Introduction
The transcription factor NF-κB regulates the
expression of hundreds of target genes involved in immune response,
inflammation, cell proliferation and survival (1–3).
Mammals express five NF-κB family proteins: NF-κB1 (p105/p50),
NF-κB2 (p100/p52), RelA (p65), RelB and c-Rel. NF-κB1 and NF-κB2
are produced as precursor proteins, p105 and p100, which are then
processed into p50 and p52, respectively. While the processing of
p105 to p50 appears to be constitutive, the processing of p100 to
p52 is regulated. The NF-κB proteins form various homo- and
hetero-dimers to generate functional NF-κB. In most normal
unstimulated cells, NF-κB dimers are retained in the cytoplasm by
IκB proteins. The IκB proteins interact, through their ankyrin
repeats, with the Rel homology region (RHD) of NF-κB dimers. Such
ankyrin repeats are also present in unprocessed NF-κB1 (p105) and
NF-κB2 (p100), which act as IκB proteins that retain the Rel
proteins in the cytoplasm (2,4,5).
Two NF-κB activation pathways, the classical and the
alternative pathways, have been extensively characterized. In the
classical NF-κB pathway (also known as the canonical pathway), the
activated IκB kinase (IKK) complex, consisting of the catalytic
subunits IKKα and IKKβ and the regulatory subunit IKKγ (also called
NEMO), phosphorylates IκB proteins. This triggers IκB
polyubiquitination and its subsequent degradation by the 26S
proteasome. IKKβ and IKKγ are essential for the classical NF-κB
activation pathway, while IKKα seems dispensable for the activation
of this pathway in response to most tested stimuli. On the other
hand, activation of the alternative NF-κB pathway (also known as
the non-canonical pathway) depends on IKKα, but neither IKKβ nor
IKKγ. In this alternative pathway, the active IKKα homodimer
induces phosphorylation of NF-κB2/p100. The phosphorylated p100 is
then polyubiquitinated and processed into p52. Activation of the
classical NF-κB pathway predominantly results in active p50/RelA
and p50/c-Rel dimers, while the alternative pathway leads to
selective release of the p52/RelB complex. The released NF-κB
dimers then translocate into the nucleus, bind target gene
promoters and activate gene transcription (2,4,5).
Numerous NF-κB-activating cascades induced by
extracellular stimuli converge on the activation of IKKα and IKKβ
(2,4,5).
This activation is regulated by the phosphorylation of two
conserved serine residues (Ser176 and 180 in IKKα and
Ser177 and 181 in IKKβ) located in their activation
loops (6–8). Despite great progress in the
understanding of signaling pathways controlling NF-κB activation,
the molecular mechanisms underlying activation of IKK complexes
induced by various signaling molecules remain to be fully
elucidated.
Protein kinase PKK (also known as DIK and RIP4) was
originally identified as a protein kinase Cβ and δ interacting
protein (9,10). We previously showed that PKK
physically interacts with co-transfected PKCβ and can be
phosphorylated by PKCβ in vitro, suggesting that it may be a
downstream target of PKCβ (10).
We also reported that PKK affects cell survival and BAFF-mediated
IKK phosphorylation and NF-κB activation in DLBCL (34). PKK belongs to the RIP kinase family
and shares high sequence homology at the N-terminal kinase domain
with other members of this gene family (9–11).
Similar to other members of the RIP family, PKK was shown to
activate NF-κB in transient transfection assays (12–14).
Moreover, catalytically inactive mutants of PKK can block NF-κB
reporter activation (13,14). These results suggest that PKK
regulates NF-κB activation. It was reported that PKK-induced NF-κB
reporter activation is inhibited by dominant-negative IKKα and IKKβ
and that PKK failed to activate the NF-κB reporter in mouse
embryonic fibroblasts (MEFs) deficient in IKKβ, suggesting that
NF-κB activation by PKK requires IKK activity (14). How PKK regulates NF-κB activation
has, however, remained elusive.
Here we have investigated the molecular mechanism by
which PKK regulates NF-κB activation. Our results indicate that PKK
activates NF-κB through both the classical and the alternative
pathways by inducing phosphorylation of both IKKα and IKKβ. In
addition, we show that PKK induces IKK activation primarily in a
kinase-dependent manner.
Materials and methods
Cell culture, antibodies, and expression
plasmids
HEK293, HEK293T and HeLa cells were cultured in
Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% of
fetal bovine serum. Human diffuse large B cell lymphoma (DLBCL)
SUDHL-6 cells were cultured in RPMI with 10% fetal bovine serum.
Antibodies against IKKα, IKKβ, p65, Rel-B, Bcl2, Mcl-1 and MCM7
were from Santa Cruz Biotechnology, Inc. Antibody specific for
human p100/p52 was from Upstate Biotechnologies. Antibodies against
IκBα, phospho-IκBα (p-Ser32), phospho-IKKα
(p-Ser180)/IKKβ (p-Ser181), and phospho-IKKα
(p-Ser176/180)/IKKβ (p-Ser177/181) were from
Cell Signaling Technology. Anti-HA tag antibody was from Covance
Research Products. Antibodies specific for γ-tubulin and Flag-tag
were from Sigma. Rabbit polyclonal anti-PKK antibody was raised
against the peptide AHINLQSLKFQGGHGPAATLL (amino acids 759–779 of
human PKK).
The pCMV5 plasmids expressing Flag-tagged human PKK,
PKK-N and the catalytically inactive mutants of Flag-PKK (D143A)
and Flag-PKK (K51R) were described previously (10,13).
Expression plasmid for the N-terminal HA-tagged p100 was kindly
provided by Dr Shao-Cong Sun. Expression plasmids for Flag-IKKα,
Flag-IKKβ, Flag-IKKα (KA) and Flag-IKKβ (KA) were kindly provided
by Dr David Goeddel (15).
Plasmids for HA-IKKα and HA-IKKβ were generous gifts from Dr
Michael Karin (8).
Transfection, immunoprecipitation,
western blot analysis and in vitro kinase assays
The transfection, preparation of cell lysates,
immunoprecipitation and western blot analysis were performed as
previously described (10,16). For in vitro kinase assays,
HEK293T cells were transfected with plasmids expressing the
indicated Flag-tagged proteins. Forty hours post-transfection, the
cell lysates were immunoprecipitated with the anti-Flag antibody
(M2). The in vitro kinase assays were carried out in the
presence of [γ-32P]ATP essentially as previously
described (10,16). The kinase reaction products were
analyzed by autoradiography following SDS-polyacrylamide gel
electrophoresis.
RNA interference
To knockdown the expression of PKK in cultured human
cell lines, we employed RNA interference with small hairpin RNAs
(shRNAs) (17,18). Two targeting sequences (shPKK-a:
5′-GCTAGTGATGCATCATATC; and shPKK-b: 5′-TACCTCACTCACGAGA ) were
selected and the PKK-specific shRNAs were expressed from the
pRetro-H1 vector (Cellogenetics Inc.). The control shRNA construct
(shControl) carries a random hairpin sequence:
5′-GTTCTCCGAACGAACGTGTCACG. To transiently suppress PKK expression,
HEK293 and HeLa cells were transfected with an shPKK and shControl.
