Introduction
Cancer incurs significant morbidity and mortality,
with >6 million deaths occurring each year worldwide (1). Until recently, cancer treatment was a
3-pronged approach, comprising surgery, radiation therapy, and
chemotherapy (2). However, cancer
treatment efficacy is frequently inadequate and disease is often
recurrent, and in such cases, virtually all patients eventually
succumb to the disease. Patients with recurrent or metastatic
disease are generally incurable and are not eligible for multimodal
curative treatment. The goals of cancer treatment are prolongation
of overall survival, palliation of existing symptoms, prevention of
new cancer-related symptoms, and improvement in the quality of life
(3). Therefore, the development of
new and effective therapeutic strategies of cancer is
necessary.
In the past 25 years, immunotherapy has been added
as an important component of cancer treatment and is expected to
provide a new strategy for cancer therapy (2). Rosenberg et al (4) introduced lymphokine-activated killer
cell (LAK) therapy as a form of immunotherapy, but frequent adverse
reactions to those cells activated with IL-2 were found and an
adequate clinical effect was not achieved, resulting in a decrease
in favor for the LAK therapy (5,6).
Recently, it has become widely accepted that cytotoxic T
lymphocytes (CTL), originally isolated from tumor-infiltrating
lymphocytes (TILs) in vitro (7), play a major role in tumor rejection
in vivo (8). This approach
involves stimulation of T lymphocytes with a specific tumor antigen
in vitro. It is known that lymphocytes rapidly express and
secrete cytokines after stimulation with appropriate antigens, and
the quantity of IFN-γ produced indicates the reactivity of
lymphocytes for tumor cells (9).
The number of tumor-specific T cells may also be enhanced by
repetitive stimulation with autologous tumor cells or antigen in
vitro. The immune system recognizes tumors, but the tumor
microenvironment generates immunosuppressive cells leading to
immune evasion of cancer. The approach to overcoming the
immunosuppressive tumor microenvironment is to generate cells in
vitro that will kill tumor cells. Cellular immunotherapy, in
the form of CTL, has been successfully used to treat
virus-associated malignancies, some hematological malignancies, and
some solid tumors (10–12). Antigen-specific CD8+ T
cell clones have also been shown to be effective for treating
malignant disease (13), implying
T-cell specificity to tumor cell antigens.
Oropharyngeal cancer is the eighth most common
cancer worldwide with over 145,500 deaths occurring every year
(14). It is widely known that
lymph node metastasis occurs at a high frequency because of the
rich lymphatic submucosal plexus of the oropharynx, resulting in
decreased survival rates. Although the conventional treatment for
oral and maxillofacial cancers is surgery, radiotherapy, and
chemotherapy, surgical treatment can often lead to severe morbidity
and decreased quality of life. Chemotherapy is also widely used in
cases of oral cancer, but the effects are limited because of lack
of selectivity, narrow therapeutic margins, and the high prevalence
of drug resistance (14). As a
result, new therapeutic options for oral and maxillofacial cancers
are urgently required. However, no studies on the use of
immunotherapy in oral and maxillofacial cancers have been reported
to date.
In this study, we applied ex vivo stimulation
of CTL using irradiated tumor cells, which were used in an attempt
to augment host immunological response, to generate killer cells
for adoptive immunotherapy. Then, we investigated the clinical
effects of CTL therapy in advanced oral and maxillofacial
cancers.
Materials and methods
Study entry criteria and patients
Patients were eligible for this study if they had
oral and maxillofacial cancers; a clinical performance status of 0,
1, or 2; if their tumor could be obtained for the CTL treatment and
was positive for HLA class I antibody. Patients were excluded from
participation if they were positive for hepatitis B or C antigens
or for HIV antibodies. Seven patients aged between 59 and 79 years
(mean age, 68.4 years) were enrolled in this study. This study was
approved by the Ethics Committee of Aichi Medical University and
written informed consent was obtained from all the patients.
Preparation of cells
Tumor samples used for the stimulation of peripheral
blood mononuclear cells (PBMCs) were obtained from each patient and
incubated for 1 h in RPMI-1640 (Nipro, Japan) containing 1,250 U/ml
penicillin, 1,250 μg/ml streptomycin and 31.25 μg/ml amphotericin
B, after which they were minced and enzymatically digested with 2%
collagenase for 1 h at 37°C in 5% CO2 in an incubator.
The tumor cells were cryopreserved at -152°C until they were used
for stimulation.
