It should be also noted that we carried out
several controls to check the specificity of the anti-NK-1 receptor
antibody [omission of the primary antibody (immunohistochemistry
and western blot analysis), preabsorption of the primary antibody
with the corresponding synthetic peptide (immunohistochemistry),
the use of human gliomas as positive controls (immunohistochemistry
and western blot analysis)]. In all cases, the results confirmed
the specificity of the anti-NK-1 receptor antibody used in this
study. This specificity was also confirmed by the results found in
BC samples, since in the same BC sample we observed cells
expressing as well as not expressing NK-1 receptors.
We also demonstrate the presence of isoforms of
different molecular weight in BC cell lines. This is in agreement
with the results of previous studies in which the presence of these
isoforms was reported in human neuroblastoma, glioma,
retinoblastoma, pancreatic, larynx, colon and gastric carcinoma
cell lines (18,32,35–37).
Currently, the functional roles of the different isoforms of the
NK-1 receptor observed in human cancer cell lines are unknown. In
any case, it should be recalled that the different human tumor cell
lines mentioned above express the same isoforms of the NK-1
receptor.
Another important result found in our study is the
involvement of the NK-1 receptor in the viability of BC cell lines.
After eliminating NK-1 receptors by a knockdown method, we observed
that this receptor plays a crucial role in the viability of BC
cells, as has been reported previously in human melanoma and acute
lymphoblastic leukemia cell lines when using the same methodology
(16,23). This means that in the future the
NK-1 receptor could well be a target for the development of new
strategies for the treatment of cancer.
In this study, we also demonstrate that BC cell
lines express mRNA for the NK-1 receptor and that the expression of
the NK-1 receptor is higher in BC cell lines (BT-474, MT-3, MCF-7
and MDA-MB-468) than in normal breast epithelial cell lines
(MCF-10A and MCF-12A). This is in agreement with previous studies.
In this sense, the expression of preprotachykinin-I and NK-1
receptors has been reported in both human BC cells (BT-474,
MDA-MB-330, T47D, ZR-75-30, DU-4475, BT-483, 184B5) and breast
biopsies (9). The authors found
that NK-1 receptor and preprotachykinin-I mRNA levels in BC cells
were significantly increased in comparison with normal cells and
that NK-1 receptor and preprotachykinin-I expression was increased
in malignant tissues, since in benign tissues NK-1 receptor and
preprotachykinin-I mRNA were not detected (9). The data provided by Singh et
al (9) and those reported here
in BC are in agreement with other studies carried out in other
cancer cell lines. Thus, it is known that NK-1 receptors are
overexpressed in primary glioblastoma, retinoblastoma, and larynx,
pancreatic, gastric and colon carcinomas (13,15,36–38)
and that tumor samples from patients with advanced tumor stages
exhibit significantly higher NK-1 receptor levels (13). Our data are also in agreement with
the study carried out by Bigioni et al (11), in which the expression of mRNA for
the NK-1 receptor in MDA-MB-231 tumor cells was demonstrated.
Moreover, it is known that there are two naturally
occurring forms of the NK-1 receptor: the full-length and the
truncated forms. It has been recently demonstrated that the
expression of the full-length NK-1 receptor is inversely associated
with proliferation, invasiveness and metastasis of MDA-MB-231 cells
and that overexpression of the truncated NK-1 receptor promotes
tumor progression and metastasis in human breast cancer (39).
The data reported above suggest that the NK-1
receptor should be considered as a target in the treatment of BC
and that by using immunohistochemical methods NK-1 receptor
visualization should facilitate the identification of tumors with a
sufficient NK-1 receptor overexpression for diagnostic and
therapeutic intervention (e.g., using NK-1 receptor
antagonists).
SP and BC cell proliferation and
migration
In the four BC cell lines studied we show that SP
exerts a mitogenic action and that SP is located in the nucleus and
in the cytoplasm of cells in BC samples. Our results are in
agreement with a previous study in which the production of SP by BC
cells was reported (9) as well as
with other studies in which SP or [Sar9,
Met(O2)11]SP, respectively, induced the
proliferation of MDA-MB-231 and T47D BC cells (10,11).