Twenty-four hours after transfection, puromycin (2 μg/ml, the
vector carries a puromycin-resistant gene) was added into the
culture medium for 4 days to select transfected (PKK knocked-down)
cells. To generate SUDHL-6 cells with PKK expression being stably
suppressed, we infected SUDHL-6 cells with retroviruses expressing
an shPKK or shControl. The transduced cells were selected with
puromycin (0.5 μg/ml) for at least two weeks.
Results
Expression of PKK can activate the
alternative NF-κB pathway as well as the classical NF-κB
pathway
It was previously shown that overexpression of PKK
activated NF-κB reporters and induced IκBα phosphorylation,
suggesting that PKK can activate the classical NF-κB pathway
(12–14). Whether PKK activates the
alternative NF-κB pathway has not been explored. To address this
issue, we transfected Flag-tagged PKK alone or together with IKKα,
which is known to be involved in activation of the alternative
pathway, into HEK293T cells and analyzed the processing of p100
(NF-κB2) to p52. As shown in Fig.
1A, while overexpression of PKK modestly increased level of
endogenous p52, co-expression of PKK with IKKα greatly increased
level of endogenous p52 with a concomitant decrease in the level of
endogenous p100 (Fig. 1).
Co-expression of PKK and IKKα also resulted in significant
processing of the exogenous p100 into p52 (Fig. 1B). These results indicate that PKK,
together with IKKα, can activate the alternative NF-κB pathway.
Consistent with the activation of the alternative pathway, nuclear
accumulation of endogenous RelB was increased following PKK
overexpression (Fig. 1C). We also
examined the effect of PKK overexpression on activation of the
classical NF-κB pathway in our assays. Overexpression of PKK
resulted in phosphorylation of endogenous IκBα (Fig. 1D), consistent with the previously
reported results (12). In
addition, we observed a concurrent decrease in the protein level of
the endogenous IκBα and an increase in nuclear translocation of p65
(Fig. 1B), hallmarks of activation
of the classical NF-κB pathway. Together, these results indicate
that PKK can induce NF-κB activation through both the classical and
the alternative pathways.
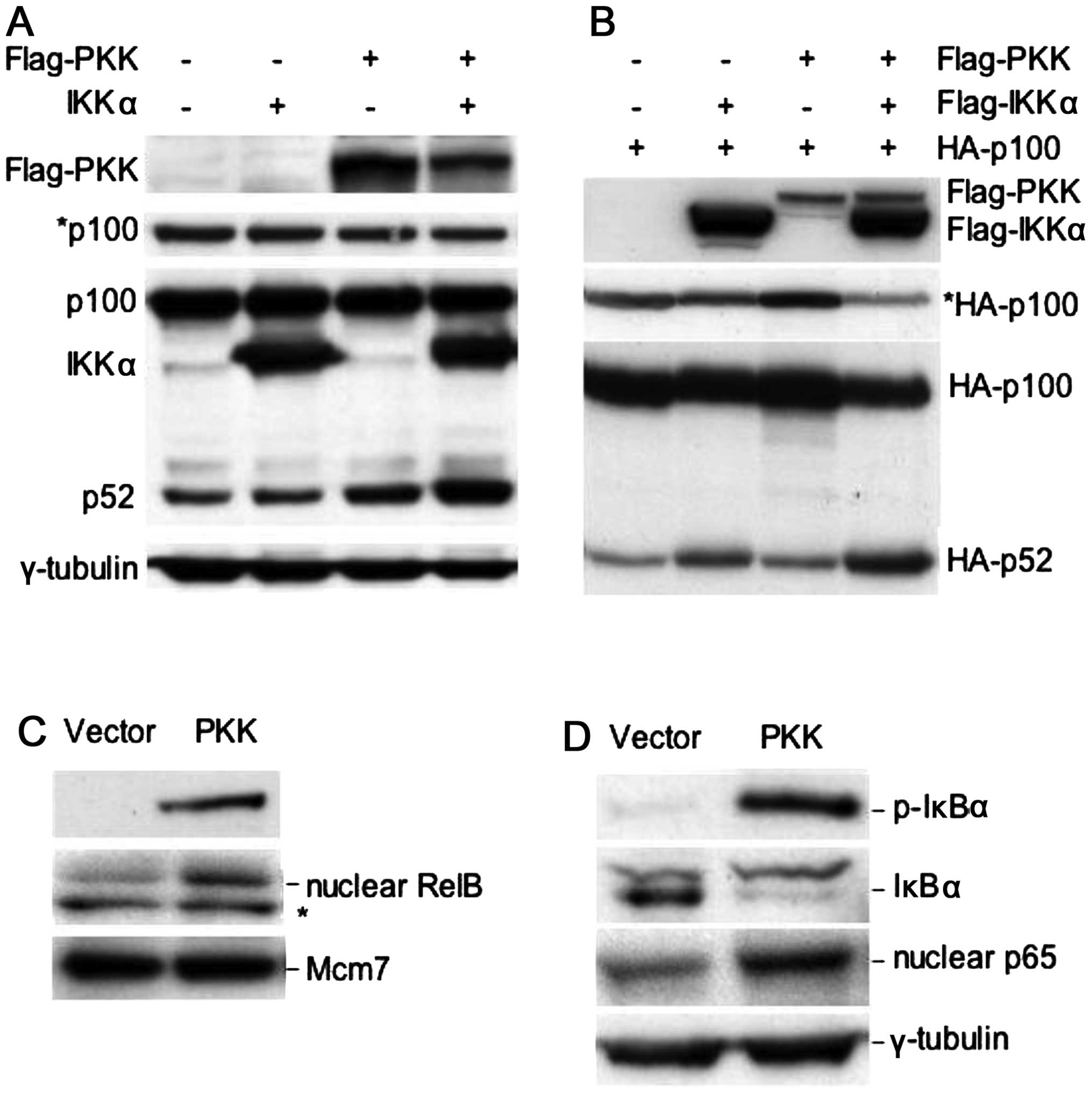 | Figure 1PKK can activate the alternative
NF-κB pathway as well as the classical NF-κB pathway. (A)
Overexpression of PKK, together with IKKα, induces processing of
endogenous p100 to p52. HEK293T cells were transfected with
plasmids expressing the indicated proteins, together with a plasmid
carrying a puromycin-resistant gene. Twenty-four hours
post-transfection, puromycin (1.5 μg/ml) was added into the culture
medium to select transfected cells for 2 days. Expression or
phosphorylation of the indicated proteins was analyzed on western
blots. *p100, a shorter exposure of the p100/p52 blot,