Peripheral blood samples (40 ml) were collected from
the patients and PBMCs were obtained by the gradient method using
Lymphosepar I (Immuno-Biological Laboratories Co., Ltd., Fujioka,
Gunma, Japan). PBMCs were incubated with TIL-Medium I
(Immuno-Biological Laboratories Co., Ltd.) containing 2.5%
autologous plasma, 50 U/ml IL-2 (Shionogi & Co., Ltd., Osaka,
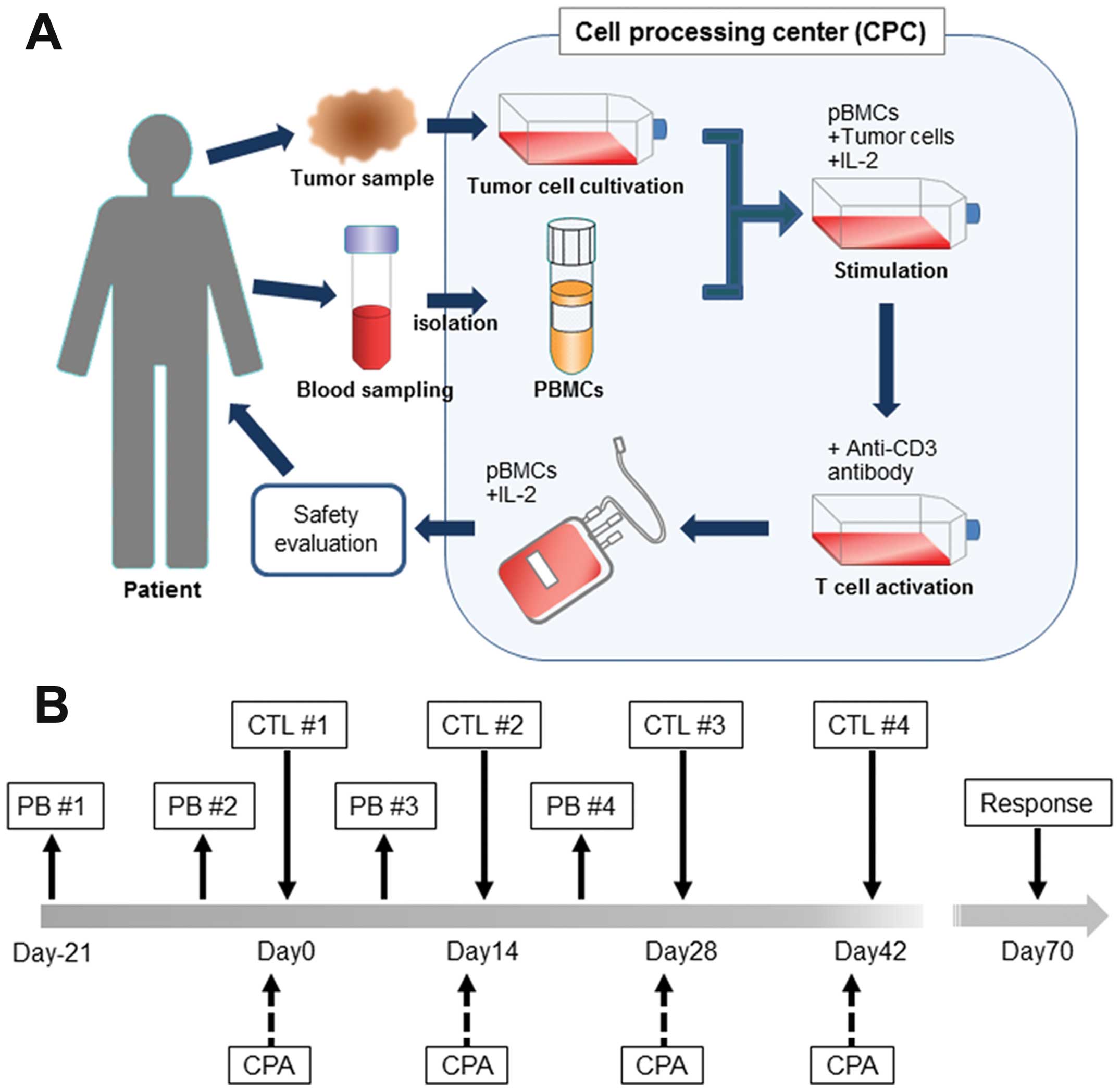
Japan), and tumor cells irradiated for 4–5 days (Fig. 1A). PBMCs stimulated with tumor
cells and IL-2 were then cultured in an anti-CD3 antibody (Janssen
Pharmaceutical, Tokyo, Japan)-coated flask for 4–6 days to activate
the T cells. At the end of this period, PBMCs were transferred into
a culture bag (GT-T610 culture bag, KB0001, Takara Bio Inc., Otsu,
Shiga, Japan) and cultured with GT-T503 culture medium (KB503S,
Takara Bio Inc.) containing 2.5% autologous plasma and 50 U/ml IL-2
for 10–12 days before use for CTL therapy. The safety of the PBMCs
was examined using the BD BACTEC™ System (Becton-Dickinson and
Company, Tokyo, Japan), MycoAlert™ (Eidia, Ibaraki, Japan), and
Endosafe-PTS (Wako, Japan).
Treatment schedule
Four infusions of CTL, scheduled at 2-week
intervals, constituted 1 cycle and were administered intravenously
(Fig. 1B). Before CTL infusion,
all patients received cyclophosphamide at a dose of 200 mg/body
(Shionogii & Co., Ltd) to inhibit the activity of regulatory T
cells. Patient response was assessed using computed tomography (CT)
and physical examination, on the basis of Response Evaluation
Criteria in Solid Tumors (RECIST) guidelines.
Phenotype assays
Phenotype analysis of infused lymphocytes as CTL was
analyzed by flow cytometry analysis with a cytomics FC500 (Beckman
Coulter, Inc., Brea, CA, USA). Cultured cells were stained with
anti-CD2/anti-CD20 (T11-RD1/B1-FITC, 6603928, Beckman Coulter,
Inc.), anti-CD4/anti-CD8 (T4-RD1/T8-FITC, 6603802, Beckman Coulter,
Inc.) and anti-CD56 (CD56-PE, N901, A07788, Beckman Coulter, Inc.)
according to the manufacturer’s instructions.
IFN-γ released from CTL in response to autologous
tumor cells was analyzed according to a previously reported method
(15). Briefly, CTL were incubated
with tumor cells overnight at 37°C in 5% CO2. The
supernatants were collected and the amount of IFN-γ was measured
using an ELISA kit (Human IFN-γ ELISA Ready-Set-Go, 88-7316-88,
eBioscience Inc., San Diego, CA, USA) according to the
manufacturer’s instructions.