These data are also in agreement with the mitogenic action exerted
by SP on other human cancer cell lines (3,14,18,23,30,35,36,40,41).
Moreover, it has been suggested that SP acts as an autocrine signal
essential for BC cell survival (24). All these findings suggest that the
peptide SP should act as a universal mitogenic agent in tumor
cells. It should also be remarked that SP is widely distributed in
the central and peripheral nervous systems and this means that a
new mechanism for the regulation of local tumor activity by
peripheral sensory nerves containing SP, through the NK-1 receptor,
should be taken into consideration. Moreover, it has been
demonstrated that SP protects against cell death (2,3,42);
that the inhibition of SP with antibodies impairs BC cell
proliferation (24); and that
psychological factors are also involved in the development and
progression of BC (43). It is
known that SP is expressed in the limbic system, this system being
involved in emotional behavior and hence this system could regulate
both the progression of cancer and the immune system, since all the
above data indicate that emotional behavior (e.g., depression)
(44) and BC might be related
through alterations in the SP/NK1 receptor system.
We report here for the first time the presence of SP
in the nucleus of BC cells. This is in agreement with other
studies, since SP has been reported in the nucleus of tumor cells
in keratocystic odontogenic tumors, oral squamous cell carcinoma
and larynx carcinoma tissues, and human normal placenta (15,31,34,45).
In all cases, the functional significance of SP in the nucleus of
tumor cells is currently unknown and in the future a possible
genetic neuromodulatory action of the peptide should be
investigated (45). It should be
remarked that we observed SP-immunoreactivity in the nucleus of
smooth muscle cells and in the endothelial cells of the small- and
medium-caliber blood vessels located in the peritumor area and that
immunoreactivity for NK-1 receptors was also observed in this area.
This suggests that the SP detected in the peritumor area could be
involved in the growth of capillary vessels and, if this were the
case, then endothelial cell proliferation could be blocked
specifically by NK-1 receptor antagonists (27), since it has been reported that SP
induces the growth of capillary vessels in vivo and the
proliferation of cultured endothelial cells in vitro
(25,28). It has been reported that an NK-1
receptor antagonist inhibited neoangiogenesis in tumor mass
(46). Our findings are in
agreement with previous studies, since in a large majority of the
tumors investigated, NK-1 receptors have been found in intra- and
peritumor blood vessels (10,23).
Moreover, it is known that endostatin (an inhibitor of
angiogenesis) inhibited the growth of breast cancer and potentiated
the antitumor effect of radiotherapy via alteration of the levels
of SP (47). The amount of SP
increased within 72 h after radiotherapy, but this increase was
inhibited when endostatin and radiotherapy were applied in
combination (47).
BC is the highest cause of cancer-related death in
females and although the reasons for such deaths may be varied,
most patients succumb to bone metastasis (12,48,49).
Tumor cell migration is a crucial requirement for the development
of metastasis and cancer progression. It has been suggested that
peptides promote bone marrow metastasis of BC cells (50). Thus, it has been reported that SP
induces the migration of tumor cells to specific organs by binding
to NK-1 receptors in cancer cells, where it can be blocked by NK-1
receptor antagonists (e.g., L-733,060) (26,51).
It has been reported that the migration of MDA-MB-468 BC cells
induced by SP was blocked by using an NK-1 receptor antagonist
(26). Moreover, it is known that
activation of the NK-1 receptor by SP induces a rapid change in
cellular shape in HEK-293 cells, including blebbing, this being a
consequence of the Rho-activated ROCK system, leading to an
increase in the phosphorylation of the myosin regulatory light
chain (MLC) (52). Membrane
blebbing is important in cell movement, cell spreading, and in
cancer cell invasion (51,52). All these data suggest that the
SP/NK-1 receptor system could play an important role in the
development of invasion and metastasis and that NK-1 receptor
antagonists could block the migration of tumor cells. Thus, in
order to prevent BC growth and spreading, the administration of the
drug aprepitant to patients during the perioperative period has
been suggested (5).