showing a decrease in the level of p100 following transfection of
PKK and IKKα. (B) PKK cooperates with IKKα to induce processing of
the co-transfected p100. HEK293T cells were transfected with the
plasmid expressing N-terminal HA-tagged p100 alone or together with
the indicated expression plasmids. Twenty-four hours
post-transfection, the protein levels of p100 and p52 were analyzed
using an anti-HA antibody. *HA-p100, a shorter exposure
of the HA-p100/HA-p52 blot, showing a decrease in the level of
HA-p100 following co-transfection with PKK and IKKα. (C)
Overexpression of PKK induces nuclear accumulation of RelB. HEK293T
cells were transfected with the plasmid vector pCMV5 or
pCMV5-Flag-PKK. Forty-eight hours post-transfection, total cellular
extracts or nuclear extracts were prepared. The levels of
transfected Flag-PKK in total cellular extracts, and the levels of
RelB and Mcm7 (which was used as the loading control for nuclear
proteins) were analyzed by western blotting. *A protein
that cross-reacts with the RelB antibody. (D) Overexpression of PKK
activates the classical NF-κB pathway. Expression of the indicated
proteins from total or nuclear extracts prepared as described in
(C) was analyzed by western blotting. p-IκBα, phosphorylated IκBα
(phospho-Ser32). |
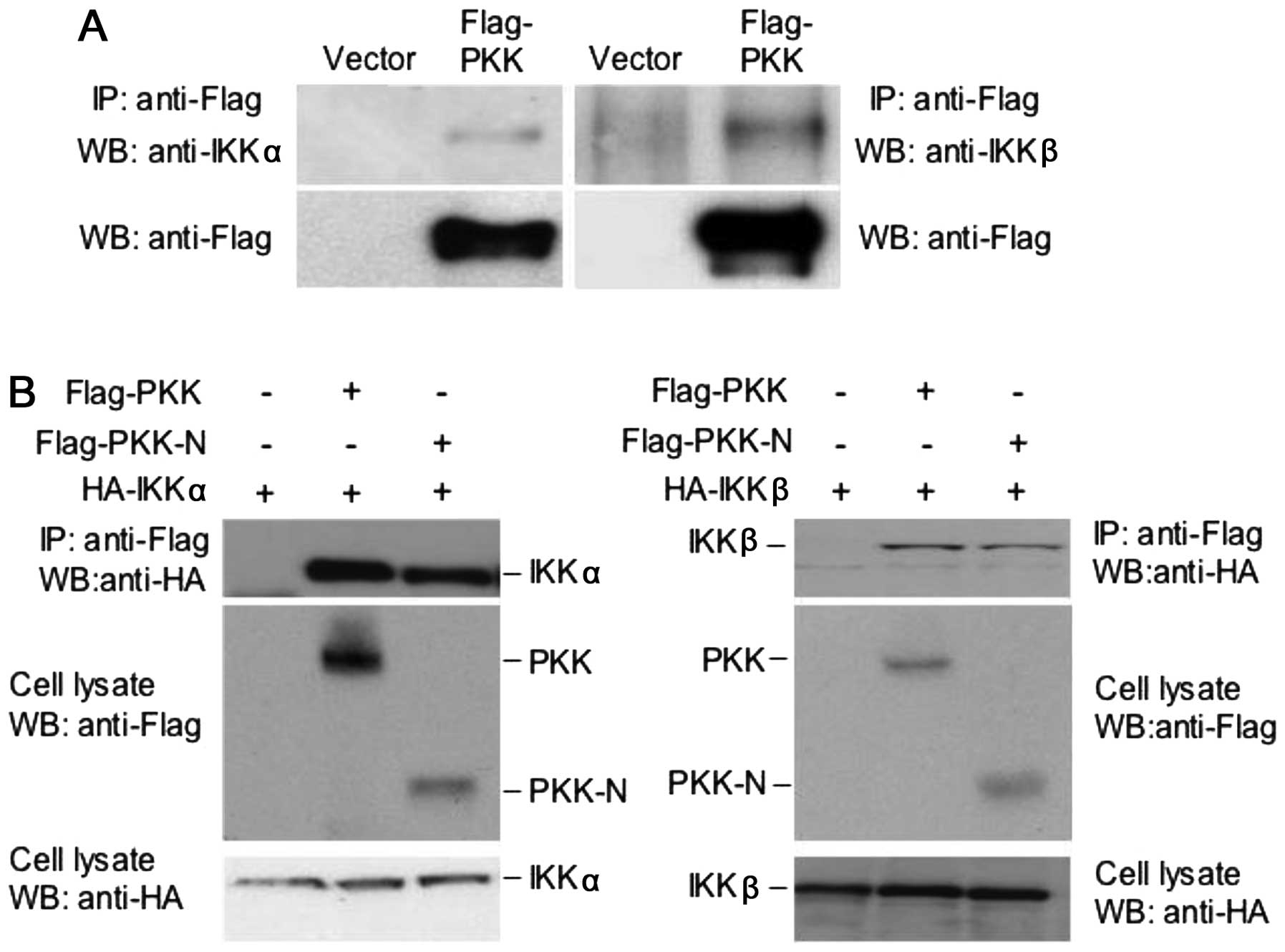
PKK interacts with IKKα and IKKβ
A crucial regulatory step in the activation of NF-κB
pathways is the activation of IKK complexes (4,5,19).
It was previously reported that activation of an NF-κB reporter by
PKK overexpression was inhibited by dominant-negative mutants of
IKKα and IKKβ and that PKK-mediated activation of the NF-κB
reporter was diminished in mouse embryo fibroblasts deficient in
IKKβ protein (14). These results
suggest that NF-κB activation induced by PKK requires IKK activity.
These observations, however, did not address directly whether PKK
works upstream of IKKs or in parallel with IKKs to induce NF-κB
activation. To investigate the relationship between PKK and IKKα
and IKKβ, we examined whether PKK interacts with IKKα and IKKβ. We
transfected HEK293T cells with the plasmid expressing Flag-tagged
full-length PKK. The interaction between Flag-PKK with the
endogenous IKKα and IKKβ was determined by co-immunoprecipitation
experiments. Data presented in Fig.
2A show that both IKKα and IKKβ are present in the PKK
immunoprecipitates, suggesting that PKK interacts with IKKα and
IKKβ in mammalian cells. While PKK-N, which contains only the
N-terminal kinase domain of PKK protein (aa1–320) and is capable of
activating NF-κB (13), interacts
with IKKα or IKKβ (Fig. 2B), the
C-terminal domain of PKK (aa461–786) showed no interaction with IKK
proteins (data not shown). These results indicate that the kinase
domain of PKK mediates its association with IKKα and IKKβ.