Results
Patient characteristics
A total of 44 T-cell infusions were administered to
7 patients (5 males and 2 females) diagnosed with oral and
maxillofacial cancers (Table I).
Five patients had squamous cell carcinoma (SCC), 1 patient had
malignant melanoma, and the remaining patient had spindle cell
sarcoma. All patients presented with stage IV disease at diagnosis.
Tumor cells from these patients expressed HLA class I antigen by
the means of immunostaining, indicating that tumor antigens could
be recognized by CTL (data not shown). All patients had received
prior treatment, including chemotherapy, radiotherapy, or surgery.
In 5 cases (cases 1, 3, 4, 6 and 7), because the patients did not
respond to previous conventional therapy, they were switched to CTL
therapy. Two patients (cases 2 and 5) received CTL therapy as
adjuvant therapy.
 | Table IPatient characteristics and clinical
summary. |
Table I
Patient characteristics and clinical
summary.
| Patient no | Age (years) | Sex | Disease site | Diagnosis
(stage) | Previous
treatments | Total no. of injected
cells ×109 (total no. of injections) | Cyclophosphamide
(mg/mm2) | Observation periods
(months) |
|---|
| 1 | 69 | M | Floor of the oral
cavity | SCC (IV) | RT, chemo | 29.4 (20) | 129 | 46 |
| 2 | 79 | M | Mandible gingiva | Malignant melanoma
(IV) | Surgery | 7.16 (4) | 129 | 60 |
| 3 | 66 | M | Maxillary
gingiva | SCC (IV) | RT, chemo,
surgery | 4.12 (4) | 119.8 | 0.5 |
| 4 | 65 | M | Oropharynx, buccal
mucosa, maxillary gingiva | SCC (IV) | RT, chemo,
surgery | 6.4 (4) | 127.4 | 3 |
| 5 | 59 | F | Tongue | SCC (IV) | RT, chemo,
surgery | 6.5 (4) | 135.1 | 58 |
| 6 | 72 | M | Tongue | SCC (IV) | Surgery | 5.38 (4) | 126.6 | 14 |
| 7 | 69 | F | Maxillary
gingiva | Spindle cell sarcoma
(IV) | Chemo, surgery | 10 (4) | 155 | 2 |
Characteristics and activity of CTL
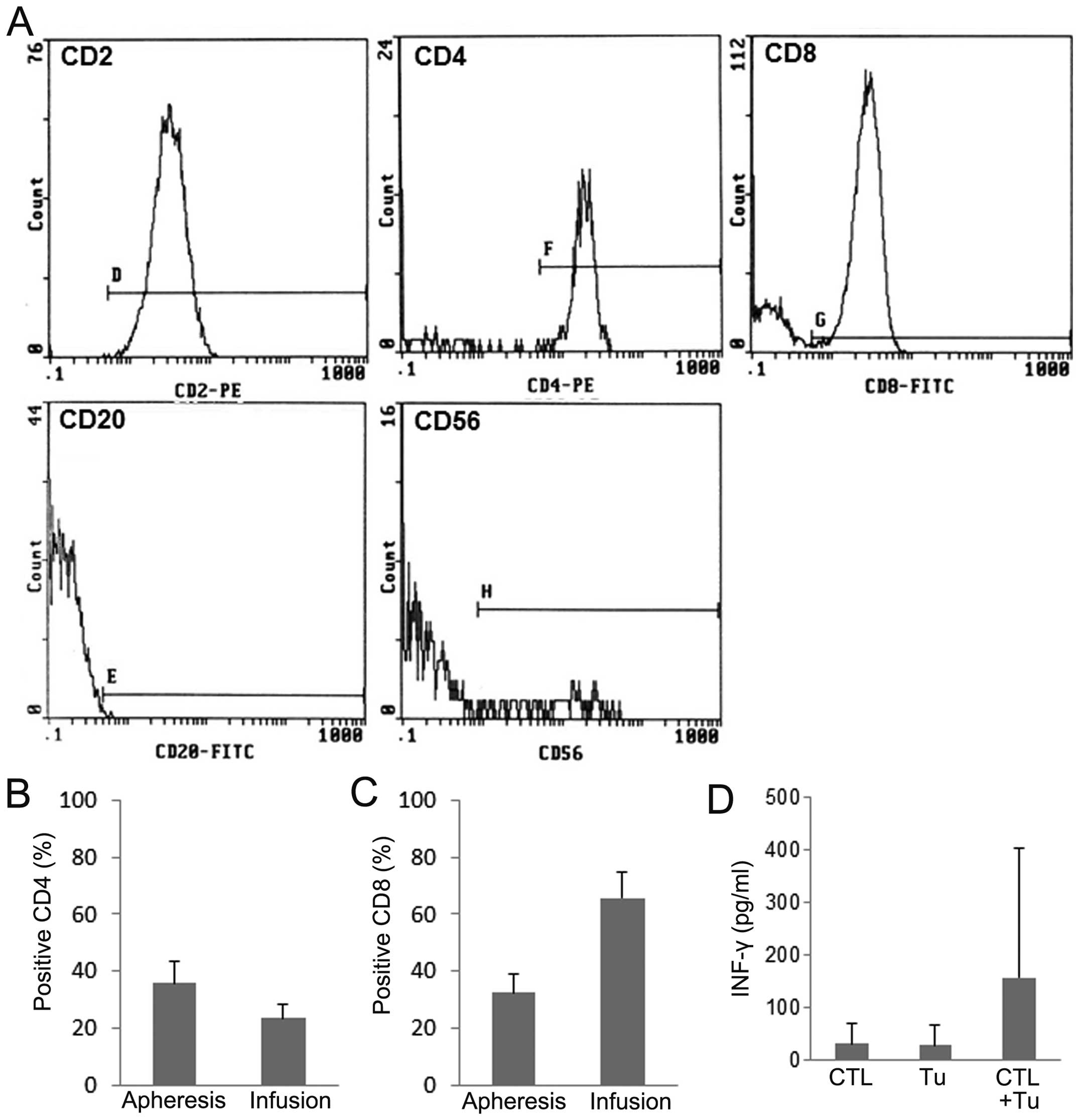
To characterize CTL, surface markers were analyzed.