NK-1 receptor antagonists and BC
We demonstrate for the first time that the NK-1
receptor antagonists L-732,138 and the drug aprepitant totally
inhibit the growth of the BC cell lines studied and that these
antagonists induce the death of BC cells by apoptosis. Our results
are in consonance with previous studies, in which the NK-1 receptor
antagonist SR-140,333 reduced the growth of the T47D cell line and
induced apoptosis in this BC cell line (10); in which the NK-1 receptor
antagonist CP-96,345 reduced BC cell (T47D, BT-474, ZR-75-30,
MDA-MB330, DU4475) proliferation (9); in which the NK-1 receptor antagonist
L-733,060 decreased cell proliferation in BC cells (24); and in which the NK-1 receptor
antagonist MEN-11467 reduced the proliferation of the MDA-MB-231
cell line (11). However, it
should be remarked that these four studies using different NK-1
receptor antagonists did not show a total inhibition of BC cell
proliferation, as reported here. It should also be noted that Singh
et al (9) administered a
low dose (1 nM) of CP-96,345, whereas we used μM concentrations of
L-733,060, L-732,138 or aprepitant. Thus, using a higher dose we
observed that all BC cells died, whereas Singh et al
(9) did not, probably due to the
lower dose used. All these data are also in agreement with a study
carried out in vivo in which it was demonstrated that the
NK-1 receptor MEN-11,467 controls the growth of BC (11). Moreover, our results are in
agreement with findings showing that NK-1 receptor antagonists
(e.g., aprepitant, L-733,060) have antitumor activity against other
human cancer cell lines, such as neuroblastoma, glioma, melanoma,
retinoblastoma, pancreas, larynx, colon and gastric carcinomas,
this antitumor activity being due to apoptosis of tumor cells
(18,20,23,30,35-37).
Additionally, in cell lines as different as those mentioned above
the same NK1 receptor antagonists elicited growth inhibition. This
observation suggests the possibility of a common mechanism for
cancer cell proliferation mediated by SP and NK1 receptors. In
addition, we have demonstrated the safety of the NK-1 receptor
antagonist aprepitant against MCF-10A and MCF-12A human breast
epithelial cell lines, since in both cell lines the IC50
was >90 μM, three times higher than the IC50 for the
BC cells studied here and higher than the IC100 for such
tumor cells (Table II).
The BC cell death observed here was due to a
specific toxic effect of the NK-1 receptor antagonists used and not
to any non-specific action of these drugs, since in the competition
experiments carried out here exogenous SP cell proliferation was
partially reverted by administration of the three NK-1 receptor
antagonists studied. This demonstrates the specificity of NK-1
receptor blockade in human BC cell lines by these antagonists. This
observation is in agreement with those reported in previous studies
in other human cancer cell lines (e.g., retinoblastoma, and
melanoma) (23,26,37).
As has been suggested previously for other tumor cells, it seems
that in BC cells the blockade of NK-1 receptors by NK-1 receptor
antagonists could inhibit both DNA synthesis and cell proliferation
through the mitogen-activated protein kinase (MAPK) pathway
(14,24). NK-1 receptor antagonists could also
inhibit the formation of a β-arrestin-containing complex that
allows the nuclear translocation of ERK1/2, inhibiting
proliferation and inducing apoptosis (53). NK-1 receptor antagonists decrease
the basal phosphorylation of Akt, indicating the presence of a
constitutively active form of NK-1 receptor for eliciting apoptosis
in tumor cells and also for causing the cleavage of caspase-3 and
proteolysis of poly(ADP-ribose) polymerase (54). The latter finding is in agreement
with our results, since we have shown that aprepitant increased
both the cleaved PARP and caspase-3 forms, indicating the
activation of apoptosis. The death of tumor cells occurs after
activation of the apoptotic machinery, and this means that the
induction of apoptosis represents an appropriate method for cancer
treatment.