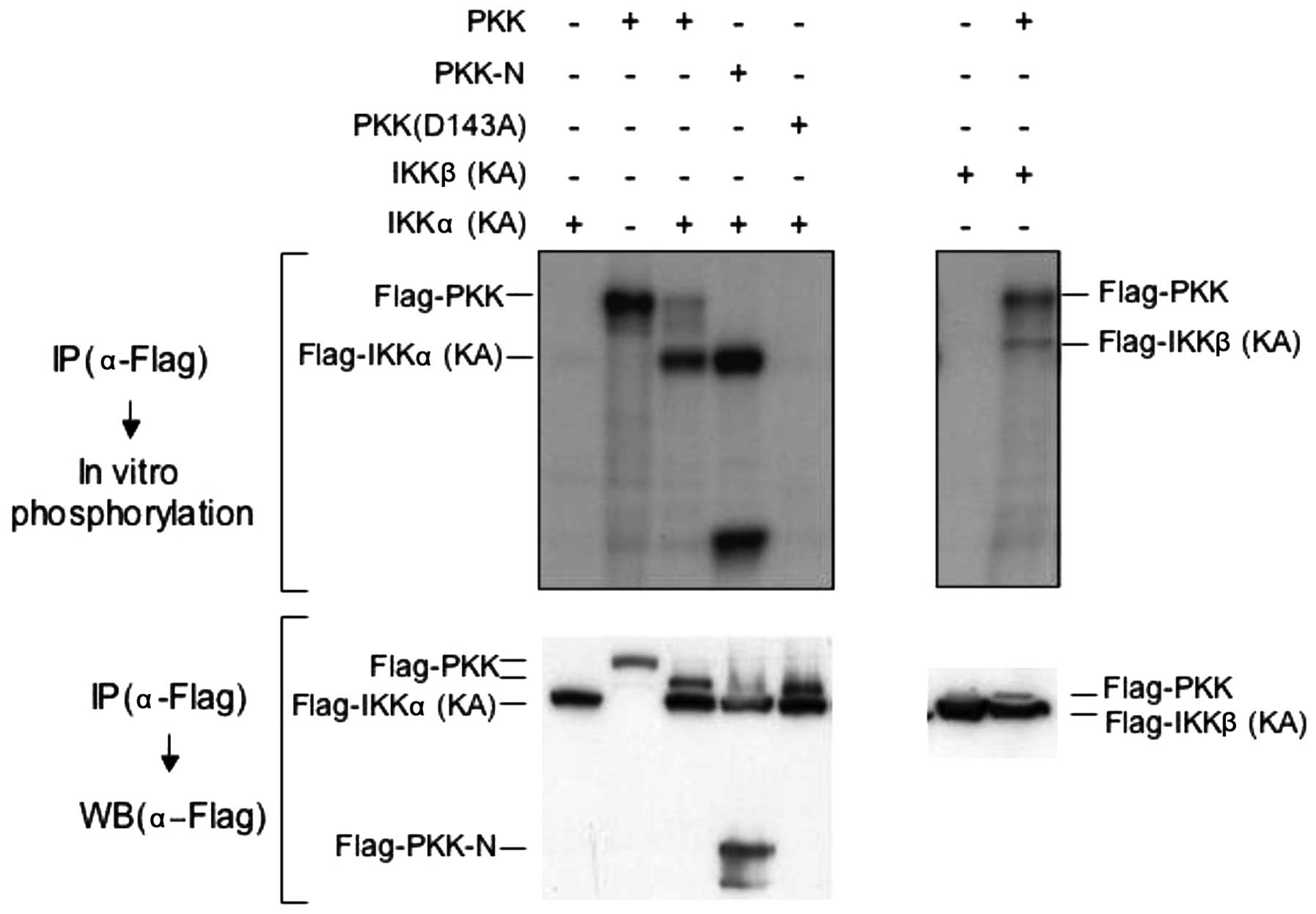
PKK induces phosphorylation of IKKα and
IKKβ in vitro and in vivo
The observation that PKK associates with IKKα and
IKKβ prompted us to explore whether PKK acts upstream of IKKs. We
first examined whether PKK can induce phosphorylation of IKKα and
IKKβ in vitro. We transfected Flag-tagged PKK with a
Flag-tagged kinase-inactive mutant of IKKα [IKKα (KA)] or IKKβ
[IKKβ (KA)] (15) in HEK293T
cells, and immunoprecipitated both PKK and IKKs with the antibody
specific for the Flag-tag. Phosphorylation of the kinase-inactive
IKK mutants in the immunoprecipitates was then carried out in
vitro in the presence of [γ-32P]ATP. The
kinase-inactive IKK mutants were used in this experiment to avoid
their autophosphorylation so that phosphorylation of IKKs induced
by PKK could be easily and unambiguously detected. As shown in
Fig. 3, both PKK and PKK-N induced
phosphorylation of IKKα and IKKβ in vitro, in addition to
their autophosphorylation as we previously reported (10). In contrast, the
catalytically-inactive PKK (D143A), although expressed at similar
levels as PKK and PKK-N, was unable to induce the phosphorylation
of co-immunoprecipitated IKKs (Fig.
3, and data not shown). Thus, PKK induces IKK phosphorylation
in a kinase-dependent manner in vitro.
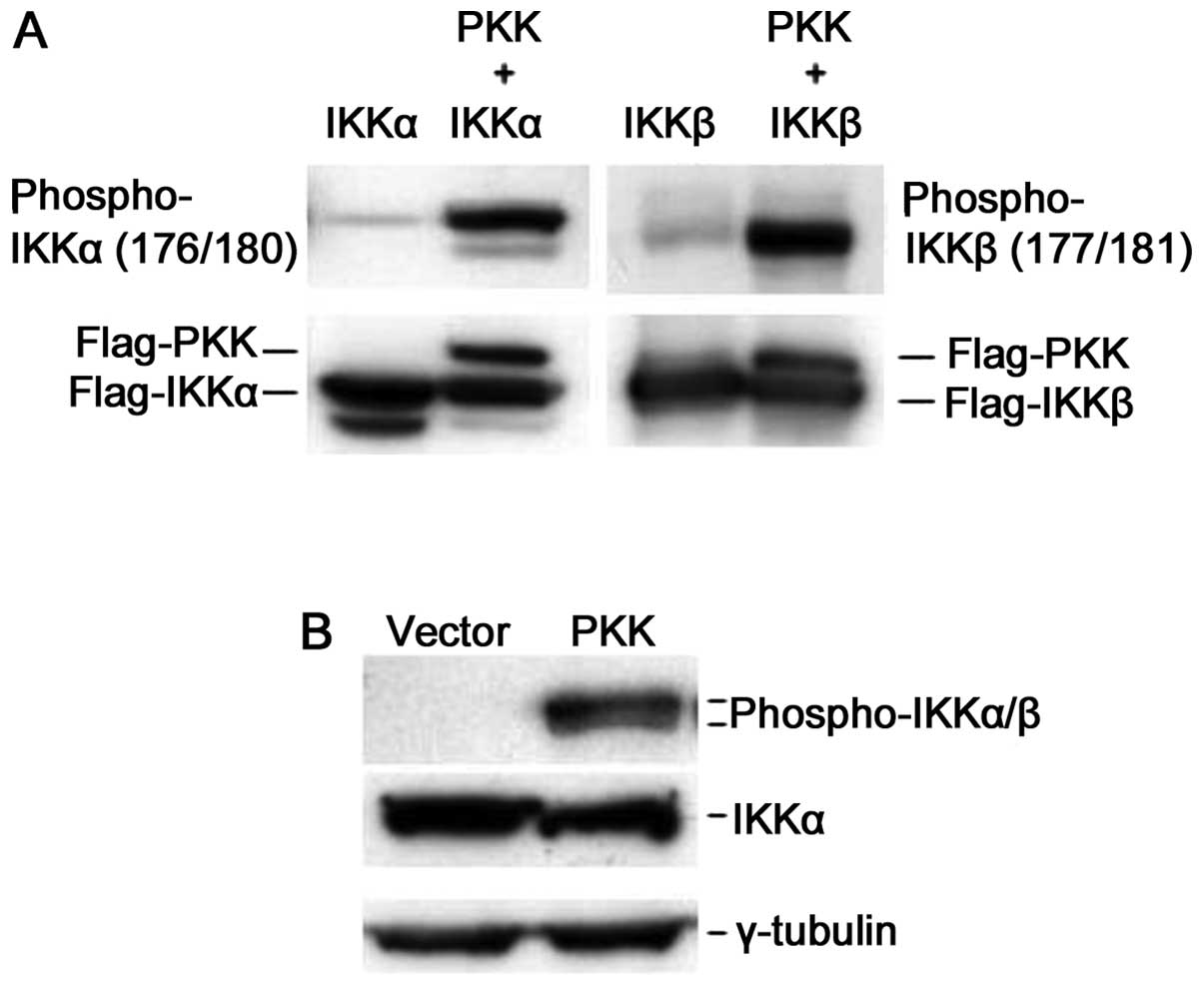
We next investigated whether expression of PKK
activates IKK in vivo. Phosphorylation of IKKα and IKKβ at
serine residues 176/180 and 177/181 (Ser176/180 and
Ser177/181), respectively, controls their activation,
and the levels of phosphorylation at these residues reflect the
relative activity of these kinases (6–8,20,21).