CTL were mostly positive for CD2 (99.3±0.3%), and partially
positive for CD56 (10.9±11.1%) (Fig.
2A). The expression of CD4, the marker of helper T cells in
CTL, decreased to 23.6±4.5%, whereas that in peripheral blood
mononuclear cells (PBMCs) before stimulation was considerably
higher (35.7±9.1%) (Fig. 2B). In
contrast, the expression of CD8, the marker of cytotoxic T cells,
in CTL increased from 32.5±6.5 to 65.6±9.3% after stimulation
(Fig. 2C). The activity of PBMCs
stimulated with autologous tumor cells and IL-2 (CTL) was assessed
by measuring IFN-γ production (Fig.
2D). CTL produced a significantly higher level of IFN-γ than
unstimulated PBMCs or tumor cells alone (Tu).
Clinical results
The total number of administered cells per patient
ranged from 4.12–29.4×109 cells. The mean dose of
cyclophosphamide received was 131.7 mg/mm2 body surface
area (range, 119.8–155 mg/mm2 body surface area). The
mean observation period was 26.2 months (range, 0.5–60 months).
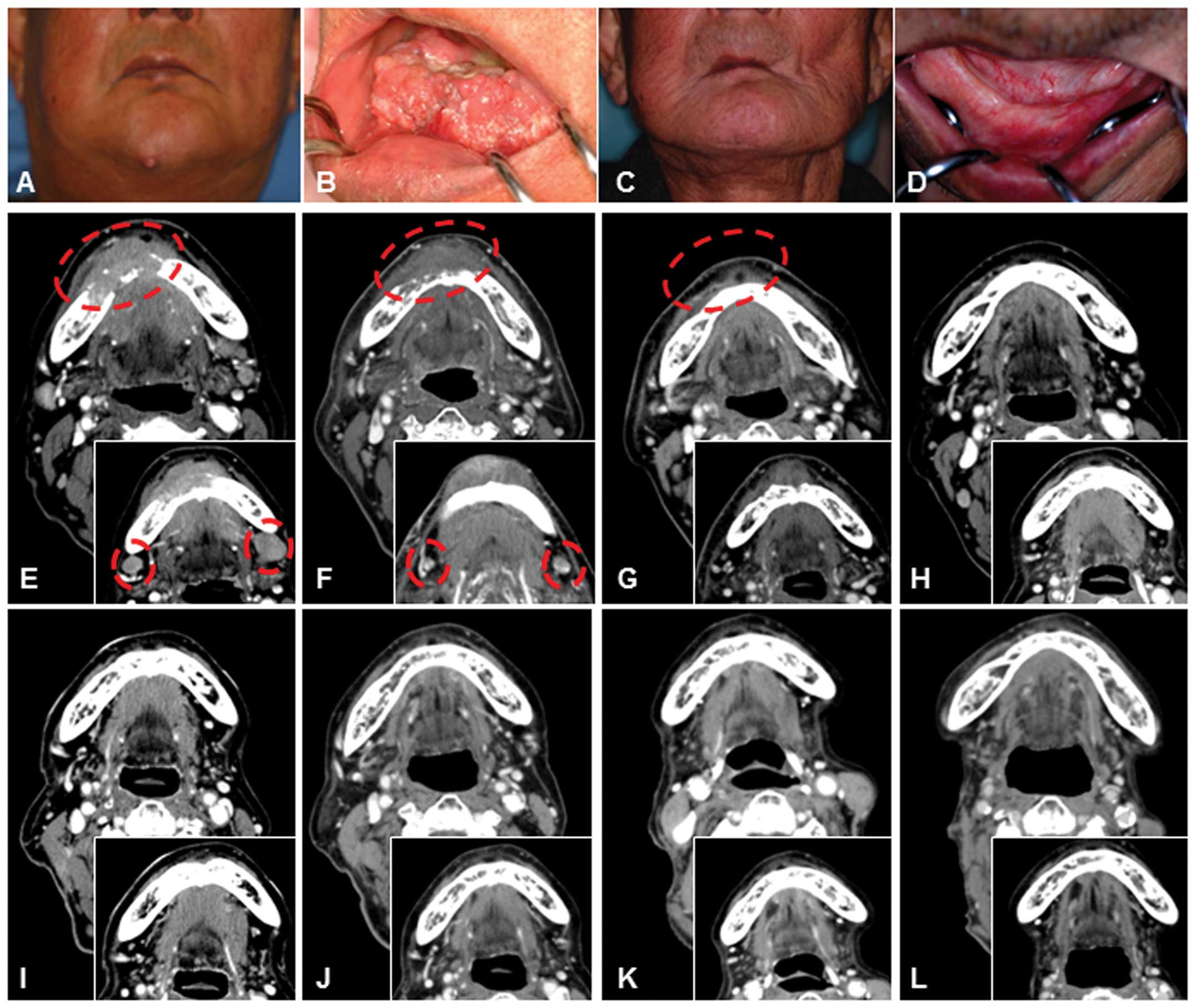
The representative patient (case 1 in Table I) attaining CR had SCC of the floor
of the oral cavity (T4N2cM0, stage IV) with lymph node metastases
at diagnosis. The patient had been treated with radiotherapy and
chemotherapy, but the disease was refractory. The patient refused
surgery, but chose CTL treatment because of the high degree of
invasiveness and the treatment burden necessitating resection as
far as the skin, including resection of most of the mandible
(Fig. 3A, B, E and F). First, the
lymph node metastatic lesions were resected before CTL treatment,
after which CTL infusion was performed by IVH injection after
culture. After the second course of CTL infusion, the tumor size
was markedly decreased (Fig. 3G).