Tumor cells need to set up strategies to neutralize
the multiple pathways leading to cell death, and it may be proposed
that at least one of the most important is the activation and/or an
increase in the phenotypic expression of the NK-1 receptor
(19). Increased NK-1 receptor
expression renders tumor cells highly dependent on the SP stimulus,
a potent mitotic signal. The increased SP-mediated mitogenic signal
could counteract the different death-signal pathways activated in
each tumor cell due to its own genetic damage, oncogene activation,
etc. Lack of this mitogenic signal after the receptor has been
blocked with the NK-1 receptor antagonist could render the balance
inside the cell favourable to apoptotic/death signals, leading to
cell death. Thus, a number of different death signals are
overridden by the SP-mediated mitotic stimulus; by cutting the
potent mitotic signal induced by SP, NK-1 receptor antagonists
leave the cell alone with its death load or at least render the
balance between life and death signals favourable to the latter
(19). This hypothesis is in
agreement with a previous study in which the ‘oncogenic addiction’
of BC cell lines to NK-1 signalling has been suggested (24).
It is known that HER2-positive cells indicate the
presence of a protein called human epidermal growth factor receptor
2 (HER2), which promotes the growth of cancer cells. HER2-positive
BC tends to be more aggressive than other types of BC (24). Moreover, it has been reported that
SP contributes to persistent HER2 activation driving malignant
progression and drug resistance in breast cancer (55). Here we report that NK-1 receptor
antagonists exert an antitumor effect against BC cell lines
overexpressing (BT-474) (HER2-positive), or not (MCF-7)
(HER2-negative), the HER2/neu gene. In approximately 1 out of all 5
BC cases, cancer cells make an excess of HER2 due to a gene
mutation. This gene mutation and the elevated levels of HER2 that
it causes can occur in many types of cancer; not only in BC.
HER2-positive cells are less responsive to hormone treatment, but
treatments that specifically target HER2 are very effective
(24).
In conclusion, we performed an in-depth study of
the involvement of the SP/NK-1 receptor system in BC. Our findings
suggest that the NK-1 receptor is a candidate target in the
treatment of BC and that NK-1 receptor antagonists (e.g.,
aprepitant) could be novel, promising antitumor drugs in BC
therapy, since they could exert an antitumor action through three
mechanisms: i) an antiproliferative effect, due to the inhibition
of tumor cell growth, inducing cell death by apoptosis; ii) an
inhibition of angiogenesis in the tumor mass; and iii) an
inhibition of tumor cell migration (invasion and metastasis). In
the future, the antitumor action of aprepitant (already available
and widely used in clinical practice) should be tested in human
clinical trials, since the safety and tolerability of this drug has
already been demonstrated. The findings described here are not
exclusive to BC, since there are sufficient data to suggest that a
common mechanism for cancer cell proliferation mediated by SP and
the NK-1 receptor occurs and that NK-1 receptor antagonists are
broad spectrum antineoplastic drugs. This should be confirmed
definitively in the coming years.
Acknowledgements
The authors wish to thank Dr José Palacios for
providing lung cancer samples, and Dr Marisa Rosso, Mr. Manuel
Sánchez and Mr. Francisco Jesus Fuentes for technical assistance
and Mr. Nicholas Skinner for supervising the English text. This
study was supported by the Consejería de Innovacion, Ciencia y
Empresa of the Regional Government of Andalucía (CTS-2247, Spain),
by the Fondo de Investigación Sanitaria, by a grant from the
Fundación Cellex, and by Redes Temáticas de Investigación en Cáncer
(RTICC, RD07/0020/2014). This study was carried out in part at the
Esther Koplowitz Centre, Barcelona (Spain). Conflict of interest:
USPTO Application no. 20090012086 ‘Use of non-peptidic NK-1
receptor antagonists for the production of apoptosis in tumor
cells’ (Miguel Muñoz). ES patent Application no. 200801071 ‘Use of
monoclonal antibodies against substance P to treat cancer’ (Vanessa
Almendro).
References
|
1
|
Ahmedin J, Freddie B, Melissa M, Jacques
F, Elizabeth W and David F: Global cancer statistics. CA Cancer J
Clin. 61:69–90. 2011. View Article : Google Scholar
|
|
2
|
Muñoz M, Rosso M and Coveñas R: The NK-1
receptor: a new target in cancer therapy. Curr Drug Targets.
12:909–921. 2011.PubMed/NCBI
|
|
3
|
Muñoz M, Rosso M and Coveñas R: A new
frontier in the treatment of cancer: NK-1 receptor antagonists.