To analyze IKK activation, we examined the phosphorylation of IKKα
and IKKβ at these serine residues following PKK overexpression. We
expressed IKKα or IKKβ alone or together with PKK in HEK293T cells,
and assayed phosphorylation of Ser176/180 and
Ser177/181 of IKKα and IKKβ, respectively, using a
commercially available antibody that is specific for both
phospho-Ser176/180 of IKKα and
phospho-Ser177/181 of IKKβ. While overexpression of IKKα
or IKKβ alone showed some basal phosphorylation at
Ser176/180 or Ser177/181, co-expression of
PKK dramatically increased phosphorylation of IKKα and IKKβ at
these serine residues (Fig. 4A).
Overexpression of PKK also induced phosphorylation of the
endogenous IKKα and IKKβ while it had no effect on the expression
levels of IKK proteins (Fig. 4B).
Taken together, these results indicate that PKK acts upstream of
IKKs by inducing their phosphorylation, and thus activation.
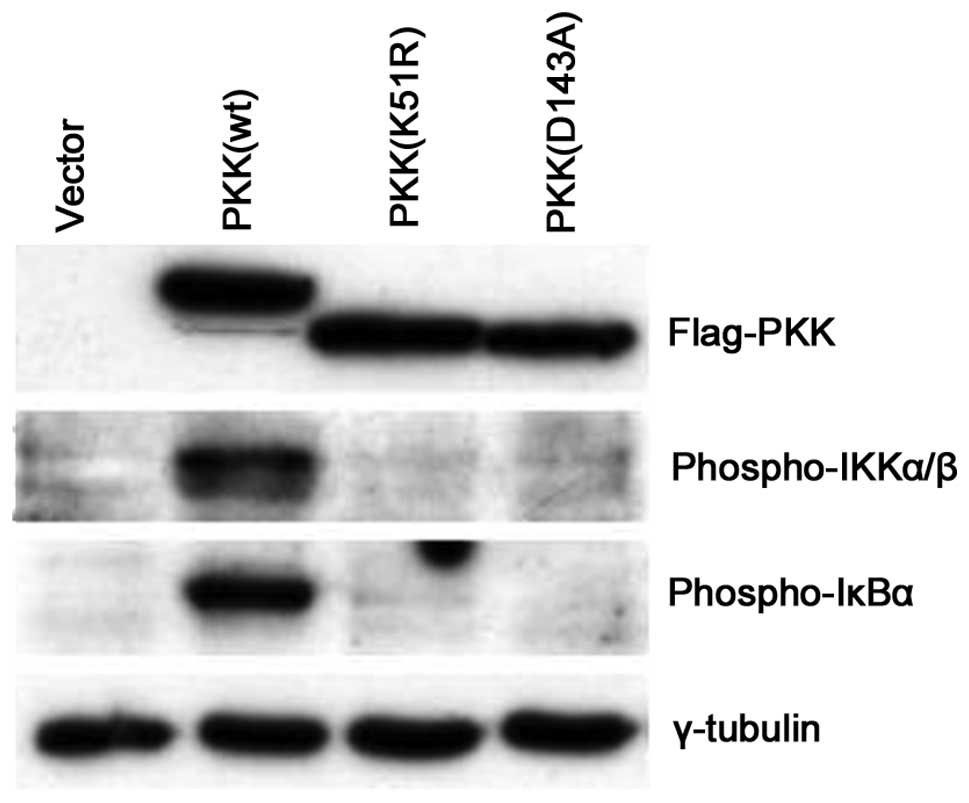
PKK induces IKK activation in a
kinase-dependent manner
It has been shown that several members of the RIP
family, including RIP, RIP2 and RIP3, activate NF-κB through a
kinase-independent mechanism (11,22–26).
It was also reported that certain kinase-inactive PKK were capable
of activating an NF-κB reporter, but at much lower efficiency than
the wild-type PKK (13). Since we
observed that a catalytically-inactive kinase failed to induce IKK
phosphorylation in vitro (Fig.
3), we investigated whether PKK induces endogenous IKK
phosphorylation in vivo in a kinase-dependent manner. In
contrast to the effect of wild-type PKK on IKK activation,
expression of two catalytically-inactive mutants of PKK (D143A and
K51R) were unable to induce IKK phosphorylation or IκBα
phosphorylation (Fig. 5). These
data suggest that PKK induces endogenous IKK activation primarily
in a kinase-dependent manner.