Because of the partial response, this patient received additional
courses of CTL therapy at 7, 11 and 18 months after the first
course of CTL infusion. Computed tomography (CT) clearly indicated
a significant reduction in tumor size followed by the complete
disappearance of the tumor (Fig. 3H
and I). Target lesions remained in stable CR for 46 months
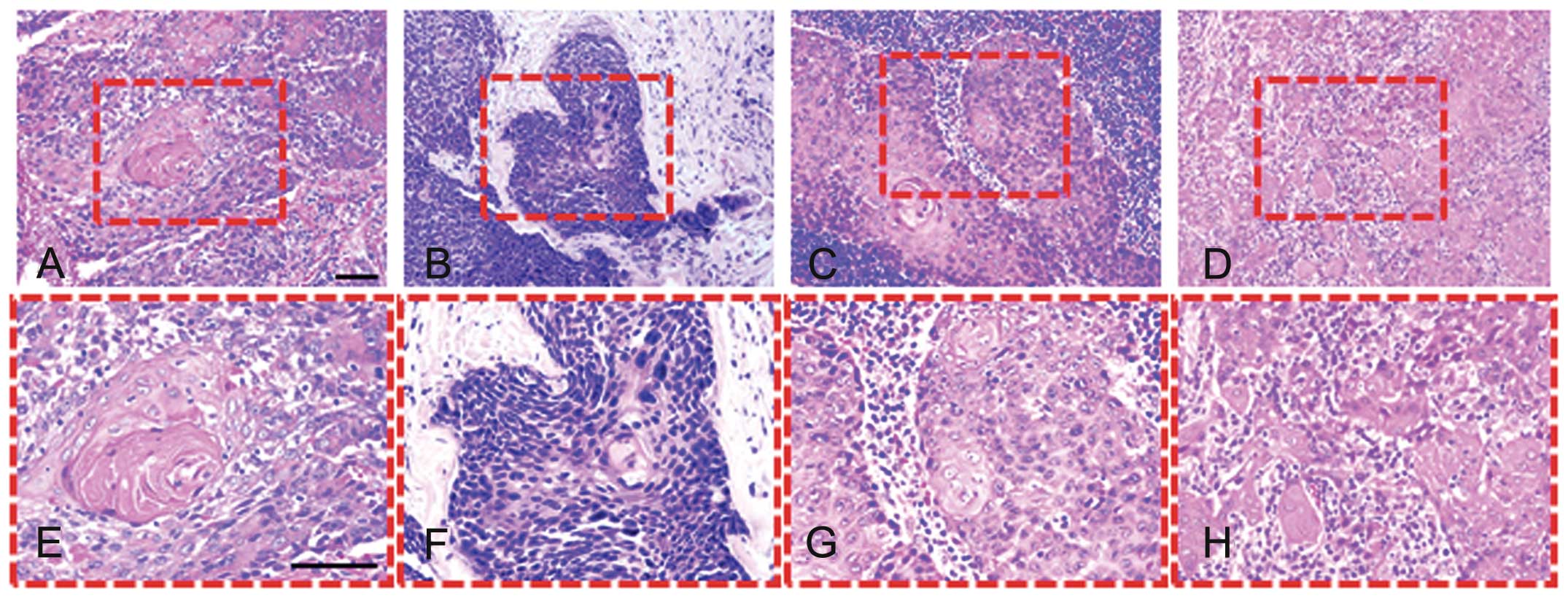
after the last course of CTL infusion (Fig. 3C, D and J-L). Histological
examination of clinical biopsy samples showed well-differentiated
SCC cells with individual cell keratinization and formation of
cancer pearls before treatment (Fig.