Curr Med Chem. 17:504–516. 2010.PubMed/NCBI
|
|
4
|
Muñoz M and Coveñas R: NK-1 receptor
antagonists: a new paradigm in pharmacological therapy. Curr Med
Chem. 17:504–513. 2011.PubMed/NCBI
|
|
5
|
Muñoz M, Rosso M, Casinello F and Coveñas
R: Paravertebral anesthesia: how substance P and the NK-1 receptor
could be involved in regional block and breast cancer recurrence.
Breast Cancer Res Treat. 122:601–603. 2010.PubMed/NCBI
|
|
6
|
Mancino M, Ametller E, Gascón P and
Almendro V: The neuronal influence on tumor progression. Biochim
Biophys Acta. 1816:105–118. 2011.PubMed/NCBI
|
|
7
|
Muñoz M, Berger M, Rosso M, et al:
Antitumor activity of neurokinin-1 receptor antagonists in MG-63
human osteosarcoma xenografts. Int J Oncol. 44:137–146.
2014.PubMed/NCBI
|
|
8
|
Berger M, Neth O, Ilmer M, et al:
Hepatoblastoma cells express truncated neurokinin-1 and can be
growth inhibited by aprepitant in vitro and in vivo. J Hepatol.
60:985–994. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Singh D, Joshi DD, Hameed M, et al:
Increased expression of preprotachykinin-I and neurokinin receptors
in human breast cancer cells: implications for bone marrow
metastasis. Proc Natl Acad Sci USA. 97:388–393. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Huang WQ, Wang JG, Chen L, Wei HJ and Chen
H: SR-140,333 counteracts NK-1 mediated cell proliferation in human
breast cancer cell line T47D. J Exp Clin Cancer Res. 29:55–62.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bigioni M, Benzo A, Irrissuto C, Maggi CA
and Goso C: Role of NK-1 and NK-2 tachykinin receptor antagonism on
the growth of human breast carcinoma cell line MDA-MB-231.
Anticancer Drugs. 16:1083–1089. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Patel HJ, Ramkissoon SH, Patel PS and
Rameshwar P: Transformation of breast cells by truncated
neurokinin-1 receptor is secondary to activation by
preprotachykinin-A peptides. Proc Natl Acad Sci USA.
102:17436–17441. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Friess H, Zhu Z, Liard V, et al:
Neurokinin-1 receptor expression and its potential effects on tumor
growth in human pancreatic cancer. Lab Invest. 83:731–742. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Luo W, Sharif TR and Sharif M: Substance
P-induced mitogenesis in human astrocytoma cells correlates with
activation of the mitogen-activated protein kinase signaling
pathway. Cancer Res. 56:4983–4991. 1996.
|
|
15
|
Esteban F, González-Moles MA, Castro D, et
al: Expression of substance P and neurokinin-1-receptor in
laryngeal cancer: linking chronic inflammation to cancer promotion
and progression. Histopathology. 54:258–260. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Muñoz M, González-Ortega A and Coveñas R:
The NK-1 receptor is expressed in human leukemia and is involved in
the antitumor action of aprepitant and other NK-1 receptor
antagonists on acute lymphoblastic leukemia cell lines. Invest New
Drugs. 30:529–540. 2010.PubMed/NCBI
|
|
17
|
Muñoz M, Pérez A, Rosso M, Zamarriego C
and Rosso R: Antitumoural action of NK1 receptor antagonist
L-733,060 on human melanoma cell lines. Melanoma Res. 14:183–188.
2004.PubMed/NCBI
|
|
18
|
Muñoz M, Rosso M, Aguilar FJ, et al: NK-1
receptor antagonists induce apoptosis and counteract substance
P-related mitogenesis in human laryngeal cancer cell line HEp-2.
Invest New Drugs. 26:111–118. 2008.PubMed/NCBI
|
|
19
|
Esteban F, Muñoz M, González-Moles MA and
Rosso M: A role for substance P in cancer promotion and
progression: a mechanism to counteract intracellular death signals
following oncogene activation or DNA damage. Cancer Metast Rev.
25:137–145. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Muñoz M and Rosso M: The NK-1 receptor
antagonist aprepitant as a broad spectrum antitumor drug. Invest
New Drugs. 28:187–193. 2010.PubMed/NCBI
|
|
21
|
Palma C, Bigioni M, Irrissuto C, et al:
Anti-tumour activity of tachykinin NK1 receptor antagonists on
human glioma U373 MG xenograft. Br J Cancer. 82:480–487.