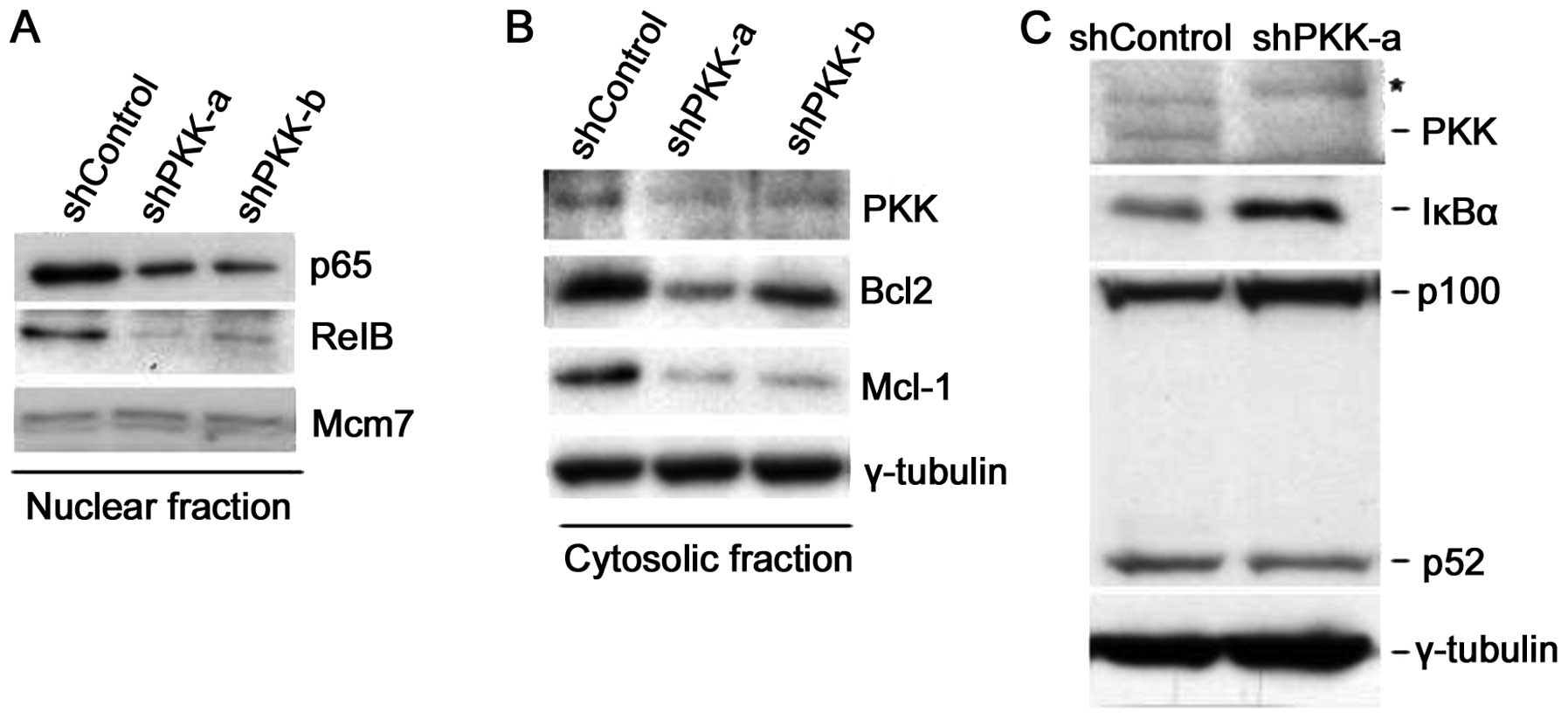
Suppression of PKK expression inhibits
NF-κB activation via inhibition of IKK phosphorylation
To determine whether endogenous PKK is involved in
IKK activation, we examined the effect of suppression of PKK
expression by RNA interference on IKK activation. To knockdown the
expression of PKK, we prepared two retroviral constructs that
express small hairpin RNAs (shRNAs) (17,18)
targeting two distinct sequences of human PKK (referred to as
shPKK-a and shPKK-b). Suppression of PKK expression by either
shPKK-α or shPKK-β in HeLa cells inhibited nuclear accumulation of
p65 and RelB (Fig. 6A), indicating
the requirement for PKK in activation of both the classical and the
alternative NF-κB pathways. More importantly, knockdown of PKK
resulted in decreased expression of Bcl2 and Mcl-1, known
transcriptional targets of NF-κB (Fig.
6B). Suppression of PKK expression by shPKK in HEK293 cells
also resulted in accumulation of IκBα and accumulation of p100 with
a concomitant decrease in the level of p52 (Fig. 6C). Together, these results support
the view that PKK regulates activation of both the classical and
the alternative NF-κB pathways in mammalian cells.
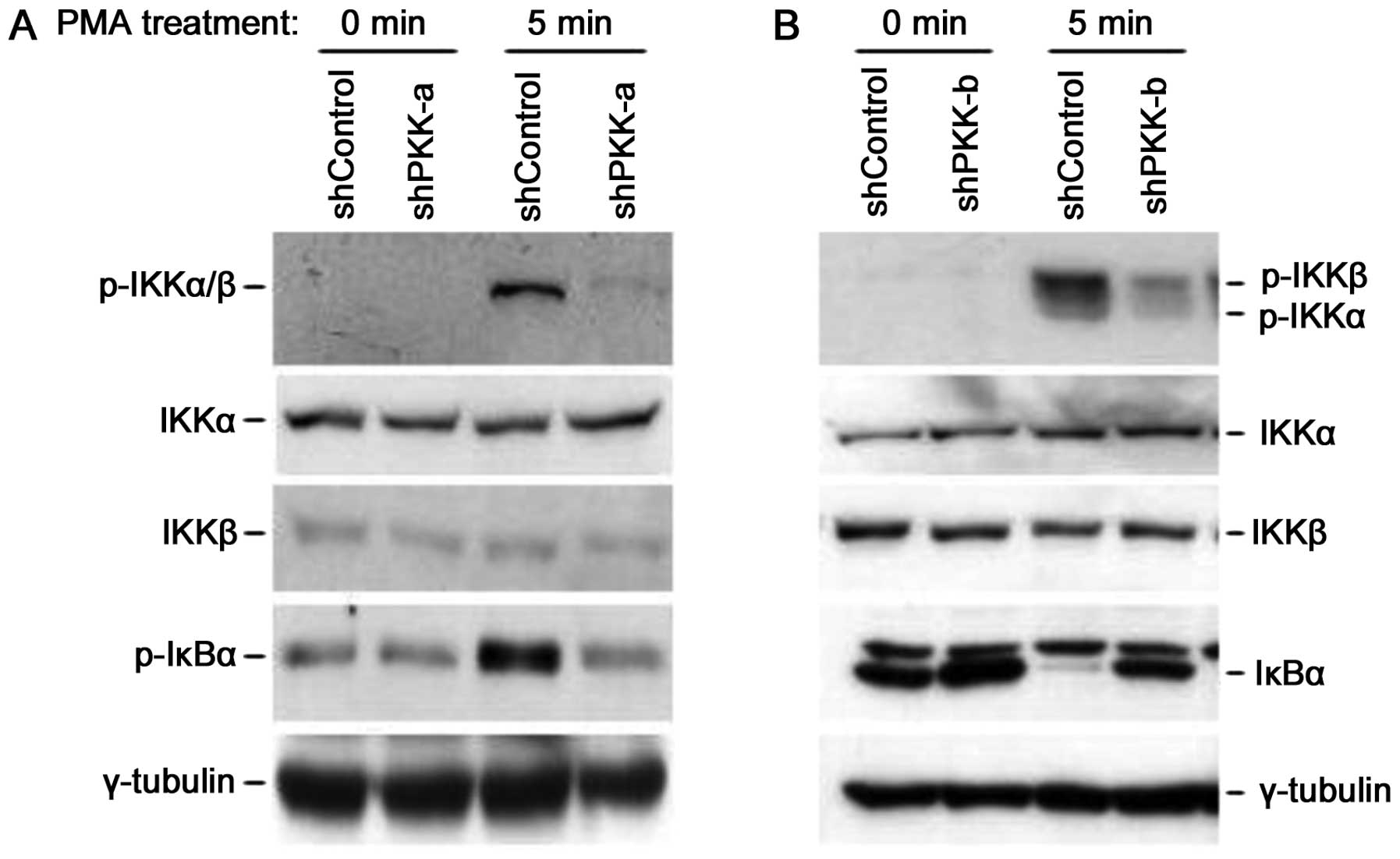
Our data from in vitro IKK phosphorylation
and PKK overexpression experiments suggest that PKK functions
upstream of IKK (Figs. 3 and
4). To further test this idea, we
investigated whether knockdown of PKK has any effect on IKK
phosphorylation. Using NF-κB reporter assays, it was previously
shown that PKK mediates NF-κB activation induced by PMA and
ionomycin, known activators of protein kinase C (PKC) in mammalian
cells (13,14). Thus, we examined whether
suppression of PKK expression inhibits IKK activation induced by
these PKC activators. As shown in Fig.