4A and E). After chemoradiotherapy, residual cancer showed a
wide pattern of alterations and the nuclei of residual neoplastic
cells were enlarged and irregular, with clumped chromatin (Fig. 4B and F). The lymph node exenterated
for the 1st cycle of CTL therapy revealed the presence of cancer
cell nests (Fig. 4C and G). Tumors
treated with CTL were heavily infiltrated with lymphocytes
(Fig. 4D and H). In addition, the
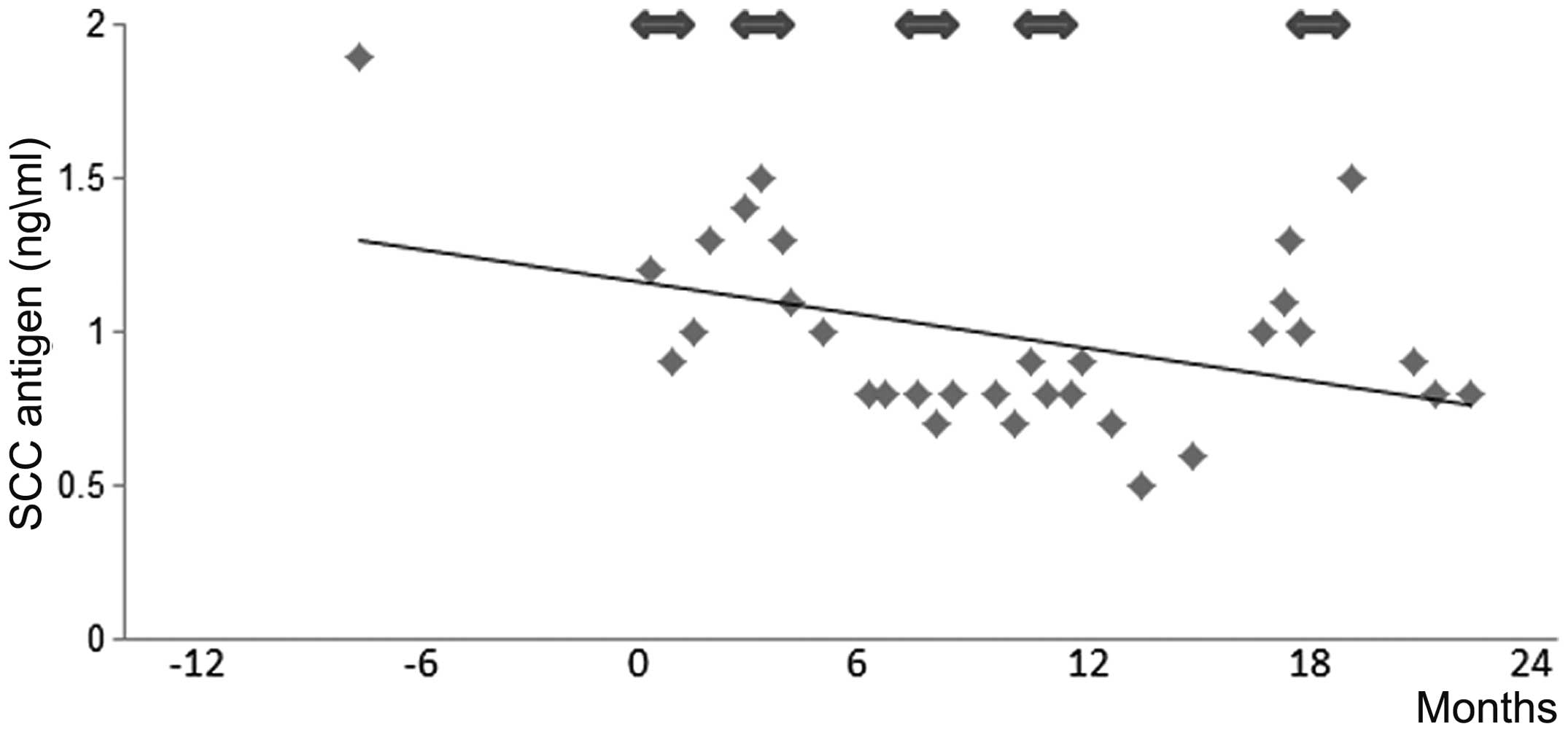
SCC antigen levels decreased after CTL treatment (Fig. 5).
Two patients (cases 2 and 5) who received CTL
therapy as adjuvant therapy showed neither recurrent disease nor
new disease lesions during the observation period. In cases 6 and
7, the target tumor lesions resulted in stable disease (SD ) after
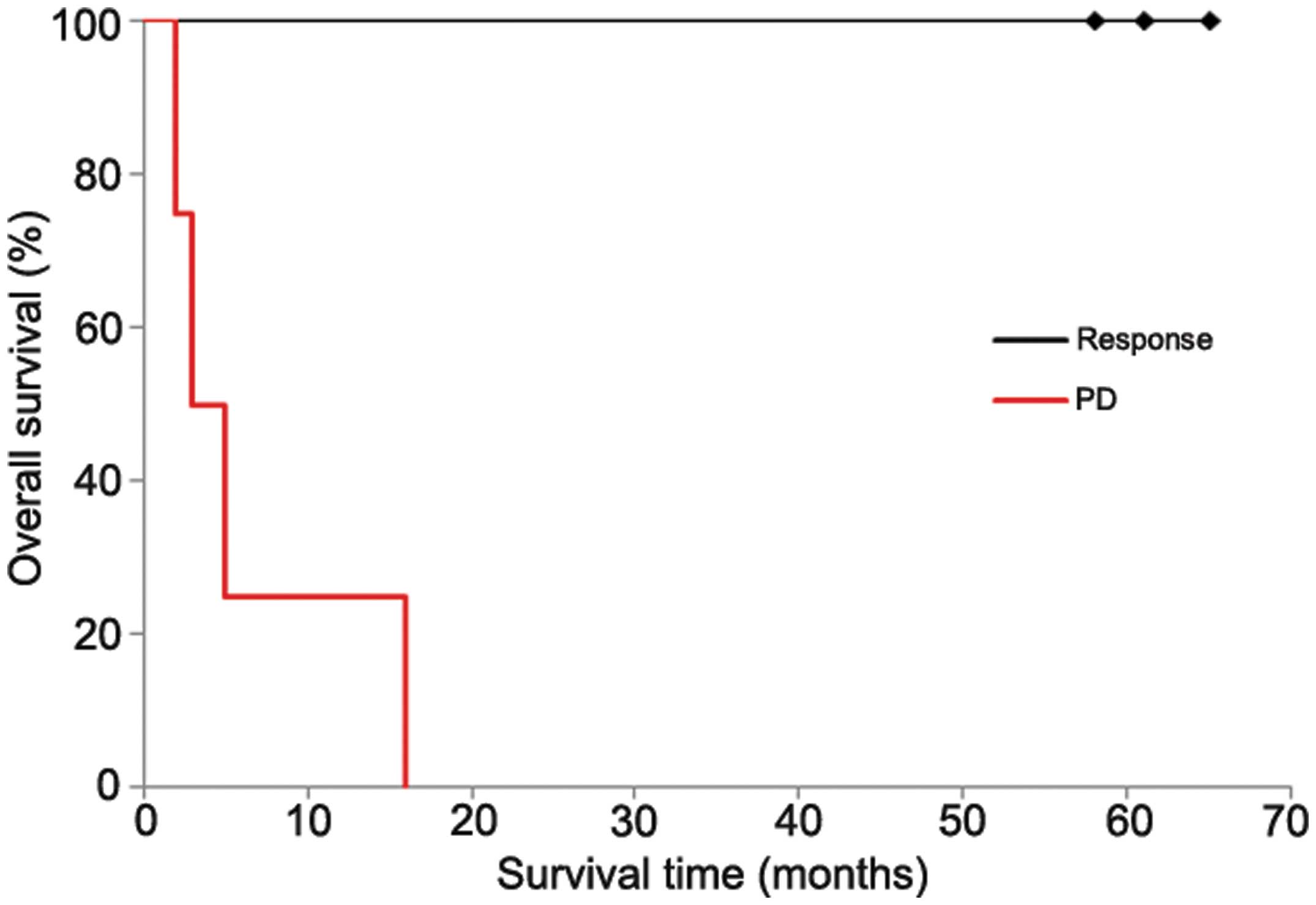
CTL treatment. The 1-year survival rates of the response group
(cases 1, 2 and 5) and the progressive disease (PD) group (cases 3,
4, 6 and 7) were 100 and 25%, respectively (Fig. 6). Moreover, no significant adverse
reactions were reported during the observation period.
Discussion
This study evaluated the clinical efficacy of
adoptive immunotherapy using specific lymphocytes activated by
irradiated autologous tumor cells, which expressed HLA class I
antigen, and used as immunogens to patient PBMCs for the treatment
of advanced oral and maxillofacial cancers. Our results with CTL
immunotherapy in 7 patients with advanced cancers showed that
adoptive cellular immunotherapy can be performed safely in such
patients. Although the cells are expected to be utilized in both
adoptive and active immunotherapies against cancer, the clinical
efficacy of immunotherapy remains to be clarified. In a previous
report, there was a tendency for CTL not to be activated from
patients with low CD8+ cell populations (16). However, in this study the
proportion of CD8+ cells in the CTL population was
increased compared to that in the PBMCs before stimulation
(Fig. 2C). Mature differentiated
CD8+ T cells and some types of CD4+ T cells
release IFN-γ, which enhances the immune response by upregulating
the expression of MHC class I molecules on both tumor cells and
tumor-resident APCs (17). Because
the percentage of CD8+ cells increased after
stimulation, higher IFN-γ production by CTL may have occurred in
the patients included in this study. The successful induction of
effective CTL from PBMCs may result in favorable clinical
results.
Many clinical studies of adoptive immunotherapy
using mostly LAK cells and TILs have shown a significant response
rate in patients with melanoma and some other cancers (17,18).