2000.PubMed/NCBI
|
|
22
|
Palma C, Nardelli F, Manzini S and Maggi
CA: Substance P activates responses correlated with tumour growth
in human glioma cell lines bearing tachykinin NK1 receptors. Br J
Cancer. 79:236–243. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Muñoz M, Rosso M, Robles-Frías MJ, et al:
The NK-1 receptor is expressed in human melanoma and is involved in
the antitumor action of the NK-1 receptor antagonist aprepitant on
melanoma cell lines. Lab Invest. 90:1259–1269. 2010.
|
|
24
|
Mayordomo C, García-Recio S, Ametller E,
et al: Targeting of substance P induces cancer cell death and
decreases the steady state of EGFR and Her2. J Cell Physiol.
227:1358–1366. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ziche M, Morbidelli L, Pacini M, et al:
Substance P stimulates neovascularization in vivo and proliferation
of cultured endothelial cells. Microvasc Res. 40:264–278. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lang K, Drell TL, Lindecke A, et al:
Induction of a meta-statogenic tumor cell type by neurotransmitters
and its pharmacological inhibition by established drugs. Int J
Cancer. 112:231–238. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fan TP, Hu DE, Guard S, Gresham GA and
Watling KJ: Stimulation of angiogenesis by substance P an
interleukin-1 in the rat and its inhibition by NK1 or interleukin-1
receptor antagonists. Br J Pharmacol. 110:43–49. 1993. View Article : Google Scholar
|
|
28
|
Seegers HC, Hood VC, Kidd BL, Cruwys SC
and Walsh DA: Enhancement of angiogenesis by endogenous substance P
release and neurokinin-1 receptors during neurogenic inflammation.
J Pharmacol Exp Ther. 306:8–12. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Muñoz M, Bernabeu-Wittel J and Coveñas R:
NK-1 as a melanoma target. Expert Opin Ther Targets. 15:889–897.
2011.
|
|
30
|
Muñoz M, Rosso M, Pérez A, et al: The NK-1
receptor is involved in the antitumoural action of L-733,060 and
the mitogenic action of substance P on neuroblastoma and glioma
cell lines. Neuropeptides. 39:427–432. 2005.PubMed/NCBI
|
|
31
|
Brener S, González-Moles MA, Tostes D, et
al: A role for the substance P/NK-1 receptor complex in cell
proliferation in oral squamous cell carcinoma. Anticancer Res.
29:2323–2329. 2009.PubMed/NCBI
|
|
32
|
Moneo V, Serelde BG, Leal JFM, et al:
Levels of p27kip1 determine Aplidin sensitivity. Mol
Cancer Ther. 6:1310–1316. 2007.
|
|
33
|
Tansky MF, Pothoulakis C and Leeman SE:
Functional consequences of alteration of N-linked glycosylation
sites on the neurokinin-1 receptor. Proc Natl Acad Sci USA.