7A, IKK phosphorylation at Ser180/181 was rapidly
increased upon PMA/ionomycin stimulation in HeLa cells expressing
shControl. Knockdown of PKK, while having no effect on the
expression levels of IKKα and IKKβ proteins, inhibited
PMA/ionomycin-induced IKK phosphorylation at Ser180/181
(Fig. 7A). As our previous studies
with transgenic mice expressing a catalytically inactive PKK
suggested that PKK may be involved in B lymphocyte development
(27), we examined whether PKK
plays a role in NF-κB activation in a B-cell line. Suppression of
PKK expression in human B-cell lymphoma SUDHL-6 cells resulted in
inhibition of phosphorylation of IKKα and IKKβ induced by
PMA/ionomycin (Fig. 7B). These
results demonstrate that PKK regulates PMA/ionomycin-induced NF-κB
activation through modulating phosphorylation of IKKα and IKKβ in
various mammalian cell lines.
Discussion
We investigated the function of PKK in NF-κB
activation. We show that PKK regulates both the classical and the
alternative NF-κB activation pathways. We also explored the
mechanism by which PKK regulates NF-κB activation. We demonstrate
that PKK induces phosphorylation of Ser176/180 and
Ser177/181 of IKKα and IKKβ, respectively. Together,
these results indicate that PKK functions upstream of IKK in NF-κB
activation pathways, extending the previous observation that
activation of an NF-κB reporter induced by PKK overexpression
requires IKKβ (14). Our finding
also provides an explanation for the earlier report that
PKK-deficient keratinocytes had similar but not identical defects
as IKKα-deficient cells in keratinocyte differentiation (28), as loss of PKK function would affect
activation of both IKKα and IKKβ.
Several members of the RIP family, such as RIP, RIP2
and RIP3, function upstream of IKK in NF-κB activation (reviewed in
ref. 11). This study identifies
PKK (RIP4) as another RIP family member that acts as an upstream
regulator of IKK activation. However, unlike the other RIP family
members, which regulate NF-κB activation in a kinase-independent
manner, PKK appears to regulate IKK and NF-κB activation
predominantly in a kinase-dependent manner. It was previously
reported that certain kinase-inactive PKK mutants could activate,
albeit at much lower efficiency than the wild-type PKK, an NF-κB
reporter in transient transfection experiments (13), suggesting that PKK may also be
capable of activating NF-κB through a kinase-independent pathway
under certain circumstances. It has been shown that RIP1 and RIP2
seem to activate NF-κB through the classical pathway (11). Here we show that PKK can regulate
the activation of both the classical and the alternative NF-κB
pathways. Thus, PKK possesses some unique features among the RIP
family members. The physiological stimuli that require PKK function
for NF-κB activation remain to be determined. As PKK interacts with
protein kinase C β and δ (9,10)
and regulates NF-κB activation induced by PMA/ionomycin (Fig. 7), known protein kinase C
activators, it is reasonable to speculate that PKK functions
downstream of protein kinase C β and δ and that PKK may play a
critical role in NF-κB activation pathways involving PKC function.
Given that PKK regulates the activation of both the classical and
the alternative NF-κB pathways in a variety of mammalian cells
(Figs. 6, 7, and data not shown), it is possible
that PKK also plays a role in NF-κB activating pathways independent
of PKC function.
Activation of IKKα and IKKβ, which is manifested by
their phosphorylation at Ser176/180 and
Ser177/181, respectively, is crucial for NF-κB
activation induced by a variety of extracellular stimuli. The
molecular mechanisms underlying IKK activation, i.e., the kinases
that directly phosphorylate IKKα and IKKβ, in response to various
extracellular signals remain to be clarified (5). We showed that PKK associates with
IKKα and IKKβ in vivo (Fig.
2), and that PKK can induce IKK phosphorylation both in
vitro and in vivo (Figs.
3 and 4). We have expressed
and purified His-tagged IKK proteins from insect Sf9 cells and were
unable to detect phosphorylation of the IKK proteins in
vitro by purified PKK expressed from a baculoviral vector in
insect Sf9 cells (data not shown). Muto et al also reported
that, as an unpublished result, purified PKK did not phosphorylate
IKKα and IKKβ in vitro (14). Thus, it is possible that PKK may
activate IKKα and IKKβ through an indirect mechanism. Additional
experiments are needed to elucidate how PKK induces activation of
IKKα and IKKβ. TGF-β activating kinase 1 (TAK1) and
NF-κB-activating kinase (NIK) are kinases that have been shown to
function upstream of IKKs, possibly through directly
phosphorylating IKK kinases (6,20).
PKK may induce activation of IKK through activating either one of
these kinases. We are currently testing these possibilities.
Overexpression of a catalytically inactive mutant of
PKK in transgenic mice inhibited the generation of pro-B cells,
suggesting a role for PKK in B cell development (27). However, mice deficient in PKK
exhibit normal B cell populations in all examined compartments
(28,29), indicating that PKK is dispensable
for B cell development in mice. In addition, activation of the
classical NF-κB pathway induced by BCR, CD40 or TLR in PKK
deficient B cells appears to be normal (29). Here we show that the knockdown of
PKK inhibits NF-κB activation in human B lymphoma cell lines
(Fig. 7B, and unpublished data).
One explanation for these apparently contradictory observations may
be that the function of PKK in B-cells can be compensated by other
members of the RIP family. Alternatively, PKK may have acquired an
essential role in NF-κB activation in malignant B lymphocytes.
Additional experiments are required to resolve these issues.
Deregulation of NF-κB activation has been associated
with numerous human diseases including cancer, chronic inflammation
and autoimmune diseases (30–33).
As PKK plays a critical role in NF-κB activation pathways,
molecular elucidation of the mechanism of PKK function may provide
new insights into mechanisms leading to abnormal NF-κB activation
in human diseases, and may also facilitate the development of
therapeutic agents for human diseases resulting from aberrant NF-κB
activation.
Acknowledgements
We thank Michael Karin, David Goeddel and Shao-Cong
Sun for plasmid constructs. This study was supported by a 2-Year
Research Grant of Pusan National University to S.W.K.
References
|
1
|
Schreiber J, Jenner RG, Murray HL, Gerber
GK, Gifford DK and Young RA: Coordinated binding of NF-kappaB
family members in the response of human cells to
lipopolysaccharide. Proc Natl Acad Sci USA. 103:5899–5904. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bonizz G and Karin M: The two NF-kappaB
activation pathways and their role in innate and adaptive immunity.