These studies also demonstrate efficacy prior to surgery (19). However, there has been no report of
a clinical study investigating CTL administration without surgery
and demonstrating obvious tumor regression of advanced oral or
maxillofacial cancers. To our knowledge, our report is the first to
show clear evidence for the potential of the application of
immunotherapy in oral and maxillofacial cancer patients. In our
study, the representative patient refused surgery and instead was
treated with CTL immunotherapy. Interestingly, the original tumor
and lymph node metastases disappeared following CTL treatment
despite the absence of surgery (Fig.
3). Histological examination showed that the efficacy of CTL
treatment, as evidenced by the fact that tumors were heavily
infiltrated and attacked by the activated lymphocytes (Fig. 4). In addition, a positive response
to CTL led to an improvement in overall survival for patients with
advanced cancer. Moreover, no significant adverse reactions were
reported during the observation period. Therefore, CTL induction
using autologous tumor cells as immunogens has potential anticancer
efficacy in refractory oral and maxillofacial cancers.
In agreement with our findings, Khammari et
al (20) reported that
adoptive immunotherapy with tumor-infiltrating lymphocytes used as
an adjuvant regimen for stage III melanoma was effective and both
relapse-free survival and overall survival were extended. Another
study revealed an apparent survival benefit for patients with
resected lung cancer who received adjuvant CTL (21). It has been reported that the rate
of local and regional recurrence of oral cancer varies from 18 to
76% according to the TNM stage and initial treatment, and
recurrence has been reported in >50% patients with advanced
clinical stages (III or IV) (22).
In this study, patients with stage IV at diagnosis who received CTL
treatment as adjuvant therapy, continue to have a good prognosis
without recurrent disease (cases 2 and 5 in Table I). These results indicate that this
treatment strategy might be effective as adjuvant treatment for
patients with advanced stage cancer.
In conclusion, adoptive immunotherapy using ex
vivo activated CTL with irradiated autologous tumor cells has
favorable clinical efficacy without side effects. Therefore, it
could form a novel treatment option that lead to improvement of
quality of life for advanced oral and maxillofacial cancers and may
be a promising approach for targeted therapy in such patients.
However, further clinical trials will be required to confirm the
present findings in a large-scale prospective clinical study.