104:10691–10696. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
González Moles MA, Mosqueda-Taylor A,
Esteban F, et al: Cell proliferation associated with actions of the
SP/NK-1 receptor complex in keratocystic odontogenic tumours. Oral
Oncol. 44:1127–1133. 2008.PubMed/NCBI
|
|
35
|
Muñoz M, Rosso M and Coveñas R: The NK-1
receptor is involved in the antitumoural action of L-733,060 and in
the mitogenic action of substance P on human pancreatic cancer cell
lines. Lett Drug Des Discov. 3:323–329. 2006.PubMed/NCBI
|
|
36
|
Rosso M, Robles-Frías MJ, Coveñas R,
Salinas-Martín MV and Muñoz M: The NK-1 receptor is involved in the
antitumor action of L-733,060 and in the mitogenic action of
substance P on human gastrointestinal cancer cell lines. Tumor
Biol. 29:245–254. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Muñoz M, Rosso M, Coveñas R, et al:
Neurokinin-1 receptors located in human retinoblastoma cell lines:
antitumor action of its antagonists, L-732,138. Invest Ophthalmol
Vis Sci. 48:2775–2781. 2007.PubMed/NCBI
|
|
38
|
Hennig IM, Laissue JA, Horisberger U and
Reubi JC: Substance-P receptors in human primary neoplasms: tumor
and vascular localization. Int J Cancer. 61:786–792. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhou Y, Zhao L, Xiong T, et al: Roles of
full-length and truncated neurokinin-1 receptors on tumor
progression and distant metastasis in human breast cancer. Breast
Cancer Res Treat. 140:49–61. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Muñoz M, Rosso M, Pérez A, et al:
Antitumor action of the neurokinin-1-receptor antagonist L-733,060
and mitogenic action of substance P on human retinoblastoma cell
lines. Invest Ophthalmol Vis Sci. 46:2567–2570. 2005.PubMed/NCBI
|
|
41
|
Muñoz M, Rosso M, González-Ortega A and
Coveñas R: The NK-1 receptor antagonist L-732,138 induces apoptosis
and counteracts substance P-related mitogenesis in human melanoma
cell lines. Cancers. 2:611–623. 2010.PubMed/NCBI
|
|
42
|
Dimri R, Sharabi Y and Shoham J: Specific
inhibition of glucocorticoid thymocyte apoptosis by substance P. J
Immunol. 164:2479–2486. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hilakivi-Clarke L, Rowland J, Clarke R and
Lippman ME: Psychosocial factors in the development and progression
of breast cancer. Breast Cancer Res Treat. 29:141–160. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
De Vane CL: Substance P: a new era, a new
role. Pharmacotherapy. 21:1061–1069. 2001.
|
|
45
|
Muñoz M, Pavón A, Rosso M, et al:
Immunolocalization of NK-1 receptor and substance P in human normal
placenta. Placenta. 31:649–651. 2010.PubMed/NCBI
|
|
46
|
Guha S, Eibl G, Kisfalvi K, et al:
Broad-spectrum G protein-coupled receptor antagonist,
[D-Arg1,DTrp5,7,9,Leu11]SP: a dual inhibitor of growth and
angiogenesis in pancreatic cancer. Cancer Res. 65:2738–2745.
2005.
|
|
47
|
Arslan Aydemir E, Simsek Oz E, Fidan
Korcum A and Fiskin K: Endostatin enhances radioresponse in breast
cancer cells via alteration of substance P levels. Oncol Lett.
2:879–886. 2011.PubMed/NCBI
|
|
48
|
Jemal A, Murray T, Samuels A, et al:
Cancer statistics, 2003. CA Cancer J Clin. 53:5–26. 2003.
View Article : Google Scholar
|
|
49
|
Mundy GR: Metastasis to bone: causes,
consequences and therapeutic opportunities. Nat Rev Cancer.
2:584–593. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
50
|
Rao G, Patel GS, Idler SP, et al:
Facilitating role of preprotachykinin- I gene in the integration of
breast cancer cells within the stromal compartment of the bone
marrow: a model of early cancer progression. Cancer Res.
64:2874–2881. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Meshki J, Douglas SD, Lai JP, et al:
Neurokinin 1 receptor mediates membrane blebbing in HEK293 cells
through a Rho/Rho-associated coiled-coil kinase dependent
mechanism. J Biol Chem. 284:9280–9289. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Fackler OT and Grosse R: Cell motility
through plasma membrane blebbing. J Cell Biol. 181:879–884. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
DeFea KA, Vaughn ZD, O’Bryan EM, et al:
The proliferative and antiapoptotic effects of substance P are
facilitated by formation of a beta-arrestin-dependent scaffolding
complex. Proc Natl Acad Sci USA. 97:11086–11091. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Akazawa T, Kwatra SG, Goldsmith LE, et al:
A constitutively active form of neurokinin 1 receptor and
neurokinin 1 receptor-mediated apoptosis in glioblastomas. J
Neurochem. 109:1079–1086. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
García-Recio S, Fuster G,
Fernández-Nogueira P, et al: Substance P autocrine signaling
contributes to persistent HER2 activation that drives malignant
progression and drug resistance in breast cancer. Cancer Res.
73:6424–6434. 2013.PubMed/NCBI
|