Trends Immunol. 25:280–288. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Aggarwal BB: Nuclear factor-kappaB: the
enemy within. Cancer Cell. 6:203–208. 2004.PubMed/NCBI
|
|
4
|
Yamamoto Y and Gaynor RB: IkappaB kinases:
key regulators of the NF-kappaB pathway. Trends Biochem Sci.
29:72–79. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hayden MS and Ghosh S: Signaling to
NF-kappaB. Genes Dev. 18:2195–2224. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ling L, Cao Z and Goeddel DV:
NF-kappaB-inducing kinase activates IKK-alpha by phosphorylation of
Ser-176. Proc Natl Acad Sci USA. 95:3792–3797. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hu Y, Baud V, Oga T, Kim KI, Yoshida K and
Karin M: IKKalpha controls formation of the epidermis independently
of NF-kappaB. Nature. 410:710–714. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Delhase M, Hayakawa M, Chen Y and Karin M:
Positive and negative regulation of IkappaB kinase activity through
IKKbeta subunit phosphorylation. Science. 284:309–313. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bahr C, Rohwer A, Stempka L, Rincke G,
Marks F and Gschwendt M: DIK, a novel protein kinase that interacts
with protein kinase Cdelta. Cloning, characterization, and gene
analysis. J Biol Chem. 275:36350–36357. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen L, Haider K, Ponda M, Cariappa A,
Rowitch D and Pillai S: Protein kinase C-associated kinase (PKK), a
novel membrane-associated, ankyrin repeat-containing protein
kinase. J Biol Chem. 276:21737–21744. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Meylan E and Tschopp J: The RIP kinases:
crucial integrators of cellular stress. Trends Biochem Sci.
30:151–159. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Meylan E, Martinon F, Thome M, Gschwendt M
and Tschopp J: RIP4 (DIK/PKK), a novel member of the RIP kinase
family, activates NF-kappa B and is processed during apoptosis.
EMBO Rep. 3:1201–1208. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Moran ST, Haider K, Ow Y, Milton P, Chen L
and Pillai S: Protein kinase C-associated kinase can activate
NFkappaB in both a kinase-dependent and a kinase-independent
manner. J Biol Chem. 278:21526–21533. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Muto A, Ruland J, McAllister-Lucas LM,
Lucas PC, Yamaoka S, Chen FF, Lin A, Mak TW, Nunez G and Inohara N:
Protein kinase C-associated kinase (PKK) mediates Bcl10-independent
NF-kappa B activation induced by phorbol ester. J Biol Chem.
277:31871–31876. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Woronicz JD, Gao X, Cao Z, Rothe M and
Goeddel DV: IkappaB kinase-beta: NF-kappaB activation and complex
formation with IkappaB kinase-alpha and NIK. Science. 278:866–869.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhao J, Dynlacht B, Imai T, Hori T and
Harlow E: Expression of NPAT, a novel substrate of cyclin E-CDK2,
promotes S-phase entry. Genes Dev. 12:456–461. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hannon GJ and Rossi JJ: Unlocking the
potential of the human genome with RNA interference. Nature.
431:371–378. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huppi K, Martin SE and Caplen NJ: Defining
and assaying RNAi in mammalian cells. Mol Cell. 17:1–10. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li ZW, Rickert RC and Karin M: Genetic
dissection of antigen receptor induced-NF-kappaB activation. Mol
Immunol. 41:701–714. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shim JH, Xiao C, Paschal AE, Bailey ST,
Rao P, Hayden MS, Lee KY, Bussey C, Steckel M, Tanaka N, Yamada G,
Akira S, Matsumoto K and Ghosh S: TA K1, but not TA B1 or TA B2,
plays an essential role in multiple signaling pathways in vivo.
Genes Dev. 19:2668–2681. 2005. View Article : Google Scholar
|
|
21
|
Saijo K, Mecklenbrauker I, Santana A,
Leitger M, Schmed C and Tarakhovsky A: Protein kinase C beta
controls nuclear factor kappaB activation in B cells through
selective regulation of the IkappaB kinase alpha. J Exp Med.
195:1647–1652. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yu PW, Huang BC, Shen M, Quast J, Chan E,
Xu X, Nolan GP, Payan DG and Luo Y: Identification of RIP3, a
RIP-like kinase that activates apoptosis and NFkappaB. Curr Biol.
9:539–542. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kasof GM, Prosser JC, Liu D, Lorenzi MV
and Gomes BC: The RIP-like kinase, RIP3, induces apoptosis and
NF-kappaB nuclear translocation and localizes to mitochondria. FEBS
Lett. 473:285–291. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
McCarthy JV, Ni J and Dixit VM: RIP2 is a
novel NF-kappaB-activating and cell death-inducing kinase. J Biol
Chem. 273:16968–16975. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kelliher MA, Grimm S, Ishida Y, Kuo F,
Stanger BZ and Leder P: The death domain kinase RIP mediates the
TNF-induced NF-kappaB signal. Immunity. 8:297–303. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hsu H, Huang J, Shu HB, Baichwal V and
Goeddel DV: TNF-dependent recruitment of the protein kinase RIP to
the TNF receptor-1 signaling complex. Immunity. 4:387–396. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cariappa A, Chen L, Haider K, Tang M,
Nebelitskiy E, Moran ST and Pillai S: A catalytically inactive form
of protein kinase C-associated kinase/receptor interacting protein
4, a protein kinase C beta-associated kinase that mediates NF-kappa
B activation, interferes with early B cell development. J Immunol.
171:1875–1880. 2003. View Article : Google Scholar
|
|
28
|
Holland P, Willis C, Kanaly S, Glaccum M,
Warren A, Charrier K, Murison J, Derry J, Virca G, Bird T and
Peschon J: RIP4 is an ankyrin repeat-containing kinase essential
for keratinocyte differentiation. Curr Biol. 12:1424–1428. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Moran ST, Cariappa A, Liu H, Boboila C,
Shi HN, Holland PM, Peschon JJ and Pillai S: Protein kinase
C-associated kinase is not required for the development of
peripheral B lymphocyte populations. Mol Immunol. 43:1694–1699.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Karin M and Greten FR: NF-kappaB: linking
inflammation and immunity to cancer development and progression.
Nat Rev Immunol. 5:749–759. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jimi E and Ghosh S: Role of nuclear
factor-kappaB in the immune system and bone. Immunol Rev.
208:80–87. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kim HJ, Hawke N and Baldwin AS: NF-kappaB
and IKK as therapeutic targets in cancer. Cell Death Differ.
13:738–747. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li Q, Withoff S and Verma IM:
Inflammation-associated cancer: NF-kappaB is the lynchpin. Trends
Immunol. 26:318–325. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kim SW, Oleksyn DW, Rossi RM, Jordan CT,
Sanz I, Chen L and Zhao J: Protein kinase C-associated kinase is
required for NF-kappaB signaling and survival in diffuse large
B-cell lymphoma cells. Blood. 111:1644–1653. 2008. View Article : Google Scholar : PubMed/NCBI
|