Acknowledgements
The authors wish to thank members of the Department
of Oral and Maxillofacial Surgery, Motoaki Uruma in the Blood
Transfusion Service Department, and Kazuo Hara in the Pathology
Department, Aichi Medical University Hospital for their help,
encouragement, and contributions to the completion of this
study.
References
|
1
|
World Health Organization. The World
Health Report 2004: Changing history. WHO; Geneva: 2004
|
|
2
|
DeVita VT Jr and Rosenberg SA: Two hundred
years of cancer research. N Engl J Med. 366:2207–2214.
2012.PubMed/NCBI
|
|
3
|
Colevas AD: Chemotherapy options for
patients with metastatic or recurrent squamous cell carcinoma of
the head and neck. J Clin Oncol. 10:2644–2652. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rosenberg SA, Lotze MT, Muul LM, et al:
Observations on the systemic administration of autologous
lymphokine-activated killer cells and recombinant interleukin-2 to
patients with metastatic cancer. N Engl J Med. 313:1485–1492. 1985.
View Article : Google Scholar
|
|
5
|
Ettinghausen SE, Puri RK and Rosenberg SA:
Increased vascular permeability in organs mediated by the systemic
administration of lymphokine-activated killer cells and recombinant
interleukin-2 in mice. J Natl Cancer Inst. 80:177–188. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Soda H, Koda K, Yasutomi J, et al:
Adoptive immunotherapy for advanced cancer patients using in vitro
activated cytotoxic T lymphocytes. J Surg Oncol. 72:211–217. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Baxevanis CN, Dedoussis GV, Papadopoulos
NG, Missitzis I, Stathopoulos GP and Papamichail M: Tumor specific
cytolysis by tumor infiltrating lymphocytes in breast cancer.
Cancer. 74:1275–1282. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Knuth A, Wolfel T and Meyer zum
Buschenfelde KH: T cell responses to human malignant tumors. Cancer
Surv. 13:39–52. 1992.PubMed/NCBI
|
|
9
|
Desombere I, Meuleman P, Rigole H, et al:
The interferon gamma secretion assay: a reliable tool to study
interferon gamma production at the single cell level. J Immunol
Methods. 286:167–185. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Straathof KC, Bollard CM, Popat U, et al:
Treatment of nasopharyngeal carcinoma with Epstein-Barr
virus-specific T lymphocytes. Blood. 105:1898–1904. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Marijt E, Wafelman A, van der Hoorn M, et
al: Phase I/II feasibility study evaluating the generation of
leukemia-reactive cytotoxic T lymphocyte lines for treatment of
patients with relapsed leukemia after allogeneic stem cell
transplantation. Haematologica. 92:72–80. 2007. View Article : Google Scholar
|
|
12
|
Toh U, Yamana H, Sueyoshi S, et al:
Locoregional cellular immunotherapy for patients with advanced
esophageal cancer. Clin Cancer Res. 6:4663–4673. 2000.PubMed/NCBI
|
|
13
|
Yee C, Thompson JA, Byrd D, et al:
Adoptive T cell therapy using antigen-specific CD8+ T
cell clones for the treatment of patients with metastatic melanoma:
in vivo persistence, migration, and antitumor effect of transferred
T cells. Proc Natl Acad Sci USA. 99:16168–16173. 2002.
|
|
14
|
da Silva SD, Hier M, Mlynarek A, Kowalski
LP and Alaoui-Jamali MA: Recurrent oral cancer: current and
emerging therapeutic approaches. Front Pharmacol.
3:1492012.PubMed/NCBI
|
|
15
|
Kawakami Y, Eliyahu S, Delgado CH, et al:
Cloning of the gene coding for a shared human melanoma antigen
recognized by autologous T cells infiltrating into tumor. Proc Natl
Acad Sci USA. 91:3515–3519. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Saitoh S, Kurisaka M, Mori K, Maeda N and
Fujimoto S: Induction of specific cytotoxic T lymphocytes against
autologous brain tumor by crossreactive allo-tumor cell
stimulation. Jpn J Cancer Res. 88:289–295. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Restifo NP, Dudley ME and Rosenberg SA:
Adoptive immunotherapy for cancer: harnessing the T cell response.
Nat Rev Immunol. 22:269–281. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rosenberg SA, Spiess P and Lafreniere R: A
new approach to the adoptive immunotherapy of cancer with
tumor-infiltrating lymphocytes. Science. 233:1318–1321. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rosenberg SA, Yang JC, Sherry RM, et al:
Durable complete responses in heavily pretreated patients with
metastatic melanoma using T-cell transfer immunotherapy. Clin
Cancer Res. 17:4550–4557. 2011. View Article : Google Scholar
|
|
20
|
Khammari A, Nguyen JM, Pandolfino MC, et
al: Long-term follow-up of patients treated by adoptive transfer of
melanoma tumor-infiltrating lymphocytes as adjuvant therapy for
stage III melanoma. Cancer Immunol Immunother. 56:1853–1860. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ratto GB, Zino P, Mirabelli S, et al: A
randomized trial of adoptive immunotherapy with tumor-infiltrating
lymphocytes and interleukin-2 versus standard therapy in the
postoperative treatment of resected nonsmall cell lung carcinoma.
Cancer. 78:244–251. 1996. View Article : Google Scholar
|
|
22
|
Agra IM, Filho JG, Martins EP and Kowalski
LP: Second salvage surgery for re-recurrent oral cavity and
oropharynx carcinoma. Head Neck. 32:997–1002. 2010. View Article : Google Scholar : PubMed/NCBI
|